Abstract
This study aimed to produce activated carbon from desilicated rice husks using various carbonization and activation methods, including a tube furnace, muffle furnace, and artisanal pyrolysis. The resulting activated carbons were characterized for their adsorptive capacity through the determination of iodine number and methylene blue adsorption; these are key indicators of specific surface area and adsorbent quality. Advanced characterization techniques were employed, such as scanning electron microscopy (SEM), which revealed a highly porous and irregular surface structure, and energy dispersive X-ray spectroscopy (EDS), confirming the effective removal of impurities and optimization of the elemental composition. Atomic force microscopy (AFM) demonstrated favorable surface roughness for adsorption processes. Among the samples, CaDH162-CADH53 exhibited the highest performance, with an iodine number of 1094.8 mg/g and a yield of 93.5%, signifying a high adsorption capacity. The activation treatments with phosphoric acid and calcium carbonate significantly improved the porous structure, further enhancing the material’s adsorptive properties. In conclusion, the activated carbons produced in this study demonstrated optimal physicochemical properties for water purification and contaminant treatment applications. These findings highlight the potential of using agricultural waste, such as rice husk, as a sustainable and scalable alternative for industrial-scale activated carbon production.
1. Introduction
Rice (Oryza sativa L.) cultivation requires the significant use of agrochemicals, resulting in large quantities of untreated residues generated during the harvest. The decomposition of these residues releases pollutants that can be transported via runoff and infiltration into groundwater—a critical freshwater source in regions where surface water is scarce [1], limiting access to quality water, damaging ecosystems, and posing risks to human health and biodiversity [2,3,4]. This highlights the urgent need for ecological and effective remediation solutions [5,6,7,8].
A variety of absorbents are available in the materials market, including silica, zeolites, polymers, clays, and activated carbon (AC). While these materials have demonstrated high efficiency in contaminant removal from water, each comes with limitations [9]. Among these, activated carbon, a highly porous material with a high carbon content, is a promising alternative. It is typically produced from lignocellulosic biomass through pyrolysis under low-oxygen conditions [5,10,11,12,13,14,15,16]. Common feedstocks for AC production include wood, various shells (e.g., coconut, orange, banana, cocoa), agricultural residues, sludge, and wastewater.
AC has gained significant attention as an effective solution for recycling bio-waste, contributing to greenhouse gas reduction and supporting the circular economy [10,17,18]. It is highly valued for its potential in adsorption processes to remove contaminants from water, particularly those originating from agro-industrial activities. This is due to its exceptional structural characteristics, wide availability, recyclability, low cost, and environmental sustainability [10,19,20]. Its adsorption performance is attributed to its porous structure, large specific surface area, cation exchange capacity, and the abundance of surface functional groups [21,22], which vary depending on the feedstock and the production conditions, such as temperature [23].
Rice-husk-derived activated carbon has demonstrated excellent results in both water and soil remediation applications [24,25]. The surface area and functional groups of AC can be further enhanced through physical, chemical, and biological treatments. Its structure, composition, and physicochemical properties can also be optimized by adjusting preparation parameters such as heating rate, temperature, and carrier gas flow rate [24]. Research has shown that rice husk AC is particularly effective in remediating water contaminated with agrochemicals and heavy metals. These contaminants not only degrade soil quality but also, through runoff and infiltration, compromise the water quality of deep wells used to supply drinking water to marginalized urban areas.
However, rice husks contain a high silica (SiO2) content that hinders the carbonization process and influences the quality of the final product. The use of calcium carbonate for its disposal is an effective strategy that combines efficiency, sustainability, and economic viability, in accordance with the current market and responsible environmental practices.
Carbonization tests were carried out and it was determined that rice husks did not carbonize at temperatures below 450 °C in a tube furnace and 350 °C in a muffle furnace. Temperatures higher than 700 °C in a tube kiln and 600 °C in a muffle furnace made ash, coinciding with the present research results. The artisanal method allows adjusting the process according to the conditions and resources available, facilitating its implementation in rural communities. Each method operates at different temperature ranges, affecting the efficiency of the process. Such analysis helps in the identification of which method produces the most efficient charcoal in terms of yield and time. In addition, such insights offer opportunities to optimize the process and encourage innovation in charcoal production.
Phosphoric acid was used in the activation, which is an effective and sustainable option. It offers significant advantages as it increases the specific surface area and modifies the physicochemical properties of the charcoal, which are crucial for optimizing its performance and capacity to adsorb heavy metals present in the water.
This study examines the influence of particle size, temperature, and processing time on the carbonization and activation of rice husk, along with the material’s adsorption capacity for iodine solution (IS) and methylene blue solution (MBS). The characteristics of the AC were analyzed using laser scattering, SEM, EDS, and AFM methods [25,26].
2. Materials and Methods
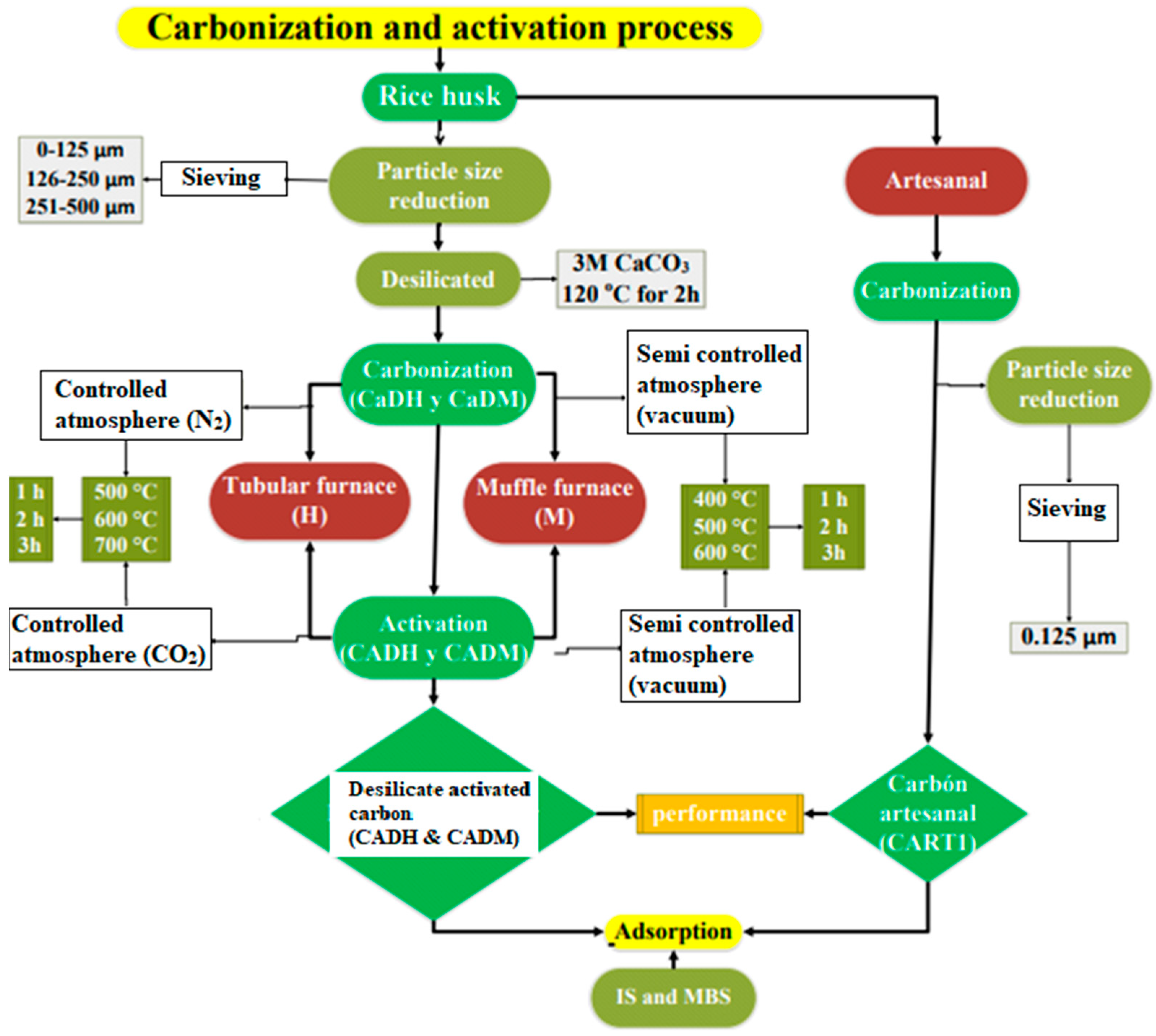
Figure 1 shows the process applied in this research for rice husk carbonization, activation, and adsorption in IS, MBS, and chromium (VI).
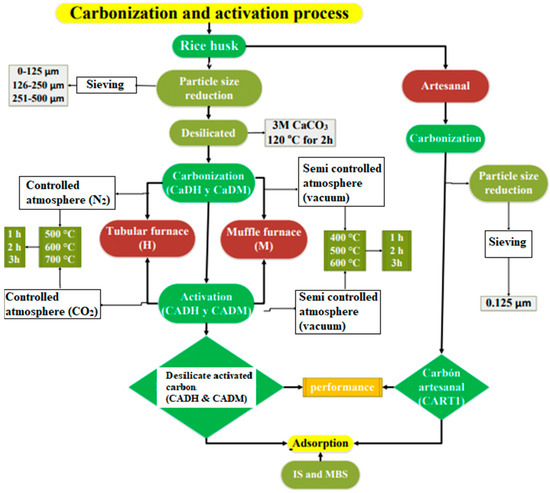
Figure 1.
Carbonization, activation, and adsorption process.
2.1. Adsorbent Preparation
The production of AC was conducted at the Materials Science Laboratory of the University of Barcelona (UB, Barcelona, Spain) and the Soils Laboratory at the State Technical University of Quevedo (UTEQ, Quevedo, Ecuador). Rice husks were ground to a particle size of less than 500 µm and pre-treated with 3M CaCO3 at 120 °C for 2 h, using a CaCO3/rice husk ratio of 5 mL/1 g to reduce silica content.
The methodology was designed to compare three different methods for the preparation of AC. In the first method, carbonization was carried out in a tubular furnace designed and built by the research team at the University of Barcelon The methodology was designed to compare three different methods for the preparation of AC. In the first method, carbonization was carried out in a reactor adapted to a tubular furnace (Nabertherm GmbH, Germany (a), manufactured by the DIOPMA Group of the University of Barcelona a (Figure 2A) at 500 °C, 600 °C, and 700 °C for 1, 2, and 3 h under a nitrogen (N2) atmosphere. The carbonized material was then activated by immersion in 85% phosphoric acid (H3PO4, Scharlab, Barcelona, Spain) at a ratio of 5 mL/g for 24 h, followed by further heat treatment at the same temperatures (500 °C, 600 °C, and 700 °C) under a carbon dioxide (CO2) atmosphere for 1, 2, and 3 h.

Figure 2.
Equipment used in the carbonization and activation process: (A) tube furnace with controlled atmosphere; (B) Landberg blue M flask Thermo Scientific; (C) artisanal method.
The second method utilized a muffle furnace (Thermo Scientific Landberg blue M), MODEL bf5194 (2012), manufactured by the Thermo Fisher Scientific company in the USA (Figure 2B) for carbonization at 400 °C, 500 °C, and 600 °C for 1, 2, and 3 h in an oxidizing atmosphere. This was followed by activation through immersion in 85% phosphoric acid (H3PO4) for 24 h at a ratio of 5 mL/g, with a subsequent activation step under an oxidizing atmosphere at 500 °C, 600 °C, and 700 °C for 1, 2, and 3 h.
Finally, a pyrolysis method was employed, mimicking the thermal treatment used for charcoal production (Figure 2C), to assess its feasibility as a low-cost artisanal alternative for preparing adsorbent materials.
2.2. Study Variables and Data Processing
This research evaluated a set of conditions (temperature and time) for the carbonization and subsequent activation of rice husks. The experiments tested a range of temperatures (500–700 °C) and durations (1–3 h). Each combination of temperature and time was repeated three times, resulting in 27 trials with a total of 81 experimental units, conducted in both a tube furnace and a muffle furnace. Additionally, a sample of charcoal produced through traditional artisanal methods, without any modifications, was included for comparison.
The resulting AC was characterized through morphological analysis, as well as gas-phase and liquid-phase adsorption studies. Adsorption tests included IS, MBS, and chromium adsorption. Statistical analyses of the treatments were performed using Statgraphics Centurion software, version 19.3 03 to determine the effects of different variables. Tukey’s test was used at a significance level (p ≤ 0.05), under a completely randomized design (CRD).
2.3. Adsorption Capacity
2.3.1. Iodine Solution
The samples with the highest performance were selected for adsorption tests, where both the iodine adsorption number (IAN) and methylene blue index (MBI) were evaluated using the methodology outlined by [25], The IAN was determined in accordance with the ASTM D4607-94 method [26], which involves treating the AC sample with 10 mL of 5% HCl. This mixture was boiled for 30 s and then allowed to cool. Afterward, 100 mL of a 0.1 N iodine solution (IS) was added, and the mixture was stirred for 30 s. The resulting solution was filtered, and 50 mL of the filtrate was titrated with 0.1 N sodium thiosulfate, using starch as an indicator.
The amount of iodine adsorbed per gram of carbon (X/M) was compared to the iodine concentration in the filtrate (C), using logarithmic scales. If the residual iodine concentration (C) falls outside the range of 0.008 to 0.04 N, the isotherm point must be repeated. A least squares regression was then applied to all three data points, with the IAN being defined as the X/M value at a residual concentration (C) of 0.02 N. The values for X/M and C were calculated using Equations (1) and (2), respectively.
where:
N1 = normality of the iodine solution (Equivalents per liter; Eq/L);
V1 = volume of iodine solution added (mL);
VHCl = volume of 5% HCl added (mL);
VF = volume of filtrate used for titration (mL);
NNa2S2O3 = normality of sodium thiosulfate solution (Equivalents per liter; Eq/L);
VNa2S2O3 = volume of sodium thiosulfate consumed (mL);
MC = mass of activated carbon (g).
Iodine number values can vary significantly based on the type of activated carbon and its intended use. However, typical ranges that are useful for analysis are as follows:
High-quality activated carbon: 900–1100 mg/g;
Good-quality activated carbon: 600–800 mg/g;
Medium-quality activated carbon: 500–600 mg/g;
Low-quality activated carbon: <500 mg/g.
2.3.2. Solution of Methylene Blue
For the methylene blue index, the adsorption capacity of the adsorbent to methylene blue solution was measured using 0.5 g of the adsorbent with the batch equilibrium method. From Equations (3) and (4), the adsorption capacity in percentage (%) and q-value were calculated. The former indicates how effective the process is in removing the contaminant from the aqueous medium, and the latter indicates the maximum amount of adsorbate that can be retained per unit mass of the adsorbent. The following equations were used:
where:
qt = amount of methylene blue adsorbed at time tt (mg/g);
C0 = initial concentration of methylene blue (mg/L);
Ct = concentration of methylene blue at time tt (mg/L);
V = volume of the solution (L);
m = mass of activated carbon used (g).
2.4. Morphological Characterization of the Adsorbent
Four samples with the best adsorption results were selected and characterized according to established procedures at the Instrumental Laboratories of the Faculty of Sciences, University of Barcelona, using laser diffraction particle size analyzer equipment 13 320 For use PN B05577AB, manufactured by Beckman Coulter, Inc. of the USA (Figure 3A), For the SEM analysis we used a JEOL JSM-5310 model, manufactured by JEOL lTTDA, of Japanese origin, equipped with an energy-dispersive X-ray spectroscopy (EDS) detector (Figure 3B). These magnifications were selected based on pore visibility, with some carbon samples requiring gold coating to improve observation (Figure 3C,D). The SEM-EDS analysis provided insights into the surface morphology of the AC, as well as the type and chemical composition of the elements present [27]. The surface characteristics were further explored using multimode atomic force microscopy (AFM, Bruker Multimode 8 with Nanoscope V controller electronics) with Nanoscope V electronics (Bruker) manufactured by JEOL lTTDA, of Japanese origin. Microzones on smooth, flat surfaces of the carbon granules were selected for detailed probing; then 2D and 3D images were captured (Figure 3E).
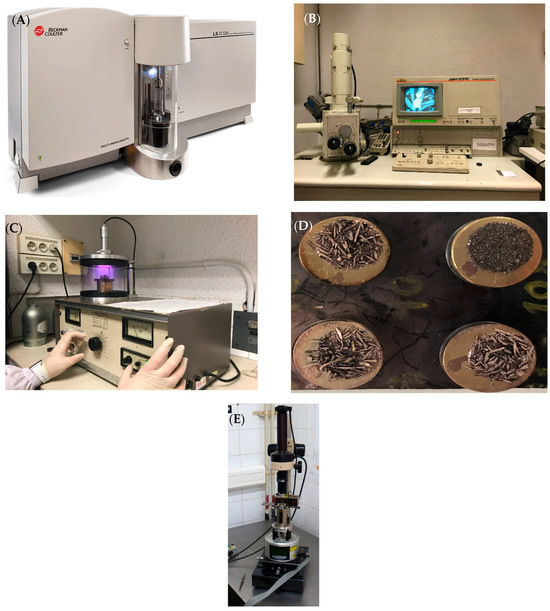
Figure 3.
(A) Laser diffraction particle size analyzer 13,320; (B) scanning electron microscope, brand JEOL, model J8M-5310, (C) gold sputtering, (D) samples before and after sputtering; (E) atomic force microscope Multimode8 Nanoscope V.
3. Results and Discussion
3.1. Carbonization
Desilicated rice husks carbonized (Table 1) in a tube furnace under a nitrogen (N2) atmosphere yielded the highest result in sample CaDH572 (77.7%), which corresponded to husks with a particle size of 251–500 µm, carbonized at 700 °C for 2 h. In contrast, the lowest yield was observed in sample CaDH273 (25.6%), consisting of husks with a particle size of 126–250 µm, carbonized at 700 °C for 3 h.

Table 1.
Yield of the carbonization process for rice husk in a tube furnace and muffle furnace.
A comparison of samples carbonized at 700 °C for 2 h in the tube furnace—CaDH572 (251–500 µm, 77.7% yield), CaDH272 (126–250 µm, 77.5% yield), and CaDH172 (0–125 µm, 48.1% yield)—highlights the influence of particle size on yield. For particle sizes ranging from 126–500 µm, yields remained consistently high at around 77%. However, reducing the particle size below 126 µm resulted in a significant drop in yield to 48%, representing a 30% decrease.
Further analysis of samples with a particle size of 251–500 µm carbonized for 2 h at different temperatures—CaDH572 (700 °C, 77.66% yield), CaDH562 (600 °C, 63.3% yield), and CaDH552 (500 °C, 59.7% yield)—indicates that 700 °C is the optimal carbonization temperature for maximizing yield in this particle size range.
Additionally, for samples with the same particle size (251–500 µm) carbonized at 700 °C, the yields at different carbonization times—CaDH571 (1 h, 35.1% yield), CaDH572 (2 h, 77.7% yield), and CaDH573 (3 h, 39.6% yield)—suggest that the optimal carbonization time is 2 h. Shorter or longer durations lead to significantly lower yields.
In the muffle furnace (Table 1), the highest yield was recorded for sample CaDM162 (92.8%), corresponding to husks with a particle size of 0–125 µm carbonized at 600 °C for 2 h. The lowest yield was observed in sample CaDM243 (47.6%), consisting of husks with a particle size of 126–250 µm carbonized at 400 °C for 3 h.
A comparison of samples carbonized at 600 °C for 2 h in the muffle furnace—CaDM562 (251–500 µm, 60.5% yield), CaDM262 (126–250 µm, 60.5% yield), and CaDM172 (0–125 µm, 92.8% yield)—demonstrates that particle sizes between 126–500 µm resulted in yields of around 60%. However, reducing the particle size below 126 µm led to a significant increase in yield to 93%, an approximate 30% increase.
Further analysis of samples with a particle size of 0–125 µm carbonized for 2 h at varying temperatures—CaDM162 (600 °C, 92.83% yield), CaDM152 (500 °C, 59.03% yield), and CaDM142 (400 °C, 72.2% yield)—shows that 600 °C is the optimal temperature for achieving the highest yield in this particle size range.
Additionally, when comparing yields at different carbonization times for samples with the same particle size (0–125 µm) carbonized at 600 °C, for example, CaDM161 (1 h, 59.8% yield), CaDM162 (2 h, 92.8% yield), and CaDM163 (3 h, 59.3% yield), it becomes evident that 2 h is the optimal carbonization time. Longer or shorter durations result in considerably lower yields.
In the artisanal carbonization method (CART1), using untreated rice husks (0–125 µm), a yield of 46.2% was achieved, meaning that from 13 lb (5.9 kg) of husks, 6.2 lb (2.8 kg) of charcoal was obtained for subsequent adsorption testing.
In a previous study, Asimbaya et al. [27] reported that carbonization yields ranging from 19.8% to 40% for various biomass sources, including orange peels, coffee husks, sugarcane bagasse, laurel, cinnamon, and eucalyptus. Yield variations are attributed to several factors, including the composition of plant material, heating rate, temperature, pressure within the carbonization equipment (tube furnace, muffle furnace, and artisanal setup), atmospheric conditions, and the use of catalysts.
Rice husk has a high silica content which influences the performance, pore formation, and specific surface area of the carbon. Its removal with calcium carbonate improves the surface structure and adsorption capacity of the resulting AC, essential for water treatment and contaminant adsorption applications.
Our findings align with the literature [28], which highlights that these factors significantly influence the sequence and kinetics of thermochemical reactions (pyrolysis), thereby affecting charcoal yield. Additionally, in other studies [29,30], it was noted that rice husks have a closed structure that hinders combustion and maintains a calorific value of 3281.6 Kcal/g. The high silica content (20%) also contributes to their low biodegradability in natural environments. The maximum combustion temperature for rice husks varies depending on their moisture content: 970 °C when dry, 650 °C when slightly moist, and up to 1000 °C when mixed with fuel. When burned, rice husks produced up to 17.8% ash, with silica content reaching 94.5%. The results obtained in this study can be explained by the composition of the plant material, the heating rate, temperature, and pressure of the carbonization equipment, as well as the ambient atmosphere and the use of catalysts, consistent with the findings reported elsewhere [29]. These factors profoundly affect the thermochemical reactions (pyrolysis), influencing the yield of the final charcoal product. The high silica content of rice husks, as highlighted by others [30,31], contributes to their challenging combustion properties and low biodegradability.
3.2. Activation
The activation of rice husk under tube furnace conditions produced varying yields (Table 2), with the highest (97.2%) obtained from sample CaDH252-CD52. This sample, consisting of rice husks sized 126–250 µm, was carbonized at 500 °C for 2 h and activated at the same temperature for another 2 h under a CO2 atmosphere. In contrast, the lowest yield (35.83%) came from sample CaDH262-CADH71, which had an identical particle size but was carbonized at 600 °C and activated at 700 °C for 2 h.

Table 2.
Yield of activation process of carbonized rice husk in tube furnace and muffle furnace.
A comparison of samples activated at 500 °C in the tube furnace—CaDH252-CADH51 (126–250 µm, 1 h, 94.5% yield), CaDH252-CD52 (126–250 µm, 2 h, 97.2% yield), and CaDH252-CD53 (126–250 µm, 3 h, 94.9% yield)—shows that activation time had minimal influence on yield, all exceeding 94%. These results suggest that for particle sizes of 126–250 µm, extending thermal treatment time does not significantly affect activation yield.
To assess the influence of temperature, samples of the same particle size (0–125 µm) carbonized at 500 °C for 2 h were activated at different temperatures for 1 h. The results with CaDH152-CADH71 (700 °C, 66.8% yield), CaDH152-CD61 (600 °C, 91.4% yield), and CaDH152-CADH51 (500 °C, 55.2% yield) indicate that 600 °C is the optimal activation temperature. Lower or higher temperatures substantially reduced yields, highlighting those temperatures above 500 °C with a heat treatment time of at least 1 h are optimal for activation using a tube furnace under a CO2 atmosphere.
Similarly, rice husks activated in a muffle furnace under oxidizing conditions (Table 2) produced a maximum yield of 92.83% in sample CaDM263-CADM61 (126–250 µm, carbonized at 600 °C for 3 h and activated at 600 °C for 1 h). The lowest yield (29.2%) was recorded in sample CaDM561-CADM63 (251–500 µm, carbonized at 600 °C for 1 h and activated at 600 °C for 3 h). When comparing treatment times at 600 °C in the muffle furnace, with CaDM263-CADM61 (1 h, 92.8% yield), CaDM263-CADM62 (2 h, 60.3% yield), and CaDM263-CADM63 (3 h, 81.6% yield), it is evident that 1 h of treatment maximized yield for 126–250 µm particle sizes, while extended activation times significantly decreased yield.
Exploring the effect of activation temperature using carbonized samples (126–250 µm, carbonized at 600 °C for 3 h) revealed that yields at different activation temperatures for 1 h were CaDM263-CADM61 (600 °C, 92.8% yield), CaDM263-CADM51 (500 °C, 51.3% yield), and CaDM263-CADM41 (400 °C, 59.8% yield), again demonstrating that 600 °C is optimal, with significantly lower yields at reduced temperatures.
Ash content analysis of the resulting AC showed notable values in samples such as CaDM541-CADM43 (67.7%), CaDM541-CADM53 (79.6%), CaDM162-CADM53 (60.4%), and CaDM542-CADM62 (61.4%).
Other researchers [31] have investigated rice husk activation under similar conditions, with activation times ranging from 1 to 3 h at temperatures between 200 and 300 °C, achieving yields exceeding 58% [31]. Our improved methodology produced AC with yields surpassing 71.1% in 58% of samples, demonstrating enhanced efficiency in AC production.
The differences in performance observed in Table 2 can be attributed to several factors: the plant material used, pretreatment methods (desilication with calcium carbonate and activation with phosphoric acid), heating intervals, temperature, choice of catalyst, and the release of volatile compounds during combustion. These findings align with previous research [32], which suggests that higher temperatures and longer exposure times contribute to a loss of volatile material. Also, the activation process develops the porous structure of the CA, and its characteristics vary depending on the method and feedstock [33]. Additional research [34,35] has also demonstrated that phosphoric acid activation can modify residual acidity, and the quality and performance of AC are strongly influenced by the properties of rice husks and the processing conditions.
ID key: CDH samples were activated in a tubular furnace and CDM samples were activated in a muffle furnace; the two numerical variables represent the temperature and time of the activation process. The first number after CDH or CDM represents the temperature with (7 for 700, 6 for 600, and 5 for 500) and the second number is the time of the activation with (1 for 1 h, 2 for 2 h, and 3 for 3 h). For example, CDM62 was activated in a muffle furnace at 600 C for 2 h.
3.3. Adsorption
3.3.1. Adsorption in Iodine Solution (IS) and Methylene Blue Solution (MBS)
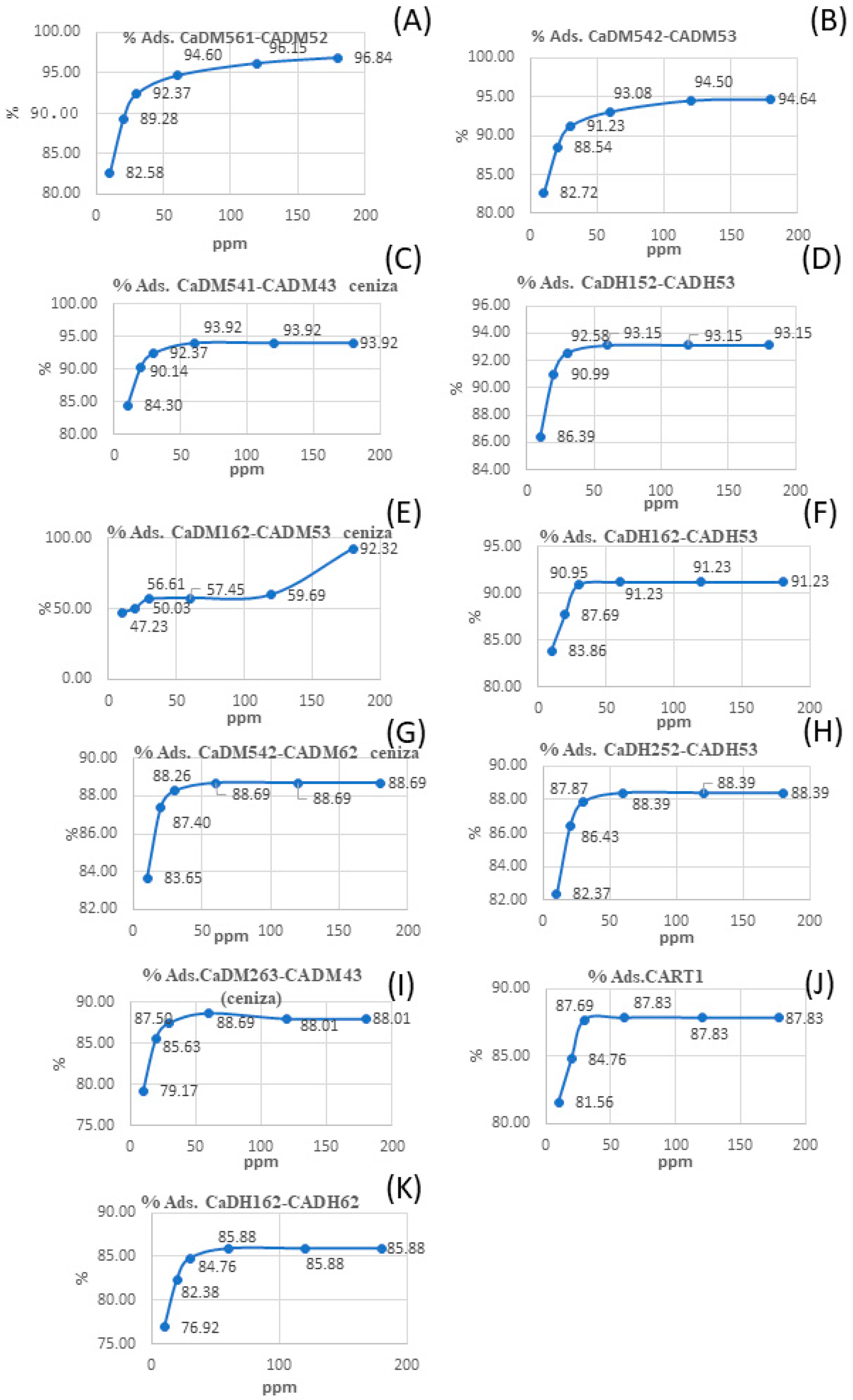
Adsorption tests were performed on all the activated carbons that obtained the highest activation yields (Table 2). The best results were obtained for CaDH162-CADH53 and CaDM561-CADM52 produced in both tube furnaces and muffle furnaces, and these were tested for adsorption in IS and MBS. In addition, these ACs were compared with the ash-containing samples CaDM162-CADM53 and CART1.
Table 3 and Figure 4 present the adsorption results in iodine solution and methylene blue solution, respectively, showing that sample CaDH162-CADH53 achieved the highest iodine adsorption value at 1094.8 mg/g. This sample was prepared using a tube furnace, carbonized at 600 °C for 2 h and activated at 500 °C for 3 h. In comparison, CaDH152-CADH53 prepared in the same furnace but carbonized at 500 °C for 2 h and activated at 500 °C for 3 h, achieved an iodine adsorption of 973 mg/g. This suggests that a higher carbonization temperature improves the iodine adsorption capacity when all other conditions remain the same.

Table 3.
Adsorption of activated carbons in iodine solution and methylene blue solution.
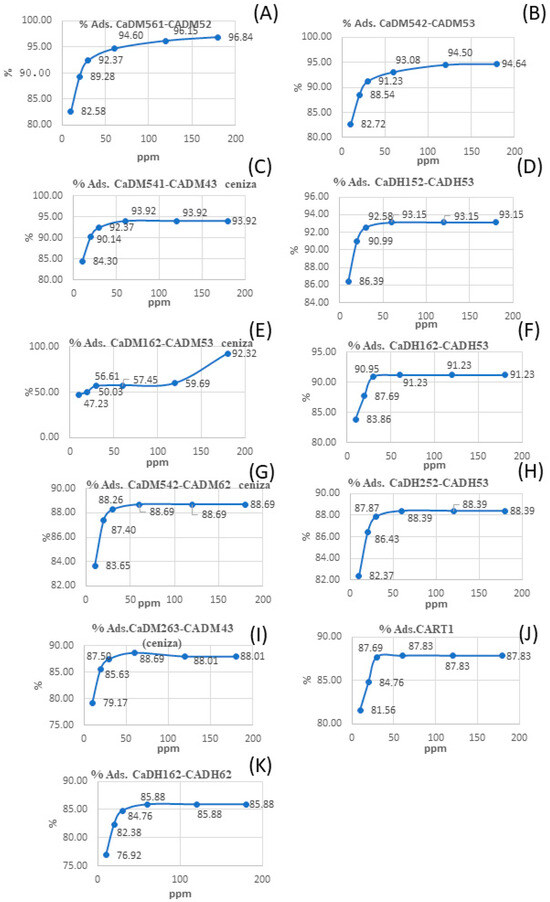
Figure 4.
Visual comparison of methylene blue adsorption (%) on various activated carbons (A–K).
For the AC prepared in the muffle furnace, CaDM542-CADM62 (Figure 4G) displayed the highest iodine adsorption at 1085.6 mg/g. This sample was carbonized at 400 °C for 2 h and activated at 600 °C for 2 h. By contrast, CaDM542-CADM53 (Figure 4B), carbonized under the same conditions but activated at 500 °C for 2 h, showed lower iodine adsorption of 964 mg/g. This indicates that higher activation temperatures in the muffle furnace reduce iodine adsorption.
Overall, the findings suggest that increasing the carbonization temperature in tube furnaces improves iodine adsorption, while in muffle furnaces, higher activation temperatures have the opposite effect.
According to the SNI (Indonesian National Standard) activated carbon standard No. 06-3730-1995, ACs with iodine adsorption values between 900 and 1100 mg/g are classified as high-quality. The CART1 (Figure 4J) sample, produced via artisanal methods, exhibited iodine adsorption values below 900 mg/g. The results in this study surpass those reported by previous researchers [27,36].
Analyzing the methylene blue adsorption values (MBS), CaDH152-CADH53 (Figure 4D), carbonized at 500 °C for 2 h and activated at 500 °C for 3 h, exhibits an adsorption capacity of 186.3 mg/g. In comparison, CaDH252-CADH53 (Figure 4H), prepared under the same carbonization and activation conditions but with a different rice husk particle size, shows a slightly lower adsorption capacity of 176.8 mg/g. These results suggest that increasing the particle size of the rice husks may result in reduced adsorption capacity for MBS and potentially other organic pollutants.
For samples prepared using the muffle furnace, CaDM542-CADM53 (Figure 4B), carbonized at 400 °C for 2 h and activated at 500 °C for 3 h, achieved a methylene blue adsorption value of 189.3 mg/g. In contrast, CaDM542-CADM62 (Figure 4G), carbonized at 400 °C for 2 h and activated at a higher temperature of 600 °C for 2 h, exhibited a lower adsorption capacity of 177.4 mg/g, suggesting that lower activation temperatures in the muffle furnace enhance methylene blue adsorption.
Notably, the CART1 (Figure 4J) sample, produced through slow pyrolysis of desilicated material without chemical activation, achieved an adsorption capacity of 175.7 mg/g, highlighting its potential for domestic water purification in vulnerable urban communities.
Figure 4 shows that most AC samples reach their maximum adsorption capacity at a methylene blue concentration of 50 ppm, consistent with previous research [37], which reported an adsorption efficiency of 99.7% at 50 ppm, decreasing slightly to 99.1% at higher concentrations. According to Hajam et al. [38], saturation occurs when the initial methylene blue concentration exceeds 50 ppm, as the active sites on the AC become insufficient to adsorb higher concentrations effectively.
The iodine number and methylene blue adsorption values of activated carbon vary significantly depending on the type of AC and the production method. Importantly, iodine values exceeding 900 mg/g are associated with proportional surface area and the presence of micropores, which facilitate iodine adsorption [39]. In contrast, methylene blue molecules, with larger cross-sectional areas, require adsorbents with both micropores and mesopores. Ebadollahzadeh and Zabihi [40] suggested that optimizing AC production enhances adsorption capacity by maximizing available surface area. Furthermore, Osman [41], highlighted that higher adsorbent dosages are necessary to accommodate higher concentrations of adsorbents.
The use of desilication agents (e.g., CaCO3), activating agents (e.g., H3PO4), and varied carbonization processes (tube furnace, muffle furnace, or artisanal methods), along with the process duration, significantly influences the adsorption capacity for iodine and methylene blue. This is attributed to the increased action of London dispersion forces, which, although weak, are enhanced under heat and pressure to improve adsorption.
Factors such as raw material type, pyrolysis conditions [42], carbon concentration, temperature, residence time, and pollutant characteristics also play crucial roles in adsorption capacity [20,43]. Higher pyrolysis temperatures, tend to reduce the functional groups on biochar surfaces [44,45], while increasing surface area, thus enhancing pollutant adsorption selectivity. Additionally, numerous studies [20,46,47] have demonstrated the importance of AC in pollution remediation, particularly in the removal of organic acids and other pollutants, with modifications necessary to improve AC performance.
3.3.2. Adsorption Isotherms
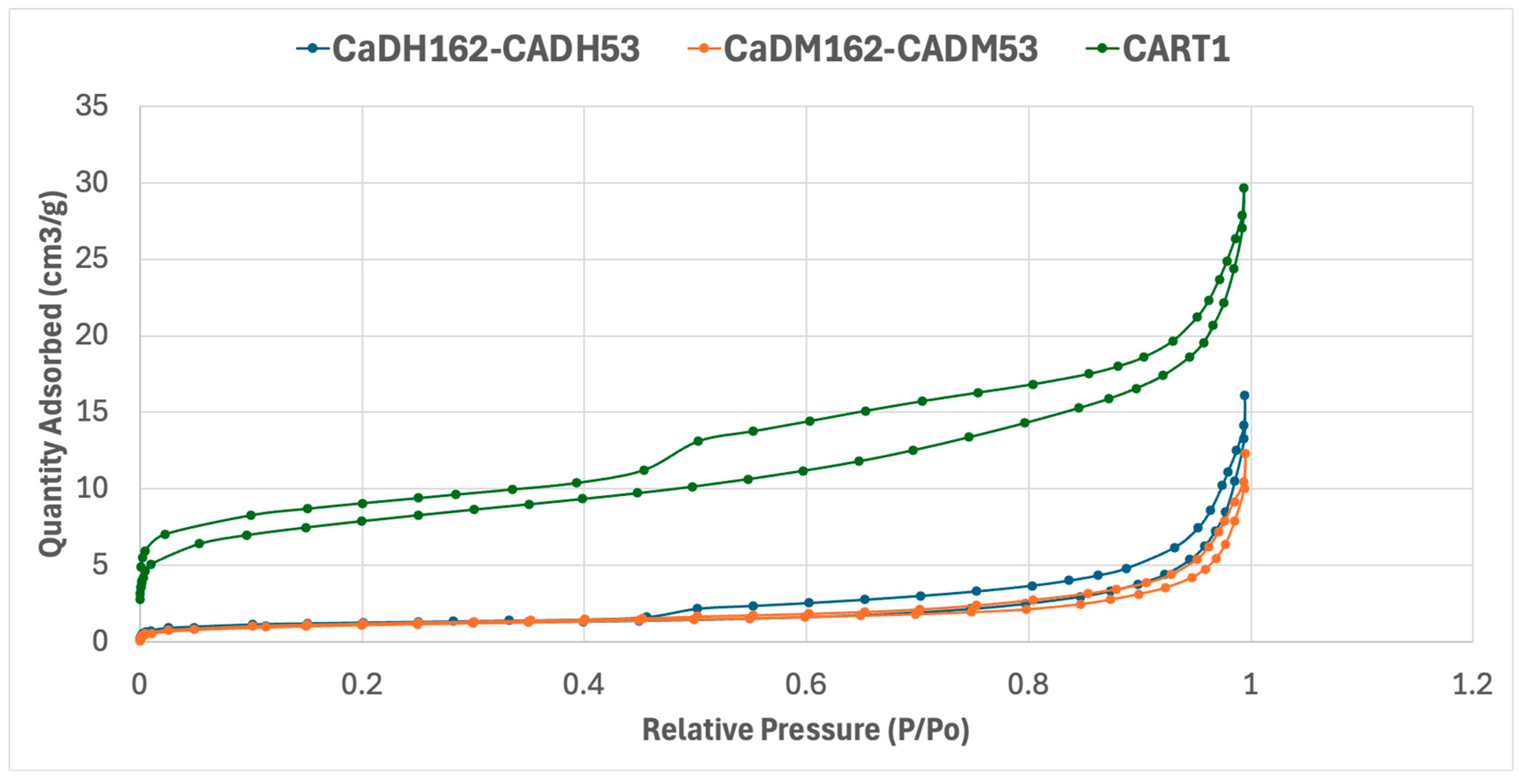
The samples with the highest adsorption capacities for IS and MBS were selected from each production method: tube furnace, muffle furnace, and artisanal furnace. The N2 adsorption–desorption results for CaDH162-CADH53, CaDM162-CADM53, and CART1 are shown in Figure 5, with surface area measurements (BET, Brunauer–Emmett–Teller) and average pore diameters calculated using the Barrett–Joyner–Halenda (BJH) model detailed in Table 4 and Figure 5.
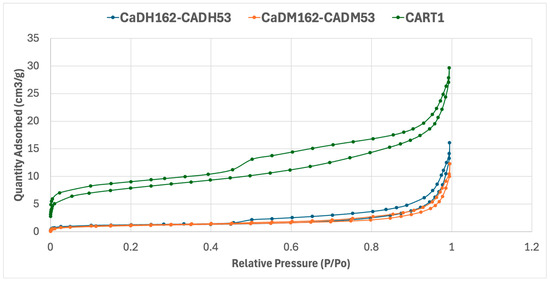
Figure 5.
Nitrogen adsorption–desorption isotherms of the materials CaDH162-CADH53, CaDM162-CADM53, and CART1.

Table 4.
Isotherm Values: BET (Brunauer–Emmett–Teller), BJH (Barrett–Joyner–Halenda) Adsorption and BJH Desorption.
Figure 5 presents the N2 adsorption–desorption isotherms for the three AC samples, all of which display Type IV isotherms with characteristic hysteresis loops. This pattern is typical of mesoporous materials, indicating that adsorption predominantly occurs through capillary condensation within the mesopores.
A key observation is that CaDM162-CADM53 and CaDH162-CADH53 exhibit similar adsorption capacities, with comparable amounts of adsorbates during the adsorption process. However, a major distinction between the ACs produced in the muffle furnace and tube furnace lies in their desorption of isotherms and hysteresis shapes.
The AC produced in the tube furnace (CaDH162-CADH53) achieves a maximum adsorbed volume of approximately 16 cm3/g, with a hysteresis loop ranging from 0.45 to 1.0 P/Po. In contrast, the muffle furnace AC reaches a slightly lower adsorbed volume of around 12 cm3/g, with a hysteresis loop spanning from 0.7 to 1.0 P/Po. The AC produced by artisanal methods (CART1) shows a significantly higher adsorption capacity, nearly doubling that of the other samples, with an adsorbed volume of 30 cm3/g and a complete hysteresis loop.
Both CaDH162-CADH53 and CaDM162-CADM53 exhibit H4-type hysteresis loops, suggesting the presence of narrow slit-like pores, internal voids with irregular shapes, and broad size distributions. These features are commonly associated with hollow spheres with ordered mesoporous silica walls. Micropores are evidenced by the initial curve at low relative pressures, while mesopores dominate the upper stages of the isotherms, consistent with the observed hysteresis behavior.
Conversely, CART1 exhibits a pronounced hysteresis between the adsorption and desorption curves, indicating a strongly bonded monolayer. This feature could pose limitations for applications requiring rapid and complete desorption. While CART1’s rich internal structure supports high adsorption capacity, its desorption challenges may impact its effectiveness in continuous regeneration processes.
CaDH162-CADH53 and CaDM162-CADM53 present similar BET surface area values of 4.14 ± 0.05 and 3.81 ± 0.07 m2/g, respectively, whereas CART1 displays a much larger BET surface area of 27.9 ± 0.2 m2/g. The disparity in the adsorbate–adsorbent system characteristics precludes direct comparison with literature values, as the porous material properties and the adsorbate’s mobility through the adsorbent’s pores significantly influence the adsorption process.
Furthermore, the average pore sizes for adsorption and desorption were calculated using the BJH model. CaDH162-CADH53 and CaDM162-CADM53 show similar average adsorption pore sizes of 240 Å and 200 Å, respectively, with desorption pore sizes of 292 Å and 221 Å. In contrast, CART1 exhibits much smaller pore sizes, with an average adsorption pore size of 65 Å and an average desorption pore size of 104 Å. The abundance of small pores in CART1 likely explains its high adsorption capacity.
These findings suggest that artisanal AC, such as CART1, hold significant potential as cost-effective adsorbent materials. Further studies should focus on the desilication of the raw material to improve charcoal production and assess whether this modification can reduce absorption time [48,49].
Surface areas of AC obtained from various raw materials, including rice husks, were reported to be in the range between 500–1000 m2/g. Even under optimum conditions, these differences are conditioned by the characteristics of the raw material, process conditions, and the effectiveness of the activating agent and desilicate with calcium carbonate, affecting its capacity to form an adequate porous network during the activation process. However, high adsorption values were obtained in iodine and methylene blue solution.
The values obtained in the iodine and methylene blue index correspond to those reported in the scientific literature and are related to the approximate size of the iodine molecule (0.335 nm) and methylene blue (180 mg/g), which facilitate adsorption on carbons with micro and mesoporous structure. In the experiment with N2, typical values (20 to 29 nm) of mesopores and atypical in the surface area (4 m2/g) were presented, possibly due to the previous process applied and the experimental conditions in the carbonization and activation.
3.4. Characterization of Adsorbents
3.4.1. Laser Scattering
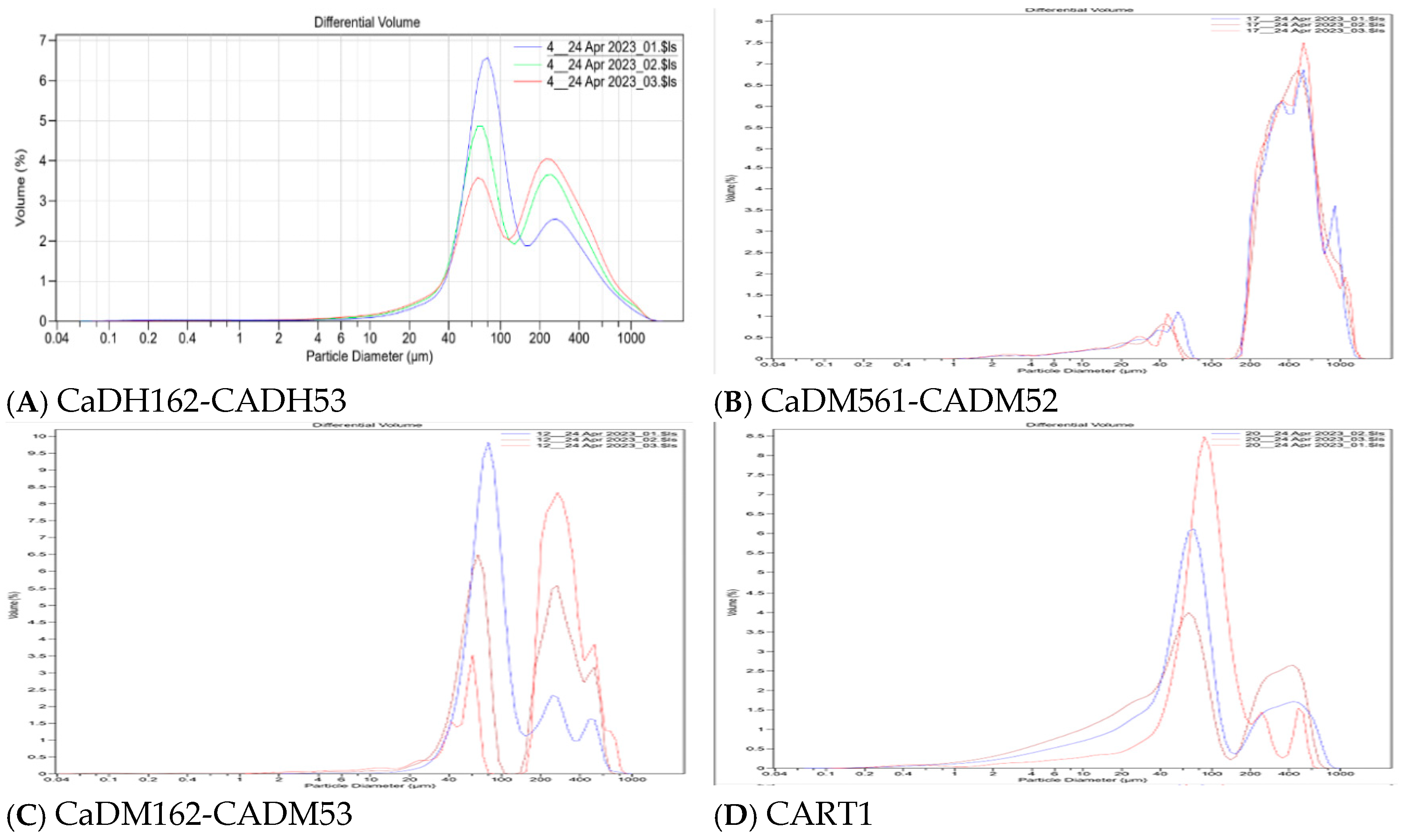
Following the methylene blue and iodine number assays, the ACs with the best adsorption performance were selected, and the particle size analysis of these ACS is presented in the next section. The laser scattering results for CaDH162-CADH53, CaDM561-CADM52, CaDM162-CADM53, and CART1 are illustrated in Figure 6.
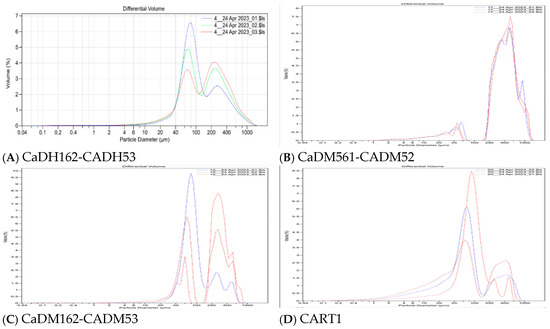
Figure 6.
Visualization of activated carbon particle size (A–D) by laser dispersion.
The relative size factor (RSF) is a key metric used to assess the uniformity and performance of AC in applications such as water treatment and air purification. A lower RSF indicates greater uniformity, resulting in more consistent adsorption processes, enhanced efficiency, and reduced operational costs. As the RSF increases, larger particles contribute less surface area per unit volume, typically reducing adsorption capacity. In contrast, smaller particles with a lower RSF accelerate the adsorption rate by shortening diffusion paths, providing quicker access to active adsorption sites. Optimizing particle size through RSF adjustments can significantly improve the overall effectiveness of AC in various applications.
Laser scattering analysis (Figure 6) of the CaDH162-CADH53 (Figure 6C) sample reveals that 10% of the particles are 44.2 µm or smaller, 50% are 130.9 µm or smaller, and 90% are 458.5 µm or smaller, resulting in an RSF of 3.2. This high RSF indicates significant variability in particle size distribution. This variability positively impacts adsorption performance, as demonstrated by the sample’s superior results in IS adsorption, while achieving over 90% adsorption efficiency in MBS. The wide range of particle sizes contributes to an optimal combined performance in adsorption tests.
For the CaDM561-CADM52 sample (Figure 6B), laser scattering analysis indicates that 10% of the particles are 99.4 µm or smaller, 50% are 395.1 µm or smaller, and 90% are 788.2 µm or smaller, with an RSF of 1.7. Although still high (>1.5), this RSF is lower than that of the previous sample, suggesting less variability in particle size. This AC demonstrated strong performance in MBS adsorption but showed lower efficiency in IS adsorption.
Laser scattering analysis of the CaDM162-CADM53 sample (Figure 6C) shows that 10% of the particles are 48.0 µm or smaller, 50% are 176.4 µm or smaller, and 90% are 410.7 µm or smaller, with an RSF of 2.1. Although still high (>1.5), this reflects a similar particle size distribution to CaDH162-CADH53, indicating comparable performance in IS and MBS adsorption tests.
For the CART1 sample (Figure 6D), analysis reveals that 10% of the particles are 15.9 µm or smaller, 50% are 71.7 µm or smaller, and 90% are 355.7 µm or smaller. The RSF of 4.5 suggests significant variability in particle size, leading to a highly non-uniform distribution. Despite this, CART1 achieved over 87% adsorption in MBS, demonstrating good performance for removing organic pollutants. This indicates that artisanal methods of AC preparation can be an effective, low-cost alternative for water purification in vulnerable communities.
The high variability in particle size can be attributed to several factors. Despite specific sieving ranges, smaller particles may agglomerate, particularly in organic materials like rice husks, which tend to absorb moisture and promote particle clustering. Additional contributing factors include variability in the raw materials used for AC production, differences in activation processes (e.g., the type of chemical agents and treatment methods), and variations in processing conditions such as temperature and contact time. Transport, handling, and storage conditions can also affect particle integrity, as well as potential chemical interactions during production or storage. It is also noteworthy that findings from previous studies [35,50,51] indicated that this particle size range may negatively impact the available surface area for adsorption, potentially leading to issues such as clogging, increased pressure drop, and reduced overall adsorption efficiency.
Studies [35,50,51] further indicated that the cellulose and silica content, along with processing temperature, significantly influenced the agglomeration of fine particles and the formation of clusters. According to research [34,50,52], high cellulose and silica levels in AC precursors could alter thermal behavior during carbonization, promoting particle agglomeration. In fine particle sizes (0–125 microns), where the surface-to-volume ratio is high, improper control of temperature and time can cause particles to fuse. Additionally, both physical and chemical activation methods can exacerbate agglomeration if factors like gas flow or humidity are not adequately managed.
These findings underscore the importance of addressing critical factors such as particle agglomeration, raw material variability, activation processes, and processing conditions. Optimizing these aspects is essential for enhancing adsorption efficiency and producing high-performance activated carbon for large-scale applications.
3.4.2. Analysis by Scanning Electron Microscopy (SEM)
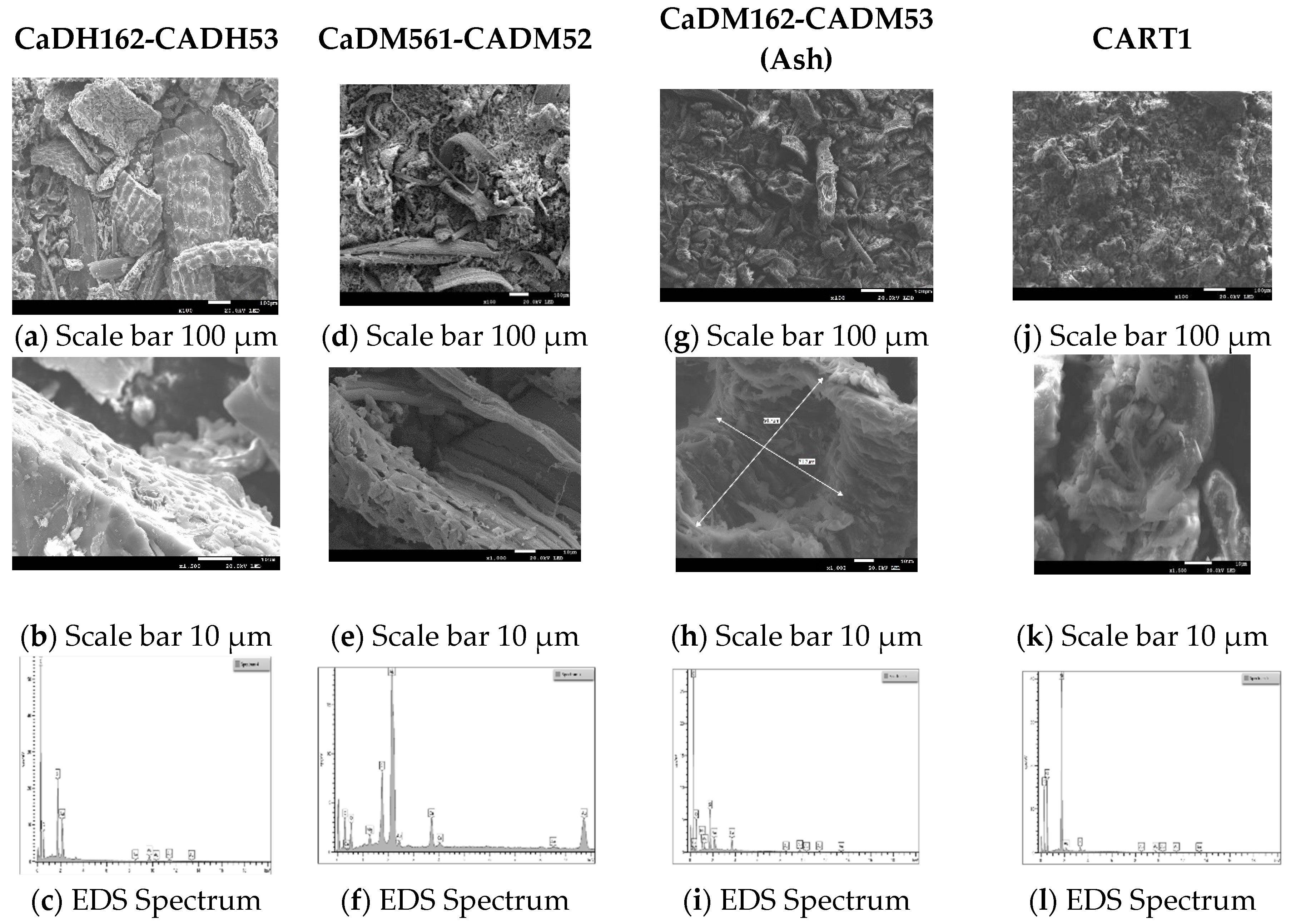
Figure 6 illustrates the morphology and chemical composition of the AC samples CaDH162-CADH53, CaDM162-CADM53, CaDM561-CADM52, and CART1.
CaDH162-CADH53 consists of rice husks with a particle size range of 0–125 µm. This sample underwent carbonization in a tube furnace at 600 °C for 2 h under a nitrogen atmosphere, followed by activation at 500 °C for 3 h using carbon dioxide. At a magnification scale of 100 µm (Figure 7a), the sample exhibits a heterogeneous texture, characterized by varying pore sizes and densely packed regions interspersed with cavities, indicative of a macroporous structure. The irregular distribution of pores suggests that the carbonization and activation processes were not entirely uniform. At a 10 µm scale with 1000× magnification (Figure 7b), the surface structure becomes more evident, revealing many clean micropores and interconnected pore networks [51].
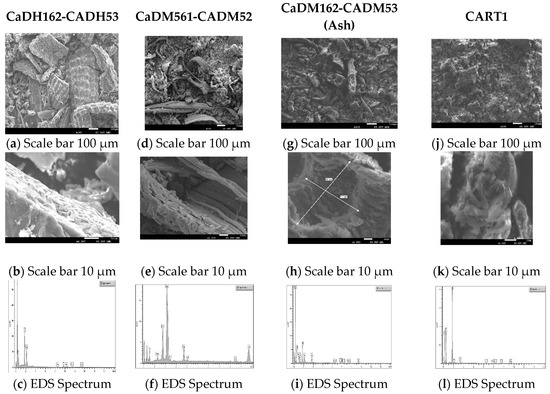
Figure 7.
Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) analysis of activated carbons (ACs) with the highest adsorption. [Activated carbons: CaDH162-CADH53, (a–c); CaDM561-CADM52, (d–f); CaDM162-CADM53, (g–i); and CART1, (j–l)].
Minor cracks and fractures on the surface may enhance the material’s specific surface area and adsorption capacity. Nanometer-scale features, including silica particles distributed within the carbon matrix, are visible, highlighting their importance in understanding surface reactivity and interaction with adsorbed contaminants. The presence of macropores with maintained micropores, as referenced in [50,53], could provide additional adsorption sites. Spectral analysis (Figure 7c) revealed the presence of carbon (77.5%), oxygen (18.4%), and silica (4.1%).
Oxygenated functional groups remaining on the carbon surface after activation, along with silica particles and a highly porous structure, can significantly enhance chemical interactions and boost adsorption capacity. These features contribute to the strong performance observed in the MBS and IS adsorption tests.
CaDM561-CADM52 micrographs revealed broad, uniform macroporous structures, indicating that carbonization preserved the biomass’s original features. At 1000× (Figure 7d) magnification, a heterogeneous surface with finer pores and irregularities at the edges was evident, suggesting the presence of an interconnected network of micro- and mesopores. At 2000× (Figure 7e) magnification, the pore structure’s complexity was further highlighted, with structural defects acting as active sites for chemical adsorption. This microstructure, with its well-distributed pores at the nanometer level, is essential for applications requiring high adsorption efficiency.
Elemental composition analysis (Figure 7f) revealed 48.4% carbon, 32.3% oxygen, 1.9% magnesium, 9.8% silica, and 7.6% calcium. The presence of silica likely enhances the material’s catalytic properties, contributing to its improved performance in methylene blue adsorption.
CaDM162-CADM53 is derived from ash, with rice husk particles in the range of 0–125 µm. This sample was carbonized in a muffle furnace at 600 °C for 2 h, followed by activation at 500 °C for 3 h in a vacuum atmosphere. At low magnification (Figure 7g), the surface appears relatively uniform, with some visible pores. However, at higher magnification (Figure 7h), a coarser texture and the presence of macropores (71.7 × 91.7 µm) become apparent, suggesting a network of interconnected pores of varying sizes. This is crucial for molecular adsorption, as smaller micropores significantly increase the surface area available for adsorption. Elemental analysis confirmed the presence (Figure 7i) of carbon (72.97%), oxygen (22.3%), sodium (0.51%), magnesium (0.5%), silica (2.4%), and calcium (1.4%).
The complex pore structure, variability in pore sizes, and high adsorption capacities (1043.2 mg/g in IS and 92.32 mg/g in MBS) suggest that CaDM162-CADM53 exhibits excellent adsorption potential for both large and small molecules. This implies its suitability for cyclic adsorption–desorption processes with high structural stability, making it ideal for industrial applications.
Micrographs of CART1 at 50× and 100× magnification (Figure 7j) arevealed a heterogeneous surface marked by irregularly distributed pores of varying sizes, a characteristic feature of carbons derived from lignocellulosic biomass. At higher magnifications (2000×) in the Figure 7k, more distinct honeycomb-like pores emerge, interconnected within a complex network. This structural configuration likely enhances the material’s adsorption capacity. With an adsorption value of 87.8 mg/g in MBS, the unique structural and compositional characteristics of CART1 indicate its significant potential for effective adsorption processes.
CART1 exhibited a composition (Figure 7l) of 51.2% carbon, 37.3% oxygen, 11.2% silica, and 0.39% potassium. The presence of these impurities can influence the material’s adsorption properties and surface reactivity, as indicated by an MBS adsorption value of 87.8 mg/g and an IS value below 900 mg/g. The combination of silica and oxygen may enhance the chemical affinity for certain adsorbed molecules, while potassium could further contribute to the material’s catalytic properties. The high silica content (11.2%) detected in CART1 suggests potential interactions with mineral components or inadequate desilication, which may affect the material’s adsorption efficiency and surface reactivity. However, additional testing is necessary to evaluate its functionality concerning heavy metals and volatile organic compounds.
3.4.3. Analysis by Atomic Force Microscopy (AFM)
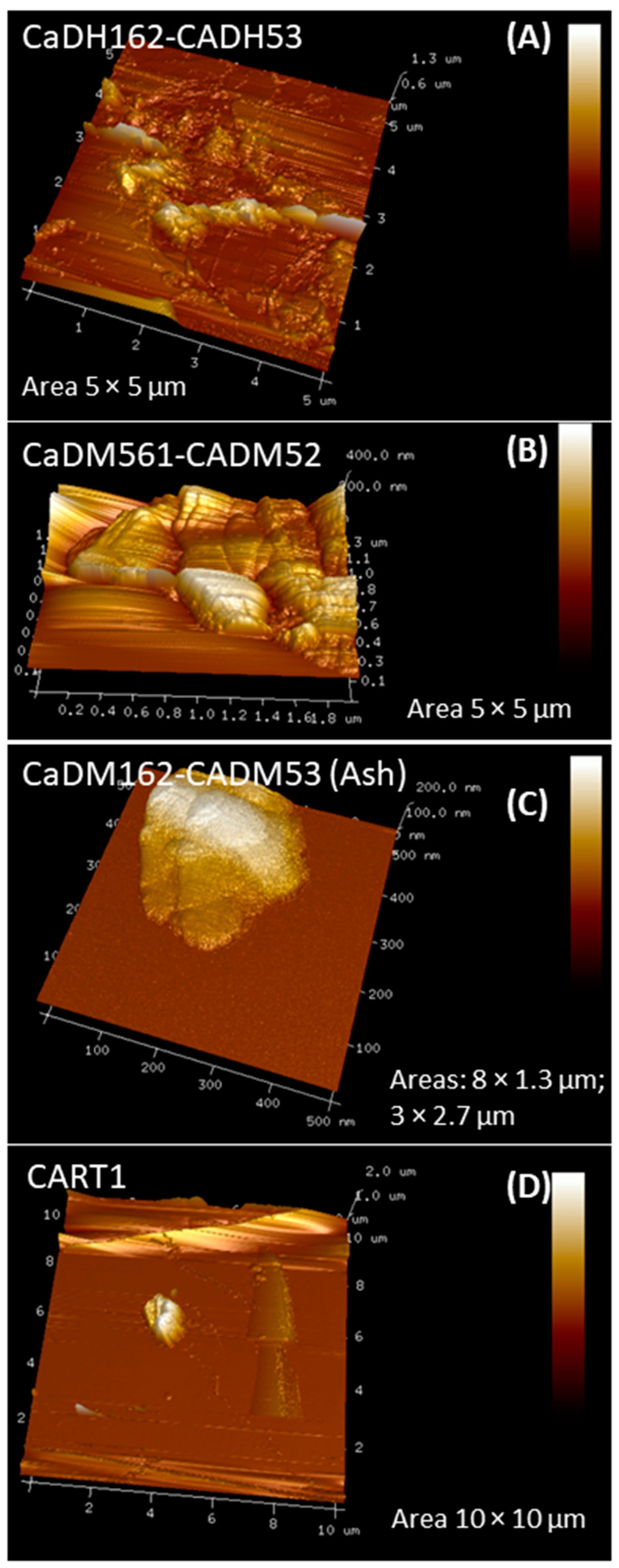
Atomic force microscopy was employed to characterize the roughness of the AC samples, with results presented in Figure 8 and roughness parameters quantified in Table 5. The low resolution observed in Figure 8 can be attributed to contaminants within the sample and external vibrations that affected the quality of the images.
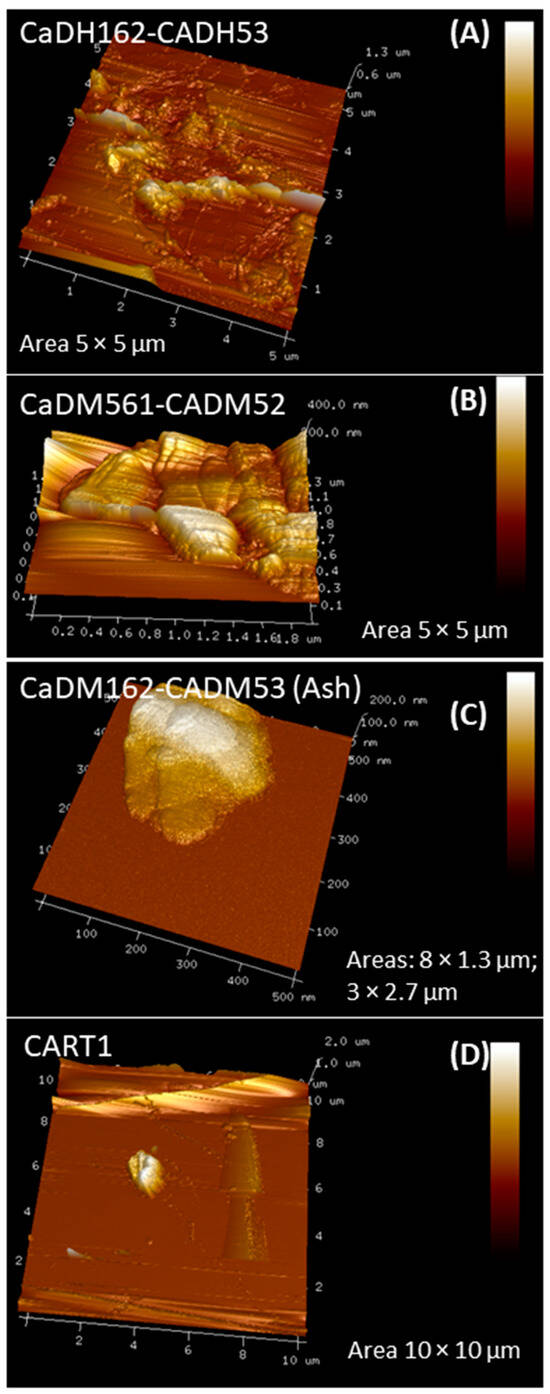
Figure 8.
Two-dimensional and three-dimensional topographic visualization of selected activated carbons (A–D) via atomic force microscopy.

Table 5.
Atomic force microscopy (AFM) analysis of selected activated carbons with highest adsorption.
The CaDH162-CADH53 sample (Figure 8A) exhibited roughness values ranging from 162 to 172 nm, with an average pore size between 0.6 and 1.3 µm. The confirmed presence of these pores significantly contributed to its superior adsorption performance, achieving values of 1094.8 mg/g in IS and 91.2 mg/g in MBS.
In contrast, the CaDM561-CADM52 sample (Figure 8B) displayed roughness values between 25.9 and 166 nm, along with porosity measurements ranging from 175 to 500 nm. The elevated pore size justified its substantial adsorption capacity of 96.8 mg/g for methylene blue.
The CaDM162-CADM53 sample (Figure 8C) showed roughness values from 77 to 162 nm and porosity between 0.6 and 1.3 µm. This sample demonstrated impressive adsorption capabilities, achieving 1043.2 mg/g in IS and 92.3 mg/g in MBS.
The CART1 sample analysis (Figure 8D) indicated roughness values of 198 to 419 nm and porosities ranging from 0.9 to 2.0 µm. Despite its high roughness, the iodine adsorption was relatively low; however, it exhibited promising results with an adsorption capacity of 87.8 mg/g for methylene blue.
The values of roughness and pore size obtained in the sample CaDH162-CADH53, differ from those obtained in the average pore diameter of 24 nm (Table 4). It is assumed that the variations detected may be due to the probe areas analyzed that presented irregular interconnected pores, with heterogeneous distribution and structural defects. In addition, although the activated carbon presents a specific average pore diameter, its roughness is the result of a combination of factors related to its pore structure, the activation method used and the intrinsic characteristics of the precursor material. These elements interact to define both the surface properties and the adsorptive capacities of the AC obtained.
4. Conclusions
- The optimal conditions for improving carbonization performance in a tube furnace were a particle size of 126–250 µm and a carbonization temperature of 600 °C for 2 h. In a muffle furnace, the best results were achieved with 0–125 µm particles at 600 °C for 2 h.
- For activation, the ideal conditions in a tube furnace involve an initial particle size of 126–250 µm, carbonized at 500 °C for 2 h, and activated at 500 °C for 2 h. In the muffle furnace, the optimal conditions were the same particle size, carbonized at 600 °C for 3 h, and activated at 600 °C for 1 h.
- Samples prepared in both tube and muffle furnaces showed similar gas adsorption characteristics. However, the AC produced via artisanal methods exhibited significantly higher nitrogen adsorption capacity.
- Increasing the carbonization temperature in tube furnaces enhances iodine adsorption, while in muffle furnaces, higher activation temperatures tend to reduce iodine adsorption.
- In the tube furnace, increasing the rice husk particle size may slightly decrease the adsorption capacity for MBS and other organic pollutants. Conversely, in the muffle furnace, lower activation temperatures enhanced methylene blue adsorption.
Author Contributions
Investigation: C.-A.M.L., G.L.A. and C.C.R.L.; Writing—original draft: C.-A.M.L. and A.-Y.V.C.; Methodology: V.-T.R.O., F.G.J. and B.H.J.; Formal analysis: V.-T.R.O., C.C.R.L., A.C.T.S., F.G.J., S.A.A. and N.R.M.; Validation: A.C.T.S.; Conceptualization: F.G.J. and B.H.J.; Writing—review & editing: S.A.A. and N.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by Agrarias, Universidad Estatal Península de Santa Elena, La Libertad, 204102, Santa Elena, Ecuador.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This work is part of the research work “Activated carbon from rice husk for the elimination of contaminants in water from deep wells” supported and financed by the State Technical University of Quevedo (Ecuador), the University of Barcelona (Spain) and the University from Granada (Spain). We thank the professionals Alda Geijo López, Loguard Rojas Uribe, and Ángel Cedeño Moreira for their support in the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Edokpayi, J.; Enitan, A.; Mutileni, N.; Odiyo, J. Water quality assessment and human risk assessment due to heavy metals in groundwater around the Muledane area of Vhembe District, Limpopo Province, South Africa. Chem. Control Mag. 2018, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Díaz, B.; Sommer-Marquez, A.O.P.E.; Gonzalez, E.B.; Ricaurte, M.; Navas, C.C. Synthesis methods, properties and modifications of biochar-based materials for wastewater treatment: A review. Resources 2024, 13, 8. [Google Scholar] [CrossRef]
- Ahmad, H.A.; Ahmad, S.; Cui, Q.; Wang, Z.; Wei, H.; Chen, X.; Ni, S.Q.; Ismail, S.; Awad, H.M.; Tawfik, A. The environmental distribution and removal of emerging pollutants, highlighting the importance of using microbes as a potential degrader: A review. Sci. Total Environ. 2022, 809, 151926. [Google Scholar] [CrossRef]
- Hannah, D.M.; Abbott, B.; Khamis, K.; Kelleher, C.; Lynch, I.; Krause, S.; Ward, A.S. Illuminating the “invisible water crisis” to address global water pollution challenges. Hydrol. Process. 2022, 36, e14525. [Google Scholar] [CrossRef]
- Babatunde, E.O.; Gurav, R.; Hwang, S.C.S. Pistia stratiotes L. Biochar for sorption removal of aqueous inorganic nitrogen. Materials 2024, 17, 3858. [Google Scholar] [CrossRef]
- Rehman, S.u.; De Castro, F.; Marini, P.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermibiocharcoal: A new approach to reduce the environmental impact of heavy metal contamination on agricultural land. Sustainability 2023, 15, 9380. [Google Scholar] [CrossRef]
- Jha, E.; Gaur, R.; Shahabuddin, S.; Tyagi, I. Biochar as a sustainable alternative and environmentally friendly adsorbent for the remediation of harmful pollutants: A comprehensive review. Toxics 2023, 2, 117. [Google Scholar] [CrossRef]
- Présiga-López, D.; Rubio-Clemente, A.; Pérez, J. Use of biochar as an alternative material for the treatment of polluted wastewater. Rev. UIS Ing. 2020, 20, 121–134. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.-C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite Waste Characterization and Use as Low-Cost, Ecofriendly, and Sustainable Material for Malachite Green and Methylene Blue Dyes Removal: Box–Behnken Design, Kinetics, and Thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Jagadeesh, N.; de Baranidharan, S. Adsorption of wastewater pollutants by biochar: A review. Hazard. Mater. Adv. Mag. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Iamsaard, K.; Weng, C.H.; Yen, L.; Tzeng, J.H.; Poonpakdee, C.; Lin, Y.T. Aadsorption of metals in pineapple leaf biochar: Key factors affecting, identification of mechanisms and assessment of regeneration. Bioresour. Technol. 2021, 344, 126131. [Google Scholar] [CrossRef]
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Mašek, O.; Parikh, S.J.; Ok, Y.S. Evaluation of biochar and its modifications for ammonium, nitrate and phosphate removal from water. Water Res. 2020, 186, 116303. [Google Scholar] [CrossRef]
- Guixiang, Z.; Zhihua, Z.; Zhu, Y. Changes in abiotic dissipation rates and bound fractions of antibiotics in biochar-amended soils. J. Clean. Prod. 2020, 256, 120314. [Google Scholar] [CrossRef]
- Fu, M.M.; Mo, C.H.; Hui, L.; Zhang, Y.N.H.W.X.; Wong, M.H. Comparación de las propiedades fisicoquímicas de los biochars e hidrochars producidos a partir de residuos alimentarios. Rev. Prod. Más Limpia 2019, 236, 2117637. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M. Effects of biochar on container substrate properties and growth of plants—A review. Horticulturae 2019, 5, 2–25. [Google Scholar] [CrossRef]
- Velázquez-Maldonado, J.; Juárez-López, P.; Anzaldo-Hernández, J.; Alejo-Santiago, G.; Valdez-Aguilar, L.A.; Alia Tejacal, I.; López-Martínez, V.; Pérez Arias, G.A.; Guillén Sánchez, D. Concentración nutrimental de biocarbón de cascarilla de arroz. Rev. Fitotec. Mex. 2019, 42, 129–136. [Google Scholar]
- Viotti, P.; Marzeddu, S.; Antonucci, A.; Décima, M.A.; Lovascio, P.; Tatti, F.; Boni, M.R. Biochar as an alternative material for the adsorption of heavy metals from groundwater: Review of a laboratory-scale experiment (column). Materials 2023, 17, 809. [Google Scholar] [CrossRef]
- Dong, M.; He, L.; Jiang, M.; Zhu, Y.; Wang, J.; Gustave, W.; Wang, S.; Deng, Y.; Zhang, X.; Wang, Z. Biochar for the Removal of Emerging Pollutants from Aquatic Systems: A Review. Int. J. Environ. Res. Public Health 2023, 20, 1679. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.; Li, G.; Tang, Y.T.; Reid, B.; Ngwabie, N.; Sun, G.X. The removal of arsenic from solution through biochar-enhanced precipitation of calcium-arsenic derivatives. Environ. Pollut. 2022, 292, 118241. [Google Scholar] [CrossRef] [PubMed]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.; Niazi, N.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G.; et al. A critical review on arsenic removal from water using biochar-based sorbents: The importance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Li, N.; Tao, J.; Yan, B.; Cui, X.; Chen, G. Adsorption of lead from aqueous solution by biochar: A review. Clean Tecnol. 2022, 4, 629–652. [Google Scholar] [CrossRef]
- Ndoun, M.; Elliott, H.; Preisendanz, H.; Williams, C.; Knopf, A.; Watson, J. Adsorption of pharmaceuticals from aqueous solutions using biochar derived from cotton gin waste and guayule bagasse. Biochar 2021, 3, 89–104. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Li, H.; Xu, D.; Li, X.; Xiang, L.; Tu, S. Review of rice husk biochar as an adsorbent for soil and water remediation. Plants 2023, 12, 1524. [Google Scholar] [CrossRef] [PubMed]
- Andalia, R.; Rahmi, R.; Julinawati, J.; Helwati, H. Isolation and characterization of cellulose from rice husk and sawdust waste with chemical method. Rev. Nat. 2020, 20, 6–9. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Determination of Iodine Number of Activated Carbon. 1999. Available online: https://cdn.standards.iteh.ai/samples/5745/e8253cc9a5ce4881916e4858e81f0bfa/ASTM-D4607-94-1999-.pdf (accessed on 1 December 2024).
- Asimbaya, C.; Rosas, N.; Endara, D.; Guerrero, V.H. Obtención de carbón activado a partir de residuos lignocelulósicos de canelo, laurel y eucalipto. Rev. Politécnica 2015, 36, 24–29. [Google Scholar]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 369–378. [Google Scholar] [CrossRef]
- Varón Camargo, J. Diseño, construcción y puesta a punto de un prototipo de quemador para la combustión continua y eficiente de la cascarilla de arroz. El Hombre Y Máquina 2005, 25, 128–135. [Google Scholar]
- Valverde, A.; Sarria, B.; Monteagudo, J.P. Análisis comparativo de las características fisicoquímicas de la cascarilla de arroz. Sci. Tech. 2007, 13, 255–260. [Google Scholar]
- Kalderis, D.K.M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Méndez, A.; Gasco, G.; Lado, M.; Paz González, A. The Effects of Feedstock, Pyrolysis Temperature, and Residence Time onthe Properties and Uses of Biochar from Broom and Time onthe Properties and Uses of Biochar from Broom and Gorce Wastes. Appl. Sci. 2024, 14, 4283. [Google Scholar] [CrossRef]
- Medina González, C.D.; Manrique-Abril, R.A. Uso del Carbón Activado Para el Tratamiento de Aguas. Revisión y Estudios de Caso. Rev. Nac. Ing. 2018, 1, 1–15. Available online: https://agenf.org/ojs/index.php/RNI/article/view/269/264 (accessed on 3 April 2024).
- Palacios-Zambrano, J.J.; Vera-Vera, Ä.R.; Arce-Santqana, I.E.; Lucero-Älvarez, M.G.; Barzola-Miranda, S.E.; Gutierrez-Lara, V.E. Análisis fisioquímico de tres variedades de carbón activado de cascarilla de arroz. Ingeniar Ing. Tecnol. E Investig. 2024, 7, 213–226. [Google Scholar] [CrossRef]
- Robinson Ubau, D.B.; Torres Martínez, D.U.; Vílchez Pérez, H.J. Uso sostenible de la cascarilla de arroz para productos de valor añadido. El Higo Rev. Científica 2022, 12, 2–27. [Google Scholar] [CrossRef]
- Sánchez, K.; Giplberto, C.; Pire, M.; Díaz, A.; Carrasquero, S. Adsorption capacity of activated carbon on total chromium from tannery waste. Rev. Técnica Fac. De Ing. Univ. Del Zulia 2013, 36, 45–52. [Google Scholar]
- Efiyanti, I.; Indrawan, D.A.; Hastuti, N.; Darmawan, S. The activated carbon produced from mayan bamboo. IOP Conf. Ser. Mater. Sci. Eng. 2020, 935, 012018. [Google Scholar] [CrossRef]
- Hajam, M.E.; Kandri, N.I.; Harrach, A.; Khomsi, A.E.; Zerouale, A. Adsorption of Methylene Blue on industrial softwood waste “Cedar” and hardwood waste “Mahogany”: Comparative study. Mater. Today Proc. 2019, 13, 812–821. [Google Scholar] [CrossRef]
- Nunes, C.A.; Guerreiro, M.C. Estimación de la supericie y el volumen de poros de carbones activados utiizando azul de metileno y número de yodo. Int. Year Chem. 2011, 34, 472–476. [Google Scholar] [CrossRef]
- Ebadollahzadeh, H.; Zabihi, M. Competitive adsorption of methylene blue and Pb (II) ions on the nano-magnetic activated carbon and alumina. Mater. Chem. Phys. 2020, 248, 122893. [Google Scholar] [CrossRef]
- Osman, Ü. Hydrogen storage capacity and methylene blue adsorption performance of activated carbon produced from Arundo donax. Mater. Chem. Phys. 2019, 237, 121858. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Perspectives on biochar and hydrocarbon production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Zhao, J.J.; Shen, X.J.; Domene, X.; Alcañiz, J.M.; Liao, X.; Palet, C. Comparison of biochars derived from different types of feedstocks and their potential for heavy metal removal in multi-metal solutions. Inf. Científicos 2019, 9, 9869. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Cao, L.; Ok, Y.S.; Cao, X. Characterization and quantification of electron donating capacity and its structure dependence in biochar derived from three waste biomasses. Chemosphere 2018, 211, 1073–1081. [Google Scholar] [CrossRef]
- Jing, F.; Pan, M.; Chen, J. Kinetic and isothermal adsorption-desorption of PAE on biochars: Effect of biomass feedstock, pyrolysis temperature and implication of desorption hysteresis mechanism. Pollut. Environ. Sci. Res. 2018, 25, 11493–11504. [Google Scholar] [CrossRef]
- Prieto García, J.O.; Curbelo Sánchez, A.; Albernas Carvajal, Y.; Rodríguez Suárez, E.; Ribalta Quezada, J.; Perez Leyva, A. Estudio de la adsorción de ácidos orgá¡nicos en ceniza de bagazo de caña de azúcar. Cent. Azúcar 2017, 44, 63–72. [Google Scholar]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Thapa, M.; Shrestha, R.G.; Maji, S.; Pradhananga, R.R.; Ariga, K. Materiales de carbono nanoporosos de gran superficie derivados de cáscara de arroz con excelentes propiedades de adsorción de yodo y azul de metileno. Repos. Inst. CONICET Digit. 2019, 5, 10. [Google Scholar] [CrossRef]
- Canh, V.D.; Tabata, S.; Yamanoi, S.; Onaka, Y.; Yokoi, T.; Furumai, H.; Katayama, H. Evaluación de adsorbentes de carbono poroso hechos a partir de cáscaras de arroz para la eliminación de virus en agua. Water 2021, 13, 1280. [Google Scholar] [CrossRef]
- Carrillo Quijano, C.C.; Albarracín Caballero, J.; Pereira Hernández, X.I. Producción de carbón activado y sílice a partir de cascarilla de arroz—Una revisión. Sci. Tech. 2013, 18, 422–429. [Google Scholar]
- Kim, J.E.; Bhatia, S.K.; Song, H.J.; Yoo, E.; Jeon, H.J.; Yoon, J.Y.; Yang, Y.; Gurav, R.; Yang, Y.H.; Kim, H.J.; et al. Adsorptive removal of tetracycline from aqueous solution using maple leaf-derived biochar. Bioresour. Technol. 2020, 306, 123092. [Google Scholar] [CrossRef]
- Arcos, C.A.; Macíaz Pinto, D.; Rodríguez Páez, J.E. La cascarilla de arroz como fuente de SiO2. Rev. Fac. Ing. Univ. Antioq. 2007, 41, 7–20. Available online: http://www.redalyc.org/articulo.oa?id=43004102 (accessed on 1 December 2024).
- Zoommicroscopio. Microscopía de Fuerza Atómica en Materiales. 2023. Available online: https://zoommicroscopio.com/microscopia-de-fuerza-atomica-en-la-investigacion-de-materiales/ (accessed on 23 January 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).