Abstract
Real winery wastewater (WW), with a high concentration of organic matter (OM), was treated using Fenton (FP), photo-Fenton (PFP), sono-Fenton (SFP), and sono-photo-Fenton processes (SPFP), with the primary objective of removing phenolic compounds (PhCs). Although beneficial to human health, these compounds are considered recalcitrant and toxic to aquatic organisms, posing significant environmental risks if discharged into water bodies. They can also reduce the efficiency of biological treatment processes. After physicochemical characterization and two hours of treatment, the removal efficiencies achieved by the FP, PFP, SFP, and SPFP processes were 29.35%, 41.30%, 28.82%, and 33.95% for PhCs; 27.88%, 31.51%, 23.19%, and 29.29% for chemical oxygen demand (COD); and 12.53%, 13.92%, 9.28%, and 10.62% for dissolved organic carbon (DOC), respectively. The degradations achieved by SFP and SPFP were lower than those of FP and PFP, respectively, due to reactions that scavenge hydroxyl radicals. Treatment of a gallic acid (GA) solution, used as a model compound for PhCs, exhibited similar trends, indicating that the lower efficiency in processes involving ultrasound is not due to the OM in the effluent, but rather the interaction between ultrasound (US) and H2O2, which reduces hydroxyl radical concentration. However, under the conditions of the wastewater used, the technologies applied did not completely reduce the parameters analyzed, being recommended as pre- or post-treatment, and combined with other processes.
1. Introduction
Winery wastewaters (WWs) are rich in natural phenolic compounds (PhCs), also known as polyphenols, which are extracted from the skin and seeds of grapes. These compounds have an aromatic structure with at least one aromatic ring bonded to hydroxyl groups [1], such as phenolic acids, stilbenes, flavonoids, anthocyanins (responsible for the color of red wine), and tannins, formed by the polymerization of flavonoids [2]. These substances are beneficial to human health due to their anti-inflammatory, antioxidant, antimicrobial, and anti-allergic properties [3]. However, when present in the natural environment through agro-industrial wastewater discharges, they can cause environmental damage by interacting with aquatic organisms [2].
Polyphenols and flavonoids in grape seed extracts have proven to inhibit the growth of bacteria and fungi because they interact with cell membranes, affect protein transport, and inhibit enzyme production in these microorganisms [4]. Low concentrations of resveratrol, the most common stilbene in wines, are toxic to the embryos of the fish species Danio rerio [5]. Additionally, polyphenols can delay and reduce the growth of plant species [6]. These examples illustrate how PhCs in wine can negatively impact the natural environment if the wastewater containing them is discharged into watercourses without proper treatment. Furthermore, PhCs are considered refractory, meaning they resist conventional degradation methods [7].
In addition to PhCs, WWs contain other substances that contribute to high organic matter (OM) concentrations, including alcohols, sugars, organic acids, and residues from yeasts and bacteria [8]. Therefore, WW treatment must focus on OM removal, with special attention to PhCs.
WWs are primarily generated during the cleaning of tanks and equipment and during the loss and disposal of wine. They are produced seasonally, because vinification is not a continuous process; it occurs in stages throughout the year, beginning with harvesting, must preparation, and fermentation, which generate large amounts of wastewater, followed by sedimentation, clarification, and filtration, which generate less wastewater, and finally stabilization and aging, where the wine remains in bottles and does not generate wastewater [9,10]. Consequently, the installation of biological treatment systems, such as the conventional activated sludge process (the most applied treatment process against WWs [11], which requires a homogeneous and constant feed to maintain the activity of microorganisms responsible for oxidation) may not be efficient for wineries. Moreover, the properties of PhCs can decrease the efficiency of biological processes [8].
Currently, wineries in the region studied hire companies specialized in effluent treatment from other regions to collect and treat the waste generated. Consequently, in addition to the cost of treatment, transportation costs increase the wineries’ expenses. Another alternative for the addition of economic and environmental values to the effluents would be the recovery of PhCs, due to their already mentioned benefits for human health. This could be conducted through extraction, adsorption or membrane filtration processes. However, according to Santos et al. [12], studies and demonstrations are still needed to make recovery feasible on a larger scale.
An alternative for the treatment of this type of wastewater is Advanced Oxidation Processes (AOPs), which rely on the generation of hydroxyl radicals (OH•), a highly oxidizing chemical species capable of degrading a wide range of organic compounds, including PhCs. These processes can transform substances into simpler, more biodegradable, and less toxic compounds, or even mineralize them into carbon dioxide, water, and other inorganic ions [13].
The Fenton process (FP) is considered one of the most efficient AOPs for treating recalcitrant compounds [14]. It is based on the generation of hydroxyl radicals through the reaction between hydrogen peroxide (H2O2) and ferrous ions (Fe2+) in an acidic medium (around pH 3), as shown in Equation (1). The process can be easily enhanced by combining it with physical agents such as ultraviolet (UV) light (photo-Fenton), electricity (electro-Fenton), and ultrasound (US) waves (sono-Fenton), leading to increased production of hydroxyl radicals. In the photo-Fenton process (PFP), ferric ions (Fe3+) in the form of [Fe(OH)]2+ react with UV light, reducing to Fe2+ (Equation (2)), which in the presence of H2O2 continues the Fenton reaction cyclically. The PFP also occurs the photolysis of H2O2, producing additional hydroxyl radicals (Equation (3)) [15].
Fe2+ + H2O2 → Fe3+ + OH• + OH−
[Fe(OH)]2+ + hν → Fe2+ + OH•
H2O2 + hν → 2 OH•
However, in the FP and its combinations, certain parameters, such as pH, H2O2, and Fe2+ concentrations, must be carefully controlled. If any of these components are excessive or insufficient, other reactions can take place, decreasing the efficiency of the processes, such as those shown in Equations (4)–(7), which promote hydroxyl radical scavenging [16,17].
OH• + H+ + e− → H2O
H2O2 + OH• → HO2• + H2O
OH• + OH• → H2O2
Fe2+ + OH• → Fe3+ + OH−
Another AOP that can be combined with the FP and PFP to increase the efficiency of these treatments is sonolysis, as in the sono-Fenton process (SFP) and the sono-photo-Fenton process (SPFP) [18,19]. The application of US waves directly to the liquid medium promote the acoustic cavitation effect, where microbubbles form and implode violently under high temperature and pressure, causing the degradation of volatile compounds that permeate into these bubbles [20], as well as the degradation of water molecules, resulting in the production of hydroxyl radicals, as shown in Equation (8) [21].
H2O + ))) → OH• + H•
Melchiors and Freire [22] reviewed WW treatment, assessing the yields of OM, color, PhCs, nitrogen, and phosphorus removals through physicochemical, biological, constructed wetlands, microbial fuel cells, membrane filtration and separation processes, and AOPs, highlighting the higher rates of COD, PhCs, and color removal by AOPs.
Several AOPs have already been applied to WW treatment, such as ozonation [23], heterogeneous photocatalysis [24,25], and the FP and its combinations [26,27,28,29,30,31], achieving high removals of PhCs and OM. However, many of these studies treated WWs with low or intermediate amounts of OM, making comparisons with WWs with high OM concentrations difficult.
Additionally, some reviews have pointed out that the SPFP can reduce the amount of iron needed and produce more hydroxyl radicals through water sonolysis, increasing degradation efficiency and reducing the process costs [18,32,33]. The FP combined with US has already been applied to the degradation of other substances, such as phenols [34], and other agro-industrial wastewaters, such as tannery [35] and cork wastewater [36], but no studies have been found on WW treatment and its PhCs degradation.
As noted by Ribeiro et al. [37], there is currently a scarcity of results, and further research using real effluents with more complex matrices, rather than synthetic compound solutions, is needed to better support the decision-making in treatment plants. Synthetic solutions, even when prepared with compounds similar to those in real wastewater, lack unidentified compounds that may interact with the reagents. This can lead to results and conclusions that do not accurately reflect practical applications of the proposed methodologies.
Thus, the aim of this study was to apply US combined with the FP and PFP in real WW, with a high OM concentration, and assess the contribution of each component—chemical (H2O2 and Fe2+) and physical (UV and US)—to the degradation of PhCs and the removal of OM and color, providing results that can help in the decision for the treatment of these types of wastewaters. Additionally, a gallic acid (GA) solution was treated by the same processes as a PhC model to eliminate the influence of OM.
2. Materials and Methods
2.1. Chemicals
The Fenton treatments were carried out using ferrous sulfate (FeSO4·7H2O, 99%) and hydrogen peroxide (H2O2, 30% w/w), both purchased from Dinâmica (São Paulo, Brazil). Sulfuric acid (H2SO4, 95% w/w), sodium hydroxide (NaOH, 99%) for pH adjustment, and GA (C7H6O5, 98%) were obtained from Vetec (São Paulo, Brazil).
2.2. Winery Wastewater
WW was collected from a winery located in São Roque, São Paulo, Brazil, during the beginning of the winemaking process (around February in the Southern Hemisphere). To characterize the effluent, analyses were performed in triplicate for total phenolic compounds (TPhCs), chemical oxygen demand (COD), dissolved organic carbon (DOC), pH, real color, turbidity, electrical conductivity (EC), and solid series (See Section 2.3).
2.3. Analytical Methods
The analyses for solid series, COD, and DOC were performed according to standard methods [38]. COD was determined using the closed reflux/colorimetric method, using a potassium dichromate (K2Cr2O7, 99%, Vetec, Brazil) standard solution and a Bell Photonics SP 2000 UV spectrophotometer (Bell Photonics, São Paulo, Brazil). DOC was measured using the high-temperature combustion method with direct injection of filtered samples (0.45 μm) into an Analytik Jena Multi N/C 3100 analyzer (Analytik Jena, Jena, Germany).
The analyses of TPhCs were conducted using a commercial Folin phenol reagent solution (Alphatec, São Paulo, Brazil), based on the reaction of the tungstophosphoric and molybdophosphoric acids with the aromatic hydroxyl groups, forming a blue color. This method is based on the same principle as the colorimetric method for tannins and lignins in standard methods [38]. The samples were measured at 760 nm, using a Hach DR 2800 spectrophotometer (HACH COMPANY World Headquarters, Loveland, CO, USA), and the results were expressed in mg of GA per liter (mgGA L−1).
Real color and turbidity were determined using Policontrol (São Paulo, Brazil) colorimeter and turbidimeter, while pH and electrical conductivity were measured with Instrutemp (São Paulo, Brazil) pH meter and conductivity meter.
To remove H2O2 prior to analyses of treated effluent, sodium sulfite (Na2SO3, 98%, Dinâmica, Brazil) was added to the samples [28].
2.4. Experimental Procedure
The treatments were performed using 150 mL of WW in a 300 mL beaker placed on a magnetic stirrer and set to 200 rpm. First, the required amount of FeSO4·7H2O was added and dissolved, followed by the addition of H2O2. The pH was maintained between 2.8 and 3.0 using sulfuric acid solution (1.0 and 0.1 mol L−1), and the treatment was carried out for 2 h.
For the application of UV light, a dark chamber, equipped with five UV lamps (28 W each) emitting at a peak wavelength of 380 nm, was built; the lamps were arranged in the shape of a cross, in the upper part of the interior of the chamber. Ultrasound (US) was applied using a 13 mm ultrasonic probe (Eco-sonics QR500, Indaiatuba, Brazil) operating at a frequency of 20 kHz and a power of 400 W, in pulsed mode (3 min on and 3 min off) throughout the 2 h treatment period. An ice bath was used to maintain the temperature at approximately 25 °C.
Optimal amounts of Fenton’s reagents were first determined using the PFP process, combining 2.0, 4.0, and 6.0 mmol L−1 of Fe2+ with 200 and 300 mmol L−1 of H2O2. Treatments with FP, SFP, and SPFP were then performed in triplicate, with TPhCs concentrations measured every 15 min and final concentrations of COD, DOC, and color recorded. Additional experiments were conducted using individual components (Fe2+, H2O2, UV, and US) and pairwise combinations (Fe2+/UV, H2O2/UV, Fe2+/US, and H2O2/US) under the same conditions to assess their effects on the processes.
Lastly, a GA solution at a concentration similar to the TPhCs concentration in the WW was treated using the four processes to evaluate the impact of the WW’s organic matrix on the removal results.
All the treatments were performed in triplicate and the results were evaluated using the Analysis of Variance (ANOVA) with the BioEstat software (Brazil), version 5.3, and Tukey’s test to compare the averages at a 95% confidence interval.
3. Results and Discussion
3.1. Winery Wastewater Characterization
The results of the WW characterization are presented in Table 1. The WW was collected at the beginning of the winemaking process, during the cleaning of vinification and storage tanks. As a result, the effluent primarily consists of residues from the finished wine and the prepared must. Due to the high content of organic compounds from the wine and grapes, the WW exhibits significant organic matter (OM), as indicated by the COD (45,221 mg L−1) and DOC (8040 mg L−1) values. These values, along with the total solids (10,912 mg L−1), are close to the maximum levels typically found for this type of effluent [9]. The WW also showed high levels of TPhCs (93.70 mgAG L−1), an acidic pH (3.36), and a red color.

Table 1.
WW characteristics.
3.2. Optimization of Fenton’s Reagents
To determine the optimal concentrations of Fenton’s reagents, which would maximize the removal of TPhCs and OM from WW, while minimizing reagent use and offering economic benefits, treatments were conducted using 2.0, 4.0, and 6.0 mmol L−1 Fe2+ and 200 and 300 mmol L−1 H2O2, combining all these concentrations, by the PFP. The results of TPhCs and COD removals are presented in Figure 1.
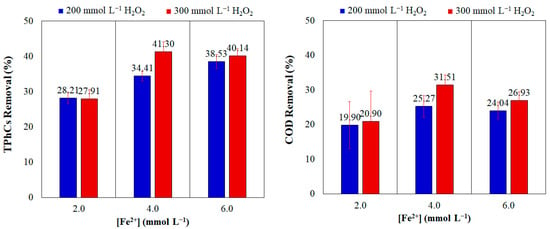
Figure 1.
Percentage removal of TPhCs and COD from WW by PFP using variable concentrations of Fenton’s reagents. (pH = 3.00; t = 120 min; v = 150 mL; T = 25 °C).
The maximum TPhCs and COD removals were achieved using 4.0 mmol L−1 Fe2+ and 300 mmol L−1 H2O2, with reductions of 41.30% and 31.51% from the initial concentrations, respectively. The results also indicated that increasing the H2O2 concentration led to higher percentage removals for all Fe2+ concentrations, except for TPhCs removal with 2.0 mmol L−1 Fe2+, due to increased hydroxyl radical generation. However, increasing the Fe2+ concentration did not consistently enhance removal and using 6.0 mmol L−1 Fe2+ resulted in lower removals than 4.0 mmol L−1 Fe2+. Therefore, to optimize PFP performance, a balance between H2O2 and Fe2+ concentrations is essential, as an excess of either reagent can lead to other reactions that decrease hydroxyl radical generation.
3.3. Winery Wastewater Treatment
Using the optimized concentrations of Fenton’s reagents, the WW was treated over 120 min with the FP, PFP, SFP, and SPFP processes. All processes successfully reduced the TPhCs concentration, with the highest degradation rates occurring in the first few minutes, followed by a slower reduction thereafter, as shown in Figure 2.
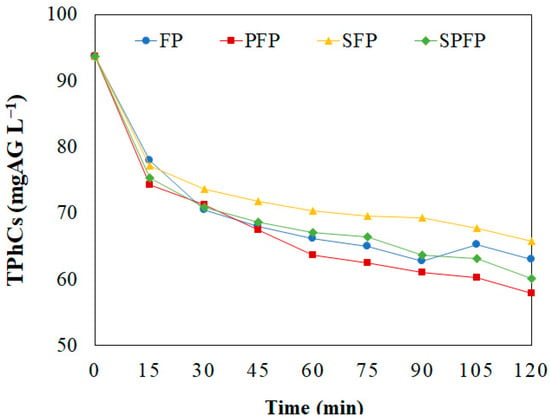
Figure 2.
TPhCs concentration in WW over time using the FP, PFP, SFP and SPFP. ([Fe2+] = 4.0 mmol L−1; [H2O2] = 300.0 mmol L−1; pH = 3.00; v = 150 mL; T = 25 °C).
With the application of these processes, it was possible to remove great amounts of COD, DOC and color, and the removal percentages of these analyzed parameters are presented in Figure 3.
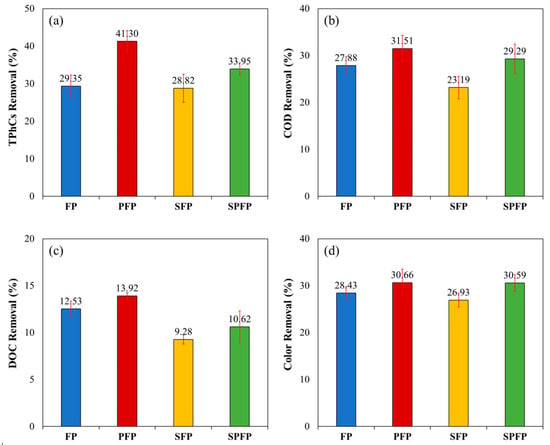
Figure 3.
Percentage removal of (a) TPhCs, (b) COD, (c) DOC and (d) color from WW by FP, PFP, SFP and SPFP. ([Fe2+] = 4.0 mmol L−1; [H2O2] = 300 mmol L−1; pH = 3.00; t = 120 min; v = 150 mL; T = 25 °C).
Comparing these results, the FP was able to remove 29.35, 27.88, 12.53, and 28.43% of the TPhCs, COD, DOC, and color, respectively. These values were lower than those observed for the PFP, which achieved removals of 41.30, 31.51, 13.92, and 30.66%. The increased efficiency of the PFP can be attributed to the application of UV light, which enhances the formation of hydroxyl radicals through the regeneration of Fe3+ to Fe2+ and H2O2 photolysis, thereby increasing the degradation of the compounds.
However, when US waves were applied in the SFP, the removal results (28.82, 23.19, 9.28, and 26.93%) were lower than those of the FP. In the SPFP, the removal results (33.95, 29.29, 10.62, and 30.59%) exceeded those of both the FP and SFP, except for the DOC removal by the FP, which remained lower than that of the PFP. This effect is caused by other reactions that occur when US is combined with H2O2, as shown in Equations (5) and (6). In the presence of H2O2, the hydroxyl radicals generated by water sonolysis can be scavenged or recombined with H2O2 [39], which reduces degradation when the H2O2 concentration is not at the optimal level [32].
Furthermore, PhCs have a high boiling point and do not volatilize into cavitation bubbles; therefore, they do not degrade by sonolysis, but only through the actions of hydroxyl radicals in the liquid phase [21]. A statistical analysis using ANOVA and Tukey’s test also indicated that, compared to untreated WW, all treatments exhibited significant differences in the removal of all parameters, with a p-value < 0.01 in all cases. For the treatments, a significant difference was observed between the PFP and SFP treatments, with a p-value of <0.01 for the removals of TPhCs, COD, and DOC; however, no significant difference was found in color removal between the treatments.
It was also noted that the COD removal percentages were higher than the DOC removal percentages, indicating that the FP and its combinations were more effective in transforming the initial compounds into more degradable substances than in mineralizing them. At the end of the treatments, the color changed from red to yellow, suggesting that PhCs, the compounds responsible for the red color of the wine and the WW, were degraded.
Compared to other studies that also treated WWs using FPs and their combinations [26,27,28,29,30,31], the removal results presented here were lower; however, these studies utilized either WWs with lower concentrations of organic matter or pre-treated wastewaters from other processes. Nevertheless, in this work, the amounts of TPhCs, COD, and DOC removed in concentration units were higher than those reported in previous research.
Nonetheless, the applied treatments are recommended as post-treatments [40], serving as a polishing step for pre-treated WWs, where the organic load and the concentration of PhCs are typically lower. However, some drawbacks need to be overcome, such as the final concentrations of iron ions and H2O2, as well as pH correction. These issues can be mitigated through the use of heterogeneous Fenton processes and homogeneous Fenton processes at circumneutral pH. Additionally, it is essential to evaluate the toxicity of the byproducts generated during the reactions.
3.4. Component Evaluation
Figure 4 shows the removal percentages of TPhCs, COD, DOC, and color with treatments using individual components (Fe2+, H2O2, UV, and US) and paired combinations (Fe2+/UV, H2O2/UV, Fe2+/US, and H2O2/US) to assess their effects on the treatments.
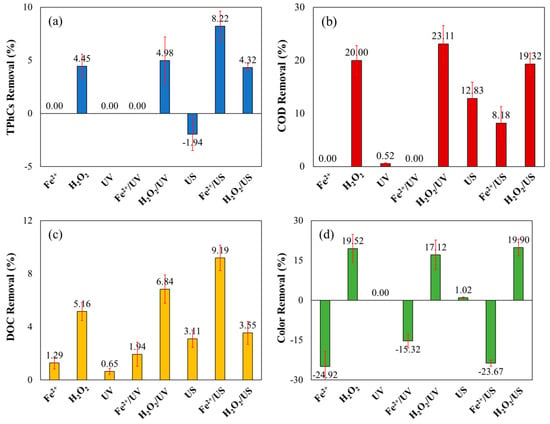
Figure 4.
Percentage removal of (a) TPhCs, (b) COD, (c) DOC and (d) color from WW using individual components and paired combinations. ([Fe2+] = 4.0 mmol L−1; [H2O2] = 300 mmol L−1; pH = 3.00; t = 120 min; v = 150 mL; T = 25 °C).
The results showed that Fe2+ alone did not change TPhCs or COD concentrations and only resulted in 1.29% DOC removal, but this value may be related to losses during sample filtration for analyses. This was expected, as iron alone does not cause significant effects. H2O2 alone removed 4.45, 20.00, and 5.16% of TPhCs, COD, and DOC, respectively, due to its oxidizing properties. The effect of the combination between these two reagents is remarkable because of the higher removal results of the FP due to the generation of hydroxyl radicals (Figure 3).
UV light alone reduced COD and DOC by only 0.52% and 0.65%, because some compounds present in the WW may be more sensitive and decomposed by the UV light, but the PhCs were not removed. Combining UV with Fe2+ removed only 1.94% of DOC, with no effect on TPhCs or COD. However, combining UV with H2O2 increased TPhCs, COD, and DOC removals to 4.98, 23.11, and 6.84%, respectively, due to enhanced hydroxyl radical generation via H2O2 photolysis.
US alone (sonolysis) removed 12.83% of COD and 3.11% of DOC, because some compounds present in the wastewater can be degraded by the application of US waves, mainly the most volatiles. However, the TPhCs concentration increased (the negative value of removal in Figure 4a), possibly because the generated hydroxyl radicals bind to the aromatic rings of PhCs and increase the regions that react to Folin’s reagent, as reported by Mosteo et al. [41].
US combined with Fe2+ improved TPhCs and DOC removals to 8.22% and 9.19%, respectively, but COD removal decreased to 8.18%. This is because hydroxyl radicals generated by US can convert into H2O2 (Equation (6)), initiating Fenton’s reaction [42]. However, US combined with H2O2 resulted in lower removals of TPhCs (4.32%), COD (19.32%), and DOC (3.55%) compared to H2O2 alone. These results show that the US addition decreases the hydroxyl radical generation, like in the SFP and SPFP, which provided lower removal results than the FP and PFP.
Finally, the results of color removal showed that the addition of only Fe2+, even when combined with UV light and US, increased the color results (indicated by negative removal values in Figure 4d) due to interactions between the wine compounds and iron, resulting in red being the final remaining color. In contrast, the addition of H2O2 alone removed 19.52% of the color, with minor variations when combined with US and UV, yielding removal percentages of 19.90% and 17.12%, respectively, leading to yellow being the final color. Therefore, the presence of H2O2 is essential for color reduction, which can be further enhanced by the FP and its combinations. UV alone did not contribute to color removal, nor did it effectively remove PhCs.
3.5. Gallic Acid Solution Treatment
The treatment of a 5.9 × 10−4 mol L−1 (100 mg L−1) GA solution with FP has been reported by Benitez et al. [43], achieving a 79% degradation in 40 min, using 2.5 × 10−5 mol L⁻1 Fe2+ and 2.5 × 10−3 mol L−1 H2O2 at pH 3.00 and a volume of 200 mL. Using these concentrations and the same treatments applied to WW, the GA concentration over 60 min of treatment (shown in Figure 5) indicates that, similarly to WW treatment, the highest degradation rate occurred in the initial minutes and decreased over time. The applied processes did not cause any differences in this behavior, the only differences presenting in the final removal percentages.
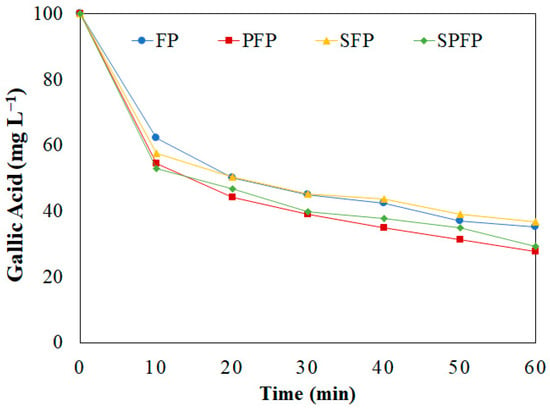
Figure 5.
GA concentration over time by the FP, PFP, SFP and SPFP. ([GA] = 100 mg L−1; [Fe2+] = 2.5 × 10−5 mol L−1; [H2O2] = 2.5 × 10−3 mol L−1; pH = 3.00; v = 200 mL; T = 25 °C).
The highest removal percentage was 72.73% (Figure 6) for the PFP, followed by 70.32, 65.00 and 63.51% for the SPFP, FP, and SFP, respectively. These removal percentages exceeded those for TPhCs obtained in WW treatment (41.30, 33.95, 29.35, and 28.82% in the same order of treatments), with an initial concentration of 93.70 mgAG L−1 of TPhCs.
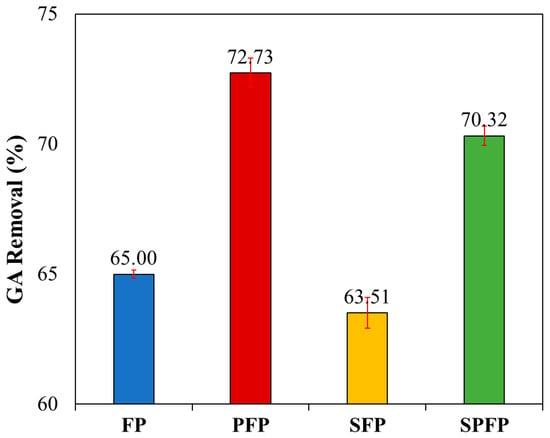
Figure 6.
GA removal percentage by the FP, PFP, SFP and SPFP. ([AG] = 100 mg L−1; [Fe2+] = 2.5 × 10−5 mol L−1; [H2O2] = 2.5 × 10−3 mol L−1; pH = 3.00; t = 60 min; v = 200 mL; T = 25 °C).
Statistical analysis revealed significant differences among treatments, with all p-values < 0.01, when comparing treatment results using ANOVA and Tukey’s test at a 95% confidence interval, indicating that the addition or absence of components affects GA degradation. These results demonstrate that the presence of other organic compounds, such as those found in WW, impacts the efficiency of TPhCs removal, since they compete for the consumption of Fenton’s reagents. Furthermore, GA degradation exhibited similar trends to TPhCs, COD, DOC, and color removals in WW. In contrast to the US application, the absence of other organic compounds did not improve the removal efficiency of SFP and SPFP, compared to FP and PFP, respectively.
The same effect was reported in the treatment of amoxicillin, which also contains a phenolic group in its structure and is considered a persistent pollutant. In the treatment of a 10 mg L−1 amoxicillin solution, complete degradation was first achieved by PFP, followed by SPFP, solar photo-Fenton, FP, and SFP, using optimized amounts of Fenton’s reagents and a 40 kHz frequency US. The authors attributed the inhibitory effect caused by the combination of US with Fenton’s reagents to the formation of species that compete for H2O2 [44].
These experiments, conducted without interference from other compounds, confirm that the applied processes are effective in degrading natural PhCs. However, US application at a frequency of 20 kHz does not yield positive effects on treatments when combined with Fenton’s reagents due to reactions that scavenge hydroxyl radicals. Moreover, the high amount of OM present in WW limits the efficiency of removals due to the amounts of Fenton’s reagents required for total degradation, which also promotes the occurrence of these undesired reactions.
4. Conclusions
The treatments using FPs and their combinations with UV light and US waves were effective in partially removing TPhCs, COD, DOC, and color from real WW. However, the removals were not as high for all of the processes due to the elevated concentration of OM, compared to other published studies utilizing lower concentrations.
The most efficient treatment was PFP, followed by SPFP, FP and SFP. The application of US did not enhance the removals, compared to the respective processes without US, due to the interaction between the generated hydroxyl radicals and the added hydrogen peroxide, which promotes the scavenging effect of hydroxyl radicals, thus reducing process efficiency.
Nonetheless, the reductions achieved can enhance the efficiency of subsequent biological treatments by decreasing the concentrations of PhCs and OM. Alternatively, these processes can be applied as post-treatments for wastewater with lower pollutant concentrations.
However, some drawbacks need to be overcome, such as the optimization and the final concentrations of iron ions and H2O2, as well as pH correction. These issues can be mitigated through the use of heterogeneous Fenton processes and homogeneous Fenton processes at circumneutral pH. Additionally, it is essential to evaluate the toxicity of the byproducts generated during the reactions, as well as the development processes for the recovery and valorization of PhCS.
Author Contributions
R.A.R.: Conceptualization, Methodology, Validation, Formal analysis, Investigation. Writing—Original Draft, Writing—Review and Editing, Visualization. M.B.M.d.C.: Methodology, Resources. P.S.T.: Conceptualization, Writing—Review and Editing, Supervision, Project Administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The authors are grateful to the Federal Institute of Education, Science and Technology of São Paulo State, Brazil and the UNESP Institute of Science and Technology of Sorocaba, Brazil, for the support and availability of its laboratories for the development of the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic compounds in water: Sources, reactivity, toxicity and treatment methods. In Phenolic Compounds: Natural Sources, Importance and Applications; Soto-Hernández, M., Ed.; IntechOpen: London, UK, 2017; pp. 419–443. [Google Scholar] [CrossRef]
- Butnariu, M.; Butu, A. Qualitative and quantitative chemical composition of wine. In Quality Control in the Beverage Industry; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 385–417. [Google Scholar] [CrossRef]
- Perveen, S.; Al-Taweel, A.M. Phenolic compounds from the natural sources and their cytotoxicity. In Phenolic Compounds: Natural Sources, Importance and Applications; Soto-Hernández, M., Ed.; IntechOpen: London, UK, 2017; pp. 29–60. [Google Scholar] [CrossRef]
- Ghouila, Z.; Laurent, S.; Boutry, S.; Elst, L.V.; Natache, F.; Muller, R.N.; Baaliouamer, A. Antioxidant, antibacterial and cell toxicity effects of polyphenols from Ahmeur Bouamer grape seed extracts. J. Fundam. Appl. Sci. 2017, 9, 392–410. [Google Scholar] [CrossRef]
- Cavalcante, A.K.; Lopes-Ferreira, M.; Rogero, S.O.; Rogero, J.R. Evaluation of resveratrol toxicity in the embryolarval stage of Danio rerio fish. Ecotoxicol. Environ. Contamin. 2017, 12, 133–139. [Google Scholar] [CrossRef]
- Mosse, K.P.M.; Patti, A.F.; Christen, E.W.; Cavagnaro, T.R. Winery wastewater inhibits seed germination and vegetative growth of common crop species. J. Hazard. Mater. 2010, 180, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Brandão, Y.B.; Oliveira, J.G.C.; Benachour, M. Phenolic Wastewaters: Definitions, sources and treatment processes. In Phenolic Compounds: Natural Sources, Importance and Applications; Soto-Hernández, M., Ed.; IntechOpen: London, UK, 2017; pp. 325–342. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S. A comprehensive approach to winery wastewater treatment: A review of the state-of-art. Desalin. Water Treat. 2016, 57, 3011–3028. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Li Puma, G.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef]
- Latessa, S.H.; Hanley, L.; Tao, W. Characteristics and practical treatment technologies of winery wastewater: A review for wastewater management at small wineries. J. Environ. Manag. 2023, 342, 118343. [Google Scholar] [CrossRef] [PubMed]
- Bolzonella, D.; Papa, M.; Da Ros, C.; Muthukumar, L.A.; Rosso, D. Winery wastewater treatment: A critical overwiew of advanced biological processes. Crit. Rev. Biotechnol. 2019, 39, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.R.F.; Rodrigues, R.P.; Quina, M.J.; Gando-Ferreira, L.M. Recovery of value-added compounds from winery wastewater: A review and bibliometric analysis. Water 2023, 15, 1110. [Google Scholar] [CrossRef]
- Ameta, S.C. Introduction. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–12. [Google Scholar] [CrossRef]
- Ameta, R.; Chohadia, A.K.; Jain, A.; Punjabi, P.B. Fenton and photo-Fenton processes. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 49–87. [Google Scholar] [CrossRef]
- Samadi, M.T.; Rezaie, A.; Ebrahimi, A.A.; Panahi, A.H.; Kargarian, K.; Abdipour, H. The utility of ultraviolet beam in advanced oxidation-reduction processes: A review on the mechanism of processes and possible production free radicals. Environ. Sci. Pollut. Res. 2024, 31, 6628–6648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Proc. Safe Environ. Protect. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Babuponusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- García-Espinoza, J.D.; Treviño-Reséndez, J.; Robles, I.; Acosta-Santoyo, G.; Godínez, L.A. A review of electro-Fenton and ultrasound processes: Towards a novel integrated technology for wastewater treatment. Environ. Sci. Pollut. Res. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ameta, S.C.; Ameta, R.; Ameta, G. Sonochemistry: An Emerging Green Technology, 1st ed.; Apple Academic Press: Toronto, ON, Canada, 2018. [Google Scholar]
- Torres-Palma, R.A.; Serna-Galvis, E.A. Sonolysis. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 177–213. [Google Scholar] [CrossRef]
- Melchiors, E.; Freire, F.B. Winery wastewater treatment: A systematic review of traditional and emerging technologies and their efficiencies. Environ. Process. 2023, 10, 43. [Google Scholar] [CrossRef]
- Lucas, M.S.; Peres, J.A.; Li Puma, G. Treatment of winery wastewater by ozone-based advanced oxidation processes (O3, O3/UV and O3/UV/H2O2) in a pilot-scale bubble column reactor and process economics. Separ. Purif. Technol. 2010, 72, 235–241. [Google Scholar] [CrossRef]
- Augustina, T.E.; Ang, H.M.; Pareek, V.K. Treatment of winery wastewater using a photocatalytic/photolytic reactor. Chem. Eng. J. 2008, 135, 151–156. [Google Scholar] [CrossRef]
- Navarro, P.; Sarasa, J.; Sierra, D.; Esteban, S.; Ovelleiro, J.L. Degradation of wine industry wastewaters by photocatalytic advanced oxidation. Water Sci. Technol. 2005, 51, 113–120. [Google Scholar] [CrossRef]
- Esteves, B.M.; Morales-Torres, S.; Maldonado-Hódar, F.J.; Madeira, L.M. Catalytic peroxidation of winery wastewater contaminants using activated carbon-supported magnetite nanoparticles. J. Water Process Eng. 2024, 58, 104772. [Google Scholar] [CrossRef]
- Ferreira, R.; Gomes, J.; Martins, R.C.; Costa, R.; Quinta-Ferreira, R.M. Winery wastewater treatment by integrating Fenton’s process with biofiltration by Corbicula fluminea. J. Chem. Technol. Biotechnol. 2018, 93, 333–339. [Google Scholar] [CrossRef]
- Santos, C.; Lucas, M.S.; Dias, A.A.; Bezerra, R.M.F.; Peres, J.A.; Sampaio, A. Winery wastewater treatment by combination of Cryptococcus laurentii and Fenton’s reaction. Chemosphere 2014, 117, 53–58. [Google Scholar] [CrossRef]
- Velegraki, T.; Mantzavinos, D. Solar photo-Fenton treatment of winery effluents in a pilot photocatalytic reactor. Catal. Today 2015, 240, 153–159. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Fatta-Kassinos, D. Solar photo-Fenton oxidation against the bioresistant fractions of winery wastewater. J. Environ. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Guimarães, V.; Lucas, M.S.; Peres, J.A. Combination of adsorption and heterogeneous photo-Fenton processes for the treatment of winery wastewater. Environ. Sci. Pollut. Res. 2019, 26, 31000–31013. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Elahinia, A.; Vasseghian, Y.; Dragoi, E.N.; Omind, F.; Khaneghah, A.M. A review on pollutants removal by sono-photo-Fenton processes. J. Environ. Chem. Eng. 2020, 8, 104330. [Google Scholar] [CrossRef]
- Liu, S.; Long, Z.; Liu, H.; Wang, Y.; Zhang, J.; Zhang, G.; Liang, J. Recent advances in ultrasound-Fenton/Fenton-like technology for degradation of aqueous organic pollutants. Chemosphere 2024, 352, 41286. [Google Scholar] [CrossRef] [PubMed]
- Yilds, S.; Sentürk, I.; Canbaz, G.T. Degradation of phenol and 4-chlorophenol from aqueous solution by Fenton, photo-Fenton, sono-Fenton, and sono-photo-Fenton methods. J. Iran. Chem. Soc. 2022, 20, 231–237. [Google Scholar] [CrossRef]
- Korpe, S.; Bethi, B.; Sonawane, S.H.; Jayakumar, K.V. Tannery wastewater treatment by cavitation combined with advanced oxidation process (AOP). Ultrason. Sonochem. 2019, 59, 104723. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yuste-Córdoba, F.J.; Cintas, P.; Wu, Z.; Boffa, L.; Mantegna, S.; Cravotto, G. Effects of ultrasonic and hydrodynamic cavitation on the treatment of cork wastewater by flocculation and Fenton processes. Ultrason. Sonochem. 2018, 40, 3–8. [Google Scholar] [CrossRef]
- Ribeiro, J.P.; Sarinho, L.; Nunes, M.I. Application of life cycle assessment to Fenton processes in wastewater treatment—A review. J. Water Process Eng. 2024, 57, 104692. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Chakma, S.; Moholkar, V.S. Physical Mechanism of sono-Fenton process. AIChE J. 2013, 59, 4303–4313. [Google Scholar] [CrossRef]
- Davididou, K.; Frontistis, Z. Advanced oxidation processes for the treatment of winery wastewater: A review and future perspectives. J. Chem. Technol. Biotechnol. 2021, 96, 2436–2450. [Google Scholar] [CrossRef]
- Mosteo, R.J.; Sarasa, J.; Ormad, M.P.; Ovelleiro, J.L. Sequential solar photo-Fenton-biological system for the treatment of winery wastewaters. J. Agric. Food Chem. 2008, 56, 7333–7338. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, K.; Nanzai, B.; Thangavadivel, K. Sonochemical degradation of aromatic compounds, surfactants and dyes in aqueous solutions. In Handbook of Ultrasonics and Sonochemistry; Ashokkumar, M., Ed.; Springer: Singapore, 2016; pp. 785–812. [Google Scholar] [CrossRef]
- Benitez, F.J.; Real, F.J.; Acero, J.L.; Leal, A.I.; Garcia, C. Gallic acid degradation in aqueous solutions by UV/H2O2 treatment, Fenton’s reagent and the photo-Fenton system. J. Hazard. Mater. 2005, 126, 31–93. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Haritash, A.K. Degradation of amoxicillin by Fenton and Fenton-integrated hybrid oxidation processes. J. Environ. Chem. Eng. 2009, 7, 102886. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).