Opoka as a Natural Material for Phosphorus Removal: Properties and Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of an Adsorbent
2.3. Determination of the Phosphorus Concentration
3. Results
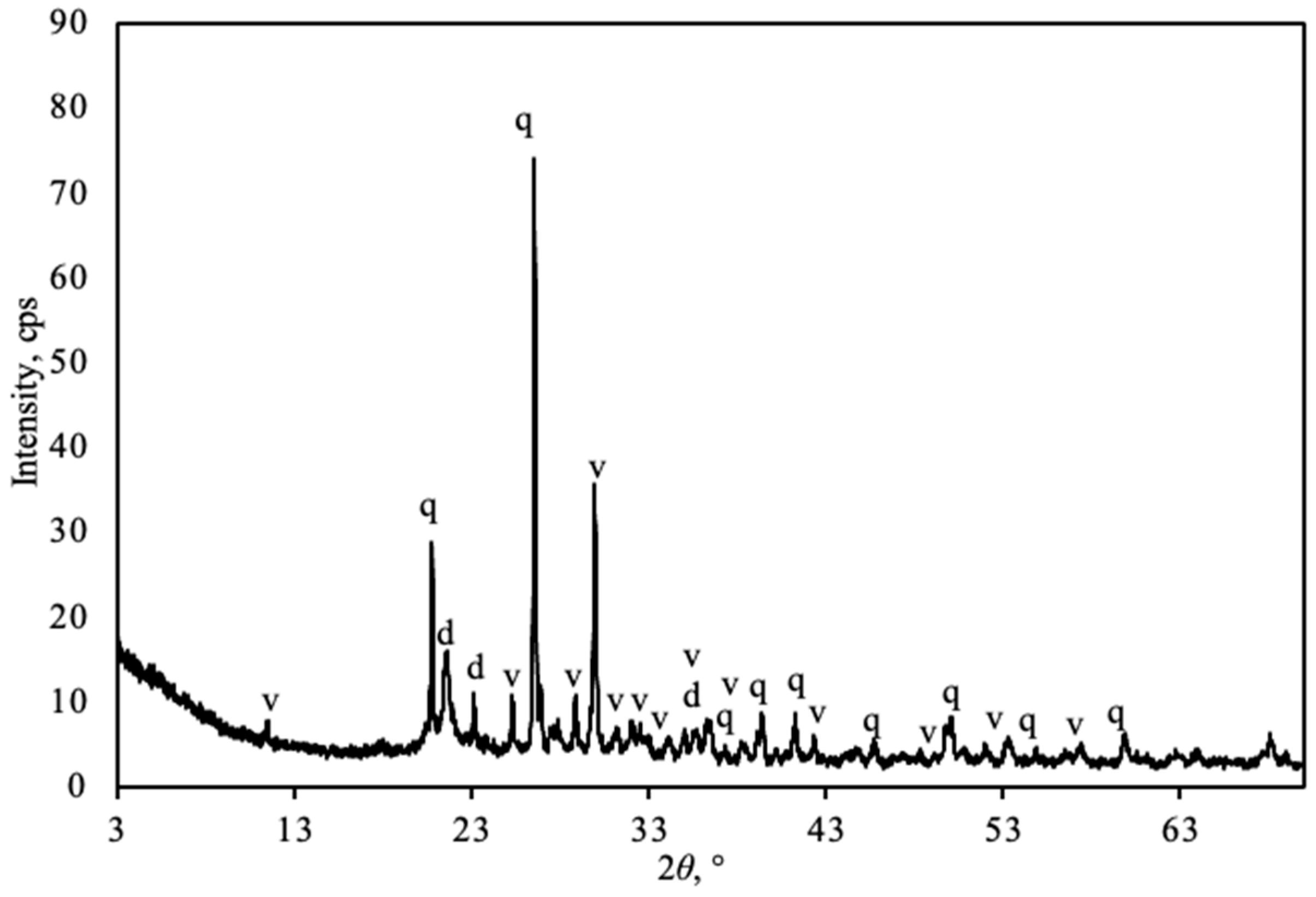
3.1. Characterization of Natural Opoka
3.2. Physical Properties
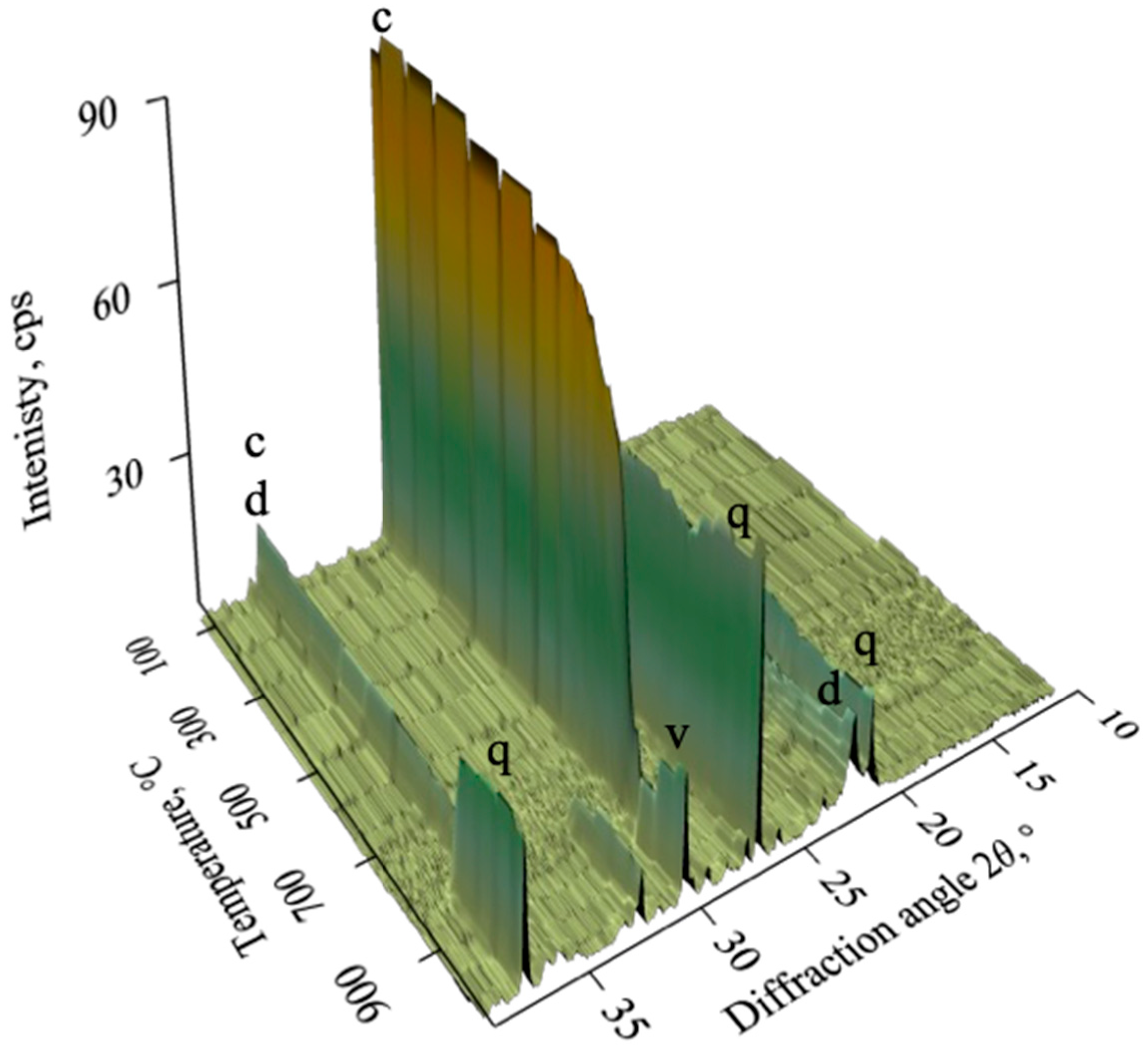
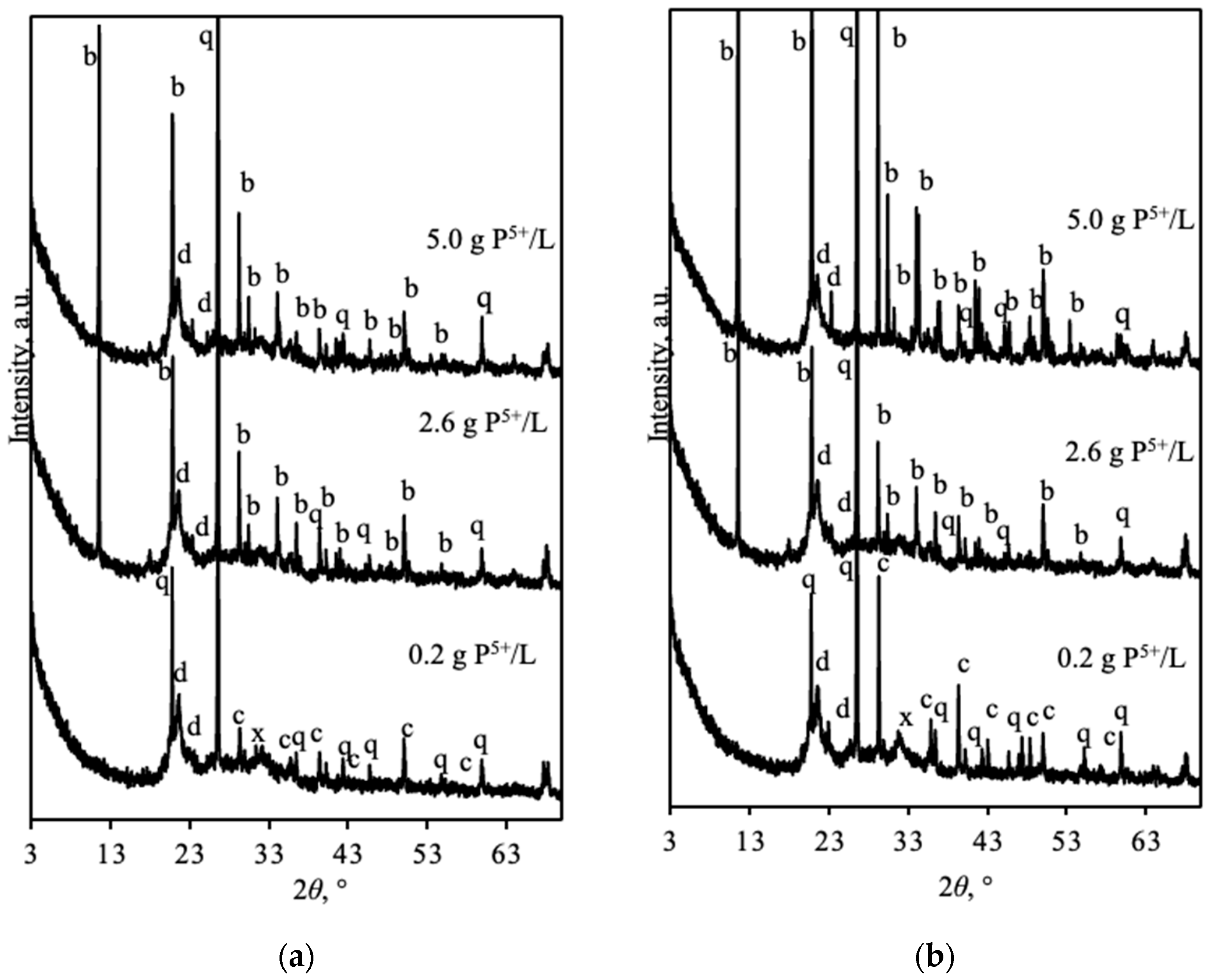
3.3. Calcination of Natural Opoka and Formation of Reactive Phases
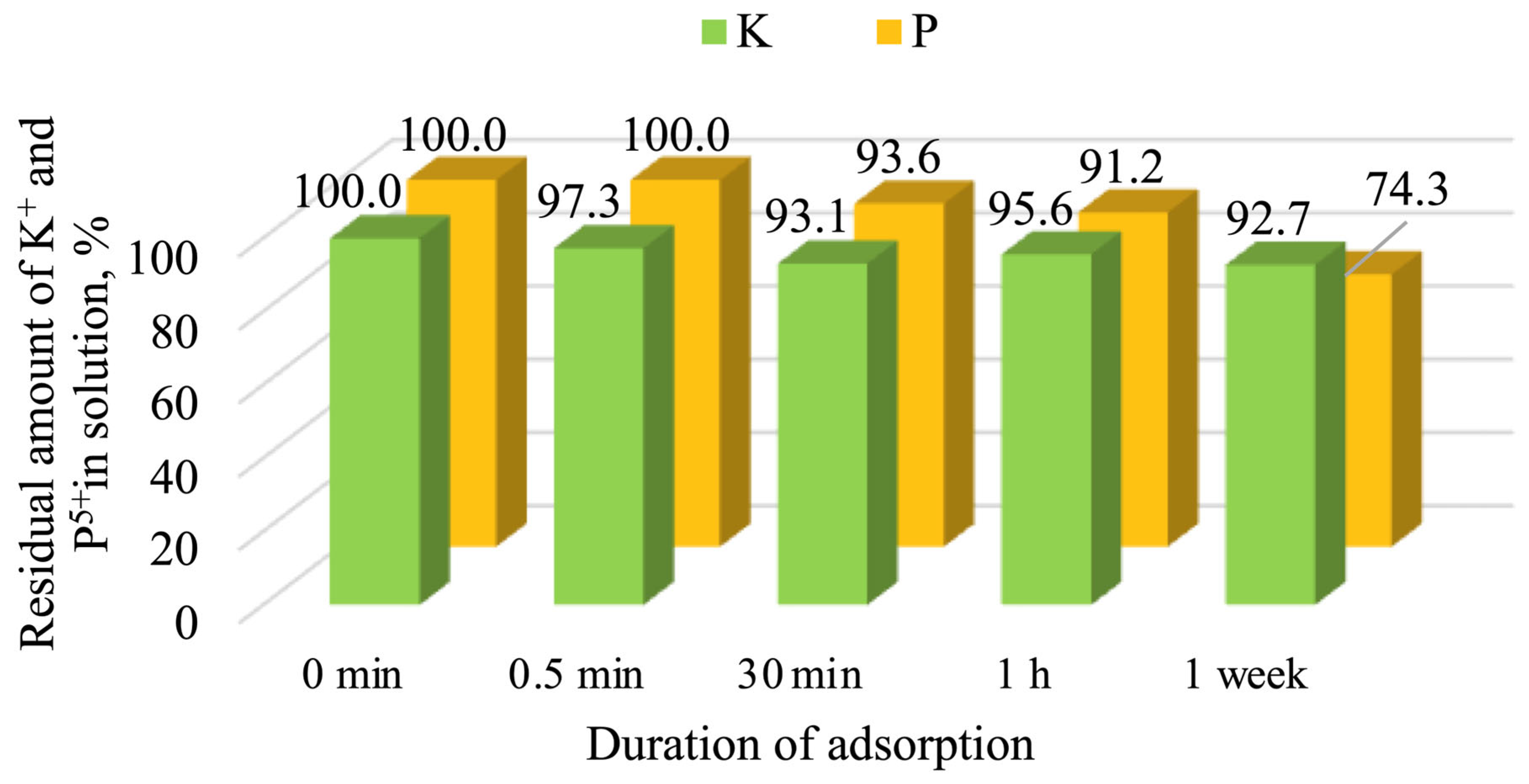
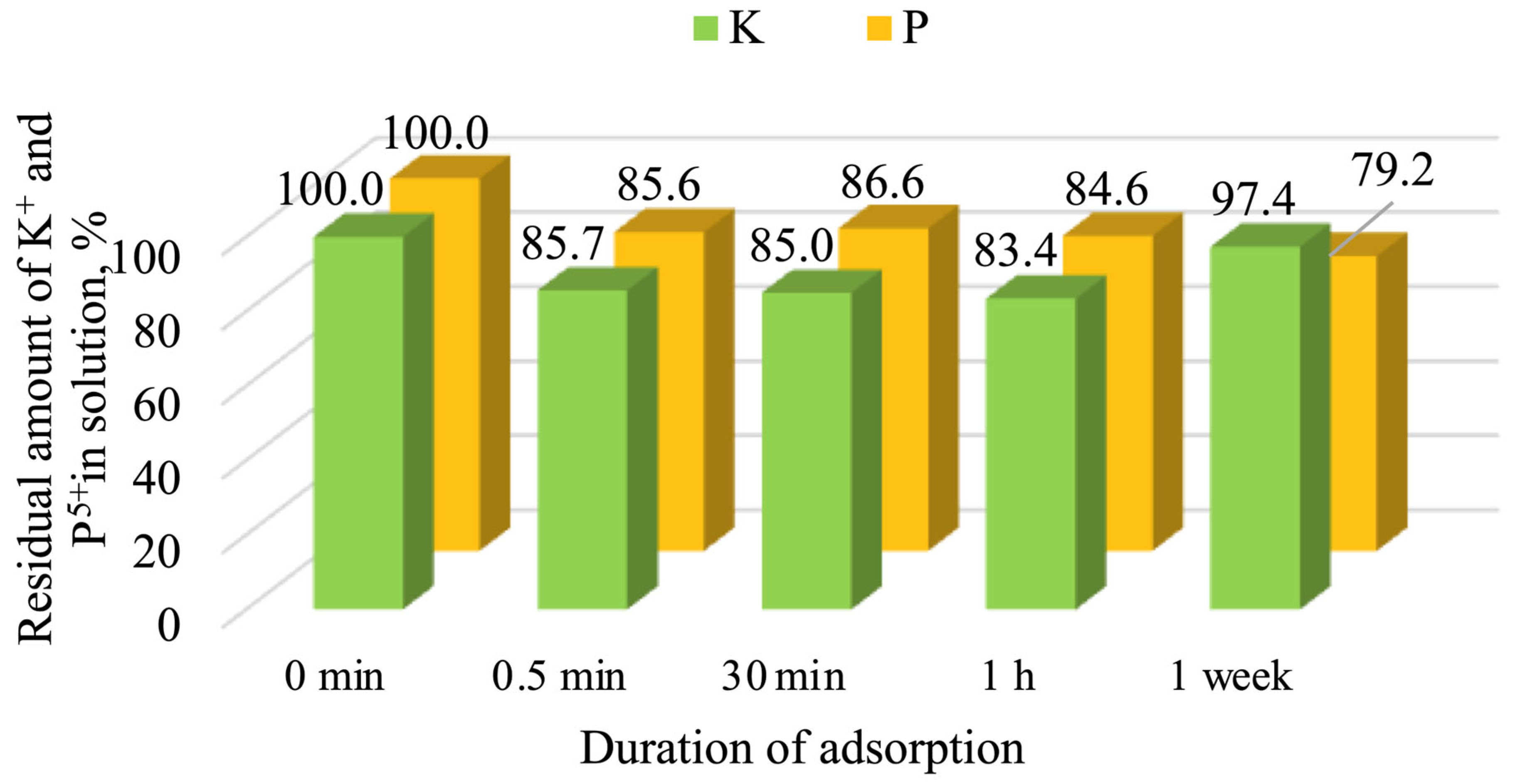
3.4. Adsorption Properties of Calcinated Opoka
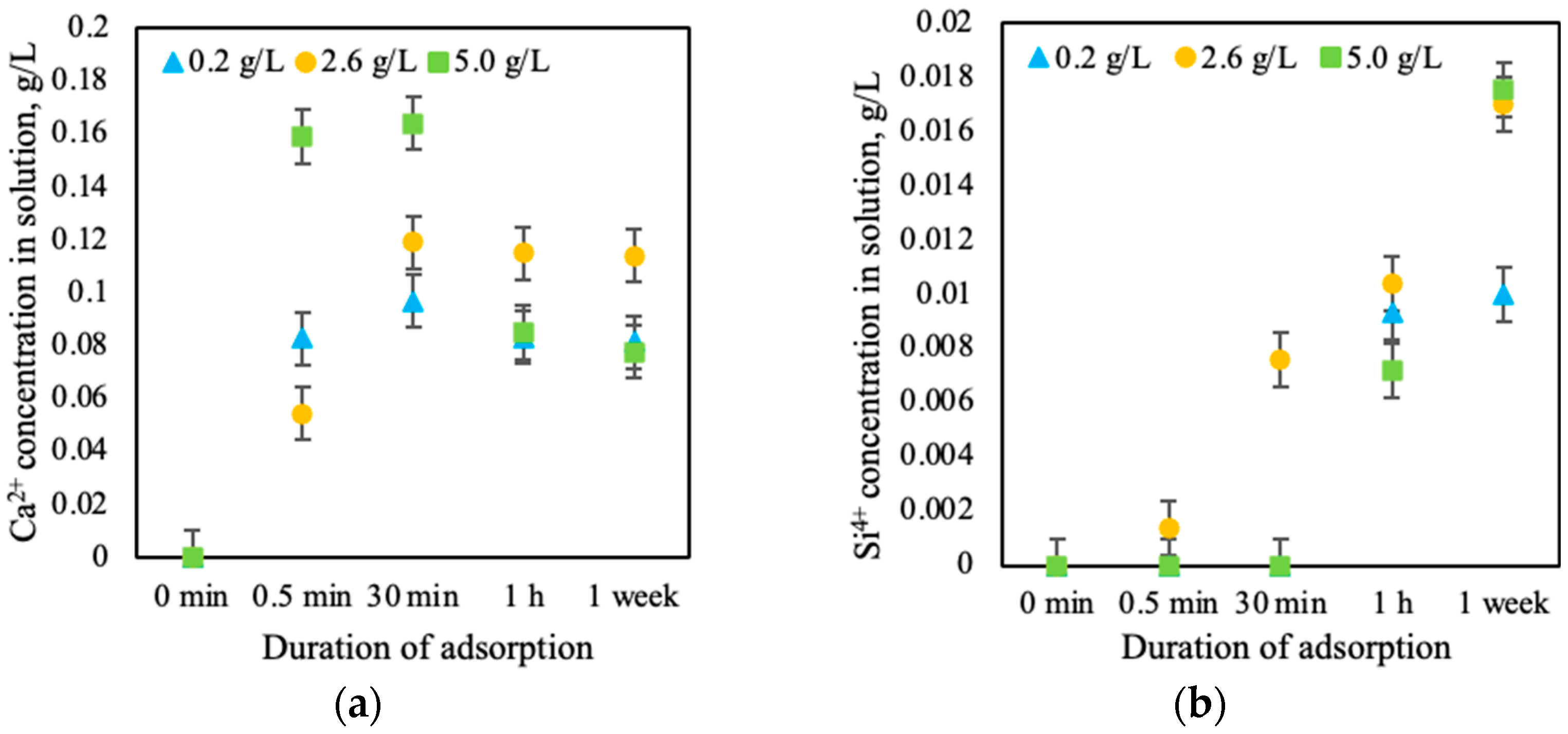
3.5. Adsorption Mechanism
3.6. Adsorption Kinetics
4. Conclusions
- 1.
- Natural opoka (SiO2—50.5%, CaO—23.3%, molar ratio CaO/SiO2 ~0.45), predominantly consisting of quartz, tridymite, and calcite minerals, is a suitable raw material for the synthesis of reactive calcium silicates. During thermal treatment at a temperature of 850 °C, calcite decomposed completely, and active CaO reacted with SiO2 by forming wollastonite.
- 2.
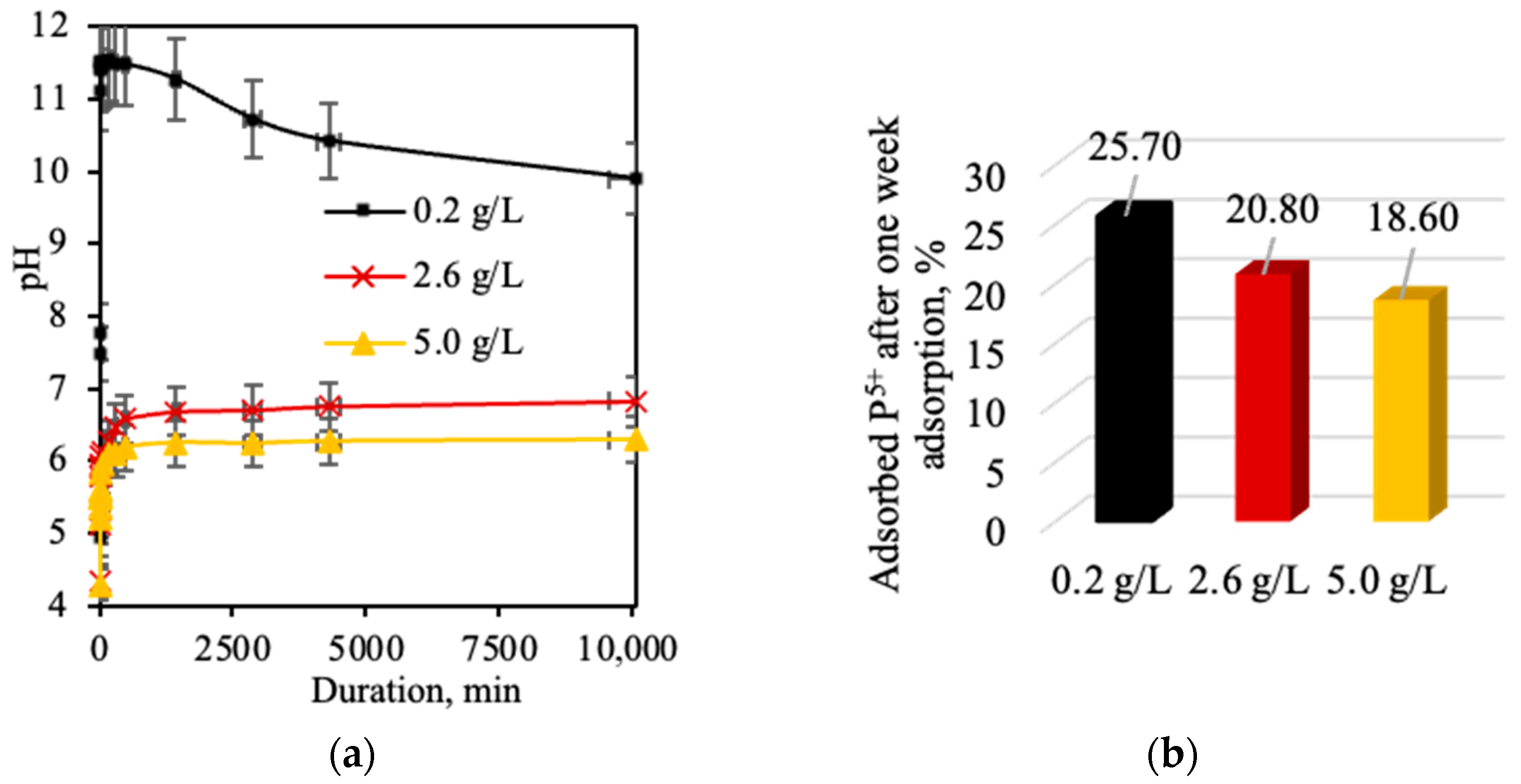
- Calcined opoka has the capacity for phosphorus ion adsorption, but as the initial solution concentration increases, the adsorption efficiency decreases: at 0.2 g of P/L, 25.7% (5.14 mg/g) was removed; at 2.6 g of P/L, 20.8% (54.08 mg/g) was removed; and at 5.0 g of P/L, only 18.6% (93.00 mg/g) was removed. Meanwhile, potassium ion adsorption was insignificant and reversible in all cases; therefore, opoka is ineffective for removing K+ from aqueous systems.
- 3.
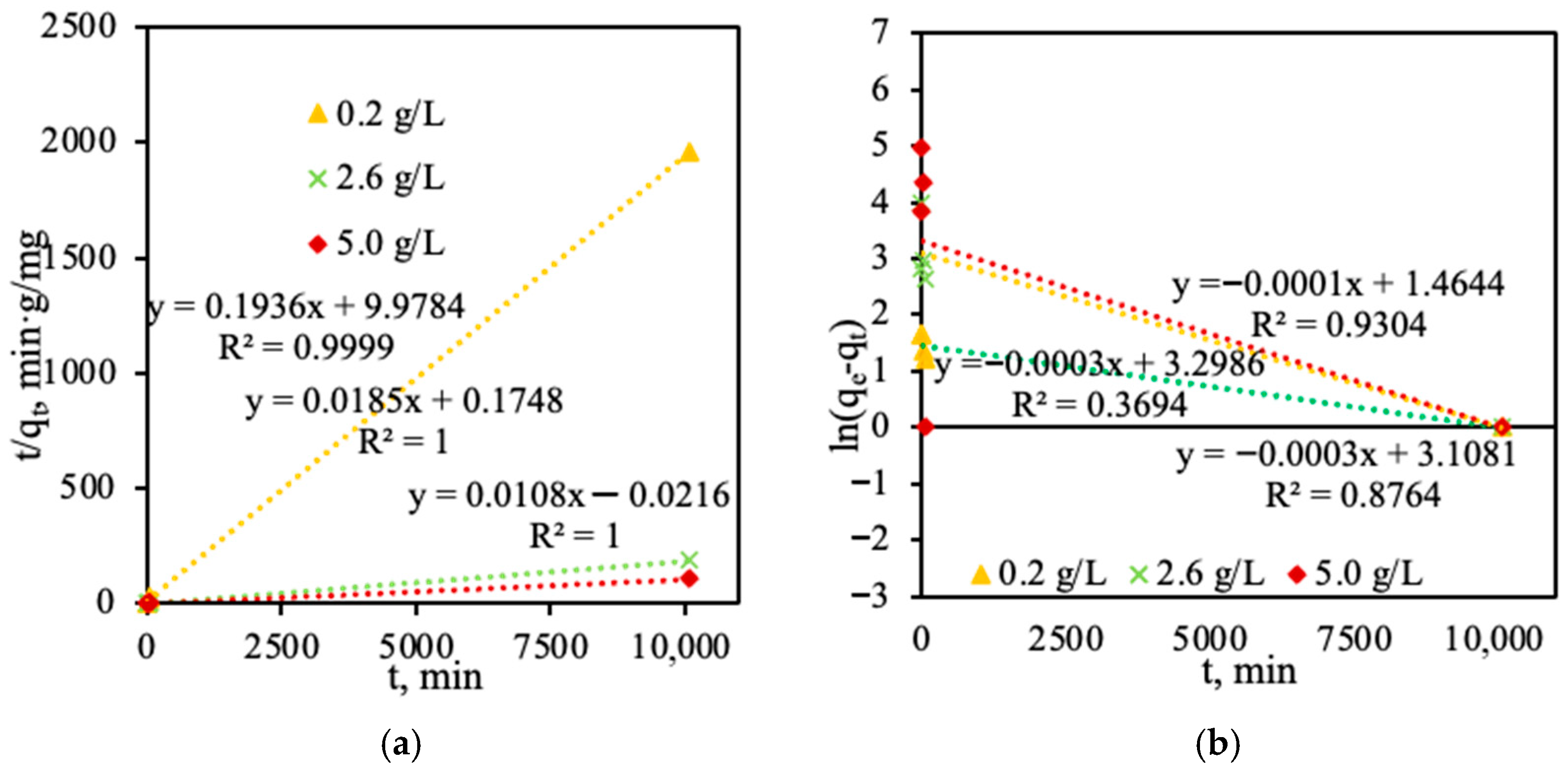
- The adsorption of phosphorus ions follows a pseudo-second-order kinetic model (R2 > 0.999), indicating chemisorption is the dominant mechanism. The adsorption rate constant (k2) decreases with increasing phosphorus concentration, whereas the adsorption capacity increases.
- 4.
- pH is a critical factor controlling both the efficiency and mechanism of the adsorption of phosphorus on calcined opoka. In an alkaline solution (pH > 7), phosphate ions (PO43− and HPO42−) react with Ca2+ to form amorphous calcium phosphate, allowing faster kinetics and higher removal efficiency. Under acidic conditions (pH < 7), adsorption occurs through brushite (CaHPO4·2H2O) crystallization, a slower and less efficient process (~18–21%).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kacprzak, M.J.; Sobik-Szołtysek, J. The opoka-rock in N and P of poultry manure management according to circular economy. J. Environ. Manag. 2022, 316, 115262. [Google Scholar] [CrossRef]
- Dragomir, V.D.; Dumitru, M. The state of the research on circular economy in the European Union: A bibliometric review. Clean. Waste Syst. 2023, 7, 100127. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate From Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020 (Text with EEA relevance). Available online: http://data.europa.eu/eli/reg/2024/1252/oj (accessed on 1 August 2025).
- Kelly, A. Towards sustainable phosphorus use in the European Union: Evaluating resource cap scenarios. J. Clean. Prod. 2025, 516, 145849. [Google Scholar] [CrossRef]
- Withers, P.; Rothwell, S.; Ross, K. Managing phosphorus input pressures for improving water quality at the catchment scale. J. Environ. Manag. 2024, 370, 122792. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewska-Madura, K.; Dondajewska, R.; Gołdyn, R.; Podsiadłowski, S. The influence of restoration measures on phosphorus internal loading from the sediments of a hypereutrophic lake. Environ. Sci. Pollut. Res. 2017, 24, 14417–14429. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wei, J.; Guo, Z.; Song, Y. Adsorption and immobilization of phosphorus from water and sediments using a lanthanum-modified natural zeolite: Performance, mechanism and effect. Sep. Purif. Technol. 2023, 329, 125187. [Google Scholar] [CrossRef]
- Forsius, M.; Posch, M.; Holmberg, M.; Vuorenmaa, J.; Kleemola, S.; Augustaitis, A.; Beudert, B.; Bochenek, W.; Clarke, N.; de Wit, H.A.; et al. Assessing critical load exceedances and ecosystem impacts of anthropogenic nitrogen and sulphur deposition at unmanaged forested catchments in Europe. Sci. Total. Environ. 2021, 753, 141791. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Sun, X.; Bol, R.; Klumpp, E.; Li, M. Organic phosphorus leaching risk from agricultural soils across China. Chem. Biol. Technol. Agric. 2022, 9, 35. [Google Scholar] [CrossRef]
- Flint, E.M.; Ascott, M.J.; Gooddy, D.C.; Stahl, M.O.; Surridge, B.W.J. Watermains Leakage and Outdoor Water Use Are Responsible for Significant Phosphorus Fluxes to the Environment Across the United States. Glob. Biogeochem. Cycles 2023, 37, e2022GB007614. [Google Scholar] [CrossRef]
- Lisi, P.J.; Hein, C.L. Eutrophication drives divergent water clarity responses to decadal variation in lake level. Limnol. Oceanogr. 2018, 64, S49–S59. [Google Scholar] [CrossRef]
- Hupfer, M.; Lewandowski, J. Oxygen Controls the Phosphorus Release from Lake Sediments—A Long-Lasting Paradigm in Limnology. Int. Rev. Hydrobiol. 2008, 93, 415–432. [Google Scholar] [CrossRef]
- Ogwu, M.C.; Patterson, M.E.; Senchak, P.A. Phosphorus mining and bioavailability for plant acquisition: Environmental sustainability perspectives. Environ. Monit. Assess. 2025, 197, 572. [Google Scholar] [CrossRef]
- Tahraoui, H.; Toumi, S.; Boudoukhani, M.; Touzout, N.; Sid, A.N.E.H.; Amrane, A.; Belhadj, A.-E.; Hadjadj, M.; Laichi, Y.; Aboumustapha, M.; et al. Evaluating the Effectiveness of Coagulation–Flocculation Treatment Using Aluminum Sulfate on a Polluted Surface Water Source: A Year-Long Study. Water 2024, 16, 400. [Google Scholar] [CrossRef]
- Karunanithi, R.; Szogi, A.A.; Bolan, N.; Naidu, R.; Loganathan, P.; Hunt, P.G.; Vanotti, M.B.; Saint, C.P.; Ok, Y.S.; Krishnamoorthy, S. Phosphorus Recovery and Reuse from Waste Streams. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 173–250. [Google Scholar] [CrossRef]
- Salkunić, A.; Vuković, J.; Smiljanić, S. Review of Technologies for the Recovery of Phosphorus from Waste Streams. Chem. Biochem. Eng. Q 2022, 36, 91–116. [Google Scholar] [CrossRef]
- Usman, M.O.; Aturagaba, G.; Ntale, M.; Nyakairu, G.W. A review of adsorption techniques for removal of phosphates from wastewater. Water Sci. Technol. 2022, 86, 3113–3132. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Vu, M.T.; Duong, H.C.; Wang, Q.; Ansari, A.; Cai, Z.; Hoang, N.B.; Nghiem, L.D. Recent technological developments and challenges for phosphorus removal and recovery toward a circular economy. Environ. Technol. Innov. 2023, 30, 103114. [Google Scholar] [CrossRef]
- Abdoli, S.; Lajayer, B.A.; Dehghanian, Z.; Bagheri, N.; Vafaei, A.H.; Chamani, M.; Rani, S.; Lin, Z.; Shu, W.; Price, G.W. A Review of the Efficiency of Phosphorus Removal and Recovery from Wastewater by Physicochemical and Biological Processes: Challenges and Opportunities. Water 2024, 16, 2507. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.; Santos, S.C.; Boaventura, R.A.; Botelho, C.M. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Mohanrasu, K.; Manivannan, A.C.; Rengarajan, H.J.R.; Kandaiah, R.; Ravindran, A.; Panneerselvan, L.; Palanisami, T.; Sathish, C.I. Eco-friendly biopolymers and composites: A sustainable development of adsorbents for the removal of pollutants from wastewater. npj Mater. Sustain. 2025, 3, 13. [Google Scholar] [CrossRef]
- Krstić, V. Role of zeolite adsorbent in water treatment. In Handbook of Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 417–481. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Wang, Z.; Lv, M.; Tang, A.; Yu, Y.; Qu, X.; Chen, Z.; Wen, Q.; Li, A. Insight into the synthesis and adsorption mechanism of adsorbents for efficient phosphate removal: Exploration from synthesis to modification. Chem. Eng. J. 2022, 442, 136147. [Google Scholar] [CrossRef]
- Awual, R.; Jyo, A.; Ihara, T.; Seko, N.; Tamada, M.; Lim, K.T. Enhanced trace phosphate removal from water by zirconium(IV) loaded fibrous adsorbent. Water Res. 2011, 45, 4592–4600. [Google Scholar] [CrossRef]
- Wang, J.; Wei, X.; Shen, Y.; Kong, H.; Wang, W.; Jr, R.L.S.; Guo, H. Mitigation of phosphorus contamination from livestock farms with La-containing hydrochar adsorbent. Sep. Purif. Technol. 2024, 359, 130465. [Google Scholar] [CrossRef]
- Rahutomo, S.; Kovar, J.L.; Thompson, M.L. Varying redox potential affects P release from stream bank sediments. PLoS ONE 2018, 13, e0209208. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.T.; Soh, L.S.; Yong, W.F. Valorization of agriculture wastes as biosorbents for adsorption of emerging pollutants: Modification, remediation and industry application. Results Eng. 2023, 17, 100960. [Google Scholar] [CrossRef]
- Luo, X.; Wu, X.; Reng, Z.; Min, X.; Xiao, X.; Luo, J. Enhancement of Phosphate Adsorption on Zirconium Hydroxide by Ammonium Modification. Ind. Eng. Chem. Res. 2017, 56, 9419–9428. [Google Scholar] [CrossRef]
- Brogowski, Z.; Renman, G. Characterization of Opoka as a Basis for Its Use in Wastewater Treatment. Pol. J. Environ. Stud. 2004, 13, 15–20. [Google Scholar]
- Johansson, L.; Gustafsson, J.P. Phosphate removal using blast furnace slags and opoka-mechanisms. Water Res. 2000, 34, 259–265. [Google Scholar] [CrossRef]
- Kacprzak, M.; Malińska, K.; Grosser, A.; Sobik-Szołtysek, J.; Wystalska, K.; Dróżdż, D.; Jasińska, A.; Meers, E. Cycles of carbon, nitrogen and phosphorus in poultry manure management technologies – environmental aspects. Crit. Rev. Environ. Sci. Technol. 2023, 53, 914–938. [Google Scholar] [CrossRef]
- Zawadzka, B.; Siwiec, T.; Marzec, M.; Jóźwiakowski, K.; Listosz, A. Meandering Flow Filter for Phosphorus Removal as a Component of Small Wastewater Treatment Plants—A Case Study. Water 2023, 15, 2703. [Google Scholar] [CrossRef]
- Bus, A.; Karczmarczyk, A. Properties of lime-siliceous rock opoka as reactive material to remove phosphorous from water and wastewater. Infrastrukt. Ekol. Teren. Wiej. 2014, 1, 227–238. [Google Scholar]
- Montayev, S.; Ongayev, M.; Begalieva, R.; Ryskaliev, M.; Denizbayev, S. Sorption Treatment and Desalination of Mineralized Water Using Opoka to Reduce Hardness and Chloride Content. Int. J. Des. Nat. Ecodynamics 2024, 19, 1733–1740. [Google Scholar] [CrossRef]
- Kalenik, M.; Chalecki, M. Investigations on the effectiveness of wastewater purification in medium sand with assisting opoka rock layer. Environ. Prot. Eng. 2021, 47, 53–65. [Google Scholar] [CrossRef]
- Bobrowska, A.; Jagoda, E.; Domonik, A.; Żurawicka, W. Conservation Treatment in the Process of Strengthening the Stone Architecture of Kazimierz Dolny (Poland). Int. J. Archit. Herit 2025, 19, 1311–1325. [Google Scholar] [CrossRef]
- Gubernat, S.; Czarnota, J.; Masłoń, A.; Koszelnik, P.; Pękala, A.; Skwarczyńska-Wojsa, A. Efficiency of phosphorus removal and recovery from wastewater using marl and travertine and their thermally treated forms. J. Water Process Eng 2023, 53, 103642. [Google Scholar] [CrossRef]
- Siauciunas, R.; Smalakys, G.; Dambrauskas, T. Porosity of Calcium Silicate Hydrates Synthesized from Natural Rocks. Materials 2021, 14, 5592. [Google Scholar] [CrossRef]
- Smalakys, G.; Siauciunas, R. Peculiarities of xonotlite synthesis from the raw materials with different SiO2 activities. J. Therm. Anal. Calorim. 2020, 142, 1671–1679. [Google Scholar] [CrossRef]
- Pekala, A. Research on Temporal Leachability of Trace Elements from Opoka-Rocks in The Aspect of Geochemical Environmental Indicators. IOP Conf. Ser. Earth Environ. Sci. 2019, 221, 012125. [Google Scholar] [CrossRef]
- Pękala, A.; Musiał, M. Modelling the Leachability of Strontium and Barium from Stone Building Materials. Materials 2021, 14, 3403. [Google Scholar] [CrossRef]
- Siauciunas, R.; Mikaliunaite, J.; Urbonas, L.; Baltakys, K. Tribochemical and thermal activation of α-c2s hydrate as precursor for cementitious binders. J. Therm. Anal. Calorim. 2014, 118, 817–823. [Google Scholar] [CrossRef]
- Duque-Redondo, E.; Yamada, K.; Arbeloa, I.L.; Manzano, H. Cs-137 immobilization in C-S-H gel nanopores. Phys. Chem. Chem. Phys. 2018, 20, 9289–9297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Z.; Wang, J.; Jiang, S.; Ma, X.; Gao, L.; Liu, H. Study on the dynamics of phosphorus adsorption and the risk of release in black soils by conservation tillage. Sci. Rep. 2025, 15, 18417. [Google Scholar] [CrossRef] [PubMed]
- Beigoli, S.; Hekmat, A.; Farzanegan, F.; Darroudi, M. Sol-gel synthesis of amorphous calcium phosphate nanoparticles in brown rice substrate and assessment of their cytotoxicity and antimicrobial activities. Avicenna J. Phytomed. 2022, 12, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ta, K.M.; Symington, A.R.; Flitcroft, J.M.; Gillie, L.J.; Cooke, D.J.; Zhu, R.; Gonçalves, M.A.; Parker, S.C.; Molinari, M. Modelling phosphate and arsenate adsorption on cerium dioxide: A density functional theory study. Appl. Surf. Sci. 2025, 708, 163619. [Google Scholar] [CrossRef]
- Kashim, M.Z.; Tsegab, H.; Rahmani, O.; Abu Bakar, Z.A.; Aminpour, S.M. Reaction Mechanism of Wollastonite In Situ Mineral Carbonation for CO2 Sequestration: Effects of Saline Conditions, Temperature, and Pressure. ACS Omega 2020, 5, 28942–28954. [Google Scholar] [CrossRef]
- Okano, K.; Miyamaru, S.; Kitao, A.; Takano, H.; Aketo, T.; Toda, M.; Honda, K.; Ohtake, H. Amorphous calcium silicate hydrates and their possible mechanism for recovering phosphate from wastewater. Sep. Purif. Technol. 2015, 144, 63–69. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Ruiz-Agudo, C.; Ibañez-Velasco, A.; Millán, R.G.-S.; Navarro, J.A.R.; Ruiz-Agudo, E.; Rodriguez-Navarro, C. The Carbonation of Wollastonite: A Model Reaction to Test Natural and Biomimetic Catalysts for Enhanced CO2 Sequestration. Minerals 2018, 8, 209. [Google Scholar] [CrossRef]
- Grinys, A.; Bocullo, V.; Gumuliauskas, A. Research of Alkali Silica Reaction in Concrete With Active Mineral Additives. J. Sustain. Arch. Civ. Eng. 2014, 6, 34–41. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Y.; Yu, H.; Xin, X.; Wei, Q.; Du, B. Adsorption of phosphate from aqueous solution by hydroxy-aluminum, hydroxy-iron and hydroxy-iron–aluminum pillared bentonites. J. Hazard. Mater. 2010, 179, 244–250. [Google Scholar] [CrossRef] [PubMed]



| Oxides, % | ||||||
|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | K2O | MgO | TiO2 |
| 53.58 | 24.72 | 4.89 | 2.55 | 1.08 | 0.57 | 0.36 |
| Oxides, % | ||||||
| SO3 | SrO | P2O5 | Na2O | ZrO2 | Other | Loss on ignition |
| 0.10 | 0.08 | 0.07 | 0.07 | 0.03 | 0.03 | 11.86 |
| Oxide | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | CO2 | H2O | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|---|
| Range (wt.%) | 24.55–73.18 | 1.01–3.33 | 0.32–1.95 | 0.80–37.98 | 0.35–0.87 | 0.23–0.99 | 6.84–29.34 | 1.48–2.90 | 9.14–31.12 |
| Average (wt.%) | 55.84 | 2.00 | 1.10 | 20.71 | 0.51 | 0.48 | 16.19 | 1.81 | 18.72 |
| Oxides, % | ||||||
|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | K2O | MgO | P2O5 |
| 62.23 | 26.25 | 5.46 | 2.63 | 1.15 | 0.70 | 0.53 |
| Oxides, % | ||||||
| TiO2 | SO3 | ZrO2 | SrO | Na2O | Other | Total |
| 0.38 | 0.11 | 0.03 | 0.09 | 0.07 | 0.03 | 100.00 |
| Temperature | R2 | qe(exp) (mg/g) | qe(cal) (mg/g) | k1 (g/(mg·min)) | k2 (g/(mg·min)) |
|---|---|---|---|---|---|
| Pseudo-first-order kinetic model | |||||
| 0.2 g of P/L | 0.93 | 5.14 | 4.33 | – | – |
| 2.6 g of P/L | 0.876 | 54.08 | 22.38 | – | – |
| 5.0 g of P/L | 0.369 | 143 | 90.97 | – | – |
| Pseudo-second-order kinetic model | |||||
| 0.2 g of P/L | 0.999 | 5.14 | 5.17 | – | 0.00281 |
| 2.6 g of P/L | 1 | 54.08 | 54.13 | – | 0.00195 |
| 5.0 g of P/L | 1 | 93.00 | 93.24 | – | 0.00000212 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svedaite, E.; Baltakys, K.; Dambrauskas, T. Opoka as a Natural Material for Phosphorus Removal: Properties and Applications. Water 2025, 17, 3017. https://doi.org/10.3390/w17203017
Svedaite E, Baltakys K, Dambrauskas T. Opoka as a Natural Material for Phosphorus Removal: Properties and Applications. Water. 2025; 17(20):3017. https://doi.org/10.3390/w17203017
Chicago/Turabian StyleSvedaite, Evelina, Kestutis Baltakys, and Tadas Dambrauskas. 2025. "Opoka as a Natural Material for Phosphorus Removal: Properties and Applications" Water 17, no. 20: 3017. https://doi.org/10.3390/w17203017
APA StyleSvedaite, E., Baltakys, K., & Dambrauskas, T. (2025). Opoka as a Natural Material for Phosphorus Removal: Properties and Applications. Water, 17(20), 3017. https://doi.org/10.3390/w17203017








