Clean and Green Bamboo Magic: Recent Advances in Heavy Metal Removal from Water by Bamboo Adsorbents
Abstract
1. Introduction
2. Bamboo-Based Adsorbents and Their Preparation
2.1. Biochar
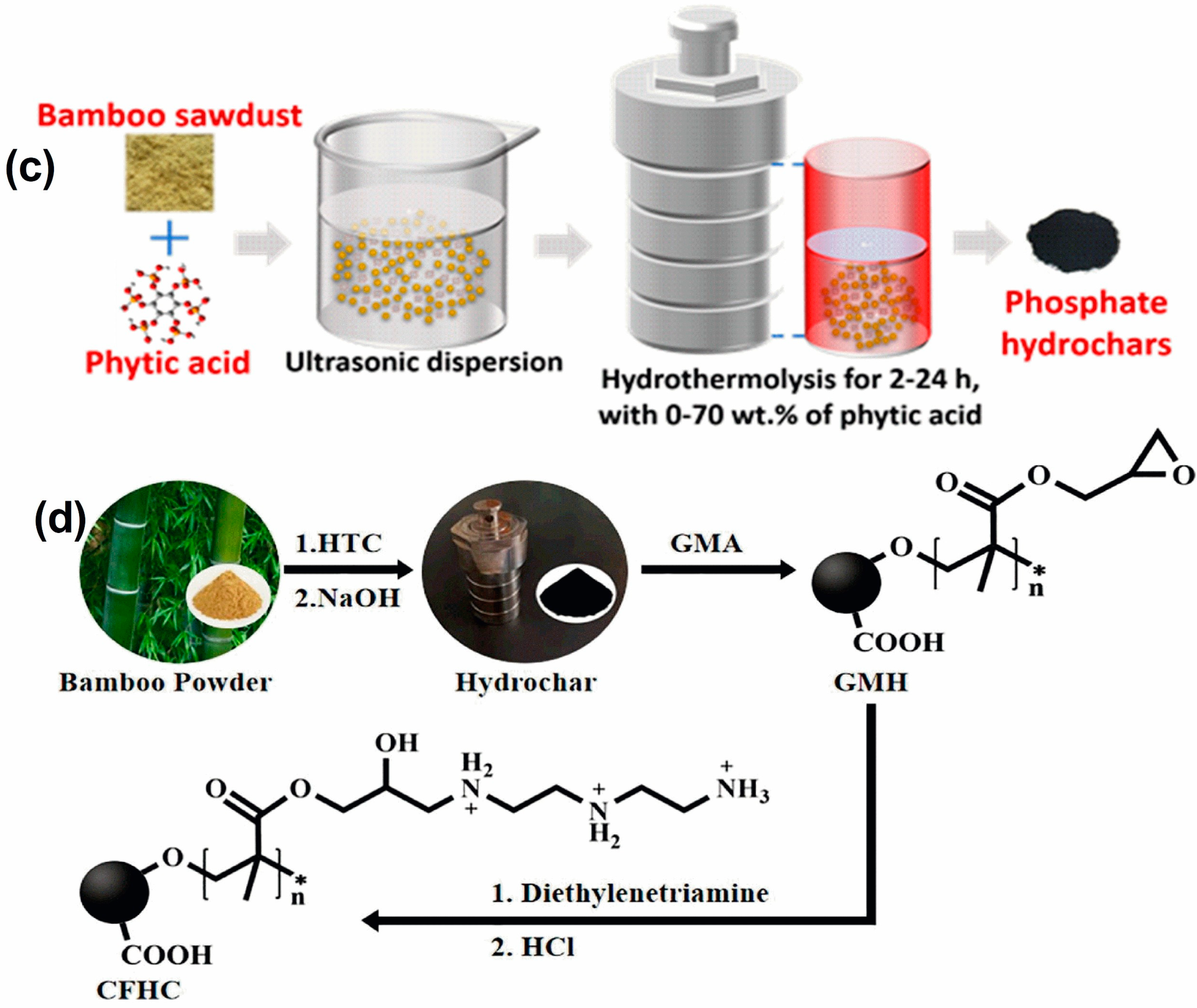
2.2. Hydrochar
2.3. Charcoal
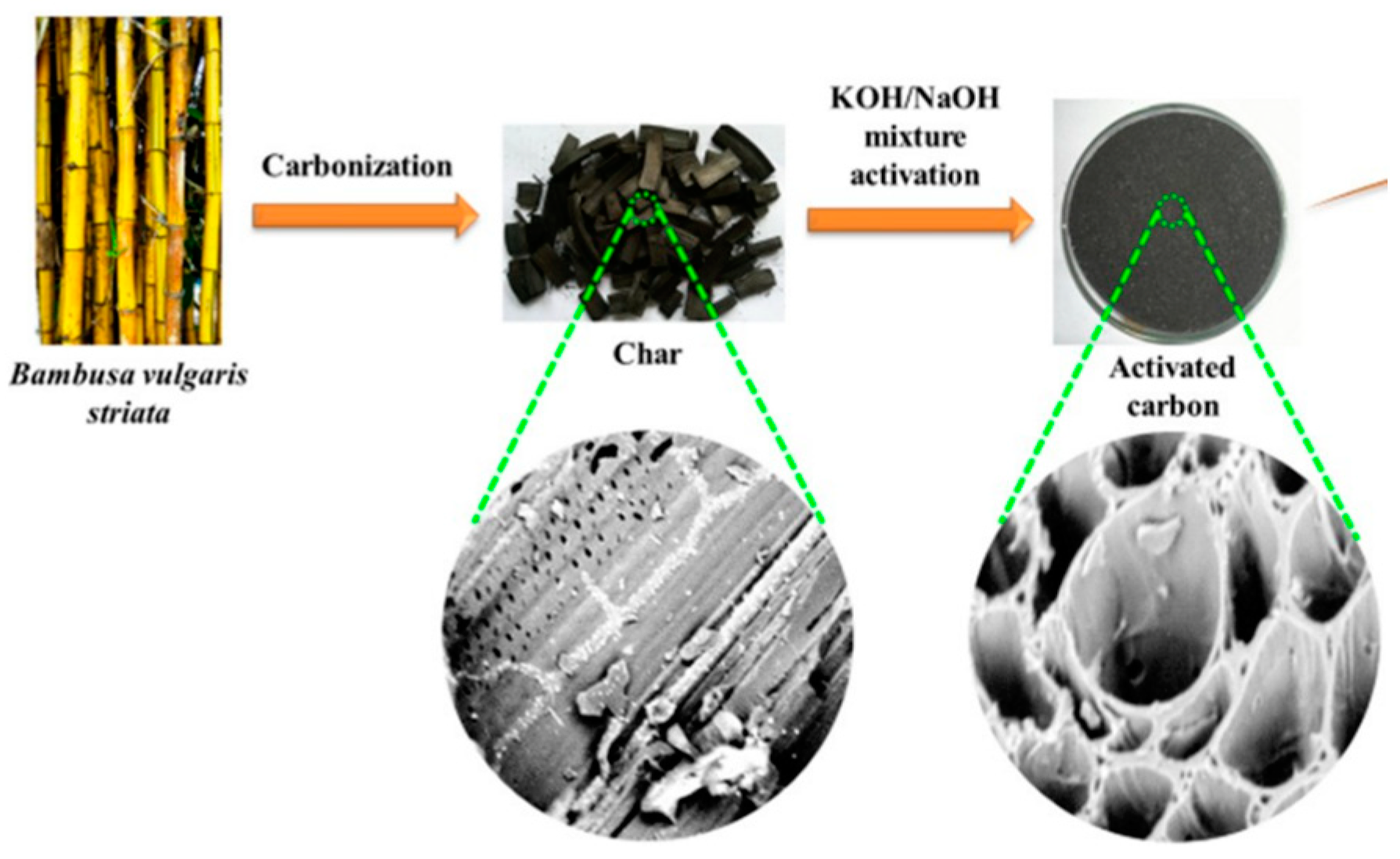
2.4. Activated Carbon
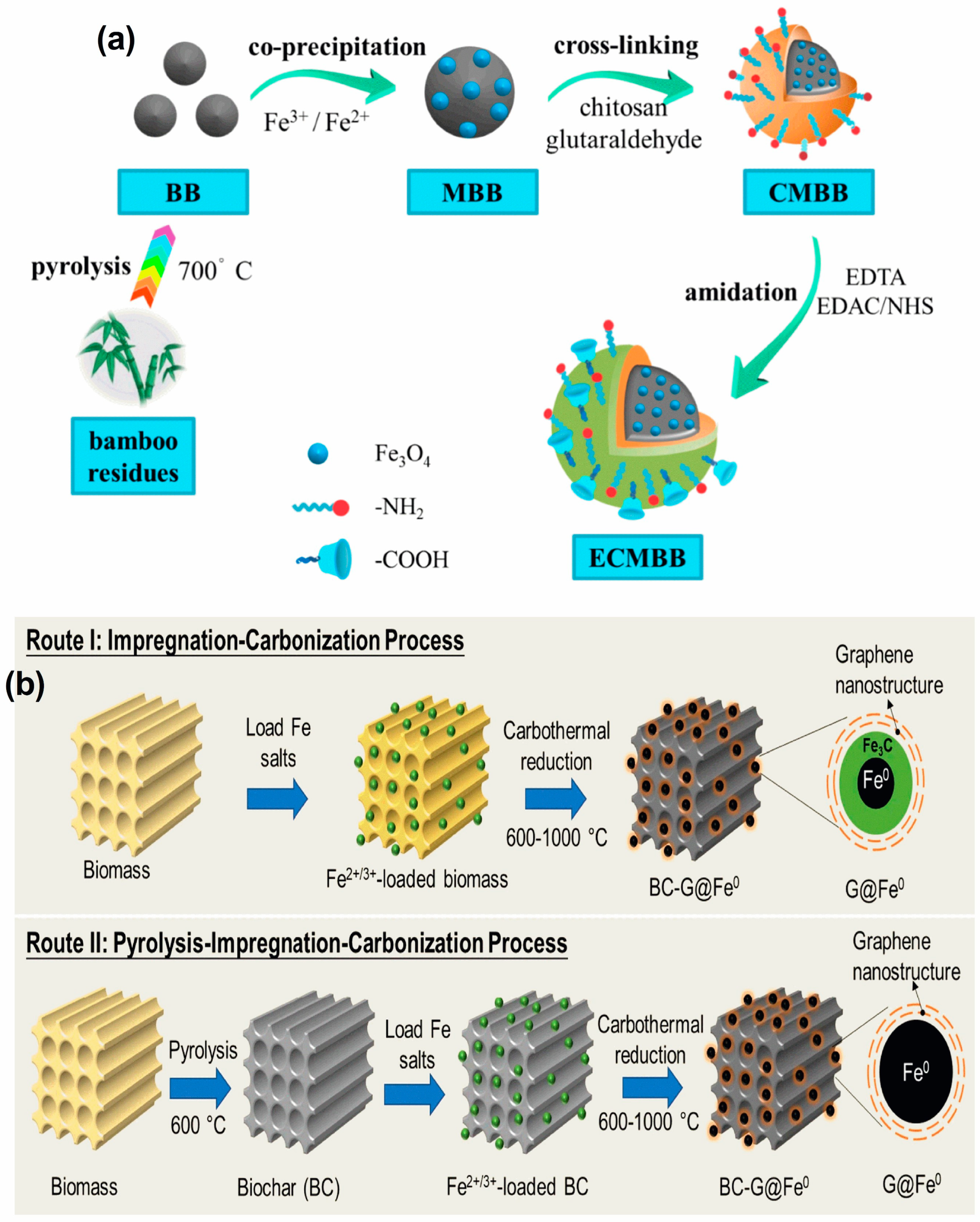
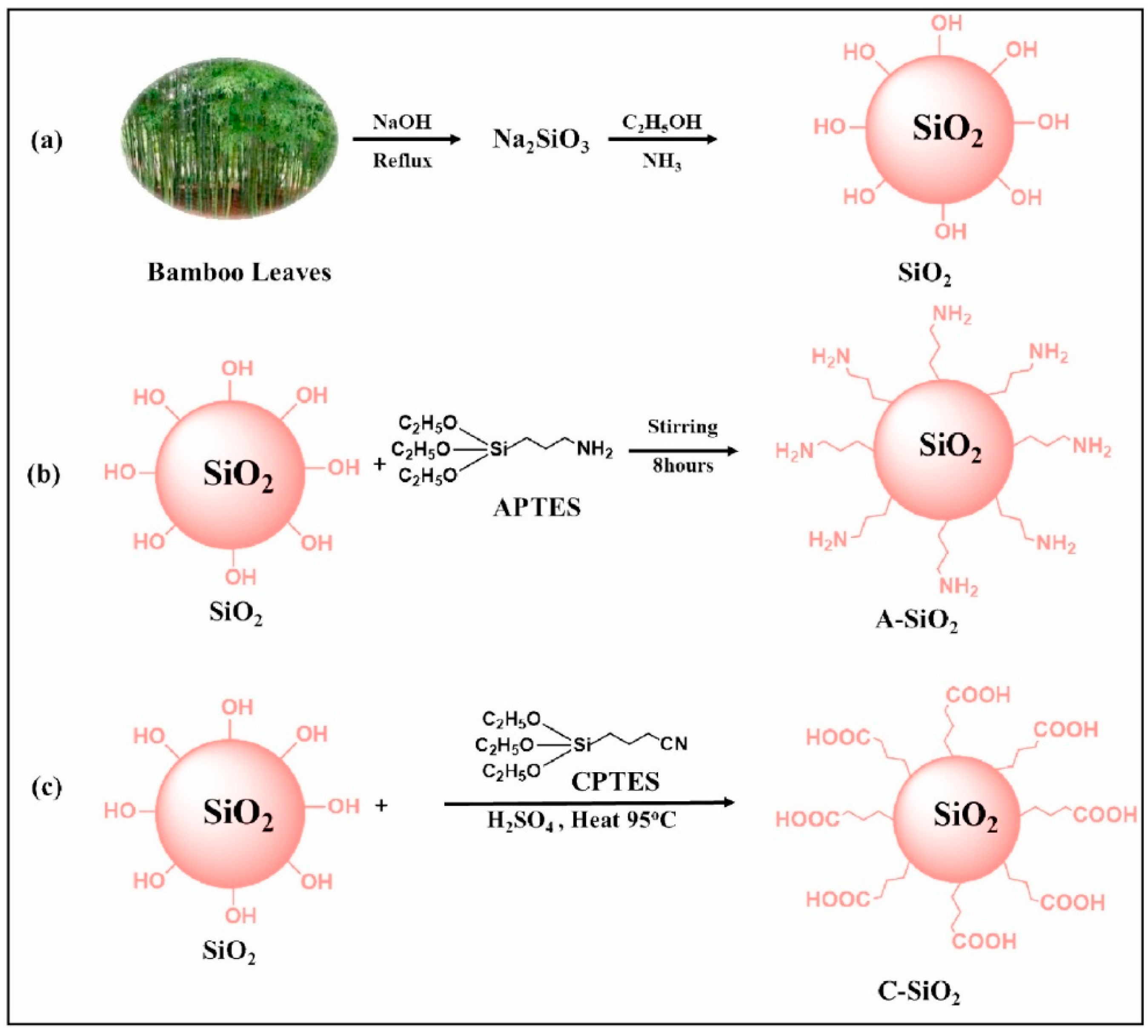
2.5. Modified Bamboo
3. Properties of Bamboo-Based Adsorbents
3.1. Physical Properties
3.1.1. Surface Area and Porosity
3.1.2. XRD Analysis
3.2. Chemical Properties
3.2.1. FTIR Analysis
3.2.2. XPS Analysis
3.2.3. CHNS Analysis
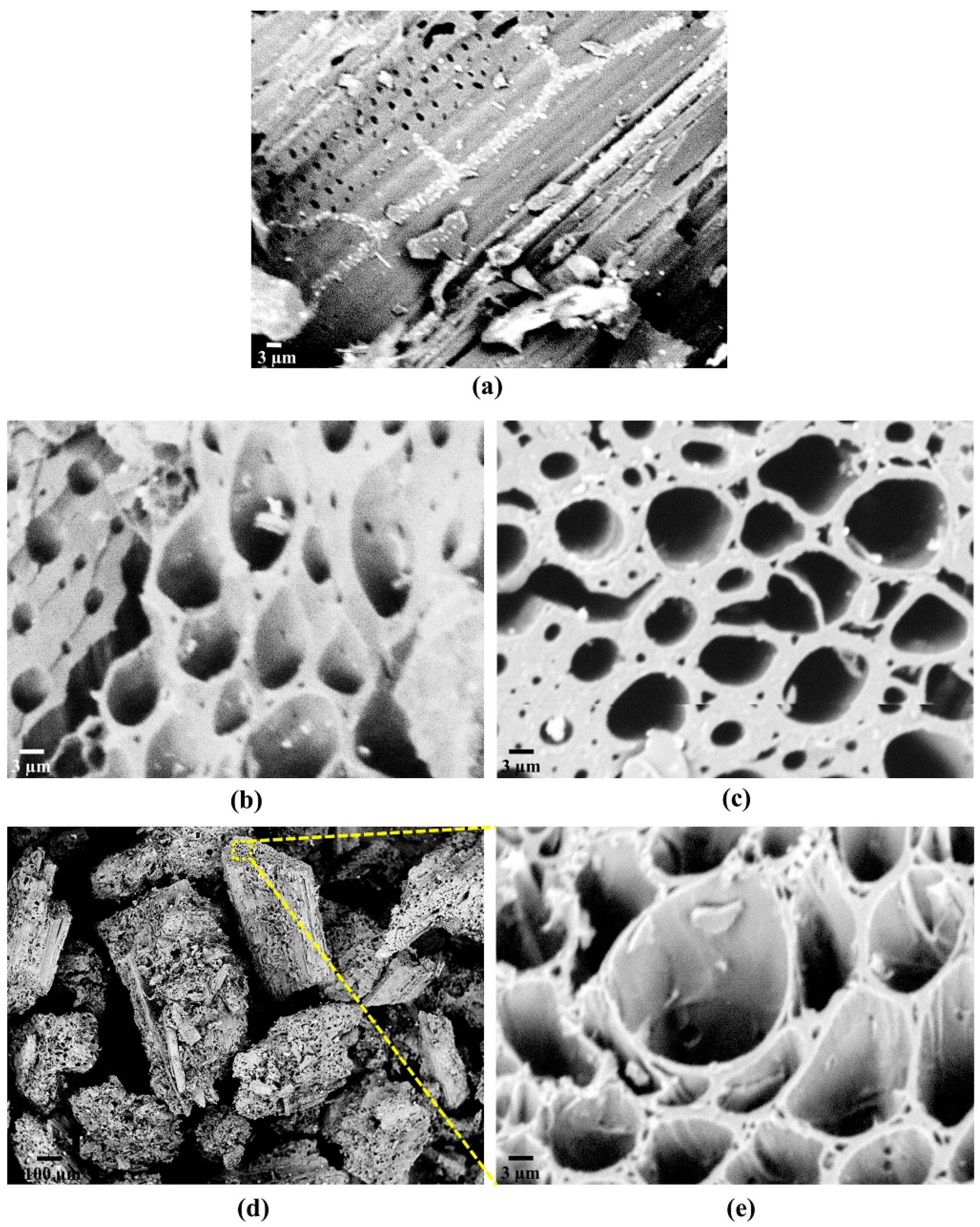
3.3. Morphological Properties
SEM Analysis
4. Application of Bamboo-Based Adsorbents for Heavy Metal Removal
4.1. Effect of pH
4.2. Effect of Contact Time
4.3. Effects of Other Anions and Cations
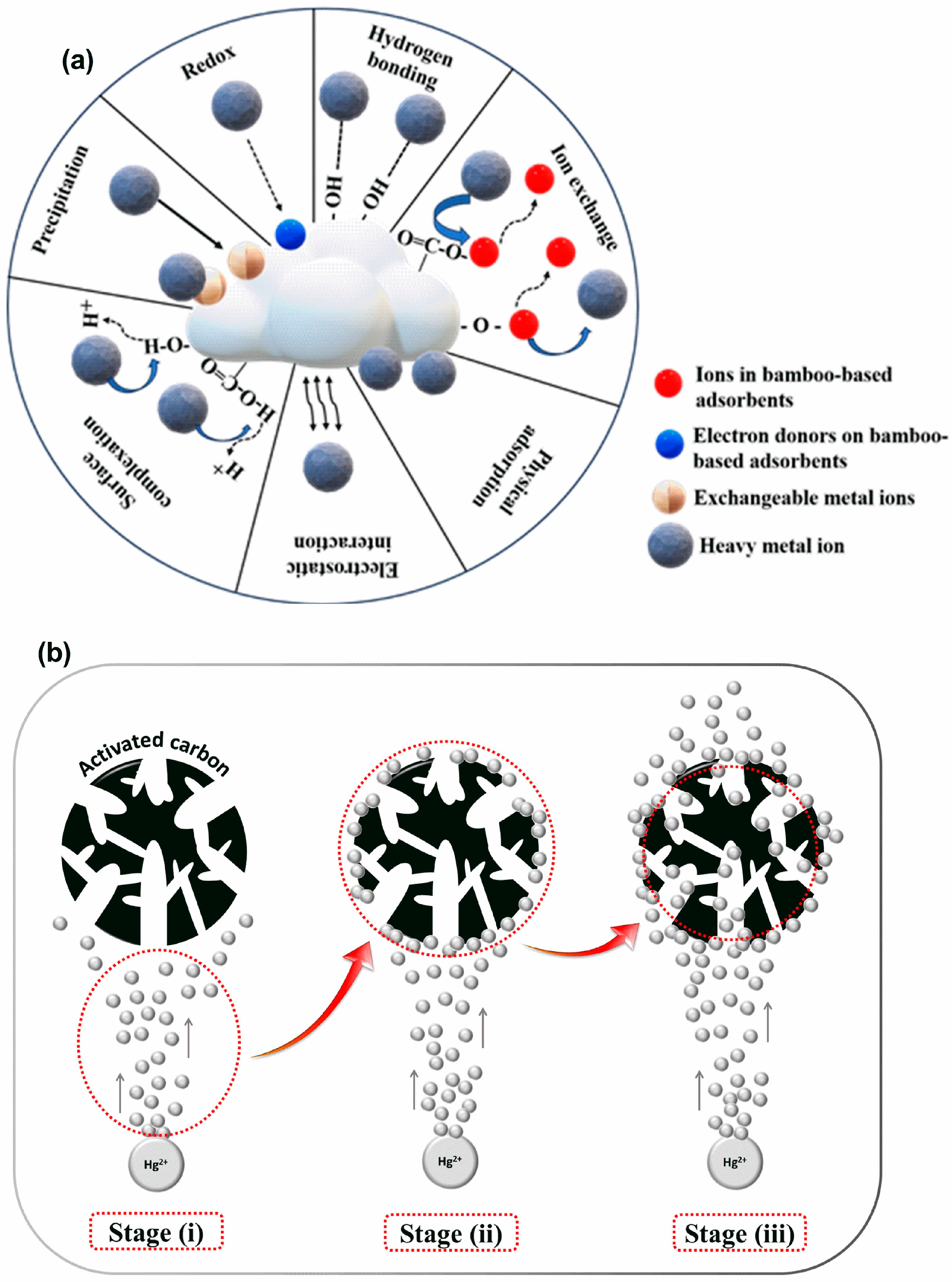
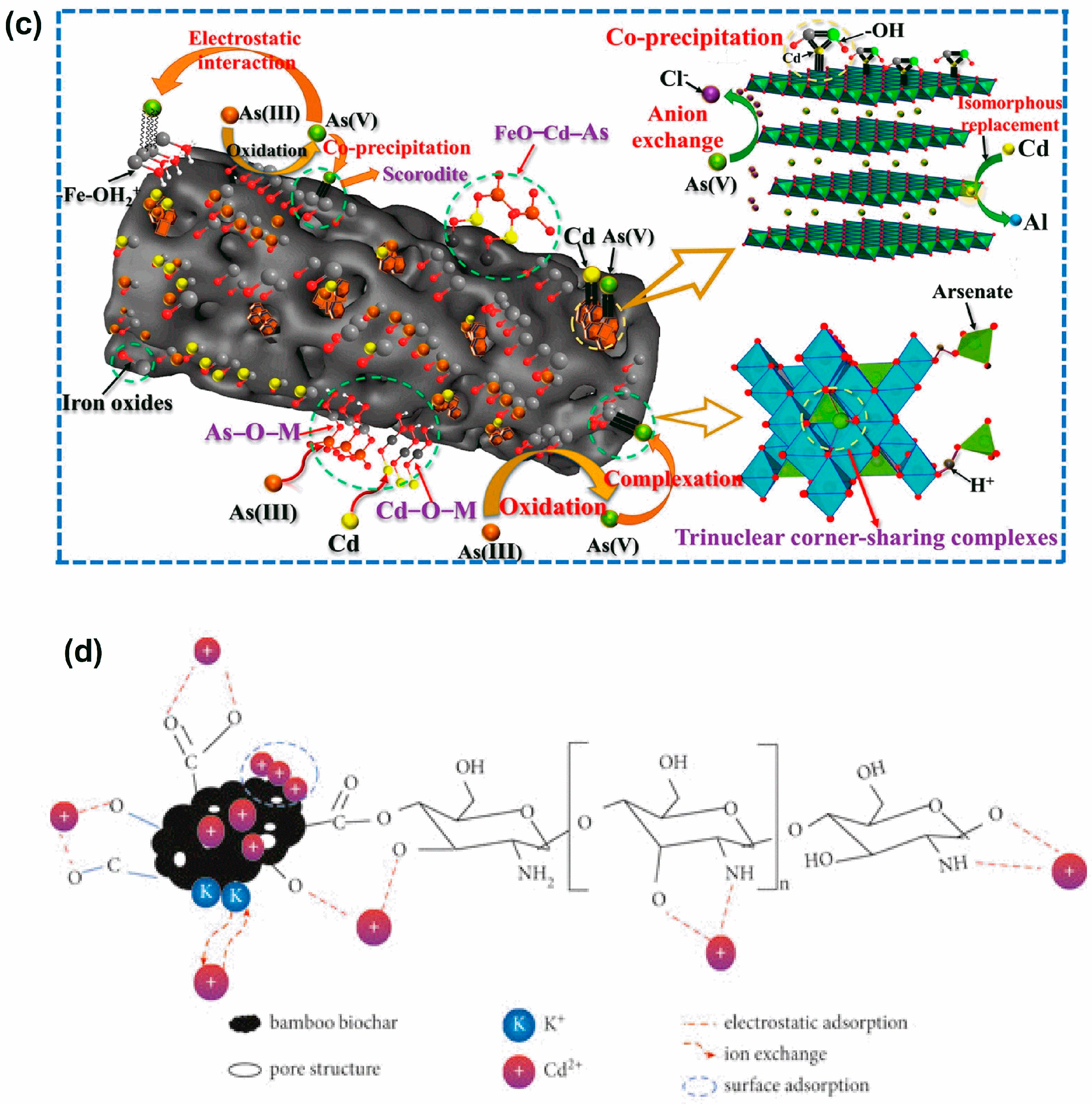
4.4. Adsorption Mechanism of Heavy Metals by Bamboo-Based Adsorbents
4.5. Reusability of the Adsorbents
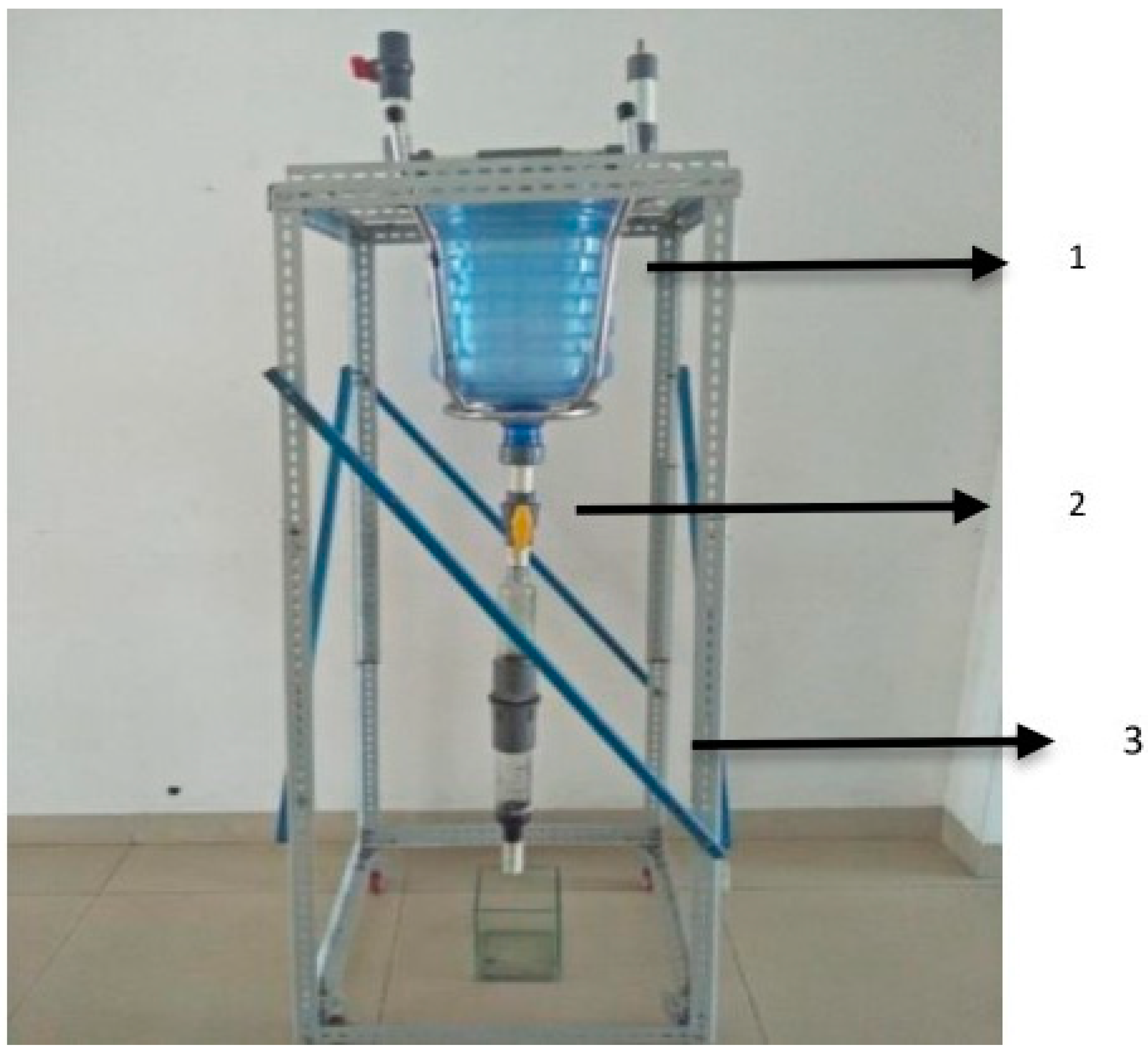
5. Water Purification Filtration Setup Using Bamboo Adsorbents
6. Comparison of Bamboo Adsorbents with Other Adsorbents
7. Disadvantages and Challenges in Practical Implementation
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Koller, M.; Saleh, H.M. Introductory Chapter: Introducing Heavy Metals. In Heavy Metals; InTech: London, UK, 2018. [Google Scholar]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.; Choudhary, S.K.; Awasthi, G.; Awasthi, K.K.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: London, UK, 2022. [Google Scholar]
- Botle, A.; Salgaonkar, S.; Tiwari, R.; Ambadekar, S.; Barabde, G.R. Brief Status of Contamination in Surface Water of Rivers of India by Heavy Metals: A Review with Pollution Indices and Health Risk Assessment. Environ. Geochem. Health 2023, 45, 2779–2801. [Google Scholar] [CrossRef]
- Elumalai, S.; Prabhu, K.; Selvan, G.P.; Ramasamy, P. Review on Heavy Metal Contaminants in Freshwater Fish in South India: Current Situation and Future Perspective. Environ. Sci. Pollut. Res. 2023, 30, 119594–119611. [Google Scholar] [CrossRef] [PubMed]
- Tessema, K.; Lemma, B.; Fetahi, T.; Kebede, E. Accumulation of Heavy Metals in the Physical and Biological Systems of Lake Koka, Ethiopia: Implications for Potential Health Risks. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2020, 25, 314–325. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Pandita, S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. A Review of Ecological Risk Assessment and Associated Health Risks with Heavy Metals in Sediment from India. Int. J. Sediment Res. 2020, 35, 516–526. [Google Scholar] [CrossRef]
- Sharma, A.; Grewal, A.S.; Sharma, D.; Srivastav, A.L. Heavy Metal Contamination in Water: Consequences on Human Health and Environment. In Metals in Water; Elsevier: Amsterdam, The Netherlands, 2023; pp. 39–52. [Google Scholar]
- Maithani, D.; Dasila, H.; Saxena, R.; Tiwari, A.; Bhatt, D.; Rawat, K.; Suyal, D.C. Heavy Metal Pollution in Water: Cause and Remediation Strategies. In Current Status of Fresh Water Microbiology; Springer Nature: Singapore, 2023; pp. 181–204. [Google Scholar]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of Heavy Metal Ions from Aqueous System by Ion-Exchange and Biosorption Methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Rahul, A.K.; Shekhar, S.; Kumar, S. Removal of Heavy Metal from Wastewater Using Ion Exchange with Membrane Filtration from Swarnamukhi River in Tirupati. Mater. Today Proc. 2023, 78, 1–6. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Lamaming, J.; Saalah, S.; Rajin, M.; Ismail, N.M.; Yaser, A.Z. A Review on Bamboo as an Adsorbent for Removal of Pollutants for Wastewater Treatment. Int. J. Chem. Eng. 2022, 2022, 7218759. [Google Scholar] [CrossRef]
- Iroegbu, A.; Ray, S. Bamboos: From Bioresource to Sustainable Materials and Chemicals. Sustainability 2021, 13, 12200. [Google Scholar] [CrossRef]
- The Global Bamboo Market. Available online: https://www.credenceresearch.com/report/bamboos-market (accessed on 17 December 2024).
- Tamang, M.; Nandy, S.; Srinet, R.; Das, A.K.; Padalia, H. Bamboo Mapping Using Earth Observation Data: A Systematic Review. J. Indian Soc. Remote Sens. 2022, 50, 2055–2072. [Google Scholar] [CrossRef]
- FAO of UN. Available online: https://www.fao.org/forest-monitoring/news-and-events/news/news-detail/Putting-bamboo-on-the-map/en (accessed on 17 December 2024).
- Wakejo, W.K.; Meshesha, B.T.; Kang, J.W.; Demesa, A.G. Bamboo Sawdust-Derived High Surface Area Activated Carbon for Remarkable Removal of Paracetamol from Aqueous Solution: Sorption Kinetics, Isotherm, Thermodynamics, and Regeneration Studies. Water Pract. Technol. 2023, 18, 1366–1388. [Google Scholar] [CrossRef]
- Jayachandran, M.; Kishore Babu, S.; Maiyalagan, T.; Rajadurai, N.; Vijayakumar, T. Activated Carbon Derived from Bamboo-Leaf with Effect of Various Aqueous Electrolytes as Electrode Material for Supercapacitor Applications. Mater. Lett. 2021, 301, 130335. [Google Scholar] [CrossRef]
- Bagbi, Y.; Yomgam, P.; Libang, E.; Boruah, B.; Kaur, J.; Jayanthi, S.; Kumar, S.; Dhania, N.K. Waste Bamboo-Derived Magnetically Separable Bamboo-Activated Carbon: From Characterization to Effective Remediation of Fluoride (F−) Ions from Water. RSC Adv. 2024, 14, 24952–24968. [Google Scholar] [CrossRef] [PubMed]
- Jayarambabu, N.; Saraswathi, K.; Akshaykranth, A.; Anitha, N.; Venkatappa Rao, T.; Kumar, R.R. Bamboo-Mediated Silver Nanoparticles Functionalized with Activated Carbon and Their Application for Non-Enzymatic Glucose Sensing. Inorg. Chem. Commun. 2023, 147, 110249. [Google Scholar] [CrossRef]
- Sema, A.I.; Bhattacharyya, J. Biochar Derived from Waste Bamboo Shoots for the Biosorptive Removal of Ferrous Ions from Aqueous Solution. J. Indian Chem. Soc. 2022, 99, 100791. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Singhwane, A.; Dhangar, M.; Mili, M.; Gorhae, N.; Naik, A.; Prashant, N.; Srivastava, A.K.; Verma, S. Bamboo for Producing Charcoal and Biochar for Versatile Applications. Biomass Convers. Biorefinery 2024, 14, 15159–15185. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and Characterization of Biochar Produced from Slow Pyrolysis of Pigeon Pea Stalk and Bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Nandana, E.; Dwivedi, A.H.; Nidheesh, P.V. Role of Biochar in Superoxide-Dominated Dye Degradation in Catalyst-Activated Peroxymonosulphate Process. Chemosphere 2024, 356, 141945. [Google Scholar] [CrossRef]
- Saini, S.; Gill, J.K.; Kaur, J.; Saikia, H.R.; Singh, N.; Kaur, I.; Katnoria, J.K. Biosorption as Environmentally Friendly Technique for Heavy Metal Removal from Wastewater. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 167–181. [Google Scholar]
- Narzary, I.; Mahato, R.K.; Middha, S.K.; Usha, T.; Goyal, A.K. Valorization of Bamboo Charcoal as a Low-Cost Adsorbent for Waste Water Treatment: A Mini Review. Adv. Bamboo Sci. 2024, 7, 100067. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Kumar, M.; Venkateshwaran, G.; Ambika, S.; Bhaskar, S.; Vinay; Ghosh, P. Conversion of Locally Available Materials to Biochar and Activated Carbon for Drinking Water Treatment. Chemosphere 2024, 353, 141566. [Google Scholar] [CrossRef]
- Ambika, S.; Kumar, M.; Pisharody, L.; Malhotra, M.; Kumar, G.; Sreedharan, V.; Singh, L.; Nidheesh, P.V.; Bhatnagar, A. Modified Biochar as a Green Adsorbent for Removal of Hexavalent Chromium from Various Environmental Matrices: Mechanisms, Methods, and Prospects. Chem. Eng. J. 2022, 439, 135716. [Google Scholar] [CrossRef]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Design, Synthesis, and Performance of Adsorbents for Heavy Metal Removal from Wastewater: A Review. J. Mater. Chem. A 2022, 10, 1047–1085. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent Advances in Heavy Metal Removal by Chitosan Based Adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent Advances in Adsorptive Removal of Heavy Metal and Metalloid Ions by Metal Oxide-Based Nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Sharma, A.; Anjana; Rana, H.; Goswami, S. A Comprehensive Review on the Heavy Metal Removal for Water Remediation by the Application of Lignocellulosic Biomass-Derived Nanocellulose. J. Polym. Environ. 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Kaur, J.; Sengupta, P.; Mukhopadhyay, S. Critical Review of Bioadsorption on Modified Cellulose and Removal of Divalent Heavy Metals (Cd, Pb, and Cu). Ind. Eng. Chem. Res. 2022, 61, 1921–1954. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of Heavy Metal Ions by Various Low-Cost Adsorbents: A Review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Mahesh, N.; Balakumar, S.; Shyamalagowri, S.; Manjunathan, J.; Pavithra, M.K.S.; Babu, P.S.; Kamaraj, M.; Govarthanan, M. Carbon-Based Adsorbents as Proficient Tools for the Removal of Heavy Metals from Aqueous Solution: A State of Art-Review Emphasizing Recent Progress and Prospects. Environ. Res. 2022, 213, 113723. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent Advances in Applications of Low-Cost Adsorbents for the Removal of Heavy Metals from Water: A Critical Review. Sep. Purif. Technol. 2022, 278, 119510. [Google Scholar] [CrossRef]
- Mariana, M.; HPS, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent Advances in Activated Carbon Modification Techniques for Enhanced Heavy Metal Adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Raninga, M.; Mudgal, A.; Patel, V.K.; Patel, J.; Kumar Sinha, M. Modification of Activated Carbon-Based Adsorbent for Removal of Industrial Dyes and Heavy Metals: A Review. Mater. Today Proc. 2023, 77, 286–294. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rawat, K.P.; Bhadauria, V.; Singh, H. Recent Trends in the Application of Modified Starch in the Adsorption of Heavy Metals from Water: A Review. Carbohydr. Polym. 2021, 269, 117763. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Adsorptive Potential of Modified Plant-Based Adsorbents for Sequestration of Dyes and Heavy Metals from Wastewater—A Review. J. Water Process Eng. 2021, 42, 102148. [Google Scholar] [CrossRef]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and Kinetics of Adsorption and Removal of Heavy Metals from Wastewater Using Nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar] [CrossRef]
- Kalderis, D.; Seifi, A.; Kieu Trang, T.; Tsubota, T.; Anastopoulos, I.; Manariotis, I.; Pashalidis, I.; Khataee, A. Bamboo-Derived Adsorbents for Environmental Remediation: A Review of Recent Progress. Environ. Res. 2023, 224, 115533. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A Review On The Comparison Between Slow Pyrolysis And Fast Pyrolysis On The Quality Of Lignocellulosic And Lignin-Based Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Phounglamcheik, A.; Wang, L.; Romar, H.; Kienzl, N.; Brostro, M.; Ramser, K.; Skreiberg, Ø.; Umeki, K. Effects of Pyrolysis Conditions and Feedstocks on the Properties and Gasi Fi Cation Reactivity of Charcoal from Woodchips. Energy Fuels 2020, 34, 8353–8365. [Google Scholar] [CrossRef]
- Erdem, A.; Dogru, M. Process Intensification: Activated Carbon Production from Biochar Produced by Gasification. Johns. Matthey Technol. Rev. 2021, 65, 352–365. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, X.; Deng, S.; Zeng, X.; Yu, Z.; Li, S.; Li, K. Waste polyethylene terephthalate (PET) plastics-derived activated carbon for CO2 capture: A route to a closed carbon loop. Green Chem. 2020, 22, 6836–6845. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of Organic and Inorganic Pollutants onto Biochars: Challenges, Operating Conditions, and Mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Zhang, X.; Bhattacharya, T.; Wang, C.; Kumar, A.; Nidheesh, P.V. Straw-Derived Biochar for the Removal of Antibiotics from Water: Adsorption and Degradation Mechanisms, Recent Advancements and Challenges. Environ. Res. 2023, 237, 116998. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of Sewage Sludge into Biochar: A Potential Resource in Water and Wastewater Treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhattacharya, T. Biochar: A Sustainable Solution. Environ. Dev. Sustain. 2021, 23, 6642–6680. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, P.; Sharma, J.; Saini, S.; Sharma, P.; Sharma, A. Rice Straw Management through Biofuel, Biochar, Mushroom Cultivation, and Paper Production to Overcome Environmental Pollution in North India. Waste Dispos. Sustain. Energy 2023, 5, 483–510. [Google Scholar] [CrossRef]
- Rubeena, K.K.; Hari Prasad Reddy, P.; Laiju, A.R.; Nidheesh, P. V Iron Impregnated Biochars as Heterogeneous Fenton Catalyst for the Degradation of Acid Red 1 Dye. J. Environ. Manag. 2018, 226, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Ghosh, G.K.; Mishra, V.K.; Choudhury, B.U.; Dutta, S.K.; Hazarika, S.; Kalita, H.; Roy, A.; Singh, N.U.; Gopi, R.; et al. Utilizing Dissimilar Feedstocks Derived Biochar Amendments to Alter Soil Biological Indicators in Acidic Soil of Northeast India. Biomass Convers. Biorefinery 2023, 13, 10203–10214. [Google Scholar] [CrossRef]
- Anand, A.; Pathak, S.; Kumar, V.; Kaushal, P. Biochar Production from Crop Residues, Its Characterization and Utilization for Electricity Generation in India. J. Clean. Prod. 2022, 368, 133074. [Google Scholar] [CrossRef]
- Nakarmi, K.J.; Daneshvar, E.; Eshaq, G.; Puro, L.; Maiti, A.; Nidheesh, P.V.; Wang, H.; Bhatnagar, A. Synthesis of Biochar from Iron-Free and Iron-Containing Microalgal Biomass for the Removal of Pharmaceuticals from Water. Environ. Res. 2022, 214, 114041. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Gopinath, A.; Ranjith, N.; Praveen Akre, A.; Sreedharan, V.; Suresh Kumar, M. Potential Role of Biochar in Advanced Oxidation Processes: A Sustainable Approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Cheng, Y.W.; Liew, R.K.; Wan Mahari, W.A.; Ong, H.C.; Chen, W.H.; Peng, W.; Park, Y.K.; Sonne, C.; Kong, S.H.; et al. Progress in the Torrefaction Technology for Upgrading Oil Palm Wastes to Energy-Dense Biochar: A Review. Renew. Sustain. Energy Rev. 2021, 151, 111645. [Google Scholar] [CrossRef]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, Economical, and Climate-Related Aspects of Biochar Production Technologies: A Literature Review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Kumar, A.; Mishra, R.; You, S.; Singh, L.; Kumar, S.; Kumar, R. Pyrolysis of Waste Biomass and Plastics for Production of Biochar and Its Use for Removal of Heavy Metals from Aqueous Solution. Bioresour. Technol. 2021, 320, 124278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, R.; Li, R.; Ali, A.; Chen, A.; Zhang, Z. Enhanced Aqueous Cr(VI) Removal Using Chitosan-Modified Magnetic Biochars Derived from Bamboo Residues. Chemosphere 2020, 261, 127694. [Google Scholar] [CrossRef]
- Pinisakul, A.; Kruatong, N.; Vinitnantharat, S.; Wilamas, P.; Neamchan, R.; Sukkhee, N.; Werner, D.; Sanghaisuk, S. Arsenic, Iron, and Manganese Adsorption in Single and Trinary Heavy Metal Solution Systems by Bamboo-Derived Biochars. C-J. Carbon Res. 2023, 9, 40. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Wang, T.; Wang, P. Adsorption of Toxic Metal Ion in Agricultural Wastewater by Torrefaction Biochar from Bamboo Shoot Shell. J. Clean. Prod. 2022, 338, 130558. [Google Scholar] [CrossRef]
- Wang, R.Z.; Huang, D.L.; Liu, Y.G.; Zhang, C.; Lai, C.; Wang, X.; Zeng, G.M.; Zhang, Q.; Gong, X.M.; Xu, P. Synergistic Removal of Copper and Tetracycline from Aqueous Solution by Steam-Activated Bamboo-Derived Biochar. J. Hazard. Mater. 2020, 384, 121470. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of Modified Biochars Derived from Bamboo Pyrolysis and Their Utilization for Target Component (Furfural) Adsorption. Energy Fuels 2014, 28, 5119–5127. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Cai, J.; Zhang, X.; Zhang, J.; Shao, J. Evaluation and Prediction of Cadmium Removal from Aqueous Solution by Phosphate-Modified Activated Bamboo Biochar. Energy Fuels 2018, 32, 4469–4477. [Google Scholar] [CrossRef]
- Wakejo, W.K.; Meshasha, B.T.; Kang, J.W.; Chebude, Y. Enhanced Ciprofloxacin Removal from Aqueous Solution Using a Chemically Modified Biochar Derived from Bamboo Sawdust: Adsorption Process Optimization with Response Surface Methodology. Adsorpt. Sci. Technol. 2022, 2022, 2699530. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Zhang, Z. A Versatile EDTA and Chitosan Bi-Functionalized Magnetic Bamboo Biochar for Simultaneous Removal of Methyl Orange and Heavy Metals from Complex Wastewater. Environ. Pollut. 2022, 293, 118517. [Google Scholar] [CrossRef]
- Karunaratne, T.N.; Nayanathara, R.M.O.; Navarathna, C.M.; Rodrigo, P.M.; Thirumalai, R.V.K.G.; Jr, C.U.P.; Kim, Y.; Mlsna, T.; Zhang, J. Pyrolytic Synthesis of Graphene—Encapsulated Zero—Valent Iron Nanoparticles Supported on Biochar for Heavy Metal Removal. Biochar 2022, 4, 70. [Google Scholar] [CrossRef]
- Zhao, X.; Li, M.; Zhai, F.; Hou, Y.; Hu, R. Phosphate Modified Hydrochars Produced via Phytic Acid-Assisted Hydrothermal Carbonization for Efficient Removal of U (VI), Pb (II) and Cd (II). J. Environ. Manag. 2021, 298, 113487. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Wu, C.; Huan, W.; Chen, L.; Li, B. Bioresource Technology Enhanced Removal of Cr (VI) by Cation Functionalized Bamboo Hydrochar. Bioresour. Technol. 2022, 347, 126703. [Google Scholar] [CrossRef] [PubMed]
- Allohverdi, T.; Mohanty, A.K.; Roy, P.; Misra, M. A Review on Current Status of Biochar Uses in Agriculture. Molecules 2021, 26, 5584. [Google Scholar] [CrossRef]
- Eshiemogie, S.O.; Iwuchukwu, F.U. Recent Advances in Hydrochar Application for the Adsorptive Removal of Wastewater Pollutants. Chem. Eng. Res. Des. 2022, 184, 419–456. [Google Scholar]
- Masoumi, S.; Borugadda, V.B.; Nanda, S.; Dalai, A.K. Hydrochar: A Review on Its Production Technologies. Catalysts 2021, 11, 939. [Google Scholar] [CrossRef]
- Shyam, S.; Arun, J.; Gopinath, K.P.; Ribhu, G.; Ashish, M.; Ajay, S. Biomass as Source for Hydrochar and Biochar Production to Recover Phosphates from Wastewater: A Review on Challenges, Commercialization, and Future Perspectives. Chemosphere 2022, 286, 131490. [Google Scholar] [CrossRef]
- Li, S.Y.; Teng, H.J.; Guo, J.Z.; Wang, Y.X.; Li, B. Enhanced Removal of Cr(VI) by Nitrogen-Doped Hydrochar Prepared from Bamboo and Ammonium Chloride. Bioresour. Technol. 2021, 342, 126028. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zimmerman, A.R.; Hu, X.; Gao, B. Chemosphere Removal of Aqueous Cr (VI) by Zn- and Al-Modi Fi Ed Hydrochar. Chemosphere 2020, 260, 127610. [Google Scholar] [CrossRef]
- Hagemann, N.; Spokas, K.; Schmidt, H.-P.; Kägi, R.; Böhler, M.; Bucheli, T. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef]
- Kabir Ahmad, R.; Anwar Sulaiman, S.; Yusup, S.; Sham Dol, S.; Inayat, M.; Aminu Umar, H. Exploring the Potential of Coconut Shell Biomass for Charcoal Production. Ain Shams Eng. J. 2022, 13, 101499. [Google Scholar] [CrossRef]
- Xiao, F.; Bedane, A.H.; Mallula, S.; Sasi, P.C.; Alinezhad, A.; Soli, D.; Hagen, Z.M.; Mann, M.D. Production of Granular Activated Carbon by Thermal Air Oxidation of Biomass Charcoal/Biochar for Water Treatment in Rural Communities: A Mechanistic Investigation. Chem. Eng. J. Adv. 2020, 4, 100035. [Google Scholar] [CrossRef]
- Maske, V.A.; Jangale, S.; Vyavahare, S.; More, A.P. Development of Coconut Shell Charcoal-Reinforced Starch Superabsorbent Composites, with the Comparative Study of Epichlorohydrin and Citric Acid as the Crosslinking Agents. Starch Stärke 2024. [Google Scholar] [CrossRef]
- Castro, J.P.; Nobre, J.R.C.; Napoli, A.; Bianchi, M.L.; Moulin, J.C.; Chiou, B.S.; Williams, T.G.; Wood, D.F.; Avena-Bustillos, R.J.; Orts, W.J.; et al. Massaranduba Sawdust: A Potential Source of Charcoal and Activated Carbon. Polymers 2019, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, C.I.; Onuegbu, T.U.; Okafor, I.F.; Igwenagu, P.C. Utilization of Agricultural Waste in the Production of Charcoal Briquette Blend as Alternative Source of Energy. IOP Conf. Ser. Earth Environ. Sci. 2023, 1178, 012015. [Google Scholar] [CrossRef]
- Bai, S.; Wang, T.; Tian, Z.; Cao, K.; Li, J. Facile Preparation of Porous Biomass Charcoal from Peanut Shell as Adsorbent. Sci. Rep. 2020, 10, 15845. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, W.; Wang, P.; Ding, Y.; Zhou, S. Adsorption of Cu (II) and Zn (II) in Aqueous Solution by Modified Bamboo Charcoal. Environ. Geochem. Health 2024, 46, 182. [Google Scholar] [CrossRef]
- Ariyadi, R.; Fatmawati, S.; Syar, N.I.; Nasir, M.; Maulina, D. Suhartono Design of Water Purification Polluted by Heavy Metal Fe with Active Charcoal Media of Palm Oil and Bamboo. J. Phys. Conf. Ser. 2021, 1796, 012054. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Lin, S. Adsorption Characteristics of Modified Bamboo Charcoal on Cu(II) and Cd(II) in Water. Toxics 2022, 10, 787. [Google Scholar] [CrossRef]
- Thotagamuge, R.; Kooh, M.R.R.; Mahadi, A.H.; Lim, C.M.; Abu, M.; Jan, A.; Hanipah, A.H.A.; Khiong, Y.Y.; Shofry, A. Copper Modified Activated Bamboo Charcoal to Enhance Adsorption of Heavy Metals from Industrial Wastewater. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100562. [Google Scholar] [CrossRef]
- Kumar, N.; Pandey, A.; Rosy; Sharma, Y.C. A Review on Sustainable Mesoporous Activated Carbon as Adsorbent for Efficient Removal of Hazardous Dyes from Industrial Wastewater. J. Water Process Eng. 2023, 54, 104054. [Google Scholar] [CrossRef]
- Mondal, M.K.; Garg, R. A Comprehensive Review on Removal of Arsenic Using Activated Carbon Prepared from Easily Available Waste Materials. Environ. Sci. Pollut. Res. 2017, 24, 13295–13306. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Trisha, M.; Kaviya Selvi, S.; Gokul, R.; Renuka, V. Recent Research Progress on Modified Activated Carbon from Biomass for the Treatment of Wastewater: A Critical Review. Environ. Qual. Manag. 2024, 33, 907–928. [Google Scholar] [CrossRef]
- Nath, H.; Das, J.; Debnath, C.; Sarkar, B.; Saxena, R.; Barma, S.D. Development of Lignocellulosic Biomass Derived Cu and Zn Doped Highly Porous Activated Carbon and Its Utilization in the Anti-Microbial Treatment. Environ. Chem. Ecotoxicol. 2023, 5, 155–164. [Google Scholar] [CrossRef]
- Singh, K.; Lohchab, R.K.; Kumari, M.; Beniwal, V. Potential of TiO 2 Loaded Almond Shell Derived Activated Carbon for Leachate Treatment: Isotherms, Kinetics, and Response Surface Methodology. Int. J. Environ. Anal. Chem. 2023, 103, 7625–7646. [Google Scholar] [CrossRef]
- Mandal, S.; Stephen, D.; Janardhanan, S.K. Activated Carbon with Composite Pore Structures Made from Peanut Shell and Areca Nut Fibers as Sustainable Adsorbent Material for the Efficient Removal of Active Pharmaceuticals from Aqueous Media. RSC Sustain. 2024, 2, 3022–3035. [Google Scholar] [CrossRef]
- Chauhan, P.R.; Raveesh, G.; Pal, K.; Goyal, R.; Tyagi, S.K. Production of Biomass Derived Highly Porous Activated Carbon: A Solution towards in-Situ Burning of Crop Residues in India. Bioresour. Technol. Reports 2023, 22, 101425. [Google Scholar] [CrossRef]
- Sahu, A.; Sen, S.; Mishra, S.C. A Comparative Study on Characterizations of Biomass Derived Activated Carbons Prepared by Both Normal and Inert Atmospheric Heating Conditions. J. Indian Chem. Soc. 2023, 100, 100943. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Singh, B.; Acharya, B. A Comprehensive Review on Activated Carbon from Pyrolysis of Lignocellulosic Biomass: An Application for Energy and the Environment. Carbon Resour. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Nam, H.; Wang, S.; Jeong, H.R. TMA and H2S Gas Removals Using Metal Loaded on Rice Husk Activated Carbon for Indoor Air Purification. Fuel 2018, 213, 186–194. [Google Scholar] [CrossRef]
- Maniarasu, R.; Rathore, S.K.; Murugan, S. Biomass-Based Activated Carbon for CO2 Adsorption–A Review. Energy Environ. 2023, 34, 1674–1721. [Google Scholar] [CrossRef]
- Ramalingam, G.; Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Hoang, T.K.A. Biomass and Waste Derived Silica, Activated Carbon and Ammonia-Based Materials for Energy-Related Applications—A Review. Fuel 2024, 355, 129490. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Ahmad, N. An Overview of Activated Carbon Preparation from Various Precursors. Sci. Res. J. 2023, 20, 51–89. [Google Scholar] [CrossRef]
- Asrat, Y.; Adugna, A.T.; Kamaraj, M.; Beyan, S.M. Adsorption Phenomenon of Arundinaria Alpina Stem-Based Activated Carbon for the Removal of Lead from Aqueous Solution. J. Chem. 2021, 2021, 5554353. [Google Scholar] [CrossRef]
- Alfatah, T.; Mistar, E.M.; Supardan, M.D. Porous Structure and Adsorptive Properties of Activated Carbon Derived from Bambusa Vulgaris Striata by Two-Stage KOH/NaOH Mixture Activation for Hg2+ Removal. J. Water Process Eng. 2021, 43, 102294. [Google Scholar] [CrossRef]
- González, P.G.; Pliego-Cuervo, Y.B. Adsorption of Cd(II), Hg(II) and Zn(II) from Aqueous Solution Using Mesoporous Activated Carbon Produced from Bambusa Vulgaris Striata. Chem. Eng. Res. Des. 2014, 92, 2715–2724. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Zheng, F.; Wang, J.; Chen, L.; Hu, P.; Zhen, Q.; Bashir, S.; Liu, J.L. Efficient Removal of Water Pollutants by Hierarchical Porous Zeolite-Activated Carbon Prepared from Coal Gangue and Bamboo. J. Clean. Prod. 2021, 325, 129322. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified Biochar: Synthesis and Mechanism for Removal of Environmental Heavy Metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Zheng, Z.; Yan, N.; Lou, Z.; Jiang, X.; Zhang, X.; Chen, S.; Xu, R.; Liu, C. Modification and Application of Bamboo-Based Materials: A Review—Part I: Modification Methods and Mechanisms. Forests 2023, 14, 2219. [Google Scholar] [CrossRef]
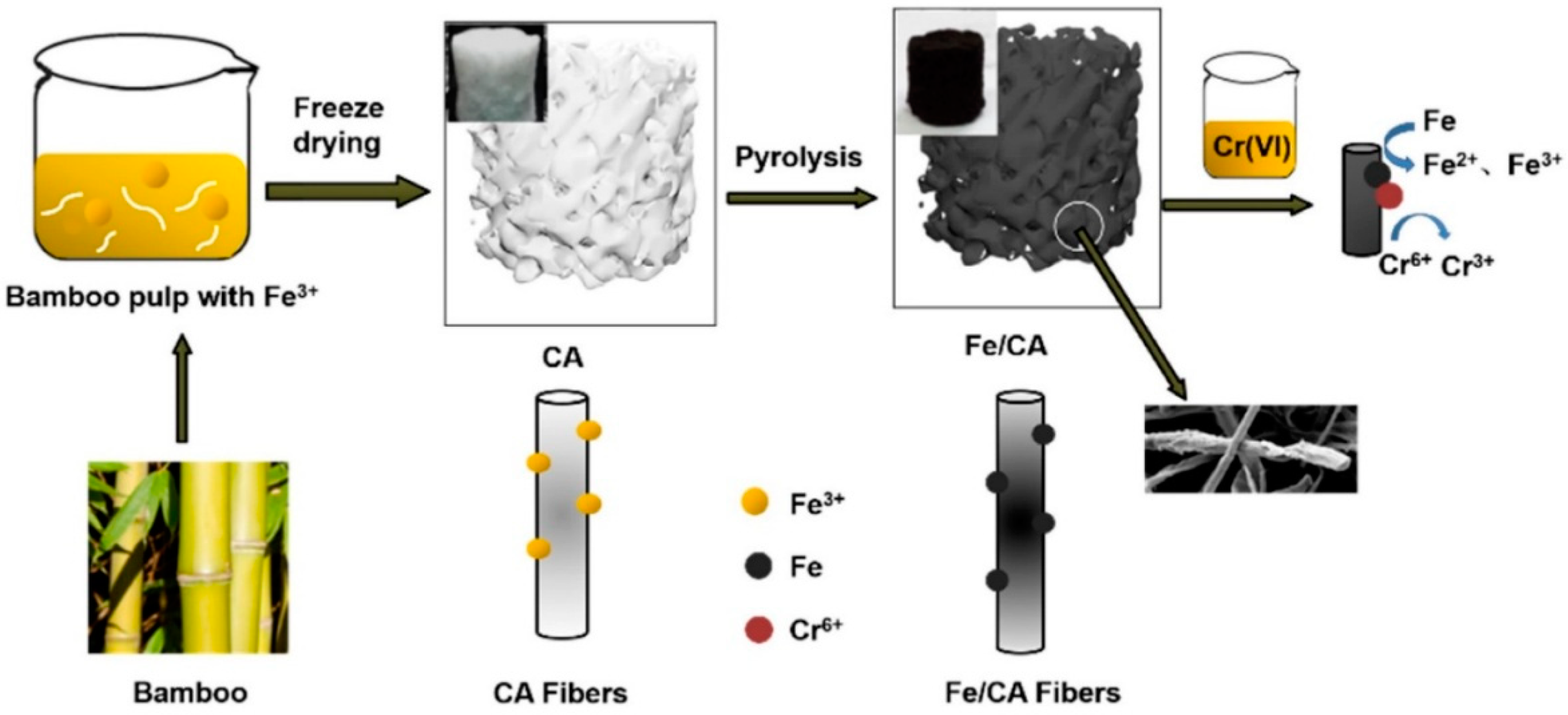
- Xue, X.; Yuan, W.; Zheng, Z.; Zhang, J.; Ao, C.; Zhao, J.; Wang, Q.; Zhang, W.; Lu, C. Iron-Loaded Carbon Aerogels Derived from Bamboo Cellulose Fibers as Efficient Adsorbents for Cr(Vi) Removal. Polymers 2021, 13, 4338. [Google Scholar] [CrossRef] [PubMed]
- Chandrawansha, S.I.; Selladurai, A. Cellulose Obtained from Banana Stem and Bamboo Stem Extraction, Modification, Characterization and Metal Adsorption Properties of Cellulose Obtained from Banana Stem and Bamboo Stem; University of Jaffna: Kokuvil East, Sri Lanka, 2024; Available online: https://vingnanam.sljol.info/articles/4241/files/66731b059e624.pdf (accessed on 17 December 2024).
- Chen, H.; Cheng, Y.; Zhu, Z.; He, H.; Zhang, L.; Li, N.; Zhu, Y. Adsorption of Pb(II) from Aqueous Solution by Mercerized Moso Bamboo Chemically Modified with Pyromellitic Dianhydride. J. Environ. Eng. 2020, 146, 04019127. [Google Scholar] [CrossRef]
- Tian, A.; Xiaojun, J.; Qingyu, L. Novel Adsorbents Based upon Carboxylic Acid-Modified Phyllostachys Pubescens Powder: Preparation, Characterization and Application for Adsorbing Lead(II) from Aqueous Solution. Sep. Sci. Technol. 2020, 55, 1249–1259. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Palai, T.; Kaushal, R. Surface Functionalization of Bamboo Leave Mediated Synthesized SiO2 Nanoparticles: Study of Adsorption Mechanism, Isotherms and Enhanced Adsorption Capacity for Removal of Cr (VI) from Aqueous Solution. Environ. Res. 2022, 214, 113761. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH Activation of Biochar: Experimental and Modeling Studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Chu, B.; Terao, K.; Amano, Y.; Machida, M. Adsorption Behavior of Cr(Vi) by n-Doped Biochar Derived from Bamboo. Water Pract. Technol. 2020, 15, 170–181. [Google Scholar] [CrossRef]
- Chu, B.; Amano, Y.; Machida, M. Preparation of Bamboo-Based Oxidized Biochar for Simultaneous Removal of Cd(II) and Cr(VI) from Aqueous Solutions. Desalin. Water Treat. 2019, 168, 269–281. [Google Scholar] [CrossRef]
- Song, W.; Zhu, M.; Zhang, S. Comparison of the Properties of Fiberboard Composites with Bamboo Green, Wood, or Their Combination as the Fibrous Raw Material. BioResources 2018, 13, 3315–3334. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Gielen, G.; Bolan, N.; Sik, Y.; Khan, N.; Xu, S.; Yuan, G.; Chen, X.; Zhang, X.; et al. Effect of Bamboo and Rice Straw Biochars on the Mobility and Redistribution of Heavy Metals (Cd, Cu, Pb and Zn) in Contaminated Soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Alchouron, J.; Navarathna, C.; Chludil, H.D.; Dewage, N.B.; Perez, F.; Hassan, E.B.; Pittman, C.U.; Vega, A.S.; Mlsna, T.E. Assessing South American Guadua Chacoensis Bamboo Biochar and Fe3O4 Nanoparticle Dispersed Analogues for Aqueous Arsenic(V) Remediation. Sci. Total Environ. 2020, 706, 135943. [Google Scholar] [CrossRef] [PubMed]
- Lyu, P.; Li, L.; Huang, X.; Wang, G.; Zhu, C. Pre-Magnetic Bamboo Biochar Cross-Linked Ca–Mg–Al Layered Double-Hydroxide Composite: High-Efficiency Removal of As(III) and Cd(II) from Aqueous Solutions and Insight into the Mechanism of Simultaneous Purification. Sci. Total Environ. 2022, 823, 153743. [Google Scholar] [CrossRef]
- Jena, L.; Soren, D.; Deheri, P.K.; Pattojoshi, P. Preparation, Characterization and Optical Properties Evaluations of Bamboo Charcoal. Curr. Res. Green Sustain. Chem. 2021, 4, 100077. [Google Scholar] [CrossRef]
- Najihah, N.; Rosli, B.; Ming, L.C.; Mahadi, A.H.; Wattanasiriwech, S.; Lim, R.C.; Kumara, N.T.R.N. Ruthenium Dye (N3) Removal from Simulated Wastewater Using Bamboo Charcoal and Activated Bamboo Charcoal. Key Eng. Mater. 2018, 765, 92–98. [Google Scholar] [CrossRef]
- Munirah, N.R.; Noriman, N.Z.; Salihin, M.Z.; Fatin, M.H.; Sam, S.T. Characteristics of Carbonized Bamboo at Various Temperatures. Appl. Mech. Mater. 2015, 755, 115–119. [Google Scholar] [CrossRef]
- Lalhruaitluanga, H.; Jayaram, K.; Prasad, M.N.V.; Kumar, K.K. Lead(II) Adsorption from Aqueous Solutions by Raw and Activated Charcoals of Melocanna Baccifera Roxburgh (Bamboo)-A Comparative Study. J. Hazard. Mater. 2010, 175, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wilamas, A.; Vinitnantharat, S.; Pinisakul, A. Manganese Adsorption onto Permanganate-Modified Bamboo Biochars from Groundwater. Sustainability 2023, 15, 6831. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Liu, H.; Zhang, Y.; Fan, Y.; Mo, S.; Li, H.; Wang, J.; Lin, H. Novel Amino-Modified Bamboo-Derived Biochar-Supported Nano-Zero-Valent Iron (AMBBC-NZVI) Composite for Efficient Cr(VI) Removal from Aqueous Solution. Environ. Sci. Pollut. Res. Int. 2023, 30, 119935–119946. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gui, C.; Ji, Y.; Li, X.; Rao, F.; Huan, W.; Li, L. Changes in Chemical and Thermal Properties of Bamboo after Delignification Treatment. Polymers 2022, 14, 2537. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, S.; Kuba, T.; Toyohara, Y.; Kamida, S.; Uchikawa, Y. Development of Ion-Exchange Properties of Bamboo Charcoal Modified with Concentrated Nitric Acid. IOP Conf. Ser. Earth Environ. Sci. 2017, 82, 012002. [Google Scholar] [CrossRef]
- Hu, H.; Sun, L.; Jiang, B.; Wu, H.; Huang, Q.; Chen, X. Low Concentration Re(VII) Recovery from Acidic Solution by Cu-Biochar Composite Prepared from Bamboo (Acidosasa longiligula) Shoot Shell. Miner. Eng. 2018, 124, 123–136. [Google Scholar] [CrossRef]
- Deepika; Reddy, S.R.; Dixit, V.; Verma, S.; Yadav, P.K. Comparative Study on Estimation of Carbon Content in Different Parts of Selected Bamboo Species. Int. J. Environ. Clim. Change 2022, 12, 908–914. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Chen, C.; Li, X.; Deng, Q.; Li, D. Mechanical, Electrical, and Thermal Properties of Highly Filled Bamboo Charcoal/Ultra-High Molecular Weight Polyethylene Composites. Polym. Compos. 2018, 39, E1858–E1866. [Google Scholar] [CrossRef]
- Mukesh, B.; Kalpit, S.; Jyeshtharaj, B.J.; Madhura, Y.; Abhishek, S. Advanced Thermo-Chemical Treatment of Waste Bambusa Vulgaris for Sustainable Resource Recovery. Mater. Res. Proc. 2023, 29, 192–200. [Google Scholar] [CrossRef]
- Saini, S.; Katnoria, J.K.; Kaur, I. A Comparative Study for Removal of Cadmium(II) Ions Using Unmodified and NTA-Modified Dendrocalamus Strictus Charcoal Powder. J. Environ. Health Sci. Eng. 2019, 17, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shang, T.; Jin, X.; Gao, J.; Zhao, Q. Study of Chromium(vi) Removal from Aqueous Solution Using Nitrogen-Enriched Activated Carbon Based Bamboo Processing Residues. RSC Adv. 2015, 5, 784–790. [Google Scholar] [CrossRef]
- Huang, A.; Bai, W.; Yang, S.; Wang, Z.; Wu, N.; Zhang, Y.; Ji, N.; Li, D. Adsorption Characteristics of Chitosan-Modified Bamboo Biochar in Cd(II) Contaminated Water. J. Chem. 2022, 2022, 6303252. [Google Scholar] [CrossRef]
- Nitayaphat, W.; Jintakosol, T. Removal of Silver(I) from Aqueous Solutions by Chitosan/Bamboo Charcoal Composite Beads. J. Clean. Prod. 2015, 87, 850–855. [Google Scholar] [CrossRef]
- Wu, J.; Ren, D.; Zhang, X.; Chen, Z.; Zhang, S.; Li, S.; Fu, L. The Adsorption Properties of Biochar Derived from Woody Plants or Bamboo for Cadmium in Aqueous Solution. Desalin. Water Treat. 2019, 160, 268–275. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, J.-Z.; Bai, L.-Q.; Chen, X.-Q.; Li, B. High Effective Adsorption of Pb(II) from Solution by Biochar Derived from Torrefaction of Ammonium Persulphate Pretreated Bamboo. Bioresour. Technol. 2021, 323, 124616. [Google Scholar] [CrossRef]
- Angthararuk, D.; Phasuk, S.; Takolpuckdee, P. Low-Cost Biochar Derived from Bamboo Waste for Removal of Heavy Metal in Aqueous Solution. J. Food Health Bioenviron. Sci. 2022, 15, 34–42. [Google Scholar]
- Tang, W.; Cai, N.; Xie, H.; Liu, Y.; Wang, Z.; Liao, Y.; Wei, T.; Zhang, C.; Fu, Z.; Yin, D. Efficient Adsorption Removal of Cd2+ from Aqueous Solutions by HNO3 Modified Bamboo-Derived Biochar. IOP Conf. Ser. Mater. Sci. Eng. 2020, 729, 012081. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, X.; Zhang, Y.; Tang, Q.; Yuan, Z.; De Hoop, C.F.; Cao, J.; Hao, S.; Liang, T.; Li, F.; et al. Bamboo-Based Biofoam Adsorbents for the Adsorption of Cationic Pollutants in Wastewater: Methylene Blue and Cu(II). ACS Omega 2021, 6, 23447–23459. [Google Scholar] [CrossRef] [PubMed]
- Sable, H.; Kumar, V.; Mishra, R.; Singh, V.; Roy, A.; Rai, A.K.; Ranjan, N.; Rustagi, S.; Pandit, S. Bamboo Stem Derived Biochar for Biosorption of Cadmium (II) Ions from Contaminated Wastewater. Environ. Nanotechno. Monit. Manag. 2024, 21, 100936. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Tu, R.; Lu, C.; He, X.; Zhang, W. Mechanically Robust, Flame-Retardant and Anti-Bacterial Nanocomposite Films Comprised of Cellulose Nanofibrils and Magnesium Hydroxide Nanoplatelets in a Regenerated Cellulose Matrix. Cellulose 2014, 21, 1859–1872. [Google Scholar] [CrossRef]
- Lou, Z.; Zhao, Z.; Li, Y.; Shan, W.; Xiong, Y.; Fang, D.; Yue, S.; Zang, S. Contribution of Tertiary Amino Groups to Re(VII) Biosorption on Modified Corn Stalk: Competitiveness and Regularity. Bioresour. Technol. 2013, 133, 546–554. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from Aqueous Solutions by Biochar Derived from KMnO4 Treated Hickory Wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Van Hien, N.; Valsami-Jones, E.; Vinh, N.C.; Phu, T.T.; Tam, N.T.T.; Lynch, I. Effectiveness of Different Biochar in Aqueous Zinc Removal: Correlation with Physicochemical Characteristics. Bioresour. Technol. Reports 2020, 11, 100466. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Zhang, H.; Liu, Q.; Yu, J.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Anti-Bacterial and Super-Hydrophilic Bamboo Charcoal with Amidoxime Modified for Efficient and Selective Uranium Extraction from Seawater. J. Colloid Interface Sci. 2021, 598, 455–463. [Google Scholar] [CrossRef]
- Chen, G.; Viengvilay, K.; Yu, W.; Mao, T.; Qu, Z.; Liang, B.; Chen, Z.; Li, Z. Effect of Different Modification Methods on the Adsorption of Manganese by Biochar from Rice Straw, Coconut Shell, and Bamboo. ACS Omega 2023, 8, 28467–28474. [Google Scholar] [CrossRef]
- Hassan, A.; Kaewsichan, L. Removal of Pb(II) from Aqueous Solutions Using Mixtures of Bamboo Biochar and Calcium Sulphate, and Hydroxyapatite and Calcium Sulphate. EnvironmentAsia 2016, 9, 37–44. [Google Scholar]
- Emmanuel, U.C.; Chukwudi, M.I.; Monday, S.S.; Anthony, A.I. Human Health Risk Assessment of Heavy Metals in Drinking Water Sources in Three Senatorial Districts of Anambra State, Nigeria. Toxicol. Rep. 2022, 9, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Alchouron, J.; Navarathna, C.; Rodrigo, P.M.; Snyder, A.; Chludil, H.D.; Vega, A.S.; Bosi, G.; Perez, F.; Mohan, D.; Pittman, C.U.; et al. Household Arsenic Contaminated Water Treatment Employing Iron Oxide/Bamboo Biochar Composite: An Approach to Technology Transfer. J. Colloid Interface Sci. 2021, 587, 767–779. [Google Scholar] [CrossRef] [PubMed]
- García-Ávila, F.; Galarza-Guamán, A.; Barros-Bermeo, M.; Alfaro-Paredes, E.A.; Avilés-Añazco, A.; Iglesias-Abad, S. Integration of High-Rate Filtration Using Waste-Derived Biochar as a Potential Sustainable Technology for Drinking Water Supply. Biochar 2023, 5, 62. [Google Scholar] [CrossRef]
- Jarawi, N.; Jusoh, I. Charcoal Properties of Malaysian Bamboo Charcoal Carbonized at 750 °C. BioResources 2023, 18, 4413–4429. [Google Scholar] [CrossRef]
- Mistar, E.M.; Alfatah, T.; Supardan, M.D. Synthesis and Characterization of Activated Carbon from Bambusa Vulgaris Striata Using Two-Step KOH Activation. J. Mater. Res. Technol. 2020, 9, 6278–6286. [Google Scholar] [CrossRef]
- Azlan Zahari, K.F.; Sahu, U.K.; Khadiran, T.; Surip, S.N.; ALOthman, Z.A.; Jawad, A.H. Mesoporous Activated Carbon from Bamboo Waste via Microwave-Assisted K2CO3 Activation: Adsorption Optimization and Mechanism for Methylene Blue Dye. Separations 2022, 9, 390. [Google Scholar] [CrossRef]
- Sadeghi, M.H.; Tofighy, M.A.; Mohammadi, T. One-Dimensional Graphene for Efficient Aqueous Heavy Metal Adsorption: Rapid Removal of Arsenic and Mercury Ions by Graphene Oxide Nanoribbons (GONRs). Chemosphere 2020, 253, 126647. [Google Scholar] [CrossRef]
- Kharrazi, S.M.; Mirghaffari, N.; Dastgerdi, M.M.; Soleimani, M. A Novel Post-Modification of Powdered Activated Carbon Prepared from Lignocellulosic Waste through Thermal Tension Treatment to Enhance the Porosity and Heavy Metals Adsorption. Powder Technol. 2020, 366, 358–368. [Google Scholar] [CrossRef]
- Mistar, E.M.; Hasmita, I.; Alfatah, T.; Muslim, A.; Supardan, M.D. Adsorption of Mercury(II) Using Activated Carbon Produced from Bambusa vulgaris Var. Striata in a Fixed-Bed Column. Sains Malays. 2019, 48, 719–725. [Google Scholar] [CrossRef]
- Xue, J.; Sun, Q.; Zhang, Y.; Mao, W.; Li, F.; Yin, C. Preparation of a Polypyrrole/Graphene Oxide Composite Electrode by Electrochemical Codeposition for Capacitor Deionization. ACS Omega 2020, 5, 10995–11004. [Google Scholar] [CrossRef]
- Rani, L.; Kaushal, J.; Srivastav, A.L.; Mahajan, P. A Critical Review on Recent Developments in MOF Adsorbents for the Elimination of Toxic Heavy Metals from Aqueous Solutions. Environ. Sci. Pollut. Res. 2020, 27, 44771–44796. [Google Scholar] [CrossRef] [PubMed]
- Davydiuk, T.; Chen, X.; Huang, L.; Shuai, Q.; Le, X.C. Removal of Inorganic Arsenic from Water Using Metal Organic Frameworks. J. Environ. Sci. 2020, 97, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Lin, S.; Liu, G.; Xuan, Y.; Gao, L.; Li, P. Functionalized Moso Bamboo Powder Adsorbent for Cd (II) Complexes with Citric Acid / Tartrate Acid: Characterization, Adsorptive Performance, and Mechanism. Environ. Eng. Res. 2022, 27, 210321. [Google Scholar] [CrossRef]
- Naseem, K.; Imran, Q.; Ur Rehman, M.Z.; Tahir, M.H.; Najeeb, J. Adsorptive Removal of Heavy Metals and Dyes from Wastewater Using Azadirachta Indica Biomass. Int. J. Environ. Sci. Technol. 2023, 20, 5799–5822. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Zhou, X.; Guo, J.; Liu, Y. Highly Efficient Removal of Cu(II), Cd(II) and Pb(II) by Carboxyl-Modified Multi-Porous Biochar. Sep. Sci. Technol. 2018, 53, 2860–2869. [Google Scholar] [CrossRef]
- Verma, M.; Lee, I.; Hong, Y.; Kumar, V.; Kim, H. Multifunctional β-Cyclodextrin-EDTA-Chitosan Polymer Adsorbent Synthesis for Simultaneous Removal of Heavy Metals and Organic Dyes from Wastewater. Environ. Pollut. 2022, 292, 118447. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, K.; Zhang, H.; Han, L.; He, Q.; Huang, F.; Yu, W.; Guo, F.; Wang, W. Chemosphere Sustainable Green Conversion of Coal Gangue Waste into Cost-Effective Porous Multimetallic Silicate Adsorbent Enables Superefficient Removal of Cd (II) and Dye. Chemosphere 2023, 324, 138287. [Google Scholar] [CrossRef]
- Jing, W.; Yin, L.; Lin, X.; Yu, Y.; Lian, D.; Shi, Z.; Chen, P.; Tang, M.; Yang, C. Simultaneous Adsorption of Cu2+ and Cd2+ by a Simple Synthesis of Environmentally Friendly Bamboo Pulp Aerogels: Adsorption Properties and Mechanisms. Polymers 2022, 14, 4909. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Xiao, Y.; Wang, G.; Fan, J.; Wan, K.; He, Q.; Gao, M.; Miao, Z. Adsorption of Heavy Metals in Water by Modifying Fe3O4 Nanoparticles with Oxidized Humic Acid. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 616, 126333. [Google Scholar] [CrossRef]
- Geng, B.; Xu, Z.; Liang, P.; Zhang, J.; Christie, P.; Liu, H.; Wu, S.; Liu, X. Three-Dimensional Macroscopic Aminosilylated Nanocellulose Aerogels as Sustainable Bio-Adsorbents for the Effective Removal of Heavy Metal Ions. Int. J. Biol. Macromol. 2021, 190, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, Z.; Ghaemy, M.; Olad, A. Removal of Heavy Metals from Polluted Water Using Magnetic Adsorbent Based on κ-Carrageenan and N-Doped Carbon Dots. Hydrometallurgy 2022, 213, 105915. [Google Scholar] [CrossRef]
- El Kaim Billah, R.; Ayouch, I.; Abdellaoui, Y.; Kassab, Z.; Khan, M.A.; Agunaou, M.; Soufiane, A.; Otero, M.; Jeon, B.-H. A Novel Chitosan/Nano-Hydroxyapatite Composite for the Adsorptive Removal of Cd(II) from Aqueous Solution. Polymers 2023, 15, 1524. [Google Scholar] [CrossRef]
- Adeiga, O.I.; Pillay, K. Adsorptive Removal of Cd(II) Ions from Water by a Cheap Lignocellulosic Adsorbent and Its Reuse as a Catalyst for the Decontamination of Sulfamethoxazole. ACS Omega 2024, 9, 38348–38358. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Yang, C.; Lin, X.; Tang, M.; Lian, D.; Yu, Y.; Liu, D. MnFe2O4-Loaded Bamboo Pulp Carbon-Based Aerogel Composite: Synthesis, Characterization and Adsorption Behavior Study for Heavy Metals Removal. RSC Adv. 2024, 14, 39995–40005. [Google Scholar] [CrossRef]
- Lu, Y.; Zeng, H.; Lin, H.; Liang, Y.; Feng, M.; Zhou, Z.; Liang, Z.; Li, H.; Chen, G. Synergistic Removal Performance and Mechanism of Cd(II) and As(III) from Irrigation Water by Iron Sulfide-Based Porous Biochar. Environ. Sci. Pollut. Res. 2024, 31, 11591–11604. [Google Scholar] [CrossRef]
- Das, J.; Saha, R.; Nath, H.; Mondal, A.; Nag, S. An Eco-Friendly Removal of Cd(II) Utilizing Banana Pseudo-Fibre and Moringa Bark as Indigenous Green Adsorbent and Modelling of Adsorption by Artificial Neural Network. Environ. Sci. Pollut. Res. 2022, 29, 86528–86549. [Google Scholar] [CrossRef] [PubMed]
- Senniappan, S.; Palanisamy, S.; Manon Mani, V.; Umesh, M.; Govindasamy, C.; Khan, M.I.; Shanmugam, S. Exploring the Adsorption Efficacy of Cassia Fistula Seed Carbon for Cd (II) Ion Removal: Comparative Study of Isotherm Models. Environ. Res. 2023, 235, 116676. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Tang, M.; Lin, X.; Yang, C.; Lian, D.; Yu, Y.; Liu, D. Microwave Irradiation-Assisted Synthesis of Anisotropic Crown Ether-Grafted Bamboo Pulp Aerogel as a Chelating Agent for Selective Adsorption of Heavy Metals (Mn+). Gels 2024, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Ramutshatsha-Makhwedzha, D.; Mbaya, R.; Mavhungu, M.L. Application of Activated Carbon Banana Peel Coated with Al2O3-Chitosan for the Adsorptive Removal of Lead and Cadmium from Wastewater. Materials 2022, 15, 860. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Long, Q.; Zhu, Y.; Lin, C.; Xu, X.; Pan, B.; Shi, W.; Guo, Y.; Deng, J.; Yao, Q.; et al. Multifunctional Self-Assembled Adsorption Microspheres Based on Waste Bamboo Shoot Shells for Multi-Pollutant Water Purification. Environ. Res. 2024, 249, 118452. [Google Scholar] [CrossRef]
- Mahanim, S.; Wan Asma, I.; Rafidah, J.; Puad, E.; Shaharuddin, H. Production of activated carbon from industrial bamboo wastes. J. Trop. For. Sci. 2011, 23, 417–424. [Google Scholar]
- Pan, C.; Zhou, G.; Shrestha, A.K.; Chen, J.; Kozak, R.; Li, N.; Li, J.; He, Y.; Sheng, C.; Wang, G. Bamboo as a Nature-Based Solution (NbS) for Climate Change Mitigation: Biomass, Products, and Carbon Credits. Climate 2023, 11, 175. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Ranaei, F.; Ahmad, Z. Application of Bamboo Plants in Nine Aspects. Sci. World J. 2020, 2020, 7284203. [Google Scholar] [CrossRef]
- Promdee, K.; Chanvidhwatanakit, J.; Satitkune, S.; Boonmee, C.; Kawichai, T.; Jarernprasert, S.; Vitidsant, T. Characterization of Carbon Materials and Differences from Activated Carbon Particle (ACP) and Coal Briquettes Product (CBP) Derived from Coconut Shell via Rotary Kiln. Renew. Sustain. Energy Rev. 2017, 75, 1175–1186. [Google Scholar] [CrossRef]
- De Magalhães, L.F.; Da Silva, G.R.; Peres, A.E.C. Zeolite Application in Wastewater Treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- León, M.; Silva, J.; Carrasco, S.; Barrientos, N. Design, Cost Estimation and Sensitivity Analysis for a Production Process of Activated Carbon Fromwaste Nutshells by Physical Activation. Processes 2020, 8, 945. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy Metal Removal from Wastewater Using Various Adsorbents: A Review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Choy, K.K.H.; Barford, J.P.; McKay, G. Production of Activated Carbon from Bamboo Scaffolding Waste—Process Design, Evaluation and Sensitivity Analysis. Chem. Eng. J. 2005, 109, 147–165. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, F.; Hu, W.; Yang, X.; Xiang, H.; Wang, G.; Fei, B.; Liu, Z. Economic Analysis of a Hypothetical Bamboo-Biochar Plant in Zhejiang Province, China. Waste Manag. Res. 2017, 35, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.M.X.; Chin, B.L.F.; Huang, M.M.; Yiin, C.L.; Kurnia, J.C.; Lam, S.S.; Tan, Y.H.; Chai, Y.H.; Rashidi, N.A. Harnessing Bamboo Waste for High-Performance Supercapacitors: A Comprehensive Review of Activation Methods and Applications. J. Energy Storage 2025, 105, 114613. [Google Scholar] [CrossRef]
- Odega, C.A.; Ayodele, O.O.; Ogutuga, S.O.; Anguruwa, G.T.; Adekunle, A.E.; Fakorede, C.O. Potential Application and Regeneration of Bamboo Biochar for Wastewater Treatment: A Review. Adv. Bamboo Sci. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of Chitosan-Based Adsorbents Used in Heavy Metal Adsorption: A Review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]


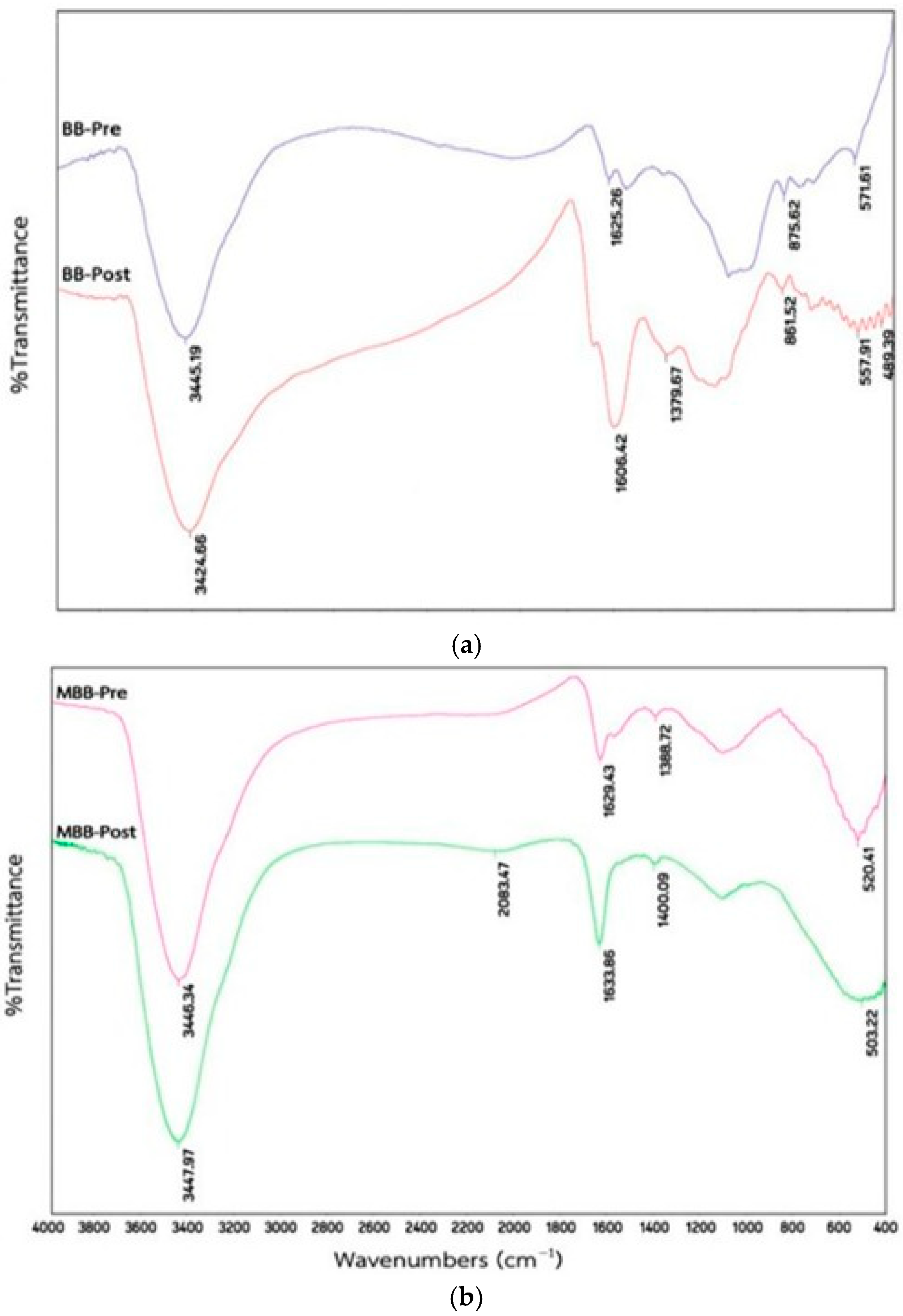


| Sr. No. | Adsorbent | FTIR Spectra | Reference |
|---|---|---|---|
| 1.→ | Bamboo cellulose-derived carbon aerogel | 2920–2850 cm−1: CH and CH2 vibration. 1083 cm−1: C–O vibration. –OH, absorbance is almost completely diminished. | [111] |
| 2.→ | Bamboo cellulose-derived iron/carbon aerogel | 3440 cm−1: –OH peak. 591 cm−1: stretching vibration of Fe–O. | |
| 3.→ | Bamboo biochar and magnetic bamboo biochar | 3677 cm−1: –OH vibration. 1733 cm−1: carboxyl C=O. 1559 cm−1: aromatic C=C. 1402 cm−1: carboxyl C–O. 1218 cm−1: vibration of C–C group. Emerging peak at 1124 cm−1: SO42−. Emerging peak 594 cm−1: Fe–O stretching modes. | [70] |
| 4.→ | Chitosan-modified magnetic bamboo biochar | 1072 cm−1: C–O–C vibration. 1031 cm−1: C–N vibration. | |
| 5.→ | EDTA and chitosan bi-functionalized magnetic bamboo biochar | 3440 cm−1: –OH stretching. and amine N–H stretching. 1631 cm−1: amide HN–C=O band. 904 cm−1: in-plane bending vibration of carboxyl –OH. Carboxyl C–O peak becomes visibly stronger. | |
| 6.→ | Bamboo biochar | 3250–3700 cm−1: stretching vibration of –OH group. 2346 cm−1: vibration of –COO group. 1690 cm−1: C–O group vibration. 1433 cm−1: –CH2 group vibration. 772 cm−1: vibration of C–H groups. 1598 cm−1: stretching vibrations of C=O and –OH. 1100 cm−1: vibration superposition of C–O and Si–O–Si. 1034 cm−1: vibration superposition of C=O and Si-O-Si. | [128] |
| 7.→ | Amino-modified bamboo biochar | Blue shift from 1598 to 1575 cm−1 and a peak broadening: overlap of the C=O stretching and –NH2 bending vibrations. Stronger peaks at 1100, 1034, and 465 cm−1 than bamboo biochar; vibration superposition of the Si–O–Si group of the grafted 3-aminopropyltriethoxysilane. | |
| 8.→ | Amino-modified bamboo-derived biochar-supported nano-zero-valent iron using FeCl3·6H2O | 580 cm−1: stretching vibration of Fe–O. The intensity of the main peaks is less than that of amino-modified bamboo biochar. | |
| 9.→ | Melocanna baccifera bamboo raw charcoal | 3447.10 cm−1: Hydroxyl group. 1743.21 cm−1: Carbonyl group. 1606.85 cm−1: C=O stretching. 1606.85 cm−1: C=O stretching. 1010.79 cm−1: C–O stretching. | [126] |
| 10.→ | Pb (II)-loaded bamboo raw charcoal | 3447.10 cm−1 peak disappeared. Peak size at 1743.21 cm−1 reduced. The peak at 1606.63 cm−1 shifted to 1608.78 cm−1. The peak at 1259.63 cm−1 shifted to 1255.77 cm−1 with a reduction in peak size. Size reduction in 1010.79 cm−1 peak. | |
| 11.→ | Pb (II)-loaded bamboo-activated charcoal | 3447.10 cm−1 peak disappeared. The peak at 1743.21 cm−1 disappeared. The peak at 1606.63 cm−1 shifted to 1595.27 cm−1. The peak at 1259.63 cm−1 shifted to 1396.59 cm−1. The peak at 1010.79 cm−1 shifted to 1020.44 cm−1. |
| Adsorbent | Heavy Metals | Temperature | pH | Contact Time | Maximum Adsorption Capacity | Efficiency | Kinetics | Isotherm | Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Chitosan/bamboo charcoal composite beads | Ag (I) | - | 6 | 180 min | 52.91 mg/g | 79.66% | - | Langmuir | - | [138] |
| Copper-impregnated biochar | Re (VII) | 25 °C | 1 | 5 h | 20.91 mg/g | >90% | Pseudo-second-order | Redlich-Peterson | Electrostatic attraction and surface complexation. | [131] |
| Phosphate-treated bamboo biochar | Cd (II) | 25 °C | 7 | - | 209.40 mg/g | 97.49% | Pseudo-first-order | Langmuir | - | [68] |
| Bamboo biochar | Cd (II) | 25 °C | 5 | 40 min | 21.45 mg/g | 94% | Ion exchange, chemisorption, electrostatic interactions, and precipitation. | [139] | ||
| Bamboo-based oxidized biochar modified by ZnCl2 and oxidized with (NH4)2S2O8 | Cd (II) | 25 °C | 6 | 180 min | 33.8 mg/g | - | Pseudo-second-order | Langmuir | Ion exchange, electrostatic interaction. | [118] |
| Bamboo-based oxidized biochar modified by ZnCl2 and oxidized with (NH4)2S2O8 | Cr (VI) | 25 °C | 6 | 180 min | 30.3 mg/g | - | Pseudo-second-order | Langmuir | Ion exchange, electrostatic interaction. | [118] |
| Steam-activated bamboo biochar | Cu (II) | 25 °C | 5 | - | 319.52 mg/g | 94.66% | - | Langmuir | Surface complexation, precipitation, cation exchange, and electrostatic interaction. | [66] |
| Bamboo biochar | Zn (II) | - | 5.5 | 240 min | 7.62 mg/g | 96% | Pseudo-second-order | Freundlich | Intraparticle diffusion, surface complexation, ion exchange, and precipitation. | [149] |
| Fe2(SO4)3 or FeSO4.7H2O modified magnetic biochar | Cr (VI) | 25 °C | 2 | 24 h | 75.8 mg/g | 90.7% | Pseudo-second-order | Langmuir | Electrostatic interaction, ion exchanges, and redox interaction. | [63] |
| Magnetic biochar modified with chitosan | Cr (VI) | 25 °C | 2 | 24 h | 127 mg/g | - | Pseudo-second-order | Langmuir | Electrostatic interaction, chelation, ion exchange, and redox interaction. | [63] |
| N-doped biochar (ZnCl2-activated) | Cr (VI) | 25 °C | 2 | 480 min | 499.1 mg/g | 86% | Pseudo-second-order | Langmuir | - | [117] |
| Fe3O4 nanoparticle-covered bamboo biochar | As (V) | 25 °C | 7 | 60 min | 90 mg/g | 100% | Pseudo-second-order | Langmuir | - | [121] |
| Fe3O4 nanoparticle-covered-activated bamboo biochar | As (V) | 25 °C | 7 | 60 min | 85 mg/g | - | Pseudo-second-order | Langmuir | - | [121] |
| HNO3 modified bamboo | Cd (II) | 35 °C | 7 | 90 min | 44.54 mg/g | 95.37% | Pseudo-second-order | Freundlich | - | [142] |
| Bamboo charcoal | U (VI) | - | 5 | 4 h | 127.65 mg/g | - | Pseudo-second-order | Freundlich | - | [150] |
| Amidoxime-modified bamboo charcoal | U (VI) | - | 7 | 4 h | 447.71 mg/g | - | Pseudo-second-order | Freundlich | - | [150] |
| Bamboo-based zeolite-activated carbon | Cu (II) | - | 6 | 210 min | 104.9 mg/g | 87.4% | Pseudo-second-order | Langmuir | Physical adsorption (Van der Waals’ force) | [108] |
| CuCl2-impregnated activated carbon | Pb (II) | - | 5.8 | 3 h | 0.460 mg/g | >96% | - | - | - | [90] |
| CuCl2-impregnated activated carbon | Zn (II) | - | 5.8 | 3 h | 0.392 mg/g | 81.3% | - | - | - | [90] |
| CuCl2-impregnated activated carbon | V | - | 5.8 | 3 h | 0.453 mg/g | 91.2% | - | - | - | [90] |
| CuCl2-impregnated activated carbon | As | - | 5.8 | 3 h | 0.408 mg/g | - | - | - | - | [90] |
| CuCl2-impregnated activated carbon | Mn | - | 5.8 | 3 h | 1.031 mg/g | - | - | - | - | [90] |
| Activated carbon activated using a KOH/NaOH mixture | Hg2+ | 25 °C | - | - | 312.7 mg/g | - | - | - | Chemisorption, surface complexation, electrostatic interaction, hydrogen bonding. | [106] |
| Biofoam (polyurethane foam matrix) adsorbent with bamboo fiber | Cu (II) | 25 °C | 7 | 24 h | 7.05 mg/g | 100% | Pseudo-second-order | Langmuir | Hydrogen bonding | [143] |
| Biofoam adsorbent with α-cellulose fiber | Cu (II) | 25 °C | 7 | 24 h | 6.11 mg/g | 95.16% | Pseudo-second-order | Freundlich | Hydrogen bonding | [143] |
| Biofoam with nanocellulose fiber | Cu (II) | 25 °C | 7 | 48 h | 4.39 mg/g | 100% | Pseudo-second-order | Langmuir | Hydrogen bonding | [143] |
| Carbon aerogels derived from bamboo cellulose fiber and loaded with iron | Cr (VI) | - | 3 | 8 min | 182 mg/g | - | Pseudo-second-order | Langmuir | Electrostatic interaction, ion exchange, surface complexation, precipitation. | [111] |
| Chitosan-modified bamboo biochar | Cd (II) | - | 7 | 120 min | 93.46 mg/g | 90.24% | - | - | Surface adsorption, electrostatic adsorption, and ion exchange. | [137] |
| KOH-activated modified bamboo charcoal | Cu (II) | 45 °C | 6 | 6 h | 39.91 mg/g | - | Pseudo-second-order | Langmuir | Chemisorption and physical adsorption. | [89] |
| KOH-activated modified bamboo charcoal | Cd (II) | 45 °C | 6 | 4 h | 51.00 mg/g | - | Pseudo-second-order | Langmuir | Chemisorption and physical adsorption | [89] |
| Sodium hydroxide-modified bamboo biochar | Mn (II) | 30 °C | 5 | 180 min | 7.89 mg/g | - | Pseudo-second-order | Langmuir | Chemisorption and physical adsorption. | [151] |
| Bamboo biochar | Mn (II) | - | 6.78 | 72 h | 0.803 mg/g | - | Pseudo-second-order | Temkin | Chemisorption and physical adsorption. | [127] |
| Potassium permanganate-modified bamboo biochar | Mn (II) | - | 6.78 | 72 h | 21.27 mg/g | - | Pseudo-second-order | Temkin | Chemisorption and ion exchange. | [127] |
| Amino-enhanced bamboo biochar reinforced by nano-zero-valent iron | Cr (VI) | 30 °C | 5 | 60 min | 63.33 mg/g | 95.3% | Pseudo-second-order | Langmuir | Electrostatic interactions, chemical reduction, surface adsorption, and co-precipitation. | [128] |
| Bamboo charcoal | Cu (II) | 25 °C | 5 | 24 h | 4.95 mg/g | - | Quasi-second-order | Langmuir | Electrostatic interaction, and ion exchange. | [87] |
| Potassium permanganate-modified bamboo charcoal | Cu (II) | 25 °C | 5 | 24 h | 9.99 mg/g | - | Quasi-second-order | Langmuir | Electrostatic interaction, and ion exchange. | [87] |
| Sodium hydroxide-modified bamboo charcoal | Cu (II) | 25 °C | 5 | 24 h | 6.24 mg/g | - | Quasi-second-order | Langmuir | Electrostatic interaction, and ion exchange. | [87] |
| Bamboo charcoal | Zn (II) | 25 °C | 7 | 24 h | 2.14 mg/g | - | Quasi-second-order | Langmuir | Electrostatic interaction, and ion exchange. | [87] |
| Potassium permanganate-modified bamboo charcoal | Zn (II) | 25 °C | 7 | 24 h | 5.85 mg/g | 95.1% | Quasi-second-order | Langmuir | Electrostatic interaction, and ion exchange. | [87] |
| Sodium hydroxide-modified bamboo charcoal | Zn (II) | 25 °C | 7 | 24 h | 5.03 mg/g | 92.9% | Quasi-second-order | Langmuir | Electrostatic interaction, and ion exchange. | [87] |
| Bamboo biochar | Cd (II) | 25 °C | 5 | 90 min | 4.53 mg/g | 90.6% | Pseudo-second-order | Langmuir | Chemisorption mechanism, complexion, and intra-particle precipitation. | [144] |
| Bamboo biochar mixed with calcium sulfate | Pb (II) | - | - | 40 min | 152.4 mg/g | 99% | Pseudo-second-order | Langmuir | - | [152] |
| Bamboo biochar | Pb (II) | - | 4 | 120 min | 41.25 mg/g | 80% | Pseudo-second-order | Langmuir | - | [141] |
| Bamboo biochar | Cu (II) | - | 5 | 120 min | 30.21 mg/g | 60% | Pseudo-second-order | Langmuir | - | [141] |
| Bamboo biochar | Zn (II) | - | 5 | 120 min | 34.48 mg/g | 70% | Pseudo-second-order | Langmuir | - | [141] |
| Adsorbent Type | Metal | Eluent | Desorption Efficiency | Regeneration Cycles | Decreased Adsorption Efficiency | References |
|---|---|---|---|---|---|---|
| Chitosan/bamboo charcoal composite beads | Ag (I) | Acetic acid | - | - | - | [138] |
| Copper-impregnated biochar | Re (VII) | KOH | >92% | 4 cycles | Adsorption capacity decreased slightly | [131] |
| Steam-activated bamboo biochar | Cu (II) | - | - | 3 cycles | 80.59% | [66] |
| Fe2(SO4)3 or FeSO4.7H2O modified magnetic biochar | Cr (VI) | Na2EDTA | - | 5 cycles | 44.1% | [63] |
| Magnetic biochar modified with chitosan | Cr (VI) | Na2EDTA | - | 5 cycles | Above 90% | [63] |
| N-doped biochar (ZnCl2 activated) | Cr (VI) | - | - | 5 cycles | 63% | [117] |
| Fe3O4 nanoparticle-covered bamboo biochar | As (V) | Potassium phosphate | 70% | - | - | [121] |
| HNO3 modified bamboo | Cd (II) | Dilute HCl | - | 4 cycles | 86.88% | [142] |
| Amidoxime-modified bamboo charcoal | U (VI) | HNO3 | - | 8 cycles | Decreased by 26.46% | [150] |
| Bamboo-based zeolite-activated carbon | Cu (II) | - | - | 5 cycles | 80.95% | [108] |
| Bamboo biochar modified with chitosan | Cd (II) | - | 65.92% | 5 cycles | 71.70% | [137] |
| Bamboo biochar, enhanced with pre-magnetic properties, cross-linked with a Ca–Mg–Al layered double hydroxide composite | As (III) | - | - | 5 cycles | 75.7% | [122] |
| Bamboo biochar, enhanced with pre-magnetic properties, cross-linked with a Ca–Mg–Al layered double hydroxide composite | Cd (II) | - | - | 5 cycles | 86.3% | [122] |
| EDTA-grafted chitosan-modified magnetic bamboo biochar | Cu (II) | - | - | 8 cycles | Decreased by 16.8% | [70] |
| EDTA-grafted chitosan-modified magnetic bamboo biochar | Zn (II) | - | - | 8 cycles | Decreased by 19.5% | [70] |
| Amino-enhanced bamboo biochar reinforced by nano-zero-valent iron | Cr (VI) | NaBH4 | - | 3 cycles | Remained above 80% | [128] |
| Potassium permanganate-modified bamboo charcoal | Cu (II) | HCl | - | 5 cycles | Decreased by 11.6% | [87] |
| Sodium hydroxide-modified bamboo charcoal | Cu (II) | HCl | - | 5 cycles | Decreased by 14.9% | [87] |
| Potassium permanganate-modified bamboo charcoal | Zn (II) | - | - | 5 cycles | 83.0% | [87] |
| Sodium hydroxide-modified bamboo charcoal | Zn (II) | - | - | 5 cycles | 77% | [87] |
| Bamboo biochar | Cd (II) | HCl (0.05 M) | 91.3% | - | - | [144] |
| Adsorbent | Preparation Method | Surface Area (m2/g) | Optimal pH | Adsorption Capacity (mg/g) | Isotherms/Kinetics | Regeneration | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Modified Fe3O4 nanoparticles with oxidized humic acid | Chemical modification | - | 5.5 | 71.43 | Langmuir isotherm Pseudo-second-order kinetics | 95% (after 4 cycles) | Cation exchange and Complexation | [171] |
| β-cyclodextrin-EDTA-chitosan polymer | EDTA-crosslinked synthesis | 2.396 | 5.14 | 202.90 | Langmuir isotherm pseudo-second-order kinetics | - | Host-guest inclusion | [168] |
| Amine-group functionalized Moso bamboo powder adsorbent with citric acid and tartrate acid | Alkaline mercerization, epoxidation, and amination | 0.463 | 4.0–10.0. | Cd(II)-citric acid:14.41 Cd(II)-tartrate acid, 7.58 | Langmuir model pseudo-second-order kinetic | Regenerated by HCl and reused at least 4 times. | Physisorption and Chemisorption | [165] |
| Carboxyl-modified palm fiber biochar | Pyrolysis | - | 4–6 | 79.9 | Langmuir isotherm Pseudo-second-order | >83% (after 5 cycles) | Physisorption and chemisorption | [167] |
| Porous multimetallic silicate adsorbent | Mineral gene reconstruction method | 582.28 | 2–9 | 168.92 | Langmuir model | >90% (after 5 cycles) | Electrostatic attraction surface complexation partial ion exchange | [169] |
| Three-dimensional macroscopic aminosilylated nanocellulose aerogels | Freeze drying process | 129.32 | 3–7 | 124.5 | Langmuir isotherm model Pseudo-second-order | 80.1% | Chemisorption | [172] |
| Pre-magnetic Fe-modified bamboo biochar crosslinked with CaMgAl-layered double hydroxide | Iron pre-magnetization followed by a hydrothermal method with CaMgAl layered double hydroxide composite | 127.45 | 5 | 320.7 | Freundlich isotherm | - | Co-precipitation and isomorphous replacement | [122] |
| Tannin/chitosan/bamboo pulp aerogel | Freeze-drying method | 137.33 | 6 | 52.52 | Langmuir isotherm pseudo-second-order kinetics | - | Electrostatic interaction and ion-exchange | [170] |
| Magnetic adsorbent developed from kappa-carrageenan, nitrogen-doped carbon dots, and Fe₃O₄ magnetic nanoparticles | Click reaction | 113.33 | 6 | 94.2 | Langmuir isotherm pseudo-second-order kinetics | - | Monolayer adsorption | [173] |
| Chitosan-modified bamboo biochar | Pyrolysis followed by composite preparation through impregnation and sonication | 0.08505 | 7 | 93.46 | Langmuir model | 65.92 (after 5 cycles) | Surface adsorption, electrostatic adsorption, and ion exchanges | [137] |
| Chitosan-/nano-hydroxyapatite composite | Alkaline hydrolysis, chemical precipitation, and solution blending | - | 5 | 126.65 | Pseudo-second-order kinetics | - | Formation of chemical bonds between Cd(II) and adsorbent | [174] |
| Rooibos tea waste adsorbent | Pulverization | 2.5108 | 7 | 7.13 | Langmuir isotherm pseudo-second-order kinetics | - | Chemical interaction between the Cd (II) ions and the adsorbent | [175] |
| Polypyrrole/graphene oxide composite electrode | Electrochemical co-deposition | 1325.4 | 7 | 41.51 | Lagergren-second-order | 94.7% (after 5 cycles) | Chemisorption | [162] |
| MnFe2O4-loaded bamboo pulp carbon-based aerogel | Freeze-drying and carbonization | >100 | 2–6 | 73.63 | Langmuir isotherm model Pseudo-first-order | - | Chemisorption and physisorption | [176] |
| Iron sulfide-based porous biochar | Pyrolysis, chemical activation, and impregnation | 37.6 | 6 | 108.8 | Langmuir isotherm model Pseudo-first-order | - | Precipitation and complexation | [177] |
| Banana pseudo-stem-derived adsorbent (BP) Moringa oleifera stem bark-derived adsorbent(MB) | Physical pre-treatment and size reduction of biomass | - | BP:5 MB:6 | BP:11.98 MB:7.04 | Freundlich isotherm pseudo-second-order | - | Ion-exchange | [178] |
| Cassia fistula seed carbon | Thermal pyrolysis | - | 6 | 68.02 | Langmuir isotherm Pseudo-first-order | - | Chemisorption | [179] |
| Crown ether-grafted bamboo pulp aerogel | microwave irradiation and directional freezing technology | 103.7 | 5 | 27.89 | Freundlich isotherm pseudo-second-order | - | Chemisorption and physisorption | [180] |
| Banana peel-activated carbon modified with Al2O3-chitosan | Activation using a solution of 1 M H2SO4 | 140.4 | 6 | 46.9 | Langmuir isotherm pseudo-second order | >80% (after 3 cycles) | Electrostatic attraction between adsorbent and Cd2+ | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negi, M.; Thankachan, V.; Rajeev, A.; Vairamuthu, M.; Arundhathi, S.; Nidheesh, P.V. Clean and Green Bamboo Magic: Recent Advances in Heavy Metal Removal from Water by Bamboo Adsorbents. Water 2025, 17, 454. https://doi.org/10.3390/w17030454
Negi M, Thankachan V, Rajeev A, Vairamuthu M, Arundhathi S, Nidheesh PV. Clean and Green Bamboo Magic: Recent Advances in Heavy Metal Removal from Water by Bamboo Adsorbents. Water. 2025; 17(3):454. https://doi.org/10.3390/w17030454
Chicago/Turabian StyleNegi, Monika, Vinju Thankachan, Arya Rajeev, M. Vairamuthu, S. Arundhathi, and P. V. Nidheesh. 2025. "Clean and Green Bamboo Magic: Recent Advances in Heavy Metal Removal from Water by Bamboo Adsorbents" Water 17, no. 3: 454. https://doi.org/10.3390/w17030454
APA StyleNegi, M., Thankachan, V., Rajeev, A., Vairamuthu, M., Arundhathi, S., & Nidheesh, P. V. (2025). Clean and Green Bamboo Magic: Recent Advances in Heavy Metal Removal from Water by Bamboo Adsorbents. Water, 17(3), 454. https://doi.org/10.3390/w17030454








