Riboflavin-Functionalized Conductive Material Enhances a Pilot-Scaled Anaerobic Digester Fed with Cattle Manure Wastewater: Synergies on Methanogenesis and Methanosarcina barkeri Enrichment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design and Operation
2.3. Standard Parametric Detection Methods
2.4. Method for Measuring Electrical Conductivity of Sludge
2.5. Microbial Community Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Performance of Different Reactors in AD Efficiency
3.2. Evolution of Internal Material Characteristics in the Reactor System
3.3. Microbial Community Structure Analysis
3.4. DIET Metabolic Profiling
3.5. Cost–Benefit Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCM-RF | Riboflavin-functionalized carbon-based conductive materials |
| DIET | Direct interspecies electron transfer |
| AD | Anaerobic digestion |
| M. barkeri | Methanosarcina barkeri |
| DSMZ | German Collection of Microorganisms and Cell Cultures |
| CC | Carbon cloth |
| GAC | Granular activated carbon |
| OLR | Organic loading rate |
| HRT | Hydraulic retention time |
| IET | Interspecies electron transfer |
| EET | Extracellular electron transfer |
| UASB | Upflow anaerobic sludge blanket |
| TS | Total solids |
| VS | Volatile solids |
| tCOD | Total chemical oxygen demand |
| OTUs | Operational taxonomic units |
References
- FAO. Livestock Primary: Cattle Population 2020, 2022. Available online: https://www.fao.org/faostat (accessed on 23 November 2024).
- Della Rosa, M.M.; Waghorn, G.C.; Vibart, R.E.; Jonker, A. An assessment of global ruminant methane-emission measurements shows bias relative to contributions of farmed species, populations and among continents. Anim. Prod. Sci. 2023, 63, 201–212. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Zheng, C.; Xu, Q.; Sun, Y.; Huang, M.; Li, L.; Yang, X.; Zhou, H.; Ma, H.; et al. Recent advances, challenges, and perspectives on carbon capture. Front. Environ. Sci. Eng. 2024, 18, 75. [Google Scholar] [CrossRef]
- Qin, C.; Xue, Q.; Zhang, J.; Lu, L.; Xiong, S.; Xiao, Y.; Zhang, X.; Wang, J. A Beautiful China Initiative Towards the Harmony between Humanity and the Nature. Front. Environ. Sci. Eng. 2024, 18, 71. [Google Scholar] [CrossRef]
- He, H.; Zeng, Y.; Dong, H.; Cui, P.; Lu, W.; Xu, H.; Qiu, B.; Sun, D.; Ma, J.; Dang, Y. Enrichment of Methanothrix species via riboflavin-loaded granular activated carbon in anaerobic digestion of high-concentration brewery wastewater amidst continuous inoculation of Methanosarcina barkeri. Water Res. 2025, 268, 122739. [Google Scholar] [CrossRef]
- Kang, H.; Lee, S.; Lim, T.; Park, J.; Kim, B.; Buffiere, P.; Park, H. Recent advances in methanogenesis through direct interspecies electron transfer via conductive materials: A molecular microbiological perspective. Bioresour. Technol. 2021, 322, 124587. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite Triggering Enhanced Direct Interspecies Electron Transfer: A Scavenger for the Blockage of Electron Transfer in Anaerobic Digestion of High-Solids Sewage Sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct Exchange of Electrons Within Aggregates of an Evolved Syntrophic Coculture of Anaerobic Bacteria. Science 2010, 330, 1413–1415. [Google Scholar] [CrossRef]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Dubey, M.; Ahmed, B.; Gahlot, P.; Khan, A.A.; Rajpal, A.; Kazmi, A.A.; Tyagi, V.K. Carbon-based conductive materials facilitated anaerobic co-digestion of agro waste under thermophilic conditions. Waste Manag. 2021, 124, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Dhar, B.R. Boosting thermophilic anaerobic digestion with conductive materials: Current outlook and future prospects. Chemosphere 2023, 343, 140175. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Bulynina, S.S.; Ziganshin, A.M. Impact of Granular Activated Carbon on Anaerobic Process and Microbial Community Structure during Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure. Sustainability 2022, 14, 447. [Google Scholar] [CrossRef]
- Zhuravleva, E.A.; Shekhurdina, S.V.; Kotova, I.B.; Loiko, N.G.; Popova, N.M.; Kryukov, E.; Kovalev, A.A.; Kovalev, D.A.; Litti, Y.V. Effects of various materials used to promote the direct interspecies electron transfer on anaerobic digestion of low-concentration swine manure. Sci. Total Environ. 2022, 839, 156073. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zheng, S.; Wang, B.; Zhang, X. Selectively facilitating the electron acceptance of methanogens by riboflavin. Renew. Energy 2022, 195, 734–741. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Ye, Y.; Chen, M.; Zhou, S. Evidence for the coexistence of direct and riboflavin-mediated interspecies electron transfer inGeobacter co-culture. Environ. Microbiol. 2020, 22, 243–254. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, H.; Chen, W.; Li, H.; Dong, H.; Wu, H.; Xu, H.; Sun, D.; Liu, X.; Li, P.; et al. Riboflavin-loaded carbon cloth aids the anaerobic digestion of cow dung by promoting direct interspecies electron transfer. Environ. Res. 2024, 241, 117660. [Google Scholar] [CrossRef]
- Rotaru, A.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- APHA; WPCF. Standard Methods for Water and Wastewater Examination, Twenty-Firsted; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Li, Y.; Zhao, J.; Achinas, S.; Zhang, Z.; Krooneman, J.; Euverink, G.J.W. The biomethanation of cow manure in a continuous anaerobic digester can be boosted via a bioaugmentation culture containing Bathyarchaeota. Sci. Total Environ. 2020, 745, 141042. [Google Scholar] [CrossRef]
- Saha, S.; Basak, B.; Hwang, J.; Salama, E.; Chatterjee, P.K.; Jeon, B. Microbial Symbiosis: A Network towards Biomethanation. Trends Microbiol. 2020, 28, 968–984. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zhao, Y.; Xu, C.; Zhang, K.; Li, J.; Yan, L.; Gu, J.; Wei, D.; Wang, W. Establishing practical strategies to run high loading corn stover anaerobic digestion: Methane production performance and microbial responses. Bioresour. Technol. 2020, 310, 123364. [Google Scholar] [CrossRef]
- He, Y.; Yang, J.; Shen, H.; Wang, L.; Gao, Z. Brazing graphite to hastelloy N superalloy using pure-Au filler metal: Bonding mechanism and joint properties. Mater. Des. 2016, 104, 1–9. [Google Scholar] [CrossRef]
- Maus, I.; Rumming, M.; Bergmann, I.; Heeg, K.; Pohl, M.; Nettmann, E.; Jaenicke, S.; Blom, J.; Pühler, A.; Schlüter, A.; et al. Characterization of Bathyarchaeota genomes assembled from metagenomes of biofilms residing in mesophilic and thermophilic biogas reactors. Biotechnol. Biofuels 2018, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Shi, Y.; Zhao, Z.; Wang, X.; Dou, M. Enhanced two-phase anaerobic digestion of waste-activated sludge by combining magnetite and zero-valent iron. Bioresour. Technol. 2020, 306, 123122. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, H.; Kim, Y.; Kim, N.; Park, H. Different contribution of exoelectrogens in methanogenesis via direct interspecies electron transfer (DIET) by the different substrate in continuous anaerobic bioreactor. Bioresour. Technol. 2022, 364, 128115. [Google Scholar] [CrossRef]
- Abid, M.; Wu, J.; Seyedsalehi, M.; Hu, Y.; Tian, G. Novel insights of impacts of solid content on high solid anaerobic digestion of cow manure: Kinetics and microbial community dynamics. Bioresour. Technol. 2021, 333, 125205. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Wu, J.; Yang, S.; Ren, N. Reflux of acidizing fluid for enhancing biomethane production from cattle manure in plug flow reactor. Bioresour. Technol. 2019, 284, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Amy Tan, G.; Jing, H.; Lee, P.; Lee, D.; Leu, S. Enhanced primary treatment for net energy production from sewage—The genetic clarification of substrate-acetate-methane pathway in anaerobic digestion. Chem. Eng. J. 2022, 431, 133416. [Google Scholar] [CrossRef]
- Guo, X.; Sun, C.; Lin, R.; Xia, A.; Huang, Y.; Zhu, X.; Show, P.; Murphy, J.D. Effects of foam nickel supplementation on anaerobic digestion: Direct interspecies electron transfer. J. Hazard. Mater. 2020, 399, 122830. [Google Scholar] [CrossRef]
- Chen, H.; Liu, G.; Wang, K.; Piao, C.; Ma, X.; Li, X. Characteristics of microbial community in EGSB system treating with oxytetracycline production wastewater. J. Environ. Manag. 2021, 295, 113055. [Google Scholar] [CrossRef]
- Jin, H.; Yao, X.; Tang, C.; Zhou, A.; Liu, W.; Ren, Y.; Li, Z.; Wang, A.; He, Z. Magnetite modified zeolite as an alternative additive to promote methane production from anaerobic digestion of waste activated sludge. Renew. Energy 2024, 224, 120181. [Google Scholar] [CrossRef]
- Dang, Y.; Sun, D.; Woodard, T.L.; Wang, L.; Nevin, K.P.; Holmes, D.E. Stimulation of the anaerobic digestion of the dry organic fraction of municipal solid waste (OFMSW) with carbon-based conductive materials. Bioresour. Technol. 2017, 238, 30–38. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, D.; Dang, Y.; Chen, H.; Zhao, Z.; Zhang, Y.; Holmes, D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016, 222, 270–276. [Google Scholar] [CrossRef]
- Venkidusamy, K.; Megharaj, M.; Schröder, U.; Karouta, F.; Mohan, S.V.; Naidu, R. Electron transport through electrically conductive nanofilaments in Rhodopseudomonas palustris strain RP2. RSC Adv. 2015, 5, 100790–100798. [Google Scholar] [CrossRef]
- Li, D.; Meng, X.; Sun, Y.; Li, X.; Liu, X.; Xie, Z.; Cao, Q. The microbial and functional reconstruction of instable syntrophic propionate-oxidizing methanogenesis by system recovering and injection modes changing. Chem. Eng. J. 2023, 455, 140736. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Bio/Technol. 2008, 7, 173–190. [Google Scholar] [CrossRef]

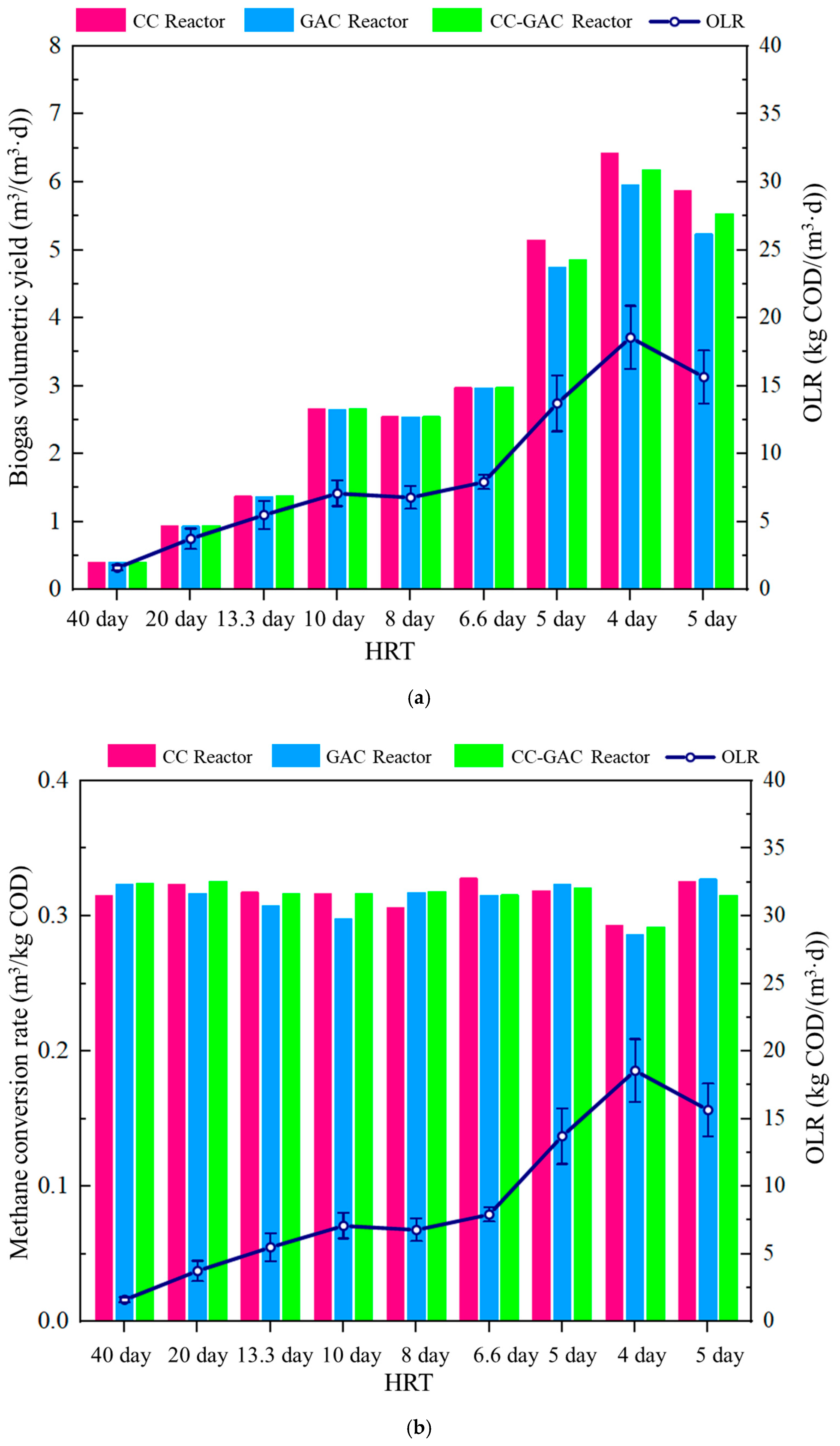
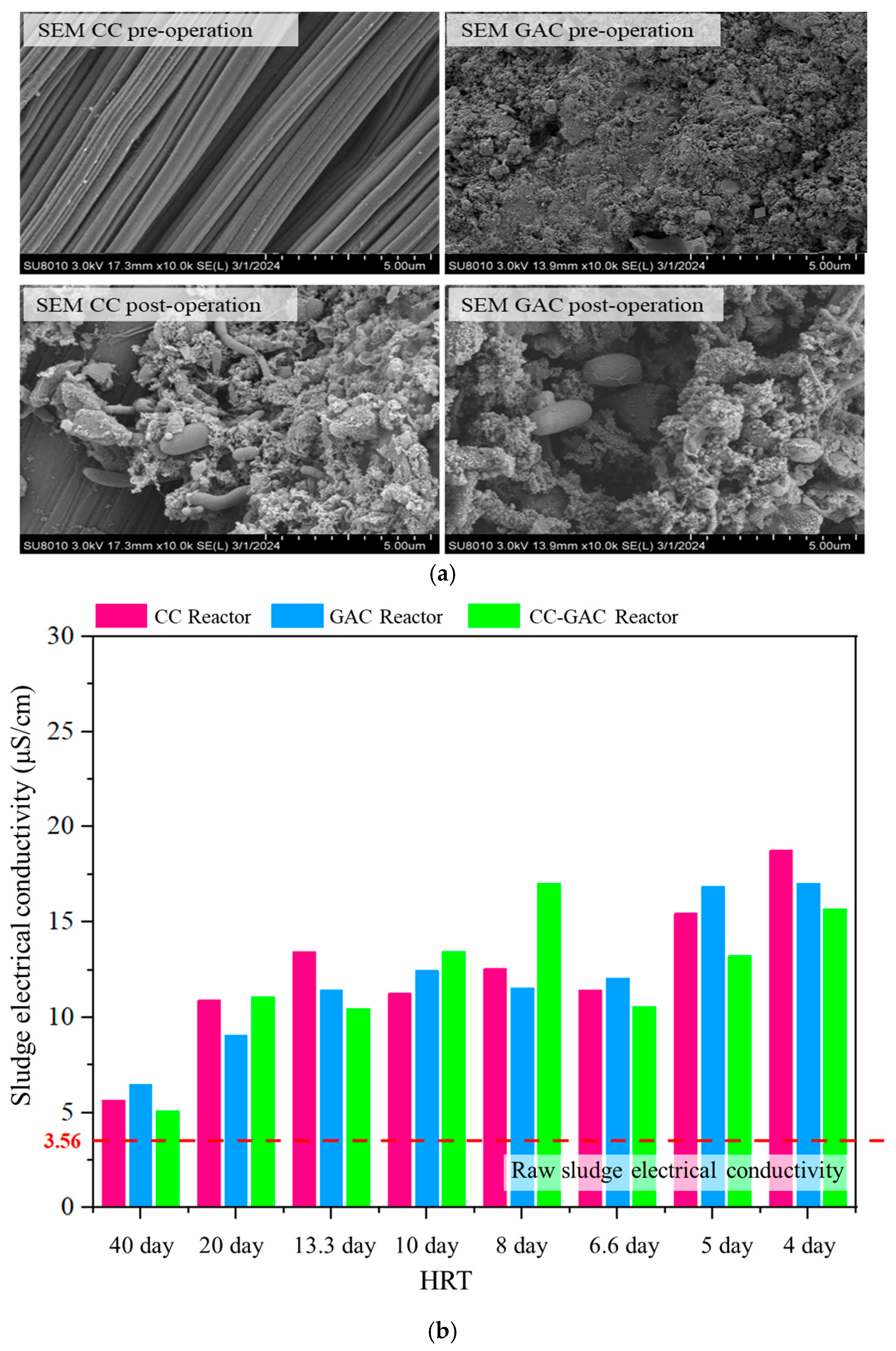
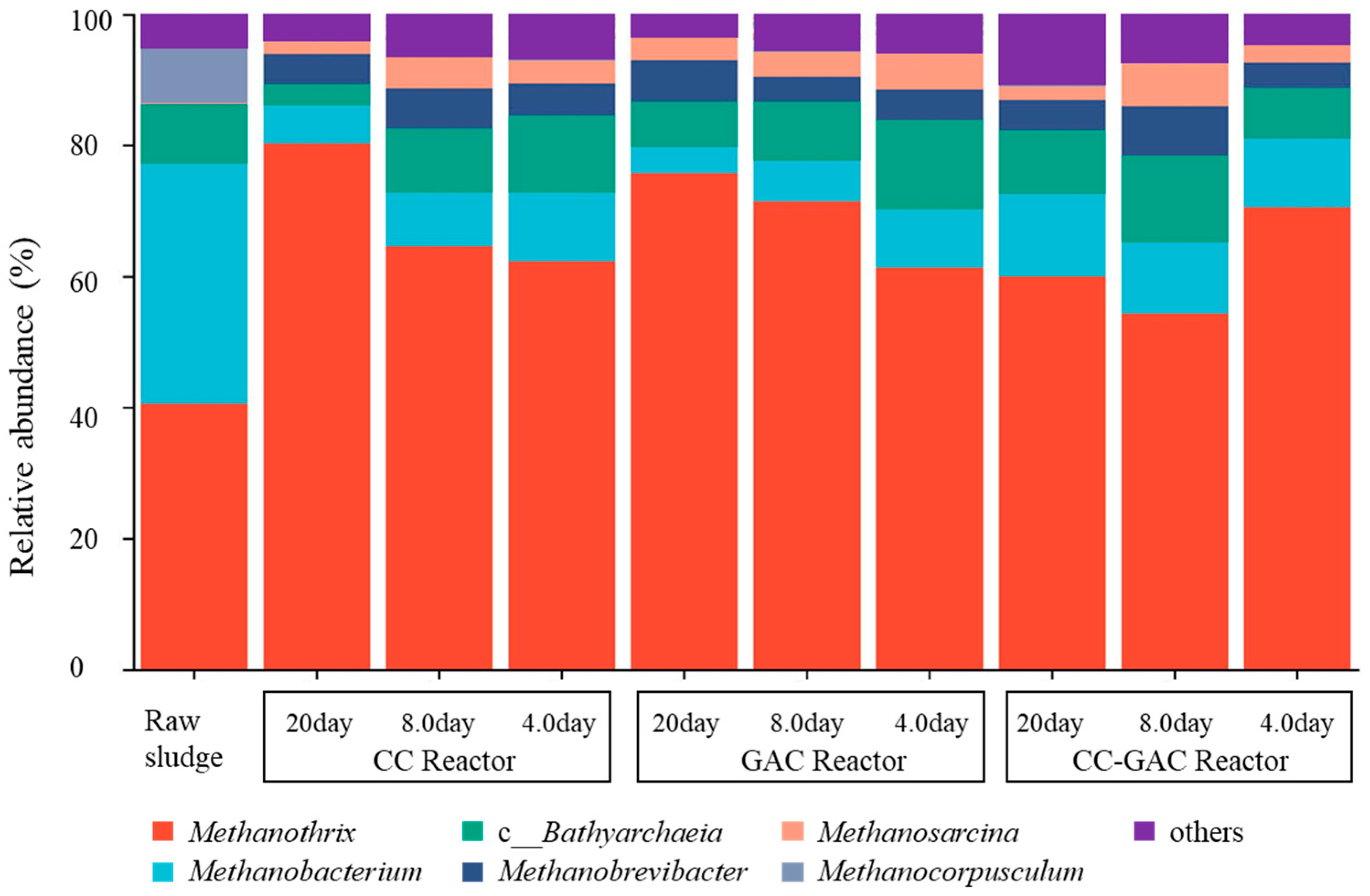


| Parameter | Cattle Manure Wastewater | Anaerobic Sludge |
|---|---|---|
| Total solids–TS (%) | 5.78 ± 0.59 | 1.20 ± 0.08 |
| Volatile solids–VS (%) | 3.98 ± 0.53 | 1.11 ± 0.10 |
| Water content (%) | 94.22 ± 0.58 | 98.20 ± 0.11 |
| pH | 8.12 ± 0.11 | 7.23 ± 0.03 |
| Total chemical oxygen demand–tCOD (g/L) | 88.76 ± 13.23 | — |
| C/N | 23.45 | — |
| H (%) | 6.46 ± 0.45 | — |
| O (%) | 33.56 ± 1.45 | — |
| HRT | Average tCOD (g/L) | OLR (kg COD/(m3·d)) |
|---|---|---|
| 40 days | 75.38 | 1.63 ± 0.18 |
| 20 days | 71.01 | 3.76 ± 0.74 |
| 13.3 days | 69.69 | 5.51 ± 1.02 |
| 10 days | 53.51 | 7.10 ± 0.94 |
| 8 days | 53.15 | 6.80 ± 0.84 |
| 6.7 days | 70.35 | 7.94 ± 0.53 |
| 5 days | 74.61 | 13.73 ± 2.06 |
| 4 days | 75.73 | 18.57 ± 2.66 |
| 5 days | 79.78 | 15.66 ± 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, G.; Zeng, Y.; Deng, Q.; Ma, J.; Dong, H.; Zhang, H.; He, H.; Xu, H.; Wu, H.; Dang, Y. Riboflavin-Functionalized Conductive Material Enhances a Pilot-Scaled Anaerobic Digester Fed with Cattle Manure Wastewater: Synergies on Methanogenesis and Methanosarcina barkeri Enrichment. Water 2025, 17, 2967. https://doi.org/10.3390/w17202967
Sun G, Zeng Y, Deng Q, Ma J, Dong H, Zhang H, He H, Xu H, Wu H, Dang Y. Riboflavin-Functionalized Conductive Material Enhances a Pilot-Scaled Anaerobic Digester Fed with Cattle Manure Wastewater: Synergies on Methanogenesis and Methanosarcina barkeri Enrichment. Water. 2025; 17(20):2967. https://doi.org/10.3390/w17202967
Chicago/Turabian StyleSun, Guangdong, Yiwei Zeng, Qingtao Deng, Jianyong Ma, He Dong, Haowen Zhang, Hao He, Haiyu Xu, Hongbin Wu, and Yan Dang. 2025. "Riboflavin-Functionalized Conductive Material Enhances a Pilot-Scaled Anaerobic Digester Fed with Cattle Manure Wastewater: Synergies on Methanogenesis and Methanosarcina barkeri Enrichment" Water 17, no. 20: 2967. https://doi.org/10.3390/w17202967
APA StyleSun, G., Zeng, Y., Deng, Q., Ma, J., Dong, H., Zhang, H., He, H., Xu, H., Wu, H., & Dang, Y. (2025). Riboflavin-Functionalized Conductive Material Enhances a Pilot-Scaled Anaerobic Digester Fed with Cattle Manure Wastewater: Synergies on Methanogenesis and Methanosarcina barkeri Enrichment. Water, 17(20), 2967. https://doi.org/10.3390/w17202967






