Abstract
Chaetoceros muelleri and Isochrysis zhanjiangensis, known for their rapid reproduction, small size, and rich nutritional content, are commonly used as feed microalgae in aquaculture. This study aimed to sterilize these microalgal species and assess the effects of antibiotics on their algal cell density. Phycospheric bacteria were isolated and identified using the spread plate method and 16S rDNA sequencing, and antibiotic susceptibility tests were conducted using four antibiotics: ampicillin, streptomycin, kanamycin, and gentamicin sulfate. A sterile system was established for C. muelleri using ampicillin, streptomycin, and gentamicin, and for I. zhanjiangensis using kanamycin, ampicillin, and streptomycin. Based on the results, antibiotics with sterilization effects were selected and added to the algal cultures. Their effects on cell density were evaluated during a six-day co-culture. Ampicillin and streptomycin effectively inhibited bacteria associated with C. muelleri, initially increasing algal cell density but later causing a decline. For I. zhanjiangensis, kanamycin and ampicillin were effective, with kanamycin significantly promoting growth throughout the cycle, achieving a 36.92% higher cell density on day six (p < 0.05).
1. Introduction
Microalgae, as primary producers, play a crucial role in global ecosystems, converting solar energy into chemical energy through photosynthesis and producing oxygen, which is essential for the survival of other organisms. Their remarkable biodiversity and adaptability enable them to thrive in various extreme environments, making them a focal point of extensive research and applications across multiple fields in recent years.
In the realm of biofuels, microalgae are promising candidates for sustainable energy production because of their high photosynthetic efficiency, rapid growth rates, and abundant lipid content [1]. Microalgae contribute to bioremediation and water purification by absorbing and degrading pollutants through biosorption [2]. Additionally, their ability to capture carbon dioxide during photosynthesis helps to mitigate greenhouse gas emissions, playing an essential role in addressing global warming and restoring aquatic ecosystems.
Microalgae, characterized by their small cell size suitable for engulfment and easily digestible cell walls, provide significant advantages for predation and digestion by aquatic animals. Studies have highlighted that microalgae are rich sources of polyunsaturated fatty acids (PUFAs), which are critical for maintaining optimal growth rates and reproductive performance in aquatic species [3]. Furthermore, the carotenoids present in microalgae have been shown to enhance the survival rate and overall antioxidant capacity of Artemia nauplii [4]. Microalgal proteins exhibit the potential to replace fishmeal-derived proteins in compound aquafeeds. Comprehensive analyses of 40 microalgae species reveal that many exhibit a similar amino acid composition, enriched with essential amino acids [5]. Additionally, microalgae contain various vital vitamins, including tocopherols, ascorbic acid, B1, B2, B3, B6, B9, B12, folic acid, pantothenic acid, nicotinic acid, inositol, and biotin, which contribute to the healthy growth of aquatic animals when used as feed [6].
Given these diverse applications, in-depth research into the biochemistry, physiology, and biotechnology of microalgae is fundamental for advancing their potential uses. However, the presence of alga-associated bacteria in microalgal environments introduces complex interactions, including symbiosis, parasitism, and competition [7], which can significantly influence algal cell growth, lipid accumulation, chlorophyll production, and protein levels [8]. Axenic (bacteria-free) cultivation methods that eliminate bacterial interference are essential to the accurate evaluation of the true application potential of microalgae and effective preservation of algal species.
This study focuses on two marine microalgal species widely used as feeds in the aquaculture industry: Chaetoceros muelleri (Mediophyceae) and I. zhanjiangensis (Coccolithophyceae). C. muelleri is a marine diatom noted for its high protein and saturated fatty acid content, rapid reproduction, and high-temperature tolerance [9], it is commonly used as feed during the mid-to-late stages of fish, shrimp, and shellfish aquaculture. I. zhanjiangensis is a unicellular alga of high economic value and characterized by its small size and lack of a cell wall, serving as an ideal initial feed for fish, shrimp, and shellfish [10]. Establishing effective axenic cultivation methods for these species is crucial for studying their nutritional components and bioactive substances and for investigating the effects of alga-associated bacteria on aquatic animals.
Antibiotics are commonly employed to control microbial contamination, inhibiting the proliferation of epiphytic bacteria in microalgal cultures. However, their effects on microalgae can be detrimental. For instance, Huang et al. successfully obtained axenic Isochrysis zhangjiangensis cultures with a combination of streptomycin, kanamycin, and gentamicin, which promote algal growth [11]. Similarly, Wang Lingling et al. achieved axenic Dunaliella (Chlorophyta) strains through sequential treatment with ampicillin, cefuroxime, and gentamicin; the strains were stable and had prolonged growth periods [12]. Conversely, Wen et al. found that gentamicin sulfate enhanced the growth of Chlorella (Chlorophyta) cells and ceftazidime, whether used alone or in combination with gentamicin sulfate, reduced algal growth rates [13]. These findings underscore the complexity of axenic treatments, as different microalgae exhibit varying levels of sensitivity to antibiotics and the inhibitory effects on epiphytic bacteria differ accordingly.
Therefore, this study aims to systematically explore effective axenic treatment methods for C. muelleri and I. zhanjiangensis and to evaluate the effects of various antibiotics with antibacterial properties on their growth. The results are expected to provide a scientific foundation for optimizing microalga cultivation strategies in the aquaculture industry and enhancing the quality and yield of microalga-based feeds. Moreover, this study will offer theoretical insights and technical support for the advancement of microalgal biotechnology, promoting the efficient utilization and industrialization of microalgal resources and contributing to the sustainable development of aquaculture and the protection of marine ecological environments.
2. Materials and Methods
2.1. Microalgal Strains and Culture Conditions
The marine microalgal strains C. muelleri and I. zhanjiangensis were obtained from the Algae Workshop of Tianjin Haisheng Aquaculture Co., Ltd., Tianjin, China. The strains were cultured in NMB3# medium, which consisted of KNO3 (100 g/L), KH2PO4 (10 g/L), FeCl3 (2 g/L), EDTA-Na2 (20 g/L), vitamin B1 (0.06 g/L), and vitamin B12 (0.01 g/L). The medium was prepared using artificial seawater with a salinity of 23 PSU. The cultures were maintained at 26 °C under a 12 h light/12 h dark cycle with a light intensity of 3000 lux. The cultures were manually shaken twice daily. All experimental procedures were performed under sterile conditions on a clean bench.
2.2. Antibiotics and Working Concentrations
The antibiotics used in this study were ampicillin, streptomycin, kanamycin, and gentamicin sulfate. The concentration gradients for each antibiotic were set at 0, 0.1, 0.25, 0.5, 1, and 5 mg/mL, based on previously published methodologies [14,15,16]. These specific concentrations were chosen to comprehensively evaluate their antibacterial effects.
2.3. Isolation and Identification of Phycospheric Bacteria
Single-bacterium colonies were obtained using the gradient dilution plate method. Initially, C. muelleri and I. zhanjiangensis were cultured separately in the NMB3# medium until they reached the logarithmic growth phase. The cultures were then diluted with sterile seawater (1:2), and the resulting solution was spread onto 2216E marine agar plates (No. HB0132, Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China). The plates were incubated at 26 °C for 5 days until single colonies became visible. Bacterial colonies exhibiting distinct phenotypes were isolated and streaked onto fresh 2216E marine agar plates three times, with each streaking followed by a 5-day incubation period at 26 °C. The purified strains were subjected to gram staining, and the results were examined under a microscope.
For molecular identification, the colonies of purified bacterial strains were suspended in sterile water to serve as templates for PCR amplification. Amplification was carried out using universal 16S rDNA primers [17] (16S-F: AGAGTTTGATCCTGGCTCA, 16S-R: GGCTACCTTGTTACGACTT). The PCR reaction mixture (total volume of 50 µL) contained 1 µL of 16S-F, 1 µL of 16S-R, 22 µL of double-distilled water, 1 µL of cDNA template, and 25 µL of PrimeSTAR Max Premix. The PCR conditions were as follows: initial denaturation at 94 °C for 3 min, followed by 32 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 5 s, and extension at 72 °C for 10 s, with a final extension at 72 °C for 7 min and storage at 12 °C. The PCR products were separated by electrophoresis on a 2% agarose gel at 120 V for 20 min. Products approximately 1500 bp in size were selected for sequencing, which was conducted by Shanghai Shenggong Bioengineering Co., Ltd., Shanghai, China. The resulting 16S rDNA sequences were compared using BLAST on NCBI, and sequences with over 98% similarity were selected. Phylogenetic trees were constructed using the neighbor-joining method in MEGA 11.0 software, and bootstrap analysis was performed using 1000 replicates.
2.4. Antibiotic Sensitivity Testing of Phycospheric Bacteria
The isolated phycospheric bacterial colonies were cultured overnight in 2216E liquid medium (No. HB0132-1, Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China) at 26 °C with shaking at 180 rpm. The following day, the bacterial cultures were spread onto 2216E solid medium plates, which were divided into six equal sections. Antibiotic sensitivity discs (6 mm in diameter) were soaked in different concentrations of antibiotics. Each plate was dedicated to a single antibiotic, and five different concentrations were tested across the sections. Each concentration was tested in triplicate, and discs soaked in sterile water served as blank controls. The plates were incubated at 26 °C for 48 h, and then the diameters of the inhibition zones were measured.
2.5. Establishment of a Sterile System
Under sterile conditions, logarithmic-phase algal cultures were inoculated into 250 mL Erlenmeyer flasks at a 25% inoculation rate. The total culture volume was 200 mL. C. muelleri had an initial density of 1.65 × 106 cells/mL, and I. zhanjiangensis had an initial density of 3.44 × 106 cells/mL. After 24 h of cultivation, antibiotics with sterilizing effects, selected based on the results in Section 2.2 and Section 2.4, were added to the C. muelleri cultures. The experimental groups were divided into two: group A received 7 mL of 10 mg/mL ampicillin, and group B received 7 mL of 25 mg/mL streptomycin. Similarly, I. zhanjiangensis treatments were divided into group C, which received 7 mL of 25 mg/mL kanamycin, and group D, which received 7 mL of 10 mg/mL ampicillin. Each group included three replicates.
2.6. Sterility Testing and Antibiotic Removal
Sterility testing was performed on the algal cultures 1 and 5 days after antibiotic addition. A 150 μL aliquot of the algal culture was spread on plates and incubated at 28 °C for 48 h. Each group included three replicates. Cultures without colonies were deemed sterile. The interference of antibiotics in subsequent experiments and residual antibiotic effects were prevented by culturing the sterile algal cultures three times. The purified sterile algae were then used in the subsequent experiments.
2.7. Effects of Antibiotics on Microalgae Growth
Antibiotics with sterilization effects were selected based on the results of Section 2.4 and Section 2.5. Under sterile conditions, logarithmic-phase algal cultures were inoculated into 250 mL Erlenmeyer flasks at a 25% inoculation rate. The total culture volume was 200 mL. C. muelleri had an initial density of 1.53 × 106 cells/mL, and I. zhanjiangensis had an initial density of 3.37 × 106 cells/mL. The selected antibiotics were added to the algal cultures as experimental groups, with the additional amount being the effective sterilization dose. The control group received an equal volume of sterile water. Each group included three replicates. The cultivation lasted for 6 days. Algal cell density was measured every 2 days using a hemocytometer.
2.8. Data Analysis
All data were analyzed using one-way analysis of variance performed on SPSS 27.0.1, and results were presented as mean ± SD (n = 3). Differences were considered statistically significant when the p value was less than 0.05. The results were plotted using Origin 2024 software.
3. Results
3.1. Isolation of Phycospheric Bacteria
The following phycospheric bacteria were successfully isolated using the streak plate method: C. muelleri strains M01, M02, M03, and M04 and I. zhanjiangensis strains J01 and J02. The characteristics of these bacterial colonies are presented in Figure 1 and Table 1.
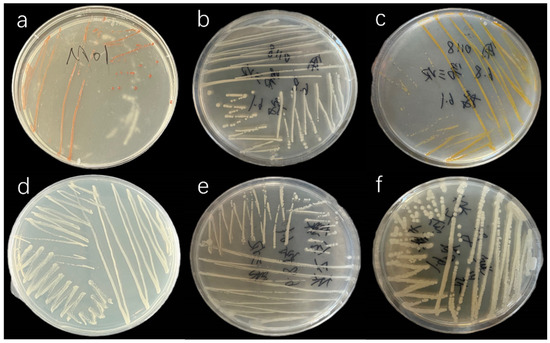
Figure 1.
Colony morphology of phycosphere bacteria: (a) M01, (b) M02, (c) M03, (d) M04, (e) J01, (f) J02.

Table 1.
Characteristics of bacterial colonies in the phycosphere.
3.2. Identification of Phycospheric Bacteria Through 16S rDNA Sequencing
The amplified 16S rDNA fragments were approximately 1400 bp in length. After sequencing, the results were assembled and analyzed with BLAST on the NCBI website. The identification results are summarized in Figure 2. Strain M01 was classified as Algoriphagus marincola with a sequence similarity of 99.72%, strains M02 and J01 were identified as Halomonas piezotolerans with a similarity of 99.58%, strain M03 was grouped with Flagellimonas marinaquae with a similarity of 99.51%, strain M04 matched Ponticoccus alexandrii with a similarity of 99.71%, and strain J02 was identified as Alteromonas abrolhosensis with a similarity of 99.15%.
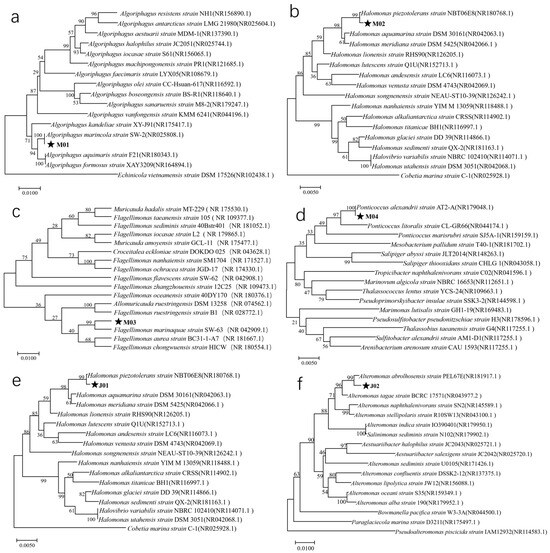
Figure 2.
Construction of a phylogenetic tree of the phycosphere bacteria based on the 16S rDNA gene sequence. (a), (b), (c), (d), (e) and (f) correspond to strains M01, M02, M03, M04, J01, and J02, respectively.
In total, four alga-associated bacterial species were isolated from C. muelleri: A. marincola, H. piezotolerans, F. marinaquae, and P. alexandrii. Additionally, two species were isolated from I. zhanjiangensis: H. piezotolerans and A. abrolhosensis.
3.3. Sensitivity of Phycospheric Bacteria to Antibiotics
The sensitivity of various phycospheric bacteria to different antibiotics is presented in Table 2. We tested varying concentrations for each antibiotic, and the final results indicated that all antibiotics exhibited the most effective antibacterial activity at a concentration of 5 mg/mL. Ampicillin exhibited strong inhibitory effects on strains M01 and M03. Streptomycin showed inhibitory activity against all the tested phycospheric bacteria. Kanamycin was effective against bacteria isolated from I. zhanjiangensis and inhibited C. muelleri strains M01 and M04. Additionally, gentamicin sulfate demonstrated inhibitory effects on all phycospheric bacteria from I. zhanjiangensis and inhibited C. muelleri strains M03 and M04.

Table 2.
Antibiotic sensitivity testing of phycosphere bacteria.
3.4. Algal Sterilization Treatment Results
After 48 h of plate culture with antibiotic-treated algal solutions, the inhibition effects were observed, which are illustrated in Figure 3. In C. muelleri group A, despite the addition of ampicillin, strains M02 and M04 persisted in substantial numbers. In group B, streptomycin was only effective in eliminating strain M01. In I. zhanjiangensis group C, kanamycin failed to effectively eliminate the bacteria. Similarly, in group D, strain J01 was still observed after the addition of ampicillin.
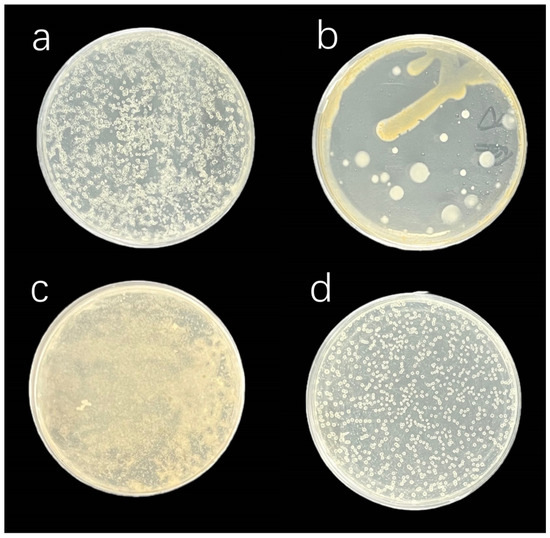
Figure 3.
Antibacterial effects of individual antibiotics. (a,b) show the addition of ampicillin and streptomycin to C. muelleri, respectively, whereas (c,d) illustrate the addition of kanamycin and ampicillin to I. zhanjiangensis, respectively.
In view of these observations and the sensitivity of the bacteria to various antibiotics, a combined treatment using ampicillin, streptomycin, kanamycin, and gentamicin sulfate was applied to the algal solutions of C. muelleri and I. zhanjiangensis, as detailed in Table 3. The selected antibiotics were continued through the first and second generations after subculturing. The final inhibition results (Figure 4) showed that no bacterial colonies were observed in C. muelleri group A or I. zhanjiangensis group A. These two sterile algal solutions were used as parental strains for three generations of subculturing, during which no bacterial colonies were observed. Finally, sterile systems were successfully established.

Table 3.
Sterilization and results for microalgae.
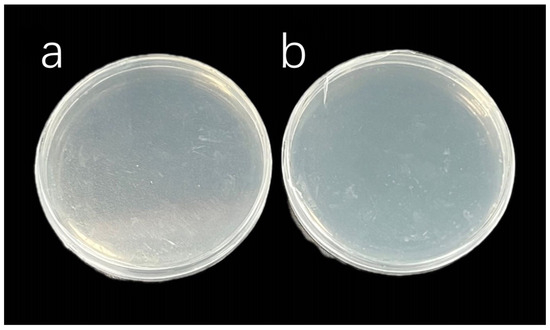
Figure 4.
Antibacterial effects after supplementing with additional antibiotics. (a,b) respectively show the sterilization results for C. muelleri and I. zhanjiangensis.
3.5. Results of the Effects of Antibiotics on Microalgae Growth
Based on the sensitivity of different phycospheric bacteria to various antibiotics, ampicillin and streptomycin were selected for the sterilization of C. muelleri, whereas kanamycin and ampicillin were selected for I. zhanjiangensis.
The effects of these antibiotics on the growth of the C. muelleri are illustrated in Figure 5. Ampicillin and streptomycin initially promoted the growth of C. muelleri, increasing cell density during the first four days. However, by day 6, both antibiotics exhibited an inhibitory effect. The ampicillin-treated group exhibited an algal cell density of 3.27 ± 0.008 × 106 cells/mL, representing a nonsignificant reduction of 5.39% compared to the control group (p > 0.05). In contrast, the streptomycin-treated group showed a significant decrease in algal cell density, measuring 3.02 ± 0.004 × 106 cells/mL, which was 12.55% lower than that of the control group (p < 0.05).
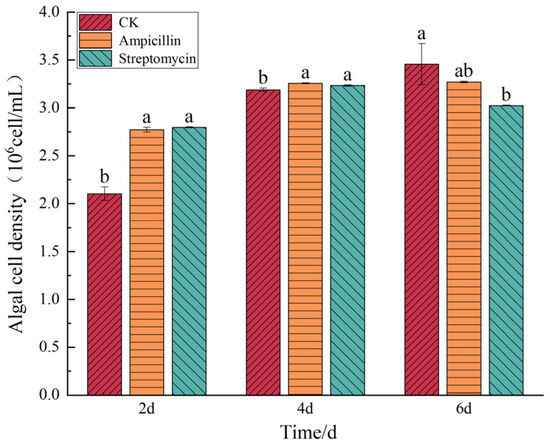
Figure 5.
Effect of antibiotics on the cell density of C. muelleri. Note: Different lowercase letters indicate significant differences between treatment groups at the same time point (p < 0.05).
The effects of these antibiotics on the growth of the I. zhanjiangensis are illustrated in Figure 6. The effects of kanamycin and ampicillin on the growth of I. zhanjiangensis varied. Kanamycin consistently promoted cell growth throughout the entire growth cycle, with a cell density of 1.25 ± 0.002 × 107 cells/mL on the sixth day, which was 36.91% higher than that of the control group (p < 0.05). By contrast, ampicillin did not significantly enhance the growth of the microalgae.
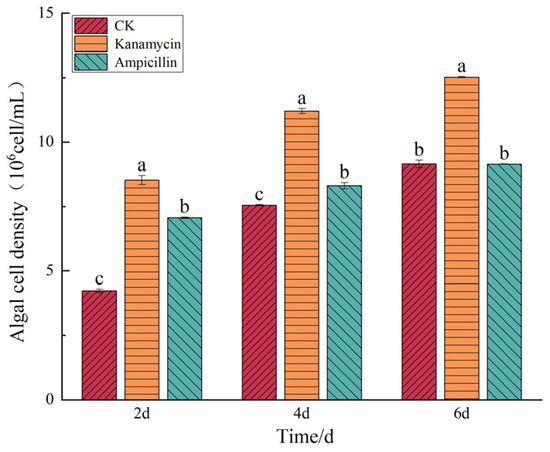
Figure 6.
Effect of antibiotics on the cell density of I. zhanjiangensis. Note: Different lowercase letters indicate significant differences between treatment groups at the same time point (p < 0.05).
4. Discussion
4.1. Composition of Phycospheric Bacteria in Two Microalgal Species
During the subculturing of microalgae, phycospheric bacteria coexist with the microalgae over extended periods, mutually influencing each other’s physiological states and metabolic activities. Algae convert solar energy into chemical energy through photosynthesis, synthesizing organic compounds [18]. Concurrently, bacteria decompose excess organic matter in the algal environment through various metabolic pathways, thereby promoting their own proliferation [19]. The composition of these bacterial communities varies depending on the species of microalgae, and interactions between different bacteria and microalgae include symbiosis, commensalism, and competition.
In this study, the alga-associated bacteria of C. muelleri and I. zhanjiangensis were isolated and purified. 16S rDNA identification revealed five distinct bacterial types and unique bacterial communities associated with each microalga. C. muelleri harbored four bacterial species: A. marincola, H. piezotolerans, F. marinaquae, and P. alexandrii. By contrast, I. zhanjiangensis was associated with two bacterial species: H. piezotolerans and A. abrolhosensis. Notably, H. piezotolerans was present in both microalgal environments, indicating its strong adaptability and resilience across different algal habitats.
A. marincola belongs to the class Cytophagia and family Cyclobacteriaceae. The genus Algoriphagus enhances the thermotolerance of microalgae, such as I. zhangjiangensis, by increasing antioxidant enzyme activities (SOD, POD, and CAT) and upregulating heat shock protein (HSP) gene expression [20]. H. piezotolerans is a Gram-negative, moderately halophilic, and piezotolerant-bacterium-resistant to oxidative stress and heavy metals. The genus Halomonas (family Halomonadaceae, class Gammaproteobacteria) utilizes diverse carbon and nitrogen sources. Genomic analyses reveal multiple toxin–antitoxin systems and a strain-specific type VI secretion system, facilitating adaptation to oligotrophic hadal environments [21]. F. marinaquae, a Gram-negative, aerobic, non-motile, rod-shaped bacterium, belongs to the genus Flagellimonas. The type species of this genus is F. eckloniae. F. maritima forms orange-colored colonies (~1 mm in diameter) on marine agar after incubation at 30 °C for 3 days [22]. P. alexandrii, a member of the Roseobacter clade, belongs to the family Rhodobacteraceae, order Rhodobacterales, and class Alphaproteobacteria. It is aerobic, Gram-negative, and non-spore-forming, forming cream-colored colonies on marine agar after 2 days of incubation at 28 °C [23]. A. abrolhosensis is a Gram-negative, rod-shaped, motile, and aerobic bacterium. Its colonies on marine agar are cream-colored, smooth, and circular after 48 h of incubation at 30 °C [24].
However, the results of this experiment differ from those obtained by Zhang Xiuxia et al., who isolated Muricauda lutimaris, Bacillus oceanisediminis, and Marinobacter salsuginis from C. muelleri [8]. These discrepancies may be due to differences in cultivation conditions.
4.2. Sterilization of Two Microalgal Species
The algal environment is highly complex, comprising various types of bacteria that differ depending on microalgal species. The diversity and abundance of these bacteria influence the effectiveness of microalgal sterilization. Owing to interactions between microalgae and bacteria, polysaccharides secreted by microalgae can encapsulate bacteria, forming a protective sheath that shields the bacteria from antibiotics [25]. This symbiotic relationship complicates the elimination of phycospheric bacteria, and a single antibiotic treatment is insufficient to completely remove them.
Han et al. produced five sterile algal strains by using a combination of ampicillin, gentamicin sulfate, kanamycin, neomycin, and streptomycin [15]. Similarly, Liu et al. achieved the complete sterilization of Chrysotila roscoffensis (Coccolithophyceae) with a combination of kanamycin, ampicillin, streptomycin, and gentamicin sulfate [26].
In this experiment, multiple antibiotics were applied to the C. muelleri culture, and successful sterilization was achieved with a combination of ampicillin, streptomycin, and gentamicin. The sterilization of I. zhanjiangensis was achieved with a combined treatment of kanamycin, ampicillin, and streptomycin.
Microalgae inherently possess the ability to purify water and eliminate antibiotics. In the study by Kiki et al., microalgae were shown to exhibit adsorption and bioaccumulation patterns toward antibiotics. Specifically, the bioaccumulation rate of macrolide antibiotics in Chlorella vulgaris was 4–5%, and Haematococcus lacustris (Chlorophyta) demonstrated the fastest degradation rate of antibiotics [27]. Scenedesmus quadricauda and Tetraselmis chui (Chlorophyta) exhibited significant adsorption capacities for tetracycline in dairy wastewater [28]. Consequently, the adsorption and degradation of antibiotics by microalgae complicate the direct removal of epiphytic bacteria during sterilization, necessitating prolonged and repeated antibiotic treatments.
In this experiment, C. muelleri and I. zhanjiangensis were subjected to multiple rounds of antibiotic treatments, and sterility was confirmed using the spread plate method. Ultimately, sterile algal strains were obtained, and the effects of antibiotics were eliminated through three successive subcultures. The entire process spanned two months, resulting in the stable growth of sterile C. muelleri and I. zhanjiangensis.
4.3. Effects of Antibiotics on the Cell Density of the Two Microalgal Species
The susceptibility of five types of phycospheric bacteria to various antibiotics was assessed. Ampicillin and streptomycin inhibited bacteria associated with C. muelleri, whereas kanamycin and ampicillin were effective against bacteria associated with I. zhanjiangensis. In view of these results, the selected antibiotics were used for the sterilization of the two microalgal species.
Different antibiotics had varying impacts on the growth of the two microalgae likely because of the elimination of either beneficial or harmful bacteria within the algal environment and thus influenced microalgal growth [29]. Antibiotics can not only alter the algal environment but also affect the physiological and biochemical composition of the microalgae [30]. Zhou Wenli et al. demonstrated that ampicillin can expose the algal cell membrane, leading to cell death [31]. Consequently, the cell density of C. muelleri and I. zhanjiangensis decreased in the later stages after the addition of ampicillin likely due to its destructive effects on algal cell structure.
Streptomycin, which is commonly used for sterilization in marine microalgae, initially increased the cell density of C. muelleri but later decreased it, eventually resulting in a density lower than that of the control group. Huang Jian et al. found that streptomycin has a toxic stimulatory effect on Chaetoceros and promotes its growth [32]. Similarly, Zhou Jing yun et al. reported that glyphosate isopropylamine salt significantly increased the cell density and chlorophyll-a content of Heterosigma akashiwo (Raphidophyceae) [33]. However, as culture time extended, factors such as pesticide degradation and microalgal adaptability restored the physiological state of the algal cells to normal levels.
Therefore, as external agents in the algal environment, antibiotics may exhibit a toxic stimulatory effect on microalgal growth. Over time, as antibiotics gradually degrade, the cell density of C. muelleri ceases to increase. Gentamicin sulfate increases the concentration of Chlorella; this effect indicates that antibiotics affect the fatty acid and amino acid concentrations of algal cells [13]. However, in the present study, the cell density of the algal cells decreased in the later stages owing to nutrient depletion in the algal solution or the degradation of the antibiotics themselves.
Wang Hongbin et al. observed that 200 μg/mL kanamycin promoted the growth of Diacronema viridis (Pavlovophyceae), and low concentrations of kanamycin enhanced the growth of Karenia mikimotoi (Dinophyceae) [34]. Liu et al. found that under high nitrogen conditions, amoxicillin enhanced the growth of Microcystis aeruginosa (Cyanophyceae) by participating in photosynthesis [35]. Consistent with these results, the present study showed that kanamycin promoted the growth of I. zhanjiangensis.
5. Conclusions
Five strains of alga-associated bacteria were isolated and purified from two microalgal species. Through 16S rDNA sequencing, the following bacteria were isolated from C. muelleri: M01 A. marincola, M02 H. piezotolerans, M03 F. marinaquae, and M04 P. alexandrii. From I. zhanjiangensis, the bacteria isolated were J01 H. piezotolerans and J02 A. abrolhosensis. The combination of ampicillin, streptomycin, and gentamicin was used in obtaining a sterile strain of C. muelleri, and I. zhanjiangensis was sterilized using the combined treatment of kanamycin, ampicillin, and streptomycin. On this basis, ampicillin and streptomycin with sterilization effects were selected and co-cultured with C. muelleri separately. Similarly, kanamycin and ampicillin were separately co-cultured with I. zhanjiangensis. Ampicillin and streptomycin exerted inhibitory effects on the cell density of C. muelleri, whereas kanamycin had a promoting effect on the cell density of I. zhanjiangensis.
Author Contributions
J.H.: investigation, formal analysis, software, visualization, and writing—original draft; S.L.: conceptualization, formal analysis, resources, and funding acquisition; H.L. and Y.L.: formal analysis; X.W.: resources; Y.G.: breeding and cultivation of materials; J.L.: conceptualization, project administration, formal analysis, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2023YFD2400800), Earmarked Fund for CARS (CARS-49), National Natural Science Foundation of China (42076121), and Tianjin Science and Technology Project (24YDTPJC00820, 24ZYCGSN01210, 23YDTPJC00560, 23ZYCGSN00040).
Data Availability Statement
Data for this research article are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, J.; Rong, J.; Zong, B. Factors in Mass Cultivation of Microalgae for Biodiesel. Chin. J. Catal. 2013, 34, 80–100. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Boopathy, R. Effect of Algal Cells on Water Pollution Control. Curr. Pollut. Rep. 2021, 7, 213–226. [Google Scholar] [CrossRef]
- Jiang, X.; Han, Q.; Gao, X.; Gao, G. Conditions Optimising on the Yield of Biomass, Total Lipid, and Valuable Fatty Acids in Two Strains of Skeletonema menzelii. Food Chem. 2016, 194, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Xu, L.; Liu, Z.; Zhou, Z.; Sun, Z. Carotenoid-Rich Microalgae Promote Growth and Health Conditions of Artemia nauplii. Aquaculture 2022, 546, 737289. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in Aquafeeds for a Sustainable Aquaculture Industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- da Silva Vaz, B.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a New Source of Bioactive Compounds in Food Supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, L.; Zhang, Y. Algae-Bacteria Interactions and Their Ecological Functions in the Ocean. Microbiol. China 2018, 45, 2043–2053. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; He, J.; Deng, X. Isolation of bacteria in phycospher from Chaetoceros muelleri and its effect on growth and metabolism of algae. Feed Res. 2022, 45, 91–96. [Google Scholar] [CrossRef]
- Su, N.; Zhang, Y.; Yang, L.; Chen, H.; Gao, Q.; Chen, C.; Yang, Y. Effects of four microalgae on the survival and growth of Acanthaster spp. larvae. J. Appl. Oceanogr. 2023, 42, 633–642. [Google Scholar]
- Zhong, J.; Lv, X.; Li, G.; Fang, S.; Wang, Y. Mass Cultivation of Feed Organism for Amphioxus in Laboratory. Chin. Wild Plant Resour. 2006, 25, 44–47, 57. [Google Scholar]
- Huang, Z.; Liu, X.; Hu, Z.; Duan, S. Effects of antibiotics on the growth of Isochrysis zhangjiangensis and axenic culture. Ecol. Sci. 2007, 26, 120–121, 125. [Google Scholar]
- Wang, L.; Liu, X.; Ma, G.; Wang, L. Axenation of Dunaliella salina and the Effects of Axenic Cultivation on the Growth of the Strain. Anhui Agric. Sci. Bull. 2019, 25, 17–21. [Google Scholar] [CrossRef]
- Wang, W.; Sheng, Y. Gene Expression Concerning Fatty Acid and Amino Acid Metabolism in Chlorella vulgaris Cultured with Antibiotics. Appl. Microbiol. Biotechnol. 2020, 104, 8025–8036. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Choi, J.S.; Kong, I.S.; Park, S.I.; Kerr, R.G.; Hong, Y.K. A Procedure for Axenic Isolation of the Marine Microalga Isochrysis galbana from Heavily Contaminated Mass Cultures. J. Appl. Phycol. 2002, 14, 385–390. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhang, L.; Yang, G.; Zhao, L.; Pan, K. A Method of Batch-Purifying Microalgae with Multiple Antibiotics at Extremely High Concentrations. Chin. J. Oceanol. Limnol. 2016, 34, 79–85. [Google Scholar] [CrossRef]
- Joo, H.-N.; Lee, C.-G. Antibiotics Addition as an Alternative Sterilization Method for Axenic Cultures in Haematococcus pluvialis. J. Ind. Eng. Chem. 2007, 13, 110–115. [Google Scholar]
- Chen, J.; Xu, J.; Zhang, S.; Liu, F.; Peng, J.; Peng, Y.; Wu, J. Nitrogen Removal Characteristics of a Novel Heterotrophic Nitrification and Aerobic Denitrification Bacteria, Alcaligenes faecalis Strain WT14. J. Environ. Manag. 2021, 282, 111961. [Google Scholar] [CrossRef]
- Kim, B.-H.; Ramanan, R.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Role of Rhizobium, a Plant Growth Promoting Bacterium, in Enhancing Algal Biomass through Mutualistic Interaction. Biomass Bioenergy 2014, 69, 95–105. [Google Scholar] [CrossRef]
- Munoz, R.; Guieysse, B. Algal-Bacterial Processes for the Treatment of Hazardous Contaminants: A Review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cao, J.; Wu, M.; Xu, Y.; Wu, Y.; Shang, K.; Ma, B.; Zhang, L.; Chen, D.; Liu, X.; et al. Enhancing the Thermotolerance of Isochrysis zhangjiangensis Through Co-Culturing with Algoriphagus marincola. Mar. Biotechnol. 2023, 25, 463–472. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Z.; Liu, Y.; Yan, F.; Cao, J.; Liu, R.; Wang, L.; Wei, Y.; Fang, J. Complete Genome Sequence of a Multiple-Stress-Tolerant Bacterium Halomonas piezotolerans NBT06E8T Isolated from a Deep-Sea Sediment Sample of the New Britain Trench. 3 Biotech 2022, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, H.; Cha, I.; Joh, K. Flagellimonas maritima sp. Nov., Isolated from Surface Seawater. Int. J. Syst. Evol. Microbiol. 2020, 70, 187–192. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Li, L.; Zhang, R.; Feng, L.; Mu, J. Ponticoccus alexandrii sp. Nov., a Novel Bacterium Isolated from the Marine Toxigenic Dinoflagellate Alexandrium minutum. Antonie Van Leeuwenhoek 2018, 111, 995–1000. [Google Scholar] [CrossRef]
- Nóbrega, M.S.; Silva, B.S.; Leomil, L.; Tschoeke, D.A.; Campeão, M.E.; Garcia, G.D.; Dias, G.A.; Vieira, V.V.; Thompson, C.C.; Thompson, F.L. Description of Alteromonas abrolhosensis sp. Nov., Isolated from Sea Water of Abrolhos Bank, Brazil. Antonie Van Leeuwenhoek 2018, 111, 1131–1138. [Google Scholar] [CrossRef]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of Cyanobacteria/Microalgae and Bacteria: Biotechnological Potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Zhang, L.; Li, X.; He, Z.; Zhou, C.; Han, J. Screening of Antibiotics to Obtain Axenic Cell Cultures of a Marine Microalga Chrysotila roscoffensis. Front. Bioeng. Biotechnol. 2023, 11, 1218031. [Google Scholar] [CrossRef] [PubMed]
- Kiki, C.; Rashid, A.; Wang, Y.; Li, Y.; Zeng, Q.; Yu, C.-P.; Sun, Q. Dissipation of Antibiotics by Microalgae: Kinetics, Identification of Transformation Products and Pathways. J. Hazard. Mater. 2020, 387, 121985. [Google Scholar] [CrossRef]
- Daneshvar, E.; Zarrinmehr, M.J.; Hashtjin, A.M.; Farhadian, O.; Bhatnagar, A. Versatile Applications of Freshwater and Marine Water Microalgae in Dairy Wastewater Treatment, Lipid Extraction and Tetracycline Biosorption. Bioresour. Technol. 2018, 268, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Scholze, M.; Grimme, L.H. The Single Substance and Mixture Toxicity of Quinolones to the Bioluminescent Bacterium Vibrio fischeri. Aquat. Toxicol. 2000, 49, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Faust, M.; Altenburger, R.; Backhaus, T.; Bödeker, W.; Scholze, M.; Grimme, L.H. Predictive Assessment of the Aquatic Toxicity of Multiple Chemical Mixtures. J. Environ. Qual. 2000, 29, 1063–1068. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Xiao, H.; Wang, R.; Qu, L.; Tang, X. Sensitivity of Several Marine Microalga to Antibiotics. J. Wuhan Univ. Nat. Sci. Ed. 2007, 53, 249–254. [Google Scholar]
- Huang, J.; Gong, X.; Tang, X.; Li, Y. Hormesis of streptomycin on 6 species of marine microalga. J. Ocean. Univ. Qingdao 2000, 30, 642–644. [Google Scholar]
- Zhou, J.; Zhang, L.; An, M.; Duan, S. Hormesis Effect of Organophosphorus Pesticide Glyphostate-Isopropylammonium on Heterosigma akashiwo. Ecol. Sci. 2012, 31, 396–400. [Google Scholar]
- Wang, H.; Cheng, M.; Yang, Y.; Li, S.; Yan, B. The Responses of Algae Pavlova viridis and Karenia mikimotoi to Three Conventional Antibiotics Stress. Fish Sci. 2012, 31, 329–332. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Zhang, J.; Gao, B. Hormesis Effects of Amoxicillin on Growth and Cellular Biosynthesis of Microcystis aeruginosa at Different Nitrogen Levels. Microb. Ecol. 2015, 69, 608–617. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).