Abstract
As a well-known photocatalyst, TiO2 still suffers from rapid electron–hole recombination and limited visible light absorption. To overcome these challenges, the combination of graphene and TiO2 has been proposed. However, traditional methods such as ball milling and hydrothermal synthesis face limitations, including high energy consumption and complex procedures. Here, we develop a simple and industrially feasible method to prepare reduced graphene oxide (rGO)-coated TiO2 nanoparticles, referred to as rGO-TiO2 composites. The optimized rGO-TiO2 composites exhibit an enhanced photocatalytic degradation of rhodamine B (RhB) under simulated sunlight conditions, about 99.95% for 4% rGO-TiO2 within 80 min. The first-order reaction rate constant (k) of 4% rGO-TiO2 (0.0867 min−1) is 5.42 times higher than that of nano TiO2 (0.0135 min−1). The key reactive species involved in the degradation process are identified. Additionally, the effects of pH and NaCl concentration on the degradation efficiency of rGO-TiO2 are also investigated. The 4% rGO-TiO2 composite exhibits an excellent photocatalytic activity within the pH range of 3.87–11.89, and the NaCl concentration does not affect its photocatalytic efficiency. After characterization, the enhanced photocatalytic activity is ascribed to the introduction of rGO and the generation of surface oxygen vacancies (OV) and Ti3+ in TiO2 crystals.
1. Introduction
Photocatalytic oxidation can be widely used to remove pollutants from wastewater [1,2,3]. Titanium dioxide (TiO2) is a typical photocatalyst, but still suffers from rapid electron–hole recombination and limited visible light absorption [4,5,6]. To overcome the aforementioned limitations of TiO2, various modification strategies have been extensively explored, such as elemental doping [7,8], semiconductor coupling [9], co-catalyst loading [10,11,12], and surface modification [13,14,15]. Regarding surface modification of TiO2, Chen et al. synthesized black TiO2 via a hydrogen thermal treatment, introducing oxygen vacancies (OV) and Ti3+ defects on the surface [13]. Similarly, Reza et al. prepared black TiO2 by sintering P25 particles at high temperatures (500–800 °C) in a vacuum [14]. With the advancement of synthesis techniques for graphene and its derived materials, the combination of graphene and TiO2 has attracted increasing attention [16,17,18,19,20,21,22,23,24]. Graphene oxides (GOs) combined with TiO2 nanoparticles through ball milling demonstrate effective adsorption of dye molecules in solution [24]. TiO2-reduced GO (rGO) composites obtained through a hydrothermal reaction have been found to achieve a 99% degradation and decolorization rate for Reactive Red 3BS dye [22]. The previously discussed methods have inherent limitations. Ball milling damages the structure due to a high mechanical stress and the prolonged ball milling time increases production cycles with a large energy consumption [25]. The hydrothermal method requires a strict control of the reaction parameters and involves complex operations [26]. To the best of our knowledge, few studies have combined rGO and TiO2 composites with modifications to the TiO2 surface.
Up to now, there are still more works on rGO-TiO2 composites to simplify the synthesis process and achieve industrial-scale production. Here, we demonstrate that rGO-TiO2 composites can be produced in two steps: (1) first, by mixing a high-concentration GO dispersion with TiO2 nanoparticles to form GO-TiO2 composites, and (2) then, by vacuum annealing them at high temperatures to obtain rGO-TiO2 composites. Rhodamine B (RhB) is selected as a representative dye pollutant for its extensive industrial usage. Furthermore, we measure the degradation efficiency of various rGO-TiO2 composites under simulated sunlight conditions to evaluate their photocatalytic activity.
2. Experimental Section
2.1. Materials
The nano-TiO2 used was obtained from vapor-phase nano-TiO2 (≥99.5%, Hifull Inc., Yichang, China). Graphite (≥99.95% metal basis, 1200 mesh, Aladdin, Shanghai, China), sulfuric acid (≥70%, Aladdin, Shanghai, China), and K2S2O8 (AR grade, ≥99.5%, Aladdin, Shanghai, China) were used to form GO. RhB (AR grade, Aladdin, Shanghai, China) was chosen as the model dye pollutant for the evaluation of the photocatalytic activity. Deionized water (DI, 18.2 MΩ·cm Millipore system) was used as a solvent.
2.2. Sample Preparation
GO suspension with a concentration of ~10 mg/mL was prepared by the modified Hummers method [27,28]. A ten-minute ultrasonic treatment with a frequency of 70 kHz was performed to enhance the dispersion of the GO sheets. A total of 5 g nano-TiO2 was weighed into a beaker, followed by the addition of 5 mL, 10 mL, 20 mL, and 25 mL of GO dispersion to obtain the GO-TiO2 mixture solution. Subsequently, the GO-TiO2 mixture solution was stirred for 3 h. The solution was separated by centrifugation at 4000 rpm and then the solid GO-TiO2 mixture was prepared by filtration. The mixtures were then dried in an oven at 80 °C for 24 h to obtain GO-TiO2 composites with mass ratios of 1%, 2%, 4%, and 5%, designated as 1% GO-TiO2, 2% GO-TiO2, 4% GO-TiO2, and 5% GO-TiO2, respectively. These composites were subsequently annealed in a vacuum furnace at 600 °C for 2 h and labeled as 1% rGO-TiO2, 2% rGO-TiO2, 4% rGO-TiO2, and 5% rGO-TiO2. The vacuum pressure in the vacuum furnace was maintained at 10−3 Pa during the annealing process. Digital photographs of nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2 composites are shown in Figure S1.
2.3. Characterization
The sample morphology was characterized using scanning electron microscopy (SEM) (LEO 1530VP, LEO Elektronenmikroskopie GmbH, Oberkochen, Germany) and transmission electron microscopy (TEM) (Tecnai G2 F20 S-TWIN, FEI, New York, NY, USA). The phase composition of the samples was characterized using an X-ray diffractometer (D8 Advance, Bruker, Rheinstetten, Germany), with a Cu Kα radiation source (λ = 1.5406 Å). Raman spectra were excited by a cw 532 nm laser line and measured by a Raman spectrometer (Horiba HR Evolution, Horiba Jobin Yvon, Palaiseau, France), using a silicon (100) wafer for calibration. The Fourier transform infrared (FTIR) spectra of the samples were recorded using a Nicolet 6700 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), operating in the range of 4000–400 cm⁻1.The diffusive reflectance and absorbance spectra of the samples in the range of 200–800 nm were measured using a UV-Vis spectrophotometer (UV3600, Shimadzu, Kyoto, Japan), while BaSO4 was used as the reference. The band gap (Eg) of the samples was determined by using a tauc plot. The specific surface area of the photocatalyst was measured using the Brunauer–Emmett–Teller (BET) method through nitrogen adsorption–desorption experiments using a Micromeritics ASAP 2020 (Micromeritics, Norcross, GA, USA). Based on the cylindrical pore model, the pore size distribution was estimated using the Barrett–Joyner–Halenda (BJH) method [29]. Chemical structure characterizations were conducted using X-ray photoelectron spectroscopy (XPS) (Escalab Xi+, Thermo Fisher Scientific, USA) with Al Kα radiation.
2.4. Evaluation of Photocatalytic Performance
The concentration of the pollutant RhB was set to 10 mg/L, and the catalyst concentration was 1 g/L. A beaker with an internal cooling reflux was used as the reactor. The experimental temperature was decided based on the temperature of the deionized water and the room temperature, about 18 °C. The EOSUN Xe-100 light source from EOS Technologies Inc. (Troy, MI, USA) was used for photocatalysis experiments. The reaction mixture was stirred using a magnetic stirrer (79-1, Jintan Xinrui Instrument Factory, Changzhou, China), and the install is shown in Figure S2. Samples of 2 mL were taken at 0, 10, 20, 30, 50, and 80 min. After centrifugation at 1080 rpm for 3 min, the supernatant was analyzed for absorbance at 554 nm by using a microplate reader (Spectramax M2e, Molecular Devices, San Jose, CA, USA), and the concentration of RhB was calculated based on the absorbance and a standard calibration curve obtained from experiments (Figure S3) [30,31]. The degradation of RhB under simulated sunlight was described using first-order chemical reaction kinetics.
3. Results and Discussion
3.1. Photocatalytic Activity
Figure 1 shows the photocatalytic activities of the samples for the removal of RhB under simulated sunlight irradiation. Compared to the photocatalytic performance of the nano-TiO2, the rGO-TiO2 composites displayed a considerable photocatalytic activity. Moreover, the photocatalytic activity of the rGO-TiO2 composites progressively improved as the rGO/TiO2 ratio increased from 1% to 4%. When the ratio further increased to 5%, the photocatalytic activity declined. The 4% rGO-TiO2 composites exhibited the best photocatalytic activity by achieving an RhB removal efficiency of 99.95% within 80 min, while the nano-TiO2 sample only achieved 66.88%. The first-order reaction rate constants (k) of these samples are listed in Table S1. The k of 4% rGO-TiO2 was 0.0867 min⁻1, 5.42 times higher than the k of the nano-TiO2 sample (0.0135 min⁻1). With an increasing rGO content in the rGO-TiO2 composites, there was an enhancement of the adsorptive properties which promoted a greater adsorption of organic pollutants on the catalyst’s surface, potentially elevating the photocatalytic efficiency [32]. However, at higher rGO concentrations, the excessive adsorption may saturate the active sites, detrimentally affecting the photocatalytic performance [33]. Concurrently, an increase in rGO may lead to aggregation, reducing the available surface area for photocatalysis and impeding the penetration of light to the TiO2 particles, thereby limiting the formation of critical electron–hole pairs [34,35].
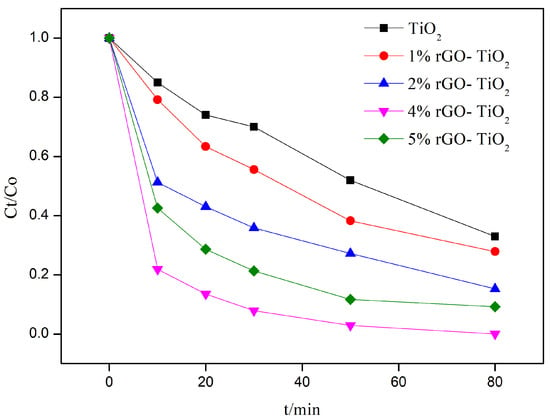
Figure 1.
Degradation efficiency of the nano-TiO2 sample and different rGO-TiO2 (1%, 2%, 4%, and 5%) samples in the RhB solution under simulated sunlight conditions (0.1448 W/cm2).
The photocatalytic activity of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2 samples are compared in Figure 2. It is apparent that the addition of GO strengthened the photocatalytic activity. Furthermore, the 4% rGO-TiO2 sample, prepared via the high-temperature vacuum annealing, exhibited the best photocatalytic activity. That is, the incorporation of GO combined with high-temperature vacuum annealing can lead to significant enhancement of the photocatalytic activity of TiO2.
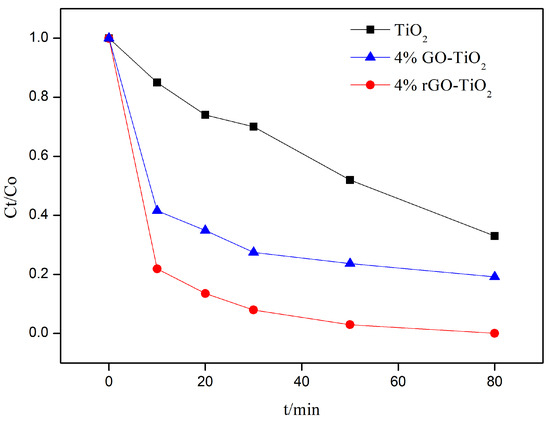
Figure 2.
Degradation efficiency of nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2 in relation to RhB under simulated sunlight conditions (0.1448 W/cm2).
To investigate the key reactive species involved in the degradation process, the following experimental studies were conducted. Generally, the main reactive species in photocatalytic processes include hydroxyl radicals (·OH), superoxide radicals (·O2), photogenerated holes (h+), and singlet oxygen (1O2) [36,37,38,39]. In this study, tertiary butyl alcohol (TBA) [37], ascorbic acid (AA) [39], disodium ethylenediaminetetraacetate (EDTA-2Na) [36], and L-histidine (L-His) [38] were used as scavengers for the reactive species, respectively, and the results are shown in Figure 3. The addition of TBA had no significant effect on the photocatalytic activity of the 4% rGO-TiO2 composite, indicating that hydroxyl radicals are not the primary reactive species. When AA, L-His, and EDTA-2Na were used, the degradation efficiency of RhB decreased to 92.03%, 82.25%, and 78.87%, respectively. These results demonstrate that h+ and 1O2 are the primary reactive species, while ·O2 plays a lesser role in the degradation process.
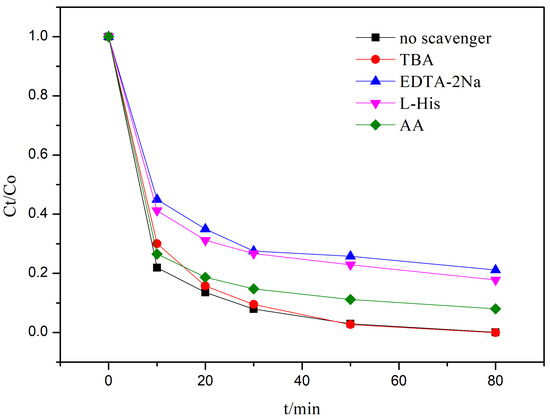
Figure 3.
Photodegradation of the RhB solution under simulated sunlight irradiation in the presence of 4% rGO-TiO2 with or without adding tertiary butyl alcohol (TBA), ascorbic acid (AA), ethylene–diaminetetra–acetic acid disodium salt (EDTA-2Na)and L-histidine (L-His).
Figure 4a,b illustrate the degradation efficiency of the 4% rGO-TiO2 in response to pH and NaCl concentration. The results indicate that the degradation efficiency of the 4% rGO-TiO2 was mildly influenced by the pH, with the highest efficiency observed under neutral conditions, followed by alkaline conditions, and the lowest under acidic conditions. At a pH of 11.89, the degradation efficiency reached 94.90% within 80 min, whereas at a pH of 2.01, it was only 80.14%. The surface charge of the 4% rGO-TiO2 and the pKa value of RhB, by modulating the electrostatic interactions between them, partially influenced the degradation process [40]. The point of zero charge (PZC) for the 4% rGO-TiO2 was calculated to be ≈3.47, and the pKa value of RhB was ≈3.72. When the pH value increased beyond PZC (pH > 3.47), the surface of the 4% rGO-TiO2 became negatively charged, while at lower pH values (pH < 3.47) the surface became positively charged. The same phenomenon is applicable to the pKa value of RhB [40,41]. At pH = 2.01, the surface of the 4% rGO-TiO2 and RhB were both positively charged, so there was electrostatic repulsion between the 4% rGO-TiO2 and RhB. However, the electrostatic forces were not the only interactions between the 4% rGO-TiO2 and the RhB; hydrogen bonding, π-π conjugation, and Van Der Waals forces also played important roles [42,43]. The O₂ preferentially adsorbs onto the negatively charged surface of the catalyst and would, subsequently, be oxidized into ·O2 [44,45,46]. Additionally, the concentration of ·O2 is considered to be pH-dependent, with alkaline conditions favoring their production, while acidic conditions inhibit it [47]. The ·O2 is an important precursor for the generation of 1O2 [48,49,50]. However, as the pH increased, both the 4% rGO-TiO2 and the RhB acquired more negative charges, leading to increased electrostatic repulsion between them. Consequently, alkaline conditions were not necessarily optimal for this process. Figure 4b demonstrates that the degradation efficiency of the 4% rGO-TiO2 composite was largely unaffected by NaCl concentrations. The 4% rGO-TiO2 composite can be used for photocatalytic degradation of high-salinity organic wastewater.
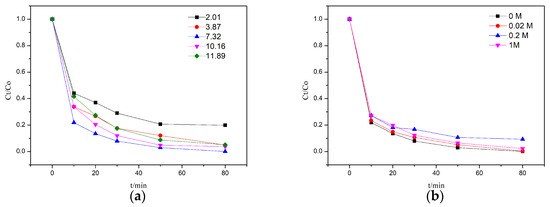
Figure 4.
The effect of pH (a) and NaCl (b) on the degradation efficiency of 4% rGO-TiO2.
3.2. Characterization Results
Figure 5 shows the TEM images of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2. The sizes of the nano-TiO2 were in the range of 10–25 nm (Figure 5a). After the incorporation of GO, the sizes joined the range of 15–30 nm (Figure 5b). Vacuum annealing was able to induce the agglomeration of the 4% rGO-TiO2 nanoparticles (Figure 5c), which is consistent with the SEM images in Figure S5. The sizes of the 4% rGO-TiO2 nanoparticles underwent an obvious growth and joined the range of 20–35 nm. That is, 4% rGO-TiO2 nanoparticles had a tendency of crystal growth by vacuum annealing. The high-magnification TEM image of the 4% rGO-TiO2 in Figure 5d shows the TiO2 nanoparticle coated with the rGO. The lattice fringes of the TiO2 nanoparticle are also clearly observed. The lattice spacing was 0.35 nm (the inset of Figure 5d), corresponding to the (101) planes of the anatase TiO2.
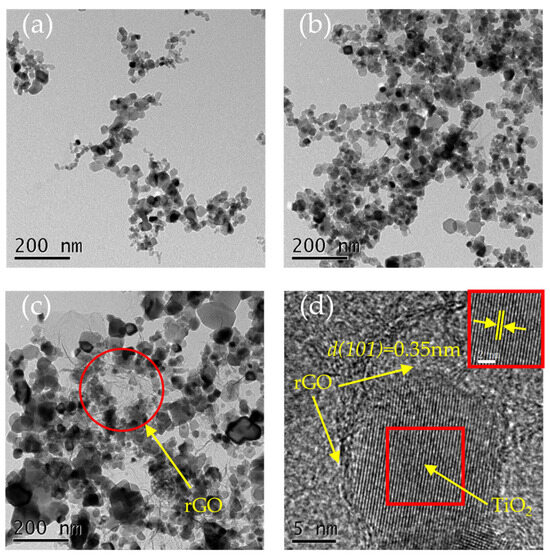
Figure 5.
TEM images of the (a) nano-TiO2, (b) 4% GO-TiO2, and (c) 4% rGO-TiO2 are shown at a scale of 200 nm, while (d) presents a high-magnification TEM image of the 4% rGO-TiO2 at a scale of 5 nm. The inset in (d) highlights the lattice spacing.
The specific surface area (SBET), pore volume, and pore size of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2 samples are shown in Table 1. In comparison with the nano-TiO2, the 4% GO-TiO2 sample had an obvious increase in SBET, pore volume, and pore size. Note that SBET decreased after vacuum annealing. This may be caused by the growth and agglomeration of TiO2 nanoparticles during the high-temperature annealing [14,51].

Table 1.
BET area, pore size, and pore volume of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2.
Figure 6 shows the XRD patterns of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2 samples. The diffraction peaks at 25.3°, 37.9°, 48.0°, 54.1°, and 62.7° corresponded to the (101), (004), (200), (105), and (204) planes of the anatase TiO2, respectively. The peaks at 27.4°, 36.1°, 41.2°, and 69.0° were attributed to the (110), (101), (111), and (301) planes of the rutile TiO2, respectively [14,52]. This indicates that the nano-TiO2 was a mixture of anatase and rutile. No diffraction peaks for GO or rGO were observed in the 4% GO-TiO2 and 4% rGO-TiO2 samples, likely because of their low concentrations [32,53]. Moreover, the diffraction peak at 24.4° for rGO was close to the anatase TiO2 (101) peak at 25.3°, a phenomenon which caused the overlapping of the rGO diffraction peak in the 4% rGO-TiO2 composites [53]. As shown in Figure 6, compared with the intensities of the anatase TiO2 diffraction peaks, the relative intensities of the rutile TiO2 diffraction peaks were enhanced after vacuum annealing, indicating that some of the anatase samples were transformed into rutile (see Table S2) [14,54,55,56]. Moreover, using the Debye–Scherrer formula [57], the average crystallite sizes shown in Table S2 increased following the addition of GO and the high-temperature annealing, a phenomenon which is in consistent with the TEM observation.
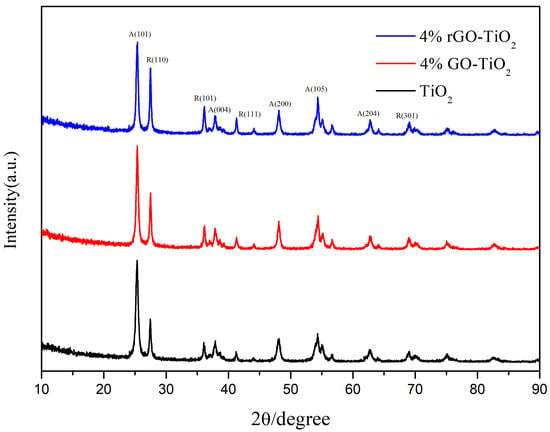
Figure 6.
XRD patterns of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2: A(101) corresponds to the (101) crystal plane of the anatase, while R(110) refers to the (110) crystal plane of the rutile, and so on for other crystal planes.
Figure 7 shows the Raman spectra of the 4% GO-TiO2 and 4% rGO-TiO2 samples. The five bands at 144 cm⁻1, 197 cm⁻1, 399 cm⁻1, 520 cm⁻1, and 637 cm⁻1 corresponded to the five active vibrational modes Eg, Eg, B1g, A1g + B1g, and Eg of the anatase TiO2, confirmed as the dominant crystalline phase, consistent with the XRD results [51,58]. The D band (1360 cm⁻1) and G band (1590 cm⁻1) characteristics of GO and rGO were prominent, indicating the existence of GO and rGO coatings on TiO2. Moreover, the ID/IG ratios of the 4% rGO-TiO2 was 0.84, lower than that of the 4% GO-TiO2 (0.89), a phenomenon which was attributed to the removal of oxygen functional groups during annealing [59]. The FTIR analysis in Figure S7 can also confirm the reduction of GO.
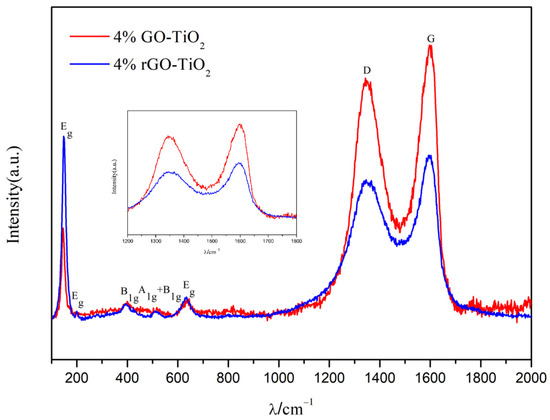
Figure 7.
Raman spectra of the 4% GO-TiO2 and 4% rGO-TiO2 samples in the range of 100 to 2000 cm⁻1. The inset highlights the differences in the D band (associated with sp3 defects) and the G band (linked to sp2 carbon) within the 1200 to 1800 cm⁻1 range. The ID/IG values of the 4% GO-TiO2 and 4% rGO-TiO2 samples are 0.89 and 0.84, respectively.
Figure 8 displays the diffusive reflectance and absorbance spectra of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2 samples. All samples exhibited strong absorption in the ultraviolet region (200−400 nm). For the 4% GO-TiO2 sample, red-shift absorbance can be observed. Furthermore, high-temperature vacuum annealing caused the more significant visible light absorption. The obvious shifts in the light absorption from UV to visible light clearly show the reduction of the band gap. Figure S7 reveals that the bandgaps of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2 samples were 3.19 eV (~388.73 nm), 2.95 eV (~420.58 nm), and 2.68 eV (~462.95 nm), respectively. That is, the incorporation of the GO or rGO coating and the high-temperature vacuum annealing can effectively narrow the bandgap, which critically influences the photocatalytic activity of catalysts [60,61,62].
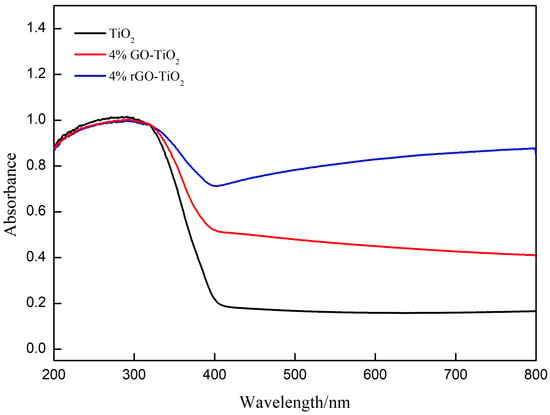
Figure 8.
Diffusive reflectance and absorbance spectra of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2.
The change in the surface chemical bonding of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2 samples was examined using XPS, as shown in Figure 9. The O 1s XPS spectra showed dramatic differences. Though the Ti-O-Ti peak (530.2 eV) was almost the same, the 4% rGO-TiO2 presented a significant enhancement of the peak (532.2 eV), indicating the formation of oxygen vacancies (OV) in the crystal surface [63,64,65]. The C 1s XPS spectra showed that the binding energies of C-C, C-O, and C=O were 284.8, 286.5, and 288.7 eV, respectively. There was no carbon in the nano-TiO2, and its C 1s peak must have come from the adventitious carbon species [63,64,65]. It should be noted that the C-O peak of the 4% rGO-TiO2 sample was more obvious, indicating that the C in rGO have a tendency to bond to the O in TiO2 during high-temperature vacuum annealing. This may also cause the formation of OV. In the Ti 2p XPS spectra of the 4% rGO-TiO2, the same shape of Ti 2p3/2 but a decrease in the 2p1/2 peak compared to the 4% GO-TiO2 were observed, which may be attributed to the partial reduction of Ti4+ to Ti3+ during the high-temperature vacuum annealing process. Further analysis in the Figure S8 revealed the successful separation of Ti3+ from Ti4+, indicating the presence of Ti3+ on the material’s surface [66,67,68]. As a result, high-temperature vacuum annealing may enhance the interfacial interaction between rGO and TiO2 and the formation of some defects (like OV and Ti3+) in the 4% rGO-TiO2, a phenomenon which may lead to the enhanced absorption (Figure 8) and narrowed bandgap (Figure S7) [14]. Thus, the 4% rGO-TiO2 demonstrated the best photocatalytic activity in all samples.
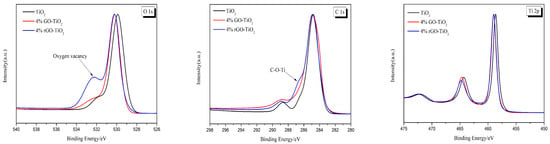
Figure 9.
XPS spectra of the nano-TiO2, 4% GO-TiO2, and 4% rGO-TiO2. The left panel shows the O 1s spectra, highlighting the oxygen vacancy peak observed at approximately 532 eV for the 4% rGO-TiO2 sample. The mid panel depicts the C 1s spectra, where the formation of the C-O-Ti bond is observed around 286–287 eV. The right panel exhibits the Ti 2p spectra. The Ti 2p3/2 peak remains unchanged, while a decrease in the Ti 2p1/2 peak is observed in the 4% rGO-TiO2 sample compared to 4% GO-TiO2.
4. Conclusions
In summary, rGO-TiO2 composites are synthesized by mixing a high-concentration GO dispersion with TiO2 nanoparticles and by subjecting the mixture to high-temperature vacuum annealing. The 4% rGO-TiO2 composite demonstrates a surprising removal efficiency of 99.95% for RhB under simulated sunlight. Moreover, the degradation of RhB using the 4% rGO-TiO2 composite is less sensitive to variations in pH and NaCl concentration. The simple preparation method, combined with the 4% rGO-TiO2 composite’s broad pH and salinity tolerance, highlights its promising potential for industrial-scale production and applications.
High-temperature vacuum annealing leads to the transformation of anatase into rutile of TiO2, an increase in the average crystallite size, and a decrease in the surface area of rGO-TiO2. These changes are harmful for photocatalysis, as larger crystallite sizes may lead to an increased recombination rate of electron–hole pairs and the rutile TiO2 exhibits an inferior photocatalytic activity compared to the anatase TiO2. Moreover, the reduction of GO and the formation of OV and Ti3+ in the 4% rGO-TiO2 composite are also analyzed. These promote the light absorption and a bandgap reduction in the rGO-TiO2 compared to TiO2 and GO-TiO2.
To optimize the photocatalytic performance of rGO-TiO2 composites, it is crucial to carefully control the temperature and duration of the high-temperature vacuum annealing process to moderate the phase transformation and the increase in crystallite size. Also, the time and temperature of the annealing process should ensure the reduction of GO and the formation of OV and Ti3+. In the future, the rGO-TiO2 composites are expected to be applied in the photocatalytic degradation of persistent organic pollutants, such as chlorobenzene, bisphenol A, and toxaphene.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w17020161/s1. Figure S1. Digital photographs of nano TiO2 (left), 4% GO-TiO2 (mid) and 4% rGO-TiO2 (right). Figure S2. Schematic diagram of photocatalytic experimental install. Figure S3. Standard calibration curve of RhB. Figure S4. First-order reaction rate constant fitting curve for the RhB degradation. Figure S5. SEM images of nano TiO2(a), 4% GO-TiO2(b) and 4% rGO-TiO2(c). Figure S6. EDS of nano TiO2 (top), 4% GO-TiO2 (mid) and 4% rGO-TiO2 (bottom). Figure S7. Bandgap of nanoTiO2, 4% GO-TiO2 and 4% rGO-TiO2. Figure S8. XPS spectra of Ti 2p peaks for 4% rGO-TiO2. Figure S9. FTIR spectra of 4% GO-TiO2 and 4% rGO-TiO2. Table S1. k value of TiO2 and different mass ratios rGO-TiO2 on RhB. Table S2. Nano TiO2, 4% GO-TiO2 and 4% rGO-TiO2 crystalline size and phase compositions.
Author Contributions
Methodology, N.X., J.H. and L.Y.; Formal analysis, NX., Y.G., Y.N. and L.Y.; Data curation, N.X., Y.G., Y.N., Y.Y., Z.Y., W.Z., R.L., X.W., H.Z., L.Z. and Y.W.; Writing—original draft, N.X.; Writing—review & editing, J.H. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 12175302, 12005284) and by the ABA Company (No. SCH1829202E).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the continuing nature of our research.
Acknowledgments
The authors from Fudan University would like to thank the ABA Company for providing financial support for this research. L.Y. and H.Z. acknowledge support from the National Natural Science Foundation of China. All the authors appreciate the staff from Shanghai Synchrotron Radiation Facility (SSRF) and User Experiment Assist System.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef]
- Dey, S.; Manna, K.; Pradhan, P.; Sarkar, A.N.; Roy, A.; Pal, S. Review of Polymeric Nanocomposites for Photocatalytic Wastewater Treatment. ACS Appl. Nano Mater. 2024, 7, 4588–4614. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Ren, Z.F.; Fan, H.J.; Yang, X.M. Elementary photocatalytic chemistry on TiO2 surfaces. Chem. Soc. Rev. 2016, 45, 3701–3730. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Hou, H.; Fang, Z.; Gao, F.; Wang, L.; Chen, D.; Yang, W. Hydrogenated TiO2 Nanorod Arrays Decorated with Carbon Quantum Dots toward Efficient Photoelectrochemical Water Splitting. Acs Appl. Mater. Interfaces 2019, 11, 19167–19175. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Duan, L.; Shen, H.; Zhang, Q.; Zhao, X. Influences of annealing atmosphere on phase transition temperature, optical properties and photocatalytic activities of TiO2 phase-junction microspheres. J. Alloys Compd. 2019, 789, 1015–1021. [Google Scholar] [CrossRef]
- Mittal, A.; Mari, B.; Sharma, S.; Kumari, V.; Maken, S.; Kumari, K.; Kumar, N. Non-metal modified TiO2: A step towards visible light photocatalysis. J. Mater. Sci. Mater. Electron. 2019, 30, 3186–3207. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Balapure, A.; Ray Dutta, J.; Ganesan, R. Recent advances in semiconductor heterojunctions: A detailed review of the fundamentals of photocatalysis, charge transfer mechanism and materials. RSC Appl. Interfaces 2024, 1, 43–69. [Google Scholar] [CrossRef]
- Jia, S.; Shu, X.; Song, H.; An, Z.; Xiang, X.; Zhang, J.; Zhu, Y.; He, J. Insights into Photocatalytic Selective Dehydrogenation of Ethanol over Au/Anatase–Rutile TiO2. Ind. Eng. Chem. Res. 2021, 60, 12282–12291. [Google Scholar] [CrossRef]
- Yu, L.; Tang, B. Photocatalytic Degradation of Phenolic Compounds from Wastewater Using Titanium dioxide@reduced Graphene Oxide (TiO2@rGO) Nanocomposites. Int. J. Electrochem. Sci. 2021, 16, 210915. [Google Scholar] [CrossRef]
- Du, X.; Luo, J.P.; Qin, Q.S.; Zhang, J.H.; Fu, D. Modified TiO2-rGO Binary Photo-Degradation Nanomaterials: Modification, Mechanism, and Perspective. Catal. Surv. Asia 2022, 26, 16–34. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Davood Abadi Farahani, M.H.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a New Type of Black TiO2 under a Vacuum Atmosphere for Sunlight Photocatalysis. Acs Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef]
- Wu, L.; Fu, C.; Huang, W. Surface chemistry of TiO2 connecting thermal catalysis and photocatalysis. Phys. Chem. Chem. Phys. 2020, 22, 9875–9909. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites.: UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, W.M.; Long, M.C.; Zhou, B.X.; Wu, Y.H.; Wu, D.Y.; Feng, Y.J. Synthesis of Visible-Light Responsive Graphene Oxide/TiO2 Composites with p/n Heterojunction. ACS Nano 2010, 4, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.Z.; Guo, S.J.; Wang, P.; Xing, L.; Fang, Y.X.; Zhai, Y.M.; Dong, S.J. One-pot, water-phase approach to high-quality graphene/TiO2 composite nanosheets. Chem. Commun. 2010, 46, 7148–7150. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, P.; Dong, S.J. Progress in graphene-based photoactive nanocomposites as a promising class of photocatalyst. Nanoscale 2012, 4, 5814–5825. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z. Comparing Graphene-TiO2 Nanowire and Graphene-TiO2 Nanoparticle Composite Photocatalysts. Acs Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Yang, J.; Zhao, D.; Chen, Y.; Cao, Y. Research on Photocatalytic Properties of TiO2-Graphene Composites with Different Morphologies. J. Mater. Eng. Perform. 2017, 26, 3263–3270. [Google Scholar] [CrossRef]
- Wu, H.; Gao, X.; Fang, J.; Liu, Q.; He, P. Preparation and photocatalytic degradation decoloring of TiO2 /reduced graphene oxide composites. J. Text. Res. 2018, 39, 78–83. [Google Scholar]
- Wang, R.; Shi, K.; Huang, D.; Zhang, J.; An, S. Synthesis and degradation kinetics of TiO2/GO composites with highly efficient activity for adsorption and photocatalytic degradation of MB. Sci. Rep. 2019, 9, 18744–18752. [Google Scholar] [CrossRef]
- Cano, F.J.; Reyes-Vallejo, O.; Ashok, A.; Olvera, M.D.; Velumani, S.; Kassiba, A. Mechanisms of dyes adsorption on titanium oxide- graphene oxide nanocomposites. Ceram. Int. 2023, 49, 21185–21205. [Google Scholar] [CrossRef]
- Joy, J.; Krishnamoorthy, A.; Tanna, A.; Kamathe, V.; Nagar, R.; Srinivasan, S. Recent Developments on the Synthesis of Nanocomposite Materials via Ball Milling Approach for Energy Storage Applications. Appl. Sci. 2022, 12, 9312. [Google Scholar] [CrossRef]
- Huang, L.; Liu, T.; Zhang, H.; Guo, W.; Zeng, W. Hydrothermal synthesis of different TiO2 nanostructures: Structure, growth and gas sensor properties. J. Mater. Sci. Mater. Electron. 2012, 23, 2024–2029. [Google Scholar] [CrossRef]
- Jiang, J.; Mu, L.H.; Qiang, Y.; Yang, Y.Z.; Wang, Z.K.; Yi, R.B.; Qiu, Y.W.; Chen, L.; Yan, L.; Fang, H.P. Unexpected Selective Absorption of Lithium in Thermally Reduced Graphene Oxide Membranes. Chin. Phys. Lett. 2021, 38, 116802. [Google Scholar] [CrossRef]
- Jiang, J.; Yan, L.; Fang, H.P. Effect of Oxide Content of Graphene Oxide Membrane on Remarkable Adsorption for Calcium Ions. Chin. Phys. Lett. 2021, 38, 106801. [Google Scholar] [CrossRef]
- Zhou, G.; Shen, L.; Xing, Z.; Kou, X.; Duan, S.; Fan, L.; Meng, H.; Xu, Q.; Zhang, X.; Li, L.; et al. Ti3+ self-doped mesoporous black TiO2/graphene assemblies for unpredicted-high solar-driven photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2017, 505, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, H.; Xu, B.; Weng, S.; Wang, S.; Tan, S.; Xie, Z.; Fang, F. FeCoNiMn/Ti electrode prepared by magnetron sputtering for efficient RhB degradation. Vacuum 2023, 214, 112186. [Google Scholar] [CrossRef]
- Quanju, Y.; Zeyan, W.; Ye, Z.; Manyun, L. Study on the performance of Cu2+ doped nano zero-valent iron for the oxidative degradation of Rhodamine B. Chem. Res. Appl. 2024, 36, 1632–1638. [Google Scholar]
- Ruidíaz-Martínez, M.; Álvarez, M.A.; López-Ramón, M.V.; Cruz-Quesada, G.; Rivera-Utrilla, J.; Sánchez-Polo, M. Hydrothermal Synthesis of rGO-TiO2 Composites as High-Performance UV Photocatalysts for Ethylparaben Degradation. Catalysts 2020, 10, 520. [Google Scholar] [CrossRef]
- Petala, A.; Noe, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Mantzavinos, D.; Kondarides, D.I. Synthesis and characterization of CoOx/BiVO4 photocatalysts for the degradation of propyl paraben. J. Hazard. Mater. 2019, 372, 52–60. [Google Scholar] [CrossRef]
- Long, M.; Qin, Y.; Chen, C.; Guo, X.; Tan, B.; Cai, W. Origin of Visible Light Photoactivity of Reduced Graphene Oxide/TiO2 by in Situ Hydrothermal Growth of Undergrown TiO2 with Graphene Oxide. J. Phys. Chem. C 2013, 117, 16734–16741. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Wang, G.; Li, L.; Wang, C.; Zhang, X. Fabrication of black TiO2/TiO2 homojunction for enhanced photocatalytic degradation. J. Mater. Sci. 2019, 54, 14320–14329. [Google Scholar] [CrossRef]
- Hu, X.; Han, W.; Zhang, M.; Li, D.; Sun, H. Enhanced adsorption and visible-light photocatalysis on TiO2 with in situ formed carbon quantum dots. Environ. Sci. Pollut. Res. Int. 2022, 29, 56379–56392. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Li, J.; Zhou, W.; Wang, Y.; Li, Q. Sulfate promotes the photocatalytic degradation of antibiotics by porphyrin MOF: The electron-donating effect of the anion. Environ. Funct. Mater. 2023, 2, 46–56. [Google Scholar] [CrossRef]
- Ma, C.; Fang, G.; Tian, J.; Zhang, G.; Yan, X. Effect of Bi/Cl atomic ratio on the photocatalytic activities of TiO2/BixOyClz composites. Acta Mater. Compos. Sin. 2024, 43, 1–10. [Google Scholar]
- Devi, S.; Kumari, S.; Sharma, A.; Dhiman, M.; Thakur, M.; Kumar, A. Boosting the photocatalytic activity of g-C3N4 via loading bio-synthesized Ag0 nanoparticles and imidazole modification for the degradation and mineralization of fluconazole. Environ. Sci. Pollut. Res. 2024, 31, 15851–15871. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Kumar, S.; Devi, J.; Sharma, A.; Kumar, A. Decoration of 1,3 oxazole modified g- C3N4 by Bio-synthesized Ag nanoparticle for the photodegradation of pharmaceutical effluent: Clotrimazole. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Donga, C.; Mishra, S.B.; Ndlovu, L.N.; Abd-El-Aziz, A.S.; Kuvarega, A.T.; Mishra, A.K. Magnetic Magnetite-Graphene Oxide (Fe3O4-GO) Nanocomposites for Removal of Dyes from Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2024, 34, 4192–4202. [Google Scholar] [CrossRef]
- Berrellez-Reyes, F.; Alvarez-Garcia, S. Insights into the Interaction of Graphene Oxide and Adsorbed RhB by Raman Spectral Deconvoluted Scanning. J. Phys. Chem. C 2019, 123, 30021–30027. [Google Scholar] [CrossRef]
- Ma, S.; Reish, M.E.; Zhang, Z.; Harrison, I.; Yates, J.T., Jr. Anatase-Selective Photoluminescence Spectroscopy of P25 TiO2 Nanoparticles: Different Effects of Oxygen Adsorption on the Band Bending of Anatase. J. Phys. Chem. C 2017, 121, 1263–1271. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Zhou, H.J.; Wang, G.Z.; Zhang, Y.X.; Zhang, H.M.; Zhao, H.J. Enhancement of the visible-light photocatalytic activity of CeO2 by chemisorbed oxygen in the selective oxidation of benzyl alcohol. New J. Chem. 2019, 43, 7355–7362. [Google Scholar] [CrossRef]
- Wang, D.; Xue, J.; Ding, X.; Wei, J.; Feng, C.; Wang, R.; Ma, P.; Wang, S.; Cao, H.; Wang, J.; et al. Neighboring Cationic Vacancy Assisted Adsorption Optimization on Single-Atom Sites for Improved Oxygen Evolution. ACS Catal. 2022, 12, 12458–12468. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, W.; Li, Y.; Dong, N.; Yu, H.; Yin, W.; Zhu, F.; Gao, B.; Xu, S. Effective inhibition of chloride ion interference in photocatalytic process by negatively charged molecularly imprinted photocatalyst: Behavior and mechanism. Water Res. 2024, 262, 122040. [Google Scholar] [CrossRef] [PubMed]
- Daimon, T.; Nosaka, Y. Formation and Behavior of Singlet Molecular Oxygen in TiO2 Photocatalysis Studied by Detection of Near-Infrared Phosphorescence. J. Phys. Chem. C 2007, 111, 4420–4424. [Google Scholar] [CrossRef]
- Buchalska, M.; Labuz, P.; Bujak, L.; Szewczyk, G.; Sarna, T.; Mackowski, S.; Macyk, W. New insight into singlet oxygen generation at surface modified nanocrystalline TiO2—The effect of near-infrared irradiation. Dalton Trans. 2013, 42, 9468–9475. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Identification and Roles of the Active Species Generated on Various Photocatalysts, in Photocatalysis and Water Purification. In Photocatalysis and Water Purification: From Fundamentals to Recent Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 1–24. [Google Scholar]
- Stengl, V.; Bakardjieva, S.; Grygar, T.M.; Bludská, J.; Kormunda, M. TiO2-graphene oxide nanocomposite as advanced photocatalytic materials. Chem. Cent. J. 2013, 7, 41. [Google Scholar] [CrossRef]
- Sukarman; Kristiawan, B.; Khoirudin; Abdulah, A.; Enoki, K.; Wijayanta, A.T. Characterization of TiO2 nanoparticles for nanomaterial applications: Crystallite size, microstrain and phase analysis using multiple techniques. Nano-Struct. Nano-Objects 2024, 38, 101168. [Google Scholar] [CrossRef]
- Trapalis, A.; Todorova, N.; Giannakopoulou, T.; Boukos, N.; Speliotis, T.; Dimotikali, D.; Yu, J. TiO2/graphene composite photocatalysts for NOx removal: A comparison of surfactant-stabilized graphene and reduced graphene oxide. Appl. Catal. B Environ. 2016, 180, 637–647. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Zhou, M.; Zhao, X. Enhanced photocatalytic activity of TiO2 powder (P25) by hydrothermal treatment. J. Mol. Catal. A Chem. 2006, 253, 112–118. [Google Scholar] [CrossRef]
- Lin, C.P.; Chen, H.; Nakaruk, A.; Koshy, P.; Sorrell, C.C. Effect of Annealing Temperature on the Photocatalytic Activity of TiO2 Thin Films. Energy Procedia 2013, 34, 627–636. [Google Scholar] [CrossRef]
- Velardi, L.; Scrimieri, L.; Serra, A.; Manno, D.; Calcagnile, L. Effect of temperature on the physical, optical and photocatalytic properties of TiO2 nanoparticles. SN Appl. Sci. 2020, 2, 707. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, L.; Guo, J. Effects of calcination on the photocatalytic properties of nanosized TiO2 powders prepared by TiCl4 hydrolysis. Appl. Catal. B Environ. 2000, 26, 207–215. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Lee, E.; Lee, D.; Yoon, J.; Yin, Y.; Lee, Y.N.; Uprety, S.; Yoon, Y.S.; Kim, D.J. Enhanced Gas-Sensing Performance of GO/TiO2 Composite by Photocatalysis. Sensors 2018, 18, 3334. [Google Scholar] [CrossRef]
- Ullah, I.; Haider, A.; Khalid, N.; Ali, S.; Ahmed, S.; Khan, Y.; Ahmed, N.; Zubair, M. Tuning the band gap of TiO2 by tungsten doping for efficient UV and visible photodegradation of Congo red dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 150–157. [Google Scholar] [CrossRef]
- Isik, M.; Delice, S.; Gasanly, N. Temperature-dependent optical properties of TiO2 nanoparticles: A study of band gap evolution. Opt. Quantum Electron. 2023, 55, 905. [Google Scholar] [CrossRef]
- Wang, G.Y.; Guo, W.J.; Xu, D.P.; Liu, D.; Qin, M.T. Graphene Oxide Hybridised TiO2 for Visible Light Photocatalytic Degradation of Phenol. Symmetry 2020, 12, 1420. [Google Scholar] [CrossRef]
- Zhang, P.; Tachikawa, T.; Fujitsuka, M.; Majima, T. Atomic Layer Deposition-Confined Nonstoichiometric TiO2 Nanocrystals with Tunneling Effects for Solar Driven Hydrogen Evolution. J. Phys. Chem. Lett. 2016, 7, 1173–1179. [Google Scholar] [CrossRef]
- Cui, Z.P.; Zhao, M.; Que, X.Y.; Wang, J.J.; Xu, Y.; Ghazzal, M.N.; Colbeau-Justin, C.; Pan, D.Q.; Wu, W.S. Facile Vacuum Annealing-Induced Modification of TiO2 with an Enhanced Photocatalytic Performance. ACS Omega 2021, 6, 27121–27128. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, M.; Li, S.; Wang, J.; Xu, Y.; Ghazzal, M.N.; Colbeau-Justin, C.; Pan, D.; Wu, W. Facile Vacuum Annealing of TiO2 with Ethanol-Induced Enhancement of Its Photocatalytic Performance under Visible Light. Ind. Eng. Chem. Res. 2022, 61, 14455–14461. [Google Scholar] [CrossRef]
- Zheng, P.; Liu, T.; Su, Y.; Zhang, L.F.; Guo, S.W. TiO2 nanotubes wrapped with reduced graphene oxide as a high-performance anode material for lithium-ion batteries. Sci. Rep. 2016, 6, 36580. [Google Scholar] [CrossRef]
- Ran, P.; Jiang, L.; Li, X.; Zuo, P.; Li, B.; Li, X.J.; Cheng, X.Y.; Zhang, J.T.; Lu, Y.F. Redox shuttle enhances nonthermal femtosecond two-photon self-doping of rGO-TiO2-x photocatalysts under visible light. J. Mater. Chem. A 2018, 6, 16430–16438. [Google Scholar] [CrossRef]
- Song, X.; Li, W.; He, D.; Wu, H.; Ke, Z.; Jiang, C.; Wang, G.; Xiao, X. The “Midas Touch” Transformation of TiO2 Nanowire Arrays during Visible Light Photoelectrochemical Performance by Carbon/Nitrogen Coimplantation. Adv. Energy Mater. 2018, 8, 1800165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).