Horizontal Flow Floating Treatment Wetlands (HFFTWs) for Reclaiming Safer Irrigation Water from Tannery Effluent
Abstract
1. Introduction
2. Materials and Methods
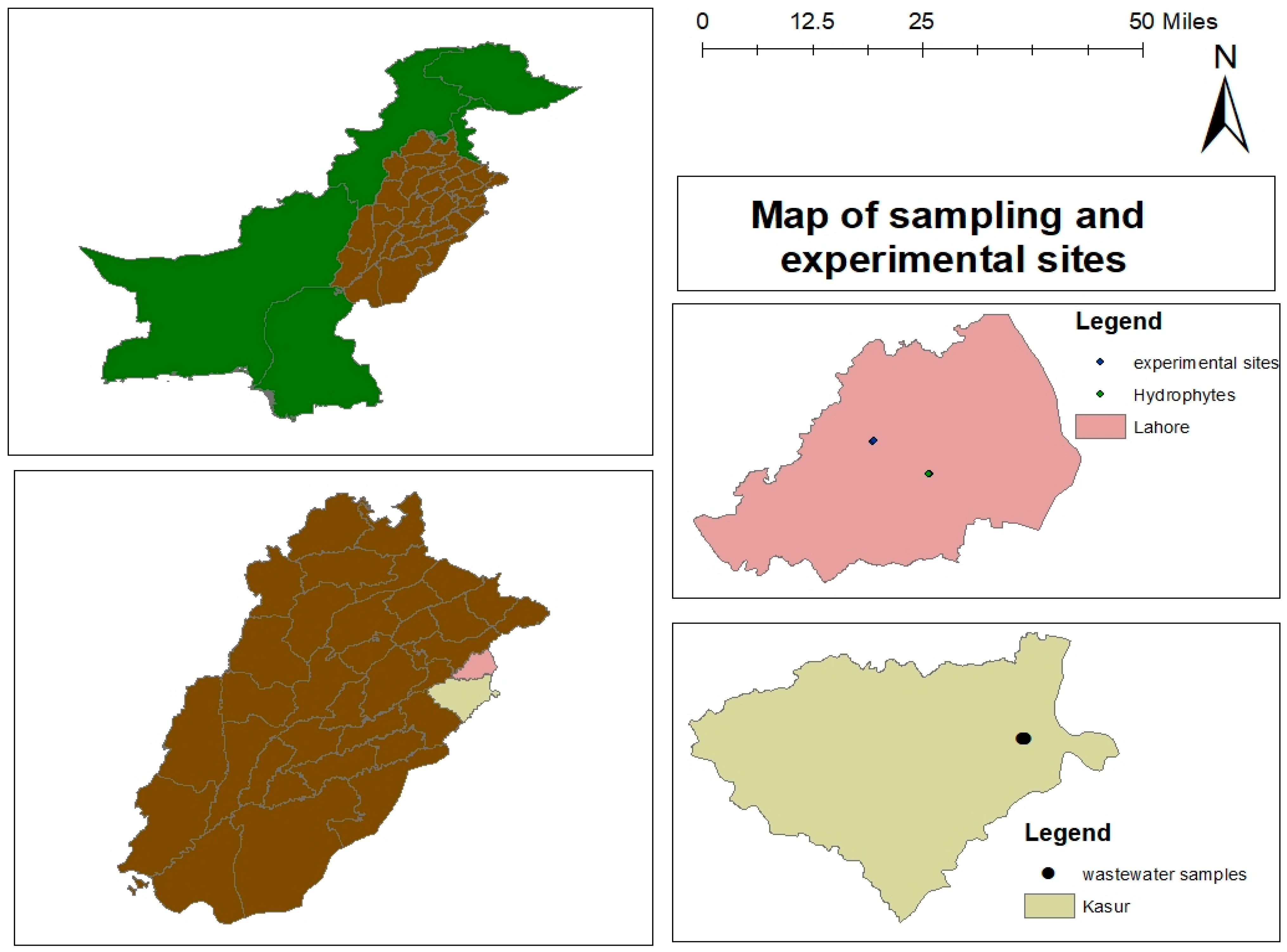
2.1. Collection of UTW
2.2. Collection of Hydrophytes, Viz. E. crassipes and P. stratiotes, Saplings for Application in HFFTW
2.3. Physicochemical and Heavy Metal Analysis of UTW at Pre- and Post-Treatment Level
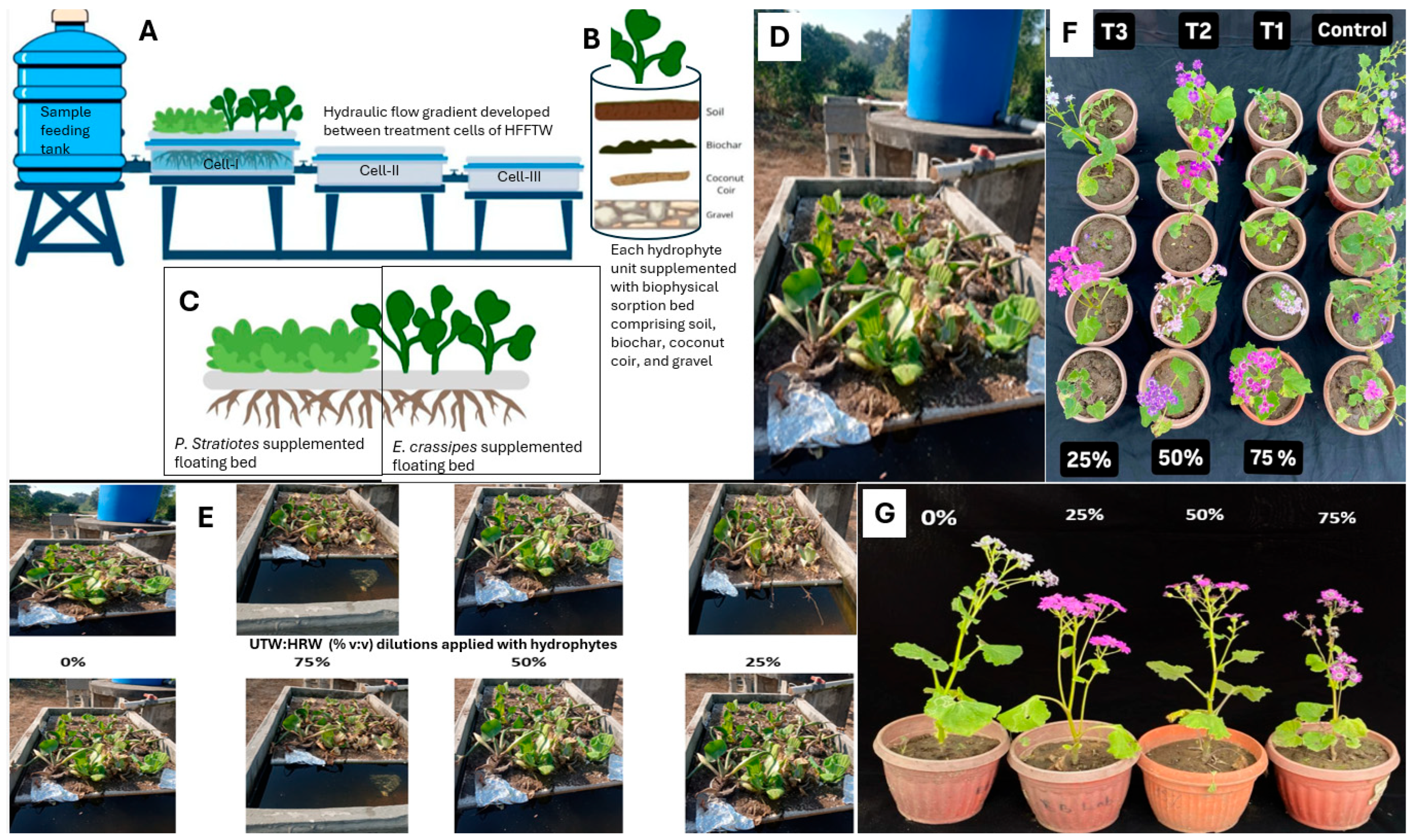
2.4. Designing and Testing HFFTW at Mesocosmic Level
2.5. Phytoremediation Performance of P. stratiotes and E. crassipes
2.6. Safer Irrigation Trials of Reclaimed Water from UTW with P. hybrida
2.7. Silting Rate (mm/hr), Evaporation Rate (mm/day), and Yield (m3/m3) of Reclaimed Water from UTW:HRW Dilutions Through PS- and EC-HFFTW Series
2.8. Data Analysis
3. Results
3.1. Pre-Treatment Characterization of UTW, HRW, and Groundwater
3.2. Pollution Reduction Potential of HFFTW Series for UTW:HRW Dilutions
3.2.1. Reduction in Physicochemical Characteristics of UTW:HRW Dilutions Under PS- and EC-HFFTW Series
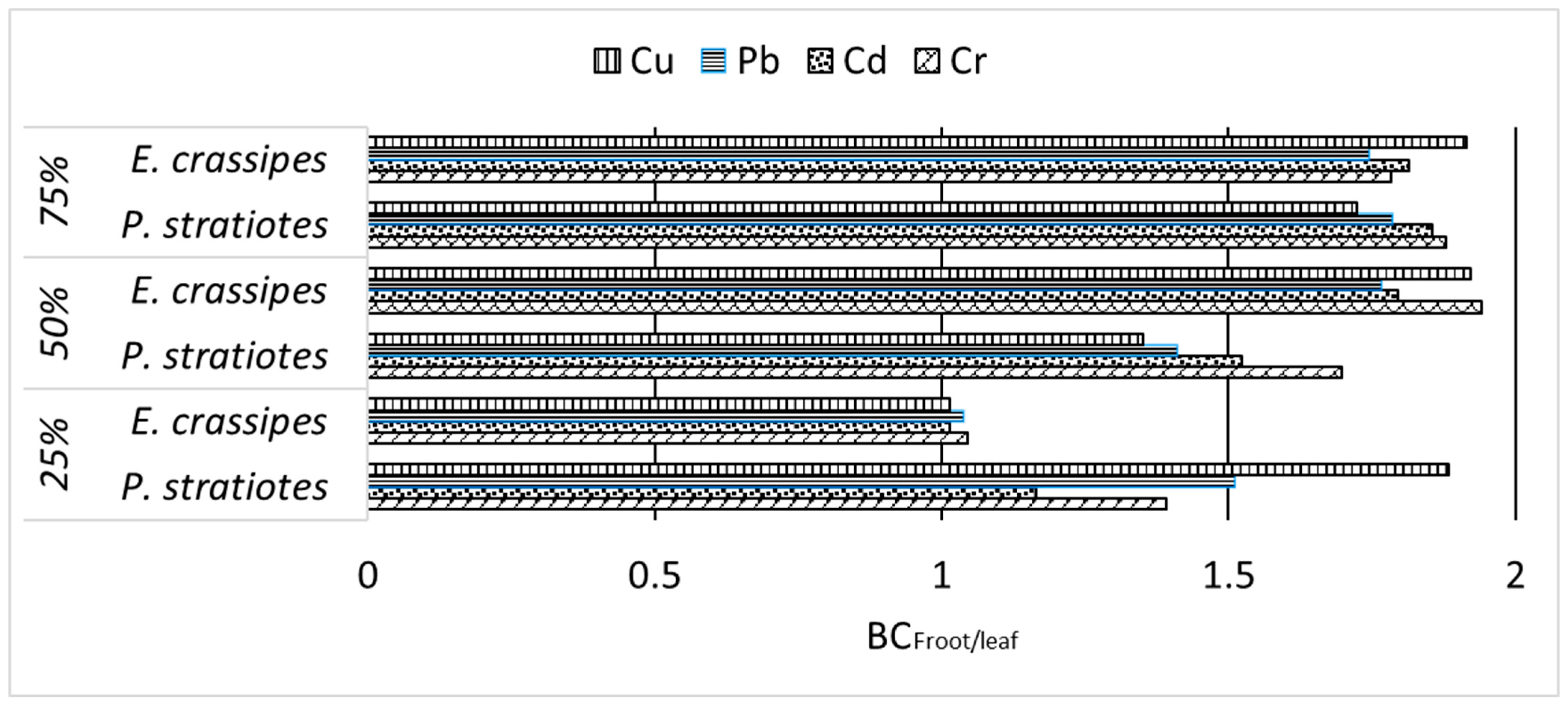
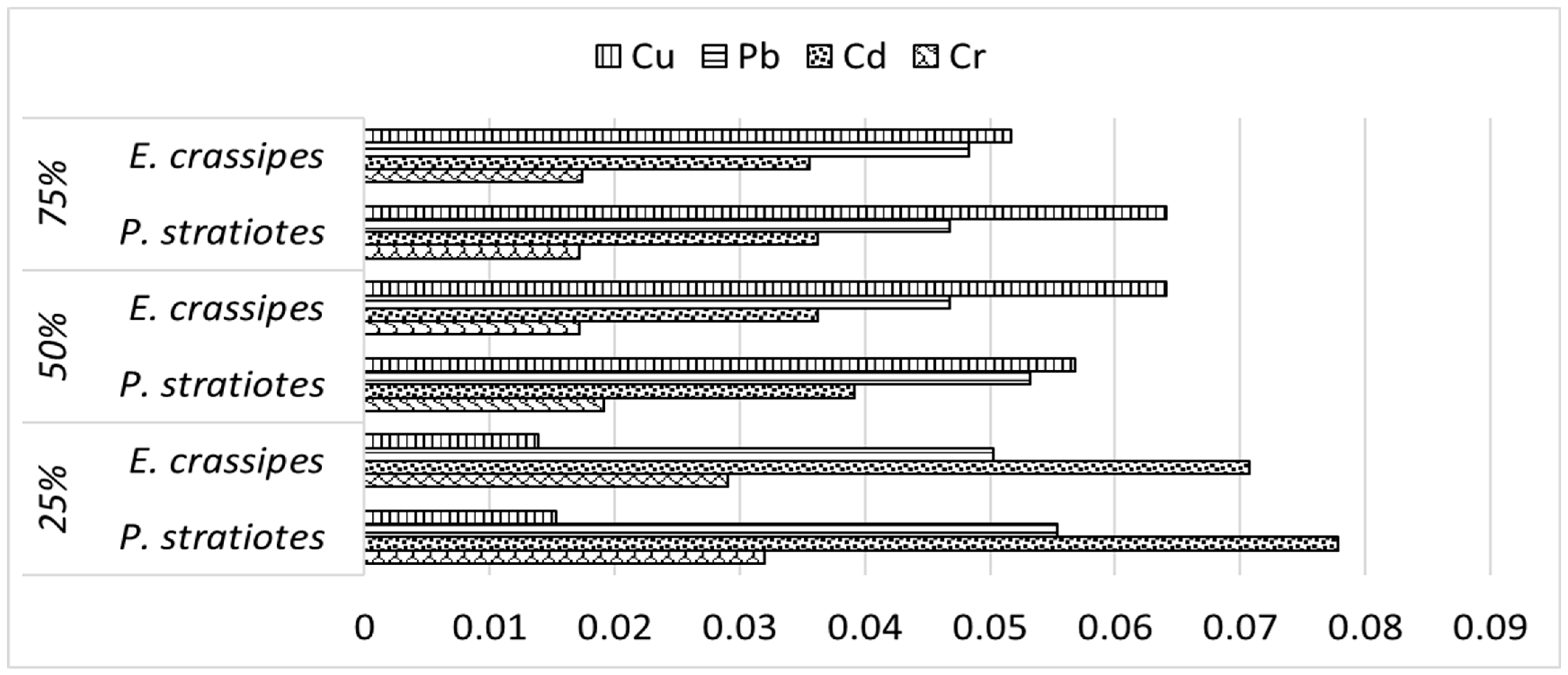
3.2.2. Heavy Metal Conc. in Roots and Shoots of P. stratiotes and E. crassipes and Their Phytoremediation Performance
3.3. Safer Irrigation Performance of P. hybrida in Reclaimed Water from UTW:HRW Dilutions Through PS- and EC-HFFTW
3.4. Variations in Silting Rate, Evaporation Rate (mm/day), and Yield of Reclaimed Water
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectrophotometer |
| APHA | American Public Health Administration |
| BAF | Bioaccumulation factor |
| BCF | Bioconcentration factor |
| BOD | Biochemical oxygen demand |
| COD | Chemical oxygen demand |
| EC | Eichhornia crassipes |
| EC-HFFTW | E. crassipes-HFFTW |
| FAO (UN) | Food and Agriculture Organization of the United Nations |
| FTWs | Floating treatment wetlands |
| HFFTWs | Horizontal flow floating treatment wetlands |
| HRW | Harvested rainwater |
| KTWMA | Kasur Tannery Waste Management Agency (Kasur, Pakistan) |
| PS | Pistia stratiotes |
| PS-HFFTW | P. stratiotes-HFFTW |
| SVI | Sludge volume index |
| TDSs | Total dissolved solids |
| Tis-r | Translocation indexshoot-root |
| TSSs | Total suspended solids |
| TTW | Treated tannery wastewater |
| TVSs | Total volatile solids |
| TWs | Treatment wetlands |
| USEPA | United States Environmental Protection Agency |
| UTW | Untreated tannery wastewater |
References
- Bocquier, P. World Urbanization Prospects. Demogr. Res. 2005, 12, 197–236. [Google Scholar] [CrossRef]
- Orimoloye, I.R.; Belle, J.A.; Olusola, A.O.; Busayo, E.T.; Ololade, O.O. Spatial Assessment of Drought Disasters, Vulnerability, Severity and Water Shortages: A Potential Drought Disaster Mitigation Strategy. Nat. Hazards 2020, 105, 2735–2754. [Google Scholar] [CrossRef]
- Rajapakse, J.; Otoo, M.; Danso, G.K. Progress in Delivering SDG6: Safe Water and Sanitation. Camb. Prism. Water 2023, 1, e6. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar, S.; Singh, D. Tannery Effluent Treatment and Its Environmental Impact: A Review of Current Practices and Emerging Technologies. Water Qual. Res. J. 2023, 58, 128–152. [Google Scholar] [CrossRef]
- Ali, H.Q.; Yasir, M.U.; Farooq, A.; Khan, M.; Salman, M.; Waqar, M. Tanneries Impact on Groundwater Quality: A Case Study of Kasur City in Pakistan. Environ. Monit. Assess. 2022, 194, 823. [Google Scholar] [CrossRef]
- Oruko, R.O.; Edokpayi, J.N.; Msagati, T.A.M.; Tavengwa, N.T.; Ogola, H.J.O.; Ijoma, G.; Odiyo, J.O. Investigating the Chromium Status, Heavy Metal Contamination, and Ecological Risk Assessment via Tannery Waste Disposal in Sub-Saharan Africa (Kenya and South Africa). Environ. Sci. Pollut. Res. 2021, 28, 42135–42149. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Hassan, A.; Riaz, M.; Khan, A.A.; Ul-Allah, S.; Shehzad, U.; Khurshid, M.; Bakhsh, A.; Shah, J.M.; Manzoor, Z. Increased Health Risk Assessment in Different Vegetables Grown under Untreated Sewerage Irrigation Regime due to Higher Heavy Metals Accumulation. Environ. Sci. Pollut. Res. 2023, 30, 86189–86201. [Google Scholar] [CrossRef] [PubMed]
- Hansen, É.; Cardoso, J.K.; Gutterres, M.; Monteiro de Aquim, P. Scale-up Testing for Reducing Pollution Load of Chemicals in Wastewater of Leather Post-Tanning. Process Saf. Environ. Prot. 2021, 155, 466–472. [Google Scholar] [CrossRef]
- Werkneh, A.A. Decentralized Constructed Wetlands for Domestic Wastewater Treatment in Developing Countries: Field-Scale Case Studies, Overall Performance and Removal Mechanisms. J. Water Process Eng. 2024, 57, 104710. [Google Scholar] [CrossRef]
- de Campos, S.X.; Soto, M. The Use of Constructed Wetlands to Treat Effluents for Water Reuse. Environments 2024, 11, 35. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Sandoval Herazo, L.C.; López-Méndez, M.C.; Sandoval-Herazo, M.; Meléndez-Armenta, R.Á.; González-Moreno, H.R.; Zamora, S. Treatment Wetlands in Mexico for Control of Wastewater Contaminants: A Review of Experiences during the Last Twenty-Two Years. Processes 2023, 11, 359. [Google Scholar] [CrossRef]
- Agaton, C.B.; Guila, P.M.C. Ecosystem Services Valuation of Constructed Wetland as a Nature-Based Solution to Wastewater Treatment. Earth 2023, 4, 78–92. [Google Scholar] [CrossRef]
- Retta, B.; Coppola, E.; Ciniglia, C.; Grilli, E. Constructed Wetlands for the Wastewater Treatment: A Review of Italian Case Studies. Appl. Sci. 2023, 13, 6211. [Google Scholar] [CrossRef]
- Kumar, S.; Sangwan, V.; Kumar, M.; Deswal, S. A Survey on Constructed Wetland Publications in the Past Three Decades. Environ. Monit. Assess. 2023, 195, 992. [Google Scholar] [CrossRef]
- Almuktar, S.A.A.A.N.; Abed, S.N.; Scholz, M. Wetlands for Wastewater Treatment and Subsequent Recycling of Treated Effluent: A Review. Environ. Sci. Pollut. Res. 2018, 25, 23595–23623. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Naveed, M.; Afzal, M.; Ashraf, S.; Ahmad, S.R.; Rehman, K.; Zahir, Z.A.; Núñez-Delgado, A. Evaluation of Toxicity on Ctenopharyngodon idella due to Tannery Effluent Remediated by Constructed Wetland Technology. Processes 2020, 8, 612. [Google Scholar] [CrossRef]
- Singh, S.; Benny, C.K.; Chakraborty, S. An Overview on the Application of Constructed Wetlands for the Treatment of Metallic Wastewater. In Biodegradation and Detoxification of Micropollutants in Industrial Wastewater; Elsevier: Amsterdam, The Netherlands, 2022; pp. 103–130. [Google Scholar] [CrossRef]
- Rabbi, F.M.; Hasan, M.K.; Rahman, M.A.; Islam, M.S.; Shohugh, P.K.; Ahmed, M.I.; Khan, M.W.; Rafi, T.; Rahman, M.M.; Rahaman, M.H.; et al. Waste-Derived Substrates in Vertical-Flow Constructed Wetlands for an Efficient Removal of High-Concentration Heavy Metals. Water Sci. Technol. 2024, 91, 21–39. [Google Scholar] [CrossRef]
- Wu, H.; Wang, R.; Yan, P.; Wu, S.; Chen, Z.; Zhao, Y.; Cheng, C.; Hu, Z.; Zhuang, L.; Guo, Z.; et al. Constructed Wetlands for Pollution Control. Nat. Rev. Earth Environ. 2023, 4, 218–234. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Zhang, M.; Zhang, G.; Hu, X.; Liu, C.; Cao, X.; Liang, W. Applications of Phytoremediation to Treat Reclaimed Water in Urban Parks Using Aquatic Macrophytes. Aquat. Ecol. 2021, 56, 75–88. [Google Scholar] [CrossRef]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C. A State-of-The-Art of Phytoremediation Approach for Sustainable Management of Heavy Metals Recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Rahi, M.A.; Faisal, A.A.H.; Naji, L.A.; Almuktar, S.A.; Abed, S.N.; Scholz, M. Biochemical Performance Modelling of Non-Vegetated and Vegetated Vertical Subsurface-Flow Constructed Wetlands Treating Municipal Wastewater in Hot and Dry Climate. J. Water Process Eng. 2020, 33, 101003. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Feng, X.; Pyo, S.-H. Current Problems and Countermeasures of Constructed Wetland for Wastewater Treatment: A Review. J. Water Process Eng. 2024, 57, 104569. [Google Scholar] [CrossRef]
- Oliveira, G.A.; Colares, G.S.; Lutterbeck, C.A.; Dell’Osbel, N.; Machado, Ê.L.; Rodrigues, L.R. Floating Treatment Wetlands in Domestic Wastewater Treatment as a Decentralized Sanitation Alternative. Sci. Total Environ. 2021, 773, 145609. [Google Scholar] [CrossRef]
- Gaballah, M.S.; Ismail, K.; Aboagye, D.; Ismail, M.M.; Sobhi, M.; Stefanakis, A.I. Effect of Design and Operational Parameters on Nutrients and Heavy Metal Removal in Pilot Floating Treatment Wetlands with Eichhornia crassipes Treating Polluted Lake Water. Environ. Sci. Pollut. Res. 2021, 28, 25664–25678. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, P.-Y.; Wang, G.-L.; Wang, J.; Zhang, Y.; Wang, S.; Yang, K.; Du, C.; Chen, H. A Review on the Removal of Heavy Metals and Metalloids by Constructed Wetlands: Bibliometric, Removal Pathways, and Key Factors. World J. Microbiol. Biotechnol. 2021, 37, 157. [Google Scholar] [CrossRef]
- Shahid, M.J.; Arslan, M.; Ali, S.; Siddique, M.; Afzal, M. Floating Wetlands: A Sustainable Tool for Wastewater Treatment. CLEAN-Soil Air Water 2018, 46, 1800120. [Google Scholar] [CrossRef]
- Mao, J.; Hu, G.; Deng, W.; Zhao, M.; Li, J. Industrial Wastewater Treatment Using Floating Wetlands: A Review. Environ. Sci. Pollut. Res. 2024, 31, 5043–5070. [Google Scholar] [CrossRef]
- Younas, A.; Kumar, L.; Deitch, M.J.; Qureshi, S.S.; Shafiq, J.; Ali Naqvi, S.; Kumar, A.; Amjad, A.Q.; Nizamuddin, S. Treatment of Industrial Wastewater in a Floating Treatment Wetland: A Case Study of Sialkot Tannery. Sustainability 2022, 14, 12854. [Google Scholar] [CrossRef]
- Arias, L.; Franco, D.; Medina, L.; Benítez, C.; Medina, L.; Villagra, V.; McGahan, S.; Mariza Duré, G.; Kurita-Oyamada, H.G. Removal of Chromium (III) and Reduction in Toxicity in a Primary Tannery Effluent Using Two Floating Macrophytes. Toxics 2024, 12, 152. [Google Scholar] [CrossRef]
- Javeed, F. Optimization of Design and Operational Parameters of Constructed Wetland System (CWS) for the Treatment of Textile and Sewage Effluents. Ph.D. Thesis, University of the Punjab, Lahore, Pakistan, 6 December 2024. [Google Scholar]
- Younas, F.; Niazi, N.K.; Bibi, I.; Afzal, M.; Hussain, K.; Shahid, M.; Aslam, Z.; Bashir, S.; Hussain, M.M.; Bundschuh, J. Constructed Wetlands as a Sustainable Technology for Wastewater Treatment with Emphasis on Chromium-Rich Tannery Wastewater. J. Hazard. Mater. 2022, 422, 126926. [Google Scholar] [CrossRef]
- Das, M.; Bramhanand, P.S.; Laxminarayana, K. Performance and Efficiency Services for the Removal of Hexavalent Chromium from Water by Common Macrophytes. Int. J. Phytoremediation 2021, 23, 1095–1103. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Priya K, S. Phytoremediation Potential of Water Hyacinth in Heavy Metal Removal in Chromium and Lead Contaminated Water. Int. J. Environ. Anal. Chem. 2021, 103, 3081–3096. [Google Scholar] [CrossRef]
- Das, E.J.; Bhuiyan, M.A.R.; Hasan, M.M. Implementation of Water Hyacinth (Eichhornia crassipes) and Water Lettuce (Pistia stratiotes) in the Re-Treatment of Conventionally Treated Pharmaceutical Wastewater: A Case Study of Radiant Pharmaceuticals Limited, Dhaka, Bangladesh. Environ. Monit. Assess. 2023, 195, 1210. [Google Scholar] [CrossRef]
- Zeb, B.S.; Mahmood, Q.; Irshad, M.; Zafar, H.; Wang, R. Sustainable Treatment of Combined Industrial Wastewater: Synergistic Phytoremediation with Eichhornia crassipes, Pistia stratiotes and Arundo donax in Biofilm Wetlands. Int. J. Phytoremediation 2024, 27, 128–134. [Google Scholar] [CrossRef]
- Samal, K.; Dash, R.R. Experiments and Modeling to Develop a Pistia stratiotes Based Floating Vegetated System (FVS) for the Removal of Heavy Metals (Pb, Zn, Cr, Cu, Ni). Sci. Total Environ. 2024, 926, 171981. [Google Scholar] [CrossRef]
- Nagial, K.S.; Kumar, N.; Poddar, A.; Shankar, V.; Rustum, R. Treated Wastewater Irrigation for Ornamental Plants: A Systematic Review. ISH J. Hydraul. Eng. 2025, 31, 123–132. [Google Scholar] [CrossRef]
- Zitácuaro-Contreras, I.; Vidal-Álvarez, M.; Hernández y Orduña, M.G.; Zamora-Castro, S.A.; Betanzo-Torres, E.A.; Marín-Muñíz, J.L.; Sandoval-Herazo, L.C. Environmental, Economic, and Social Potentialities of Ornamental Vegetation Cultivated in Constructed Wetlands of Mexico. Sustainability 2021, 13, 6267. [Google Scholar] [CrossRef]
- Lipps, W.C.; Braun-Howland, E.B.; Baxter, T.E. (Eds.) Standard Methods for the Examination of Water and WastewaterTM, 24th ed.; APHA Press: Washington DC, USA, 2023; p. 1516. ISBN 9780875532998. [Google Scholar]
- ASTM D1252-06(2020); Standard Test Methods for Chemical Oxygen Demand (Dichromate Oxygen Demand) of Water. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://store.astm.org/d1252-06r20.html (accessed on 22 July 2025).
- Javeed, F.; Bareen, F.; Shafiq, M.; Nazir, A.; Scholz, M. Optimization of Selected Parameters in Vertical, Horizontal, and Hybrid Surface Flow Constructed Wetland Systems for Improving the Treatment Efficiency of Textile and Sewage Effluents. Water 2025, 17, 402. [Google Scholar] [CrossRef]
- Telliard, W.A. Method 1684: Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids; USEPA-821-R-01-015; United States Environmental Protection Agency: Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/method_1684_draft_2001.pdf (accessed on 22 July 2025).
- USEPA. SW-846 Test Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; United States Environmental Protection Agency: Washington, DC, USA, 1996. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/3052.pdf (accessed on 22 July 2025).
- Javeed, F.; Nazir, A.; Firdaus-e-Bareen; Shafiq, M.; Scholz, M. Industrial Water Treatment within a Wetland Planted with Hemarthria compressa and Subsequent Effluent Reuse to Grow Abelmoschus esculentus. J. Water Process Eng. 2022, 45, 102511. [Google Scholar] [CrossRef]
- Mahtab, S.; Nazir, A.; Shafiq, M. Coupling of Phycoremediation and Phytoremediation Technologies to Treat Tannery Effluents with Rainwater Dilutions. Int. J. Phytoremediation 2024, 26, 1667–1675. [Google Scholar] [CrossRef]
- Government of Pakistan, Ministry of Environment, Local Government and Rural Development. Statutory Notification S.R.O. 549(I)/2000, National Environmental Quality Standards (NEQS); The Gazette of Pakistan: Islamabad, Pakistan, 2000. Available online: https://environment.gov.pk/SiteImage/Misc/files/Rules/SRO549I2000NEQS.pdf (accessed on 22 July 2025).
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Guidelines for the Safe Use and Reuse of Water in Food Production and Processing (Codex Alimentarius Guideline CXG 100–2023); Codex Alimentarius Commission: Rome, Italy, 2023; Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/jp/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B100-2023%252FCXG_100e.pdf (accessed on 22 July 2025).
- Sahreen, S.; Mukhtar, H. Floating Treatment Wetlands (FTW) for Sustainable Industrial Wastewater Treatment. In Microbial Bioremediation and Multiomics Technologies for Sustainable Development: Recent Trends; Royal Society of Chemistry: London, UK, 2024; pp. 291–318. [Google Scholar] [CrossRef]
- Wei, F.; Shahid, M.J.; Alnusairi, G.S.H.; Afzal, M.; Khan, A.; El-Esawi, M.A.; Abbas, Z.; Wei, K.; Zaheer, I.E.; Rizwan, M.; et al. Implementation of Floating Treatment Wetlands for Textile Wastewater Management: A Review. Sustainability 2020, 12, 5801. [Google Scholar] [CrossRef]
- Afzal, M.; Arslan, M.; Müller, J.A.; Shabir, G.; Islam, E.; Tahseen, R.; Anwar-ul-Haq, M.; Hashmat, A.J.; Iqbal, S.; Khan, Q.M. Floating Treatment Wetlands as a Suitable Option for Large-Scale Wastewater Treatment. Nat. Sustain. 2019, 2, 863–871. [Google Scholar] [CrossRef]
- Rizvi, Z.F.; Jamal, M.; Parveen, H.; Sarfraz, W.; Nasreen, S.; Khalid, N.; Muzammil, K. Phytoremediation Potential of Pistia stratiotes, Eichhornia crassipes, and Typha latifolia for Chromium with Stimulation of Secondary Metabolites. Heliyon 2024, 10, e29078. [Google Scholar] [CrossRef]
- Tanpichai, S.; Biswas, S.K.; Witayakran, S.; Yano, H. Water Hyacinth: A Sustainable Lignin-Poor Cellulose Source for the Production of Cellulose Nanofibers. ACS Sustain. Chem. Eng. 2019, 7, 18884–18893. [Google Scholar] [CrossRef]
- Kumar, A.; Devnani, G.L.; Pal, D.B. Thermal Kinetic Analysis and Characterization of Water Hyacinth Biomass for Renewable Energy Application. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Kings, A.J.; Suyambulingam, I.; Narayanaperumal, S.; Miriam, L.M.; Divakaran, D.; Murali, A.; Han, S.S. Exploring Biomass Derived Microcrystalline Cellulose from the Waste Aquatic Plant Pistia stratiotes: A Comprehensive Characterization for Polymer Composite Reinforcement. Int. J. Biol. Macromol. 2025, 300, 140217. [Google Scholar] [CrossRef]
- Lee, L.T.; Blatchley, E.R. Long-Term Monitoring of Water and Air Quality at an Indoor Pool Facility during Modifications of Water Treatment. Water 2022, 14, 335. [Google Scholar] [CrossRef]
- Petrov, D.S.; Korotaeva, A.E.; Pashkevich, M.A.; Chukaeva, M.A. Assessment of Heavy Metal Accumulation Potential of Aquatic Plants for Bioindication and Bioremediation of Aquatic Environment. Environ. Monit. Assess. 2022, 195, 122. [Google Scholar] [CrossRef] [PubMed]



| HFFTW Series | HFFTW Treatment Cells | UTW:HRW Treated | Safer Irrigation Trials of Reclaimed Water from UTW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell-I | Cell-II | Cell-III | |||||||
| Hydrophyte | E. crassipes | EC-HFFTW | 0 | UTW:HRW Dilutions (% v:v) |  | P. hybrida | |||
| EC-HFFTW | 25 | P. hybrida | |||||||
| EC-HFFTW | 50 | P. hybrida | |||||||
| EC-HFFTW | 75 | P. hybrida | |||||||
| P. stratiotes | PS-HFFTW | 0 | UTW:HRW Dilutions (% v:v) | P. hybrida | |||||
| PS-HFFTW | 25 | P. hybrida | |||||||
| PS-HFFTW | 50 | P. hybrida | |||||||
| PS-HFFTW | 75 | P. hybrida | |||||||
| Physicochemical Properties | UTW:HRW Dilutions (% v:v) | HRW | Ground Water | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | ||||
| pH | 5.6 ± 0.01 | 6.5 ± 0.02 | 7.4 ± 0.01 | 7.9 ± 0.03 | 8.9 ± 0.01 | 5.6 ± 0.01 | 7.6 ± 0.02 | |
| EC (µS/cm) | 69.63 ± 1.1 | 198.3 ± 2.3 | 389.8 ± 2.7 | 572.9 ± 3.1 | 13,993 ± 14 | 69.63 ± 0.6 | 861 ± 2.9 | |
| NaCl (%) | 0.0009 ± 0.001 | 0.04429 ± 0.002 | 0.07154 ± 0.03 | 0.31 ± 0.09 | 0.93 ± 0.1 | 0.0009 ± 0.003 | 0.013 ± 0.002 | |
| TDS | mg/L | 67 ± 2.1 | 449.76 ± 4.3 | 726.54 ± 6.1 | 3148.3 ± 8.6 | 9445 ± 12 | 67 ± 2.1 | 581 ± 7.6 |
| TSS | 34 ± 1.1 | 24.29 ± 0.8 | 39.23 ± 1.4 | 170 ± 2.1 | 510 ± 2.8 | 131 ± 1.3 | 61 ± 2.1 | |
| TVS | BDL | 22.52 ± 0.7 | 36.38 ± 0.9 | 157.67 ± 2.1 | 473 ± 5 | 1.43 ± 0.12 | BDL | |
| SVI | BDL | 68.76 ± 0.7 | 54.15 ± 0.8 | 41.33 ± 2.1 | 38.2 ± 2.7 | BDL | BDL | |
| HCO3− | 11.25 ± 0.8 | 9.67 ± 0.6 | 15.62 ± 1.1 | 67.67 ± 1.8 | 203 ± 2.7 | 11.25 ± 0.7 | 73.4 ± 1.9 | |
| Cl− | 35.97 ± 0.9 | 266.14 ± 4.2 | 429.92 ± 8.1 | 1863 ± 9.2 | 5589 ± 13.4 | 35.97 ± 0.9 | 21.8 ± 0.8 | |
| BOD5 | 0.38 ± 0.01 | 66 ± 2.1 | 106.62 ± 2.5 | 462 ± 7.9 | 1386 ± 8.1 | 0.38 ± 0.001 | 1.94 ± 0.11 | |
| COD | 3.81 ± 0.8 | 204.6 ± 2.8 | 330.5 ± 4.9 | 1432 ± 7.4 | 4297 ± 12.9 | 3.81 ± 0.09 | 7.8 ± 0.72 | |
| Cr | BDL | 140.9 ± 1.5 | 305.2 ± 2.8 | 404.8 ± 3.3 | 549.4 ± 2.9 | BDL | 0.068 ± 0.008 | |
| Cd | BDL | 45 ± 0.91 | 87.86 ± 1.1 | 140.6 ± 1.3 | 184.5 ± 2.1 | BDL | 0.046 ± 0.005 | |
| Pb | BDL | 36.14 ± 0.85 | 77.7 ± 0.98 | 117.5 ± 1.89 | 155.4 ± 1.89 | BDL | 0.035 ± 0.002 | |
| Cu | BDL | 32.65 ± 0.63 | 63.79 ± 0.93 | 96.76 ± 1.4 | 124.4 ± 1.63 | BDL | 0.893 ± 0.071 | |
| Physicochemical Characteristics | UTW:HRW Dilutions (% v:v) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||||||
| Pre- | Post- | RE (%) | Pre- | Post- | RE (%) | Pre- | Post- | RE (%) | ||
| pH | 6.5 | 7.1 | −92 | 7.4 | 7.15 | 34 | 7.9 | 7.48 | 53 | |
| EC (µS/cm) | 198.3 | 156 | 21 | 389.8 | 313.2 | 20 | 572.9 | 373.45 | 35 | |
| NaCl (%) | 0.0439 | 0.0221 | 50 | 0.0709 | 0.0349 | 51 | 0.3074 | 0.0828 | 73 | |
| TDS | mg/L | 449.76 | 374 | 17 | 726.54 | 473.31 | 35 | 3148 | 679.4 | 78 |
| TSS | 24.29 | 32 | −32 | 39.23 | 24.52 | 37 | 170 | 52.1 | 69 | |
| TVS | 22.52 | 17.4 | 23 | 36.38 | 19.65 | 46 | 157.67 | 67.6 | 57 | |
| SVI | 68.76 | 52.32 | 26 | 54.15 | 47.54 | 15 | 41.33 | 29.8 | 28 | |
| HCO3− | 9.67 | 7.21 | 25 | 15.62 | 8.78 | 44 | 67.67 | 13.65 | 80 | |
| Cl− | 266.14 | 134.1 | 50 | 429.92 | 211.65 | 51 | 1863 | 501.9 | 73 | |
| BOD5 | 66 | 24.4 | 63 | 106.62 | 46.81 | 56 | 462 | 94.73 | 79 | |
| COD | 204.6 | 81.1 | 60 | 330.5 | 143.89 | 56 | 1432 | 311.89 | 78 | |
| Cr | 140.9 | 4.23 | 97 | 305.2 | 13.21 | 96 | 404.8 | 19.81 | 95 | |
| Cd | 45 | 4.23 | 91 | 87.86 | 9.66 | 89 | 140.6 | 17.4 | 88 | |
| Pb | 36.14 | 4.23 | 88 | 77.7 | 4.66 | 94 | 117.5 | 8.9 | 92 | |
| Cu | 32.65 | 0.33 | 99 | 63.79 | 3.05 | 95 | 96.76 | 5.04 | 95 | |
| Physicochemical Characteristics | UTW:HRW Dilutions (% v:v) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||||||
| Pre- | Post- | RE (%) | Pre- | Post- | RE (%) | Pre- | Post- | RE (%) | ||
| pH | 6.5 | 6.99 | −75 | 7.4 | 7.19 | 28 | 7.9 | 7.26 | 81 | |
| EC (µS/cm) | 198.3 | 148.5 | 25 | 389.8 | 281.3 | 29.8 | 572.9 | 361.4 | 37 | |
| NaCl (%) | 0.0439 | 0.0184 | 58 | 0.0709 | 0.0310 | 56 | 0.3074 | 0.0764 | 75 | |
| TDS | mg/L | 449.76 | 324.8 | 28 | 726.54 | 411.4 | 43 | 3148 | 679.4 | 78 |
| TSS | 24.29 | 29.3 | −21 | 39.23 | 19.78 | 50 | 170 | 52.1 | 69 | |
| TVS | 22.52 | 14.76 | 34 | 36.38 | 15.64 | 57 | 157.67 | 67.6 | 57 | |
| SVI | 68.76 | 49.93 | 36 | 54.15 | 45.91 | 15 | 41.33 | 26.9 | 35 | |
| HCO3− | 9.67 | 6.89 | 29 | 15.62 | 6.49 | 56.2 | 67.67 | 13.65 | 80 | |
| Cl− | 266.14 | 111.8 | 58 | 429.92 | 187.9 | 56 | 1863 | 463.2 | 75 | |
| BOD5 | 66 | 21.3 | 68 | 106.62 | 41.59 | 61 | 462 | 89.78 | 81 | |
| COD | 204.6 | 68.89 | 66 | 330.5 | 117.81 | 64 | 1432 | 256.8 | 82 | |
| Cr | 140.9 | 3.76 | 97 | 305.2 | 11.75 | 96 | 404.8 | 17.63 | 96 | |
| Cd | 45 | 3.8 | 92 | 87.86 | 8.60 | 90 | 140.6 | 15.5 | 89 | |
| Pb | 36.14 | 3.76 | 90 | 77.7 | 4.15 | 95 | 117.5 | 7.9 | 93 | |
| Cu | 32.65 | 0.29 | 99 | 63.79 | 2.72 | 96 | 96.76 | 4.48 | 95 | |
| Physicochemical Characteristics | UTW:HRW Dilutions (% v:v) | ||||||
|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | |||||
| PS-HFFTW | EC-HFFTW | PS-HFFTW | EC-HFFTW | PS-HFFTW | EC-HFFTW | ||
| pH | −92.3 | −75.4 | 33.8 | 28.4 | 53.2 | 81 | |
| EC (µS/cm) | 21.3 | 25.1 | 19.7 | 27.8 | 34.8 | 36.9 | |
| NaCl (%) | 49.6 | 58 | 50.8 | 56.3 | 73.1 | 75.1 | |
| TDS | mg/L | 16.8 | 27.8 | 34.9 | 43.4 | 78.4 | 78.4 |
| TSS | −31.7 | −20.6 | 37.5 | 49.6 | 69.4 | 69.4 | |
| TVS | 22.7 | 34.5 | 46 | 57 | 57.1 | 57.1 | |
| SVI | 39.3 | 43.7 | 46.7 | 58.2 | 56.1 | 56.1 | |
| HCO3− | 25.4 | 28.7 | 43.8 | 58.5 | 79.8 | 79.8 | |
| Cl− | 49.6 | 58 | 50.8 | 56.3 | 73.1 | 75.1 | |
| BOD5 | 63 | 67.7 | 56.1 | 61 | 79.5 | 80.6 | |
| COD | 60.4 | 66.3 | 56.5 | 64.4 | 78.2 | 82.1 | |
| Cr | 97.0 | 97.3 | 95.7 | 96.1 | 95.1 | 95.6 | |
| Cd | 90.6 | 91.6 | 89.0 | 90.2 | 87.6 | 89.0 | |
| Pb | 88.3 | 89.6 | 94.0 | 94.7 | 92.4 | 93.3 | |
| Cu | 99.0 | 99.1 | 95.2 | 95.7 | 94.8 | 95.4 | |
| Metals | Plant Attribute | P. stratiotes | E. crassipes |
|---|---|---|---|
| Cr | Shoot (mg/kg) | 85.2 ± 0.66 | 98.6 ± 0.86 |
| Root (mg/kg) | 30.5 ± 0.6 | 54.3 ± 0.76 | |
| TEroot-shoot | 2.79 | 1.82 | |
| Cd | Shoot (mg/kg) | 45.1 ± 0.67 | 58.8 ± 0.75 |
| Root (mg/kg) | 13.6 ± 0.62 | 31.7 ± 0.64 | |
| TEroot-shoot | 3.12 | 1.85 | |
| Pb | Shoot (mg/kg) | 49.2 ± 0.73 | 49.7 ± 0.83 |
| Root (mg/kg) | 21.7 ± 0.36 | 28.3 ± 0.6 | |
| TEroot-shoot | 2.27 | 1.76 | |
| Cu | Shoot (mg/kg) | 51.2 ± 0.59 | 35.2 ± 1.01 |
| Root (mg/kg) | 24.5 ± 1.12 | 18.3 ± 0.56 | |
| TEroot-shoot | 2.09 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, A.; Tanveer, H.; Shafiq, M.; Ihsan, M.; Maqbool, T.; Scholz, M. Horizontal Flow Floating Treatment Wetlands (HFFTWs) for Reclaiming Safer Irrigation Water from Tannery Effluent. Water 2025, 17, 2720. https://doi.org/10.3390/w17182720
Nazir A, Tanveer H, Shafiq M, Ihsan M, Maqbool T, Scholz M. Horizontal Flow Floating Treatment Wetlands (HFFTWs) for Reclaiming Safer Irrigation Water from Tannery Effluent. Water. 2025; 17(18):2720. https://doi.org/10.3390/w17182720
Chicago/Turabian StyleNazir, Aisha, Haiqa Tanveer, Muhammad Shafiq, Muhammad Ihsan, Tasmia Maqbool, and Micklas Scholz. 2025. "Horizontal Flow Floating Treatment Wetlands (HFFTWs) for Reclaiming Safer Irrigation Water from Tannery Effluent" Water 17, no. 18: 2720. https://doi.org/10.3390/w17182720
APA StyleNazir, A., Tanveer, H., Shafiq, M., Ihsan, M., Maqbool, T., & Scholz, M. (2025). Horizontal Flow Floating Treatment Wetlands (HFFTWs) for Reclaiming Safer Irrigation Water from Tannery Effluent. Water, 17(18), 2720. https://doi.org/10.3390/w17182720






