Genetic Mechanism of Geothermal Water in Typical Structural Belts from the Altay and Tianshan to the Kunlun Mountains in Xinjiang: Evidence from Hydrogeochemistry and δ2H–δ18O Isotopes
Abstract
1. Introduction
2. Geothermal Geological Setting
3. Materials and Methods
3.1. Sampling and Laboratory Analysis
3.2. Processing and Analysis of Data
4. Results and Discussion
4.1. Hydrogeochemical Characteristics and HCA of Geothermal Water
4.2. Analysis of the Hydrochemical Genesis of Geothermal Water
4.3. Geothermal Water H-O Isotope Characteristics and Identification of Recharge Sources
4.4. Heat Source and Geothermal Gradient Characteristics
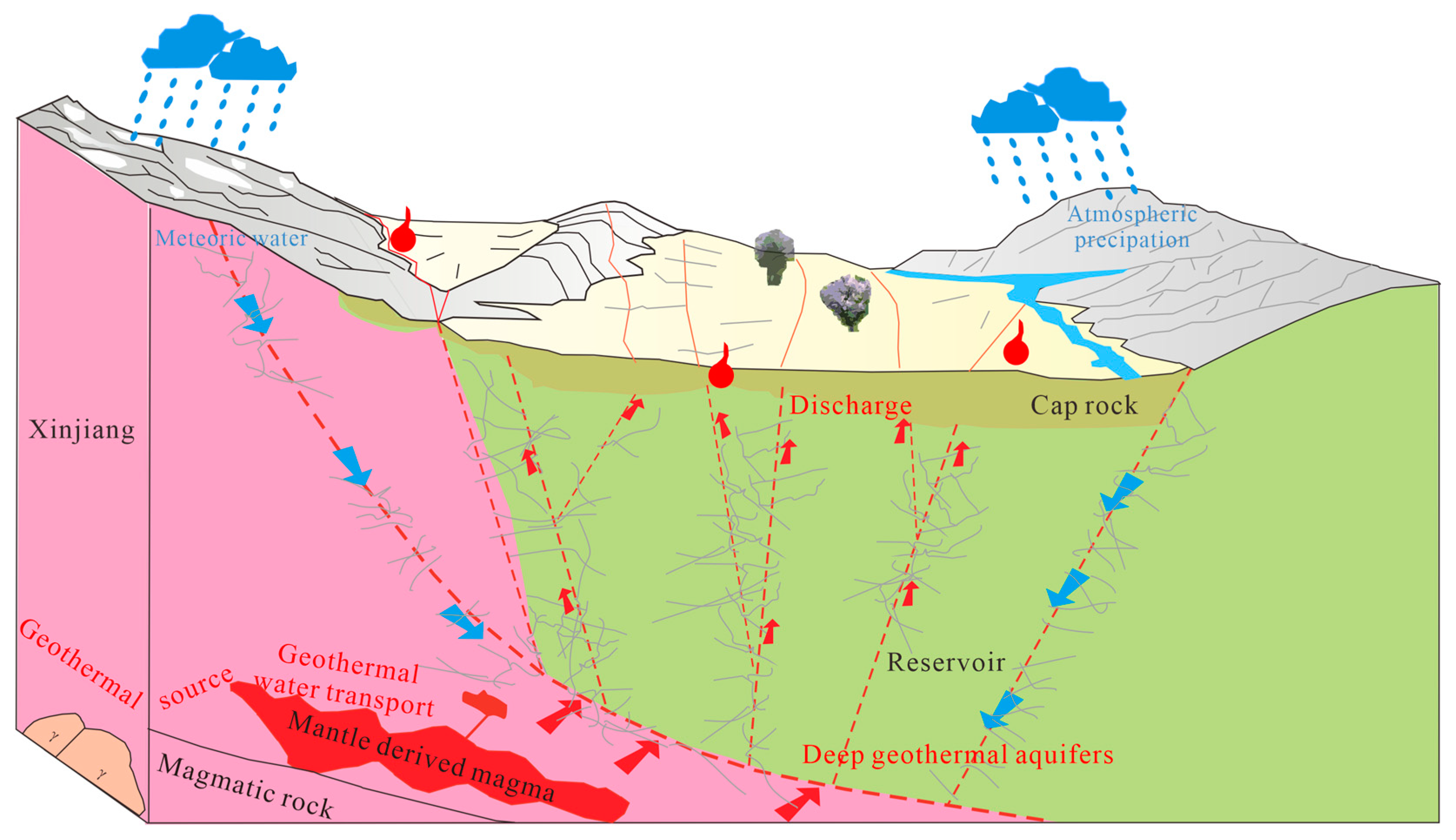
4.5. Geothermal Reservoir Characteristics and Conceptual Model
5. Conclusions
- (1)
- The temperature of geothermal springs and geothermal wells in Xinjiang ranges from 3.8 to 162 °C. Deep acidic rock masses and surrounding rocks are the heat sources. Magmatic activity or radioactive elements in acidic granite bodies provide heat sources for hot springs. The hydrochemical types of geothermal waters are mainly SO4·Cl-Na and SO4-Na, showing weak alkalinity. Hierarchical cluster analysis simplifies the complexity of water sample analysis. Ion ratio analysis shows that these geothermal waters are affected by alternating water–rock interaction and cation adsorption.
- (2)
- Combining isotopic characteristics and exploration data, it is determined that the source of geothermal water recharge is meteoric water. High-temperature geothermal waters are mostly distributed near deep fault tectonic zones. The heat storage type in the study area is determined to be a deep circulation convection-type zonal heat storage.
- (3)
- The geothermal genesis model in the study area is that atmospheric precipitation recharges groundwater along deep faults. Groundwater, through deep circulation, transports heat energy from the deep crust and upper mantle to shallow heat reservoirs, forming a zonal distribution of geothermal fields. The development and utilization of geothermal resources in this area is promising, and it is expected that higher-quality medium-temperature geothermal resources will be discovered.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Yao, R.; Deng, C.; Huang, X.; Lv, G.; Li, X.; Wang, Y. Genetic mechanism of geothermal water in the sandstone reservoir of western Sichuan, SW China: Evidence from hydrochemistry and δD–δ18O isotopes. Ore Geol. Rev. 2024, 167, 105994. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Z.; Yu, Q.; Yan, X.; Qi, K.; Wang, Z.; Lan, H.; Jia, M.; Liu, Y.; Wu, H. Hydrogeochemistry and genetic mechanisms of the geothermal system in the Xi’an depression of the southern Weihe Basin, China. Geothermics 2024, 122, 103090. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, H.Z.; Chen, K. Geothermal Geology Characteristic and Origin Analysis of Shilin Basin in Yunnan Province. Adv. Mater. Res. 2013, 779–780, 1449–1452. [Google Scholar] [CrossRef]
- Şanliyüksel Yücel, D.; Özden, S.; Marmara, H. Hydrogeochemical and isotopic monitoring of the Kestanbol geothermal field (Northwestern Turkey) and its relationship with seismic activity. Turk. J. Earth Sci. 2021, 30, 1112–1133. [Google Scholar] [CrossRef]
- Banerjee, A.; Person, M.; Hofstra, A.; Sweetkind, D.; Cohen, D.; Sabin, A.; Unruh, J.; Zyvoloski, G.; Gable, C.W.; Crossey, L.; et al. Deep permeable fault—Controlled helium transport and limited mantle flux in two extensional geothermal systems in the Great Basin, United States. Geol. Soc. Am. 2011, 39, 195–198. [Google Scholar] [CrossRef]
- Toro Vivanco, D.I.; Kleine-Marshall, B.I.; Smit, M.J.; Gunnarsdóttir, S.H.; Franzson, H.; Stefánsson, A. Lithogeochemistry of the Nesjavellir geothermal field, SW Iceland—Implications for elemental mass movement in active geothermal systems. Geothermics 2024, 120, 103013. [Google Scholar] [CrossRef]
- Abdel Hafeez, T.; Abdelwahhab, M.; Elmahdy, M. Geothermal application of spectral gamma ray logging in the South Kansas Subsurface, USA. Appl. Radiat. Isot. 2019, 154, 108904. [Google Scholar] [CrossRef]
- Guo, Q. Hydrogeochemistry of high-temperature geothermal systems in China: A review. Appl. Geochem. 2012, 27, 1887–1898. [Google Scholar] [CrossRef]
- Li, Q.; Hao, Y.; Liu, C.; Huang, J.; Yuan, X. Representative High-Temperature Hydrothermal Activities in the Himalaya Geothermal Belt (HGB): A Review and Future Perspectives. Water 2024, 16, 1378. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Z.; Wang, G.; Mao, T.; Li, C.; Zhou, Y.; Xie, H.; Xiang, T. Hydrogeochemical signatures origin of a karst geothermal reservoir–the Sinian Dengying Formation in northern Guizhou, China. Geosci. J. 2024, 28, 107–123. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Y. Rheological characteristics of lithospheric mantle in the Pamir Plateau and temperature structure of its northeastern margin: Results of the Third Xinjiang Comprehensive Scientific Expedition on Geothermal Science. Chin. J. Geophys. 2025, 68, 199–212. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, K.; Chang, J.; He, G.; Chen, F.; Chen, L.; Liu, Z.; Huang, Y. Formation mechanism and evaluation of geothermal resources in Yanqi Qikexing town, Xinjiang. Sci. Rep. 2025, 15, 7006. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Li, P.; Wang, L.; He, X.; Zhang, L. Hydrochemical characteristics, hydrochemical processes and recharge sources of the geothermal systems in Lanzhou City, northwestern China. Urban Clim. 2022, 43, 101152. [Google Scholar] [CrossRef]
- Han, S.; Nan, D.; Liu, Z.; Gesang, N.; Bianma, C.; Zhao, H.; Zheng, Y.; Xiao, P. Hydrochemical Characteristics and Formation Mechanism of Geothermal Fluids in Zuogong County, Southeastern Tibet. Water 2024, 16, 2852. [Google Scholar] [CrossRef]
- Shang, J.; Liu, M.; Cao, Y.; Shi, H.; Wei, X. Trace element geochemistry of high-temperature geothermal waters in the Yunnan-Tibet geothermal province, Southwest China. Appl. Geochem. 2024, 162, 105910. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Chen, A. The Geochemical Characteristics and Genesis Mechanisms of the Zaozigou Geothermal Field. Sustainability 2024, 16, 6790. [Google Scholar] [CrossRef]
- Yan, X.; Wei, S.; Zhang, W.; Liu, F.; Liao, Y. Reservoirs and Hydrogeochemical Characterizations of the Yanggao Geothermal Field in Shanxi Province, China. Water 2024, 16, 669. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Liang, L.; Zhu, Z.; Zhang, A. Constraining the properties of the heat sources of high-temperature hydrogeothermal systems: Evidence from the lithium concentrations of geothermal waters. J. Hydrol. 2024, 640, 131696. [Google Scholar] [CrossRef]
- Chen, W.; Liu, P.; Luo, Y.; Li, B.; Peng, J.; Jin, X. Behavior of Sb and As in the hydrogeochemistry of adjacent karst underground river systems and the responses of such systems to mining activities. Sci. Total Environ. 2023, 857, 159411. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, X.; Wang, C.; Li, J.; He, M.; Tian, J.; Saimaiernaji, K.; Zhu, C.; Yan, W.; Ma, R.; et al. Hydrogeochemical characterization and precursor anomalies of hot springs in the North Tianshan orogen. Appl. Geochem. 2023, 158, 105813. [Google Scholar] [CrossRef]
- Peng, Y.W.; Gu, X.X.; Su, J.; Zhang, Y.; Wang, J.; Wang, X.; Chen, X.; Song, M.; Shu, Z. Geology, geochemistry and genesis of Tabei: A newly identified intermediate-sulphidation epithermalPb–Zn deposit adjacent to low-sulphidation Au deposit in the Tulasu Basin, Chinese Western Tianshan. Geol. J. 2023, 58, 583–604. [Google Scholar] [CrossRef]
- Li, Y.; Pang, Z.; Yang, F.; Yuan, L.; Tang, P. Hydrogeochemical characteristics and genesis of the high-temperature geothermal system in the Tashkorgan basin of the Pamir syntax, western China. J. Asian Earth Sci. 2017, 149, 134–144. [Google Scholar] [CrossRef]
- Li, X.; Huang, X.; Liao, X.; Zhang, Y. Hydrogeochemical Characteristics and Conceptual Model of the Geothermal Waters in the Xianshuihe Fault Zone, Southwestern China. Int. J. Environ. Res. Public Health 2020, 17, 500. [Google Scholar] [CrossRef]
- Yin, Z.; Li, X.; Huang, C.; Chen, W.; Hou, B.; Li, X.; Han, W.; Hou, P.; Han, J.; Ren, C.; et al. Analysis of the Formation Mechanism of Medium and Low-Temperature Geothermal Water in Wuhan Based on Hydrochemical Characteristics. Water 2023, 15, 227. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Zhang, W.; Ma, F.; Zhu, X.; Yue, G.; Yu, M. Characteristics of the Rongcheng Bulge Geothermal Field and the Evolution of Geothermal Fluids, Xiong’an New Area, China. Water 2022, 14, 2468. [Google Scholar] [CrossRef]
- Gao, G.; Kang, G.; Bai, C.; Li, G. Distribution of the crustal magnetic anomaly and geological structure in Xinjiang, China. J. Asian Earth Sci. 2013, 77, 12–20. [Google Scholar] [CrossRef]
- Laurent Charvet, S.; Charvet, J.; Shu, L.; Ma, R.; Lu, H. Palaeozoic late collisional strike-slip deformations in Tianshan and Altay, Eastern Xinjiang, NW China. Terra Nova 2002, 14, 249–256. [Google Scholar] [CrossRef]
- Chen, W.; Liu, P.; Xu, Z.; Luo, Y.; Zhang, Y.; Wang, Y. Heavy metals’ release behavior in antimony tailings dump and its response to different rainfall conditions. J. Geochem. Explor. 2025, 278, 107846. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, P.; Dor, J.; Zhang, H. Multi-isotopes (H, O, Sr, and Li) and element geochemistry constrain the formation of Kongchutso helium-rich geothermal field in western Tibet, China. Geothermics 2024, 120, 102986. [Google Scholar] [CrossRef]
- Liu, P.; Hoth, N.; Drebenstedt, C.; Sun, Y.; Xu, Z. Hydro-geochemical paths of multi-layer groundwater system in coal mining regions—Using multivariate statistics and geochemical modeling approaches. Sci. Total Environ. 2017, 601–602, 1–14. [Google Scholar] [CrossRef]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, A.; Li, X.; Zhou, J.; Pan, G.; He, N. Response of antimony and arsenic in karst aquifers and groundwater geochemistry to the influence of mine activities at the world’s largest antimony mine, central China. J. Hydrol. 2021, 603, 127131. [Google Scholar] [CrossRef]
- Bahrami, M.; Khaksar, E.; Khaksar, E. Spatial variation assessment of groundwater quality using multivariate statistical analysis(Case Study: Fasa Plain, Iran). J. Groundw. Sci. Eng. 2020, 8, 230–243. [Google Scholar] [CrossRef]
- Ali, S.; Shekhar, S.; Bhattacharya, P.; Verma, G.; Chandrasekhar, T.; Chandrashekhar, A. Elevated fluoride in groundwater of Siwani Block, Western Haryana, India: A potential concern for sustainable water supplies for drinking and irrigation. Groundw. Sustain. Dev. 2018, 7, 410–420. [Google Scholar] [CrossRef]
- Chen, J.; Qian, H.; Gao, Y.; Wang, H.; Zhang, M. Insights into hydrological and hydrochemical processes in response to water replenishment for lakes in arid regions. J. Hydrol. 2020, 581, 124386. [Google Scholar] [CrossRef]
- Palmer, M.J.; Chételat, J.; Richardson, M.; Jamieson, H.E.; Galloway, J.M. Seasonal variation of arsenic and antimony in surface waters of small subarctic lakes impacted by legacy mining pollution near Yellowknife, NT, Canada. Sci. Total Environ. 2019, 684, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Fu, Z.; Zhang, Z.; Wang, H.; Liu, S.; Feng, W.; Zhao, X.; Giesy, J.P. Synthesis of Fe3O4 magnetic nanoparticles coated with cationic surfactants and their applications in Sb(V) removal from water. Sci. Total Environ. 2020, 710, 136302. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Das, A.; Sarma, K.P.; Kumar, M. Provenance, prevalence and health perspective of co-occurrences of arsenic, fluoride and uranium in the aquifers of the Brahmaputra River floodplain. Chemosphere 2018, 194, 755–772. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, Y.; Ren, T.; Han, X.; Wu, X. Hydrochemical Characteristics and Formation Mechanism of Neogene Geothermal Water in the Zhangye–Minle Basin. Water 2025, 17, 2641. [Google Scholar] [CrossRef]
- Duan, R.; Chang, L.; Gu, X.; Li, X.; You, X.; Zhang, Q.; Wang, Q. The Hydrogeochemical Processes of Groundwater in the Bieletan Area, the Western Potash Production Region in China. Water 2024, 16, 1833. [Google Scholar] [CrossRef]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]

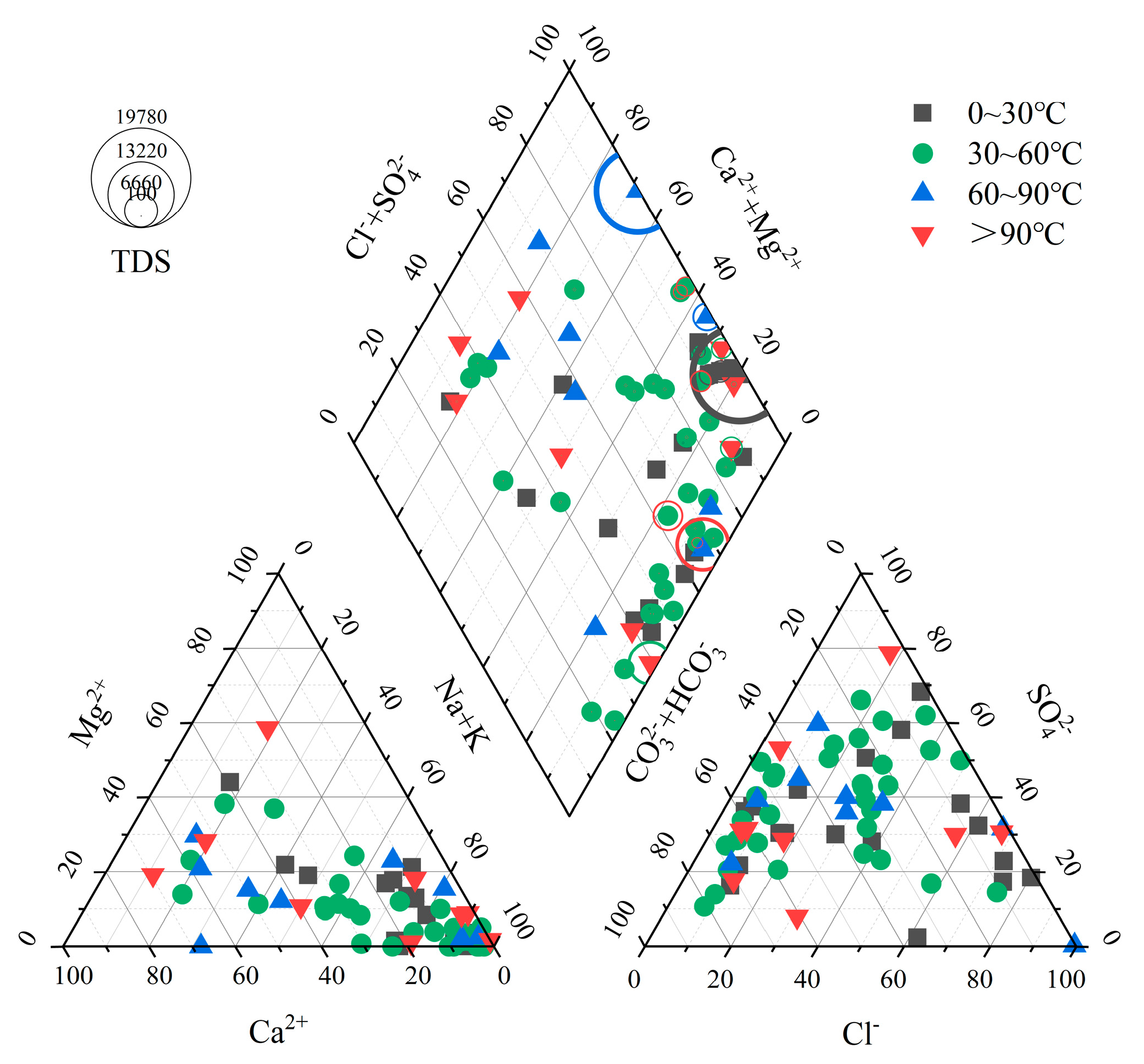

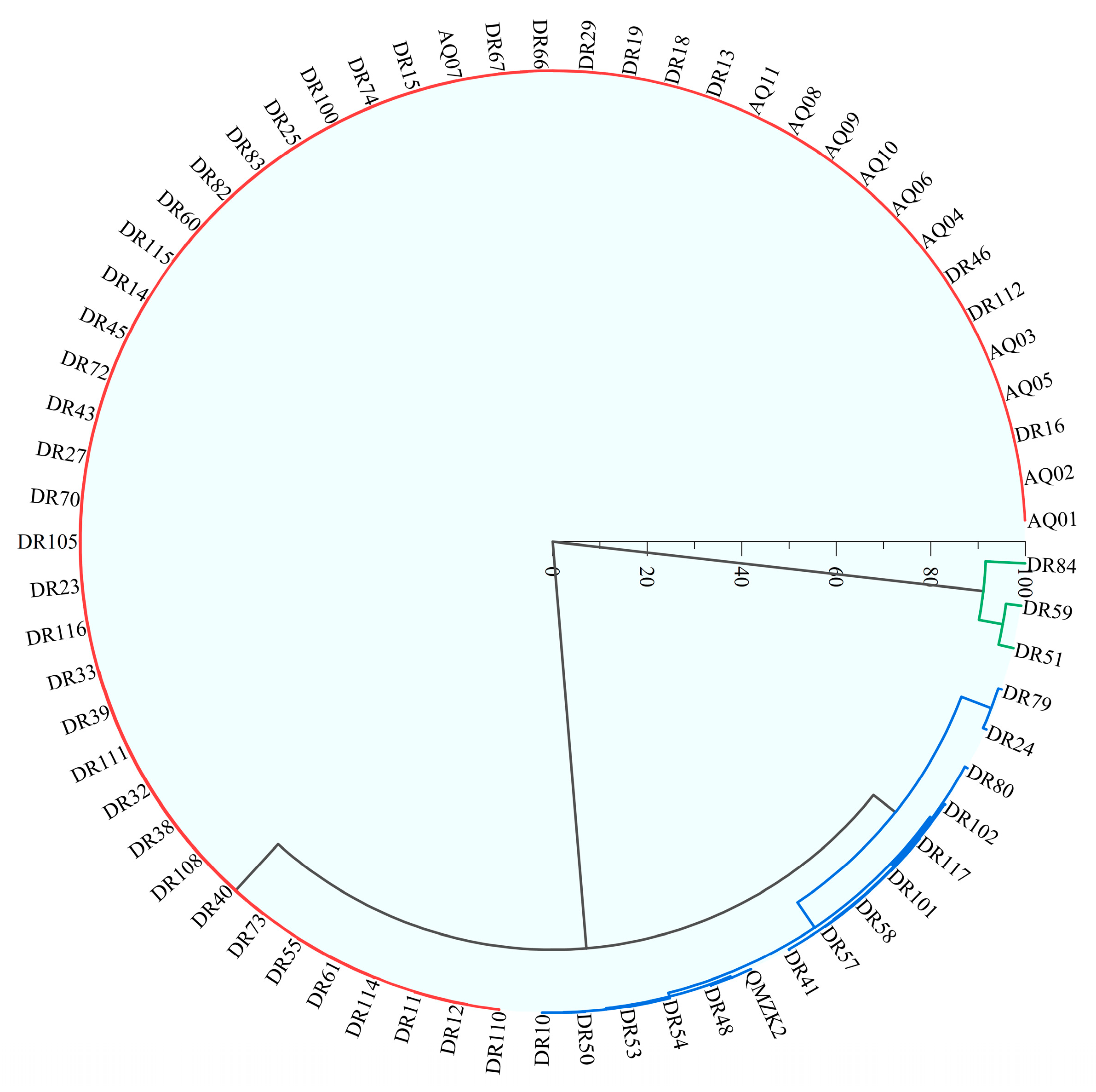
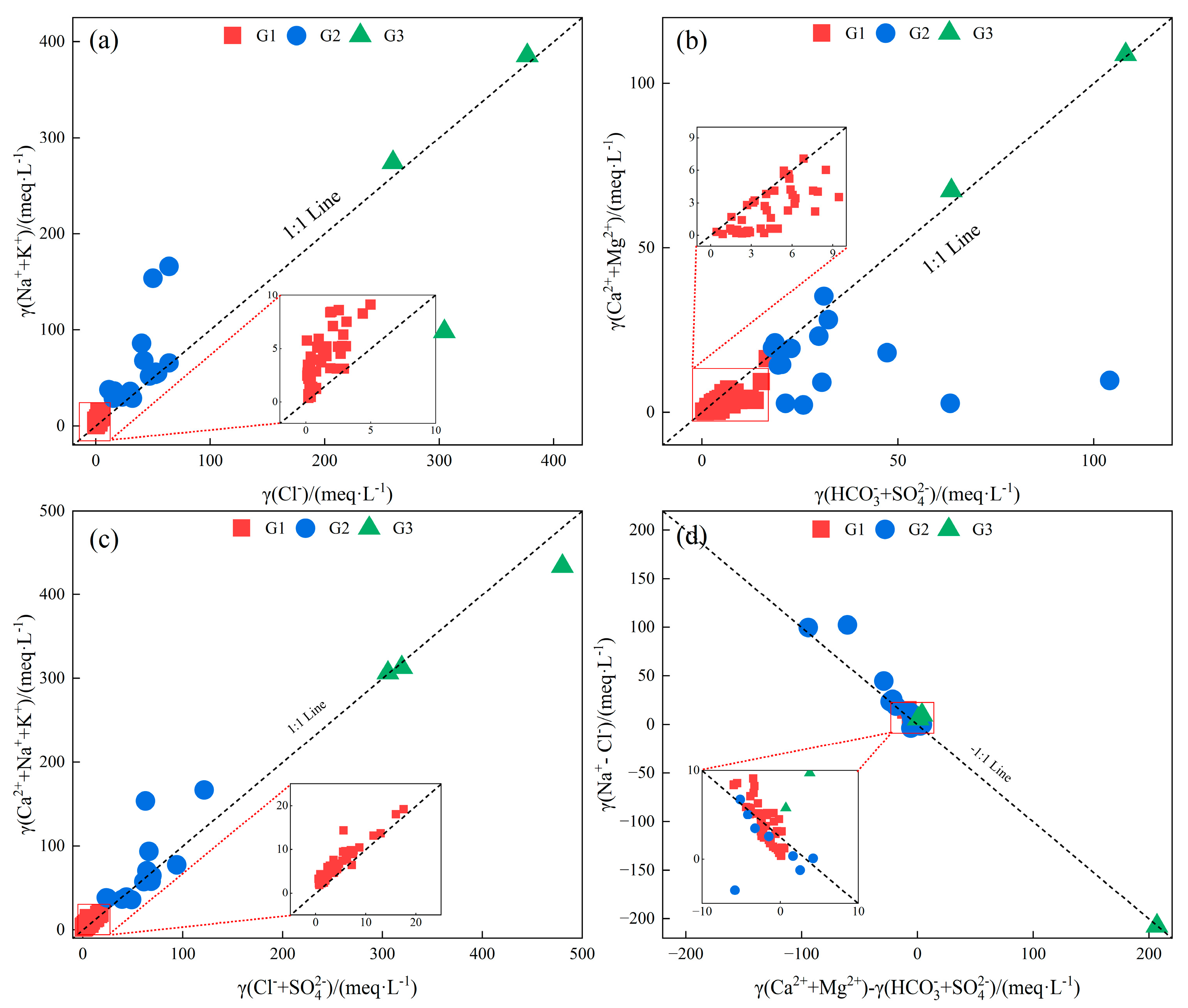
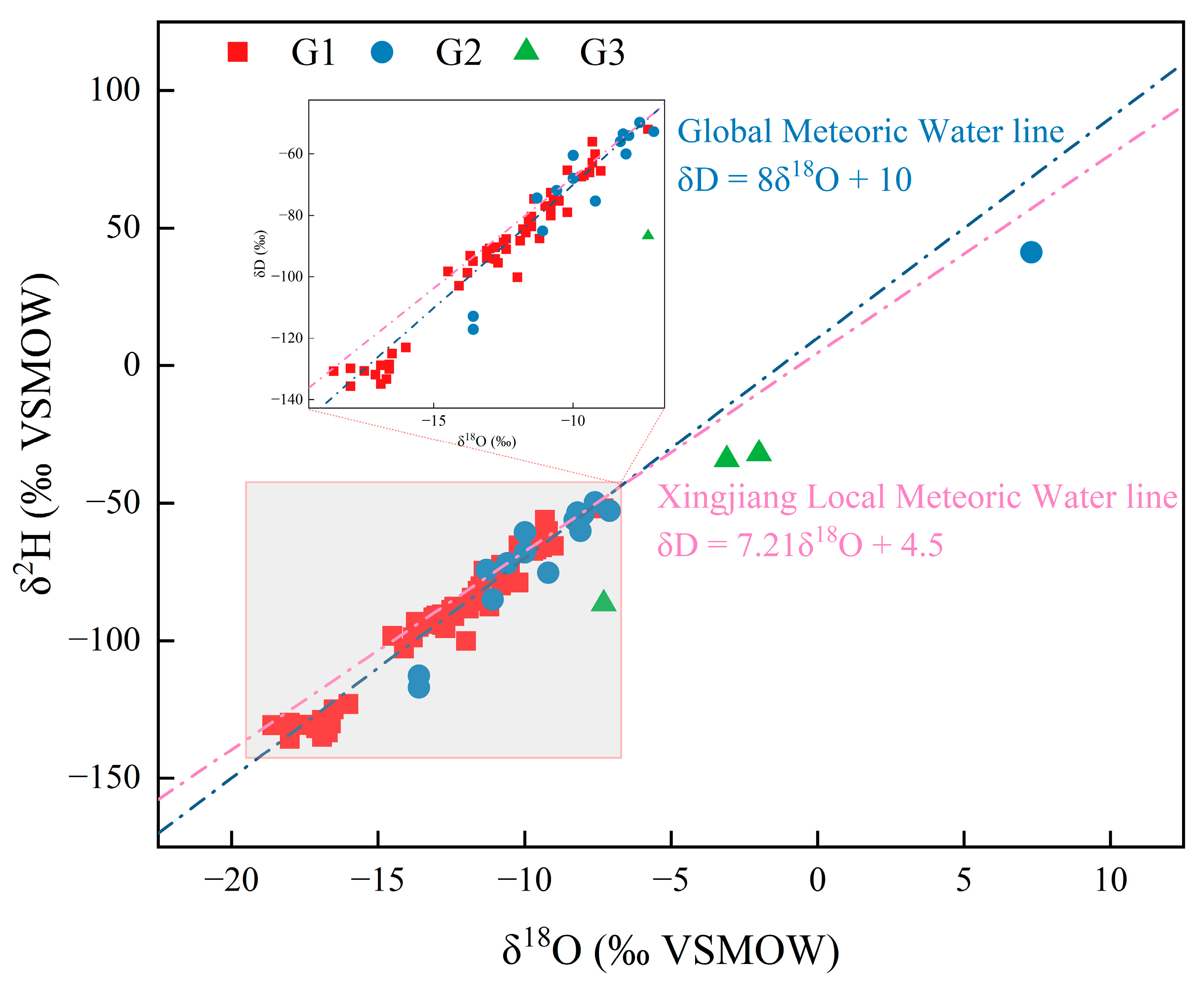
| Statistical Value | pH | K++Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3−+CO32− | TDS |
|---|---|---|---|---|---|---|---|---|
| Max | 9.95 | 8919.7 | 4168.3 | 729.0 | 13,382.4 | 4947.1 | 4485.6 | 29,094.2 |
| Min | 7.27 | 8.6 | 1.2 | 0 | 3.5 | 4.8 | 15.2 | 111.2 |
| Mean | 8.53 | 652.9 | 143.7 | 53.2 | 825.7 | 436.6 | 308.1 | 2279.0 |
| CV/% | 5.81 | 222 | 357 | 209 | 282 | 180 | 220 | 213 |
| Region | Dominant Water Chemistry Type (*n = Number of Samples) |
|---|---|
| Ili | SO4·HCO3·Cl-Na·Ca*4, SO4·Cl-Na·Ca*2, SO4·Cl-Na*2, SO4·Cl-Na*2, HCO3-Na, SO4-Na·Ca, SO4·HCO3-Na, SO4·HCO3-Ca·Mg |
| Altay | SO4·HCO3-Na*4, HCO3-Na·Ca |
| Tacheng | SO4·Cl-Na, SO4-Na, HCO3·SO4-Ca, CO3-Na |
| Bozhou | SO4-Na*2, SO4·HCO3-Na |
| Changji | SO4·HCO3·Cl-Na·Ca, Cl·CO3-Na, Cl-Na, SO4·HCO3-Ca, Cl-Ca·Na, SO4·HCO3-Ca, SO4·HCO3-Na |
| Urumqi | HCO3·Cl·CO3-Na |
| Turpan | HCO3·SO4-Na·Ca |
| Bazhou | SO4·Cl-Na·Ca*2, Cl·SO4-Na*2, Cl-Na |
| Aksu | HCO3-Ca, HCO3·Cl-Na, SO4·HCO3-Na·Ca·Mg, SO4·Cl-Na, SO4·Cl-Na |
| Kezhou | Cl-Na*4, SO4·HCO3-Na*2, SO4·HCO3-Na·Ca, Cl·SO4·HCO3-Na, HCO3·Na·Ca, Cl·SO4-Na, Cl·SO4·HCO3-Na, HCO3-Ca·Mg |
| Kashgar | SO4·HCO3-Na*3, SO4·HCO3-Na·Ca*2, Cl·SO4·HCO3-Na, Cl·SO4-Na, SO4-Na·Ca, SO4-Na, HCO3-Mg, HCO3-Na |
| Hotan | Cl·SO4·HCO3-Na, Cl-Na, SO4·HCO3-Ca·Mg |
| Grouping Details | K++Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− + CO32− | TDS | pH | |
|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Min | 8.60 | 1.20 | 0.00 | 3.50 | 4.80 | 15.20 | 111.20 | 7.67 |
| Max | 349.10 | 210.40 | 69.30 | 189.70 | 629.20 | 701.70 | 1219.60 | 9.95 | |
| Mean | 102.14 | 3568 | 10.10 | 49.13 | 134.21 | 138.85 | 405.92 | 8.48 | |
| CV (%) | 71 | 109 | 148 | 96 | 100 | 82 | 67 | 5 | |
| Group 2 | Min | 669.30 | 0.00 | 9.70 | 407.70 | 417.90 | 67.10 | 2266.75 | 7.34 |
| Max | 3859.70 | 438.90 | 279.40 | 2268.80 | 2761.70 | 4485.60 | 10,802.60 | 9.34 | |
| Mean | 1481.42 | 145.36 | 103.60 | 1403.82 | 1029.13 | 904.23 | 4695.74 | 8.09 | |
| CV (%) | 66 | 80 | 63 | 44 | 58 | 144 | 51 | 6 | |
| Group 3 | Min | 2275.10 | 781.60 | 0.00 | 9217.00 | 38.40 | 51.90 | 17,319.95 | 7.27 |
| Max | 8919.70 | 4168.30 | 729.00 | 13,382.40 | 4947.10 | 308.20 | 29,094.20 | 7.76 | |
| Mean | 5858.00 | 1970.60 | 356.40 | 11,137.20 | 2614.43 | 203.43 | 22,038.35 | 7.52 | |
| CV (%) | 57 | 97 | 102 | 19 | 94 | 66 | 28 | 3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, D.; Li, Y.; Qi, Z.; Qi, X.; Ma, C. Genetic Mechanism of Geothermal Water in Typical Structural Belts from the Altay and Tianshan to the Kunlun Mountains in Xinjiang: Evidence from Hydrogeochemistry and δ2H–δ18O Isotopes. Water 2025, 17, 2946. https://doi.org/10.3390/w17202946
Hu D, Li Y, Qi Z, Qi X, Ma C. Genetic Mechanism of Geothermal Water in Typical Structural Belts from the Altay and Tianshan to the Kunlun Mountains in Xinjiang: Evidence from Hydrogeochemistry and δ2H–δ18O Isotopes. Water. 2025; 17(20):2946. https://doi.org/10.3390/w17202946
Chicago/Turabian StyleHu, Dongqiang, Yanjun Li, Zhilon Qi, Xinghua Qi, and Changqiang Ma. 2025. "Genetic Mechanism of Geothermal Water in Typical Structural Belts from the Altay and Tianshan to the Kunlun Mountains in Xinjiang: Evidence from Hydrogeochemistry and δ2H–δ18O Isotopes" Water 17, no. 20: 2946. https://doi.org/10.3390/w17202946
APA StyleHu, D., Li, Y., Qi, Z., Qi, X., & Ma, C. (2025). Genetic Mechanism of Geothermal Water in Typical Structural Belts from the Altay and Tianshan to the Kunlun Mountains in Xinjiang: Evidence from Hydrogeochemistry and δ2H–δ18O Isotopes. Water, 17(20), 2946. https://doi.org/10.3390/w17202946






