Abstract
The co-contamination of nitrate nitrogen (NO3−-N) and tetracycline (TC) in aquaculture water has caused serious environmental and health problems. Bioremediation is a promising approach for the removal of NO3−-N and TC. However, free bacteria are sensitive to environmental variation, limiting its application. In this study, a bacterial strain with high NO3−-N and TC degradation ability, Bacillus cereus W2, was isolated and immobilized on wheat straw biochar by an adsorption method. The effect of immobilization conditions, including biochar dosage, inoculum amount, and immobilization time on NO3−-N and TC removal was explored. The degradation abilities of the biochar-immobilized Bacillus cereus W2 under different nitrate and TC concentrations was investigated. Results showed that the prepared biochar had abundant functional groups such as -COOH, -OH, -C=C-OH, etc., which have good affinity for microbial cell membranes and are conducive to the adhesion and proliferation of microbial cells. The highest NO3−-N and TC removal efficiencies of 99.50% and 78.60% after 24 h were obtained under a biochar dosage of 4 mg·mL−1, microbe inoculation amount of 40%, and immobilization time of 24 h. The immobilized Bacillus cereus W2 performed better NO3−-N and TC removal than the free cells under different initial NO3−-N and TC concentrations. The enhanced removal of NO3−-N by the biochar-immobilized Bacillus cereus W2 may be attributed to the promoted expression level of functional genes involved in denitrification (nirS, norB, and nosZ). The biochar-immobilized Bacillus cereus W2 demonstrates potential for treating various nitrate-antibiotic co-contaminated wastewaters, including those from livestock farming, aquaculture systems, and pharmaceutical industries.
1. Introduction
Tetracycline (TC) is one of the most widely used antibiotics in the world due to its low cost and high efficacy [1,2]. In aquaculture, it is usually used to prevent or treat infectious diseases, promote growth, and reduce nutrient consumption [3,4]. About 75% of TC used in aquaculture is released into the aquatic environment in active form because of incomplete absorption or metabolism [5]. These residues inhibit the growth and development of aquatic species, threaten the aquatic ecosystems, and impact human health through the food chain [6,7]. Meanwhile, the high-density, intensive, and large-scale aquaculture model leads to high input [8]. Most of the input is freed into water as feed waste and excreta. For example, large amounts of nitrogen (72–88%) is discharged into aquatic ecosystems [9], and nitrate nitrogen (NO3−-N) pollution is becoming increasingly severe, leading to acute environmental and health problems. NO3−-N and substantial quantities of unmetabolized TC are frequently detected in effluent discharges of aquaculture. The co-contamination of NO3−-N and TC is a tricky problem needing to be solved.
The removal of NO3−-N and TC by microorganisms is a cost-effective, efficient and environmentally friendly method [10,11,12]. The simultaneous removal of NO3−-N and TC was achieved by microorganisms according to previous studies [13,14]. However, in practical applications, biodegradation by microorganisms may have several problems, such as low bacterial survival rates, intense competition with indigenous microorganisms, and reduced degradation activity. Microbial immobilization technology (MIT) confined free microorganisms in a specific spatial area while maintaining their activity by chemical, physical, and other methods. It has the advantages of high cell density, good stability, excellent reusability, and resistance to toxic compounds [15,16]. Therefore, MIT is a promising biological technology for wastewater treatment [17]. The immobilization carriers played a vital role in MIT. The carriers serve as substrates for the bacteria growth and reproduction, affecting the bacterial stability, contaminant removal efficiencies, and costs of MIT [18]. The widely used synthetic polymer carriers (polyvinyl alcohol, polyurethane, polyethylene, polyacrylamide, et al.) often face problems such as complex preparation, high cost, and poor mass transfer performance [19]. Thus, there is a need to investigate more support materials with high performance.
Biochar has attracted increasing attention due to its physical and chemical properties and cost effectiveness [20]. The high porosity, good mass transfer performance, and abundant functional groups of biochar is suitable for the habitation of microorganisms [21], making it an ideal carrier for microbial immobilization. It also has the advantages of being easily available, low-cost, eco-friendly, and easy to use [19]. Biochar-based microbial immobilization has gained significant attention for its application in removing diverse contaminants from water systems, including nitrogen [22], heavy metal [23], Phenol [24], polycyclic aromatic hydrocarbon [25], and antibiotics [26]. Previous studies have reported that biochar can provide electron donors to promote nitrate reduction and increase the abundance of denitrifying bacteria to promote denitrification [27,28]. Yu et al. [29] reported a remarkable 11.43-fold increase of biochar-immobilized consortia in TC removal compared with free consortia. Most studies mainly focused on the single pollution of NO3−-N or antibiotics. Moreover, the toxic effects of TC on denitrification microorganisms were reported [30]. Thus, the simultaneous removal of NO3−-N and TC using biochar-immobilized bacteria remains limited and requires further in-depth investigation. There is a need to isolate and identify the microbial strains capable of simultaneously removing NO3−-N and TC. And the immobilization of these functional microorganisms with biochar needs further investigation.
In this study, a denitrifier with TC degradation ability was isolated from the laboratory-immobilized microbial reactor. The wheat straw biochar-immobilized microbial agent was prepared by an adsorption method for the simultaneous removal of NO3−-N and TC. The objectives of the study are as follows: (1) determine the effect of preparation conditions including biochar dosage, inoculum amount, and immobilization time on nitrate and TC removal; (2) investigate the performance of biochar-immobilized bacteria under varying pollution concentrations; and (3) quantify the functional genes correlated with the degradation process by real-time quantitative polymerase chain reaction (qPCR) to reveal the mechanisms underlying this process. This study provides a better understanding about the preparation and application of biochar-immobilized functional microbial strains for simultaneous removal of NO3−-N and TC, which will facilitate the treatment of wastewaters from livestock farming, aquaculture systems, and pharmaceutical industries.
2. Materials and Methods
2.1. Isolation and Identification of the Bacterium
The strain was isolated from a laboratory-immobilized microbial reactor. The biofilm was enriched and cultured in Luria-Bertani (LB) medium (10 g·L−1 tryptone, 5 g·L−1 yeast extract, 10 g·L−1 sodium chloride) for 12 h at 30 °C. Then, 1 mL of bacterial solution was transferred to a nitrate medium with a TC concentration of 5 m g·L−1, incubated at 30 °C. Nitrate medium was composed of NaNO3 (0.607 g·L−1), CH3COONa (0.641 g·L−1), Na2HPO4 (1.21 g·L−1), KH2PO4 (0.743 g·L−1), CaCl2 (0.04 g·L−1), MgSO4·7H2O (0.01 g·L−1), and trace element solution (1 mg·L−1). The composition of the trace element solution is listed in Table S1. After several consecutive transfers, 1 mL of bacterial solution was diluted and spread onto LB solid medium via gradient dilution. Finally, one strain named W2 was obtained.
The strain was sequenced and identified by 16S rDNA, and the extracted DNA was amplified by polymerase chain reaction (PCR) using primers 27F (AGAGTTGATCMTGCTCAG) and 1492R (GGTTACCTTGTTACGACTT). The purified DNA was sent to Nanjing Jisihuiyuan Biotechnology Co., Ltd (Nanjing, China). for sequencing. The sequencing results were aligned using Nucleotide BLAST. A 16S rDNA phylogenetic tree was constructed using the adjacency method by MEGA software (Mega Limited, Auckland, New Zealand, https://www.megasoftware.net).
2.2. Preparation and Characterization of Biochar
The wheat straw was cut into fragments of about 10 cm in length after air drying and then carbonized in a carbonization furnace. The furnace was heated at a rate of 8.5 °C per minute until the maximum target temperature of 300 °C was reached, and it remained for 10 h. The prepared biochar (BC) was sieved through a 100-mesh sieve, then dried and stored for further use. The surface morphology characteristics of biochar were observed by scanning electron microscopy (SEM, SU-8100, Hitachi, Japan); the pore characteristics and specific surface area of biochar were determined using a specific surface and porosity analyzer (Kubo-X1000, Builder, Beijing, China) and an N2 adsorption–desorption isotherm test by Brunauer–Emmett–Teller (BET). The functional groups of biochar were determined using Fourier transform infrared spectroscopy (FTIR, TENSOR 27, Bruker, Bremen, Germany).
2.3. Immobilization of W2 on Biochar
The biochar-immobilized bacteria W2 (IW2) were prepared using an adsorption method. The strain was cultivated at 30 °C and 120 rpm using LB liquid medium for 12 h, and then 40 mL of bacterial solution (OD600 = 1) was centrifuged (6000 rpm, 5 min, 4 °C) to obtain the cell pellets. The cell pellets and 0.4 mg of biochar were added into a 250 mL conical flask containing 100 mL of phosphate-buffered saline (PBS). The biochar particles and cell pellets were cultured together at 30 °C and 120 rpm for 24 h. The biochar-based immobilized bacteria were obtained by centrifugation and discarding the supernatant. After immobilization, the biochar-immobilized bacteria were characterized by SEM and FTIR.
2.4. Effect of Immobilization Conditions
Three immobilization factors were investigated using a single-factor experiment: the biochar dosage (mass of biochar per unit volume of nitrate medium: 0, 2, 4, 8, and 12 mg·mL−1), bacterial inoculation amount (5%, 10%, 20%, 40%, and 60%), and immobilization time (6 h, 12 h, 24 h, and 36 h). The basic immobilization conditions were as described above. The prepared biochar-based immobilized bacteria were added into 200 mL of nitrate medium with a TC concentration of 5 mg·L−1 to investigate their degradation properties. All treatments were prepared in triplicate. Water samples were collected after different culture times to measure NO3−-N and TC concentrations. The samples were filtered through 0.45 μm film before tested.
2.5. Simultaneous Removal of NO3−-N and TC Under Different Concentrations
The degradation ability of the biochar-immobilized bacteria under different initial nitrate and TC concentrations were investigated. Four initial NO3−-N and TC concentrations (nitrate 50 mg·L−1 with TC 2.5 mg·L−1, nitrate 100 mg·L−1 with TC 5 mg·L−1, nitrate 200 mg·L−1 with TC 10 mg·L−1, and nitrate 400 mg·L−1 with TC 20 mg·L−1) were set; 40 mL of bacterial solution (OD600 = 1) was centrifuged and inoculated into 100 mL of nitrate medium to make a free cell culture. The biochar-immobilized particles prepared under the optimal conditions were washed twice with sterile distilled water to remove free cells and then added into the flask with 100 mL of nitrate medium. Meanwhile, the sterilized biochar as control experiment was conducted under the same conditions. All of the flasks were cultured at 30 °C, and water samples were collected after 12 h to measure NO3−-N and TC concentrations. The samples were filtered through 0.45 μm film before testing. The following formula was used to calculate the degradation rate:
where D (%) is the degradation rate, is the initial concentration of the pollutants, and is the concentration of pollutants at time t.
2.6. Quantification of Gene Expressions
To reveal the molecular mechanism of the NO3−-N removal process, the bacteria cells were harvested from the free and immobilized cell bath experiments by centrifugation after 24 h of incubation. The harvested cells were washed 3 times using PBS solution. The total RNA was isolated by employing a total RNA extraction kit (Solarbio, Beijing, China) following the manufacturer’s standard protocol. The expression levels of functional genes relating to denitrification (narG, nirS, norB, and nosZ) in strain Bacillus cereus W2 were measured by reverse transcription and real-time quantitative polymerase chain reaction (RT-qPCR). The primers studied are listed in Table S2.
2.7. Analysis Methods
The detection method for NO3−-N was carried out in accordance with Chinese Standard HJ/T 346-2007 [31]. TC was determined using high-performance liquid chromatography (Agilent 1260, Agilent, Sanda Clara, CA, USA). The water samples were filtered through 0.45 μm film before testing. A C18 column (5 μm, 4.6 mm × 150 mm; ZORBAX Eclipse XDB) was used with a column temperature of 30 °C. The mobile phase was acetonitrile and 0.01 M oxalic acid solutions (30%:70%) at a flow rate of mL·min−1. The detection wavelength was 360 nm, and 20 μL of samples was injected. Each sample was tested in triplicate to ensure reproducibility.
3. Results and Discussion
3.1. Biochar-Immobilized Bacteria W2
After screening and domestication, a strain named W2 with the ability to degrade NO3−-N and TC was obtained. As shown in Figure 1a, the strain was rod-shaped with an average size of approximately (1.5–2.5) µm × (0.3–0.5) µm. A 16S rDNA sequence analysis of strain W2 showed a similarity of 99.72% to the strain Bacillus cereus based on a BLAST search. The neighbor-joining tree method was used to analyze the phylogenetic position of strain W2 using MEGA software (Mega Limited, Auckland, New Zealand). Figure 1b shows the phylogenetic tree of strain W2. The results indicated that strain W2 was closely related to a number of Bacillus strains.
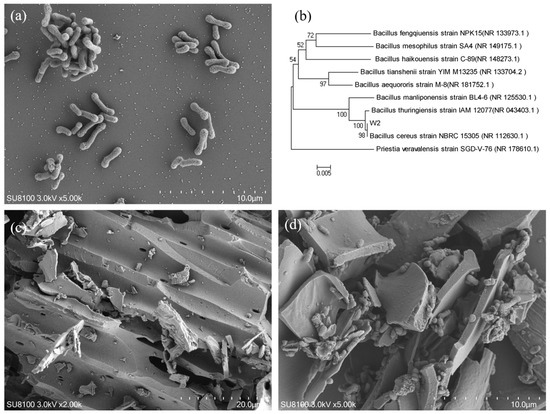
Figure 1.
SEM image (×5000 times) (a) and phylogenetic tree (b) of strain W2, SEM image of BC (×2000 times), (c) and IW2 (×5000 times) (d).
The surface structure of wheat straw biochar prepared at 300 °C is shown in Figure 1c. It can be seen that its carbonization degree is low, the surface is smooth, and the structure is relatively complete. The BET results showed that the average pore size was 10.44 nm, and the specific surface area was 10.66 m2·g−1. According to a previous study, a larger pore size facilitates the transfer of nutrients between microorganisms and the environment, which is beneficial for the growth and reproduction of microorganisms [32]. The pore size of the prepared wheat straw biochar was mesoporous, which was conducive to the entry and attachment of microorganisms. The biochar provided sufficient habitats for microbes, which was beneficial for improving the resistance to toxic compounds and enhancing the viability. As shown in Figure 1d, a large number of bacteria were adsorbed on the surface and interior pores of the biochar and strongly adsorbed after immobilization. Previous studies demonstrate that microbial immobilization on biochar primarily occurs through physical adsorption and electrostatic interactions [33].
The FTIR spectra of strain W2, biochar (BC), and biochar-immobilized W2 (IW2) are shown in Figure 2. The absorption peaks at 3427 cm−1 of W2, BC, and IW2 were a characteristic peak generated by the stretching vibration of -OH [34]. The peaks at 2960 cm−1 of W2 and IW2 were attributed to the stretching vibrations of –CH [35]. The absorption peaks at 2360 cm−1 were the characteristic peak of C = C in aromatic rings in W2, BC, and IW2. The absorption peaks at 1640 cm−1 and 1400 cm−1 were mainly generated by the stretching vibrations of C=O [36] in W2, BC, and IW2. The peaks around 1530 cm−1 were assigned to the bending vibration of –CN/-NH bond [37] in W2 and IW2. The peaks at 1000–1250 cm−1 were attributed to the stretching vibration of C–O in W2, BC, and IW2 [38]. Previous research has shown that functional groups such as COOH, -OH, and -C=C-OH in biochar have good affinity for microbial cell membranes [39], which is beneficial for the adhesion and proliferation of microbial cells. The IW2 contained the functional groups of W2 and BC, which means that the bacteria were successfully immobilized on the biochar.
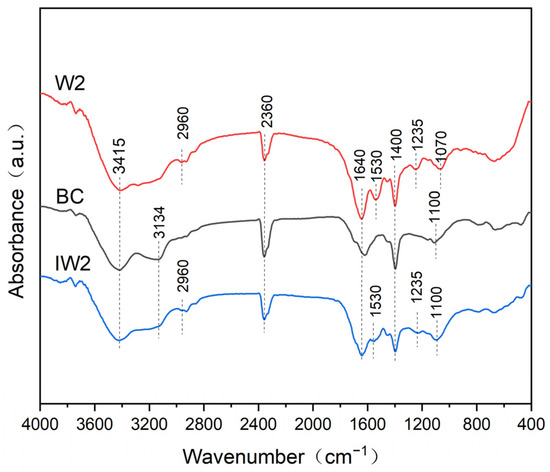
Figure 2.
FTIR spectra of W2, BC, and IW2.
3.2. Effect of Immobilization Conditions
3.2.1. Effect of Biochar Dosage
As shown in Figure 3a, after 12 h of degradation, the NO3−-N removal efficiency increased from 71.96% to 98.16% when the biochar dosage increased from 0 to 4 mg·mL−1 and then gradually decreased from 98.16% to 89.55% when the biochar dosage increased from 4 to 12 mg·mL−1. After 24 h, the NO3−-N was almost 100% removed by the immobilized Bacillus cereus W2 (groups with a biochar dosage of 2, 4, 8, and 12 mg·mL−1), while the removal efficiency by free bacteria (groups with a biochar dosage of 0 mg·mL−1) was 93.50%. The degradation of TC under different dosage conditions showed similar trends (Figure 3b). After 24 h, as the dosage increased from 0 to 4 mg·mL−1, the TC removal efficiency increased from 69.03% to 78.34% and then decreased to 75.10% when the biochar dosage increased to 12 mg·mL−1. The addition of biochar enhanced the NO3−-N and TC degradation ability of bacteria. On one hand, biochar provides a suitable living environment for bacteria, increasing their abundance and activity, which is beneficial for bacteria to resist pollutant loads [40]. On the other hand, the surface of biochar has abundant active functional groups that provide reaction sites for electron transfer, which can improve the system’s electron transfer ability and facilitate the degradation of NO3−-N and TC [32]. The research by Yu et al. [29] revealed that biochar promoted TC degradation by improving the microbial growth and catalase activities of immobilized microbial communities. The biomass adsorbed by biochar per unit mass increased with the biochar under relatively low dosage. However, collisions may occur between biochar particles, causing the microorganisms originally adsorbed on the surface of biochar to detach when too much biochar was added and resulting in the decrease of the biomass adsorbed by biochar per unit mass.
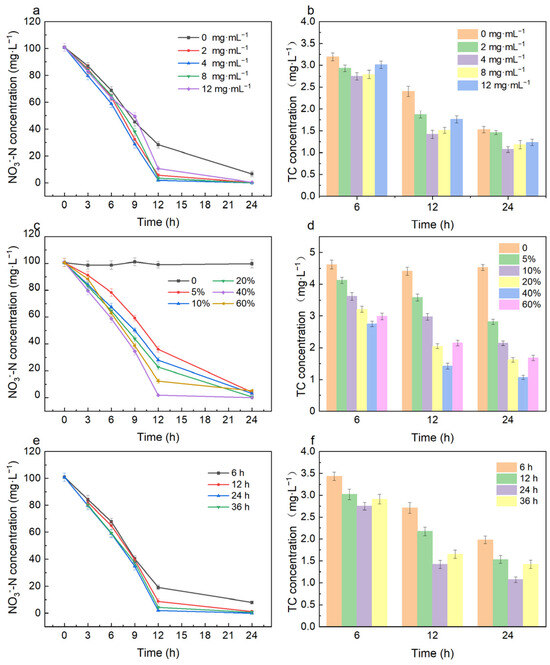
Figure 3.
Effect of preparation condition on the degradation properties: effect of biochar dosage on the degradation of NO3−-N (a) and TC (b); effect of bacterial dosage on the degradation of NO3−-N (c) and TC (d); effect of immobilization time on the degradation of NO3−-N (e) and TC (f).
3.2.2. Effect of Inoculum Amount
The degradation of NO3−-N and TC by the immobilized Bacillus cereus W2 prepared under different inoculum amounts is shown in Figure 3c,d. It can be seen that the removal of NO3−-N and TC was mainly due to microbial degradation rather than adsorption by biochar (less than 10%). The NO3−-N and TC removal efficiency increased with increasing inoculum amounts (0 to 40%) and then slightly decreased with the increase in inoculum amounts (40 to 60%). The maximum NO3−-N and TC removal efficiency was obtained when the inoculum amount was 40%, which was 99.87% and 78.34%, respectively. The toxic effects of pollutants on microorganisms lead to a decrease in microbial activity. The increased inoculum amount enhanced the activity of microbes. However, with the increase in immobilized microorganisms, the competition for limited nutrients leads to bacterial decay, and the aggregation among microorganisms limits the increase in effective surface area and binding sites [41].
3.2.3. Effect of Immobilization Time
The degradation of NO3−-N and TC by the immobilized Bacillus cereus W2 prepared under different immobilization times is shown in Figure 3e,f. In Figure 3e, after 12 h of degradation, the removal rate of NO3−-N under different immobilization times is shown in the following order: 24 h (98.15%) > 36 h (5.69%) > 12 h (91.34%) > 6 h (81.01%). The NO3−-N and TC removal efficiency showed similar trends. They increased with the increasing immobilization time (6 to 24 h) and gradually decreased with the increased immobilization time (24 to 36 h). The maximum NO3−-N and TC removal efficiency was obtained at the immobilization time of 24 h after 24 h of degradation, which was 99.87% and 78.34%, respectively. The adsorption of microorganisms by biochar was a dynamic process. The microorganisms attached to biochar were unstable and prone to detachment when the immobilization time was short. With the extension of immobilization time, microorganisms obtain sufficient nutrients and reproduce in large quantities, and the number of microorganisms absorbed on biochar reaches its maximum value. However, the bacteria may enter the decay stage, resulting in a small number of immobilized active bacteria, when the immobilization time was long [42].
3.3. Simultaneous Removal of Nitrate and TC Under Different Concentrations
Figure 4 shows the NO3−-N and TC removal efficiency under different initial NO3−-N and TC concentrations. As shown in Figure 4a, the adsorption of NO3−-N by the biochar only accounts for less than 10% under different initial concentrations. The NO3−-N removal efficiency decreased from 92.30% to 11.58% for free bacteria (W2) and from 99.07% to 34.97% for biochar-immobilized bacteria (IW2) with the NO3−-N concentration increased from 50 to 400 mg·L−1 after 24 h. Zhang et al. [32] pointed out that the longer lag phases of bacteria and toxic effect caused by high nitrate concentration resulted in the reduced nitrate removal rate. The nitrate removal efficiency was enhanced by the immobilized bacterial cells, especially in the high concentrations. As shown in Figure 4b, the TC removal efficiency decreased from 71.60% to 38.78% for free bacteria (W2) with the TC concentration increased from 2.5 to 20 mg·L−1 after 12 h of incubation. The initial TC concentration showed little effect on the TC removal efficiency of biochar-immobilized bacteria (IW2) within the tested concentration ranges. The immobilized bacteria showed significantly higher TC removal efficiencies than free ones, which was related to the adsorption and protection of bacteria by BC [43]. Previous studies also reported the simultaneous removal of NO3−-N and TC by immobilized microorganisms (Table 1). It can be seen that loofah-based systems demonstrate comparatively lower removal efficiencies (91.97% for NO3−-N and 57.39% for TC) [44], and polyurethane foam (PUF) achieves a comparable performance to the current study (99.18% NO3−-N and 77.30% TC removal) [45]. However, PUF’s potential environmental risks necessitate alternative solutions. The developed biochar-based system addresses these limitations by combining enhanced removal efficiency (99.50% for NO3−-N and 78.60% for TC) with environmental sustainability, offering a technically superior and ecologically sound approach for simultaneous NO3−-N and TC removal.
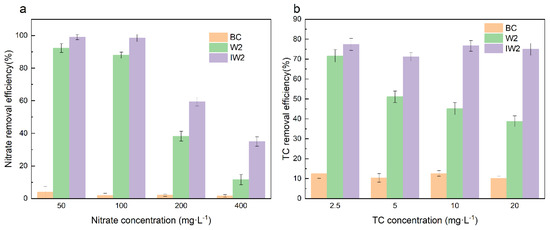
Figure 4.
The NO3−-N (a) and TC (b) removal efficiency under different initial NO3−-N and TC concentrations.

Table 1.
Comparison of the relative studies.
3.4. The Expression of Functional Genes
The expression level of denitrification function genes was shown in Figure 5. As shown in the figure, the expression levels of narG showed no significant differences between W2 and IW2. The expression levels of nirS, norB, and nosZ of the immobilized W2 cells were up-regulated by 1.83 times, 3.47 times, and 3.72 times of the free ones, respectively. The up-regulated denitrification functional genes led to an improved denitrification performance. It was reported that the up-regulation of gene expression by biochar may be attributed to the following reasons: (1) biochar’s adsorption capacity enhances nitrate accumulation near immobilized bacterial cells, facilitating substrate transport and inducing denitrification gene upregulation [32]. (2) The release of soluble organic compounds and essential nutrients from biochar potentially contains bioactive components that promote the denitrification gene expression [43].
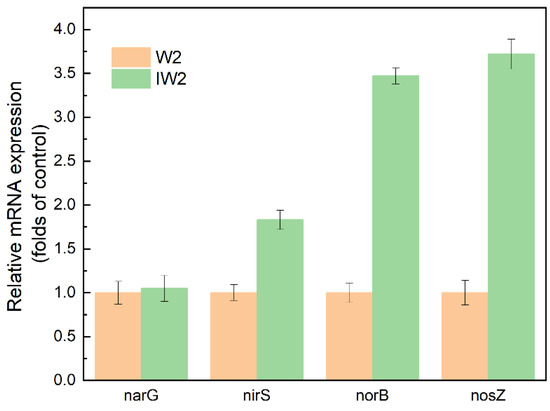
Figure 5.
The expression level of denitrification function genes.
4. Conclusions
In this study, wheat straw biochar, which had abundant functional groups such as -COOH, -OH, and -C=C-OH was prepared at 300 °C. A bacterial strain with high NO3−-N and TC degradation ability, Bacillus cereus W2, was isolated and immobilized on wheat straw biochar by an adsorption method. The highest NO3−-N and TC removal efficiencies of 99.50% and 78.60% after 24 h were obtained under a biochar dosage of 4 mg·mL−1, microbe inoculation amount of 40%, and immobilization time of 24 h. The immobilized Bacillus cereus W2 performed higher NO3−-N and TC removal efficiency than the free bacterial cells under different initial NO3−-N and TC conditions. The enhanced removal of NO3−-N by the biochar-immobilized Bacillus cereus W2 may be attributed to the promoted expression level of functional genes involved in denitrification (nirS, norB, nosZ). These findings demonstrate the potential of biochar-immobilized Bacillus cereus W2 for addressing nitrate-antibiotic co-contamination in diverse wastewater streams, including livestock, aquaculture, and pharmaceutical effluents. Further investigations should focus on three critical aspects: the reusability of the immobilized bacteria, the fate of TC in the immobilized system, and performance evaluation through pilot-scale or full-scale implementation in real-world wastewater treatment scenarios.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17030380/s1, Table S1: Composition of trace element solution; Table S2: The primers’ sequence.
Author Contributions
J.X.: Conceptualization, Methodology, Validation, Resource, Formal Analysis, Investigation, Data Curation, Writing—Original Draft, and Writing—Review and Editing; X.L. contributed equally with J.X.; L.W.: Methodology, Validation, Data Curation, and Project Administration; X.Z.: Methodology, Data Curation, Supervision, and Project Administration; W.X.: Conceptualization, Writing—Review and Editing, Supervision, and Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Open Fund of Xinjiang Biomass Solid Waste Resources Technology and Engineering Center] grant number [KSUGCZX202304], [Youth Fund of Nanjing Institute of Technology] grant number [QKJ202203], and [Industry-University-Research Collaboration Project of Jiangsu Province] grant number [BY20230601].
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Xinyue Lu was employed by the company CNOOC Gas & Power Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Antos, J.; Piosik, M.; Ginter-Kramarczyk, D.; Zembrzuska, J.; Kruszelnicka, I. Tetracyclines contamination in European aquatic environments: A comprehensive review of occurrence, fate, and removal techniques. Chemosphere 2024, 353, 141519. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhi, D.; Zhou, H.; He, X.; Zhang, D. Evaluating tetracycline degradation pathway and intermediate toxicity during the electrochemical oxidation over a Ti/Ti4O7 anode. Water Res. 2018, 137, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The Impact of Tetracycline Pollution on the Aquatic Environment and Removal Strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. Growth promotion and gut microbiota: Insights from antibiotic use. Environ. Microbiol. 2015, 7, 2216–2227. [Google Scholar] [CrossRef]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Effects of carbon source, nitrogen source, and natural algal powder-derived carbon source on biodegradation of tetracycline (TEC). Bioresour. Technol. 2019, 288, 121567. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2021, 753, 141975. [Google Scholar] [CrossRef]
- Ohore, O.E.; Zhang, S.; Guo, S.; Manirakiza, B.; Addo, F.G.; Zhang, W. The fate of tetracycline in vegetated mesocosmic wetlands and its impact on the water quality and epiphytic microbes. J. Hazard. Mater. 2021, 417, 126148. [Google Scholar] [CrossRef]
- Wang, J.; Beusen, A.; Liu, X.; Bouwman, A.F. Aquaculture Production is a Large, Spatially Concentrated Source of Nutrients in Chinese Freshwater and Coastal Seas. Environ. Sci. Technol. 2020, 54, 1464–1474. [Google Scholar] [CrossRef]
- Zhang, Y.; Bleeker, A.; Liu, J. Nutrient discharge from China’s aquaculture industry and associated environmental impacts. Environ. Res. Lett. 2015, 10, 45002. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, X.; Feng, F.; Xu, X. Biodegradation of tetracycline by the yeast strain Trichosporon mycotoxinivorans XPY-10. Prep. Biochem. Biotech. 2016, 46, 15–22. [Google Scholar] [CrossRef]
- Shao, S.; Hu, Y.; Cheng, J.; Chen, Y. Biodegradation mechanism of tetracycline (TEC) by strain Klebsiella sp. SQY5 as revealed through products analysis and genomics. Ecotoxicol. Environ. Saf. 2019, 185, 109676. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Dai, X.; Chai, X. Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes. Sci. Total Environ. 2018, 634, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ali, A.; Su, J.; Xu, L.; Wang, X.; Liang, E. Simultaneous removal of nitrate, tetracycline, and Pb(II) by iron oxidizing strain Zoogloea sp. FY6: Performance and mechanism. Bioresour. Technol. 2022, 360, 127569. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Ali, A.; Su, J.; Wen, Q.; Bai, Y.; Gao, Z. Simultaneous removal of nitrate, manganese, and tetracycline by Zoogloea sp. MFQ7: Adsorption mechanism of tetracycline by biological precipitation. Bioresour. Technol. 2021, 340, 125690. [Google Scholar] [CrossRef]
- Wu, C.; Zhi, D.; Yao, B.; Zhou, Y.; Yang, Y.; Zhou, Y. Immobilization of microbes on biochar for water and soil remediation: A review. Environ. Res. 2022, 212, 113226. [Google Scholar] [CrossRef]
- Lou, L.; Huang, Q.; Lou, Y.; Lu, J.; Hu, B.; Lin, Q. Adsorption and degradation in the removal of nonylphenol from water by cells immobilized on biochar. Chemosphere 2019, 228, 676–684. [Google Scholar] [CrossRef]
- Wang, C.; Ren, J.; Qiao, X.; Habib, M. Ammonium removal efficiency of biochar-based heterotrophic nitrifying bacteria immobilization body in water solution. Environ. Eng. Res. 2021, 26, 190451. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z.; Zhang, G.; Qin, L.; Fang, J. Application progress of microbial immobilization technology based on biomass materials. BioResources 2021, 16, 8509–8524. [Google Scholar] [CrossRef]
- Hou, L.; Hu, K.; Huang, F.; Pan, Z.; Jia, X.; Liu, W.; Yao, X.; Yang, Z.; Tang, P.; Li, J. Advances in immobilized microbial technology and its application to wastewater treatment: A review. Bioresour. Technol. 2024, 413, 131518. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, Y.; Hou, Y.; Arp, H.P.H.; Reid, B.J.; Cai, C. Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar. Chemosphere 2017, 182, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fu, G.; Pang, W.; Tang, J.; Guo, Z.; Hu, Z. Biochar immobilized bacteria enhances nitrogen removal capability of tidal flow constructed wetlands. Sci. Total Environ. 2022, 836, 155728. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Ke, Y.; Xue, B.; Zhang, Z.; Zhu, X. Immobilized sulfate reducing bacteria (SRB) enhanced passivation performance of biochar for Zn. Sci. Total Environ. 2023, 892, 164556. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xiao, J.; Li, M.; Wu, J.; Zhang, T. Degradation of Phenol by Immobilized Alcaligenes faecalis Strain JH1 in Fe3O4-Modified Biochar from Pharmaceutical Residues. Water 2023, 15, 4084. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Zhu, M.; Yu, Y.; Lu, G.; Dang, Z. Co-metabolic and biochar-promoted biodegradation of mixed PAHs by highly efficient microbial consortium QY1. J. Environ. Sci. 2021, 107, 65–76. [Google Scholar] [CrossRef]
- Xia, M.; Niu, Q.; Qu, X.; Zhang, C.; Qu, X.; Li, H.; Yang, C. Simultaneous adsorption and biodegradation of oxytetracycline in wastewater by Mycolicibacterium sp. immobilized on magnetic biochar. Environ. Pollut. 2023, 339, 122728. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, F.; Yang, C.; Su, X.; Guo, F.; Xu, Q.; Peng, G.; He, Q.; Chen, Y. Highly efficient nitrate removal in a heterotrophic denitrification system amended with redox-active biochar: A molecular and electrochemical mechanism. Bioresour. Technol. 2019, 275, 297–306. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Z.; Zhang, Z.; Zhang, R. Redox-active reactions in denitrification provided by biochars pyrolyzed at different temperatures. Sci. Total Environ. 2018, 615, 1547–1556. [Google Scholar] [CrossRef]
- Yu, X.; Bai, M.; Li, X.; Yang, P.; Wang, Q.; Wang, Z.; Weng, L.; Ye, H. Tetracycline removal by immobilized indigenous bacterial consortium using biochar and biomass: Removal performance and mechanisms. Bioresour. Technol. 2024, 413, 131463. [Google Scholar] [CrossRef]
- Shu, Y.; Liang, D. Effect of tetracycline on nitrogen removal in Moving Bed Biofilm Reactor (MBBR) System. PLoS ONE 2022, 17, e0261306. [Google Scholar] [CrossRef]
- HJ/T 346-2007; Water Quality—Determination of Nitrate-Nitrogen—Ultraviolet Spectrophotometry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2007.
- Zhang, W.; Shen, J.; Zhang, H.; Zheng, C.; Wei, R.; Gao, Y.; Yang, L. Efficient nitrate removal by Pseudomonas mendocina GL6 immobilized on biochar. Bioresour. Technol. 2021, 320, 124324. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Deng, J.; Liang, J.; Jiang, L.; Arslan, M.; Gamal El-Din, M.; Wang, X.; Chen, C. Biochar immobilized petroleum degrading consortium for enhanced granulation and treatment of synthetic oil refinery wastewater. Bioresour. Technol. Rep. 2022, 17, 100909. [Google Scholar] [CrossRef]
- Shi, J.; Han, H.; Xu, C. A novel enhanced anaerobic biodegradation method using biochar and Fe(OH)3@biochar for the removal of nitrogen heterocyclic compounds from coal gasification wastewater. Sci. Total Environ. 2019, 697, 134052. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Cheng, J.; Huang, H.; Xiong, S.; Gao, J.; Zhang, J.; Feng, S. Optimization of cadmium biosorption by Shewanella putrefaciens using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2019, 175, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiao, J.; Zeng, Z.; Zhang, T.; Ren, Y. Study on the biodegradation of phenol by Alcaligenes faecalis JH1 immobilized in rice husk biochar. Front. Environ. Sci. 2023, 11, 1294791. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Dong, Y.; Li, B.; Liu, C.; Yin, T. Mechanisms underlying enhanced bioremediation of sulfamethoxazole and zinc(II) by Bacillus sp. SDB4 immobilized on biochar. J. Clean. Prod. 2022, 370, 133483. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, J.; Deng, Y.; Tian, Y.; Zhang, G.; Liao, J.; Yang, J.; Yang, Y.; Liu, N.; Sun, Q. Uranium(Ⅵ) adsorption from aqueous solutions by microorganism-graphene oxide composites via an immobilization approach. J. Clean. Prod. 2019, 236, 117624. [Google Scholar] [CrossRef]
- Song, J.; Li, M.; Wang, C.; Fan, Y.; Li, Y.; Wang, Y.; Zhang, W.; Li, H.; Wang, H. Enhanced treatment of landfill leachate by biochar-based aerobic denitrifying bacteria functional microbial materials: Preparation and performance. Front. Microbiol. 2023, 14, 1139650. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Zhang, J.; Li, C.; Wu, H. Dynamic variation in nitrogen removal of constructed wetlands modified by biochar for treating secondary livestock effluent under varying oxygen supplying conditions. J. Environ. Manag. 2020, 260, 110152. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Wang, S.; Leng, S. Effective removal of chlortetracycline and treatment of simulated sewage by Bacillus cereus LZ01 immobilized on erding medicine residues biochar. Biomass Convers. Biorefin. 2024, 14, 2281–2291. [Google Scholar] [CrossRef]
- Liu, F.; Liu, H.; Zhu, H.; Xie, Y.; Zhang, D.; Cheng, Y.; Zhang, J.; Feng, R.; Yang, S. Remediation of petroleum hydrocarbon-contaminated groundwater by biochar-based immobilized bacteria. Biochem. Eng. J. 2023, 197, 108987. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Wu, X.; Long, Y.; An, H.; Pan, X.; Li, M.; Dong, F.; Zheng, Y. Rapid degradation of dimethomorph in polluted water and soil by Bacillus cereus WL08 immobilized on bamboo charcoal–sodium alginate. J. Hazard. Mater. 2020, 398, 122806. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into Multiple and Multilevel Structures of Biochars and Their Potential Environmental Applications: A Critical Review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Su, J.; Ali, A.; Wen, Q.; Chang, Q.; Gao, Z.; Wang, Y. Efficient removal of nitrate, manganese, and tetracycline in a novel loofah immobilized bioreactor: Performance, microbial diversity, and functional genes. Bioresour. Technol. 2022, 344, 126228. [Google Scholar] [CrossRef]
- Xu, W.; Luo, M.; Lu, X.; Ye, Z.; Jeong, T. Simultaneous Removal of Nitrate and Tetracycline by an Up-Flow Immobilized Biofilter. Water 2022, 14, 2595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).