Combined Efficacy of Silver, Copper, and Hypochlorite Ions for Vector Control of Juvenile Aedes aegypti in Household Water Storage Containers
Abstract
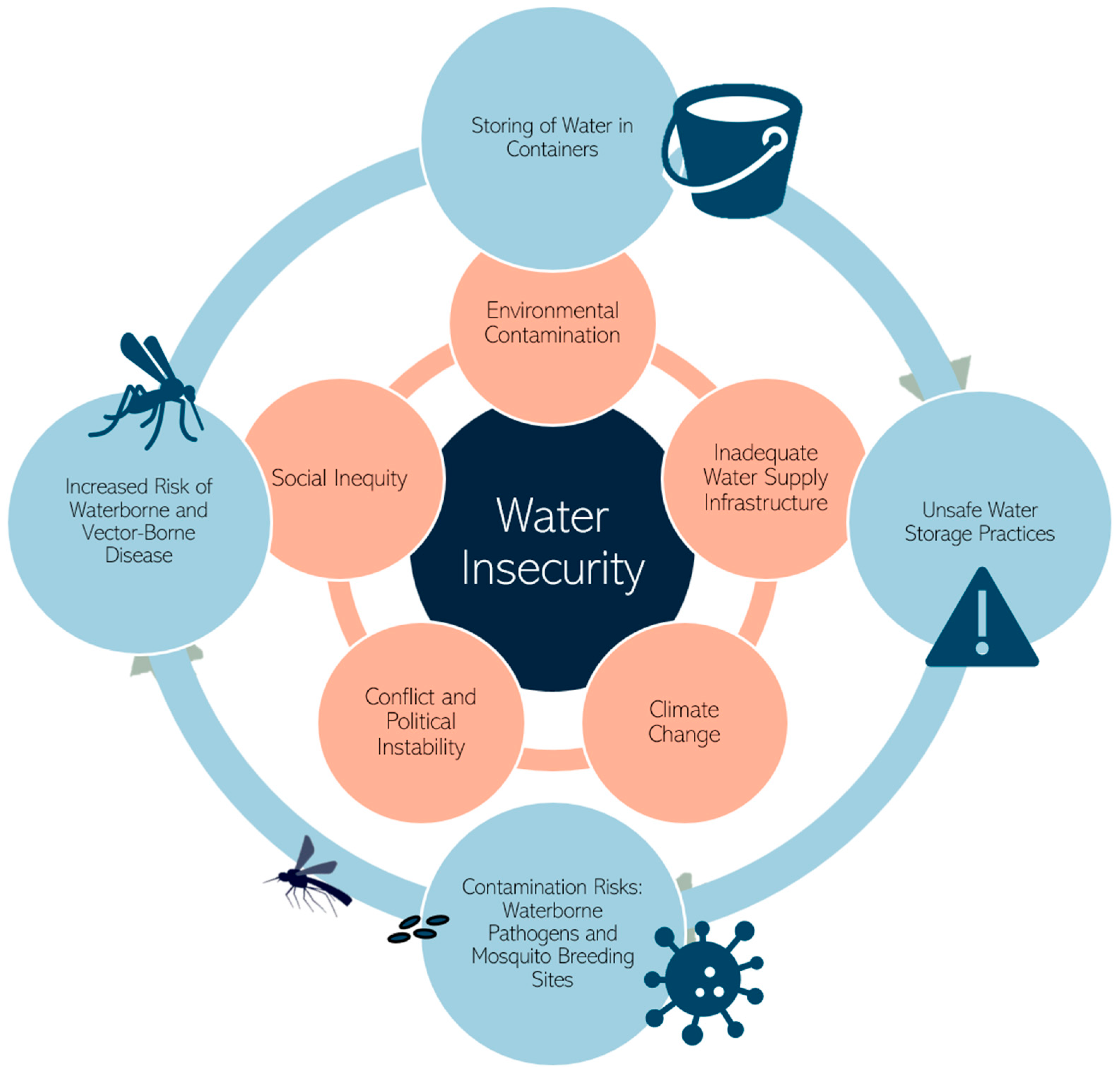
1. Introduction
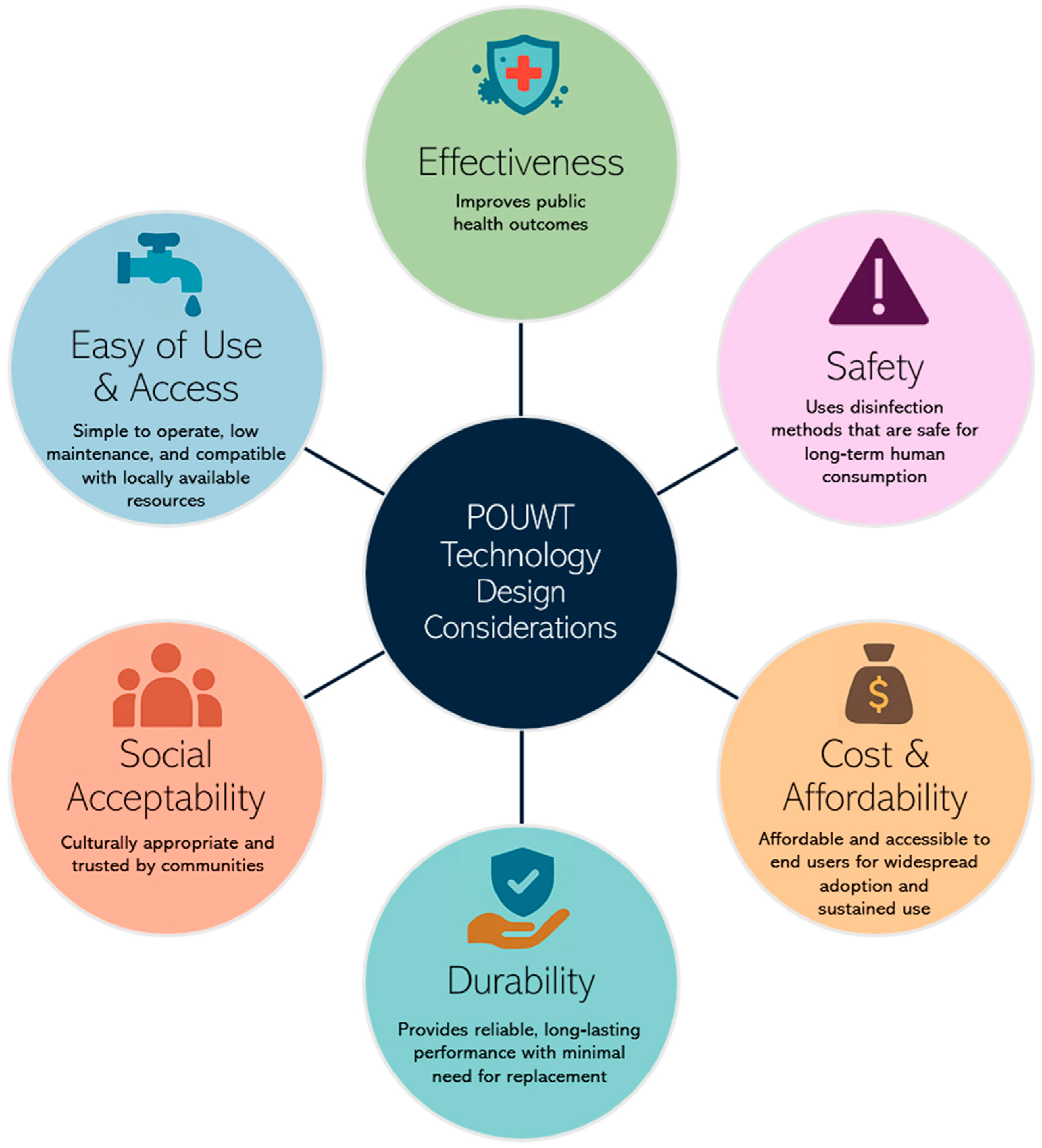
- Circumstance (e.g., local water source type, availability of replacement parts, ease of operation, cultural acceptance, and cost);
2. Materials and Methods
2.1. Culturing and Rearing
2.2. Test Concentrations for Treatment Scenarios
Preparing Stock Solutions
2.3. Survival Bioassays: Evaluation of Dose Response to Water Treatment Disinfectants
2.3.1. Experimental Setup
2.3.2. Data Analysis
3. Results and Discussion
3.1. Late Third Instar Experiments
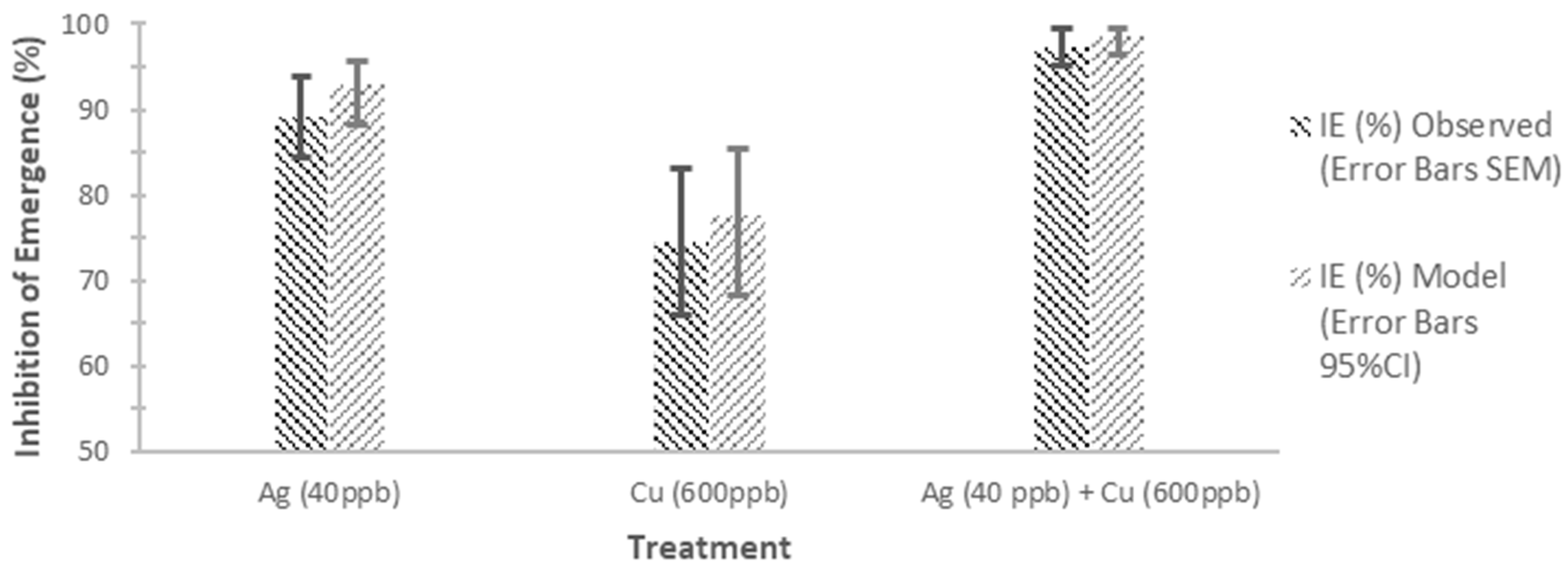
3.1.1. Silver + Copper Exposure to Older Instar
3.1.2. Silver + Chlorine Exposure to Older Instar
3.1.3. Chlorine + Copper Exposure to Older Instar
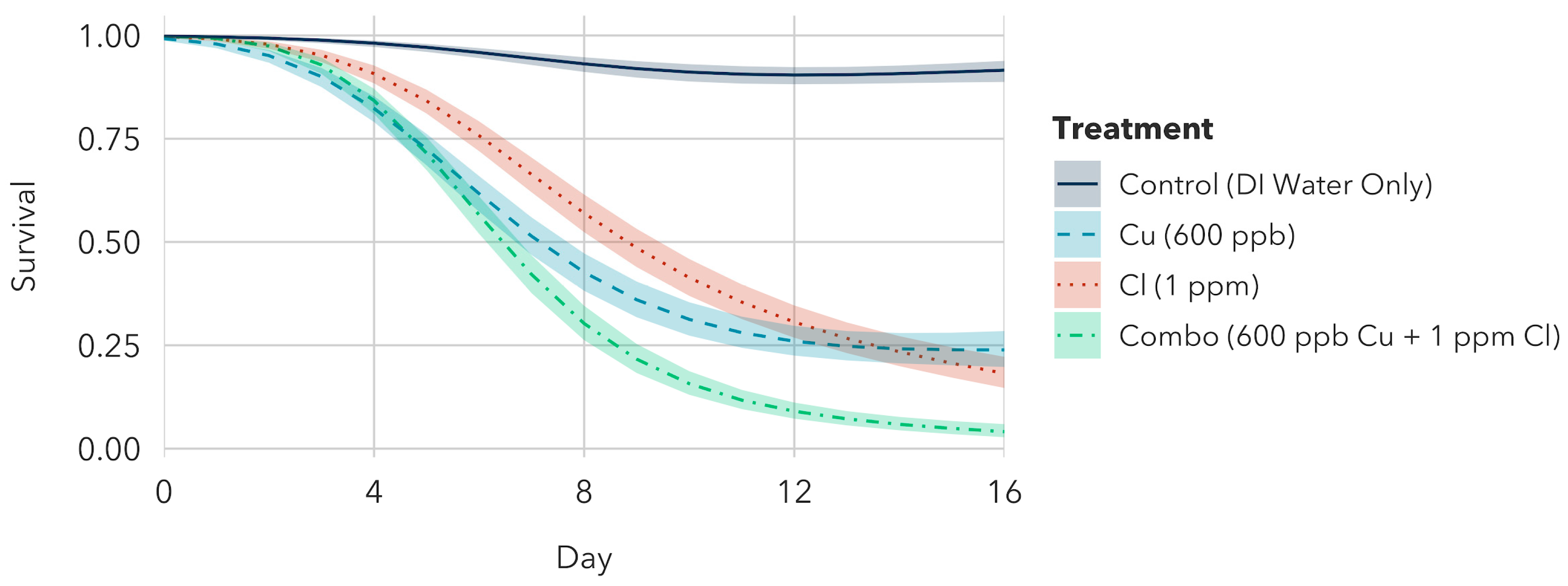
3.1.4. Silver + Chlorine + Copper Exposure to Older Instar
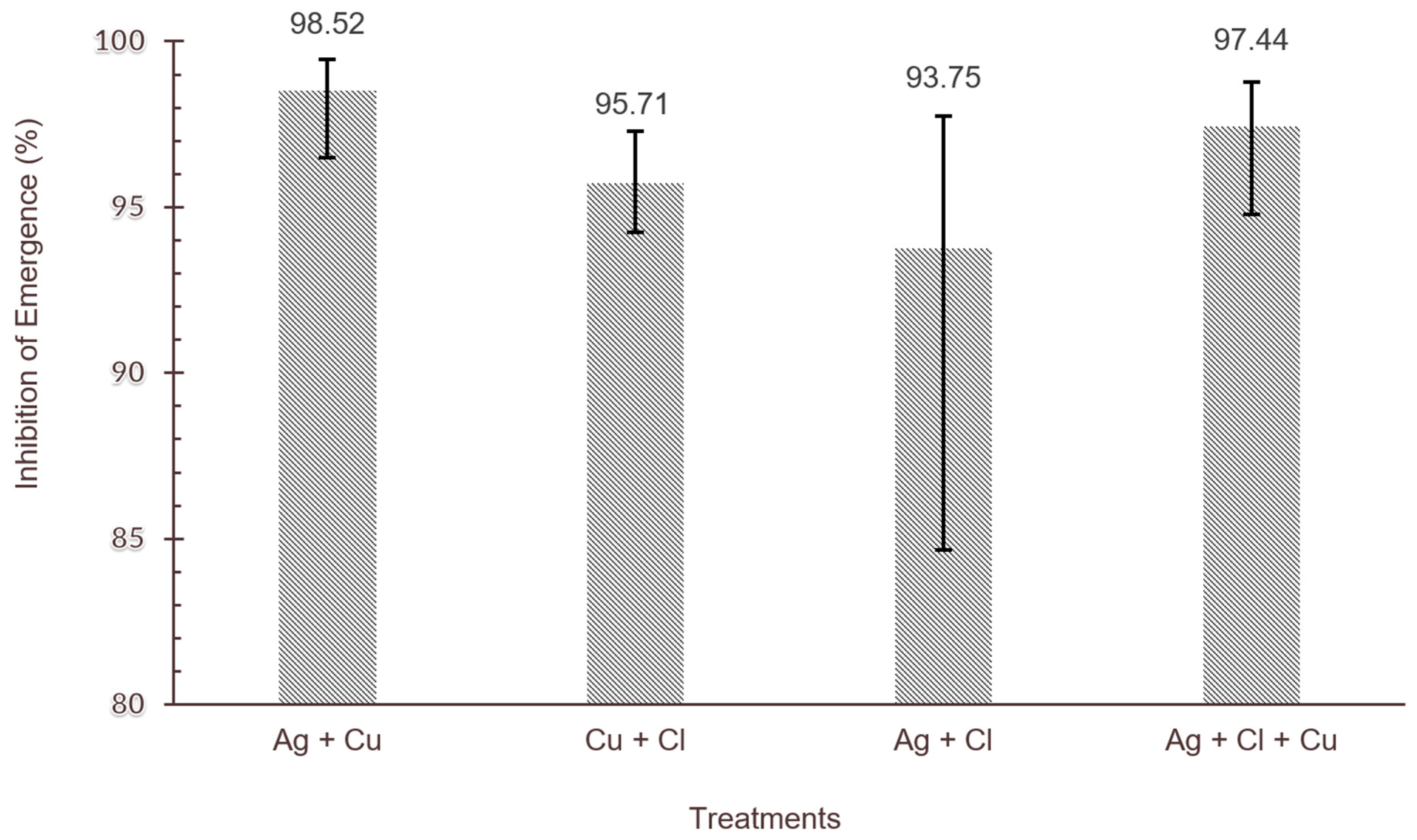
3.1.5. Comparing Efficacy of Water Disinfectants in Combination Exposure to Older Instar
3.2. Younger Instar Experiments
3.2.1. Silver + Copper Exposure to Younger Instar
3.2.2. Silver + Chlorine Exposure to Younger Instar
3.2.3. Chlorine + Copper Exposure to Younger Instar
3.2.4. Comparing Efficacy of Water Disinfectants in Combination Exposure to Younger Instar
3.3. Exploring Chemical Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 30 June 2025).
- World Health Organization (WHO). Global Vector Control Response 2017–2030; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151297-8. [Google Scholar]
- Torto, B.; Tchouassi, D.P. Grand Challenges in Vector-Borne Disease Control Targeting Vectors. Front. Trop. Dis. 2021, 1, 635356. [Google Scholar] [CrossRef]
- Chilakam, N.; Lakshminarayanan, V.; Keremutt, S.; Rajendran, A.; Thunga, G.; Poojari, P.G.; Rashid, M.; Mukherjee, N.; Bhattacharya, P.; John, D. Economic Burden of Mosquito-Borne Diseases in Low- and Middle-Income Countries: Protocol for a Systematic Review. JMIR Res. Protoc. 2023, 12, e50985. [Google Scholar] [CrossRef]
- Chapter 2—Mosquito-Borne Diseases. In Zika Virus Disease; Qureshi, A.I., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 27–45. ISBN 978-0-12-812365-2. [Google Scholar]
- Overgaard, H.J.; Dada, N.; Lenhart, A.; Stenström, T.A.B.; Alexander, N. Integrated Disease Management: Arboviral Infections and Waterborne Diarrhoea. Bull. World Health Organ. 2021, 99, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Akanda, A.S.; Johnson, K.; Ginsberg, H.S.; Couret, J. Prioritizing Water Security in the Management of Vector-Borne Diseases: Lessons From Oaxaca, Mexico. GeoHealth 2020, 4, e2019GH000201. [Google Scholar] [CrossRef]
- Venkataramanan, V.; Collins, S.M.; Clark, K.A.; Yeam, J.; Nowakowski, V.G.; Young, S.L. Coping Strategies for Individual and Household-Level Water Insecurity: A Systematic Review. WIREs Water 2020, 7, e1477. [Google Scholar] [CrossRef]
- Barrera, R.; Avila, J.; González-Téllez, S. Unreliable Supply of Potable Water and Elevated Aedes aegypti Larval Indices: A Causal Relationship? J. Am. Mosq. Control Assoc. 1993, 9, 189–195. [Google Scholar]
- Turner, S.S.; Smith, J.A.; Howle, S.L.; Hancock, P.I.; Brett, K.; Davis, J.; Bruno, L.M.; Cecchetti, V.; Ford, C. Analyzing the Efficacy of Water Treatment Disinfectants as Vector Control: The Larvicidal Effects of Silver Nitrate, Copper Sulfate Pentahydrate, and Sodium Hypochlorite on Juvenile Aedes aegypti. Water 2025, 17, 348. [Google Scholar] [CrossRef]
- Padmanabha, H.; Soto, E.; Mosquera, M.; Lord, C.C.; Lounibos, L.P. Ecological Links between Water Storage Behaviors and Aedes aegypti Production: Implications for Dengue Vector Control in Variable Climates. EcoHealth 2010, 7, 78–90. [Google Scholar] [CrossRef]
- Wright, J.; Gundry, S.; Conroy, R. Household Drinking Water in Developing Countries: A Systematic Review of Microbiological Contamination between Source and Point-of-Use. Trop. Med. Int. Health TMIH 2004, 9, 106–117. [Google Scholar] [CrossRef]
- Hoekstra, A.Y.; Buurman, J.; Ginkel, K.C.H. van Urban Water Security: A Review. Environ. Res. Lett. 2018, 13, 053002. [Google Scholar] [CrossRef]
- Deshpande, A.; Miller-Petrie, M.K.; Lindstedt, P.A.; Baumann, M.M.; Johnson, K.B.; Blacker, B.F.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; Abdollahpour, I.; et al. Mapping Geographical Inequalities in Access to Drinking Water and Sanitation Facilities in Low-Income and Middle-Income Countries, 2000–2017. Lancet Glob. Health 2020, 8, e1162–e1185. [Google Scholar] [CrossRef] [PubMed]
- Delpla, I.; Jung, A.-V.; Baures, E.; Clement, M.; Thomas, O. Impacts of Climate Change on Surface Water Quality in Relation to Drinking Water Production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef]
- Falkenmark, M. The Greatest Water Problem: The Inability to Link Environmental Security, Water Security and Food Security. Int. J. Water Resour. Dev. 2001, 17, 539–554. [Google Scholar] [CrossRef]
- United Nations-Water Summary Progress Update 2021: SDG 6—Water and Sanitation for All. Available online: https://www.unwater.org/publications/summary-progress-update-2021-sdg-6-water-and-sanitation-all (accessed on 10 May 2024).
- Stoler, J.; Brewis, A.; Kangmennang, J.; Keough, S.B.; Pearson, A.L.; Rosinger, A.Y.; Stauber, C.; Stevenson, E.G. Connecting the Dots between Climate Change, Household Water Insecurity, and Migration. Curr. Opin. Environ. Sustain. 2021, 51, 36–41. [Google Scholar] [CrossRef]
- Gosling, S.N.; Arnell, N.W. A Global Assessment of the Impact of Climate Change on Water Scarcity. Clim. Change 2016, 134, 371–385. [Google Scholar] [CrossRef]
- Levy, B.S. Increasing Risks for Armed Conflict: Climate Change, Food and Water Insecurity, and Forced Displacement. Int. J. Health Serv. Plan. Adm. Eval. 2019, 49, 682–691. [Google Scholar] [CrossRef]
- Hidalgo, J.P.; Boelens, R.; Vos, J. De-Colonizing Water. Dispossession, Water Insecurity, and Indigenous Claims for Resources, Authority, and Territory. Water Hist. 2017, 9, 67–85. [Google Scholar] [CrossRef]
- Deitz, S.; Meehan, K. Plumbing Poverty: Mapping Hot Spots of Racial and Geographic Inequality in U.S. Household Water Insecurity. Ann. Am. Assoc. Geogr. 2019, 109, 1092–1109. [Google Scholar] [CrossRef]
- Ezbakhe, F.; Giné-Garriga, R.; Pérez-Foguet, A. Leaving No One behind: Evaluating Access to Water, Sanitation and Hygiene for Vulnerable and Marginalized Groups. Sci. Total Environ. 2019, 683, 537–546. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Results of Round II of the WHO Household Water Treatment Evaluation Scheme; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151603-7. [Google Scholar]
- Pinchoff, J.; Silva, M.; Spielman, K.; Hutchinson, P. Use of Effective Lids Reduces Presence of Mosquito Larvae in Household Water Storage Containers in Urban and Peri-Urban Zika Risk Areas of Guatemala, Honduras, and El Salvador. Parasit. Vectors 2021, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Vannavong, N.; Seidu, R.; Stenström, T.-A.; Dada, N.; Overgaard, H.J. Effects of Socio-Demographic Characteristics and Household Water Management on Aedes aegypti Production in Suburban and Rural Villages in Laos and Thailand. Parasit. Vectors 2017, 10, 170. [Google Scholar] [CrossRef]
- Ehdaie, B.; Rento, C.T.; Son, V.; Turner, S.S.; Samie, A.; Dillingham, R.A.; Smith, J.A. Evaluation of a Silver-Embedded Ceramic Tablet as a Primary and Secondary Point-of-Use Water Purification Technology in Limpopo Province, S. Africa. PLoS ONE 2017, 12, e0169502. [Google Scholar] [CrossRef]
- Fewtrell, L.; Kaufmann, R.B.; Kay, D.; Enanoria, W.; Haller, L.; Colford, J.M. Water, Sanitation, and Hygiene Interventions to Reduce Diarrhoea in Less Developed Countries: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2005, 5, 42–52. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Managing Water in the Home Accelerated Health Gains from Improved Water Supply; Sobsey, M.D., World Health Organization, Eds.; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- National Sanitation Foundation. NSF Standards for Water Treatment Systems. Available online: https://www.nsf.org/consumer-resources/articles/standards-water-treatment-systems (accessed on 15 August 2025).
- Pooi, C.K.; Ng, H.Y. Review of Low-Cost Point-of-Use Water Treatment Systems for Developing Communities. Npj Clean Water 2018, 1, 11. [Google Scholar] [CrossRef]
- Clasen, T.F.; Alexander, K.T.; Sinclair, D.; Boisson, S.; Peletz, R.; Chang, H.H.; Majorin, F.; Cairncross, S. Interventions to Improve Water Quality for Preventing Diarrhoea. Cochrane Database Syst. Rev. 2015, 2015, CD004794. [Google Scholar] [CrossRef]
- Nakada, L.Y.K.; Franco, R.M.B.; da Silva Fiuza, V.R.; dos Santos, L.U.; Branco, N.; Guimarães, J.R. Pre-Ozonation of Source Water: Assessment of Efficacy against Giardia Duodenalis Cysts and Effects on Natural Organic Matter. Chemosphere 2019, 214, 764–770. [Google Scholar] [CrossRef]
- Rose, J.B.; Huffman, D.E.; Gennaccaro, A. Risk and Control of Waterborne Cryptosporidiosis. FEMS Microbiol. Rev. 2002, 26, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of Ozone, Chlorine Dioxide, Chlorine, and Monochloramine on Cryptosporidium Parvum Oocyst Viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar] [CrossRef]
- Sobsey, M.D. Inactivation of Health-Related Microorganisms in Water by Disinfection Processes. Water Sci. Technol. 1989, 21, 179–195. [Google Scholar] [CrossRef]
- Mostafa, S.; Camacho-Galván, M.N.; Castelán-Martínez, H.E.; Galdos-Balzategui, A.; Reygadas, F. A Hybrid Centralized-Point-of-Use Drinking Water Treatment System in a Rural Community in Chiapas, Mexico. Environ. Eng. Sci. 2021, 38, 418–429. [Google Scholar] [CrossRef]
- Bruchet, A.; Duguet, J.P. Role of Oxidants and Disinfectants on the Removal, Masking and Generation of Tastes and Odours. Water Sci. Technol. 2004, 49, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Mitch, W.A. Drinking Water Disinfection Byproducts (DBPs) and Human Health Effects: Multidisciplinary Challenges and Opportunities. Environ. Sci. Technol. 2018, 52, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, Y.; Tang, S.; Yang, H.; Xie, Y.F. Disinfection Byproducts in Drinking Water and Regulatory Compliance: A Critical Review. Front. Environ. Sci. Eng. 2015, 9, 3–15. [Google Scholar] [CrossRef]
- Estrella-You, A.; Harris, J.; Singh, R.; Smith, J. Inactivation of Waterborne Pathogens by Copper and Silver Ions, Free Chlorine, and N-Chloramines in Point-of-Use Technology: A Review. In Water Purification: Processes, Applications and Health Effects; LeBlanc, P., Ed.; Water Purification; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2022; pp. 1–88. ISBN 978-1-68507-622-1. [Google Scholar]
- Singh, R.; Rento, C.; Son, V.; Turner, S.; Smith, J.A. Optimization of Silver Ion Release from Silver-Ceramic Porous Media for Household Level Water Purification. Water 2019, 11, 816. [Google Scholar] [CrossRef]
- Pathak, S.P.; Gopal, K. Evaluation of Bactericidal Efficacy of Silver Ions on Escherichia Coli for Drinking Water Disinfection. Environ. Sci. Pollut. Res. 2012, 19, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Swathy, J.R.; Sankar, M.U.; Chaudhary, A.; Aigal, S.; Anshup; Pradeep, T. Antimicrobial Silver: An Unprecedented Anion Effect. Sci. Rep. 2014, 4, 7161. [Google Scholar] [CrossRef]
- Cameron, P.; Gaiser, B.K.; Bhandari, B.; Bartley, P.M.; Katzer, F.; Bridle, H. Silver Nanoparticles Decrease the Viability of Cryptosporidium Parvum Oocysts. Appl. Environ. Microbiol. 2016, 82, 431–437. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- Sudha, V.B.P.; Ganesan, S.; Pazhani, G.P.; Ramamurthy, T.; Nair, G.B.; Venkatasubramanian, P. Storing Drinking-Water in Copper Pots Kills Contaminating Diarrhoeagenic Bacteria. J. Health Popul. Nutr. 2012, 30, 17–21. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial Applications of Copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef]
- Patil, R.A.; Ahmad, D.; Kausley, S.B.; Balkunde, P.L.; Malhotra, C.P. A Compact Point-of-Use Water Purification Cartridge for Household Use in Developing Countries. J. Water Health 2014, 13, 91–102. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Johnston, M.D.; Hanlon, G.W.; Denyer, S.P. Theory of Antimicrobial Combinations: Biocide Mixtures—Synergy or Addition? J. Appl. Microbiol. 2003, 94, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Estrella-You, A.; Smith, J.A. Synergistic Bacterial Inactivation by Silver Ions and Free Chlorine in Natural Waters. J. Environ. Eng. 2022, 148, 04022072. [Google Scholar] [CrossRef]
- Soliman, M.Y.M.; Medema, G.; Bonilla, B.E.; Brouns, S.J.J.; van Halem, D. Inactivation of RNA and DNA Viruses in Water by Copper and Silver Ions and Their Synergistic Effect. Water Res. X 2020, 9, 100077. [Google Scholar] [CrossRef] [PubMed]
- Landeen, L.K.; Yahya, M.T.; Gerba, C.P. Efficacy of Copper and Silver Ions and Reduced Levels of Free Chlorine in Inactivation of Legionella Pneumophila. Appl. Environ. Microbiol. 1989, 55, 3045–3050. [Google Scholar] [CrossRef]
- Estrella-You, A.; Duti, I.J.; Luo, Q.; Harris, J.D.; Letteri, R.A.; Smith, J.A. N-Chloramine Functionalized Polymer Gels for Point-of-Use Water Disinfection. Water 2024, 16, 3128. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA) EPA Method 6020B (SW-846): Inductively Coupled Plasma—Mass Spectrometry. Available online: https://www.epa.gov/esam/epa-method-6020b-sw-846-inductively-coupled-plasma-mass-spectrometry (accessed on 17 August 2025).
- World Health Organization. World Health Organization Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Ngonzi, A.J.; Muyaga, L.L.; Ngowo, H.; Urio, N.; Vianney, J.-M.; Lwetoijera, D.W. Susceptibility Status of Major Malaria Vectors to Novaluron, an Insect Growth Regulator South-Eastern Tanzania. Pan Afr. Med. J. 2022, 41, 273. [Google Scholar] [CrossRef]
- Jacobs, E.; Chrissian, C.; Rankin-Turner, S.; Wear, M.; Camacho, E.; Broderick, N.A.; McMeniman, C.J.; Stark, R.E.; Casadevall, A. Cuticular Profiling of Insecticide Resistant Aedes aegypti. Sci. Rep. 2023, 13, 10154. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Li, Y.; Ju, Y.; Zhang, W.; Wang, Y. Insect Cuticle and Insecticide Development. Arch. Insect Biochem. Physiol. 2023, 114, e22057. [Google Scholar] [CrossRef]
- Mireji, P.O.; Keating, J.; Hassanali, A.; Impoinvil, D.E.; Mbogo, C.M.; Njeri, M.; Nyambaka, H.; Kenya, E.; Githure, J.I.; Beier, J.C. Expression of Metallothionein and α-Tubulin in Heavy Metal-Tolerant Anopheles Gambiae Sensu Stricto (Diptera: Culicidae). Ecotoxicol. Environ. Saf. 2010, 73, 46–50. [Google Scholar] [CrossRef]
- Chain, F.J.J.; Finlayson, S.; Crease, T.; Cristescu, M. Variation in Transcriptional Responses to Copper Exposure across Daphnia PULEX Lineages. Aquat. Toxicol. 2019, 210, 85–97. [Google Scholar] [CrossRef]
- Bossuyt, B.T.A.; Janssen, C.R. Acclimation of Daphnia Magna to Environmentally Realistic Copper Concentrations. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2003, 136, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Sobsey, M.D.; Stauber, C.E.; Casanova, L.M.; Brown, J.M.; Elliott, M.A. Point of Use Household Drinking Water Filtration: A Practical, Effective Solution for Providing Sustained Access to Safe Drinking Water in the Developing World. Environ. Sci. Technol. 2008, 42, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Lantagne, D.S.; Quick, R.; Mintz, E.D. Household Water Treatment and Safe Storage Options in Developing Countries: A Review of Current Implementation Practices; Woodrow Wilson International Center for Scholars: Washington, DC, USA, 2010. [Google Scholar]
- Perez, M.H.; Noriega, F.G. Aedes aegypti Pharate 1st Instar Quiescence Affects Larval Fitness and Metal Tolerance. J. Insect Physiol. 2012, 58, 824–829. [Google Scholar] [CrossRef] [PubMed]





| Product | Manufacturer | Disinfectant | Evaluation |
|---|---|---|---|
| Aquatabs® & Aquatabs Flo | Medentech Ltd. (Wexford, Ireland) | Chlorine in the form of sodium dichloroiso-cyanurate (NaDCC) as the active ingredient | Met World Health Organization’s (WHO) performance criteria, receiving the designation of “targeted protection (bacteria and viruses only)” |
| H2gO Purifier | Aqua Research, LLC (Albuquerque, NM, USA) | Electrolytic chlorine generator: splits salt (NaCl) to chlorine (Cl2) and hydrogen peroxide (H2O2) | Met WHO’s performance criteria, receiving the designation of “targeted protection (bacteria and viruses only)” |
| H2O ResQ Copper-Silver Ion Water Storage Treatment Drops | Legacy Food Storage (Salt Lake City, UT, USA) | Copper–silver ion formula | No third-party certifications (e.g., WHO or National Sanitation Foundation [NSF]) found. |
| MadiDrop® | Silivhere Technologies Inc. (Charlottesville, VA, USA) | Releases ionic silver | Certified for drinking water treatment by NSF International. |
| Oasis Water Purification Tablets | Hydrachem Ltd. (Billingshurst, England) | Chlorine in the form of sodium dichloroisocyanurate (NaDCC) as the active ingredient | Met WHO’s performance criteria, receiving the designation of “targeted protection (bacteria and viruses only)” |
| P&G Purifier of Water Packets | Procter & Gamble (Cincinnati, OH, USA) | Calcium hypochlorite (chlorine disinfectant) + ferric sulfate (coagulant/flocculant) | Met WHO’s performance criteria; provides comprehensive protection by combining coagulation, flocculation, and disinfection |
| Disinfectant | Drinking Water Quality Guideline (DWQG) | Concentrations Tested (μg/L) | ||
|---|---|---|---|---|
| High (80–95% of DWQG) | Mid (40–50% of DWQG) | Low (20–25% of DWQG) | ||
| Silver (Ag): | US EPA and WHO: 100 μg/L | 20 | 40 | 80 |
| Copper (Cu): | US EPA: 1300 μg/L WHO: 2000 μg/L | 300 | 600 | 1200 |
| Free Chlorine (OCl−/HOCl): | US EPA: 4000 μg/L WHO: 2000 μg/L free chlorine dose for clear water (<10 NTU) and 4000 μg/L for turbid water (≥10 NTU) for POUWT | 500 | 1000 | 2000 |
| Water Disinfectants | Silver (Ag) AgNO3 | Copper (Cu) CuSO4·5H2O | Free Chlorine (HOCl/OCl−) NaOCl |
|---|---|---|---|
| Silver | 40 ppb Ag | 600 ppb Cu + 40 ppb Ag | 1 ppm Cl + 40 ppb Ag |
| Copper | - | 600 ppb Cu | 1 ppm Cl + 600 ppb Cu |
| Chlorine | - | - | 1 ppm Cl |
| Treatment | Ag (40 ppb) | Cu (600 ppb) | Ag (40 ppb) + Cu (600 ppb) | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | ||||||||||||
| Variable | mean | sd | sem | mean | sd | sem | mean | sd | sem | mean | sd | sem |
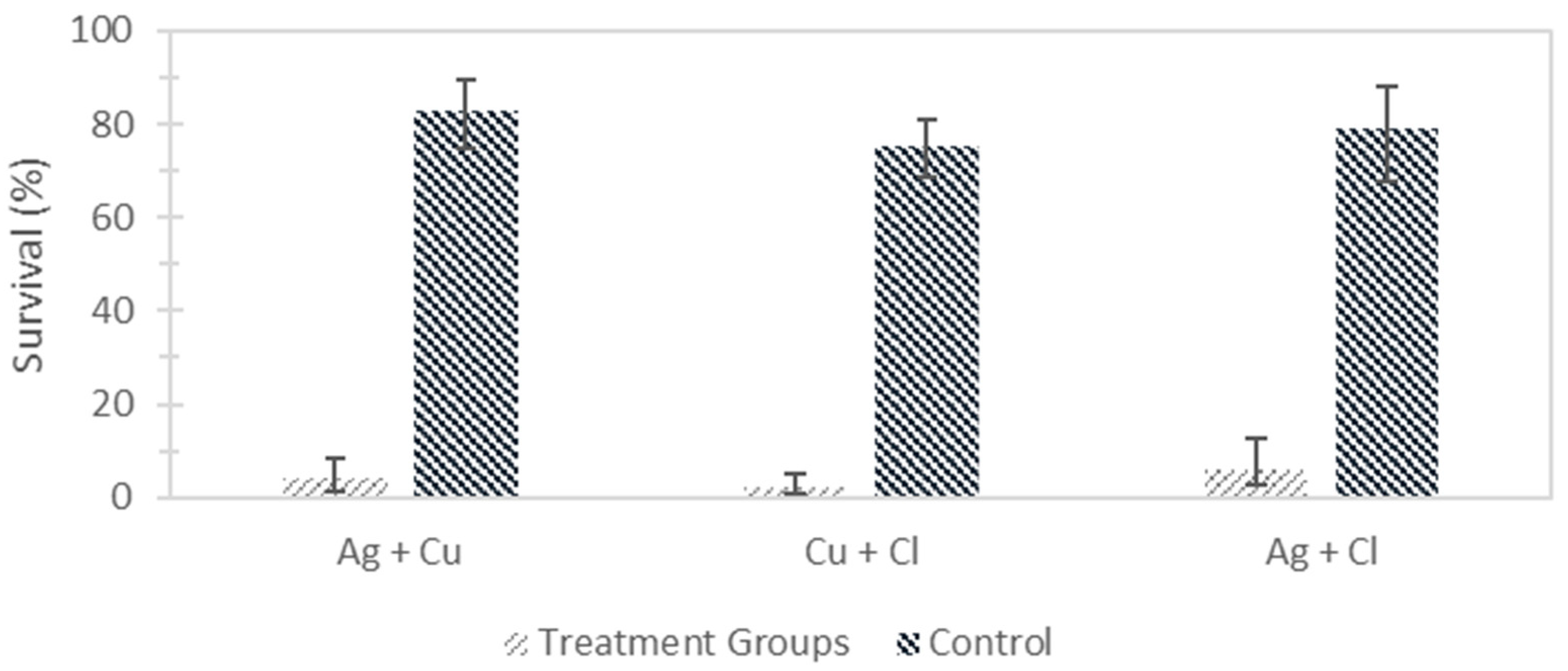
| Survival (%) | 11.23 | 7.74 | 3.87 | 29.19 | 14.78 | 7.39 | 3.25 | 4.11 | 2.06 | 91.33 | 1.72 | 0.86 |
| Emerg (%) | 9.94 | 8.80 | 4.40 | 22.00 | 13.90 | 6.95 | 2.52 | 4.10 | 2.05 | 88.33 | 5.03 | 2.52 |
| IE (%) | 89.01 | 9.47 | 4.74 | 74.47 | 17.08 | 8.54 | 97.29 | 4.38 | 2.19 | |||
| Model | ||||||||||||
| Variable | Predicted mean [95% Confidence Interval] | |||||||||||
| Survival (%) | 10.00 [6.31, 14.68] | 29.53 [23.09, 37.71] | 3.53 [1.87, 5.33] | 92.07 [86.79, 95.58] | ||||||||
| Emerg (%) | 7.15 [3.90, 1 4.09] | 22.47 [13.50, 33.15] | 1.48 [0.39, 2.94] | 91.81 [85.28,95.8] | ||||||||
| IE (%) | 92.85 [88.24, 95.68] | 77.53 [68.19, 85.34] | 98.52 [96.50, 99.47] | |||||||||
| Treatment | Ag (40 ppb) | Free Chlorine (1 ppm) | Ag (40 ppb) + Free Chlorine (1 ppm) | Control |
|---|---|---|---|---|
| Variable | Predicted Mean [95% Confidence Interval] | |||
| Survival (%) | 17.30 [12.03, 23.11] | 29.26 [23.25, 36.70] | 7.38 [4.49, 11.36] | 94.42 [90.99, 96.72] |
| Emerg (%) | 11.69 [4.42, 24.67] | 31.65 [21.95, 37.68] | 6.25 [1.83, 15.35] | 94.75 [85.62, 98.53] |
| IE (%) | 88.31 [78.05, 94.66] | 68.35 [51.93, 82.01] | 93.75 [84.65, 97.76] | |
| Treatment | Cu (600 ppb) | Free Chlorine (1 ppm) | Ag (40 ppb) + Cl (1 ppm) | Control |
|---|---|---|---|---|
| Variable | Predicted Mean [95% Confidence Interval] | |||
| Survival (%) | 19.87 [16.51, 24.48] | 26.08 [21.84, 31.84] | 4.48 [3.19, 6.36] | 91.59 [88.75, 93.85] |
| Emerg (%) | 22.67 [19.19, 27.77] | 15.75 [12.87, 19.09] | 4.29 [2.66, 6.06] | 93.12 [90.89, 94.90] |
| IE (%) | 84.25 [81.08, 87.78] | 77.33 [72.57, 81.12] | 95.71 [94.25, 97.28] | |
| Treatment | Ag (40 ppb) | Cu (600 ppb) | Ag (40 ppb) + Cu (600 ppb) | Control |
|---|---|---|---|---|
| Time (h) | Predicted Mean [95% Confidence Interval] | |||
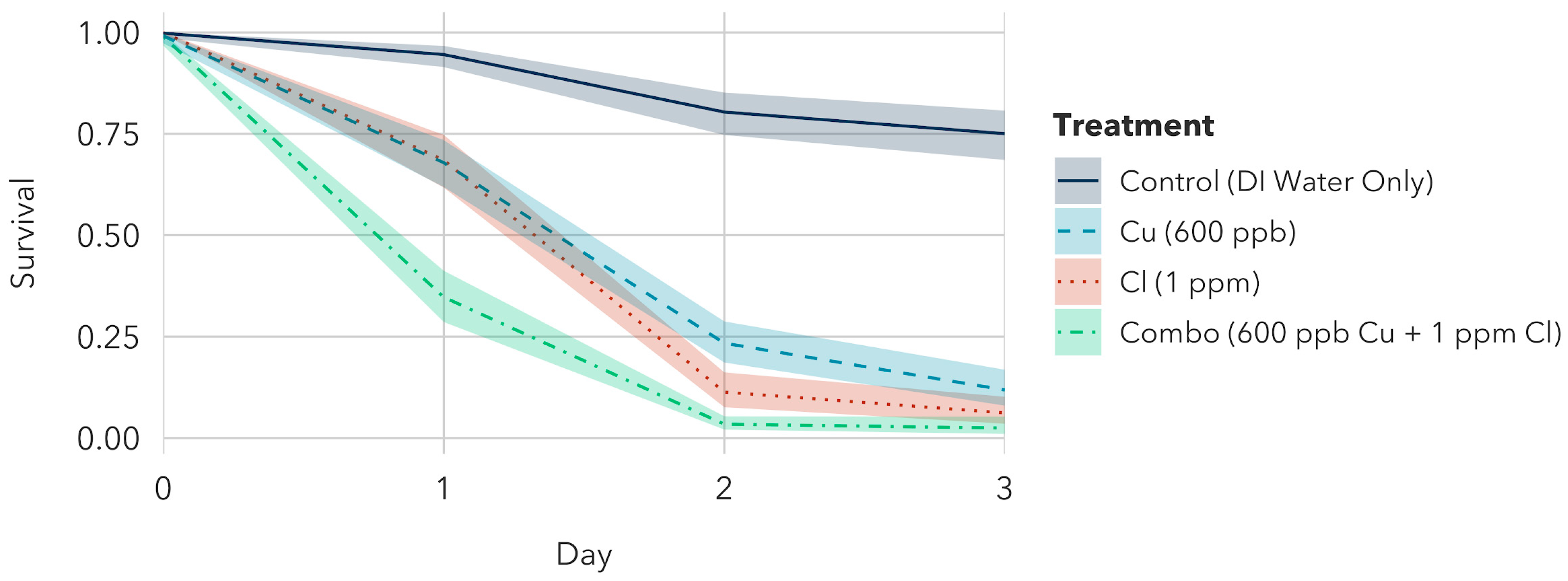
| 24 | 88.50 [82.32, 92.97] | 84.20 [76.42, 90.07] | 79.73 [70.86, 86.75] | 97.58 [94.92, 98.96] |
| 48 | 53.34 [43.08, 63.37] | 34.49 [25.27, 44.74] | 13.74 [8.28, 21.25] | 90.60 [84.61, 94.66] |
| 72 | 23.28 [15.69, 32.57] | 17.14 [10.83, 25.41] | 3.33 [1.35, 7.26] | 82.97 [74.73, 89.25] |
| Treatment | Ag (40 ppb) | Cl (1 ppm) | Ag (40 ppb) + Cl (1 ppm) | Control |
|---|---|---|---|---|
| Time (h) | Predicted Mean [95% Confidence Interval] | |||
| 24 | 83.81 [73.88, 90.89] | 69.37 [56.61, 80.13] | 64.79 [51.61, 76.40] | 95.17 [90.17, 97.89] |
| 48 | 46.51 [33.46, 59.95] | 21.53 [12.91, 32.80] | 16.64 [9.45, 26.65] | 84.74 [74.89, 91.61] |
| 72 | 30.31 [19.30, 43.48] | 8.99 [4.26, 16.82] | 6.22 [2.67, 12.68] | 79.17 [67.55, 87.89] |
| Treatment | Cu (600 ppb) | Cl (1 ppm) | Cu (600 ppb) + Cl (1 ppm) | Control |
|---|---|---|---|---|
| Time (h) | Predicted Mean [95% Confidence Interval] | |||
| 24 | 67.88 [61.89, 73.44] | 68.53 [61.74, 74.74] | 34.71 [28.60, 41.25] | 94.54 [91.47, 96.67] |
| 48 | 23.38 [18.62, 28.75] | 11.32 [7.61, 16.19] | 3.41 [2.08, 5.36] | 80.37 [74.74, 85.17] |
| 72 | 11.83 [7.98, 16.84] | 6.18 [3.53, 10.18] | 2.44 [1.01, 5.31] | 75.04 [68.55, 80.73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, S.S.; Smith, J.A.; Brett, K.; Hancock, P.I.; Howle, S.L.; Cecchetti, V.; Bruno, L.M.; Davis, J.; Ford, C. Combined Efficacy of Silver, Copper, and Hypochlorite Ions for Vector Control of Juvenile Aedes aegypti in Household Water Storage Containers. Water 2025, 17, 2754. https://doi.org/10.3390/w17182754
Turner SS, Smith JA, Brett K, Hancock PI, Howle SL, Cecchetti V, Bruno LM, Davis J, Ford C. Combined Efficacy of Silver, Copper, and Hypochlorite Ions for Vector Control of Juvenile Aedes aegypti in Household Water Storage Containers. Water. 2025; 17(18):2754. https://doi.org/10.3390/w17182754
Chicago/Turabian StyleTurner, Sydney S., James A. Smith, Karin Brett, Patrick I. Hancock, Sophie L. Howle, Victoria Cecchetti, Lorin M. Bruno, Julia Davis, and Clay Ford. 2025. "Combined Efficacy of Silver, Copper, and Hypochlorite Ions for Vector Control of Juvenile Aedes aegypti in Household Water Storage Containers" Water 17, no. 18: 2754. https://doi.org/10.3390/w17182754
APA StyleTurner, S. S., Smith, J. A., Brett, K., Hancock, P. I., Howle, S. L., Cecchetti, V., Bruno, L. M., Davis, J., & Ford, C. (2025). Combined Efficacy of Silver, Copper, and Hypochlorite Ions for Vector Control of Juvenile Aedes aegypti in Household Water Storage Containers. Water, 17(18), 2754. https://doi.org/10.3390/w17182754








