1. Introduction
Chemical weathering is a fundamental process in Earth’s geochemical cycles, acting as a natural regulator of atmospheric carbon dioxide (CO
2) levels and shaping the carbon cycle over geological timescales [
1]. Changes in Earth’s climate are linked to the levels of atmospheric carbon dioxide (CO
2), which are ultimately regulated by terrestrial silicate weathering, which controls the carbon removal from the ocean–atmosphere system [
2]. The rate of chemical weathering is influenced by the concentration of CO
2 in the atmosphere, with feedback mechanisms linking weathering rates to the global carbon budget [
3,
4,
5,
6,
7,
8]. Weathering of silicate minerals is especially significant, as it acts as a long-term sink for atmospheric CO
2 [
1,
8,
9,
10,
11]. Global CO
2 consumption by chemical weathering is 237 Mt C per year, with a high proportion (63%) from silicate weathering [
7]. Silicate weathering generates bicarbonate (HCO
3−), which accounts for approximately half of the total atmospheric and soil CO
2 consumed during mineral dissolution [
12,
13].
Dissolved inorganic carbon (DIC) species exhibit pH-dependent distributions: carbonic acid dominates below pH 6, bicarbonate is most predominant between pH 6.4 and 10.3, and carbonate becoming significant only at high pH values above 10.3 [
14]. Partial pressure of CO
2 (pCO
2) in aquatic systems is a key variable in chemical weathering processes, as it directly influences the concentration of DIC and governs the rate of weathering [
13]. Soil CO
2 levels are typically 100 times higher than atmospheric levels, with groundwater and river systems also showing elevated concentrations due to interactions with terrestrial carbon pools [
15]. The largest global carbon pool resides in the oceans (~38,000 Gt C), while terrestrial soils contain approximately 1500 Gt C [
16]. Anthropogenic activities, including deforestation and fossil fuel combustion, release an estimated 7.3 Gt C/year into the atmosphere, impacting carbon dynamics in aquatic systems [
17], The net cumulative emissions of CO
2 from 1850 to 2019 were 2400 ± 240 Gt CO
2 and of these 58% occurred between 1850 and 1989 and 42% from 1990 to 2019 [
18].
The Himalayan region has garnered significant attention due to its unique geotectonic setting and its role in global carbon cycling. Studies have linked enhanced silicate weathering in the Himalayas to tectonic uplift and global cooling during the Cenozoic era [
19,
20]. However, recent studies suggest that only a small fraction of CO
2 consumption in the Himalayas contributes to global cooling [
21,
22]. Recently, Bhatt et al. (2018) [
23] hypothesized that pyrite oxidation compensates for the idealized CO
2 consumption by silicate weathering along the Langtang–Narayani river system in the central Himalaya and later confirmed this based on sulfur isotope data, indicating that weathering exerts minimal impact on pCO
2 over timescales of >5–10 kyr and less than 10 Myr [
24]. Rivers originating in the Himalayas transport substantial organic and inorganic carbon to the oceans, playing a vital role in the global carbon budget [
6,
25]. In the Langtang–Narayani river system, hydrothermal activity and erosion-driven processes have been shown to influence carbon fluxes, with silicate weathering accounting for 10% of annual silicate alkalinity flux [
26].
Surface waters are normally supersaturated with carbon dioxide with respect to atmospheric concentration [
13,
27,
28,
29,
30,
31,
32,
33]. In about 95% of 6708 stream and river sampling locations, a median pCO
2 value greater than atmospheric values were reported, and in a similar way pCO
2 observation of 20,623 sites from 7939 lakes and reservoirs also observed supersaturation [
29]. Regnier et al. (2013) [
30] compiled pCO
2 values for about 12,000 sampling locations and found that 96% of inland waters were supersaturated and 82% showed double the concentration of the atmosphere. Lauerwald et al. (2015) [
31] documented the first global maps of partial pressure of carbon dioxide (pCO
2) in rivers of stream orders three and higher and estimated an average pCO
2 of 2400 μatm at the global scale. Global analysis of pCO
2 for rivers and streams with a catchment area of less than 500 km
2 reveals high maximum pCO
2 values, showing three times higher values in headwater streams than the global average [
34]. Xiao et al. (2020) [
33] found a mean annual average pCO
2 value of 778 μatm at the eutrophic lake Taihu in China, with the highest value reported being 1000 μatm at the river mouth. Bhatt and McDowell (2007) [
13] observed 1568 μatm in tropical streams and an average pCO
2 value of up to 12,938 μatm in lower source points within the Rio Icacos basin, one of the critical zone observatories (CZO) sites of the US in Puerto Rico.
The normally high pCO
2 values from the Langtang–Narayani river system is comparable with global observations indicating the supersaturation of surface waters worldwide, except for exceptionally high values from mid-mountain sites (LNS-5-LNS7) due to metamorphic activities in the region [
27,
29,
31]. More than 15 times higher pCO
2 than the atmospheric value was reported in the River Dee in northern Scotland by Dawson et al. in 1995 [
35]. The Amazon, the largest river in the world, is supersaturated with carbon dioxide due to high respiration relative to primary production [
36]. Higher carbon dioxide in surface waters is due to inputs of organic and inorganic carbon from terrestrial sources [
27]. Tovas et al. (2023) [
32] documented the 779,186 pCO
2 estimates from 35,855 sites across 48 US states and found that flowing freshwater was mostly supersaturated and rising pCO
2 affects a wide range of ecosystems and organisms. Decomposition of organic carbon acts as a primary driver of CO
2 emission from the lake to the atmosphere and reduction in CO
2 emission is due to eutrophication in aquatic ecosystems, which increases primary production and hence the eutrophic lake acts as sink for CO
2 [
37].
Decarbonation hot springs within the mountainous regions play a vital role in the Earth’s carbon budget [
38]. Wang et al. (2024) [
39] documented metamorphic decarbonation as a major source of carbon flux in the southern Tibetan region, responsible for 82% of total dissolved carbon in spring water and estimated a carbon flux of 2.7 to 4.5 × 10
12 mol yr
−1 for the entire Himalayan Orogenic belt. France-Lanord and Derry (1997) [
40] documented that the dominant effect of Neogene Himalayan erosion increased the amount of organic carbon in the sedimentary reservoir, which has had a significantly larger effect on the carbon cycle than the weathering of aluminosilicate by a factor of two to three. Later, Eveans et al. (2008) [
41] reported a four-fold higher rate of CO
2 degassing in geothermal systems than from the chemical weathering uptake of CO
2 in the Narayani basin of the central Nepal Himalaya. The substantial production of CO
2 from metamorphic decarbonation reactions in the mid-mountain region of Nepal is attributed to the Himalayan collision [
41,
42]. Earlier studies have documented higher CO
2 production and emissions due to human activities and climate change or warming [
30,
33,
37,
43]. Mineral weathering and biological activity appear to control the dissolved inorganic carbon (DIC) flux in river basins [
44].
While studies have documented bicarbonate concentration variations in Himalayan river systems [
23,
45,
46,
47,
48], the controlling factors of all inorganic carbon species and their fluxes remain poorly understood. This study aims to evaluate the spatial and temporal variations and fluxes of inorganic carbon species along the Langtang–Narayani river system in the central Nepal Himalaya. Additionally, it seeks to elucidate the mechanisms regulating inorganic carbon dynamics within this high-altitude Himalayan drainage network through the application of empirical modeling.
4. Results and Discussion
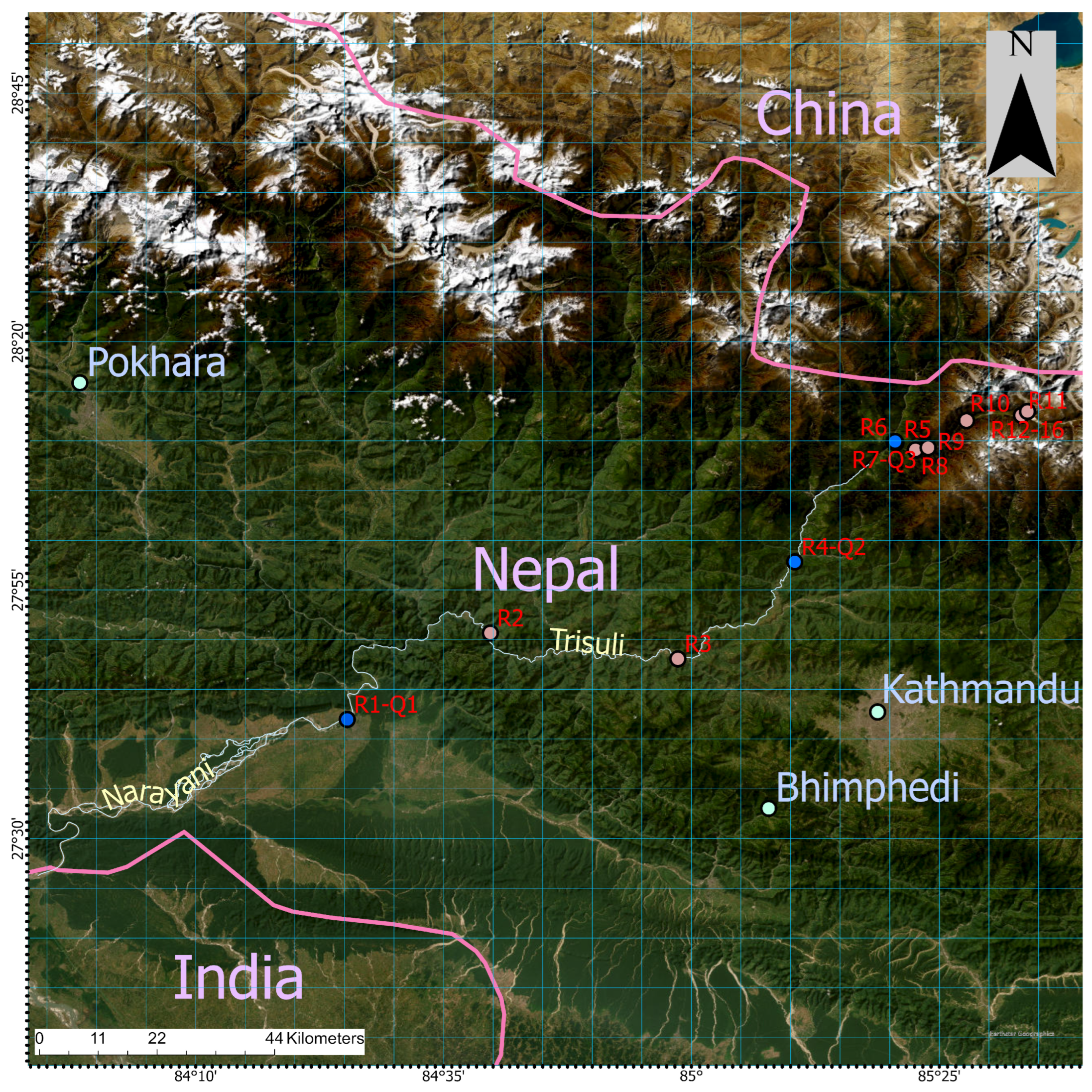
Detailed site descriptions including drainage basins, vegetation cover, geographic coordinates, elevation, water temperature, and pH along the Langtang–Narayani river system with sample locations (LNS-1 to LNS-16) are presented in
Table 1. The Langtang River flows through diverse landscapes and eventually merges into Narayani River. The system is characterized by steep gradients, varied vegetation cover, and significant elevation changes (169 m to 3889 m), making it a valuable site for investigating ecological and biogeochemical dynamics. Samples from lower elevations (LNS-1 to LNS-7) were collected monthly, while those from higher elevations (LNS-8 to LNS-16) were collected once every two months due to difficulty accessing sampling sites. The sites range from 169 m at Narayanghat to 3989 m at the Lirung outlet, highlighting diverse vegetation types from tropical forests in the lowlands to sub-alpine shrubs in the high mountains. Water temperature decreases and pH generally increases with elevation, reflecting the transition from warm, tropical regions to colder, high-altitude environments (
Table 1). High pH values were observed from Kyangjin at Langtang to Khimjung meltwater.
The average concentrations of inorganic carbon species along the Langtang–Narayani river system in central Nepal, measured from fall 2010 to fall of 2011, are presented in
Table 2. The data clearly shows the decrease in inorganic carbon species with an increase in elevation. The partial pressure of carbon dioxide (pCO
2) for seven low-elevation monitoring stations (LNS-1 to LNS-7) is several-fold higher than atmospheric values, whereas the other seven high-elevation monitoring stations (LNS-8 to LNS-16) exhibit lower pCO
2 than atmospheric values. Up to nearly 27-fold higher pCO
2 than the atmospheric value was measured at site LNS-6. Exceptionally very high pCO
2 levels at certain mid-elevation sites (e.g., LNS-5 to LNS-7) suggest localized environmental factors, primarily relating to metamorphic activities and the presence of hot springs in the region. These findings provide insight into the spatial variability of carbon species in this Himalayan river system. Waters from low-elevation sites are supersaturated with carbon dioxide, whereas the waters of high-elevation sites are undersaturated. Our observed pCO
2 values are comparable with global and regional freshwater pCO
2 values except from mid-mountain section due to metamorphic activities [
27,
28,
29,
30,
31,
32,
33].
Carbon concentrations were higher at lower-elevation sites, such as LNS-1, compared to higher-elevation sites like LNS-15 (
Table 2). Notably, pCO
2 values decrease with increasing elevation, transitioning from tropical to alpine environments. This pattern highlights the influence of altitude, temperature, and vegetation cover on the inorganic carbon dynamics of the river system.
4.1. Spatial Variation of Inorganic Carbon Species
4.1.1. Variation of Average DIC Along Langtang–Narayani
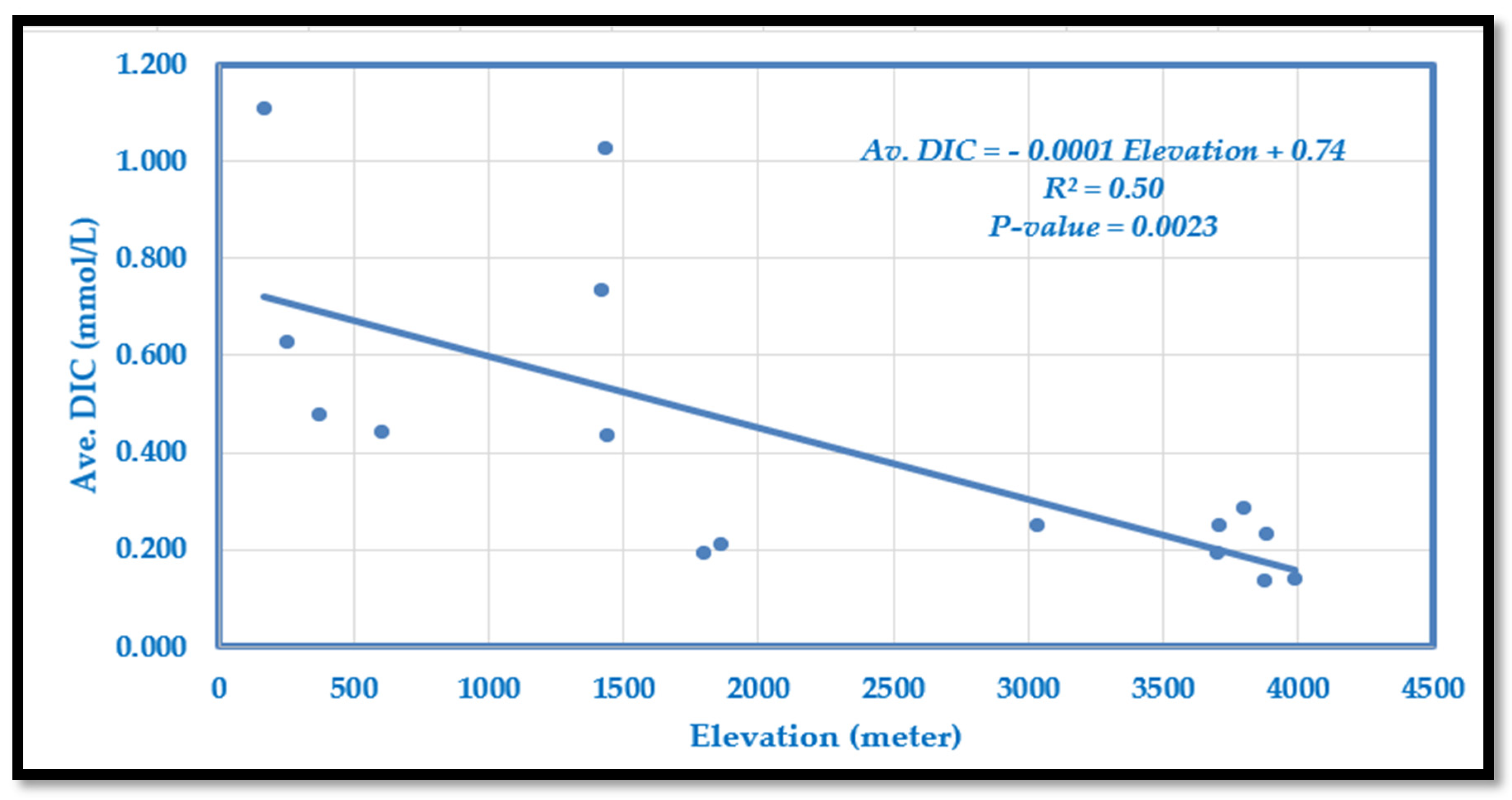
Dissolved inorganic carbon (DIC) ranges from 0.133 mmol/L at high elevations to 1.108 mmol/L in lowland Tarai (
Table 2). DIC generally decreases with elevation, with some mid-mountain anomalies due to metamorphic activity (
Figure 2). The relationship is described by the following model:
The slope indicates a decrease of 0.1 mmol/L per 1000 m rise in elevation. The model explains 50% of the variation (R2 = 0.50, p = 0.0023), showing a moderate but significant negative correlation between DIC and elevation.
Table 3 summarizes linear regression models predicting average dissolved inorganic carbon (DIC) using variables such as elevation, water temperature (WT), alkalinity, CO
2, HCO
3−, pCO
2, and CO
32−. Model fits (R
2) range from 0.50 for elevation to 1.00 for HCO
3−, with the multivariate Model #7 providing a perfect fit (R
2 = 1.00).
Model #7 shows that DIC decreases with rising temperature (−0.001 mmol/L per °C) and higher pH (−0.009 mmol/L per unit). In contrast, DIC increases with CO2 (+0.367 mmol/L per mmol/L), HCO3− (+1.048 mmol/L per mmol/L), and pCO2 (small positive effect). These results highlight bicarbonate and CO2 as the strongest predictors of DIC, while temperature and pH act as negative influences.
4.1.2. Variation of Average Alkalinity Along Langtang–Narayani
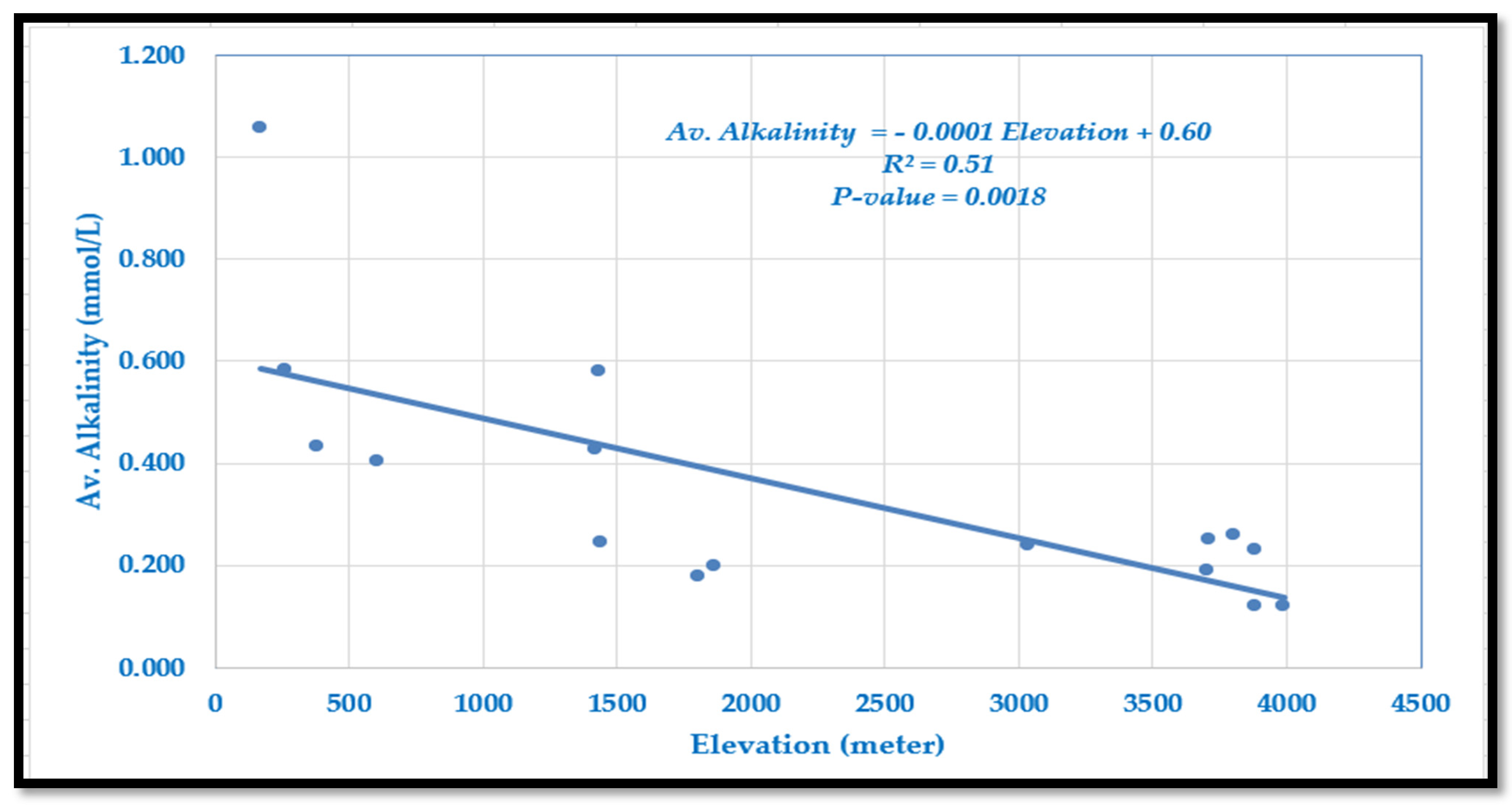
Alkalinity in the Langtang–Narayani river system ranges from 0.120 mmol/L at high elevations to 1.059 mmol/L in lowland Tarai (
Figure 3). Alkalinity generally decreases with elevation, with some site-specific deviations. The relationship is described by the following model:
The slope indicates a decline of 0.1 mmol/L per 1000 m increase in elevation. The model explains 51% of the variation (R2 = 0.51, p = 0.0018), showing a moderate and statistically significant negative correlation. This pattern likely reflects reduced chemical weathering, lower bicarbonate concentrations, and limited ion dissolution at higher altitudes.
4.1.3. Variation of Average CO2 Along Langtang–Narayani
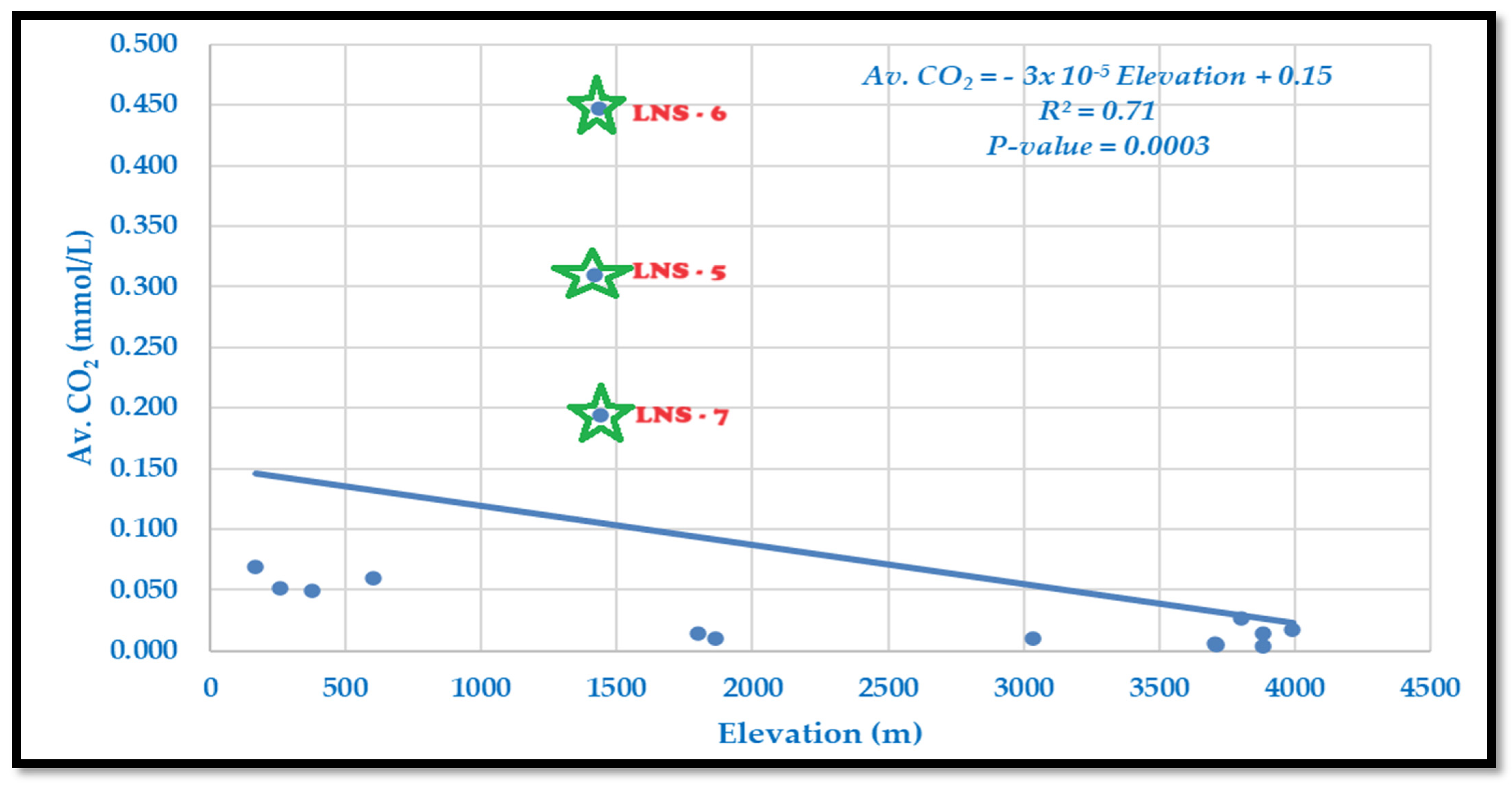
Dissolved CO
2 ranges from 0.004 mmol/L at high elevations to 0.448 mmol/L at mid-elevations, with mid- and lowland sites often showing supersaturation relative to air (
Table 2,
Figure 4). Carbon dioxide decreases with increasing elevation, except for a few mid-mountain sites due to metamorphic activities, e.g., LNS-5 to LNS-7 (
Figure 4). Mantle degassing at hotspots, subduction zone volcanoes, metamorphosis of carbonate rocks into silicate rocks, and oxidative weathering are thought to be the major sources of CO
2 [
62]. We found supersaturated waters with CO
2 in mid-mountain and low-elevation sites but undersaturated waters in high-elevation Himalayan sites. Rivers are mostly supersaturated with CO
2 relative to ambient air and considered as a net source of CO
2 to the atmosphere [
3,
63,
64]. The relationship is described by the following model:
The slope indicates a decline of 0.03 mmol/L per 1000 m rise in elevation. The model explains 71% of the variation (R2 = 0.71, p = 0.0003), showing a strong and significant negative correlation. Higher CO2 in lowlands likely reflects slower flow, greater biological activity, and soil respiration, while lower CO2 levels at high elevations align with cooler temperatures, faster runoff, and reduced organic input.
Table 4 shows a collection of regression models that predict the average carbon dioxide (CO
2) concentration based on various independent variables, such as elevation, water temperature (WT), pH, alkalinity (Alk), dissolved inorganic carbon (DIC), bicarbonate (HCO
3−), partial pressure of CO
2 (pCO
2), and carbonate (CO
32−). Each model provides a predictive equation alongside the R
2 value, indicating the proportion of variance explained. The R
2 values range from 51% (CO
32−) to 99% (a multivariate model incorporating elevation, CO
32−, and pCO
2). This table highlights the effectiveness of single-variable models and the significant improvement in prediction accuracy when multiple variables are combined, as seen in Model #7.
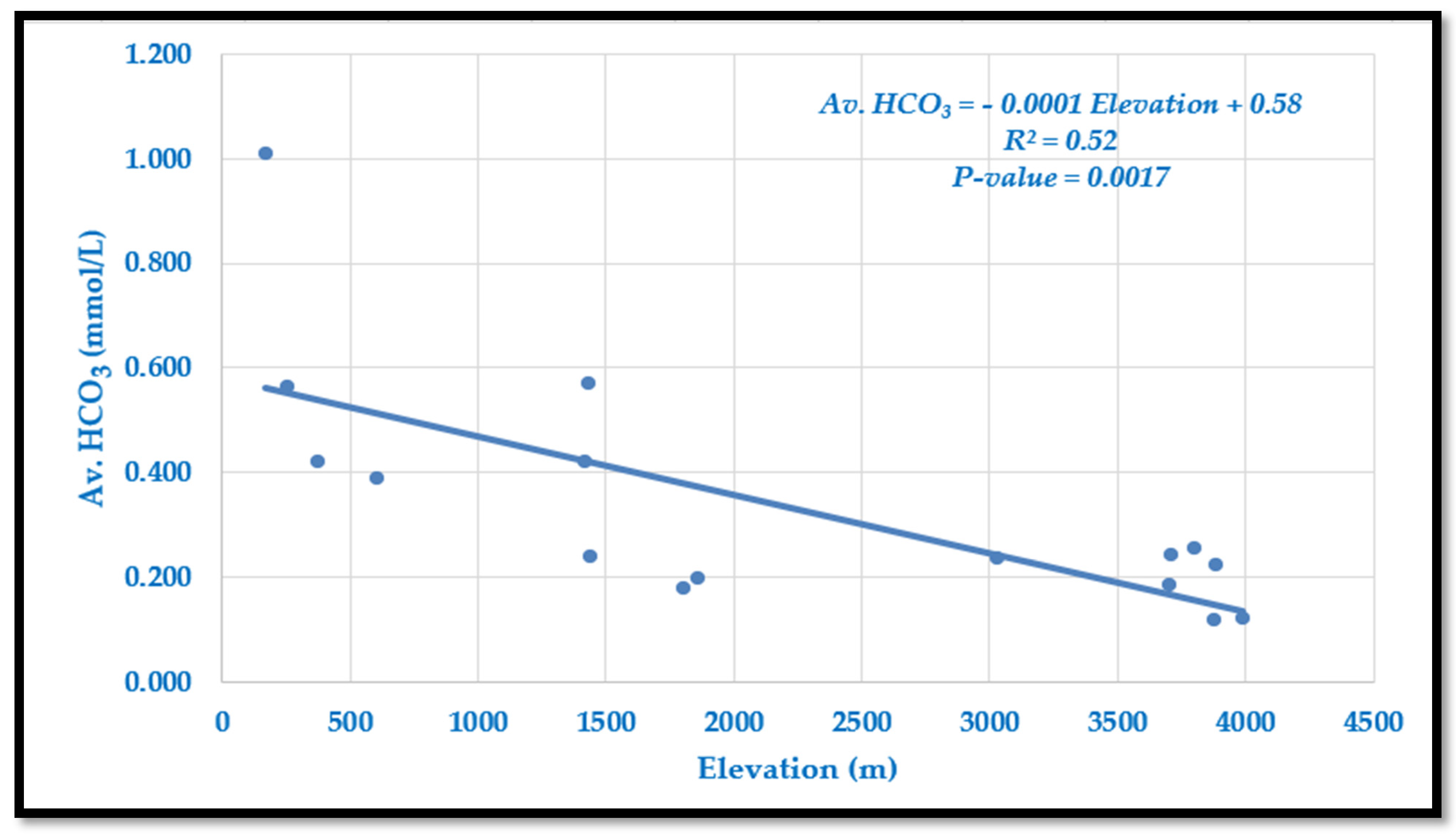
4.1.4. Variation of Average HCO3− Along the Langtang–Narayani River System
Bicarbonate (HCO
3−) ranges from 0.118 mmol/L at high elevations to 1.008 mmol/L in lowland Tarai (
Table 2,
Figure 5). Concentrations generally decrease with elevation, though mid-mountain sites (LNS-5 and LNS-6) show higher values due to enhanced weathering from metamorphic activity. The relationship is described by the following model:
The slope indicates a decrease of 0.1 mmol/L per 1000 m increase in elevation. The model explains 52% of the variation (R2 = 0.52, p = 0.0017), showing a moderate and significant negative correlation. This pattern reflects reduced weathering and CO2 input at high altitudes, while higher concentrations in lowlands are linked to high extent of chemical weathering due to favorable condition, soil CO2 input, abundant availability of partially weathered materials transported from high elevation, and longer water–rock interaction times.
Table 5 displays regression models for predicting average bicarbonate (HCO
3−) concentrations using various independent variables such as elevation, alkalinity (Alk), dissolved inorganic carbon (DIC), carbon dioxide (CO
2), partial pressure of CO
2 (pCO
2), and carbonate (CO
32−). Each model provides a predictive equation along with its R
2 value, which indicates the percentage of variance explained. The R
2 values range from 52% (elevation) to 100% (models involving Alk or the combination of pH, Alk, and CO
32−). The table highlights the strong predictive power of Alk, and the enhanced accuracy achieved through multivariate models, as seen in Model #7.
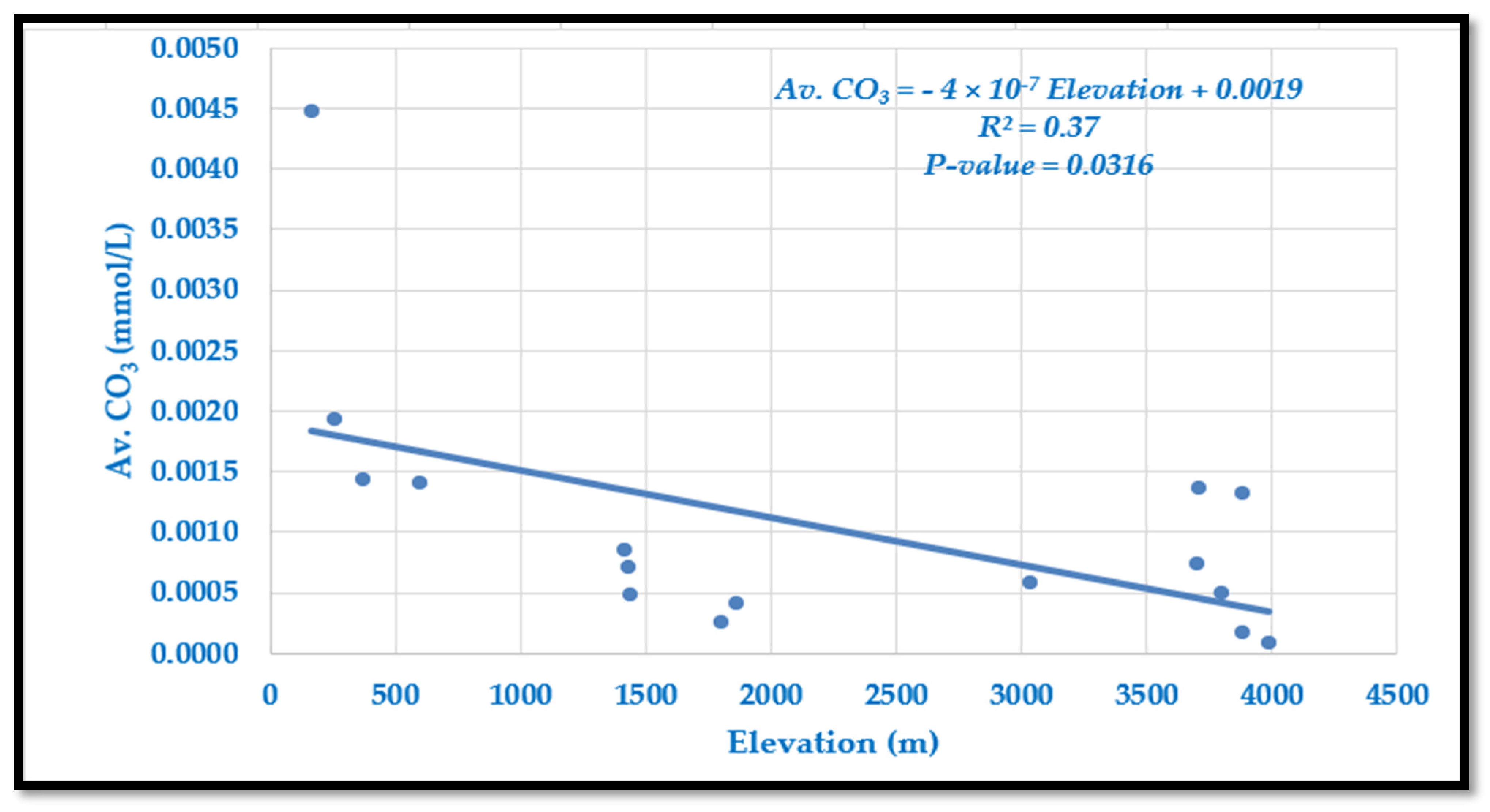
4.1.5. Variation of Average Carbonate (CO32−) Along Langtang–Narayani
Carbonate (CO
3) concentrations vary from 0.083 μmol/L at high elevations to 4.474 μmol/L in lowland Tarai (
Table 2,
Figure 6). Overall, CO
32− decreases with elevation, though occasional site-specific deviations occur. The relationship is captured by the following model:
The slope reflects only a minimal decline in carbonate with altitude, and the model explains 37% of the variation (R2 = 0.37, p = 0.0316), indicating a weak but statistically significant correlation. Compared with bicarbonate, carbonate forms a much smaller fraction of the buffering system, which explains its limited variability. Slightly higher concentrations in lowlands likely result from slower flows and greater rock–water interaction, while colder, fast-flowing highland streams restrict carbonate dissolution and shift the equilibrium toward bicarbonate.
Table 6 highlights the regression models for predicting average carbonate (CO
32−) concentrations based on variables such as elevation, alkalinity (Alk), dissolved inorganic carbon (DIC), carbon dioxide (CO
2), bicarbonate (HCO
3−), partial pressure of CO
2 (pCO
2), and pH. The table presents predictive equations and corresponding R
2 values, which range from 37% (elevation) to 98% (a multivariate model using pH and HCO
3−). Alk, DIC, and HCO
3− individually demonstrate strong predictive power with R
2 values above 90%, while combining pH and HCO
3− in Model #7 yields the highest accuracy. The table highlights the relative contributions of each variable to the prediction of CO
32− concentrations.
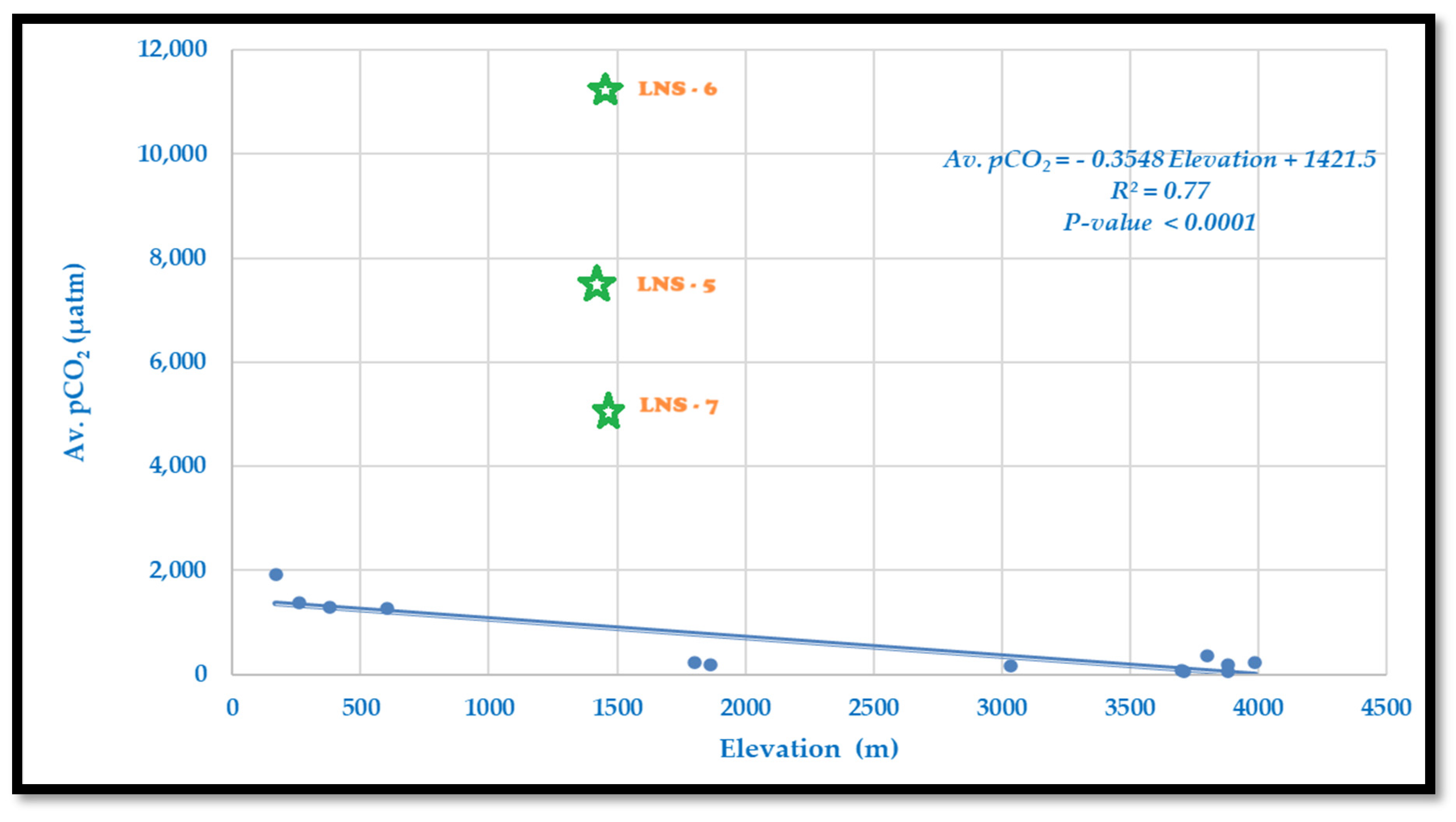
4.1.6. Variation of the Partial Pressure of Carbon Dioxide (pCO2) Along Langtang–Narayani
Partial pressure of carbon dioxide (pCO
2) shows a wide variation along the Langtang–Narayani river system in central Himalaya which ranges from 11,349 μatm at mid-elevation in Syabrubenshi to 56 μatm at high elevation in Khimjung glacier meltwater (
Table 2). The observed pCO
2 values from mid-mountain sites in our studied area appeared much higher than the reported pCO
2 from lakes, streams, and rivers of local and global data sets, although these reported pCO
2 values are also several-fold higher than the atmospheric value (supersaturated) [
13,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37]. The observed pCO
2 in river water from a metamorphic site in the Himalaya basin is 4.7-fold higher than global streams and rivers [
31], 14.6-fold higher than the Taihu lake in China [
33], 7-fold higher than the pCO
2 from Rio Icacos mainstem, 1.7-fold higher than the upper source points, and comparable with the lower source points in the flood-plain area within the tropical rainforest along the Rio Icacos basin, Luquillo Critical Zone Observatory in Puerto Rico [
13]. Metamorphic decarbonation appeared as a dominant source of carbon flux in the southern Tibetan region [
39]. Eveans et al. (2008) [
41] found a 4-fold higher rate of CO
2 degassing in geothermal systems than from the weathering uptake from the central Nepal Himalaya. Such wide variations of pCO
2 in surface waters reflect the importance of metamorphic activities in carbon dynamics.
The pCO
2 decreases with increasing elevation, except for a few mid-mountain sites due to metamorphic activities, e.g., LNS-5 to LNS-7, which are considered as an outlier (
Figure 7). We found the descriptive model of the given linear equation as follows:
Equation (6) describes the relationship between the average partial pressure of carbon dioxide (pCO2) in river water (in micro atmospheres, µatm) and elevation (in meters). Below is a detailed analysis of this relationship. The slope of −0.3548 indicates that for every 1 m increase in elevation, the average pCO2 decreases by approximately 0.3548 µatm. This suggests a significant reduction in dissolved pCO2 concentrations at higher elevations, likely driven by environmental factors such as water turbulence, temperature changes, and gas exchange rates. The intercept of 1421.5 µatm represents the predicted average pCO2 at sea level (elevation = 0 m). This relatively high value reflects the abundance of pCO2 in lowland waters, where biological and chemical processes contribute to its accumulation. The R2 value of 0.77 indicates that 77% of the variability in average pCO2 is explained by elevation. This strong correlation suggests that elevation is a dominant factor influencing pCO2 levels in the studied river system.
The decline in pCO
2 with elevation can be attributed to turbulent water flow at higher elevations, which increases surface area and speeds up CO
2 degassing into the atmosphere, and colder water temperatures at higher altitudes, which reduces microbial respiration, resulting in less CO
2 production. Liu et al. (2022) [
65] highlights the importance of hydrology to transfer terrestrial carbon to the atmosphere through global drainage networks, and the authors also described soil respiration rate as the best predictor for riverine pCO
2 linking between soil carbon dynamics and watershed carbon loss through CO
2 evasion from the water surface. Organic matter decomposition, a key source of CO
2, is often lower at higher elevations due to limited vegetation and slower biological activity. Elevated pCO
2 levels at low altitudes are likely influenced by greater plant and microbial respiration in lowland regions, contributing to higher CO
2 production. The terrestrial respiration and aquatic respiration both contribute equally to pCO
2 efflux, based on the research from Swedish watersheds [
28]. Lowland rivers may receive groundwater enriched in CO
2, further increasing pCO
2 levels. The strong R
2 value highlights elevation as a critical factor in controlling riverine pCO
2 variability. The analysis yielded a
p-value of <0.0001, providing strong evidence against the null hypothesis (H
0) of no relationship between pCO
2. This result supports the inclusion of elevation as a predictor in the model. This is consistent with the expectation that elevation influences water turbulence, temperature, and interactions with atmospheric CO
2.
Table 7 provides regression models predicting average partial pressure of carbon dioxide (pCO
2) using various independent variables, including elevation, water temperature (WT), alkalinity (Alk), dissolved inorganic carbon (DIC), bicarbonate (HCO
3−), carbon dioxide (CO
2), and carbonate (CO
32−). The models present predictive equations along with R
2 values, which indicate the proportion of variance explained by each model. The R
2 values range from 64% (CO
32−) to 99% (a multivariate model with WT, CO
2, and CO
32−). Among individual predictors, CO
2 exhibits the strongest predictive power with R
2 = 97%. The multivariate model (Model #7) achieves the highest accuracy, highlighting the combined influence of WT, CO
2, and CO
32− in estimating pCO
2.
4.2. Descriptive Correlation Analysis of Measured Parameters
Table 8 presents the results of a Pearson’s correlation analysis examining the relationships between elevation and key carbonate chemistry variables (Alk, DIC, CO
2, HCO
3−, CO
32−, and pCO
2) along the Langtang–Narayani river system.
4.2.1. Influence of Elevation on Other Parameters
Negative correlations were observed for the water temperature, alkalinity, DIC, HCO3−, CO32−, CO2, and pCO2, such as with water temperature (r = −0.971, p < 0.01); higher elevations correspond to significantly cooler water temperatures, consistent with typical climatic patterns. With alkalinity (r = −0.735, p < 0.01), DIC (r = −0.751, p < 0.01), HCO3− (r = −0.737, p < 0.01), and CO32− (r = −0.610, p < 0.05), these parameters decrease at higher altitudes, reflecting the influence of temperature and pressure on carbonate equilibria and mineral dissolution rates. With CO2 (r = −0.843, p < 0.01) and pCO2 (r = −0.876, p < 0.01), the reduction in dissolved CO2 with elevation may be tied to changes in gas solubility and atmospheric pressure.
4.2.2. Carbonate Chemistry Interactions
Highly interrelated parameters include alkalinity and HCO3− (r = 1.000, p < 0.01), and these variables are essentially identical under the studied conditions. DIC and HCO3− (r = 0.999, p < 0.01) showing strong coupling highlights the dominance of bicarbonate buffering in the carbonate system. CO2 and pCO2 coupling (r = 0.983, p < 0.01) indicates tight regulation between dissolved CO2 and its partial pressure. CO32− shows strong positive correlations with alkalinity (r = 0.964, p < 0.01) and DIC (r = 0.954, p < 0.01), reflecting its dependence on the overall carbonate equilibrium. The CO32− shows moderate correlation with CO2 (r = 0.713, p < 0.01), suggesting interplay between dissolved CO2 and carbonate ion concentrations.
The strong negative correlations of elevation with temperature, alkalinity, and carbonate chemistry parameters emphasize the role of altitude in regulating geochemical processes. Temperature strongly influences carbonate system dynamics, promoting higher dissolved carbon and alkalinity at lower elevations. Tight coupling among alkalinity, DIC, and HCO3− highlights the buffering role of bicarbonates, while the pH–carbonate relationship reinforces the balance between acidity and dissolved carbon species. These relationships reflect the interconnected influence of physical and chemical factors on water composition. Future research could explore the specific drivers of variability (e.g., biological activity or mineral weathering) in different altitudinal and thermal contexts.
4.3. Seasonal Variations of Inorganic Carbon Species Along Langtang–Narayani
Seasonal variation of inorganic carbon species measured along the Langtang–Narayani river system in central Nepal Himalaya from fall 2010 to fall 2011 are presented in
Table 9. The data includes alkalinity, dissolved inorganic carbon (DIC), carbon dioxide (CO
2), bicarbonate (HCO
3−), carbonate (CO
32−), and partial pressure of CO
2 (pCO
2) across 16 sampling locations during pre-monsoon, monsoon, and post-monsoon periods. Measurements are reported as means with standard deviations.
Table 9 reveals the substantial seasonal and spatial variability of carbon species including pCO
2 across seasons, reflecting the significant role of climatic, hydrological, and geochemical processes in the river system.
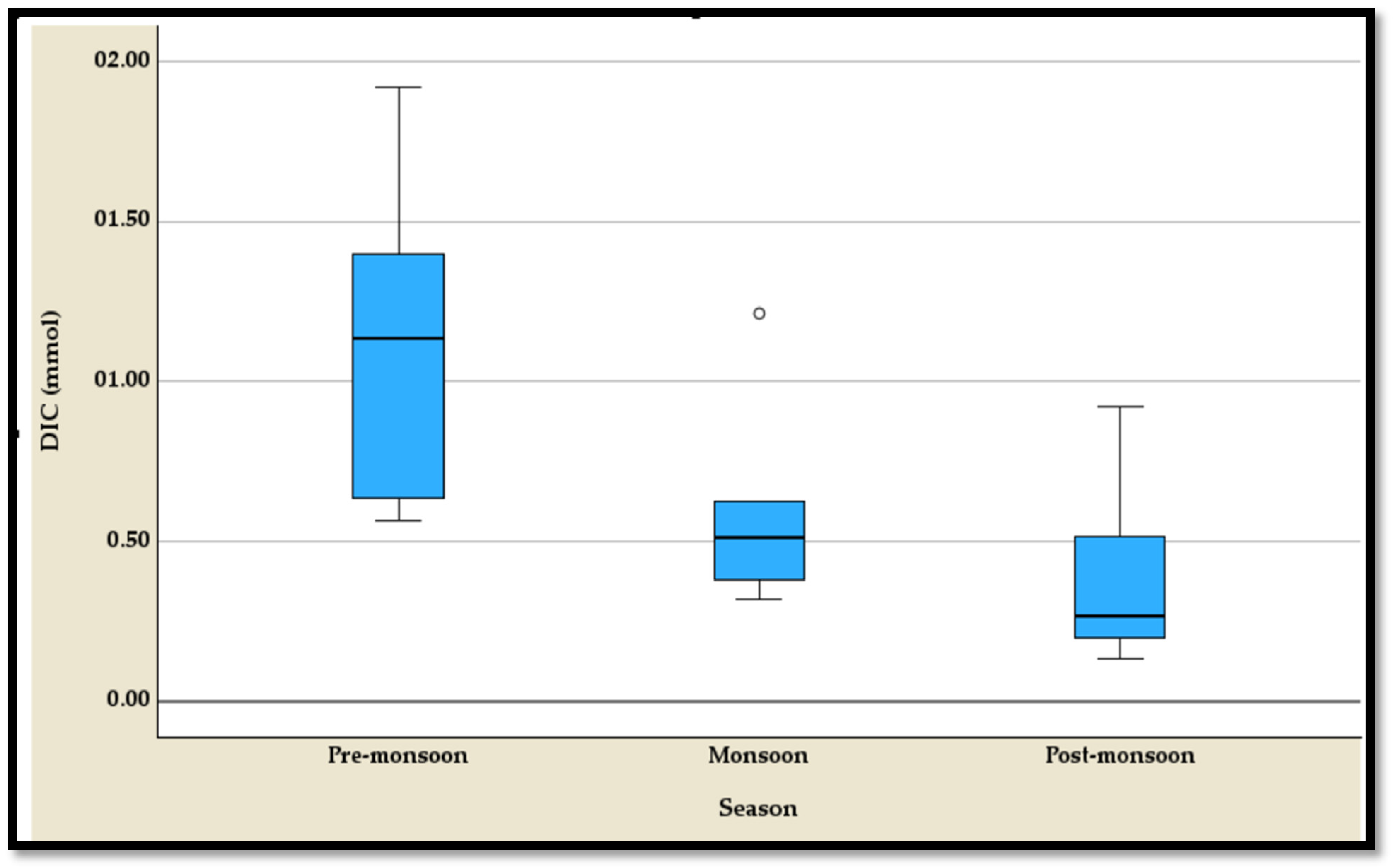
4.3.1. The Temporal Variation of the DIC
The temporal variation of the dissolved inorganic carbon (DIC) exhibits distinct seasonal patterns, as highlighted by the five-number summary and boxplot (
Table S1;
Figure 8). During the pre-monsoon season, DIC values are the highest, ranging from a minimum of 0.564 mmol to a maximum of 1.917 mmol, with a median of 1.135 mmol (
Table S1). This season shows a wide spread of values, indicating elevated carbon concentrations. In contrast, the monsoon season experiences a significant drop in DIC levels, with a narrower range from 0.317 mmol to 1.212 mmol and a median of 0.511 mmol (
Table S1). However, an outlier is observed in this season, reflecting occasional deviations from typical concentrations. The post-monsoon season records the lowest DIC levels overall, ranging from 0.133 mmol to 0.921 mmol and a median of 0.267 mmol, suggesting a significant depletion in dissolved carbon post rainfall (
Table S1). The progression from pre-monsoon to post-monsoon illustrates a clear decline in DIC, likely driven by seasonal hydrological and ecological dynamics.
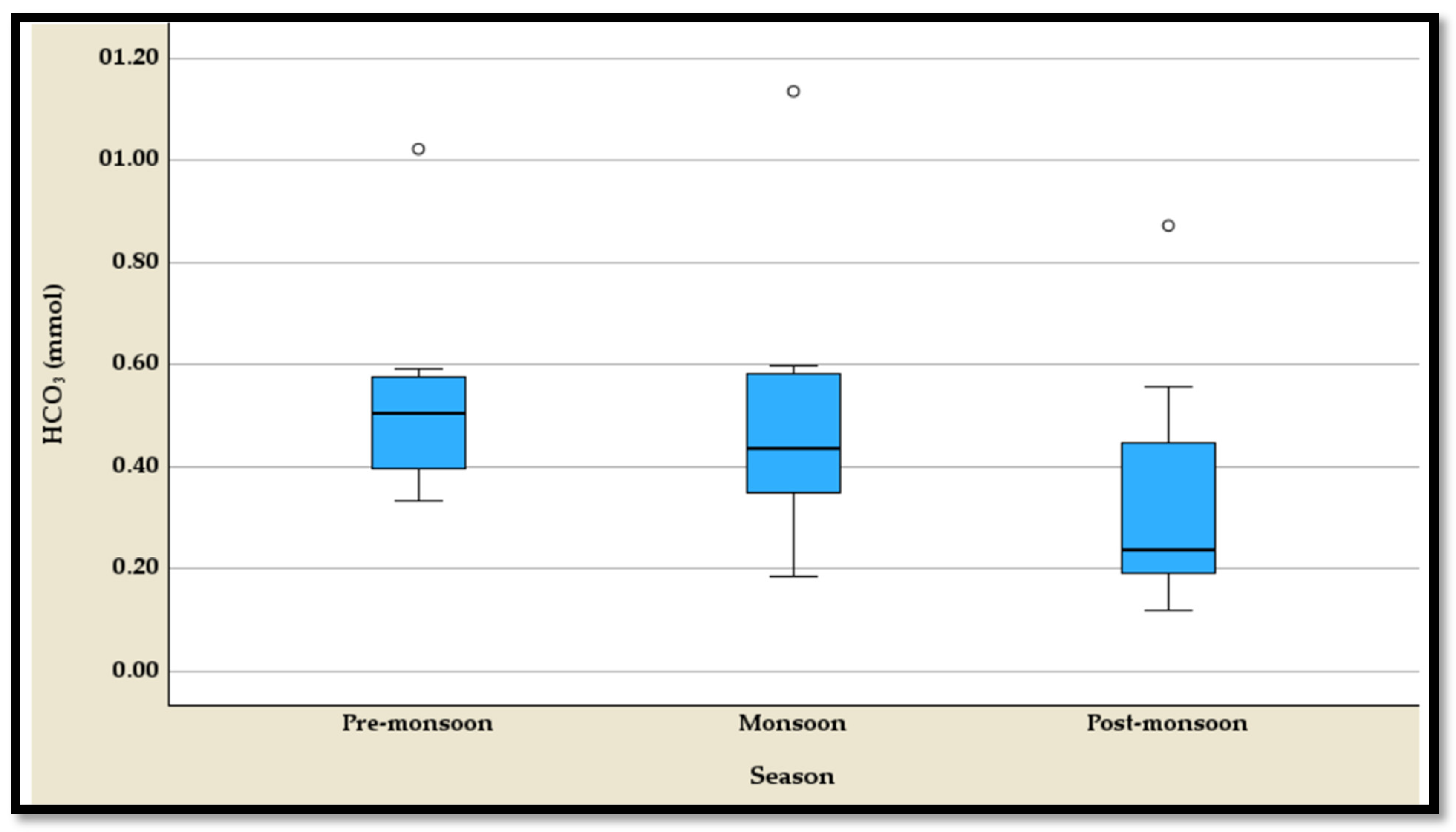
4.3.2. The Temporal Variation of the HCO3−
The temporal variation of bicarbonate (HCO
3−) concentrations reveals distinct seasonal differences, as shown by the five-number summary and corresponding boxplots, each displaying an outlier (
Table S2,
Figure 9). During the pre-monsoon season, HCO
3− levels are the highest, ranging from 0.334 mmol to 1.022 mmol, with a median of 0.506 mmol (
Table S2). This season exhibits relatively higher concentrations, reflecting increased bicarbonate availability. The monsoon season shows a slight reduction in HCO
3− levels, with a range from 0.186 mmol to 1.135 mmol and a median of 0.434 mmol, though the interquartile range (Q
1 to Q
3) is comparable to the pre-monsoon period (
Table S2). The post-monsoon season has the lowest HCO
3− concentrations, spanning from 0.118 mmol to 0.872 mmol, with a median of 0.238 mmol, indicating significant bicarbonate depletion after the monsoon (
Table S2). Each season features an outlier, suggesting occasional anomalous variations in HCO
3− levels that deviate from the typical seasonal patterns. This trend reflects the interplay of hydrological and biogeochemical processes influencing bicarbonate dynamics throughout the year.
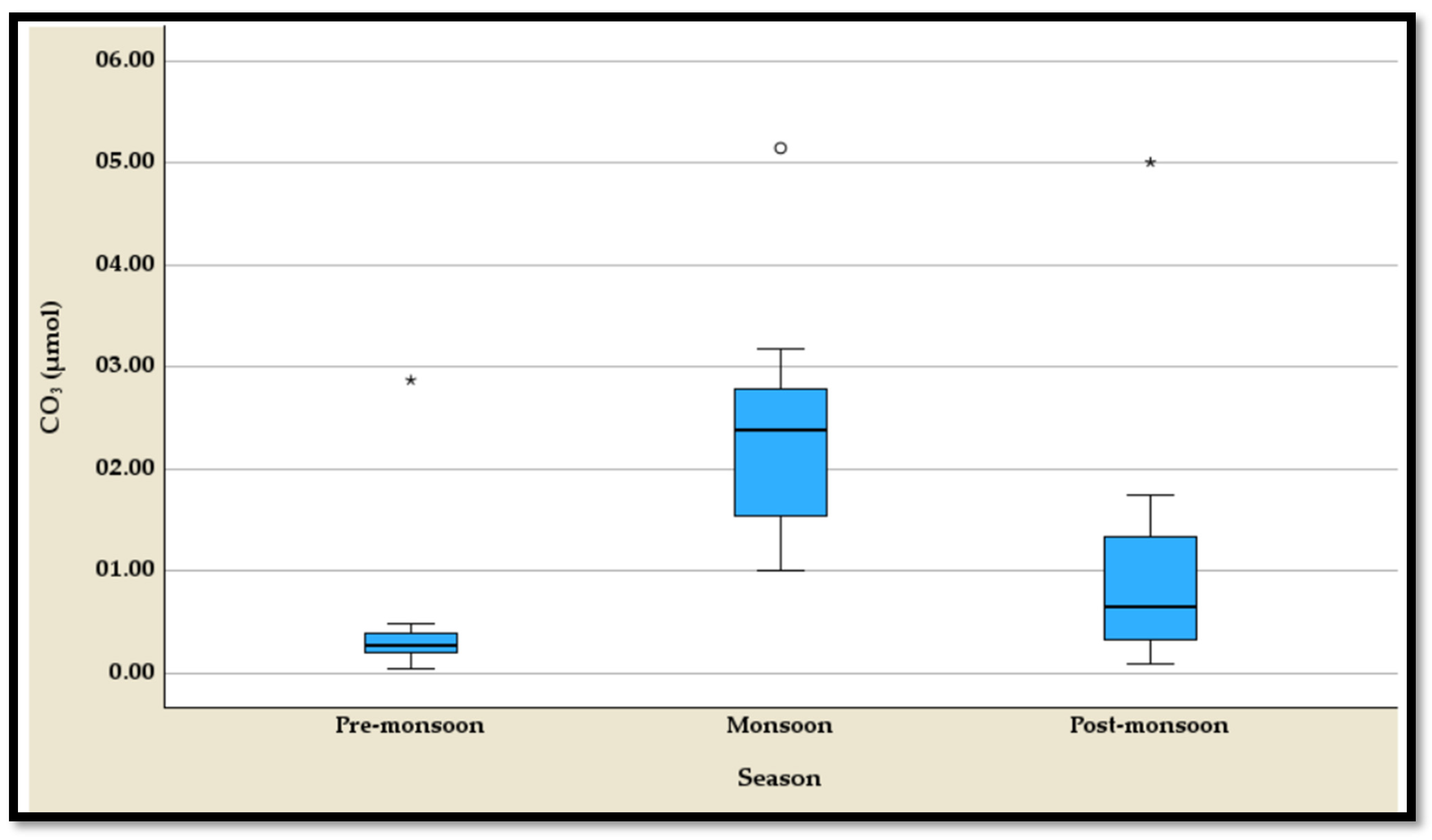
4.3.3. The Temporal Variation of the CO32−
The temporal variation of carbonate (CO
32−) concentrations demonstrates notable seasonal differences, as revealed by the five-number summary and boxplots, with each season exhibiting an outlier (
Table S3,
Figure 10). During the pre-monsoon season, CO
32− levels are relatively low, ranging from 0.040 µmol to 0.491 µmol, with a median of 0.252 µmol (
Table S3). This season reflects the lowest overall carbonate concentrations, suggesting limited carbonate availability. In stark contrast, the monsoon season exhibits a significant increase, with values ranging from 1.011 µmol to 3.170 µmol and a median of 2.159 µmol (
Table S3). This marked elevation highlights the impact of monsoonal processes on carbonate levels. The post-monsoon season shows intermediate carbonate concentrations, with a range from 0.083 µmol to 1.747 µmol and a median of 0.577 µmol, indicating a reduction compared to the monsoon season but higher levels than pre-monsoon (
Table S3). The presence of outliers in all three seasons suggests episodic deviations in carbonate concentrations, likely influenced by localized or transient environmental factors. These seasonal patterns reflect dynamic carbonate chemistry shaped by precipitation, runoff, and ecological interactions.
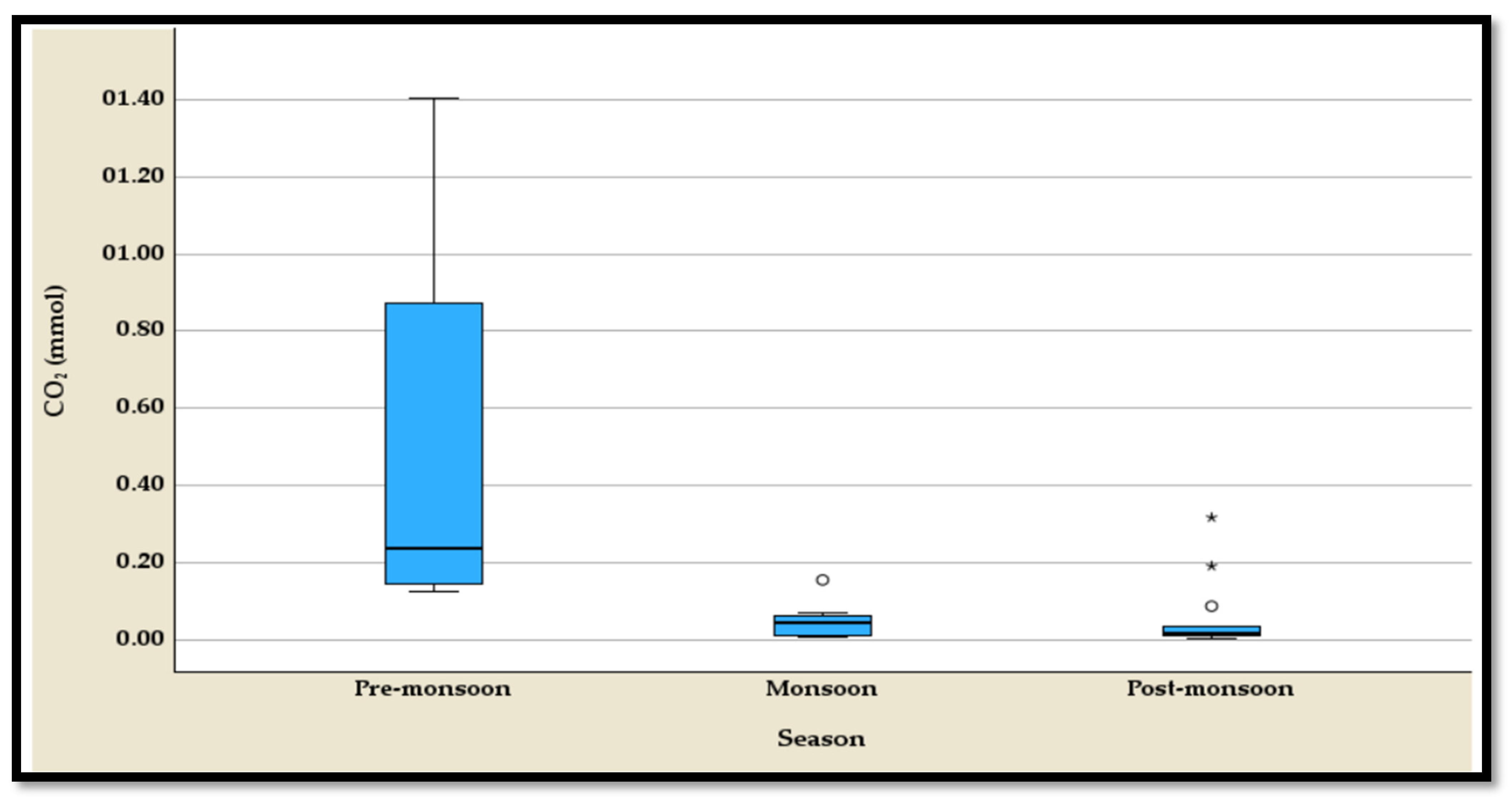
4.3.4. The Temporal Variation of the CO2
The temporal variation of carbon dioxide (CO
2) concentrations highlights pronounced seasonal differences, as evidenced by the five-number summary and boxplots (
Table S4,
Figure 11). During the pre-monsoon season, CO
2 levels are the highest, ranging from 0.125 mmol to 1.405 mmol, with a median of 0.236 mmol (
Table S4). This season shows a wide interquartile range, reflecting elevated and variable CO
2 concentrations without any outliers. In the monsoon season, CO
2 levels drop significantly, with a range from 0.006 mmol to 0.155 mmol and a median of 0.046 mmol (
Table S4). This sharp decline suggests enhanced CO
2 dissolution or reduced production during monsoonal rainfall, although outliers are observed, indicating occasional deviations. The post-monsoon season records the lowest median CO
2 concentration (0.019 mmol) and a range from 0.004 mmol to 0.317 mmol (
Table S4). Despite the overall decrease, outliers are present, reflecting episodic CO
2 increases possibly due to localized post-monsoonal processes. These seasonal patterns underscore the dynamic interplay of hydrological, atmospheric, and biological factors governing CO
2 levels.
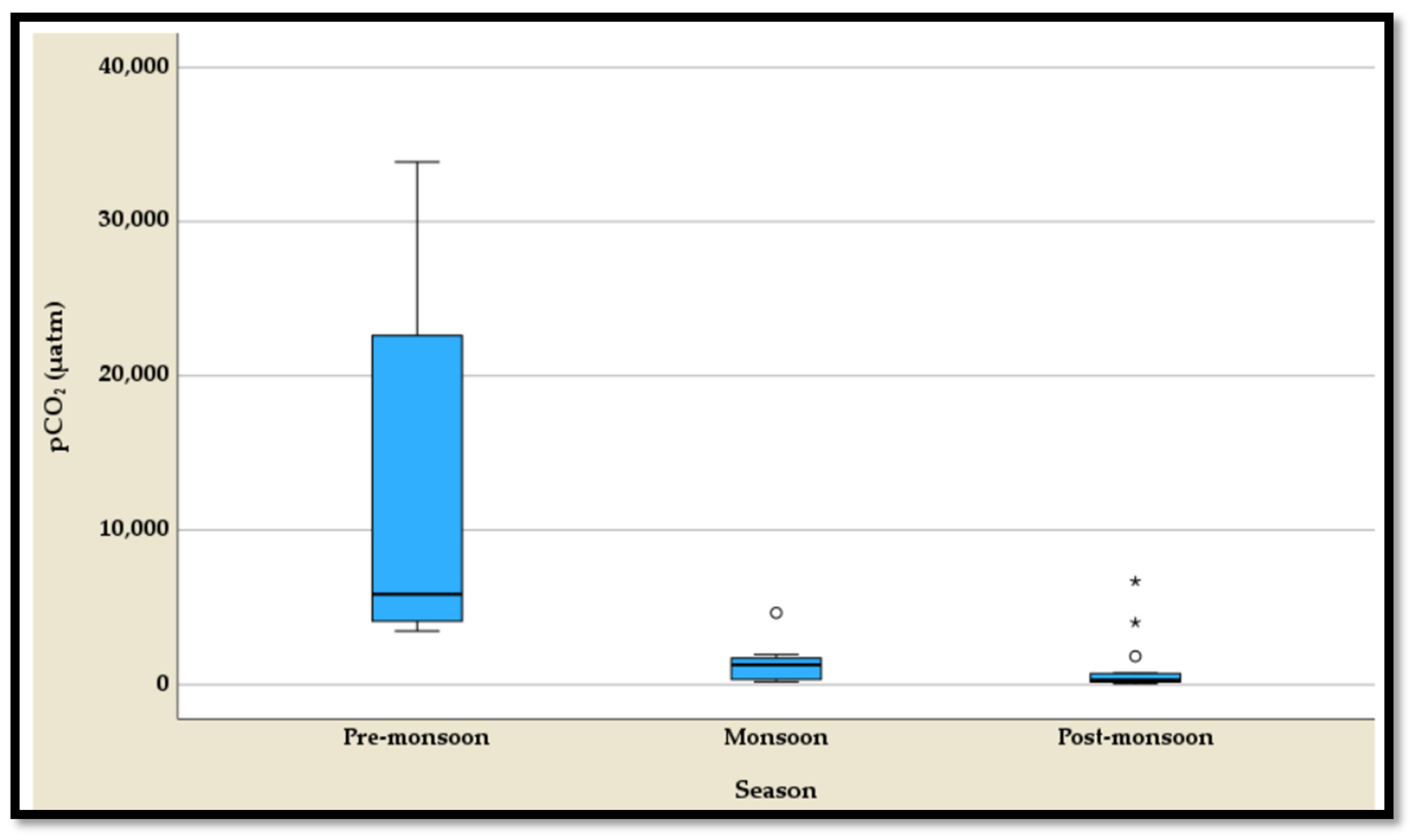
4.3.5. The Temporal Variation of the pCO2
The temporal variation of partial pressure of carbon dioxide (pCO
2) shows distinct seasonal patterns, as highlighted by the five-number summary and boxplots, with outliers observed in the monsoon and post-monsoon seasons but not in the pre-monsoon season (
Table S5,
Figure 12). During the pre-monsoon season, pCO
2 levels are substantially higher, ranging from 3465 µatm to 33,869 µatm, with a median of 5836 µatm (
Table S5). This season exhibits the highest variability and concentrations, likely driven by elevated biological or atmospheric CO
2 contributions. In contrast, the monsoon season experiences a dramatic reduction in pCO
2 levels, ranging from 185 µatm to 4637 µatm, with a median of 1283 µatm (
Table S5). Outliers in this season suggest occasional localized surges in pCO
2 despite the overall decline. The post-monsoon season records the lowest pCO
2 levels, ranging from 56 atm to 6673 µatm, with a median of 305 µatm (
Table S5). While the levels are more stable compared to the monsoon season, outliers highlight sporadic increases. These seasonal trends reflect the influence of monsoonal precipitation, runoff, and associated biogeochemical processes on pCO
2 dynamics.
Water levels increase drastically along the Himalaya river system during monsoon months (June–September) due to extreme rainfall, rapid glacier melting, and a much higher groundwater recharge rate [
61]. The highest discharge occurs during the summer months (June to September), followed by post-monsoon months (October to January) and the lowest during the pre-monsoon months (February to May) [
23]. More than 80% of solute load transport from Himalayan river system to the global ocean occurs during monsoon, reflecting seasonal control on chemicals and suspended sediment transport. The proportion of pCO
2 released was 78.6% during pre-monsoon, 17.3% during monsoon, and 4.1% during post-monsoon along the Himalayan drainage network (
Figure 12;
Table S5). This variation is attributed to difference in microbial activities, residence time of water, and variation in dissolution rate due to abundant hydrolysis conditions with the availability of fresh reactive mineral surfaces during monsoon months. Variation in discharge, microbial activities, and terrestrial and aquatic respiration are all factors that influence carbon dynamics, including pCO
2, in different landscapes [
28,
65]. The dissolution rate is less during pre-monsoon, so less bicarbonate is produced and more pCO
2 is available in water, while during monsoon enhanced weathering produces tremendous amounts of bicarbonate as more pCO
2 is consumed during fast weathering of carbonate and aluminosilicate dissolution, hence pCO
2 appeared low. A similar relationship between pCO
2 and SiO
2 was observed within the Rio Icacos watershed in the tropical rainforest in Puerto Rico [
13].
4.4. Export of Inorganic Carbon Species from Different Transects of Langtang–Narayani
Annual export of inorganic carbon species at three transects of the Langtang–Narayani river system in central Nepal Himalaya during 2010–2011 is presented in
Table 10. The transects range from low altitude (Narayani at 169 m) to high altitude (Langtang at 3710 m). Reported values include dissolved inorganic carbon (DIC), bicarbonate (HCO
3−), carbon dioxide (CO
2), and carbonate (CO
32−), expressed in tons km
−2 yr
−1. The estimated DIC export rates were at 93.66, 37.81, and 12.59-tons km
−2 yr
−1 from the Narayani River in the lowlands, the Trisuli River in the middle mountains, and the Langtang River in the high Himalayas, respectively. These fluxes are calculated based on the average concentrations of inorganic carbon species and average annual discharge of the same year from all three monitoring stations to present the exact transport rate. Earlier, Bhatt et al. (2018) [
23] documented annual river runoff and export of all water constituents for each station with the number of years of available average discharge data, including percentage of runoff and export by season. The Himalayan river system, including the Ganga river system, is a critical region, having significantly large inputs of carbon into the Bay of Bengal [
30]. Freshwater atmosphere flux of CO
2 primarily from rivers and streams contributes about 560 Mt C per year [
64,
66]. Rivers are considered as a biogeochemical reactor from which substantial net fluxes of CO
2 move to the atmosphere [
29,
67]. The data reveal a significant decrease in inorganic carbon exports with increasing altitude, with Narayani exhibiting the highest export and Langtang the lowest.
The dissolved inorganic carbon (DIC) export rate is much higher than the cationic denudation after sea-salt correction (75.48 tons km
−2 yr
−1), silica denudation (12.72 tons km
−2 yr
−1), and dissolved organic carbon (DOC) export rate (2.62 tons km
−2 yr
−1) at the low-elevation site at Narayanghat in the Narayani river system, a major tributary of the Ganga river system, where the weathering advance rate is reported as 201.12 mm kyr
−1, several-fold higher than the world average denudation rate (Bhatt et al., 2018) [
23]. The contribution of bicarbonate, carbon dioxide, and carbonate export rate to total DIC export rate is 97.9%, 1.46%, and 0.52%, respectively, at the high-elevation Langtang Himalaya region while the contribution of bicarbonate, carbon dioxide, and carbonate export rate to total DIC export rate is 94.9%, 4.68%, and 0.42%, respectively, at the low-elevation Narayani River at Narayanghat. These findings indicate substantial transport of dissolved inorganic carbon transport from the Himalayan river system to the global ocean.
5. Conclusions
This study investigates the intricate relationships between elevation and inorganic carbon species such as dissolved inorganic carbon (DIC), bicarbonate (HCO3−), carbonate (CO32−), carbon dioxide (CO2), and partial pressure of carbon dioxide (pCO2) dynamics along the Langtang–Narayani river system in central Nepal Himalaya, providing valuable insights into the geochemical and hydrological processes shaping the riverine environment. Bicarbonate, carbonate, and DIC declined significantly with altitude, indicating a reduced chemical weathering rate due to colder temperatures, less runoff, lower biological activity, and diminished CO2 availability. We have also observed a significant reduction in CO2 and pCO2, primarily because of enhanced CO2 degassing in more turbulent waters and reduced microbial respiration in the colder, less biologically active environment in higher elevation of the Himalayas. The strong correlations between temperature, alkalinity, bicarbonate, and DIC emphasize the role of thermal conditions in controlling carbonate system dynamics. We observed pCO2 values of up to 33,869 μatm during pre-monsoon period in the mid-elevation site, suggesting waters are supersaturated with carbon dioxide in this region due to metamorphic activities or the presence of hot springs in the region. High-elevation sites are undersaturated with pCO2. The variation in dynamics of inorganic carbon species along the Langtang–Narayani river system in the central Himalaya is primarily due to variation in climatic, biological, and hydrological factors.