Abstract
Adsorbents derived from bamboo, such as biochar, charcoal, activated carbon, and chemically modified bamboo, are recognized for their efficiency and cost-effectiveness in removing heavy metals from water. Despite this, there remains a gap in applying bamboo-based adsorbents for treating heavy metal-contaminated water sources, particularly regarding their physicochemical properties, adsorption mechanisms, and modifications. This review highlights the influence of factors such as specific surface area, pore distribution, pH, cation exchange capacity, elemental composition, and surface functional groups on the ability of bamboo adsorbents to adsorb heavy metals. It also discusses recent advancements in enhancing the properties of bamboo adsorbents through physical and chemical modifications and examines how variables like adsorbent dosage, water pH, temperature, initial concentrations of cations, and heavy metals affect heavy metal removal. The review categorizes the mechanisms of heavy metal adsorption into surface complexation, physical adsorption, electrostatic interaction, ion exchange, precipitation, and redox effect. While bamboo-based adsorbents have shown higher sorption capacity in laboratory settings, there is a need for more comprehensive studies to optimize their performance, scalability, and cost-effectiveness in real-world applications.
Keywords:
bamboo; heavy metal contamination; water treatment; adsorption; mechanism; biochar; activated carbon 1. Introduction
Heavy metals refer to naturally occurring elements with high atomic weights or densities of at least 5 g/cm3 [1]. These metals contaminate water environments through both natural processes, like the weathering of rocks and volcanic activity, and human activities, including industrial discharges, mining operations, agricultural runoff, urban runoff, and improper waste disposal (Figure 1) [2,3,4]. Once these heavy metals are widely spread in the water ecosystem, they pose significant risks to aquatic life by causing toxicity, disrupting growth and reproduction, and leading to bioaccumulation and biomagnification [5], which can further affect the entire food chain. For humans, consuming contaminated water can result in severe health issues, such as kidney damage, neurological disorders, and cancer [6,7].
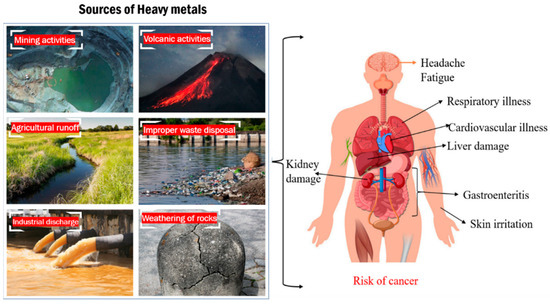
Figure 1.
Sources of heavy metals and their impacts on humans.
The contamination of natural water bodies, such as lakes and rivers, by heavy metals is a significant global issue. It is estimated that around 40% of the world’s lakes and rivers are polluted with toxic metals such as lead, mercury, arsenic, and cadmium [8]. These toxic substances present serious health risks due to their harmful effects. Lead exposure can cause cognitive deficits and kidney damage. Mercury can lead to nervous system disorders and gastrointestinal issues. Arsenic exposure is linked to skin lesions and an increased risk of cancers. Cadmium can cause respiratory issues and bone damage. The severity of these effects depends on the level and duration of exposure. Efforts to remediate and manage this contamination are crucial to protect these vital water resources and ensure the safety of ecosystems and communities [2].
Traditional methods for removing heavy metals from water, such as chemical precipitation, ion exchange, membrane filtration, electrodialysis, phytoremediation, coagulation and flocculation, and distillation, often involve high costs and complex operations [9,10]. Hence, in recent years, there has been a growing interest in using natural and sustainable materials for water treatment. Among these, adsorption is particularly effective due to its simplicity, high removal efficiency, wide applicability across different pH levels, and cost-effectiveness. Adsorbents like activated carbon, biochar, and specialized materials can be reused, making the process sustainable and economically viable [11]. The key factor in this adsorption technology is choosing the right adsorbents. An effective adsorbent should possess a large specific surface area, a rapid adsorption rate, and a short equilibrium time. Among these, bamboo-based adsorbents have emerged as a promising solution due to their unique properties and environmental benefits [12].
Bamboo is a fast-growing, renewable resource abundantly available in many parts of the world [13]. A significant growth in its global production and utilization was observed. As of 2021, the global bamboo market was valued at approximately USD 59.30 billion and is projected to grow at a compound annual growth rate (CAGR) of 4.5% from 2022 to 2030. In 2023, the market size reached USD 75.10 billion and is expected to expand to USD 123.16 billion by 2032, driven by increasing environmental concerns and the adoption of sustainable materials [14]. China leads in bamboo production, holding a 75% market share and employing over 35 million people in the industry. India follows closely, with bamboo covering around 15 million hectares of land [15]. Globally, bamboo covers about 35 million hectares across Africa, Asia, and America [16]. This rapid growth rate and ability to thrive in diverse climatic conditions make it an attractive raw material for various applications, including water treatment. The structure of bamboo, characterized by its high cellulose and lignin content, provides a robust framework that can be converted into effective adsorbents [12].
Bamboo-based adsorbents, compared to coconut shell- and rice husk-derived adsorbents, offer high adsorption capacity, versatility, and sustainability. However, bamboo-based adsorbents face processing challenges, including the need for chemical activation, which can be complex and costly. Coconut shell-based adsorbents, known for their high adsorption efficiency and renewable nature, are versatile but may be limited due to their availability and cost. Rice husk-derived adsorbents are abundant, cost-effective, and environmentally beneficial, yet they generally have lower adsorption capacities and processing challenges. Despite bamboo’s advantages, it remains underutilized in industrial-scale water treatment due to a lack of awareness, processing complexity, market preferences for established materials, and regulatory hurdles. Addressing these issues could enhance the adoption of bamboo-based adsorbents in sustainable water treatment processes [17].
Bamboo can be processed into various forms of adsorbents, such as activated carbon [18,19,20], biochar [21,22,23,24], and charcoal [25,26]. The conversion process typically involves carbonization and activation, which enhance the surface area and porosity of the material, making it highly effective in adsorbing contaminants [27,28]. Using bamboo-based adsorbents for water treatment offers several environmental and economic advantages. Bamboo is a sustainable and eco-friendly material that reduces the reliance on synthetic adsorbents and minimizes the environmental footprint of water treatment processes. Additionally, the low cost and availability of bamboo make it an economically viable option for large-scale applications, particularly in developing regions where water contamination is a critical issue.
Recent reviews on heavy metal removal by adsorption have focused on the application of polymers [29,30,31], metals and metal compounds [30,32], cellulose [33,34] industrial by-products and wastes [29,30,35], carbon materials [30,36,37], layered double hydroxide [38], natural minerals [29,30], agricultural waste [30,39], modified activated carbon [40,41], modified starch [42], modified plant-based materials [43] and nanomaterial [29,44] adsorbents. To the best of our knowledge, published reviews that purely focus on the removal of heavy metals by bamboo adsorbents are limited. The review article by Lamaming et al. [12] detailed the performance of bamboo biochar, aerogel, and activated carbon in removing various pollutants. The review article by Kalderis and co-workers [45] provided insights into the capacities of raw bamboo, bamboo biochar, and activated carbon for removing nutrients and heavy metals from water, and for the removal of gaseous pollutants.
The present review offers a novel perspective on the application of bamboo-based materials as highly efficient and sustainable adsorbents for heavy metal removal from contaminated water. By focusing on bamboo’s inherent abundance, renewability, and eco-friendliness, this review highlights its potential as a viable alternative to conventional, resource-intensive adsorbents. Furthermore, this review delves into a wide array of modifications and treatment methods ranging from traditional carbonization and pyrolysis to advanced techniques, such as potassium permanganate and chitosan modifications, demonstrating the versatility of bamboo in addressing diverse heavy metals like Pb (II), Cd (II), Cr (VI), and others. The comprehensive coverage of various heavy metals underlines the adaptability of bamboo-based adsorbents to a broad spectrum of environmental contaminants, presenting a sustainable solution for water purification. This review also explores the potential for innovation in the field, paving the way for further research and the development of novel, high-performance bamboo-based materials that could revolutionize the way we address water contamination challenges globally.
2. Bamboo-Based Adsorbents and Their Preparation
Bamboo-based adsorbents, including bamboo biochar, charcoal, activated carbon, and modified bamboo, are increasingly recognized for their efficiency in diverse adsorption applications due to their unique characteristics and sustainability. Derived from the fast-growing bamboo plant, these adsorbents are characterized by their porous structure, abundance of functional groups, and high surface areas, which enhance their ability to capture pollutants. These bamboo-based adsorbents are used to eliminate contaminants such as dyes, heavy metals, and organic pollutants from water. These materials are derived from bamboo biomass through processes like pyrolysis, carbonization, and torrefaction, which enhance their porous structure and surface area. The sustainable nature of bamboo, combined with its versatile applications in water purification and air filtration, positions bamboo-based adsorbents as promising solutions for various environmental challenges. The effectiveness of bamboo-based adsorbents can be attributed to their rich array of functional groups, such as carboxyl, hydroxyl, and phenolic groups, which enhance their binding capabilities. Additionally, the modification of bamboo through chemical or physical treatments can further increase its adsorption capacity and selectivity for specific pollutants.
The production of biochar, charcoal, and activated carbon mainly involves pyrolysis, but the process parameters and subsequent treatments differ considerably. Biochar is synthesized through slow pyrolysis, which involves heating the biomass at lower temperatures, generally ranging from 300 to 700 °C, in the absence of oxygen [46]. Charcoal is produced through a similar carbonization method at a slightly higher temperature range of 400–800 °C [47]. The synthesis of charcoal also includes additional steps like pre-carbonization or calcination. Activated carbon is obtained from biomass or carbon-rich material by pyrolysis followed by activation. Activation takes place at higher temperatures (above 600 °C) in the presence of activating agents [48,49]. This activation creates a highly porous structure and enhances its surface area.
2.1. Biochar
Biochar is a potential adsorbent for water and wastewater treatment, efficiently removing various contaminants, including heavy metals, nutrients, and organic pollutants. [50,51,52]. It is a stable, carbon-rich product derived from thermal processing of any kind of biomass or carbonaceous feedstocks [53]. Biochar can be produced from a variety of feedstocks like rice straw [54], rice husk [55], black gram [56], crop residues [57], Lantana camara [56], coir pith [55], pine needle [56], microalgal biomass [58], maize stalk [56], etc.
Biochar is produced by various thermochemical conversions like torrefaction, flash carbonization, hydrothermal carbonization, gasification, and pyrolysis [53,59]. Torrefaction is performed at a temperature range of 200–300 °C in an inert environment [60]. In the process of pyrolysis, the biomass is heated in an oxygen-free environment at a temperature ranging from 300 to 900 °C, where the biomass is heated over its thermal stability limit, which leads to the formation of more stable products [53]. Depending upon the reaction time of pyrolysis materials, there are two types of pyrolysis: fast pyrolysis and slow pyrolysis [61]. In fast pyrolysis, biomass is quickly heated to temperatures between 600 and 1000 °C in an oxygen-free environment, with a rapid heating rate of >10 °C per minute. Slow pyrolysis involves the gradual heating of biomass to temperatures between 300 °C and 700 °C over several hours, with a low heating rate of 0.1 to 10 °C per minute [46]. Pyrolysis is the most widely used and effective technique for the preparation of biochar.
Bamboo biochar is an extensively used bamboo-based adsorbent for the removal of heavy metals from water. Its efficiency for the removal of Cr increased with pyrolysis temperature as reported by Singh et al. [62]. The Cr sorption capacity of bamboo biochar improved with its conversion to magnetic bamboo biochar using the co-precipitation method [63]. Chitosan-impregnated and iron-impregnated biochars exhibited comparatively higher adsorption capacity for Mn (II), As (V), and Fe (II), from contaminated water [64]. It was also reported that torrefaction can potentially increase the ability of lignocellulose materials to adsorb toxic metals [65].
It was reported that physical and chemical modification of bamboo biochar improved its heavy metal sorption capacity. Wang et al. synthesized steam-activated bamboo-based biochar [66], whereas Li et al. prepared modified bamboo biochar through bamboo pyrolysis followed by chemical modification, where the raw biochar samples were subjected to oxidation treatment with HNO3 and KMnO4, followed by base treatment with NaOH and heat treatment [67]. In another study, biochar was produced by pyrolyzing bamboo particles that had been impregnated with phosphate (Na2HPO4) [68]. Chemical modification of bamboo sawdust was carried out using ferric chloride and potassium hydroxide, and modified bamboo biochar was then produced by carbonizing the treated sawdust at 700 °C for 1 h [69].
Bamboo biochar was synthesized by pyrolyzing bamboo sawdust. This bamboo biochar was used to produce magnetic bamboo biochar (MBB) through the co-precipitation method; through a glutaraldehyde cross-linking method, chitosan-modified magnetic bamboo biochar (CMBB) was prepared. To increase the Cd(II) and Zn(II) removal efficiencies, EDTA was grafted onto CMBB to create EDTA and chitosan bi-functionalized magnetic bamboo biochar adsorbent (ECMBB) using a solution of EDTA disodium salt (Na2EDTA), N-(3-Dimethylaminopropyl)-N′-ethyl carbodiimide hydrochloride (EDAC), and N-Hydroxy succinimide (NHS) (Figure 2a) [70]. In the first method, biochar (BC)-supported graphene-encapsulated zero-valent iron nanoparticle composites (BC-G@Fe0) were prepared through direct carbonization of unpyrolyzed bamboo particles that had been impregnated with Fe ions from Fe(NO3)3. The BC-G@Fe0, which has high efficiency in removing Pb2+, Cu2+, and Ag+ ions, was prepared using an alternative method. This involved pyrolyzing bamboo particles to produce biochar, followed by impregnating the biochar with Fe ions and carbonization (Figure 2b) [71].
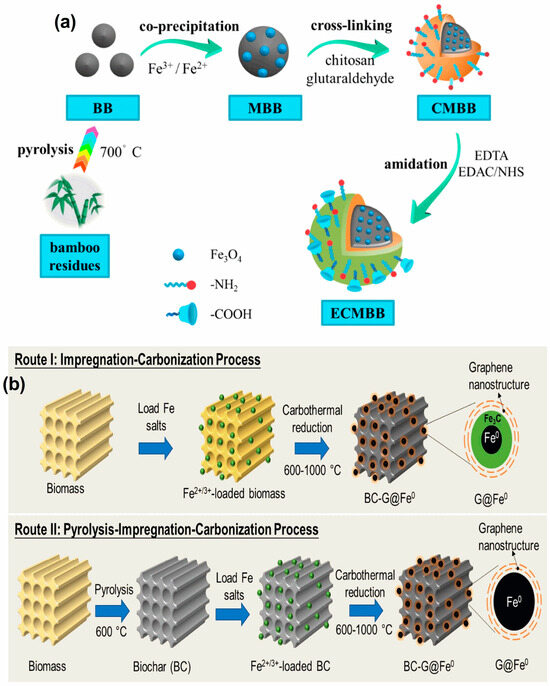
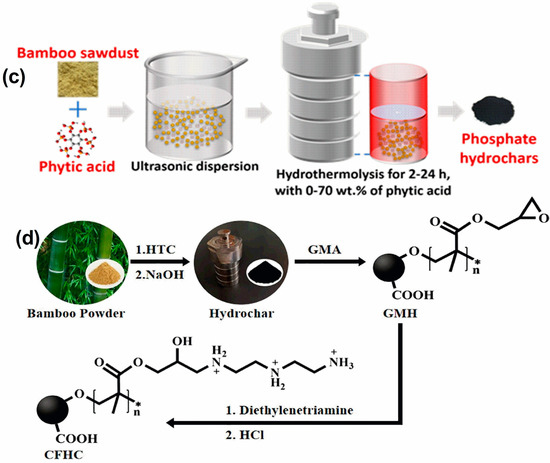
Figure 2.
(a) Schematic diagram for the synthesis of magnetic bamboo biochar (MBB), chitosan-modified magnetic bamboo biochar (CMBB), EDTA, and chitosan bi-functionalized magnetic bamboo biochar adsorbent (ECMBB). Reprinted with permission of Elsevier Ltd. from Reference [70]. (b) Diagrammatic representation of two suggested carbothermal reduction methods for the preparation of BC-G@Fe0 [71]. (c) Diagrammatic representation of the phosphate-modified HTBs synthesis. Reprinted with permission of Elsevier Ltd. from Reference [72]. (d) Synthesis pathway of cation-functionalized hydrochar (CFHC). Reprinted with permission of Elsevier Ltd. from Reference [73].
2.2. Hydrochar
Hydrothermal carbonization, or wet pyrolysis, produces a solid product, hydrochar [74]. Hydrochars are a unique type of biochar produced through hydrothermal processes like hydrothermal carbonization (HTC) and hydrothermal liquefaction (HTL). Due to their favorable pore volume, surface area, regenerative capacity, and high efficiency, hydrochars are a promising option for remediating various contaminants [75].
HTC generally occurs at temperatures between 180 °C and 240 °C for durations of 5 to 240 min under subcritical water pressures. Since the carbon conversion is lower, hydrochar exhibits higher atomic ratios of hydrogen to carbon and oxygen to carbon compared to biochar [76]. The typical product distribution from hydrothermal carbonization on a dry basis of the input feedstock consists of 2–5% gases, 10–20% liquid, and 40–70% hydrochar [77]. HTL is performed at higher temperatures, typically ranging from 250 to 375 °C and at pressure ranging from 10 to 22 MPa. Biocrude oil is the primary product produced and hydrochar and syngas are produced in smaller amounts during the HTL [75].
Polyethyleneimine-modified hydrochar (PEI-HC) was produced through the hydrothermal carbonization (HTC) of methyl acrylate and bamboo, with the addition of ammonium persulfate as an initiator. The resulting hydrochar was then modified with polyethyleneimine (PEI) and used for the treatment of Cr(VI). Characterization results indicated that PEI was effectively grafted onto the hydrochar, and the PEI-HC contained abundant nitrogen and oxygen functional groups, which contributed to its strong Cr(VI) adsorption capacity [78]. Two modified hydrochars were generated by co-hydrothermal carbonization of bamboo sawdust with zinc chloride (ZnCl2) or aluminum chloride (AlCl3) at 200 °C for 7 h. Compared to the unmodified hydrochar, the modified hydrochar demonstrated higher surface area, pore structure, and aromaticity, and was more efficient in removing Cr(VI) from aqueous solutions [79]. Phosphate-modified hydrothermal biochars/hydrochars, which could capture U(VI), Pb(II), and Cd(II) efficiently, were produced by subjecting bamboo sawdust to hydrothermal carbonization for different durations while incorporating phytic acid solution (0–70 wt%) in varying amounts (Figure 2c) [72]. A cation-functionalized hydrochar (CFHC) containing –N+H2R was synthesized through the grafting polymerization of glycidyl methacrylate (GMA) onto bamboo hydrochar using benzoyl peroxide as an initiator. This was followed by amination of the introduced epoxy group with diethylenetriamine and treatment with hydrochloric acid (Figure 2d) [73].
2.3. Charcoal
Charcoal is a carbonized wood that is mostly utilized as a fuel or a reductant in industrial settings [80]. Compared to raw biomass, charcoal has advantages as an energy-dense fuel. Charcoal finds applications in pharmaceuticals, cosmetics, fertilizers, animal feed, power generation, water purification, and filtration [81]. Sources of charcoal include almond shells, walnut shells [82], coconut shells [83], sawdust [84], cashew leaves, cassava stems [85], etc. Charcoal can be prepared by a high-temperature pyrolysis process [86]. The stages in the preparation of bamboo charcoal include pre-carbonization, carbonization, calcination, etc. [87]. Bamboo is a valuable starting material for the production of charcoal owing to its excellent adsorption properties, huge surface area, and highly porous structure. Since bamboo charcoal has four times the adsorption rate and ten times the surface area of regular charcoal, bamboo charcoal is recommended for a variety of applications, including as an adsorbent material, blood purifier, anode for dye-sensitive solar panels, and water purifier [22].
To improve the adsorption capacity of charcoal, researchers modified the charcoal as in the case of bamboo charcoal, which was physically activated for the removal of iron from water [88]. Zn2+ and Cu2+ removal from water by charcoal was improved after its modification with potassium permanganate and sodium hydroxide. The increased adsorption capacity after modification is due to the introduction of additional functional groups, such as carboxyl (C=O) and hydroxyl (C–OH), which enhance metal ion interactions. The formation of Mn–O bonds also improves adsorption by providing more active sites for metal ion binding [87]. Similarly, acid-base surface modification with HNO3 and KOH improved the Cd (II) and Cu (II) sorption capacity of bamboo charcoal [89]. Copper-impregnated HCl-activated charcoal effectively removes many heavy metals like Cd, Pb, As, Cr, etc. [90].
2.4. Activated Carbon
Activated Carbon (AC) is a highly porous, dark, and tasteless solid carbonaceous material with a high surface area that possesses exceptional adsorption capabilities [41,91,92,93]. There are a plethora of base materials available for the synthesis of activated carbon like rubber wood sawdust [94], almond shell [95], peanut shell [96], ramie fiber [94], crop residue [97], Calotropis gigantea stem [98], and areca husk [94]. Raw materials with high carbon content are preferable for activated carbon production. Activated carbon is widely utilized for water and wastewater treatment [27,99], air purification [100,101], and energy applications [102].
During the synthesis of activated carbon, firstly, the raw materials undergo preprocessing/pretreatment steps like washing, drying, size reduction, etc. These pretreated materials are then subjected to thermal conversion. The most relevant thermal conversion methods are pyrolysis and carbonization. The activation of carbon enhances its properties, mainly its porosity/pore structure. Types of activation include physical, chemical, and physiochemical activation [103,104].
Arundinaria alpine species were used for the preparation of bamboo-activated carbon by Asrat et al. [105] and it was used for lead removal from an aqueous solution. Alfatah et al. [106] synthesized activated carbon, from Bambusa vulgaris, with an extensive surface area through a two-stage activation process with a KOH/NaOH mixture, for the removal of Hg2+. Bamboo was carbonized at 500 °C in a nitrogen flow and the resulting chars were mixed with KOH/NaOH at various impregnation ratios of 1:1 (AC-1), 1:2 (AC-2), and 1:3 (AC-3). After drying, the mixture underwent pyrolysis at 800 °C and was washed with 0.1 M HCl to remove residues. The high Hg2+ removal can be attributed to the unique pore structure and functional active sites of the synthesized bamboo-activated carbon. The SEM images of both char and the synthesized activated carbon are shown in Figure 3. Bambusa vulgaris was used for the preparation of physically activated carbon adsorbent aimed at removing heavy metals like Zn (II), Cd (II), and Hg (II) from water. Steam was used for activation at 650 °C for 2 h [107]. Li et al. synthesized a composite of zeolite-activated carbon was synthesized from coal gangue to remove Cu2+ and rhodamine-B. The study states that bamboo-based zeolite-activated carbon has plenty of macropores, micropores, and mesopores, which is attributed to its efficiency in adsorbing heavy metals and other pollutants [108].
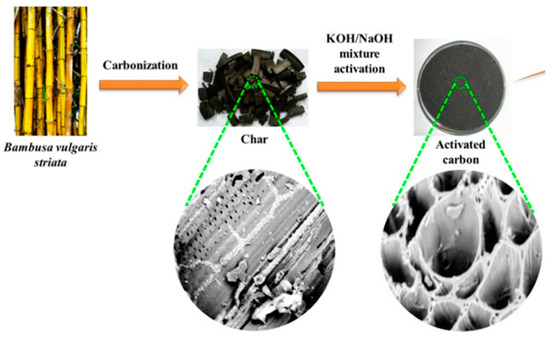
Figure 3.
SEM micrographs of char and synthesized activated carbon. Reprinted with permission of Elsevier Ltd., Amsterdam, The Netherlands, from Reference [106].
2.5. Modified Bamboo
In order to overcome the inherent limitations of raw bamboo, such as its lower adsorptive capacity, lower porosity, and limited surface functionalization, modified bamboo is effectively synthesized and used as an adsorbent for heavy metal removal. Modifications to bamboo-based adsorbents predominantly concentrate on expanding their surface area, enhancing porosity, altering functional groups, etc., to improve their efficiency in removing impurities like heavy metals from water and soil [109]. There are many methods for modifying bamboo adsorbents, including physical (e.g., activation and hydrothermal treatments, etc.), chemical (acid treatment, base treatment, chitosan coating, surface functionalization, etc.), biological, and physicochemical treatments [109,110]. The modification technique should be chosen by considering its advantages and drawbacks as well as suitability, cost-effectiveness, and environmental impact [110].
In general, chemical modification of bamboo is preferred for decontaminating heavy metals from water. Modifying bamboo with Fe(NO3)3·9H2O improved Cr sorption capacity. Bamboo pulp was suspended in deionized water, mixed with Fe(NO3)3·9H2O, freeze-dried to form the Fe(NO3)3/cellulose hybrid aerogel (Fe/CA), and then subjected to a controlled pyrolysis process (Figure 4) [111]. The adsorbent’s numerous interconnected pores ultimately resulted in a high adsorption capacity of Cr (VI); a significant part of the adsorbed Cr (VI) ions had been reduced to Cr (III), which is less toxic and a necessary nutrient for metabolizing sugar and fat in the human body [111]. Chandrawansha and Selladurai [112] modified bamboo stems into cellulose glycine hydrogels for the adsorption of metals like Cd2+ and Cr3+. The hydrogels adsorbed divalent cations rather than monovalent and trivalent cations. Among all the hydrogels, one with 10% glycine exhibited more efficiency in heavy metal adsorption [112].
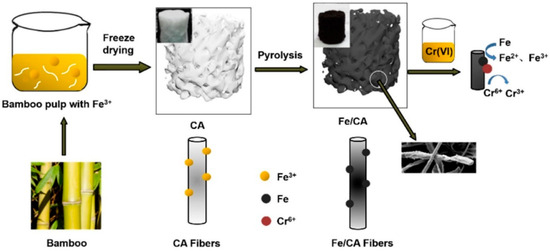
Figure 4.
Diagram for the iron/carbon aerogel (Fe/CA) synthesis process (adapted from Reference [111]).
Chemical modifications of Moso bamboo using pyromellitic dianhydride were done by Chen et al. [113]. The highest yield mass percent gain (mpg) of 18.6% was achieved when 5.0 g of pyromellitic dianhydride (PMDA) was used in the modification, and the maximum Pb (II) equilibrium adsorption capacity was observed at a pH of 5.0. The experimental results further confirmed that the equilibrium adsorption capacity of chemically modified Moso bamboo adsorbent was 2.7 times that of activated carbon [113]. Tartaric acid (TA), oxalic acid (OA), and citric acid (CA) were used for the modification of dried Phyllostachys pubescens powder [114]. Citric acid-modified Phyllostachys pubescens powder was identified as the most efficient adsorbent with the maximum adsorption capacity of 161.031 mg/g, followed by tartaric acid-modified and oxalic acid-modified P. pubescens powder. The study demonstrated that citric acid modification significantly enhanced the specific surface area and pore size of the adsorbent, thereby optimizing its structural properties to facilitate a more efficient adsorption process [114].
Silica nanoparticles generated from bamboo leaves were also used for heavy metal removal. Bamboo leaves were dried and refluxed with NaOH to form sodium silicate, and then mixed with ethanol and ammonia to synthesize SiO2 nanoparticles, which were separated, washed, and dried. The synthesized SiO2 nanoparticles were dispersed in ethanol, sonicated, treated with 1 mL of 3-aminopropyltriethoxy silane (APTES), stirred, centrifuged, and dried to obtain NH2-functionalized SiO2 nanoparticles (A-SiO2 NPs). SiO2 nanoparticles were mixed with toluene and water, treated with 2 mL of 3-cyanopropyltriethoxy silane (CPTES), refluxed, filtered, and washed with toluene; again, the dried particles were refluxed in sulfuric acid and dried to obtain COOH functionalized SiO2 NPs (C–SiO2 NPs) (Figure 5) [115].
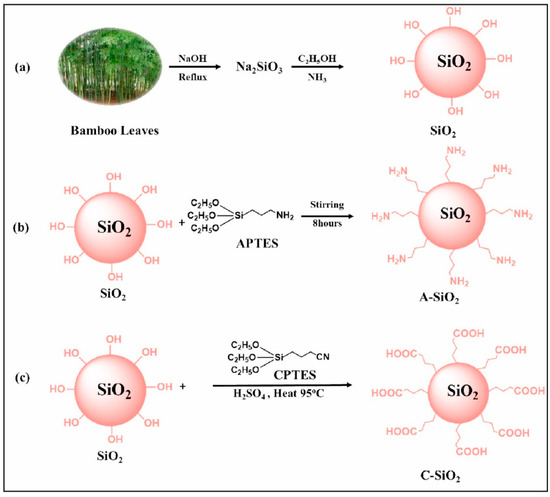
Figure 5.
Illustrations for (a) the green synthesis of SiO2 NPs, (b) amine functionalization of SiO2 NPs to A-SiO2 NPs, and (c) carboxylic functionalization of SiO2 NPs to C–SiO2 NPs. Reprinted with permission of Elsevier Inc. from Reference [115].
3. Properties of Bamboo-Based Adsorbents
Bamboo-based adsorbents, with their unique physical, chemical, and morphological properties, are highly versatile and used in various applications. Prior to investigating the mechanisms underlying bamboo-based adsorbents’ ability to remove metals, their characteristics need to be described. The properties can be evaluated by using various tools such as BET analysis, CHNS, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD) analysis, X-ray photoelectron spectroscopy (XPS) analysis, etc. (Table S1).
3.1. Physical Properties
3.1.1. Surface Area and Porosity
The surface area and porosity of bamboo-based adsorbents play a crucial role in their heavy metal adsorption effectiveness. The Brunauer–Emmett–Teller analysis is used to determine the surface area and pore size distribution of the adsorbents. In general, the pore volume and surface area of bamboo increased with its conversion to biochar, charcoal, or activated carbon. Using a two-stage KOH/NaOH activation process [106], Bambusa vulgaris striata was converted into activated carbon with a markedly enlarged surface area, rising from 553 to 1108 m2/g as the KOH/NaOH impregnation ratio rose from 1:1 to 1:3. The total pore volume nearly doubled, from 0.394 to 0.634 cm3/g at the 1:3 ratio. Additionally, microporosity increased from 62.4% to 84.5%. Steam activation enhances the overall pore volume in biochar samples, which supports pollutant adsorption [116]. The surface area and pore volume exhibited by biochar and steam-activated biochar were 1.22, 2.12 m2 g−1, and 0.01, 0.02 × 10−1 cm3 g−1, respectively [66]. At the same time, the specific surface area of bamboo biochar initially decreased with NH3 introduction but increased with longer retention times [117]. The oxidation of biochar with (NH4)2S2O2 resulted in the reduction of the specific surface area from 1726 to 928 m2 g−1 and pore volume from 1.61 to 0.47 cm3 g−1. This occurred because an increase in oxidation time leads to the higher loading of oxygen-containing functional groups onto the surface of the biochar [118]. The BET analysis of KMnO4-modified bamboo charcoal [87] also indicated that charcoal modified with 0.06 mol/L KMnO4 exhibited a 6.38% decrease in surface area relative to virgin bamboo charcoal due to the partial blockage of micropores from particulate matter formed during KMnO4 treatment and the collapse of the pore structure after modification.
3.1.2. XRD Analysis
The X-ray diffraction (XRD) analysis of ‘bamboo green’ revealed distinct peaks at 2θ angles of 16°, 22°, and 35°, indicating a crystalline structure associated with the native cellulose. Additionally, a peak at 2θ = 26° was observed in the diffraction pattern, attributed to the presence of silica [119]. The presence of SiO2 was also observed in bamboo biochar [120,121]. XRD analysis revealed a broad peak at 2θ = 22.7° for raw biochar and activated biochar, indicating a more carbonaceous structure after the pyrolysis [121]. The broad diffraction peaks at around 23° and 43° in biochar indicated an amorphous carbon crystallization [122]. XRD patterns of bamboo charcoal (Figure 6) subjected to carbonization at 500 °C and 660 °C exhibited two wide peaks at 2θ values of 12° and 22°, indicating the presence of amorphous carbon [123]. Furthermore, the detection of distinct peaks at 2θ values of 28.4° and 40.5° verifies the presence of crystalline graphite structures in the material [123]. In parallel, Najihah et al. reported two broad peaks of low intensity at approximately 23° and 44°, further indicating that bamboo charcoal and activated bamboo charcoal are predominantly amorphous in nature [124].
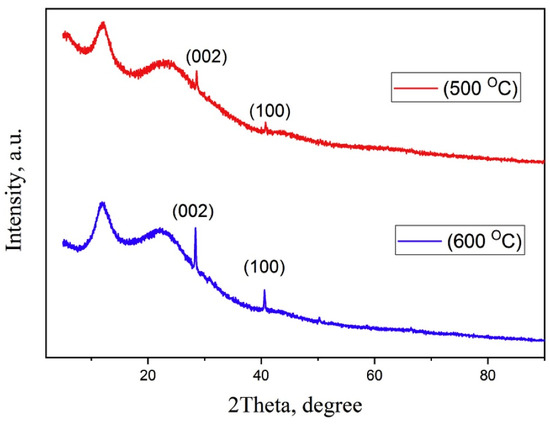
Figure 6.
XRD spectra of bamboo charcoal at different temperatures (adapted from Reference [123]).
3.2. Chemical Properties
3.2.1. FTIR Analysis
In the FTIR analysis of raw bamboo, distinct peaks were identified at 3314, 2916, 1713, 1237, and 1034 cm−1, corresponding to the stretching vibrations of the O-H functional group, asymmetric C-H stretching, C=O stretching, and C-O stretching, respectively [125]. The dried charcoal showed a broad band at 3447.10 cm−1 (hydroxyl group), a peak at 1743.21 cm−1 (carbonyl), and peaks at 1606.85 cm−1 (C=O stretching), 1259.63 cm−1 (C–H bending), and 1010.79 cm−1 (C–O stretching) [126]. The FTIR spectrum of bamboo biochar (Figure 7) exhibited peaks at 3445.19 cm−1 (O–H stretching), 1625.26 cm−1 (C=C stretching), and 875.62 cm−1 (C–H bending) [127]. After Mn adsorption, these peaks moved to 1602.42 cm−1, 861.52 cm−1, and 577.91 cm−1, indicating reduced O–H and C=C groups and suggesting coordination with Mn2+ ions, along with a new peak at 489.39 cm−1. Potassium permanganate-modified bamboo biochar displayed a peak at 1388.72 cm−1 for −OH stretching and at 520.41 cm−1 for Mn–O stretching. Shifts to 3447.97 cm−1, 1400.09 cm−1, and 503.22 cm−1 further indicate enhanced Mn-binding to the −OH group, highlighting changes in functional groups due to the modification [127].
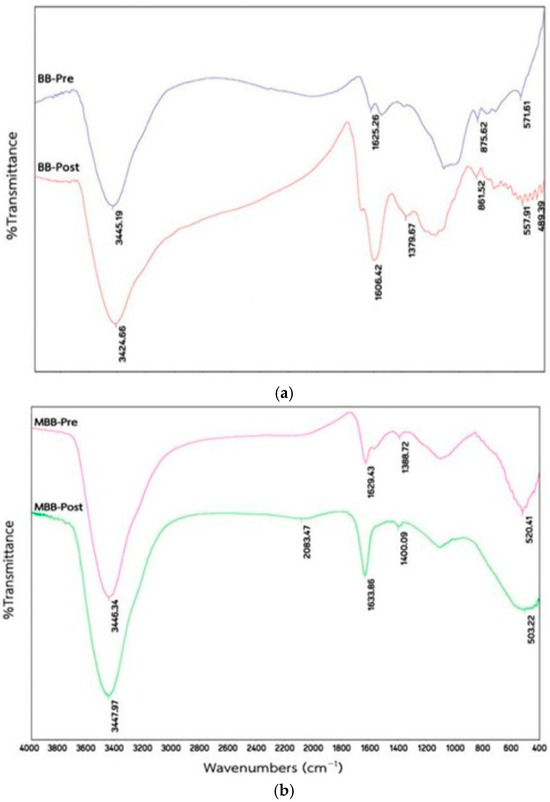
Figure 7.
FTIR spectra of bamboo biochar (a) and potassium permanganate-modified bamboo biochar (b) before (purple) and after Mn adsorption (green) (adapted from Reference [127]).
FTIR analysis of activated carbons revealed stretching vibrations of −OH groups, asymmetric CH stretching in methylene and methyl groups at 2850–2950 cm−1, C≡C groups at 2275–2375 cm−1, C=O groups at 1700–1750 cm−1, and C=C groups at 1500–1550 cm−1 [106]. The highest peak intensities were noted for activated carbon at a char-to-hydroxide ratio of 1:3; activated carbon at ratios of 1:2 exhibited slightly higher peak intensities than activated carbon at ratios of 1:1 [106]. This variation is likely attributed to the differing impregnation ratios of the activating agents, which influenced the surface chemistry of the activated carbon. Within the functional groups, O–H groups displayed the highest transmittance, suggesting that phenols are the most significant functional group in the lignocellulosic biomass precursor [106]. Table 1 details the changes in spectra due to various types of modifications.

Table 1.
Changes in spectra due to modifications.
3.2.2. XPS Analysis
Bamboo primarily consists of cellulose, hemicellulose, lignin, and some extractives, with carbon (C), hydrogen (H), and oxygen (O) as its main elements. Analysis of C1 and O1 peak intensities and shifts revealed the chemical properties of bamboo surfaces. The C1 peaks were associated with functional groups such as C–C, C–H (C1), C–O (C2), O–C–O, and C=O (C3), and O–C=O (C4), while the O1 peaks were associated with O–C=O (O1) and C–O (O2), categorized by binding energy levels. In the C1 spectra, C1 peaks mainly originated from lignin and extracts (C–C, C–H), while C2, C3, and C4 were derived from cellulose and hemicellulose. In the O1 spectra, the O1 peak was associated with lignin and extracts, and the O2 peak was associated with hemicellulose and cellulose [129].
The conversion of bamboo to charcoal, activated carbon, or biochar improved its properties (Figure 8). The deconvolution of C1 spectra identified six peaks in bamboo charcoal that corresponded to different carbon functionalities [130]: graphitic carbon (284.4 eV), C–C (285.0 eV), C–O (286.5 eV), C=O (288.0 eV), COO− (288.9 eV), and π-π* (290.2 eV), with respective percentages of 71.12%, 4.98%, 15.09%, 0.52%, 2.70%, and 5.59%. Activation with concentrated HNO3 significantly increased the proportions of aldehyde/ketone (C=O) and carboxyl (COO–) groups, raising their contents from 0.52% and 2.70% in bamboo charcoal to 6.79% and 6.86% in nitric acid activated charcoal. A substantial amount of surface carbon in a graphite configuration was observed, with C−O being the dominant oxygen-containing functional group. The notable enhancement of oxygen-containing groups (C=O and COO–) on the bamboo charcoal surface suggests its potential as an effective adsorbent for eliminating heavy metals and radioactive materials in aqueous solutions via ion exchange processes [130].
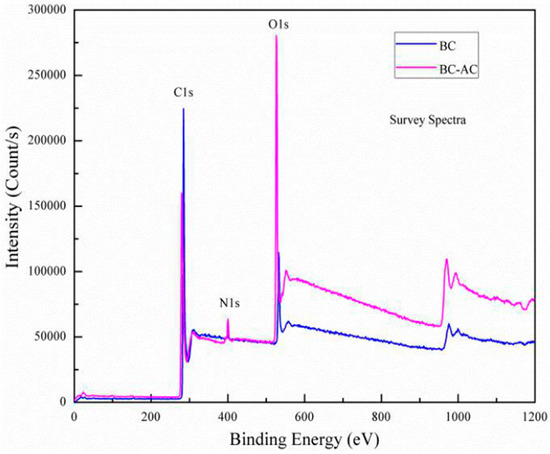
Figure 8.
XPS spectrum of bamboo charcoal and bamboo charcoal-activated charcoal (adapted from Reference [130]).
Treatment or modification of bamboo adsorbents also significantly changes the functional groups on the surface. For example, the proportion of carboxyl C=O groups in bamboo biochar increased significantly from 18.5% to 35% after EDTA modification [70]. The (NH4)2S2O8 oxidized biochar was enriched in –COOH functional groups, along with a smaller presence of −OH groups [118]. In Cu-coated bamboo-based biochar composites, the Cu 2p3/2 spectra can be deconvoluted into three distinct peaks at 932.40 eV, 933.01 eV, and 934.60 eV, representing Cu+ (49%), Cu2+ (35%), and Cu0 (16%), respectively, indicating that Cu2O is the predominant metal oxide in the copper-coated biochar composite [131].
3.2.3. CHNS Analysis
The CHNS (carbon, hydrogen, nitrogen, and sulfur) analysis provides valuable insights into the elemental composition of a compound. Deepika et al. [132] conducted a comparative analysis of the carbon content across various components of different bamboo species. The CHNS analysis of Bambusa balcooa exhibited carbon composition values of 45.2% in the leaf, 48% in the rhizome, and 49.1% in the culm. Dendrocalamus asper showed values of 45.9% in the leaf, 47.1% in the rhizome, and 49.7% in the culm. Dendrocalamus strictus had carbon composition values of 44.7, 45.9, and 48.4% in the leaf, rhizome, and culm, respectively. For Bambusa tulda, the carbon content values were 43.6% (leaf), 46.8% (rhizome), and 48% (culm), while Bambusa vulgaris exhibited composition values of 44.7% in the leaf, 42.8% in the rhizome, and 47.2% in the culm [132].
Li et al. reported that the chemical composition values of raw biochar included 86.69 wt% carbon, 0.71 wt% hydrogen, 0.64 wt% nitrogen, 0.06 wt% sulfur, and 6.07 wt% oxygen. In contrast, for biochar carbonized at 1000 °C, the composition values changed to 89.08 wt% carbon, 0.56 wt% hydrogen, 0.43 wt% nitrogen, 0.07 wt% sulfur, and 3.90 wt% oxygen. The fixed carbon content of biochar carbonized at 1000 °C was higher than that of the original biochar [133]. Mukesh et al. explored a high carbon content of 78.60% and other elemental compositions, including 1.92 wt% hydrogen, 1.823 wt% nitrogen, and 0.028 wt% sulfur for bamboo biochar [134]. The carbon–nitrogen and hydrogen contents in the charcoal powder were measured at 52.10%, 11.20%, and 8.04%, respectively [135]. Bamboo-activated carbon has an elemental composition of 90.59 wt% carbon, 0.524 wt% hydrogen, 0.491 wt% nitrogen, and 6.05 wt% oxygen [136].
3.3. Morphological Properties
SEM Analysis
SEM analysis shows how the adsorbents’ surfaces are characterized in terms of their structures and shapes. Bamboo displayed smooth cell wall surfaces with an orderly arrangement of pores [129]. The smooth surface of the bamboo biochar, characterized by a dense pore structure and many small particles, likely resulted from the degradation of bamboo tissue during high-temperature pyrolysis [137]. The activated carbon produced through KOH/NaOH chemical activation exhibited well-defined pores. Activated carbon at a KOH/NaOH impregnation ratio of 1:2 demonstrated superior pore development relative to an impregnation ratio of 1:1. Activated carbon achieved an optimized pore structure at an impregnation ratio of 1:3, showcasing an ideal porous carbon skeleton with an appropriate micro–meso porosity framework [106] (Figure 9). Pure activated carbon derived from bamboo powder displayed irregular shapes and a pronounced porous structure, with a significant abundance of pores distributed across a wide range of sizes, including both mesopores and macropores [108].
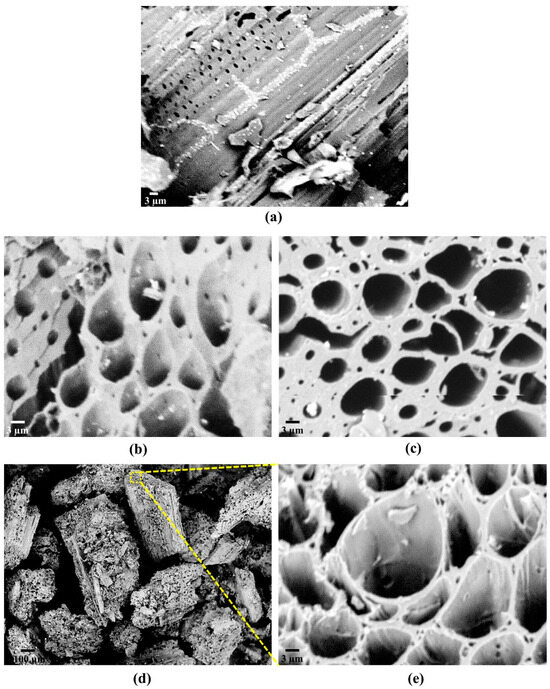
Figure 9.
SEM images of (a) char of Bambusa vulgaris striata and activated carbon at KOH/NaOH ratio (b) 1:1, (c)1:2, (d,e) 1:3. Reprinted with permission of Elsevier Ltd. from Reference [106].
In contrast, the chitosan-modified bamboo biochar exhibited a rough surface, possibly due to the uneven stacking of bamboo charcoal particles [137]. Similarly, chitosan charcoal composite beads [138] also displayed a rough surface due to the irregular stacking of bamboo charcoal particles. KMnO4-modified bamboo charcoal displayed a comparatively rougher surface with particles of Mn present, suggesting that MnOx was likely loaded on the surface of the bamboo charcoal [87].
4. Application of Bamboo-Based Adsorbents for Heavy Metal Removal
4.1. Effect of pH
The pH of the solution has a significant impact on the adsorption process. It can affect the surface charge of the adsorbent and the ionization state of heavy metal ions, affecting the adsorption rate [90,111,139,140,141]. Due to the abundance of hydronium ions (H+) at a low pH, the surface of the bamboo-based adsorbents becomes highly protonated. This low pH leads to the protonation of functional groups like hydroxyl (–OH) groups or carboxyl groups (–COOH), creating a positively charged surface. This protonation may enhance the adsorption of anions through mechanisms like hydrogen bonding and electrostatic attraction. It may also generate electrostatic repulsion against cations, thus lowering the overall adsorption efficiency.
As the pH increases, protonation of the adsorbent surface decreases, which leads to partial deprotonation and the development of negatively charged sites, such as deprotonated carboxyl (–COO−) and hydroxyl groups. This strengthens the electrostatic interaction between the metal cations and the adsorbent, increasing cation adsorption. At higher pH levels, the adsorbent surface becomes more negatively charged due to further deprotonation of functional groups, enhancing the adsorption of cations, but reducing the capacity for anions due to electrostatic repulsion. However, at a slightly acidic to neutral pH, the formation of insoluble metal hydroxide precipitates decreases the availability of free ions for adsorption. Cadmium and copper ions precipitate at pH levels higher than 7 [87,118,139,142,143]. Silver ions begin to precipitate at pH 6 [138], whereas zinc ions do so at pH 8 [87].
Cadmium was mainly found as Cd(OH)2 when the pH was above 8, as Cd(OH)+ when the pH ranged between 7 to 8, and as Cd2+ when the pH was below 7 (Figure S1). The optimum pH for Cd2+ ions removal was reported to be 5 for bamboo biochar [139] and bamboo stem-derived biochar [144], whereas it was reported as 7 for HNO3-modified bamboo biochar [142] and chitosan-modified bamboo biochar [137]. For Cd2+ removal, using chitosan-modified bamboo biochar, adsorption rates increased from 66.78% to 90.66% as pH went from 3 to 7 [137]. Lyu et al. reported pH 4.5 as the optimum pH for Cd2+ removal using bamboo biochar, enhanced with pre-magnetic properties, and cross-linked with a Ca–Mg–Al layered double hydroxide composite [122].
As the pH increased, the copper (Cu2+) adsorption capacity also increased and the optimal results were obtained at pH 6 using bamboo charcoal and its modifications (KMnO4 and NaOH); pH 5 was preferred to prevent Cu(OH)2 precipitation [87]. Qui et al. reported 100% Cu(II) removal efficiency at pH 7 when bamboo fiber-embedded biofoam was used [143]. The adsorption capacity of composite beads of chitosan/bamboo charcoal for Ag2+ peaked at pH 6 [138]. Ma et al. reported that a pH of 7 is optimal for the adsorption of Zn2+ using bamboo charcoal and its modifications (KMnO4 and NaOH) [87].
Cr (VI) ions were primarily found as Cr2O72−, HCrO4−, and HCr2O7− when pH was less than 6.8; above pH 6.8, CrO42− became the predominant species [145]. Under an acidic environment, H+ ions can increase the oxidation ability of Cr (VI) [111]. The efficiency of bamboo-based oxidized biochar for the adsorption of Cr reduced as the pH climbed from 2 to 7 [118]. Similarly, for iron/carbon aerogel made from bamboo cellulose fiber, the maximum Cr (VI) adsorption occurred at a pH of 3, with a significant decline in adsorption capacity and an increase in the pH [111]. As the pH rose from 2 to 9, the adsorption of Cr (VI) onto N-doped biochar decreased [117].
The predominant form of arsenate at pH 5 was H2AsO4−, while at pH 9, it was HAsO42−. Within the pH range of 5 to 9, the iron-modified bamboo biochar demonstrated 100% removal of As (V) [121]. The iron-modified bamboo biochar and bamboo biochar, enhanced with pre-magnetic properties and cross-linked with a Ca–Mg–Al layered double hydroxide composite, demonstrated a high As (III) adsorption capacity between pH 2 and 3, and the removal rates were >70% and >99%, respectively. As the pH increased from 3 to 6, the removal efficiency gradually declined until it plateaued [122]. In an acidic solution, Re (VII) primarily occurred as the ReO4− anionic [146]. Hu et al. observed the optimal pH of 1 for Re (VII) adsorption for both copper-impregnated and pristine bamboo biochar. In the copper-impregnated bamboo biochar, the formation of new copper-bearing minerals led to an increase in surface area and total pore volume compared to the pristine bamboo biochar. A sharp decrease in biochar adsorption capacity was observed as the pH increased from 1 to 3, followed by a slow decline with a further increase in pH [131]. Figure S2 shows the heavy metal adsorption capacities of various bamboo adsorbents at different pH values.
4.2. Effect of Contact Time
The efficiency of adsorbent removal increased as the contact time increased. However, the rate of heavy metal removal decreased as time elapsed. Carbon aerogels derived from bamboo cellulose fibers and loaded with iron were used for the removal of Cr ions. It was observed that within 3 min, adsorption quickly reached 80% of its maximum capacity, and equilibrium was achieved after 8 min [111]. The rapid adsorption of Zn (II), Pb (II), and Cu (II) occurred within the first 120 min, achieving removal rates of 70%, 83%, and 60% respectively, which was followed by a slower adsorption phase, eventually reaching equilibrium at 240 min [141]. When the contact duration was extended from 5 to 120 min, the adsorption rate for removal of Cd (II) using bamboo biochar modified with chitosan increased from 81.54% to 90.24% [137].
The influence of contact time on adsorption can be explained as follows: the amount of adsorption initially increases because the adsorbent surface has a sufficient number of adsorption sites, a suitable pore structure, and functional groups. As adsorption continues, the active sites on the adsorbent gradually become occupied by heavy metal ions. This results in the further filling of the pore structure and the formation of complexes between the functional groups and the heavy metal ions, which stabilize the carbon surface. As a result, the adsorption rate decreases due to the reduction in the concentration of heavy metal ions in the solution [89]. Moreover, as the adsorption time increases, it becomes more difficult for adsorption to occur as the adsorbent surface becomes saturated with metal ions, leading to repulsive interactions within the solution. Ultimately, after a certain period, the adsorption capacity for metal ions stabilizes.
4.3. Effects of Other Anions and Cations
Ions are ubiquitous in the environment, occurring in streams of natural water as well as wastewater, and they have the potential to impact the heavy metal adsorption capacity of bamboo adsorbents. It was found that NaCl has a significant effect on the Cu2+ adsorption of steam-activated bamboo biochar and Cd (II), Zn (II) sorption using EDTA-grafted chitosan-modified magnetic bamboo charcoal [66,70]. The adsorption capacity of the bamboo adsorbents decreased with NaCl concentrations. At a 0.1 M NaCl concentration, the adsorption values of Cd (II) and Zn (II) on EDTA-grafted chitosan-modified magnetic bamboo charcoal decreased by 38.4% and 39.9%, respectively [70]. The reductions are likely due to the competition between Na+ ions and metal ions (Cu2+) for the same binding sites [147]. Furthermore, the addition of Na+ may create an electrostatic screening effect that hinders the electrostatic interaction between the adsorbent and adsorbate. Additionally, there is also competition between anions (Cl−) and metal ion (Cu2+) species for active sites through charge diffusion and competitive adsorption mechanisms [66]. Additionally, it was noted that for steam-activated bamboo biochar, other coexisting ions such as Pb(NO3)2, MgCl2, Na2CO3, CaCl2, and KH2PO4 had a more significant impact on Cu2+ adsorption compared to NaCl. This could be attributed to their superior screening effects and their ability to occupy more active adsorption sites that were originally available for Cu2+ [66].
As the concentration of NaNO3 increased from 0 to 0.04 mol/L, the ability of bamboo biochar treated with (NH4)2S2O8 to adsorb Pb (II) decreased from 175.25 to 146.56 mg/g [140]. The removal of Cd (II) using bamboo biochar, enhanced with pre-magnetic properties, cross-linked with a Ca–Mg–Al layered double hydroxide composite, was minimally affected by anions such as Cl−, NO3−, SO42−, and CO32−. However, cations like Ca2+, K+, and Na+ demonstrated greater inhibitory effects, although the removal rate remained above 60% [122]. This can be due to the competition of adsorption sites between Cd2+ ions and cations. A similar trend was observed for the removal of Cd2+ using bamboo stem biochar. The Cd2+ removal efficiency rapidly dropped to 32.36% from 82.41% due to the presence of Pb2+ as an interfering ion [144].
The ability of chitosan-modified magnetic biochar to adsorb Cr (VI) decreased as the concentration of NO3− increased, with a 38.3% reduction seen at 0.1 M NO3− [63]. In comparison, magnetic bamboo biochar experienced only a 10% reduction under the same conditions. As the ionic strength increased, the NO3− and Cr (VI) ions started competing for the adsorption sites, which was the main reason for the reduction in Cr (VI) removal. Coexisting anions had a notable impact on reducing Cr (VI) removal in magnetic biochar modified with chitosan, with the order of influence being Cl− < Ca2+ < PO43− < Fe3+ < SO42−. On the other hand, magnetic bamboo biochar showed limited effects from these coexisting ions. The introduction of Ca2+ and Fe3+ led to a reduction in Cr (VI) removal by forming compounds with Cr (VI) oxyanions [63]. The presence of Ca2+, K+, Na+, NO3−, and Cl−, even at greater concentrations, did not significantly impact the adsorption of As (III) onto the bamboo biochar Ca–Mg–Al layered double hydroxide composite. The composite had high selectivity for As (III), with a very minimal influence of SO42− and CO32− [122].
4.4. Adsorption Mechanism of Heavy Metals by Bamboo-Based Adsorbents
Understanding how the heavy metals are adsorbed and removed on different bamboo-based adsorbents is crucial for modifying and improving their adsorption capacity. Bamboo-based adsorbents have surfaced as a viable alternative because of their distinct characteristics, including large surface area and functional groups like amino, hydroxyl, and carboxylic groups, which can bind with heavy metal ions. Additionally, their environmentally friendly and affordable attributes make them a promising choice [148]. Each heavy metal has a different dominant mechanism due to the complexity of the adsorption process and the magnitude of factors that influence it. The existing research indicated that physical adsorption, electrostatic adsorption, oxidation, precipitation, ion exchange, reduction, hydrogen bonding, and complexation are important adsorption mechanisms, as shown in Figure 10a. However, several mechanisms work together for the adsorption of heavy metals by biochar, rather than being solely responsible for it [63].
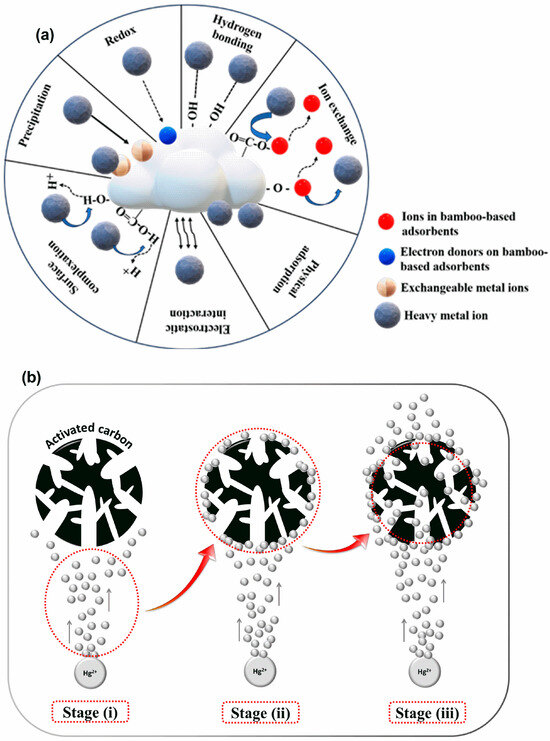
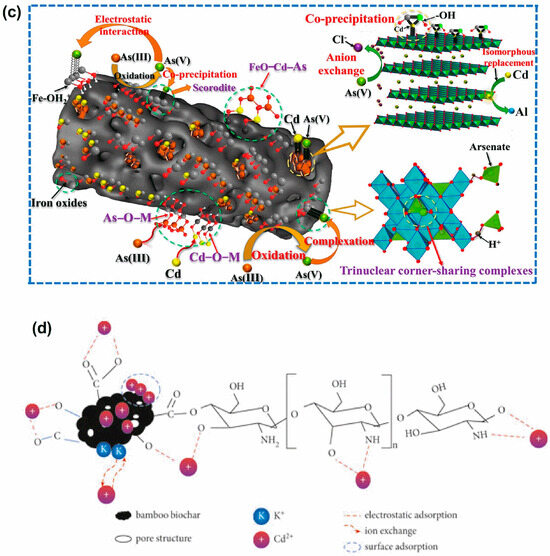
Figure 10.
Adsorption mechanisms of bamboo-based adsorbents for heavy metal removal: (a) General adsorption mechanism for bamboo-based adsorbents, (b) mechanism of Hg2+ removal using activated carbon produced through KOH/NaOH chemical activation. Reprinted with permission of Elsevier Ltd. from Reference [106], (c) removal of As(III) and Cd(II) via bamboo biochar (Fe-BC@LDH). Reprinted with permission of Elsevier B.V. from Reference [122] and (d) Cd(II) removal from biochar modified with chitosan (CBB) [137].
Several studies have investigated the adsorption capacities and mechanisms of bamboo-based adsorbents for removing various heavy metals, including copper [66,87,108,143], lead [140], chromium [63,111,128], cadmium [118,139,144], rhenium [131], zinc [87,149], manganese [127], and mercury [106]. Cr (VI) removal by amino-enhanced bamboo biochar reinforced by nano-zero-valent iron (AMBBX-nZVI) likely involves electrostatic interactions, chemical reduction, surface adsorption, and co-precipitation [128]. Under an acidic environment, the amino groups (-NH2) on the biochar are protonated to –NH3+, facilitating the adsorption of the negatively charged Cr (VI) species onto AMBBX-nZVI. Adsorbed Cr (VI) is subsequently reduced to Cr (III) by Fe0 along with its conversion to Fe (II). While the majority of Fe (II) forms an iron oxide on the biochar surface, a small fraction is released into the solution, further reducing Cr (VI) to Cr (III) and leading to the formation of Fe (III). Cr (III) and Fe (III) subsequently form Cr (III)-Fe (III) hydroxide coprecipitates (CrxFe1−x(OH)3 or CrxFe1−xOOH), which are deposited on the AMBBX-nZVI surface [128]. Bamboo biochar, enhanced with pre-magnetic properties and cross-linked with a Ca–Mg–Al layered double hydroxide composite (Fe-BC@LDH), was employed for the removal of As(III) and Cd(II) from wastewater. The Fe-BC@LDH surface is uniformly coated with iron oxides, Fe2O3 and FeOOH, which act as oxidizing agents to convert As3+ into the less toxic As5+. Due to the good crystallization of Fe3O4 on the Fe-BC@LDH surface, As3+ initially adsorbs, forming monodentate bonds with iron. Once oxidized, As5+ forms stable bi- or tridentate corner-sharing complexes with Fe3O4 [122] (Figure 10c).
At the same time, free FeO− ions on the surface electrostatically attract Cd2+ ions, leading to the formation of FeOCd+ complexes that precipitate as stable FeOCdOH ligands via inner-sphere complexation. The iron and oxygen atoms on the surface act as Lewis acids, facilitating chemisorption with arsenite, which serves as a Lewis base, resulting in the formation of covalent inner-sphere Fe-O-As complexes [122] (Figure 10c).
The removal of Cu2+ ions using bamboo-based adsorbents has been primarily attributed to physical adsorption mechanisms. Bamboo-based zeolite-activated carbon adsorbs Cu2+ through van der Waals forces, enabling rapid and efficient adsorption, as well as good desorption and re-adsorption capabilities [108]. Similarly, Moso bamboo-based biofoam facilitated Cu2+ removal through hydrogen bonding interactions between the hydroxyl groups of the biofoam and the Cu2+ ions [143]. However, Cu2+ removal using steam-activated biochar is mainly by chemical adsorption mechanisms, such as surface complexation, in which Cu2+ ions interact with functional groups containing oxygen on the biochar surface. The XPS results made it clear that Cu2+ binds to the biochar by complexation, as evidenced by the rise in metal oxide content and the subsequent decrease in the C-OH group following Cu2+ adsorption. Furthermore, the influence of pH on Cu2+ removal suggests that electrostatic interactions were involved in its removal [66].
Intraparticle diffusion, precipitation with inorganic groups, and complexation with organic surface groups are the key mechanisms governing the adsorption of Zn2+ onto bamboo biochar. The precipitation of Zn2+ was mainly facilitated by CO32− groups present in the bamboo biochar, as indicated by FTIR data, and was influenced by the alkaline pH of the biochar [149]. The adsorption of Re (VII) ions using copper-impregnated biochar showed that the primary mechanism for Re (VII) removal was the complexation reaction. FTIR analysis demonstrated that Cu+/Cu2+ species on the surface of the Cu-biochar may interact with Re (VII) ions to form CuReO4 or Cu(ReO4)2 complexes [131].
Activated carbon (AC), produced through KOH/NaOH chemical activation, was utilized for Hg2+ removal, with the removal mechanism governed by three stages: (i) the transport of Hg2+ from the solution to the film layer surrounding the adsorbent, (ii) diffusion of Hg2+ from the film to the AC surface, and (iii) the migration of Hg2+ from the surface into the internal pores, where it binds to active sites within the adsorbent. The homogeneous binding of Hg2+ ions is facilitated by the highly porous structure of activated carbon, resulting from intra- or inter-molecular cross-linking of its carbon atoms. These cross-links generate new binding sites within the micropores, and the increased surface area of the micropore walls further enhances adsorption capacity. The oxygen-containing functional groups on the surface of activated carbon, such as hydroxyl (O–H), carbonyl (C=O), and alkoxy (C–O), interact with the mercury ions through electrostatic attraction and form chemical bonds. This leads to a complexation reaction, where the functional groups react with Hg2+ to form stable complexes such as (–O)2Hg, (–COO)2Hg, and (–CO)2Hg. Another involved interaction is hydrogen bonding between acceptor and donor groups on the AC-Hg2+ system [106] (Figure 10b).
The removal of Cd (II) ions from the biochar modified with chitosan (CBB) involves a complex mechanism that mainly involves electrostatic interactions, ion exchange processes, and surface adsorption. The modification provides the biochar with a porous structure and a variety of active functional groups, such as C=O, –NH2, –COOH, and –OH, which together enhance its surface adsorption capacity. Additionally, the abundant –COOH and –OH functional groups on the surface of CBB can alter the surface zeta potentials by losing H+ ions, forming charged functional groups, and making the adsorbent surface more negatively charged. This, in turn, strengthens the electrostatic adsorption of Cd (II) [137] (Figure 10d).
The adsorption behaviors of various heavy metals onto bamboo-based adsorbents can be well understood through kinetic and isotherm studies. The kinetic analysis reveals that the majority of adsorption processes follow pseudo-second-order kinetics, indicating that chemisorption is the rate-limiting step and the adsorption rate depends primarily on the availability of binding sites rather than the metal ion concentration in solution. This suggests strong chemical interactions between the adsorbent surface and metal ions. The equilibrium data predominantly fit the Langmuir isotherm model, with some cases following Freundlich or other models. This indicates monolayer adsorption on homogeneous surfaces with energetically equivalent binding sites and minimal interaction between adsorbed molecules. In cases where Freundlich isotherm fit well, particularly for Zn (II) and U(VI) adsorption, it suggests the presence of heterogeneous surfaces with multiple layers of adsorption and varying site energies. The combination of pseudo-second-order kinetics and Langmuir isotherm behavior strongly suggests that the primary mechanism of heavy metal removal involves specific chemical interactions between the metal ions and active sites on the bamboo-based adsorbents, rather than simple physical adsorption. This is further supported by the various mechanisms reported in experimental studies, including ion exchange, surface complexation, and electrostatic interactions, which all point to strong chemical binding between the adsorbent surface and metal ions. Table 2 compares various bamboo-based adsorbents for heavy metal removal, outlining their raw materials, target metals, removal efficiency, contact time, adsorption capacity, and the key mechanisms involved.

Table 2.
Summary of bamboo-based adsorbents for heavy metal removal: key parameters and adsorption mechanisms.
Surface modifications of bamboo-based adsorbents significantly enhance their heavy metal removal capabilities through various chemical and physical treatments. Chemical modifications such as amidoxime functionalization, chitosan grafting, and metal oxide impregnation substantially improve adsorption capacities compared to unmodified materials. These enhancements can be attributed to the introduction of additional functional groups, increased surface area, improved pore structure, and the creation of new binding sites. Physical modifications like steam activation have also proven effective in improving metal uptake capacity. The incorporation of magnetic properties through iron oxide modifications enhances adsorption capacity. Chemical activation using alkali treatments effectively develops the pore structure and surface functionality. These various modification strategies demonstrate the versatility of bamboo-based materials and their potential for optimization in heavy metal removal applications, with modified materials consistently showing superior performance compared to their unmodified counterparts.
4.5. Reusability of the Adsorbents
Regeneration and desorption studies are crucial for assessing the recovery and reusability of metal-laden adsorbents, offering essential insights that contribute to environmental protection and cost reduction. The capability to regenerate and reuse an adsorbent is a key factor in determining its practical viability for real-world applications. Chitosan/bamboo charcoal composite beads achieved 31.76% desorption of Ag+ ions in 180 min using a 10 mM acetic acid solution [138]. Similarly, 0.1 mol/L KOH at 25 °C successfully desorbed over 92% of Re (VII) ions from copper-impregnated bamboo biochar due to the action of OH− ions in displacing rhenium anions [131]. In another study, Fe3O4 nanoparticle-coated bamboo biochar lost nearly 70% of adsorbed As (V) when treated with 1 M potassium phosphate, as arsenates and phosphates competed for adsorption sites [121]. Desorption of Cd2+ ions from bamboo-derived biochar was 87.9% with 0.01 M HCl, increased to 91.3% with 0.05 M HCl, but dropped to 77% when using 0.1 M HCl [144].
Numerous research studies have highlighted the reusability of bamboo-based adsorbents and their significant potential for the removal of heavy metals from aqueous solutions. Copper-impregnated bamboo biochar showed only a slight decrease in adsorption capacity for Re (VII) after four adsorption–desorption cycles [131]. Similarly, the removal efficiency of Cu2+ by steam-activated bamboo biochar decreased to 80.59% from 94.66% after three cycles, indicating good reusability [66]. A comparison showed that chitosan-modified magnetic bamboo biochar retained over 90% of its Cr (VI) removal efficiency across five cycles. In contrast, non-modified magnetic bamboo biochar experienced a significant decline in efficiency, dropping from 90.7% to 44.1% when treated with Na2EDTA. This highlights that chitosan modification substantially enhances regeneration efficiency [63]. ZnCl2-activated NH3-doped bamboo biochar retained 63% of its initial Cr (VI) adsorption capacity (338.7 mg/g) after five regeneration cycles [117]. HNO3-modified bamboo biochar, regenerated through reflux in dilute HCl, decreased Cd2+ removal efficiency from 95.33% to 90.36% after three cycles, further reducing to 86.88% in the fourth cycle [142]. Similarly, amidoxime-modified bamboo charcoal lost 26.46% of its adsorption capacity for U (VI) removal after eight cycles [150]. Additionally, after five cycles, bamboo-based zeolite-activated carbon maintained 80.9% of initial adsorption efficiency (87.4%) for Cu2+ [108].
Bamboo biochar, enhanced with pre-magnetic properties, cross-linked with a Ca–Mg–Al layered double hydroxide composite initially removed As (III) and Cd (II) with efficiencies of 98.7% and 99.6%, respectively, but these declined to 75.7% and 86.3% after five cycles [122]. Bamboo biochar modified with chitosan achieved a Cd (II) adsorption rate of 71.7% and a desorption rate of 65.92% after five cycles [137]. Magnetic bamboo biochar enhanced with EDTA and chitosan retained 83.2% of its initial Cd (II) (34.5 mg/g) adsorption capacity and 80.5% for Zn (II) (32.9 mg/g) after eight cycles [70]. Amino-enhanced bamboo biochar reinforced by nano-zero-valent iron maintained Cr (VI) removal efficiency above 80% after three regeneration cycles using 1 M NaBH4 [128].
Several critical factors influence the regeneration efficiency of bamboo-based adsorbents, including the type and concentration of desorbing agents, surface modifications, and operational conditions. The choice of desorption agent significantly impacts recovery—for instance, HCl concentration affects Cd2+ desorption rates, with 0.05 M showing optimal results. Surface modifications, particularly chitosan treatment, substantially improve regeneration efficiency and maintain removal capacity across multiple cycles. The number of regeneration cycles also plays a crucial role, as most of the adsorbents show a gradual decline in performance over successive cycles, although the rate of decline varies based on the specific modification and target metal ion. Temperature, contact time, and the presence of competing ions in the desorption medium further affect the regeneration process and subsequent adsorption efficiency. The desorption and regeneration efficiency of various bamboo-based adsorbents for heavy metal removal, including the types of adsorbents, target metals, elution agents, and the corresponding efficiencies after multiple regeneration cycles, are summarized in Table 3.

Table 3.
An overview of the types of adsorbents, raw materials, and performance parameters used in metal adsorption studies.
5. Water Purification Filtration Setup Using Bamboo Adsorbents
Direct exposure to heavy metals in drinking water beyond the allowable tolerable limit can adversely affect human health [153]. Hence, a suitable filtration system is essential for the removal of heavy metals from water before its consumption. Ariyadi et al. explored the design of a water purification system using active charcoal media derived from bamboo to remove heavy metal pollution, specifically iron. The water purifier contained two parts: one buffer part and the second purifier part. The purifier was built with a 61 cm × 61 cm × 150 cm iron frame, two glass bottles connected by 2-inch pipes, and a 20-liter water reservoir (Figure 11). The experiment was repeated using different length variations of activated charcoal filling, as follows: 5 cm, 10 cm, and 15 cm. The initial concentration of Fe at the inlet was 5.59 mg/L, which was reduced to <0.014 mg/L at the outlet. The results showed that the changes in filling length did not significantly influence the adsorption results. However, the adsorption capacity of activated charcoal is more effective for filling lengths of more than 5 cm [88].
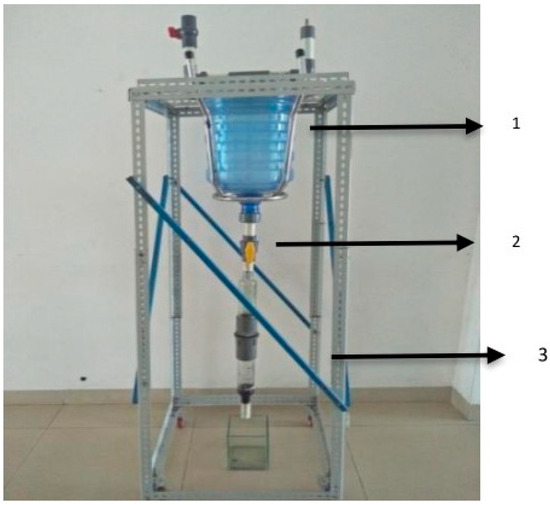
Figure 11.
Water purifier equipment (1. Water storage tube; 2. Water purifier tube; 3. Trestle/a stand for the water purifier equipment (Adapted from Reference [88]).
A cartridge-based water treatment device for domestic use, utilizing iron oxide-infused bamboo biochar (Fe-BC), has been proposed to decontaminate arsenic-contaminated water. The system features Fe-BC (specific amount) packed inside a cloth bag, which can be installed either between the built-in covers of a polypropylene cartridge (Figure 12a) or within a two-piece funnel-type cartridge (Figure 12b,c). Arsenic-contaminated water is introduced into the cartridge, where it flows through the biochar composite, allowing the arsenic to be adsorbed, resulting in arsenic-free drinking water. The cloth-bag system represents the minimal viable product, providing a low-cost and user-friendly option that can be easily manufactured, distributed, and integrated into daily routines. According to Alchouron et al., this is an initial design that has not yet undergone complete validation [154].
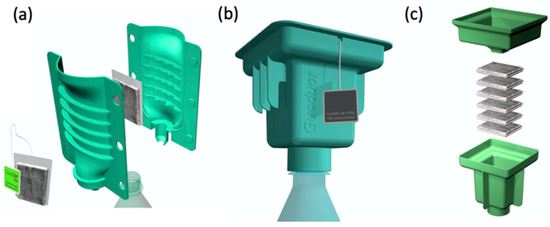
Figure 12.
Proposed designs for household arsenic-safe water provision. BC-Fe is packed inside cloth bags and inserted between polypropylene cartridge covers (a) or inside a two funnel-type piece cartridge (b,c). Reprinted with permission of Elsevier Inc. from Reference [154].
A gravity-fed filtration system [155] was constructed using individual acrylic columns (1.8 m height, 10 cm diameter). Each column contained a 30 cm gravel support layer overlaid with 60 cm of filter media, allowing for a 90 cm maximum head loss. The system could process different water sources including raw water, flocculated water, and settled water, with flow rates controlled by inlet valves. The system was tested with different filter media including biochar produced from bamboo through slow pyrolysis, sand, and anthracite. It was operated at a 180 m3/m2/d filtration rate for heavy metal removal assessment using solutions containing copper, iron, and aluminum. The biochar filter demonstrated superior performance in metal removal compared to other media, achieving removal efficiencies of 95.7% for iron, 90.7% for aluminum, and 75.9% for copper [155].
6. Comparison of Bamboo Adsorbents with Other Adsorbents
The major environmental challenge of removing heavy metal from aqueous solutions led to the advancement in various adsorbent materials in recent years. These materials are advantageous because of their large surface area-to-mass ratio, which makes adsorbents efficient while considering a broad variety of substances, mainly heavy metals and other pollutants [137,156]. Bamboo-derived adsorbents have demonstrated a strong ability to adsorb various heavy metals, including arsenic (As) [154], chromium (Cr2+) [78], cadmium (Cd2+) [142], mercury (Hg2+) [106], etc. The adsorption capacity of bamboo-derived materials can be significantly enhanced through various processes such as carbonization and activation resulting in values comparable to or slightly lower than activated carbon [157,158]. To evaluate the true potential of bamboo-based adsorbents, their comparison with other commonly used adsorbents is crucial.
Sadeghi et al. developed an adsorbent that shows remarkable effectiveness, for Hg(II) having an adsorption capacity of 155.61 mg/g at a pH of 6.0 [159]. Similarly, Kharrazi et al. developed a KOH-activated adsorbent with adsorption capacities of 232.56 mg/g and 113.63 mg/g respectively, which effectively removed chromium (Cr6+) and lead (Pb3+) at a pH of 2.0 [160]. As an illustration of the competitiveness of bamboo-derived materials, Mistar et al. reported that activated carbon derived from Bambusa vulgaris var. Striata has a remarkable adsorption capacity of 218.08 mg/g for Hg2+ [161]. Synthetic nanomaterials such as graphene oxides [162] or metal-organic frameworks [163,164] usually have high adsorption capacities, but they are typically more expensive and less sustainable.
The natural polymer chitosan (CS) is broadly used for its adsorptive characteristics. Even so, it has several drawbacks, such as low mechanical strength, instability, difficulties with regeneration due to its minimal surface area, low porosity, and interesting mass transfer resistance [31]. In contrast, adsorbents derived from bamboo offer a viable substitute that exhibits comparable or better performance qualities.
Table 4 presents a compilation of different adsorbents used for cadmium removal, providing their preparation methods, adsorption capacities, and regeneration efficiencies. Bamboo-based adsorbents, like amine-functionalized bamboo powder (AMBP) and chitosan-modified bamboo biochar (CBB), exhibit remarkable effectiveness. AMBP, synthesized through mercerization, epoxidation, and amination, achieves a cadmium adsorption capacity of 14.41 mg/g at a pH of 4–10 with a sustained regeneration over four cycles. CBB exhibits an adsorption capacity of 93.46 mg/g and retains 71.7% efficiency after five cycles, indicating the material’s durability and use [137,165]. Because of the Fe-BC@LDH composite’s synergistic effects with stacked double hydroxides, bamboo biochar performs even better, obtaining a remarkable capacity of 320.7 mg/g [122]. Bamboo-based adsorbents routinely exhibit competitive performance relative to other adsorbents such as neem biomass (79.9 mg/g) [166], palm fiber biochar (79.9 mg/g) [167], and magnetic composites (94.2 mg/g) [122]. For instance, Fe-BC@LDH surpasses the β-cyclodextrin-EDTA-chitosan polymer (202.9 mg/g) [168] and green porous silicate adsorbents (168.92 mg/g) [169], highlighting the superior adsorption ability of bamboo composites. Additionally, tannin/chitosan/bamboo pulp aerogels (TCPA) achieve 52.52 mg/g sorption capacity, indicating the efficiency of bamboo when incorporated into aerogel structures [170]. In terms of mechanisms, bamboo-based adsorbents efficiently capture cadmium ions by using chemisorption, ion exchange, electrostatic interactions, and surface complexation. These adsorbents usually show significant adsorbate–adsorbent interactions and monolayer adsorption by fitting into pseudo-second-order kinetic and Langmuir isotherm models. The adaptability, high surface area, and regeneration capability of bamboo biochar composites further support their appropriateness for extensive environmental remediation.

Table 4.
Performance of adsorbents for the removal of Cd(II) from an aqueous solution.
Materials made from bamboo are superior in terms of sustainability as well as performance. They provide an environmentally beneficial substitute for conventional adsorbents, which can be more expensive and less long-lasting because they are renewable, biodegradable, and non-toxic. For example, compared to synthetic nanomaterials, the production of biochar microspheres derived from bamboo shells is substantially less expensive [182]. Interestingly, adsorbents derived from bamboo frequently exhibit a reduced environmental impact, which makes them a desirable choice for environmentally friendly water treatment technologies.
Bamboo grows rapidly, flourishes in different climates, and regenerates easily. Bamboo-based adsorbents are cost-effective, as leftover bamboo from industries like furniture, paper production, and construction can be reused, reducing its cost [45,183,184,185]. Conventional adsorbents like activated carbon are produced from raw materials such as coal or wood. The processing of these materials is expensive, which will contribute to the overall cost of activated carbon. The material cost of coconut shell-activated carbon is higher compared to wood-based variants [186]. Another class of adsorbents like zeolites are obtained through the mining of natural deposits or by artificial methods. But for both cases, it involves the availability of significant raw materials and processing expenses [187]. Compared with conventional adsorbents, carbonization and activation techniques used in the production of bamboo-based adsorbents are generally less energy-intensive and use fewer chemicals. For example, carbonization and steam activation can be used to create activated carbon from bamboo residues; the ideal activation temperature is 800 °C for 120 min [183]. When compared to traditional adsorbent production processes that require chemical impregnation or steam activation, the chemical activation of bamboo employing mild chemicals can, in fact, lower production expenses. The production of activated carbon from bamboo waste provided a comprehensive economic analysis, estimating internal return rates for independent and integrated production plants to be 13.0% and 20.1%, respectively [188].
The prices of commercial activated carbon and bioadsorbents were compared in another study. According to the research, the price of bioadsorbents ranges from INR 4.4 to INR 36.89/kg, whereas commercial activated carbon costs INR 500/kg. This significant cost difference highlights the financial benefit of employing bioadsorbents, such as activated carbon generated from bamboo, for the removal of heavy metals [189]. A feasibility study evaluated the production of activated carbon from bamboo scaffolding waste with a throughput of 30 tons per day [190]. Two scenarios were compared: a stand-alone carbonization plant with a total capital investment of USD 7,430,465 and an integrated plant at USD 6,430,203, saving USD 1 million in capital costs by integrating the bamboo carbonization plant into other major processing facilities. Annual production costs are USD 2,829,678 for the stand-alone plant and USD 2.5 million for the integrated setup. The stand-alone plant offers a return on investment (ROI) of 18.2%, an internal rate of return (IRR) of 13.0%, and a 6-year payback period. The integrated plant improves profitability with a 26.1% ROI, a 20.1% IRR, and a 4-year payback period, highlighting significant economic advantages [190]. Zhang et al. assessed the economic viability of building and operating an industrial plant, yielding 500 tons per year of bamboo biochar. The payback period is 2.58 years, the IRR is 38.8%, and the net present value (NPV) is USD 486,700, surpassing the forestry industry benchmark of 11%. This indicates higher profits and a superior ROI for bamboo biochar production [191].
7. Disadvantages and Challenges in Practical Implementation
The bamboo family, part of the Poaceae family and subfamily Bambusoideae, consists of evergreen, perennial plants. Known for its high strength-to-weight ratio, bamboo is a versatile building material, with some species capable of growing up to 90 cm in just 24 h due to its unique rhizome system [166]. Bamboo charcoal, with a larger pore surface area than wood charcoal, is more effective at cleaning water [111]. Additionally, bamboo biochar, which has higher carbon content, hydrophobicity, and aromaticity than rice husk biochar, is ideal for heavy metal removal due to its low humification [142]. However, premature, or improper cutting of bamboo reduces the overall biomass, ultimately diminishing the ecosystem’s ability to sequester carbon [167]. These unique properties make bamboo a promising material for water purification, though sustainable management practices are crucial to maximizing its environmental benefits and ensuring long-term carbon sequestration.
Bamboo is an eco-friendly adsorbent due to its quick growth cycles and smaller environmental impact compared to traditional materials like wood or coal. It can sequester carbon, benefiting the environment. However, improper management can lead to land-use changes or deforestation, making sustainable cultivation and harvesting crucial [192]. Disposal of adsorbents saturated with heavy metals needs careful consideration to avoid secondary pollution. Traditional methods release energy-intensive and potentially hazardous contaminants into the environment during regeneration [45]. According to some studies, bamboo adsorbents have high removal efficiency for some metals, but other metals might not be as well adsorbed, which would restrict their usefulness in practical situations. Research is needed to standardize bamboo-based adsorbents, particularly in size, surface area, and pore structure, to ensure consistent results on large scales. Bamboo adsorbents lack uniformity in preparation and performance compared to conventional adsorbents. Despite their economic and environmental benefits, they face obstacles like large-scale efficiency, regeneration costs, restricted adsorption capacity, quality control, etc. To make bamboo-based adsorbents more suitable for industrial applications, further investigation and technical developments are needed to optimize regeneration processes and increase adsorption capacity, and standardization.
Another challenge in the application of bamboo-based adsorbent is the disposal of chemicals used for the preparation and regeneration of adsorbent. The ecological impacts of modified bamboo-based adsorbents, particularly the release of residual chemicals during desorption or regeneration, require thorough evaluation. A key concern is the release of residual chemicals from modified bamboo-based adsorbents during desorption or regeneration, which can harm ecosystems if spent adsorbents are improperly handled.
Adsorbent regeneration methods include biological, thermal, and chemical techniques, each with unique advantages and limitations. Biological regeneration, suitable for biodegradable contaminants, fully restores adsorption capacity but is slow and prone to pore fouling. Thermal regeneration, widely used in industrial applications, is effective but requires high temperatures, is costly, and can release harmful gases while degrading adsorbents. Chemical regeneration is fast and cost-effective but may alter adsorbent properties, produce waste, and recover adsorption capacity incompletely. Factors such as adsorbate type, adsorbent properties, and process conditions influence the effectiveness of each method [193,194]. Even though the amount of chemicals used for adsorbent preparation and regeneration is minimal, it should be properly disposed of as per the rules. Efforts should be made for the recovery of chemicals so that the cost of treatment can be reduced.
8. Conclusions
Different types of bamboo-based adsorbents like bamboo biochar, bamboo charcoal, bamboo activated carbon, and modified bamboo can effectively adsorb heavy metals from water. These bamboo-based adsorbents can be synthesized by various methods like pyrolysis, carbonization, torrefaction, etc. Depending upon the preparation methods and modification process, the properties of the adsorbent can be enhanced and the adsorption capacity can be increased, making bamboo-based adsorbents a potential and effective solution for heavy metal contamination. These adsorbents exhibit strong potential for the removal of heavy metals and other contaminants from water because of their unique physical, chemical, and morphological properties. Incorporating bamboo charcoal into chitosan-based composites or activating it with steam, KOH/NaOH mixtures or nitrogen doping can expand pore volume and specific surface area, thereby improving adsorption rates. However, treatments like KMnO4 or ZnCl2 followed by oxidation may reduce surface area and porosity. Functional groups like hydroxyl, carboxyl, and carbonyl introduced through chemical modifications, play a key role in metal ion binding, improving adsorption capabilities which can be identified by using FTIR. Additionally, the surface morphology of bamboo adsorbents, ranging from smooth to rough, can be optimized using various modification methods, by using SEM analysis.
Bamboo-based adsorbents showed considerable efficacy in removing heavy metals; with their adsorption capacity being influenced by factors such as functional groups present on the adsorbent surface, solution pH, the presence of competing ions, and contact time. pH influences the ionization of metal ions and the surface charge of the adsorbent, which affects the adsorption capacity of the adsorbent. As contact time increases, the removal efficiency of the adsorbent increases, ultimately reaching an equilibrium stage. Moreover, the removal of metal ions can be impeded by competing ions. Even with the presence of competing ions, many bamboo-based adsorbents showed strong performance. They also exhibit favorable reusability, with effective regeneration and desorption abilities. The reported mechanisms were reduction, electrostatic adsorption, precipitation, ion exchange, hydrogen bonding, physical adsorption, complexation, and chemisorption.
The superior performance of bamboo adsorbents can be combined with other water treatment methods. Ti-Sn/BC and Ti-Sn-Ce/BC particle electrodes, a new class of bamboo biochar (BC) electrodes, were successfully prepared using bamboo pyrolysis and sol–gel methods and were utilized in a three-dimensional electrochemical reaction system (3ders) for the efficient treatment of coking wastewater [164]. Luo et al. synthesized bamboo hydrochar (BHC), cornstalk hydrochar (CHC), and pine wood hydrochar (PHC) by one-step hydrothermal carbonization and used them as dual-functional adsorbent/photo reductors to remove Cr (VI) from water. BHC eliminated almost 100% of Cr (VI) in 60 min, while CHC and PHC achieved 90% removal in the same timeframe [31]. Bamboo-based activated carbon, due to its high electrical conductivity, hierarchical pore structure, and large surface area, is a promising candidate for seawater desalination. A composite of acidified bamboo-based activated carbon (BACa) and manganese dioxide (MnO2) was synthesized using a simple co-precipitation method and used as a high-performance electrode material for capacitive deionization [165].
The abundant availability, affordability, and environmental sustainability of bamboo-based adsorbents make them an efficient alternative to prevalent adsorbents. Environmental friendliness and regeneration efficiency are two benefits of using adsorbents based on bamboo. However, some issues must be resolved, like sluggish equilibrium rates and reduced regeneration efficiency after several cycles. To enhance their effectiveness and increase their range of applications in various environmental settings, future research should concentrate on improving the characteristics of adsorbents derived from bamboo.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w17030454/s1, Figure S1: Cadmium speciation distribution in aqueous solutions. Reprinted with permission of Elsevier Inc. from the reference [18]; Figure S2: Adsorption capacities of bamboo adsorbents for various heavy metals at different pH levels; Table S1: Summary of physical, chemical, and morphological properties of bamboo-based adsorbents for heavy metal removal.
Author Contributions
Conceptualization, P.V.N.; writing—original draft preparation, M.N., V.T., A.R., M.V. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
References
- Koller, M.; Saleh, H.M. Introductory Chapter: Introducing Heavy Metals. In Heavy Metals; InTech: London, UK, 2018. [Google Scholar]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022. [Google Scholar]
- Botle, A.; Salgaonkar, S.; Tiwari, R.; Ambadekar, S.; Barabde, G.R. Brief Status of Contamination in Surface Water of Rivers of India by Heavy Metals: A Review with Pollution Indices and Health Risk Assessment. Environ. Geochem. Health 2023, 45, 2779–2801. [Google Scholar] [CrossRef]
- Elumalai, S.; Prabhu, K.; Selvan, G.P.; Ramasamy, P. Review on Heavy Metal Contaminants in Freshwater Fish in South India: Current Situation and Future Perspective. Environ. Sci. Pollut. Res. 2023, 30, 119594–119611. [Google Scholar] [CrossRef] [PubMed]
- Tessema, K.; Lemma, B.; Fetahi, T.; Kebede, E. Accumulation of Heavy Metals in the Physical and Biological Systems of Lake Koka, Ethiopia: Implications for Potential Health Risks. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2020, 25, 314–325. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Pandita, S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. A Review of Ecological Risk Assessment and Associated Health Risks with Heavy Metals in Sediment from India. Int. J. Sediment Res. 2020, 35, 516–526. [Google Scholar] [CrossRef]
- Sharma, A.; Grewal, A.S.; Sharma, D.; Srivastav, A.L. Heavy Metal Contamination in Water: Consequences on Human Health and Environment. In Metals in Water; Elsevier: Amsterdam, The Netherlands, 2023; pp. 39–52. [Google Scholar]
- Maithani, D.; Dasila, H.; Saxena, R.; Tiwari, A.; Bhatt, D.; Rawat, K.; Suyal, D.C. Heavy Metal Pollution in Water: Cause and Remediation Strategies. In Current Status of Fresh Water Microbiology; Springer Nature: Singapore, 2023; pp. 181–204. [Google Scholar]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of Heavy Metal Ions from Aqueous System by Ion-Exchange and Biosorption Methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Rahul, A.K.; Shekhar, S.; Kumar, S. Removal of Heavy Metal from Wastewater Using Ion Exchange with Membrane Filtration from Swarnamukhi River in Tirupati. Mater. Today Proc. 2023, 78, 1–6. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Lamaming, J.; Saalah, S.; Rajin, M.; Ismail, N.M.; Yaser, A.Z. A Review on Bamboo as an Adsorbent for Removal of Pollutants for Wastewater Treatment. Int. J. Chem. Eng. 2022, 2022, 7218759. [Google Scholar] [CrossRef]
- Iroegbu, A.; Ray, S. Bamboos: From Bioresource to Sustainable Materials and Chemicals. Sustainability 2021, 13, 12200. [Google Scholar] [CrossRef]
- The Global Bamboo Market. Available online: https://www.credenceresearch.com/report/bamboos-market (accessed on 17 December 2024).
- Tamang, M.; Nandy, S.; Srinet, R.; Das, A.K.; Padalia, H. Bamboo Mapping Using Earth Observation Data: A Systematic Review. J. Indian Soc. Remote Sens. 2022, 50, 2055–2072. [Google Scholar] [CrossRef]
- FAO of UN. Available online: https://www.fao.org/forest-monitoring/news-and-events/news/news-detail/Putting-bamboo-on-the-map/en (accessed on 17 December 2024).
- Wakejo, W.K.; Meshesha, B.T.; Kang, J.W.; Demesa, A.G. Bamboo Sawdust-Derived High Surface Area Activated Carbon for Remarkable Removal of Paracetamol from Aqueous Solution: Sorption Kinetics, Isotherm, Thermodynamics, and Regeneration Studies. Water Pract. Technol. 2023, 18, 1366–1388. [Google Scholar] [CrossRef]
- Jayachandran, M.; Kishore Babu, S.; Maiyalagan, T.; Rajadurai, N.; Vijayakumar, T. Activated Carbon Derived from Bamboo-Leaf with Effect of Various Aqueous Electrolytes as Electrode Material for Supercapacitor Applications. Mater. Lett. 2021, 301, 130335. [Google Scholar] [CrossRef]
- Bagbi, Y.; Yomgam, P.; Libang, E.; Boruah, B.; Kaur, J.; Jayanthi, S.; Kumar, S.; Dhania, N.K. Waste Bamboo-Derived Magnetically Separable Bamboo-Activated Carbon: From Characterization to Effective Remediation of Fluoride (F−) Ions from Water. RSC Adv. 2024, 14, 24952–24968. [Google Scholar] [CrossRef] [PubMed]
- Jayarambabu, N.; Saraswathi, K.; Akshaykranth, A.; Anitha, N.; Venkatappa Rao, T.; Kumar, R.R. Bamboo-Mediated Silver Nanoparticles Functionalized with Activated Carbon and Their Application for Non-Enzymatic Glucose Sensing. Inorg. Chem. Commun. 2023, 147, 110249. [Google Scholar] [CrossRef]
- Sema, A.I.; Bhattacharyya, J. Biochar Derived from Waste Bamboo Shoots for the Biosorptive Removal of Ferrous Ions from Aqueous Solution. J. Indian Chem. Soc. 2022, 99, 100791. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Singhwane, A.; Dhangar, M.; Mili, M.; Gorhae, N.; Naik, A.; Prashant, N.; Srivastava, A.K.; Verma, S. Bamboo for Producing Charcoal and Biochar for Versatile Applications. Biomass Convers. Biorefinery 2024, 14, 15159–15185. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and Characterization of Biochar Produced from Slow Pyrolysis of Pigeon Pea Stalk and Bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Nandana, E.; Dwivedi, A.H.; Nidheesh, P.V. Role of Biochar in Superoxide-Dominated Dye Degradation in Catalyst-Activated Peroxymonosulphate Process. Chemosphere 2024, 356, 141945. [Google Scholar] [CrossRef]
- Saini, S.; Gill, J.K.; Kaur, J.; Saikia, H.R.; Singh, N.; Kaur, I.; Katnoria, J.K. Biosorption as Environmentally Friendly Technique for Heavy Metal Removal from Wastewater. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 167–181. [Google Scholar]
- Narzary, I.; Mahato, R.K.; Middha, S.K.; Usha, T.; Goyal, A.K. Valorization of Bamboo Charcoal as a Low-Cost Adsorbent for Waste Water Treatment: A Mini Review. Adv. Bamboo Sci. 2024, 7, 100067. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Kumar, M.; Venkateshwaran, G.; Ambika, S.; Bhaskar, S.; Vinay; Ghosh, P. Conversion of Locally Available Materials to Biochar and Activated Carbon for Drinking Water Treatment. Chemosphere 2024, 353, 141566. [Google Scholar] [CrossRef]
- Ambika, S.; Kumar, M.; Pisharody, L.; Malhotra, M.; Kumar, G.; Sreedharan, V.; Singh, L.; Nidheesh, P.V.; Bhatnagar, A. Modified Biochar as a Green Adsorbent for Removal of Hexavalent Chromium from Various Environmental Matrices: Mechanisms, Methods, and Prospects. Chem. Eng. J. 2022, 439, 135716. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Design, Synthesis, and Performance of Adsorbents for Heavy Metal Removal from Wastewater: A Review. J. Mater. Chem. A 2022, 10, 1047–1085. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent Advances in Heavy Metal Removal by Chitosan Based Adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent Advances in Adsorptive Removal of Heavy Metal and Metalloid Ions by Metal Oxide-Based Nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Sharma, A.; Anjana; Rana, H.; Goswami, S. A Comprehensive Review on the Heavy Metal Removal for Water Remediation by the Application of Lignocellulosic Biomass-Derived Nanocellulose. J. Polym. Environ. 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical Review of Bioadsorption on Modified Cellulose and Removal of Divalent Heavy Metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of Heavy Metal Ions by Various Low-Cost Adsorbents: A Review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Mahesh, N.; Balakumar, S.; Shyamalagowri, S.; Manjunathan, J.; Pavithra, M.K.S.; Babu, P.S.; Kamaraj, M.; Govarthanan, M. Carbon-Based Adsorbents as Proficient Tools for the Removal of Heavy Metals from Aqueous Solution: A State of Art-Review Emphasizing Recent Progress and Prospects. Environ. Res. 2022, 213, 113723. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent Advances in Applications of Low-Cost Adsorbents for the Removal of Heavy Metals from Water: A Critical Review. Sep. Purif. Technol. 2022, 278, 119510. [Google Scholar] [CrossRef]
- Mariana, M.; HPS, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent Advances in Activated Carbon Modification Techniques for Enhanced Heavy Metal Adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Raninga, M.; Mudgal, A.; Patel, V.K.; Patel, J.; Kumar Sinha, M. Modification of Activated Carbon-Based Adsorbent for Removal of Industrial Dyes and Heavy Metals: A Review. Mater. Today Proc. 2023, 77, 286–294. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rawat, K.P.; Bhadauria, V.; Singh, H. Recent Trends in the Application of Modified Starch in the Adsorption of Heavy Metals from Water: A Review. Carbohydr. Polym. 2021, 269, 117763. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Adsorptive Potential of Modified Plant-Based Adsorbents for Sequestration of Dyes and Heavy Metals from Wastewater—A Review. J. Water Process Eng. 2021, 42, 102148. [Google Scholar] [CrossRef]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and Kinetics of Adsorption and Removal of Heavy Metals from Wastewater Using Nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar] [CrossRef]
- Kalderis, D.; Seifi, A.; Kieu Trang, T.; Tsubota, T.; Anastopoulos, I.; Manariotis, I.; Pashalidis, I.; Khataee, A. Bamboo-Derived Adsorbents for Environmental Remediation: A Review of Recent Progress. Environ. Res. 2023, 224, 115533. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review On The Comparison Between Slow Pyrolysis And Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Phounglamcheik, A.; Wang, L.; Romar, H.; Kienzl, N.; Brostro, M.; Ramser, K.; Skreiberg, Ø.; Umeki, K. Effects of Pyrolysis Conditions and Feedstocks on the Properties and Gasi Fi Cation Reactivity of Charcoal from Woodchips. Energy Fuels 2020, 34, 8353–8365. [Google Scholar] [CrossRef]
- Erdem, A.; Dogru, M. Process Intensification: Activated Carbon Production from Biochar Produced by Gasification. Johns. Matthey Technol. Rev. 2021, 65, 352–365. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, X.; Deng, S.; Zeng, X.; Yu, Z.; Li, S.; Li, K. Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: A route to a closed carbon loop. Green Chem. 2020, 22, 6836–6845. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of Organic and Inorganic Pollutants onto Biochars: Challenges, Operating Conditions, and Mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Zhang, X.; Bhattacharya, T.; Wang, C.; Kumar, A.; Nidheesh, P.V. Straw-Derived Biochar for the Removal of Antibiotics from Water: Adsorption and Degradation Mechanisms, Recent Advancements and Challenges. Environ. Res. 2023, 237, 116998. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of Sewage Sludge into Biochar: A Potential Resource in Water and Wastewater Treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhattacharya, T. Biochar: A Sustainable Solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, P.; Sharma, J.; Saini, S.; Sharma, P.; Sharma, A. Rice Straw Management through Biofuel, Biochar, Mushroom Cultivation, and Paper Production to Overcome Environmental Pollution in North India. Waste Dispos. Sustain. Energy 2023, 5, 483–510. [Google Scholar] [CrossRef]
- Rubeena, K.K.; Hari Prasad Reddy, P.; Laiju, A.R.; Nidheesh, P. V Iron Impregnated Biochars as Heterogeneous Fenton Catalyst for the Degradation of Acid Red 1 Dye. J. Environ. Manag. 2018, 226, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Ghosh, G.K.; Mishra, V.K.; Choudhury, B.U.; Dutta, S.K.; Hazarika, S.; Kalita, H.; Roy, A.; Singh, N.U.; Gopi, R.; et al. Utilizing Dissimilar Feedstocks Derived Biochar Amendments to Alter Soil Biological Indicators in Acidic Soil of Northeast India. Biomass Convers. Biorefinery 2023, 13, 10203–10214. [Google Scholar] [CrossRef]
- Anand, A.; Pathak, S.; Kumar, V.; Kaushal, P. Biochar Production from Crop Residues, Its Characterization and Utilization for Electricity Generation in India. J. Clean. Prod. 2022, 368, 133074. [Google Scholar] [CrossRef]
- Nakarmi, K.J.; Daneshvar, E.; Eshaq, G.; Puro, L.; Maiti, A.; Nidheesh, P.V.; Wang, H.; Bhatnagar, A. Synthesis of Biochar from Iron-Free and Iron-Containing Microalgal Biomass for the Removal of Pharmaceuticals from Water. Environ. Res. 2022, 214, 114041. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Gopinath, A.; Ranjith, N.; Praveen Akre, A.; Sreedharan, V.; Suresh Kumar, M. Potential Role of Biochar in Advanced Oxidation Processes: A Sustainable Approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Cheng, Y.W.; Liew, R.K.; Wan Mahari, W.A.; Ong, H.C.; Chen, W.H.; Peng, W.; Park, Y.K.; Sonne, C.; Kong, S.H.; et al. Progress in the Torrefaction Technology for Upgrading Oil Palm Wastes to Energy-Dense Biochar: A Review. Renew. Sustain. Energy Rev. 2021, 151, 111645. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, Economical, and Climate-Related Aspects of Biochar Production Technologies: A Literature Review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Kumar, A.; Mishra, R.; You, S.; Singh, L.; Kumar, S.; Kumar, R. Pyrolysis of Waste Biomass and Plastics for Production of Biochar and Its Use for Removal of Heavy Metals from Aqueous Solution. Bioresour. Technol. 2021, 320, 124278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, R.; Li, R.; Ali, A.; Chen, A.; Zhang, Z. Enhanced Aqueous Cr(VI) Removal Using Chitosan-Modified Magnetic Biochars Derived from Bamboo Residues. Chemosphere 2020, 261, 127694. [Google Scholar] [CrossRef]
- Pinisakul, A.; Kruatong, N.; Vinitnantharat, S.; Wilamas, P.; Neamchan, R.; Sukkhee, N.; Werner, D.; Sanghaisuk, S. Arsenic, Iron, and Manganese Adsorption in Single and Trinary Heavy Metal Solution Systems by Bamboo-Derived Biochars. C-J. Carbon Res. 2023, 9, 40. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Wang, T.; Wang, P. Adsorption of Toxic Metal Ion in Agricultural Wastewater by Torrefaction Biochar from Bamboo Shoot Shell. J. Clean. Prod. 2022, 338, 130558. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.M.; Zhang, Q.; Gong, X.M.; Xu, P. Synergistic Removal of Copper and Tetracycline from Aqueous Solution by Steam-Activated Bamboo-Derived Biochar. J. Hazard. Mater. 2020, 384, 121470. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of Modified Biochars Derived from Bamboo Pyrolysis and Their Utilization for Target Component (Furfural) Adsorption. Energy Fuels 2014, 28, 5119–5127. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Cai, J.; Zhang, X.; Zhang, J.; Shao, J. Evaluation and Prediction of Cadmium Removal from Aqueous Solution by Phosphate-Modified Activated Bamboo Biochar. Energy Fuels 2018, 32, 4469–4477. [Google Scholar] [CrossRef]
- Wakejo, W.K.; Meshasha, B.T.; Kang, J.W.; Chebude, Y. Enhanced Ciprofloxacin Removal from Aqueous Solution Using a Chemically Modified Biochar Derived from Bamboo Sawdust: Adsorption Process Optimization with Response Surface Methodology. Adsorpt. Sci. Technol. 2022, 2022, 2699530. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Zhang, Z. A Versatile EDTA and Chitosan Bi-Functionalized Magnetic Bamboo Biochar for Simultaneous Removal of Methyl Orange and Heavy Metals from Complex Wastewater. Environ. Pollut. 2022, 293, 118517. [Google Scholar] [CrossRef]
- Karunaratne, T.N.; Nayanathara, R.M.O.; Navarathna, C.M.; Rodrigo, P.M.; Thirumalai, R.V.K.G.; Jr, C.U.P.; Kim, Y.; Mlsna, T.; Zhang, J. Pyrolytic Synthesis of Graphene—Encapsulated Zero—Valent Iron Nanoparticles Supported on Biochar for Heavy Metal Removal. Biochar 2022, 4, 70. [Google Scholar] [CrossRef]
- Zhao, X.; Li, M.; Zhai, F.; Hou, Y.; Hu, R. Phosphate Modified Hydrochars Produced via Phytic Acid-Assisted Hydrothermal Carbonization for Efficient Removal of U (VI), Pb (II) and Cd (II). J. Environ. Manag. 2021, 298, 113487. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Wu, C.; Huan, W.; Chen, L.; Li, B. Bioresource Technology Enhanced Removal of Cr (VI) by Cation Functionalized Bamboo Hydrochar. Bioresour. Technol. 2022, 347, 126703. [Google Scholar] [CrossRef] [PubMed]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A Review on Current Status of Biochar Uses in Agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef]
- Eshiemogie, S.O.; Iwuchukwu, F.U. Recent Advances in Hydrochar Application for the Adsorptive Removal of Wastewater Pollutants. Chem. Eng. Res. Des. 2022, 184, 419–456. [Google Scholar]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Shyam, S.; Arun, J.; Gopinath, K.P.; Ribhu, G.; Ashish, M.; Ajay, S. Biomass as Source for Hydrochar and Biochar Production to Recover Phosphates from Wastewater: A Review on Challenges, Commercialization, and Future Perspectives. Chemosphere 2022, 286, 131490. [Google Scholar] [CrossRef]
- Li, S.Y.; Teng, H.J.; Guo, J.Z.; Wang, Y.X.; Li, B. Enhanced Removal of Cr(VI) by Nitrogen-Doped Hydrochar Prepared from Bamboo and Ammonium Chloride. Bioresour. Technol. 2021, 342, 126028. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zimmerman, A.R.; Hu, X.; Gao, B. Chemosphere Removal of Aqueous Cr (VI) by Zn- and Al-Modi Fi Ed Hydrochar. Chemosphere 2020, 260, 127610. [Google Scholar] [CrossRef]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.; Bucheli, T. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef]
- Kabir Ahmad, R.; Anwar Sulaiman, S.; Yusup, S.; Sham Dol, S.; Inayat, M.; Aminu Umar, H. Exploring the Potential of Coconut Shell Biomass for Charcoal Production. Ain Shams Eng. J. 2022, 13, 101499. [Google Scholar] [CrossRef]
- Xiao, F.; Bedane, A.H.; Mallula, S.; Sasi, P.C.; Alinezhad, A.; Soli, D.; Hagen, Z.M.; Mann, M.D. Production of Granular Activated Carbon by Thermal Air Oxidation of Biomass Charcoal/Biochar for Water Treatment in Rural Communities: A Mechanistic Investigation. Chem. Eng. J. Adv. 2020, 4, 100035. [Google Scholar] [CrossRef]
- Maske, V.A.; Jangale, S.; Vyavahare, S.; More, A.P. Development of Coconut Shell Charcoal-Reinforced Starch Superabsorbent Composites, with the Comparative Study of Epichlorohydrin and Citric Acid as the Crosslinking Agents. Starch Stärke 2024. [Google Scholar] [CrossRef]
- Castro, J.P.; Nobre, J.R.C.; Napoli, A.; Bianchi, M.L.; Moulin, J.C.; Chiou, B.S.; Williams, T.G.; Wood, D.F.; Avena-Bustillos, R.J.; Orts, W.J.; et al. Massaranduba Sawdust: A Potential Source of Charcoal and Activated Carbon. Polymers 2019, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, C.I.; Onuegbu, T.U.; Okafor, I.F.; Igwenagu, P.C. Utilization of Agricultural Waste in the Production of Charcoal Briquette Blend as Alternative Source of Energy. IOP Conf. Ser. Earth Environ. Sci. 2023, 1178, 012015. [Google Scholar] [CrossRef]
- Bai, S.; Wang, T.; Tian, Z.; Cao, K.; Li, J. Facile Preparation of Porous Biomass Charcoal from Peanut Shell as Adsorbent. Sci. Rep. 2020, 10, 15845. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, W.; Wang, P.; Ding, Y.; Zhou, S. Adsorption of Cu (II) and Zn (II) in Aqueous Solution by Modified Bamboo Charcoal. Environ. Geochem. Health 2024, 46, 182. [Google Scholar] [CrossRef]
- Ariyadi, R.; Fatmawati, S.; Syar, N.I.; Nasir, M.; Maulina, D. Suhartono Design of Water Purification Polluted by Heavy Metal Fe with Active Charcoal Media of Palm Oil and Bamboo. J. Phys. Conf. Ser. 2021, 1796, 012054. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Lin, S. Adsorption Characteristics of Modified Bamboo Charcoal on Cu(II) and Cd(II) in Water. Toxics 2022, 10, 787. [Google Scholar] [CrossRef]
- Thotagamuge, R.; Kooh, M.R.R.; Mahadi, A.H.; Lim, C.M.; Abu, M.; Jan, A.; Hanipah, A.H.A.; Khiong, Y.Y.; Shofry, A. Copper Modified Activated Bamboo Charcoal to Enhance Adsorption of Heavy Metals from Industrial Wastewater. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100562. [Google Scholar] [CrossRef]
- Kumar, N.; Pandey, A.; Rosy; Sharma, Y.C. A Review on Sustainable Mesoporous Activated Carbon as Adsorbent for Efficient Removal of Hazardous Dyes from Industrial Wastewater. J. Water Process Eng. 2023, 54, 104054. [Google Scholar] [CrossRef]
- Mondal, M.K.; Garg, R. A Comprehensive Review on Removal of Arsenic Using Activated Carbon Prepared from Easily Available Waste Materials. Environ. Sci. Pollut. Res. 2017, 24, 13295–13306. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Trisha, M.; Kaviya Selvi, S.; Gokul, R.; Renuka, V. Recent Research Progress on Modified Activated Carbon from Biomass for the Treatment of Wastewater: A Critical Review. Environ. Qual. Manag. 2024, 33, 907–928. [Google Scholar] [CrossRef]
- Nath, H.; Das, J.; Debnath, C.; Sarkar, B.; Saxena, R.; Barma, S.D. Development of Lignocellulosic Biomass Derived Cu and Zn Doped Highly Porous Activated Carbon and Its Utilization in the Anti-Microbial Treatment. Environ. Chem. Ecotoxicol. 2023, 5, 155–164. [Google Scholar] [CrossRef]
- Singh, K.; Lohchab, R.K.; Kumari, M.; Beniwal, V. Potential of TiO 2 Loaded Almond Shell Derived Activated Carbon for Leachate Treatment: Isotherms, Kinetics, and Response Surface Methodology. Int. J. Environ. Anal. Chem. 2023, 103, 7625–7646. [Google Scholar] [CrossRef]
- Mandal, S.; Stephen, D.; Janardhanan, S.K. Activated Carbon with Composite Pore Structures Made from Peanut Shell and Areca Nut Fibers as Sustainable Adsorbent Material for the Efficient Removal of Active Pharmaceuticals from Aqueous Media. RSC Sustain. 2024, 2, 3022–3035. [Google Scholar] [CrossRef]
- Chauhan, P.R.; Raveesh, G.; Pal, K.; Goyal, R.; Tyagi, S.K. Production of Biomass Derived Highly Porous Activated Carbon: A Solution towards in-Situ Burning of Crop Residues in India. Bioresour. Technol. Reports 2023, 22, 101425. [Google Scholar] [CrossRef]
- Sahu, A.; Sen, S.; Mishra, S.C. A Comparative Study on Characterizations of Biomass Derived Activated Carbons Prepared by Both Normal and Inert Atmospheric Heating Conditions. J. Indian Chem. Soc. 2023, 100, 100943. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Singh, B.; Acharya, B. A Comprehensive Review on Activated Carbon from Pyrolysis of Lignocellulosic Biomass: An Application for Energy and the Environment. Carbon Resour. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Nam, H.; Wang, S.; Jeong, H.R. TMA and H2S Gas Removals Using Metal Loaded on Rice Husk Activated Carbon for Indoor Air Purification. Fuel 2018, 213, 186–194. [Google Scholar] [CrossRef]
- Maniarasu, R.; Rathore, S.K.; Murugan, S. Biomass-Based Activated Carbon for CO2 Adsorption–A Review. Energy Environ. 2023, 34, 1674–1721. [Google Scholar] [CrossRef]
- Ramalingam, G.; Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Hoang, T.K.A. Biomass and Waste Derived Silica, Activated Carbon and Ammonia-Based Materials for Energy-Related Applications—A Review. Fuel 2024, 355, 129490. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Ahmad, N. An Overview of Activated Carbon Preparation from Various Precursors. Sci. Res. J. 2023, 20, 51–89. [Google Scholar] [CrossRef]
- Asrat, Y.; Adugna, A.T.; Kamaraj, M.; Beyan, S.M. Adsorption Phenomenon of Arundinaria Alpina Stem-Based Activated Carbon for the Removal of Lead from Aqueous Solution. J. Chem. 2021, 2021, 5554353. [Google Scholar] [CrossRef]
- Alfatah, T.; Mistar, E.M.; Supardan, M.D. Porous Structure and Adsorptive Properties of Activated Carbon Derived from Bambusa Vulgaris Striata by Two-Stage KOH/NaOH Mixture Activation for Hg2+ Removal. J. Water Process Eng. 2021, 43, 102294. [Google Scholar] [CrossRef]
- González, P.G.; Pliego-Cuervo, Y.B. Adsorption of Cd(II), Hg(II) and Zn(II) from Aqueous Solution Using Mesoporous Activated Carbon Produced from Bambusa Vulgaris Striata. Chem. Eng. Res. Des. 2014, 92, 2715–2724. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Zheng, F.; Wang, J.; Chen, L.; Hu, P.; Zhen, Q.; Bashir, S.; Liu, J.L. Efficient Removal of Water Pollutants by Hierarchical Porous Zeolite-Activated Carbon Prepared from Coal Gangue and Bamboo. J. Clean. Prod. 2021, 325, 129322. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified Biochar: Synthesis and Mechanism for Removal of Environmental Heavy Metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Zheng, Z.; Yan, N.; Lou, Z.; Jiang, X.; Zhang, X.; Chen, S.; Xu, R.; Liu, C. Modification and Application of Bamboo-Based Materials: A Review—Part I: Modification Methods and Mechanisms. Forests 2023, 14, 2219. [Google Scholar] [CrossRef]
- Xue, X.; Yuan, W.; Zheng, Z.; Zhang, J.; Ao, C.; Zhao, J.; Wang, Q.; Zhang, W.; Lu, C. Iron-Loaded Carbon Aerogels Derived from Bamboo Cellulose Fibers as Efficient Adsorbents for Cr(Vi) Removal. Polymers 2021, 13, 4338. [Google Scholar] [CrossRef] [PubMed]
- Chandrawansha, S.I.; Selladurai, A. Cellulose Obtained from Banana Stem and Bamboo Stem Extraction, Modification, Characterization and Metal Adsorption Properties of Cellulose Obtained from Banana Stem and Bamboo Stem; University of Jaffna: Kokuvil East, Sri Lanka, 2024; Available online: https://vingnanam.sljol.info/articles/4241/files/66731b059e624.pdf (accessed on 17 December 2024).
- Chen, H.; Cheng, Y.; Zhu, Z.; He, H.; Zhang, L.; Li, N.; Zhu, Y. Adsorption of Pb(II) from Aqueous Solution by Mercerized Moso Bamboo Chemically Modified with Pyromellitic Dianhydride. J. Environ. Eng. 2020, 146, 04019127. [Google Scholar] [CrossRef]
- Tian, A.; Xiaojun, J.; Qingyu, L. Novel Adsorbents Based upon Carboxylic Acid-Modified Phyllostachys Pubescens Powder: Preparation, Characterization and Application for Adsorbing Lead(II) from Aqueous Solution. Sep. Sci. Technol. 2020, 55, 1249–1259. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Palai, T.; Kaushal, R. Surface Functionalization of Bamboo Leave Mediated Synthesized SiO2 Nanoparticles: Study of Adsorption Mechanism, Isotherms and Enhanced Adsorption Capacity for Removal of Cr (VI) from Aqueous Solution. Environ. Res. 2022, 214, 113761. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH Activation of Biochar: Experimental and Modeling Studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Chu, B.; Terao, K.; Amano, Y.; Machida, M. Adsorption Behavior of Cr(Vi) by n-Doped Biochar Derived from Bamboo. Water Pract. Technol. 2020, 15, 170–181. [Google Scholar] [CrossRef]
- Chu, B.; Amano, Y.; Machida, M. Preparation of Bamboo-Based Oxidized Biochar for Simultaneous Removal of Cd(II) and Cr(VI) from Aqueous Solutions. Desalin. Water Treat. 2019, 168, 269–281. [Google Scholar] [CrossRef]
- Song, W.; Zhu, M.; Zhang, S. Comparison of the Properties of Fiberboard Composites with Bamboo Green, Wood, or Their Combination as the Fibrous Raw Material. BioResources 2018, 13, 3315–3334. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Sik, Y.; Khan, N.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of Bamboo and Rice Straw Biochars on the Mobility and Redistribution of Heavy Metals (Cd, Cu, Pb and Zn) in Contaminated Soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Alchouron, J.; Navarathna, C.; Chludil, H.D.; Dewage, N.B.; Perez, F.; Hassan, E.B.; Pittman, C.U.; Vega, A.S.; Mlsna, T.E. Assessing South American Guadua Chacoensis Bamboo Biochar and Fe3O4 Nanoparticle Dispersed Analogues for Aqueous Arsenic(V) Remediation. Sci. Total Environ. 2020, 706, 135943. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Li, L.; Huang, X.; Wang, G.; Zhu, C. Pre-Magnetic Bamboo Biochar Cross-Linked Ca–Mg–Al Layered Double-Hydroxide Composite: High-Efficiency Removal of As(III) and Cd(II) from Aqueous Solutions and Insight into the Mechanism of Simultaneous Purification. Sci. Total Environ. 2022, 823, 153743. [Google Scholar] [CrossRef]
- Jena, L.; Soren, D.; Deheri, P.K.; Pattojoshi, P. Preparation, Characterization and Optical Properties Evaluations of Bamboo Charcoal. Curr. Res. Green Sustain. Chem. 2021, 4, 100077. [Google Scholar] [CrossRef]
- Najihah, N.; Rosli, B.; Ming, L.C.; Mahadi, A.H.; Wattanasiriwech, S.; Lim, R.C.; Kumara, N.T.R.N. Ruthenium Dye (N3) Removal from Simulated Wastewater Using Bamboo Charcoal and Activated Bamboo Charcoal. Key Eng. Mater. 2018, 765, 92–98. [Google Scholar] [CrossRef]
- Munirah, N.R.; Noriman, N.Z.; Salihin, M.Z.; Fatin, M.H.; Sam, S.T. Characteristics of Carbonized Bamboo at Various Temperatures. Appl. Mech. Mater. 2015, 755, 115–119. [Google Scholar] [CrossRef]
- Lalhruaitluanga, H.; Jayaram, K.; Prasad, M.N.V.; Kumar, K.K. Lead(II) Adsorption from Aqueous Solutions by Raw and Activated Charcoals of Melocanna Baccifera Roxburgh (Bamboo)-A Comparative Study. J. Hazard. Mater. 2010, 175, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wilamas, A.; Vinitnantharat, S.; Pinisakul, A. Manganese Adsorption onto Permanganate-Modified Bamboo Biochars from Groundwater. Sustainability 2023, 15, 6831. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Liu, H.; Zhang, Y.; Fan, Y.; Mo, S.; Li, H.; Wang, J.; Lin, H. Novel Amino-Modified Bamboo-Derived Biochar-Supported Nano-Zero-Valent Iron (AMBBC-NZVI) Composite for Efficient Cr(VI) Removal from Aqueous Solution. Environ. Sci. Pollut. Res. Int. 2023, 30, 119935–119946. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gui, C.; Ji, Y.; Li, X.; Rao, F.; Huan, W.; Li, L. Changes in Chemical and Thermal Properties of Bamboo after Delignification Treatment. Polymers 2022, 14, 2537. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, S.; Kuba, T.; Toyohara, Y.; Kamida, S.; Uchikawa, Y. Development of Ion-Exchange Properties of Bamboo Charcoal Modified with Concentrated Nitric Acid. IOP Conf. Ser. Earth Environ. Sci. 2017, 82, 012002. [Google Scholar] [CrossRef]
- Hu, H.; Sun, L.; Jiang, B.; Wu, H.; Huang, Q.; Chen, X. Low Concentration Re(VII) Recovery from Acidic Solution by Cu-Biochar Composite Prepared from Bamboo (Acidosasa longiligula) Shoot Shell. Miner. Eng. 2018, 124, 123–136. [Google Scholar] [CrossRef]
- Deepika; Reddy, S.R.; Dixit, V.; Verma, S.; Yadav, P.K. Comparative Study on Estimation of Carbon Content in Different Parts of Selected Bamboo Species. Int. J. Environ. Clim. Change 2022, 12, 908–914. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Chen, C.; Li, X.; Deng, Q.; Li, D. Mechanical, Electrical, and Thermal Properties of Highly Filled Bamboo Charcoal/Ultra-High Molecular Weight Polyethylene Composites. Polym. Compos. 2018, 39, E1858–E1866. [Google Scholar] [CrossRef]
- Mukesh, B.; Kalpit, S.; Jyeshtharaj, B.J.; Madhura, Y.; Abhishek, S. Advanced Thermo-Chemical Treatment of Waste Bambusa Vulgaris for Sustainable Resource Recovery. Mater. Res. Proc. 2023, 29, 192–200. [Google Scholar] [CrossRef]
- Saini, S.; Katnoria, J.K.; Kaur, I. A Comparative Study for Removal of Cadmium(II) Ions Using Unmodified and NTA-Modified Dendrocalamus Strictus Charcoal Powder. J. Environ. Health Sci. Eng. 2019, 17, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shang, T.; Jin, X.; Gao, J.; Zhao, Q. Study of Chromium(vi) Removal from Aqueous Solution Using Nitrogen-Enriched Activated Carbon Based Bamboo Processing Residues. RSC Adv. 2015, 5, 784–790. [Google Scholar] [CrossRef]
- Huang, A.; Bai, W.; Yang, S.; Wang, Z.; Wu, N.; Zhang, Y.; Ji, N.; Li, D. Adsorption Characteristics of Chitosan-Modified Bamboo Biochar in Cd(II) Contaminated Water. J. Chem. 2022, 2022, 6303252. [Google Scholar] [CrossRef]
- Nitayaphat, W.; Jintakosol, T. Removal of Silver(I) from Aqueous Solutions by Chitosan/Bamboo Charcoal Composite Beads. J. Clean. Prod. 2015, 87, 850–855. [Google Scholar] [CrossRef]
- Wu, J.; Ren, D.; Zhang, X.; Chen, Z.; Zhang, S.; Li, S.; Fu, L. The Adsorption Properties of Biochar Derived from Woody Plants or Bamboo for Cadmium in Aqueous Solution. Desalin. Water Treat. 2019, 160, 268–275. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, J.-Z.; Bai, L.-Q.; Chen, X.-Q.; Li, B. High Effective Adsorption of Pb(II) from Solution by Biochar Derived from Torrefaction of Ammonium Persulphate Pretreated Bamboo. Bioresour. Technol. 2021, 323, 124616. [Google Scholar] [CrossRef]
- Angthararuk, D.; Phasuk, S.; Takolpuckdee, P. Low-Cost Biochar Derived from Bamboo Waste for Removal of Heavy Metal in Aqueous Solution. J. Food Health Bioenviron. Sci. 2022, 15, 34–42. [Google Scholar]
- Tang, W.; Cai, N.; Xie, H.; Liu, Y.; Wang, Z.; Liao, Y.; Wei, T.; Zhang, C.; Fu, Z.; Yin, D. Efficient Adsorption Removal of Cd2+ from Aqueous Solutions by HNO3 Modified Bamboo-Derived Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2020, 729, 012081. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, X.; Zhang, Y.; Tang, Q.; Yuan, Z.; De Hoop, C.F.; Cao, J.; Hao, S.; Liang, T.; Li, F.; et al. Bamboo-Based Biofoam Adsorbents for the Adsorption of Cationic Pollutants in Wastewater: Methylene Blue and Cu(II). ACS Omega 2021, 6, 23447–23459. [Google Scholar] [CrossRef] [PubMed]
- Sable, H.; Kumar, V.; Mishra, R.; Singh, V.; Roy, A.; Rai, A.K.; Ranjan, N.; Rustagi, S.; Pandit, S. Bamboo Stem Derived Biochar for Biosorption of Cadmium (II) Ions from Contaminated Wastewater. Environ. Nanotechno. Monit. Manag. 2024, 21, 100936. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Tu, R.; Lu, C.; He, X.; Zhang, W. Mechanically Robust, Flame-Retardant and Anti-Bacterial Nanocomposite Films Comprised of Cellulose Nanofibrils and Magnesium Hydroxide Nanoplatelets in a Regenerated Cellulose Matrix. Cellulose 2014, 21, 1859–1872. [Google Scholar] [CrossRef]
- Lou, Z.; Zhao, Z.; Li, Y.; Shan, W.; Xiong, Y.; Fang, D.; Yue, S.; Zang, S. Contribution of Tertiary Amino Groups to Re(VII) Biosorption on Modified Corn Stalk: Competitiveness and Regularity. Bioresour. Technol. 2013, 133, 546–554. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from Aqueous Solutions by Biochar Derived from KMnO4 Treated Hickory Wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Van Hien, N.; Valsami-Jones, E.; Vinh, N.C.; Phu, T.T.; Tam, N.T.T.; Lynch, I. Effectiveness of Different Biochar in Aqueous Zinc Removal: Correlation with Physicochemical Characteristics. Bioresour. Technol. Reports 2020, 11, 100466. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Zhang, H.; Liu, Q.; Yu, J.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Anti-Bacterial and Super-Hydrophilic Bamboo Charcoal with Amidoxime Modified for Efficient and Selective Uranium Extraction from Seawater. J. Colloid Interface Sci. 2021, 598, 455–463. [Google Scholar] [CrossRef]
- Chen, G.; Viengvilay, K.; Yu, W.; Mao, T.; Qu, Z.; Liang, B.; Chen, Z.; Li, Z. Effect of Different Modification Methods on the Adsorption of Manganese by Biochar from Rice Straw, Coconut Shell, and Bamboo. ACS Omega 2023, 8, 28467–28474. [Google Scholar] [CrossRef]
- Hassan, A.; Kaewsichan, L. Removal of Pb(II) from Aqueous Solutions Using Mixtures of Bamboo Biochar and Calcium Sulphate, and Hydroxyapatite and Calcium Sulphate. EnvironmentAsia 2016, 9, 37–44. [Google Scholar]
- Emmanuel, U.C.; Chukwudi, M.I.; Monday, S.S.; Anthony, A.I. Human Health Risk Assessment of Heavy Metals in Drinking Water Sources in Three Senatorial Districts of Anambra State, Nigeria. Toxicol. Rep. 2022, 9, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Alchouron, J.; Navarathna, C.; Rodrigo, P.M.; Snyder, A.; Chludil, H.D.; Vega, A.S.; Bosi, G.; Perez, F.; Mohan, D.; Pittman, C.U.; et al. Household Arsenic Contaminated Water Treatment Employing Iron Oxide/Bamboo Biochar Composite: An Approach to Technology Transfer. J. Colloid Interface Sci. 2021, 587, 767–779. [Google Scholar] [CrossRef] [PubMed]
- García-Ávila, F.; Galarza-Guamán, A.; Barros-Bermeo, M.; Alfaro-Paredes, E.A.; Avilés-Añazco, A.; Iglesias-Abad, S. Integration of High-Rate Filtration Using Waste-Derived Biochar as a Potential Sustainable Technology for Drinking Water Supply. Biochar 2023, 5, 62. [Google Scholar] [CrossRef]
- Jarawi, N.; Jusoh, I. Charcoal Properties of Malaysian Bamboo Charcoal Carbonized at 750 °C. BioResources 2023, 18, 4413–4429. [Google Scholar] [CrossRef]
- Mistar, E.M.; Alfatah, T.; Supardan, M.D. Synthesis and Characterization of Activated Carbon from Bambusa Vulgaris Striata Using Two-Step KOH Activation. J. Mater. Res. Technol. 2020, 9, 6278–6286. [Google Scholar] [CrossRef]
- Azlan Zahari, K.F.; Sahu, U.K.; Khadiran, T.; Surip, S.N.; ALOthman, Z.A.; Jawad, A.H. Mesoporous Activated Carbon from Bamboo Waste via Microwave-Assisted K2CO3 Activation: Adsorption Optimization and Mechanism for Methylene Blue Dye. Separations 2022, 9, 390. [Google Scholar] [CrossRef]
- Sadeghi, M.H.; Tofighy, M.A.; Mohammadi, T. One-Dimensional Graphene for Efficient Aqueous Heavy Metal Adsorption: Rapid Removal of Arsenic and Mercury Ions by Graphene Oxide Nanoribbons (GONRs). Chemosphere 2020, 253, 126647. [Google Scholar] [CrossRef]
- Kharrazi, S.M.; Mirghaffari, N.; Dastgerdi, M.M.; Soleimani, M. A Novel Post-Modification of Powdered Activated Carbon Prepared from Lignocellulosic Waste through Thermal Tension Treatment to Enhance the Porosity and Heavy Metals Adsorption. Powder Technol. 2020, 366, 358–368. [Google Scholar] [CrossRef]
- Mistar, E.M.; Hasmita, I.; Alfatah, T.; Muslim, A.; Supardan, M.D. Adsorption of Mercury(II) Using Activated Carbon Produced from Bambusa vulgaris Var. Striata in a Fixed-Bed Column. Sains Malays. 2019, 48, 719–725. [Google Scholar] [CrossRef]
- Xue, J.; Sun, Q.; Zhang, Y.; Mao, W.; Li, F.; Yin, C. Preparation of a Polypyrrole/Graphene Oxide Composite Electrode by Electrochemical Codeposition for Capacitor Deionization. ACS Omega 2020, 5, 10995–11004. [Google Scholar] [CrossRef]
- Rani, L.; Kaushal, J.; Srivastav, A.L.; Mahajan, P. A Critical Review on Recent Developments in MOF Adsorbents for the Elimination of Toxic Heavy Metals from Aqueous Solutions. Environ. Sci. Pollut. Res. 2020, 27, 44771–44796. [Google Scholar] [CrossRef] [PubMed]
- Davydiuk, T.; Chen, X.; Huang, L.; Shuai, Q.; Le, X.C. Removal of Inorganic Arsenic from Water Using Metal Organic Frameworks. J. Environ. Sci. 2020, 97, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lin, S.; Liu, G.; Xuan, Y.; Gao, L.; Li, P. Functionalized Moso Bamboo Powder Adsorbent for Cd (II) Complexes with Citric Acid / Tartrate Acid: Characterization, Adsorptive Performance, and Mechanism. Environ. Eng. Res. 2022, 27, 210321. [Google Scholar] [CrossRef]
- Naseem, K.; Imran, Q.; Ur Rehman, M.Z.; Tahir, M.H.; Najeeb, J. Adsorptive Removal of Heavy Metals and Dyes from Wastewater Using Azadirachta Indica Biomass. Int. J. Environ. Sci. Technol. 2023, 20, 5799–5822. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Zhou, X.; Guo, J.; Liu, Y. Highly Efficient Removal of Cu(II), Cd(II) and Pb(II) by Carboxyl-Modified Multi-Porous Biochar. Sep. Sci. Technol. 2018, 53, 2860–2869. [Google Scholar] [CrossRef]
- Verma, M.; Lee, I.; Hong, Y.; Kumar, V.; Kim, H. Multifunctional β-Cyclodextrin-EDTA-Chitosan Polymer Adsorbent Synthesis for Simultaneous Removal of Heavy Metals and Organic Dyes from Wastewater. Environ. Pollut. 2022, 292, 118447. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, K.; Zhang, H.; Han, L.; He, Q.; Huang, F.; Yu, W.; Guo, F.; Wang, W. Chemosphere Sustainable Green Conversion of Coal Gangue Waste into Cost-Effective Porous Multimetallic Silicate Adsorbent Enables Superefficient Removal of Cd (II) and Dye. Chemosphere 2023, 324, 138287. [Google Scholar] [CrossRef]
- Jing, W.; Yin, L.; Lin, X.; Yu, Y.; Lian, D.; Shi, Z.; Chen, P.; Tang, M.; Yang, C. Simultaneous Adsorption of Cu2+ and Cd2+ by a Simple Synthesis of Environmentally Friendly Bamboo Pulp Aerogels: Adsorption Properties and Mechanisms. Polymers 2022, 14, 4909. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Xiao, Y.; Wang, G.; Fan, J.; Wan, K.; He, Q.; Gao, M.; Miao, Z. Adsorption of Heavy Metals in Water by Modifying Fe3O4 Nanoparticles with Oxidized Humic Acid. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 616, 126333. [Google Scholar] [CrossRef]
- Geng, B.; Xu, Z.; Liang, P.; Zhang, J.; Christie, P.; Liu, H.; Wu, S.; Liu, X. Three-Dimensional Macroscopic Aminosilylated Nanocellulose Aerogels as Sustainable Bio-Adsorbents for the Effective Removal of Heavy Metal Ions. Int. J. Biol. Macromol. 2021, 190, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Ghaemy, M.; Olad, A. Removal of Heavy Metals from Polluted Water Using Magnetic Adsorbent Based on κ-Carrageenan and N-Doped Carbon Dots. Hydrometallurgy 2022, 213, 105915. [Google Scholar] [CrossRef]
- El Kaim Billah, R.; Ayouch, I.; Abdellaoui, Y.; Kassab, Z.; Khan, M.A.; Agunaou, M.; Soufiane, A.; Otero, M.; Jeon, B.-H. A Novel Chitosan/Nano-Hydroxyapatite Composite for the Adsorptive Removal of Cd(II) from Aqueous Solution. Polymers 2023, 15, 1524. [Google Scholar] [CrossRef]
- Adeiga, O.I.; Pillay, K. Adsorptive Removal of Cd(II) Ions from Water by a Cheap Lignocellulosic Adsorbent and Its Reuse as a Catalyst for the Decontamination of Sulfamethoxazole. ACS Omega 2024, 9, 38348–38358. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Yang, C.; Lin, X.; Tang, M.; Lian, D.; Yu, Y.; Liu, D. MnFe2O4-Loaded Bamboo Pulp Carbon-Based Aerogel Composite: Synthesis, Characterization and Adsorption Behavior Study for Heavy Metals Removal. RSC Adv. 2024, 14, 39995–40005. [Google Scholar] [CrossRef]
- Lu, Y.; Zeng, H.; Lin, H.; Liang, Y.; Feng, M.; Zhou, Z.; Liang, Z.; Li, H.; Chen, G. Synergistic Removal Performance and Mechanism of Cd(II) and As(III) from Irrigation Water by Iron Sulfide-Based Porous Biochar. Environ. Sci. Pollut. Res. 2024, 31, 11591–11604. [Google Scholar] [CrossRef]
- Das, J.; Saha, R.; Nath, H.; Mondal, A.; Nag, S. An Eco-Friendly Removal of Cd(II) Utilizing Banana Pseudo-Fibre and Moringa Bark as Indigenous Green Adsorbent and Modelling of Adsorption by Artificial Neural Network. Environ. Sci. Pollut. Res. 2022, 29, 86528–86549. [Google Scholar] [CrossRef] [PubMed]
- Senniappan, S.; Palanisamy, S.; Manon Mani, V.; Umesh, M.; Govindasamy, C.; Khan, M.I.; Shanmugam, S. Exploring the Adsorption Efficacy of Cassia Fistula Seed Carbon for Cd (II) Ion Removal: Comparative Study of Isotherm Models. Environ. Res. 2023, 235, 116676. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Tang, M.; Lin, X.; Yang, C.; Lian, D.; Yu, Y.; Liu, D. Microwave Irradiation-Assisted Synthesis of Anisotropic Crown Ether-Grafted Bamboo Pulp Aerogel as a Chelating Agent for Selective Adsorption of Heavy Metals (Mn+). Gels 2024, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Ramutshatsha-Makhwedzha, D.; Mbaya, R.; Mavhungu, M.L. Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater. Materials 2022, 15, 860. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Long, Q.; Zhu, Y.; Lin, C.; Xu, X.; Pan, B.; Shi, W.; Guo, Y.; Deng, J.; Yao, Q.; et al. Multifunctional Self-Assembled Adsorption Microspheres Based on Waste Bamboo Shoot Shells for Multi-Pollutant Water Purification. Environ. Res. 2024, 249, 118452. [Google Scholar] [CrossRef]
- Mahanim, S.; Wan Asma, I.; Rafidah, J.; Puad, E.; Shaharuddin, H. Production of activated carbon from industrial bamboo wastes. J. Trop. For. Sci. 2011, 23, 417–424. [Google Scholar]
- Pan, C.; Zhou, G.; Shrestha, A.K.; Chen, J.; Kozak, R.; Li, N.; Li, J.; He, Y.; Sheng, C.; Wang, G. Bamboo as a Nature-Based Solution (NbS) for Climate Change Mitigation: Biomass, Products, and Carbon Credits. Climate 2023, 11, 175. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Ranaei, F.; Ahmad, Z. Application of Bamboo Plants in Nine Aspects. Sci. World J. 2020, 2020, 7284203. [Google Scholar] [CrossRef]
- Promdee, K.; Chanvidhwatanakit, J.; Satitkune, S.; Boonmee, C.; Kawichai, T.; Jarernprasert, S.; Vitidsant, T. Characterization of Carbon Materials and Differences from Activated Carbon Particle (ACP) and Coal Briquettes Product (CBP) Derived from Coconut Shell via Rotary Kiln. Renew. Sustain. Energy Rev. 2017, 75, 1175–1186. [Google Scholar] [CrossRef]
- De Magalhães, L.F.; Da Silva, G.R.; Peres, A.E.C. Zeolite Application in Wastewater Treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- León, M.; Silva, J.; Carrasco, S.; Barrientos, N. Design, Cost Estimation and Sensitivity Analysis for a Production Process of Activated Carbon Fromwaste Nutshells by Physical Activation. Processes 2020, 8, 945. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy Metal Removal from Wastewater Using Various Adsorbents: A Review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Choy, K.K.H.; Barford, J.P.; McKay, G. Production of Activated Carbon from Bamboo Scaffolding Waste—Process Design, Evaluation and Sensitivity Analysis. Chem. Eng. J. 2005, 109, 147–165. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, F.; Hu, W.; Yang, X.; Xiang, H.; Wang, G.; Fei, B.; Liu, Z. Economic Analysis of a Hypothetical Bamboo-Biochar Plant in Zhejiang Province, China. Waste Manag. Res. 2017, 35, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.M.X.; Chin, B.L.F.; Huang, M.M.; Yiin, C.L.; Kurnia, J.C.; Lam, S.S.; Tan, Y.H.; Chai, Y.H.; Rashidi, N.A. Harnessing Bamboo Waste for High-Performance Supercapacitors: A Comprehensive Review of Activation Methods and Applications. J. Energy Storage 2025, 105, 114613. [Google Scholar] [CrossRef]
- Odega, C.A.; Ayodele, O.O.; Ogutuga, S.O.; Anguruwa, G.T.; Adekunle, A.E.; Fakorede, C.O. Potential Application and Regeneration of Bamboo Biochar for Wastewater Treatment: A Review. Adv. Bamboo Sci. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of Chitosan-Based Adsorbents Used in Heavy Metal Adsorption: A Review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).