Pre-Treatment and Characterization of Water Hyacinth Biomass (WHB) for Enhanced Xylose Production Using Dilute Alkali Treatment Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Equipment
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Pre-Treatment of WHB
2.2.3. Effect of Process Parameters on Xylose Production
2.2.4. Spectrophotometric Determination of Xylose
2.2.5. Surface Morphology Observation
2.2.6. XRD and Crystallinity Index Measurement
2.2.7. FTIR Analysis
3. Results and Discussion
3.1. Spectrophotometric Analysis of Hydrolysate
3.2. Effect of Process Parameters on Xylose Production
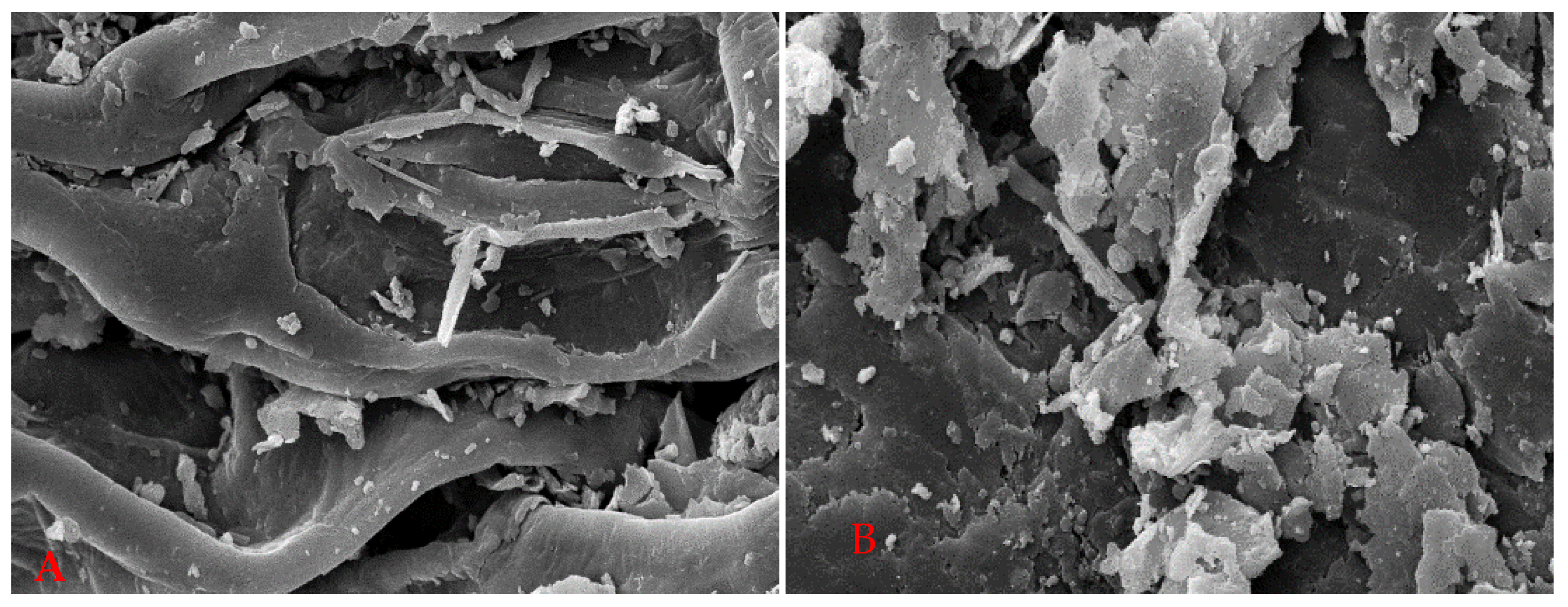
3.3. FE-SEM Analysis
3.4. XRD Analysis
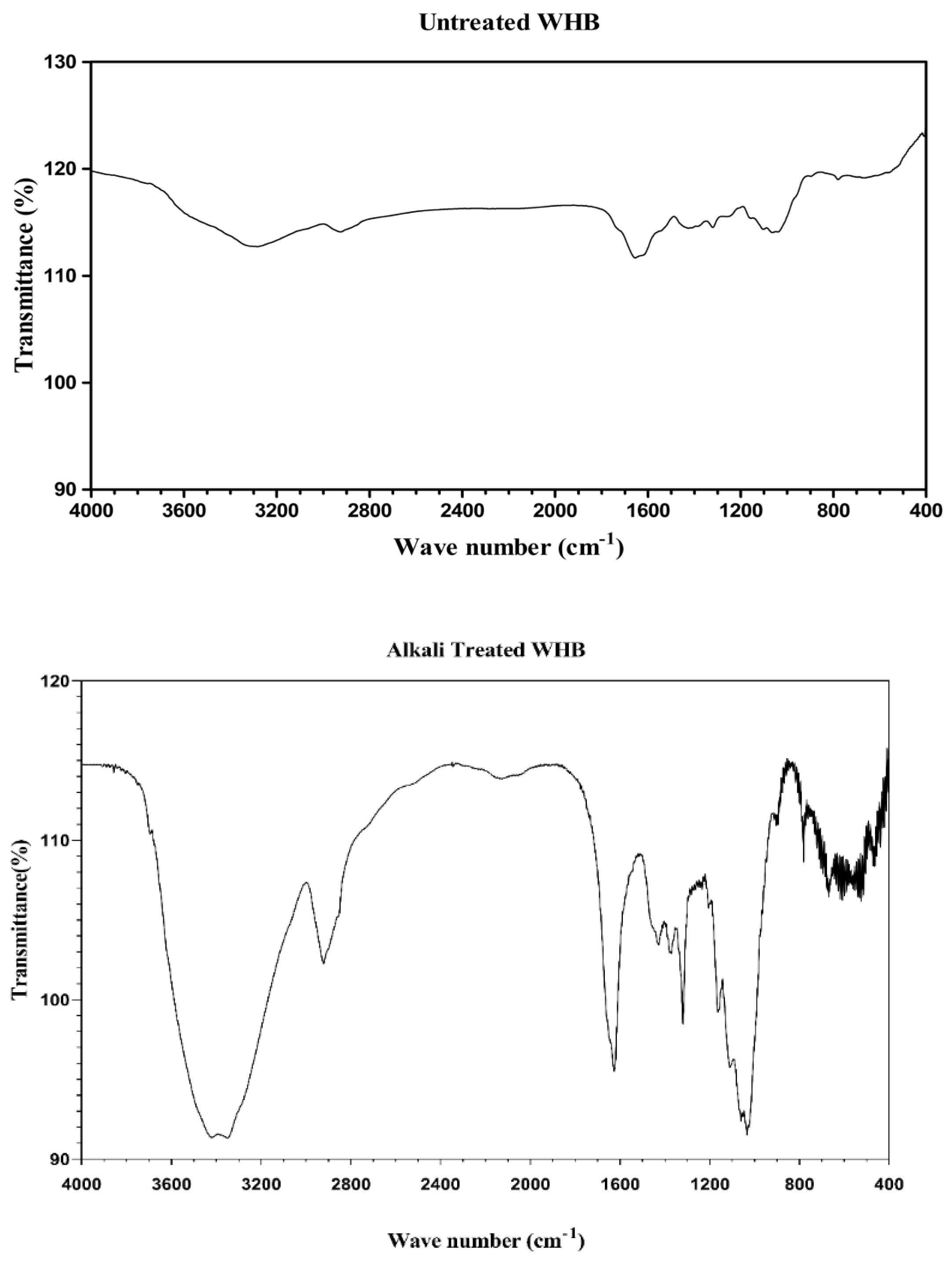
3.5. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carneiro, M.T.; Barros, A.Z.B.; Morais, A.I.S.; Melo, A.L.F.C.; Bezerra, R.D.S.; Osajima, J.A.; Silva-Filho, E.C. Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study. Polymers 2022, 14, 2732. [Google Scholar] [CrossRef] [PubMed]
- Ilo, O.P.; Simatele, M.D.; Nkomo, S.L.; Mkhize, N.M.; Prabhu, N.G. The Benefits of Water Hyacinth (Eichhornia crassipes) for Southern Africa: A Review. Sustainability 2020, 12, 9222. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, X.; Chen, X.; Tian, Y.; Zhou, Y.; Lu, X.; Huang, T. Conversion of Water Hyacinth to Value-Added Fuel via Hydrothermal Carbonization. Energy 2020, 197, 117193. [Google Scholar] [CrossRef]
- Singh, A.; Bishnoi, N.R. Comparative Study of Various Pretreatment Techniques for Ethanol Production from Water Hyacinth. Ind. Crops Prod. 2013, 44, 283–289. [Google Scholar] [CrossRef]
- Jin, X.; Chen, X.; Shi, C.; Li, M.; Guan, Y.; Yu, C.Y.; Yamada, T.; Sacks, E.J.; Peng, J. Determination of Hemicellulose, Cellulose and Lignin Content Using Visible and near Infrared Spectroscopy in Miscanthus Sinensis. Bioresour. Technol. 2017, 241, 603–609. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, Y.; Han, H.; Weng, C. Enhancing Bioethanol Production from Water Hyacinth by New Combined Pretreatment Methods. Bioresour. Technol. 2018, 251, 358–363. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, F.; Xue, S. The Economic Feasibility of the Valorization of Water Hyacinth for Bioethanol Production. Sustainability 2019, 11, 905. [Google Scholar] [CrossRef]
- Arcaño, Y.D.; García, O.D.V.; Mandelli, D.; Carvalho, W.A.; Pontes, L.A.M. Xylitol: A Review on the Progress and Challenges of Its Production by Chemical Route. Catal. Today 2020, 344, 2–14. [Google Scholar] [CrossRef]
- Dey, A.; Das, S.; Das, K.; Ghosh, P.; Ganguly, A.; Chatterjee, P.K.; Chatterjee, K. Studies on the Optimization of Phenolics during Production of Xylitol from Water Hyacinth. Eur. J. Biotechnol. Biosci. 2015, 25, 25–33. [Google Scholar]
- Dalli, S.S.; Patel, M.; Rakshit, S.K. Development and Evaluation of Poplar Hemicellulose Prehydrolysate Upstream Processes for the Enhanced Fermentative Production of Xylitol. Biomass Bioenergy 2017, 105, 402–410. [Google Scholar] [CrossRef]
- Chinwatpaiboon, P.; Savarajara, A.; Luengnaruemitchai, A. Enzymatic Hydrolysate of Water Hyacinth with NaOH Pretreatment for Biobutanol Production via ABE Fermentation by Clostridium Beijerinckii JCM 8026. Biomass Bioenergy 2023, 173, 106782. [Google Scholar] [CrossRef]
- Packiam, K.K.; Murugesan, B.; Sundaramoorthy, P.M.K.; Srinivasan, H.; Dhanasekaran, K. Extraction, Purification and Characterization of Nanocrystalline Cellulose from Eichhornia crassipes (Mart.) Solms: A Common Aquatic Weed Water Hyacinth. J. Nat. Fibers 2022, 19, 7424–7435. [Google Scholar] [CrossRef]
- Singh, J.K.; Chaurasia, B.; Dubey, A.; Noguera, A.M.F.; Gupta, A.; Kothari, R.; Upadhyaya, C.P.; Kumar, A.; Hashem, A.; Alqarawi, A.A.; et al. Biological Characterization and Instrumental Analytical Comparison of Two Biorefining Pretreatments for Water Hyacinth (Eichhornia crassipes) Biomass Hydrolysis. Sustainability 2020, 13, 245. [Google Scholar] [CrossRef]
- Rezania, S.; Alizadeh, H.; Cho, J.; Darajeh, N.; Park, J.; Hashemi, B.; Din, M.F.M.; Krishnan, S.; Yadav, K.K.; Gupta, N.; et al. Changes in Composition and Structure of Water Hyacinth Based on Various Pretreatment Methods. Bioresources 2019, 14, 6088–6099. [Google Scholar] [CrossRef]
- Bronzato, G.R.F.; Ziegler, S.M.; Silva, R.C.; Cesarino, I.; Leão, A.L. Characterization of the Pre-Treated Biomass of Eichhornia crassipes (Water Hyacinth) for the Second Generation Ethanol Production. Mol. Cryst. Liq. Cryst. 2017, 655, 224–235. [Google Scholar] [CrossRef]
- S, L.M.; Baas-López, J.M.; Barbosa, R.; Pacheco, D.; Escobar, B. Activated Carbon from Water Hyacinth as Electrocatalyst for Oxygen Reduction Reaction in an Alkaline Fuel Cell. Int. J. Hydrog. Energy 2021, 46, 25995–26004. [Google Scholar] [CrossRef]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal Crystallinity Index Revisited by the Simulation of X-Ray Diffraction Patterns of Cotton Cellulose Iβ and Cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Tanpichai, S.; Biswas, S.K.; Witayakran, S.; Yano, H. Water Hyacinth: A Sustainable Lignin-Poor Cellulose Source for the Production of Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 18884–18893. [Google Scholar] [CrossRef]
- Pham, P.J.; Hernandez, R.; French, W.T.; Estill, B.G.; Mondala, A.H. A Spectrophotometric Method for Quantitative Determination of Xylose in Fermentation Medium. Biomass Bioenergy 2011, 35, 2814–2821. [Google Scholar] [CrossRef]
- Dey, A.; Ting, Y.-P. Characterization of Water Hyacinth Biomass and Microbial Degradation of the Biomass under Solid State Fermentation Using a Lignocellulolytic Fungus (Alterneria Spp NITDS1). J. Chem. Biol. Phys. Sci. 2014, 4, 2279–2293. [Google Scholar]
- Asrofi, M.; Abral, H.; Kasim, A.; Pratoto, A. XRD and FTIR Studies of Nanocrystalline Cellulose from Water Hyacinth (Eichornia crassipes) Fiber. J. Metastable Nanocrystalline Mater. 2017, 29, 9–16. [Google Scholar] [CrossRef]
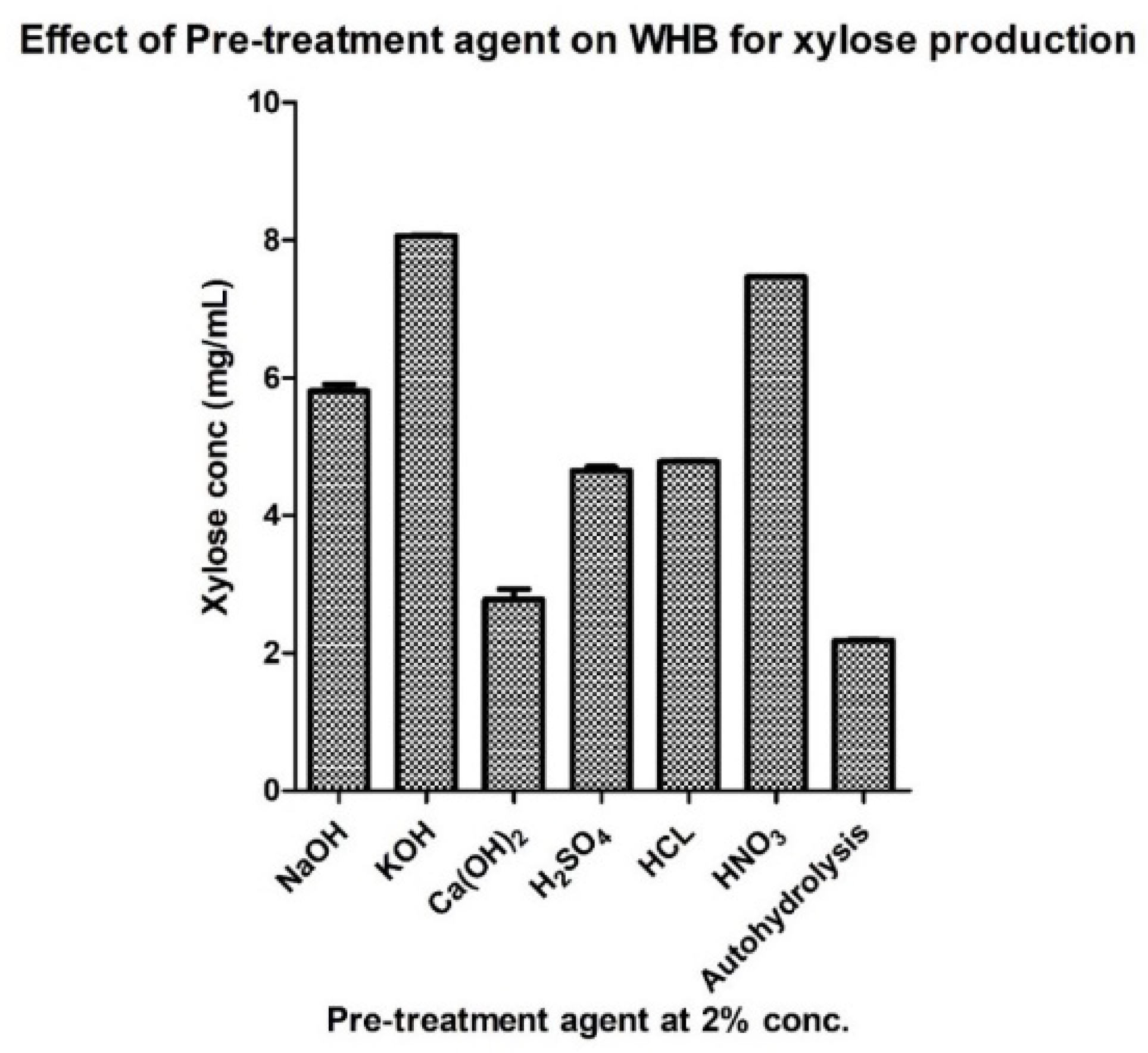




| Sr. No | Pre-Treatment Agent (2%) | Xylose Conc. (mg/mL) |
|---|---|---|
| 1 | NaOH | 5.8029 |
| 2 | KOH | 8.0587 |
| 3 | Ca(OH)2 | 2.7739 |
| 4 | H2SO4 | 4.6469 |
| 5 | HCl | 4.7827 |
| 6 | HNO3 | 7.4625 |
| 7 | Autohydrolysis | 2.1785 |
| Sr. No | KOH Conc. (%) | Xylose Conc. (mg/mL) |
|---|---|---|
| 1 | 1 | 2.0329 |
| 2 | 2 | 4.0641 |
| 3 | 3 | 4.4130 |
| 4 | 4 | 3.3116 |
| 5 | 5 | 3.3715 |
| 6 | 6 | 3.7323 |
| 7 | 7 | 3.7221 |
| 8 | 8 | 3.6636 |
| 9 | 9 | 3.5469 |
| 10 | 10 | 3.4883 |
| Sr. No | Pre-Treatment Time (min.) | Xylose Conc. (mg/mL) |
|---|---|---|
| 1 | 10 | 3.0943 |
| 2 | 20 | 3.1833 |
| 3 | 30 | 2.8463 |
| 4 | 40 | 2.7646 |
| 5 | 60 | 2.2213 |
| Sr. No | WHB Conc. (%) | Xylose Conc. (mg/mL) |
|---|---|---|
| 1 | 1 | 5.6307 |
| 2 | 2 | 6.4549 |
| 3 | 3 | 5.4647 |
| 4 | 4 | 4.4746 |
| 5 | 5 | 3.9528 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, R.H.; Dey, A. Pre-Treatment and Characterization of Water Hyacinth Biomass (WHB) for Enhanced Xylose Production Using Dilute Alkali Treatment Method. Water 2025, 17, 301. https://doi.org/10.3390/w17030301
Jadhav RH, Dey A. Pre-Treatment and Characterization of Water Hyacinth Biomass (WHB) for Enhanced Xylose Production Using Dilute Alkali Treatment Method. Water. 2025; 17(3):301. https://doi.org/10.3390/w17030301
Chicago/Turabian StyleJadhav, Rohan Harsh, and Apurba Dey. 2025. "Pre-Treatment and Characterization of Water Hyacinth Biomass (WHB) for Enhanced Xylose Production Using Dilute Alkali Treatment Method" Water 17, no. 3: 301. https://doi.org/10.3390/w17030301
APA StyleJadhav, R. H., & Dey, A. (2025). Pre-Treatment and Characterization of Water Hyacinth Biomass (WHB) for Enhanced Xylose Production Using Dilute Alkali Treatment Method. Water, 17(3), 301. https://doi.org/10.3390/w17030301








