Radionuclide Distribution and Hydrochemical Controls in Groundwater of the Nile Valley, Upper Egypt: Health and Environmental Implications
Abstract
1. Introduction
2. Experimental Procedures
2.1. Description of the Study Area
2.2. Field Sampling
2.3. Laboratory Analyses
2.3.1. Radiometric Analysis
2.3.2. Hydrochemical Analysis
2.4. Data Processing and Statistical Analysis
2.4.1. Statistical and Multivariate Analysis
2.4.2. Geospatial Modeling
3. Results
3.1. Descriptive Statistics of Measured Parameters
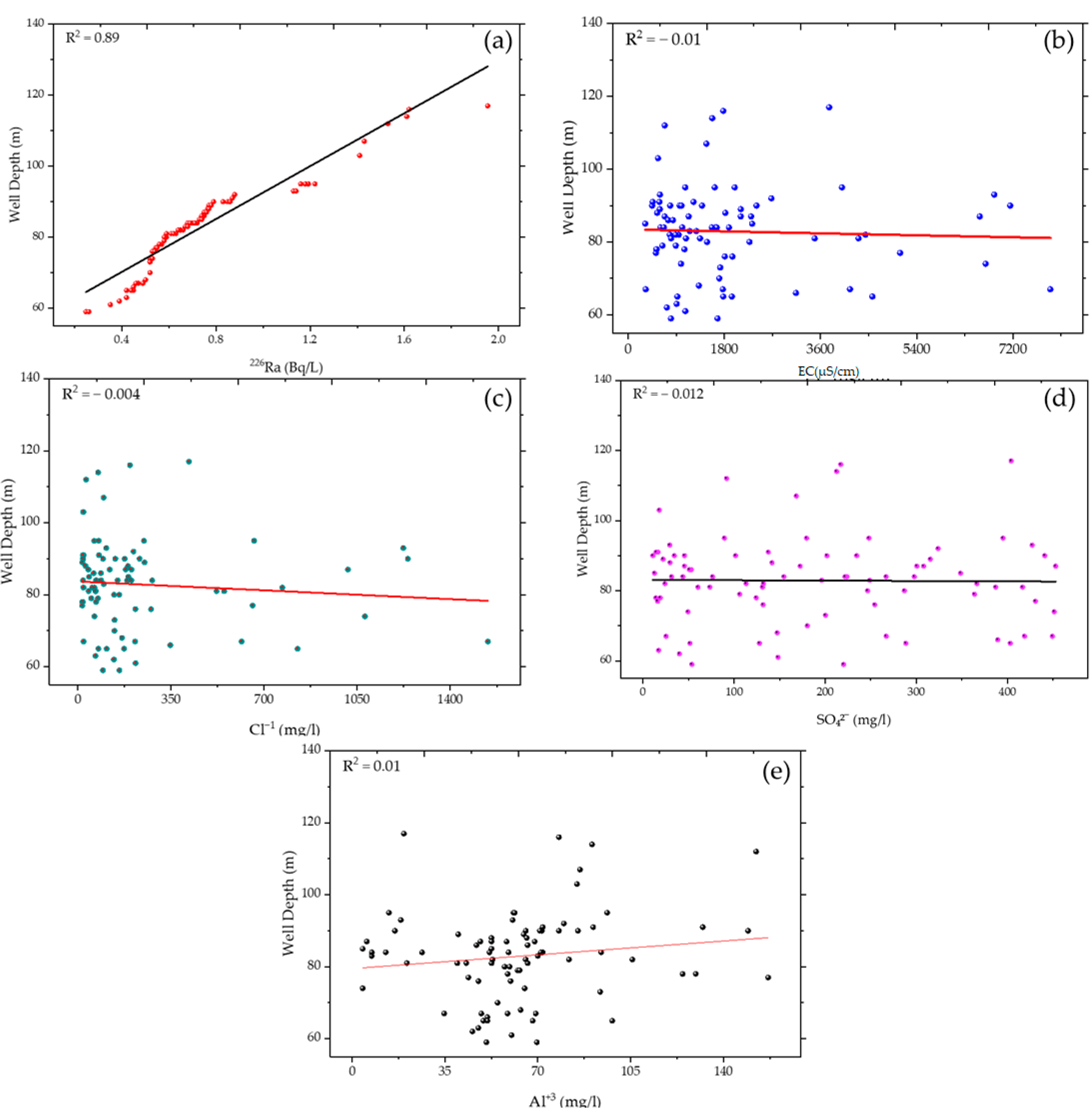
3.2. Depth-Dependent Variation of Key Radionuclides and Hydrochemical Parameters
3.3. Spatial Distribution of Radionuclide Activity Concentrations
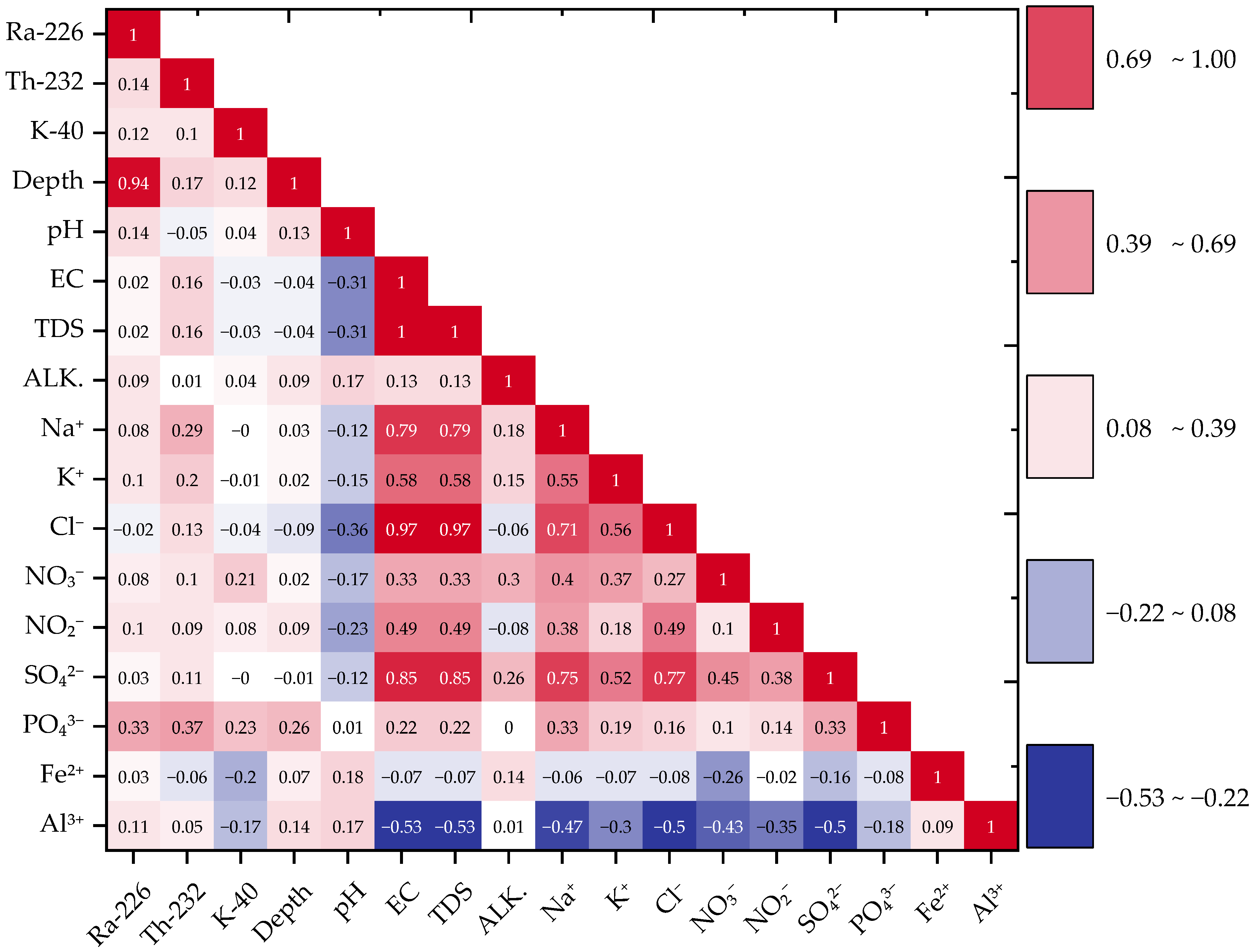
3.4. Inter-Variable Correlations in Groundwater Chemistry
3.5. Principal Component Analysis
3.6. Radiological Health Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; WHO: Geneva, Switzerland, 2022.
- Abdelkareem, M.; El-Baz, F. Analyses of optical images and radar data reveal structural features and predict groundwater accumulations in the central Eastern Desert of Egypt. Arab. J. Geosci. 2014, 8, 2653–2666. [Google Scholar] [CrossRef]
- Alezabawy, A.K.; Eissa, M.; Salem, Z.E.-S. Hydrogeochemical and isotopic investigations of groundwater in the reclaimed desert located between EL Nasr canal and Mariut Tableland, NW Coast, Egypt. Sci. Rep. 2024, 14, 21172. [Google Scholar] [CrossRef]
- Ray, C.; Shamrukh, M. NATO Science for Peace and Security Series-C: Environmental Security. In Riverbank Filtration for Water Security in Desert Countries; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Zhuo, W.; Iida, T.; Yang, X. Occurrence of 222Rn, 226Ra, 228Ra and U in groundwater in Fujian Province, China. J. Environ. Radioact. 2001, 53, 111–120. [Google Scholar] [CrossRef]
- Saqan, S.; Kullab, M.; Ismail, A. Radionuclides in hot mineral spring waters in Jordan. J. Environ. Radioact. 2001, 52, 99–107. [Google Scholar] [CrossRef]
- El-Din, K.S.; Ali, K.; Harb, S.; Abbady, A.E.-B. Measurement of 222Rn concentration levels in drinking water samples from Qena city (Egypt) and evaluation of the annual effective doses. Int. J. Radiat. Res. 2020, 18, 227–233. [Google Scholar]
- Din, K.S.; Ali, K.; Harb, S.; Abbady, A.B. Natural radionuclides in groundwater from Qena governorate, Egypt. Environ. Forensics 2021, 22, 48–55. [Google Scholar]
- Ahmed, N.K. Natural Radioactivity of Ground and Drinking Water in Some Areas in Upper Egypt. Arab. J. Nucl. Sci. Appl. 2004, 36, 571–580. [Google Scholar]
- Carvalho, F.; Chambers, D.; Fernandes, S.; Fesenko, S.; Goulet, R.; Howard, B.; Kim, C.K.; Martin, P.; Moore, W.S.; Phaneuf, M.; et al. The Environmental Behaviour of Radium: Revised Edition; International Atomic Energy Agency: Vienna, Austria, 2014; 267p.
- Pérez-Moreno, S.; Guerrero, J.; Mosqueda, F.; Gázquez, M.; Bolívar, J. Hydrochemical behavior of long-lived natural radionuclides in Spanish groundwaters. CATENA 2020, 191, 104558. [Google Scholar] [CrossRef]
- Vengosh, A.; Coyte, R.M.; Podgorski, J.; Johnson, T.M. A critical review on the occurrence and distribution of the uranium- and thorium-decay nuclides and their effect on the quality of groundwater. Sci. Total Environ. 2022, 808, 151914. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Szabo, Z.; Jurgens, B.C. Radium mobility and the age of groundwater in public-drinking-water supplies from the Cambrian-Ordovician aquifer system, north-central USA. Appl. Geochem. 2018, 89, 34–48. [Google Scholar] [CrossRef]
- Faraj, T.; Ragab, A.; El Alfy, M. Geochemical and hydrogeological factors influencing high levels of radium contamination in groundwater in arid regions. Environ. Res. 2020, 184, 109303. [Google Scholar] [CrossRef]
- Erőss, A.; Mádl-Szőnyi, J.; Surbeck, H.; Horváth, Á.; Goldscheider, N.; Csoma, A.É. Radionuclides as natural tracers for the characterization of fluids in regional discharge areas, Buda Thermal Karst, Hungary. J. Hydrol. 2012, 426–427, 124–137. [Google Scholar] [CrossRef]
- Gainon, F.; Surbeck, H.; Zwahlen, F. Natural radionuclides in groundwater as pollutants and as useful tracers. In Proceedings of the 12th Symposium on Water Rock Interaction, Kunming, China, 31 July–5 August 2007. [Google Scholar]
- Gainon, F.; Goldscheider, N.; Surbeck, H. Conceptual model for the origin of high radon levels in spring waters–the example of the St. Placidus spring, Grisons, Swiss Alps. Swiss J. Geosci. 2007, 100, 251–262. [Google Scholar] [CrossRef]
- Tóth, J. Groundwater as a geological agent: An overview of the causes, processes, and manifestations. Hydrogeol. J. 1999, 7, 1–14. [Google Scholar] [CrossRef]
- Canu, I.G.; Laurent, O.; Pires, N.; Laurier, D.; Dublineau, I. Health effects of naturally radioactive water ingestion: The need for enhanced studies. Environ. Health Perspect. 2011, 119, 1676–1680. [Google Scholar] [CrossRef]
- World Health Organization’s (WHO). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO Library Cataloguing-in-Publication Data: Geneva, Switzerland, 2017. [Google Scholar]
- Kitto, M.E.; Kim, M.S. Naturally occurring radionuclides in community water supplies of New York state. Health Phys. 2005, 88, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.M.; Godoy, M.L. Natural radioactivity in Brazilian groundwater. J. Environ. Radioact. 2006, 85, 71–83. [Google Scholar] [CrossRef]
- Salonen, L. 238U series radionuclides as a source of increased radioactivity in groundwater originating from Finnish bedrock. In Future Groundwater Resources at Risk; Soveri, J., Suokko, T., Eds.; International Association of Hydrological Sciences Publications: Oxfordshire, UK, 1994; pp. 71–84. [Google Scholar]
- M. Isam Salih, M.; BL Pettersson, H.; Lund, E. Uranium and thorium series radionuclides in drinking water from drilled bedrock wells: Correlation to geology and bedrock radioactivity and dose estimation. Radiat. Prot. Dosim. 2002, 102, 249–258. [Google Scholar] [CrossRef] [PubMed]
- El-Mageed, A.I.A.; El-Kamel, A.E.-H.; Abbady, A.E.-B.; Harb, S.; Saleh, I.I. Natural radioactivity of ground and hot spring water in some areas in Yemen. Desalination 2013, 321, 28–31. [Google Scholar] [CrossRef]
- Said, R. The Geology of Egypt; CRC Press: Boca Raton, FL, USA, 2017; p. 734. [Google Scholar]
- Ahmed, A.A.; Fogg, G.E. The impact of groundwater and agricultural expansion on the archaeological sites at Luxor, Egypt. J. Afr. Earth Sci. 2014, 95, 93–401. [Google Scholar] [CrossRef]
- Razik, T.A.; Razvaliaev, A.V. On the Tectonic Origin of the Nile Valley between Idfu and Qena. Egypt. J. Geol. 1972, 16, 235–244. [Google Scholar]
- El Hosary, M.F.M. Hydrogeological and Hydrochemical Studies on Luxor Area, Southern Egypt. Master’s Thesis, Ain Shams University, Cairo, Egypt, 1994; p. 121. [Google Scholar]
- Said, R. The Geology of Egypt; Taylor & Francis: Oxfordshire, UK, 1990; p. 734. [Google Scholar]
- Said, R. The Geology of Egypt; Elsevier Publishing Company: New York, NY, USA, 1962; p. 377.
- Egyptian Ministry of Water Resources and Irrigation (MWRI). MWRI, Copyright © 2020–2024 Designed by Mic-MWRI. Available online: https://www.mwri.gov.eg/ (accessed on 1 December 2024).
- Research Institute for Groundwater (RIGW). Ministry of public works and water resources, Egypt. In Detailed Hydrogeological Maps of Luxor and Esna Areas (1:100.000); RIGW: Cairo, Egypt, 1997. [Google Scholar]
- Knoll, G.F. Radiation Detection and Measurement, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 857. [Google Scholar]
- Ali, K.; Abu-Taleb, A.; Abbady, A.E.-B.; Harb, S. Radiological risks in Nasser lake water and their health and environmental implications. Sci. Rep. 2025, 15, 14545. [Google Scholar] [CrossRef]
- Harb, S. On the Human Radiation Exposure as Derived from the Analysis of Natural and Man-Made Radionuclides in Soil. Ph.D. Thesis, Institute for Radiation Protection and Radioecology, ZSR. Hannover University, Hannover, Germany, 2004. [Google Scholar]
- Ali, K.; Abbady, A.E.-B.; Abu-Taleb, A.; Harb, S. Assessment of radioactive substance transfer and its ecological and health impacts on the Nasser Lake ecosystem. Sci. Rep. 2025, 15, 26115. [Google Scholar] [CrossRef] [PubMed]
- Abbady, A.G.E.; Yousef, A.M.M.; Ali, K. Natural Radioactivity and Health Risks in Southeastern Desert, Egypt: A Forensic Evaluation of Wadi Um-Sleimat, Jebel El-Erediya, and Wadi Um-Had. Environ. Forensics 2025, 1–15. [Google Scholar] [CrossRef]
- Harb, S.; El-Kamel, A.H.; El-Mageed, A.I.A.; Abbady, A.; Rashed, W. Radioactivity Levels and Soil-to-Plant Transfer Factor of Natural Radionuclides from Protectorate Area in Aswan, Egypt. World J. Nucl. Sci. Technol. 2014, 4, 7–15. [Google Scholar] [CrossRef]
- El-Arabi, A.E.-G.; Khalifa, N.; El-Din, K.S. Natural radionuclides and dose estimation in natural water resources from Elba protective area, Egypt. Radiat. Prot. Dosim. 2006, 121, 284–292. [Google Scholar] [CrossRef]
- El-Gamal, H.; Sefelnasr, A.; Salaheldin, G. Determination of Natural Radionuclides for Water Resources on the West Bank of the Nile River, Assiut Governorate, Egypt. Water 2019, 11, 311. [Google Scholar] [CrossRef]
- Currie, L.A.; Currie. Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]
- ISO 5667-3:2024; Water Quality—Sampling. Part 3: Preservation and Handling of Water Samples. International Organization for Standardization (ISO): Geneva, Switzerland, 2024.
- ISO/IEC 17025; Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Ahmed, M.; Chen, Y.; Khalil, M. Isotopic composition of groundwater resources in arid environments. J. Hydrol. 2022, 609, 127773. [Google Scholar] [CrossRef]
- Dabous, A.A.; Osmond, J.K. Uranium isotopic study of artesian and pluvial contributions to the Nubian Aquifer, Western Desert, Egypt. J. Hydrol. 2001, 243, 242–253. [Google Scholar] [CrossRef]
- El-Aassy, I.E.; El-Feky, M.G.; Issa, F.A.; Ibrahim, N.M.; Desouky, O.A.; Khattab, M.R. Uranium and 234U/238U isotopic ratios in some groundwater wells at Southwestern Sinai, Egypt. J. Radioanal. Nucl. Chem. 2015, 303, 357–362. [Google Scholar] [CrossRef]
- Sherif, M.I.; Lin, J.; Poghosyan, A.; Abouelmagd, A.; Sultan, M.I.; Sturchio, N.C. Geological and hydrogeochemical controls on radium isotopes in groundwater of the Sinai Peninsula, Egypt. Sci. Total Environ. 2018, 613–614, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Kiro, Y.; Weinstein, Y.; Starinsky, A.; Yechieli, Y. Application of radon and radium isotopes to groundwater flow dynamics: An example from the Dead Sea. Chem. Geol. 2015, 411, 155–171. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Specification of Radionuclide Content in Commodities Requiring Regulation for Purposes of Radiation Protection; IAEA: Vienna, Austria, 2002.
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation; UN Publications: New York, NY, USA, 2000. [Google Scholar]
- Nicolov, V.P.; Georgescu, P.L.; Iticescu, C.; Moraru, D.I.; Pintilie, A.G. The assessment of the annual effective dose due to ingestion of radionuclides from drinking water consumption: Calculation methods. J. Radioanal. Nucl. Chem. 2020, 327, 49–58. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Report to the General Assembly: Volume 1 Sources of Ionizing Radiation; UN Publications: New York, NY, USA, 2000. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation-UNSCEAR. Report to the General Assembly, with Scientifc Annexes Annex B: Exposures of the Public and Workers from Various Sources of Radiation; UN Publications: New York, NY, USA, 2008. [Google Scholar]
- Eckerman, K.; Harrison, J.; Menzel, H.-G.; Clement, C.H. ICRP Publication 119: Compendium of Dose Coefficients Based on ICRP Publication 60; International Commission on Radiological Protection (ICRP): Ottawa, ON, Canada, 2012. [Google Scholar]
- International Atomic Energy Agency (IAEA). Safety Standards for Protecting People and the Environment, Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards; IAEA: Vienna, Austria, 2014.


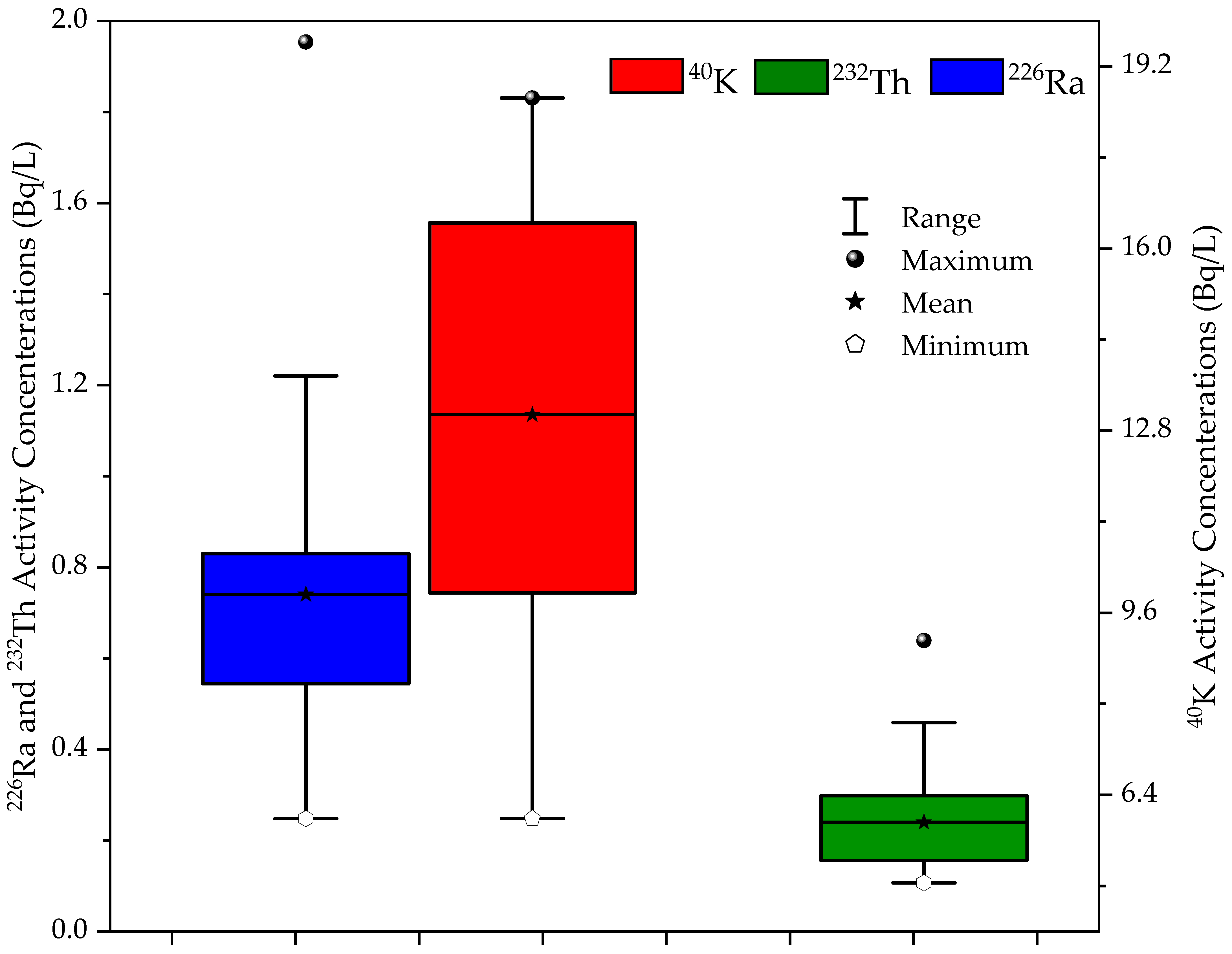










| Parameter | Mean | Std. Dev | Min | Max | CV (%) |
|---|---|---|---|---|---|
| Radioactive elements (Bq/L) | |||||
| 226Ra | 0.74 | 0.3 | 0.25 | 2 | 42.05 |
| 232Th | 0.24 | 0.1 | 0.11 | 0.6 | 42.08 |
| 40K | 13 | 4 | 6 | 19 | 27.09 |
| Physicochemical properties | |||||
| pH | 7.59 | 0.2 | 7.1 | 8.4 | 3.2 |
| EC (µS/cm) | 1871.3 | 1676.1 | 318 | 7900 | 89.6 |
| TDS (mg/L) | 1234.7 | 1106.3 | 209 | 5214 | 89.6 |
| Hydrochemical properties (mg/L) | |||||
| Total Hardness | 460.9 | 317.2 | 159.7 | 1790.9 | 68.8 |
| Alkalinity | 363.4 | 148.1 | 99.5 | 965.2 | 40.8 |
| Major cations (mg/L) | |||||
| Na+ | 261.2 | 181.9 | 2.9 | 679.6 | 69.7 |
| K+ | 13.2 | 6.3 | 4.1 | 35.1 | 47.6 |
| Cl− | 225.1 | 306.7 | 16.6 | 1541.3 | 136.2 |
| NO3− | 6.9 | 4.6 | 0.1 | 12.4 | 66.1 |
| NO2− | 0.1 | 0.3 | 0.01 | 2.3 | 300 |
| SO42− | 184.5 | 140.5 | 10.7 | 453 | 76.2 |
| PO43− | 0.9 | 0.3 | 0.2 | 1.5 | 33.2 |
| Trace elements (mg/L) | |||||
| Fe2+ | 0.03 | 0.02 | 0 | 0.1 | 78.3 |
| Al3+ | 62.4 | 31.7 | 4 | 156.9 | 50.7 |
| Relationship | Correlation Coefficient (r) | Parameter Type |
|---|---|---|
| 226Ra ↔ Depth | 0.94 *** | Radionuclide ↔ Well Depth |
| 226Ra ↔ PO43− | 0.33 * | Radionuclide ↔ Anion |
| 232Th ↔ PO43− | 0.37 * | Radionuclide ↔ Anion |
| 40K ↔ PO43− | 0.23 * | Radionuclide ↔ Anion |
| EC ↔ TDS | 1.00 *** | Major ion ↔ TDS |
| Cl− ↔ TDS | 0.97 *** | Anion ↔ TDS |
| SO42− ↔ EC | 0.85 *** | Anion ↔ Conductivity |
| Na+ ↔ SO42− | 0.75 *** | Cation ↔ Anion |
| Cl− ↔ Na+ | 0.71 *** | Anion ↔ Cation |
| NO3− ↔ SO42− | 0.45 ** | Nitrate ↔ Sulfate |
| Al3+ ↔ EC/TDS | −0.53 | Trace metal ↔ Salinity indicator |
| Category | Min | Max | Mean | Std. Dev | Acceptable Dose Limit [20,56] |
|---|---|---|---|---|---|
| Annual Effective Dose (µSv/y) | |||||
| Adults (>17 years) | 48.18 | 292.65 | 131.24 | 46.61 | 100 |
| Children (2–7 years) | 68.15 | 444.35 | 190.05 | 70.33 | 200 |
| Infants (<2 years) | 42.12 | 280.86 | 118.37 | 44.41 | 260 |
| Category | Min | Max | Mean | Std. Dev | Acceptable Dose Limit [20,56] |
|---|---|---|---|---|---|
| Cancer Risk (Mortality) (×10−4) | |||||
| Male | 0.79 | 6.26 | 2.37 | 0.99 | <1 × 10−4 |
| Female | 0.83 | 6.56 | 2.48 | 1.04 | |
| Cancer Risk (Morbidity) (×10−4) | |||||
| Male | 1.15 | 9.08 | 3.44 | 1.45 | <1 × 10−4 |
| Female | 1.21 | 9.51 | 3.60 | 1.52 | |
| This Study | WHO [20] | IAEA [57] | Regional Studies | ||||
|---|---|---|---|---|---|---|---|
| Avg. | Area | Ref. | |||||
| 226Ra (Bq/L) | 0.74 ± 0.3 | ≤1 (alpha) | ≤1 | 0.54 | Qena | [8] | |
| 1.50 | Safaga-Quseir | [9] | |||||
| 0.59 | Naser Lake | [35] | |||||
| 0.20 | Assiut | [41] | |||||
| 232Th (Bq/L) | 0.24 ± 0.10 | ≤0.1 (beta) | ≤1 | 0.36 | Qena | [8] | |
| 0.50 | Safaga-Quseir | [9] | |||||
| 0.36 | Naser Lake | [35] | |||||
| 0.08 | Assiut | [41] | |||||
| 40K (Bq/L) | 13 ± 3.54 | 10 | 10 | 5.10 | Qena | [8] | |
| 8.60 | Naser Lake | [35] | |||||
| 6.80 | Assiut | [41] | |||||
| AFD (µSv/y) | Adults | 131.24 ± 46.61 | <100 | <1000 | – | ||
| Children | 190.05 ± 70.33 | <200 | – | – | |||
| Infants | 118.37 ± 44.41 | <260 | – | – | |||
| CR (Mortality, ×10−4) | 2.37–2.48 | <1 × 10−4 | – | – | |||
| CR (Morbidity, ×10−4) | 3.44–3.60 | <1 × 10−4 | – | – | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, K.; Matar, Z.S.; Walther, C.; Salah El-Din, K.; Harb, S.; Kilany, M.; Moubark, K. Radionuclide Distribution and Hydrochemical Controls in Groundwater of the Nile Valley, Upper Egypt: Health and Environmental Implications. Water 2025, 17, 2730. https://doi.org/10.3390/w17182730
Ali K, Matar ZS, Walther C, Salah El-Din K, Harb S, Kilany M, Moubark K. Radionuclide Distribution and Hydrochemical Controls in Groundwater of the Nile Valley, Upper Egypt: Health and Environmental Implications. Water. 2025; 17(18):2730. https://doi.org/10.3390/w17182730
Chicago/Turabian StyleAli, Khaled, Zinab S. Matar, Clemens Walther, Khaled Salah El-Din, Shaban Harb, Mahmoud Kilany, and Karem Moubark. 2025. "Radionuclide Distribution and Hydrochemical Controls in Groundwater of the Nile Valley, Upper Egypt: Health and Environmental Implications" Water 17, no. 18: 2730. https://doi.org/10.3390/w17182730
APA StyleAli, K., Matar, Z. S., Walther, C., Salah El-Din, K., Harb, S., Kilany, M., & Moubark, K. (2025). Radionuclide Distribution and Hydrochemical Controls in Groundwater of the Nile Valley, Upper Egypt: Health and Environmental Implications. Water, 17(18), 2730. https://doi.org/10.3390/w17182730







