1. Introduction
The integration of circular economy principles into zero-waste policies contributes directly to mitigating the adverse effects of anthropogenic climate change [
1]. Particularly important in this context is the need to intensify efforts aimed at water conservation and recovery, as both water scarcity, and the disruption of hydrological systems are becoming increasingly apparent worldwide—not only in the Global South, but also across the Northern Hemisphere, affecting both rural areas and expanding urban centers [
2,
3]. The expanding awareness of environmental issues among the public not only shapes consumer behavior but can also facilitate the adoption of legal frameworks aimed at lowering climate stress over time [
4,
5].
Agriculture is one of the most water-intensive sectors of the economy and plays a crucial role in the design, planning, and implementation of measures aimed at water conservation [
6,
7]. According to data from the European Environment Agency, the average annual water use for agricultural purposes across the EEA-39 countries between 2008 and 2017 was 92 billion m
3. In 2017 alone, the EU-28 member states used an average of 50 billion m
3 of water per year, while Turkey reported usage of approximately 40 billion m
3 [
8]. In the European Union, the adoption of diverse water-saving solutions has been facilitated through the Common Agricultural Policy, including measures such as irrigation automation, precision farming, the reuse of treated water, the promotion of less invasive cultivation methods, and the use of crops with increased resilience to water stress [
9].
The processing of freshly harvested fruits and vegetables is recognized as a relevant niche for implementing circular economy solutions, particularly through the reuse of water employed in raw material washing [
10,
11]. This issue is particularly important given that wash water is often discharged as wastewater and not reused. However, this approach is primarily driven by concerns over preventing microbiological cross-contamination, which may lead to outbreaks that pose risks to public health and compromise the quality of the final product. An alternative strategy involves the use of conventional chemical disinfection, typically based on chlorine compounds such as sodium and calcium hypochlorite [
12]. However, the use of this conventional approach may lead to the formation of harmful disinfectant byproducts (DBPs), some of which exhibit carcinogenic, cytotoxic, teratogenic, or neurotoxic properties (
Table 1). In the early cleaning stages at agri-food-processing facilities, fruits and vegetables often carry soil particles, dust, and remnants of dead organic matter. These materials can react with disinfectants, which raises concerns about the potential formation of toxic or otherwise undesirable compounds [
13].
Due to the wide availability and convenience of chemical disinfection, alternative methods—such as mechanical filtration using carbon, sand, or silica filters, UV irradiation, or cold plasma—tend to attract less interest among producers [
11,
21]. The technical problem addressed in this study is the lack of alternative, non-invasive methods for the effective disinfection of wastewater generated in the fresh-cut industry.
In recent years, there has been growing interest in the use of less invasive methods involving natural plant extracts, which may offer a more appealing alternative [
22]. Research on the use of plant-based raw materials as natural preservatives, and antimicrobials has gained importance in response to the growing demand for safe and environmentally friendly alternatives to synthetic additives [
23,
24]. Plant extracts, due to their antibacterial and antifungal properties, are used not only in the food industry, but also in pharmaceuticals and cosmetology [
25,
26,
27,
28,
29]. The active compounds found in plant extracts—such as flavonoids, alkaloids, and tannins—exhibit diverse antimicrobial mechanisms. Flavonoids, for example, can inhibit DNA gyrase activity, destabilize bacterial cell membranes, and interfere with cellular energy metabolism [
30]. Alkaloids, on the other hand, have been shown to inhibit bacterial cell wall formation, alter membrane permeability, suppress the synthesis of proteins and nucleic acids, and disrupt various metabolic pathways [
31]. Tannins exert antibacterial activity through several mechanisms, including iron chelation, disruption of the bacterial cell membrane, inhibition of cell wall synthesis and fatty acid biosynthesis. The compounds also demonstrate antivirulence effects, such as the suppression of quorum sensing and biofilm formation [
32]. In light of the above, the use of plant extracts in water sanitization processes represents a promising area of research, as their antimicrobial properties may serve as a natural alternative to conventional disinfectants while simultaneously reducing the formation of harmful disinfection byproducts [
33].
The research hypothesis assumes that the use of a multilayer filtration bed composed of hydrogel infused with natural plant extracts, ion-exchange resin, and activated carbon significantly enhances the efficiency of pathogen removal from wastewater compared to single-layer filtration systems.
The aim of this study was to experimentally evaluate the efficiency of a pilot-scale filtration bed designed for the treatment of wastewater under semi-technical conditions. The research focused on assessing the effectiveness of pathogen removal using different bed configurations, which included hydrogel infused with natural plant extracts, an ion-exchange resin layer, and an activated carbon layer. The objective was to determine which configuration offers the highest microbiological purification efficiency while maintaining operational stability and demonstrating potential for application in environmental engineering practice. The second phase of the study involved preliminary validation on an industrial scale. The most effective configuration identified during the pilot tests was applied to the treatment of real process water collected from a fruit processing facility.
2. Materials and Methods
2.1. Preparation of Filter Material
Based on previous research, the selected plant material included bark of
Quercus robur,
Salix alba, and
Betula pendula, stems of
Rubus idaeus, leaves of
Camellia sinensis, and flowers of
Tilia cordata [
34].
Hydroethanolic extracts (70%) were prepared from the selected plant material. For this purpose, 25 g of cleaned and dried plant material (pre-dried for 12 h at 30 °C) were ground into a fine powder and then soaked in 250 mL of solvent. The mixtures were shaken for 24 h at 150 rpm, then filtered using standard laboratory-grade filter paper. The filtered extracts (150 mL) were concentrated threefold to 50 mL using a high-efficiency rotary evaporator (Laborota 4000, Heidolph, Schwabach, Germany) at 65 °C and 90 rpm. To ensure sterility, the extracts were further filtered through 25 mm syringe filters with a pore size of 0.22 µm.
Subsequently, the extracts were applied for hydrogel hydration to prepare the material for further analysis (
Figure 1).
The remaining filtration materials included ion-exchange resin and activated carbon. According to the manufacturer Formaster S.A., Kielce, Poland), the macroporous resin removes calcium and magnesium ions from water through ion exchange, while the activated carbon, derived from coconut shells, effectively adsorbs chlorine and other contaminants due to its high porosity, thereby improving the taste and odor of the water. According to the manufacturer, the service life of activated carbon cartridges is estimated at 4–6 months, while ion-exchange resin cartridges lose efficiency once their exchange capacity is exhausted. For medium-hard water this corresponds to approximately 700–1000 L, with higher capacities for lower hardness. In this study, these values were considered as reference information, while the experimental evaluation focused on microbiological performance.
2.2. Filtration System
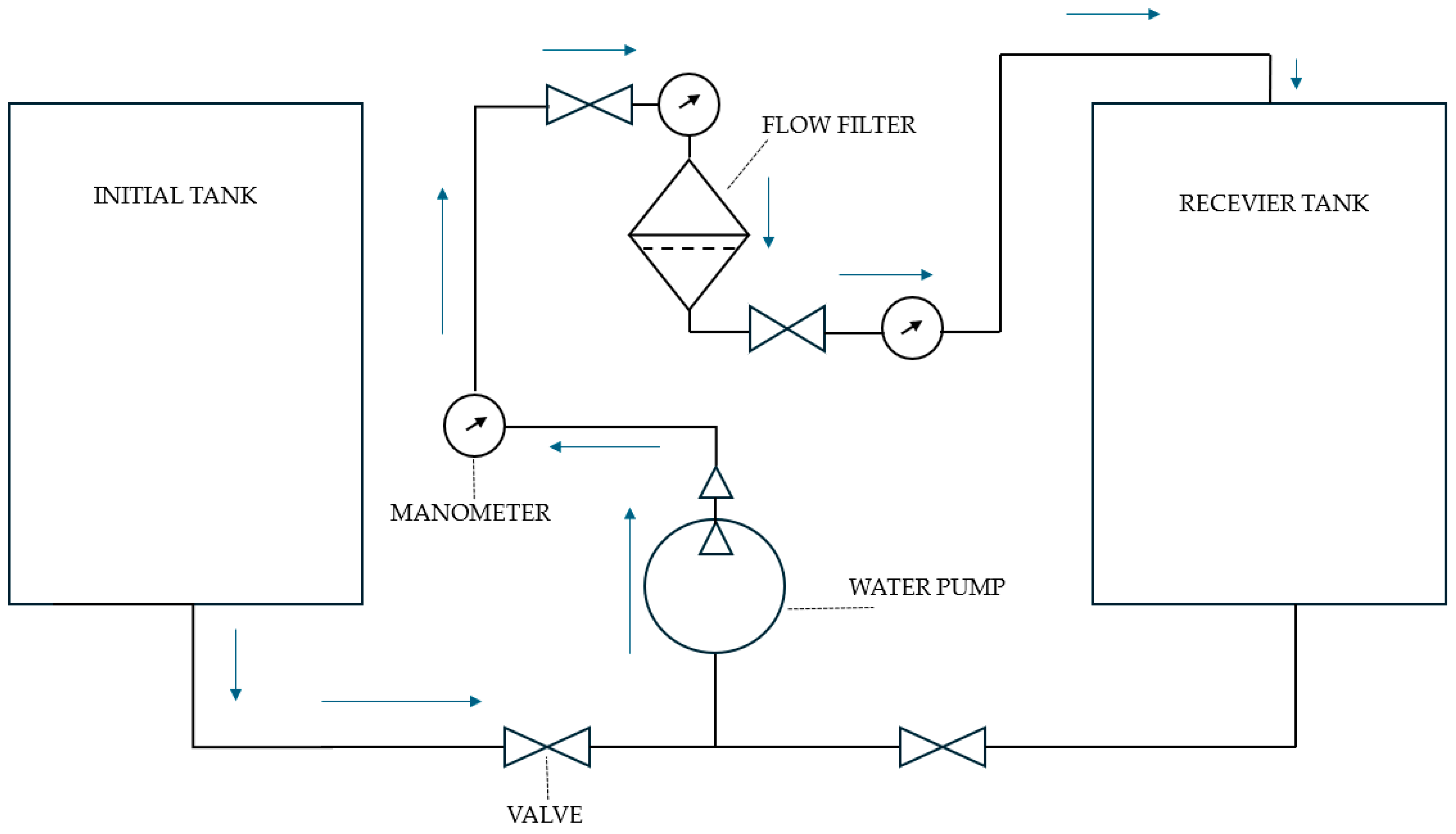
The filtration system (
Figure 2) consisted of an initial tank containing 25 L of inoculated water with bacteria at a specified concentration, a water pump ensuring constant flow, control valves regulating both the direction and intensity of flow, manometers monitoring pressure at various stages, and a main flow-through filter filled with a suitable filtration medium—activated carbon with ion-exchange resin, hydrogel, or a mixture of these, depending on the setup. Filtered water was collected in a receiving tank for further analysis.
2.3. Bacterial Inoculum
As identified in prior studies, the test strains comprised opportunistic pathogens isolated from wash waters in agri-food processing facilities. These included
Pseudomonas aeruginosa,
Klebsiella oxytoca,
Klebsiella pneumoniae,
Proteus vulgaris,
Serratia marcescens, and
Enterococcus faecalis [
34,
35]. To prepare the bacterial inoculum, strains stored in Brain Heart Infusion (BHI) broth (Merck, Darmstadt, Germany) with glycerol at −20 °C were revived on general-purpose medium (Tryptic Soy Agar, TSA; Merck, Germany) and subcultured twice. Bacteria were subsequently collected with a sterile inoculation loop and suspended in 9 mL of sterile 0.9% sodium chloride solution. The suspension was adjusted to a concentration of 1.0–2.0 × 10
8 CFU/mL, consistent with the cell density typically applied in antimicrobial susceptibility assays. A 1 mL aliquot from each bacterial suspension was inoculated into 250 mL of sterile Nutrient Broth (Merck, Germany) and mixed thoroughly to achieve uniform dispersion of the cells. The same procedure was carried out for each strain tested.
To prepare the inoculum for further testing, a total of 1.5 L of bacterial culture (6 × 250 mL), each at a concentration of 2 × 108 CFU/mL, was added to 25 L of sterile water. This resulted in a final bacterial concentration of approximately 1.2 × 107 CFU/mL. The actual concentration was confirmed by determining colony-forming units (CFU/mL) in samples collected immediately after inoculation.
2.4. Testing Procedure
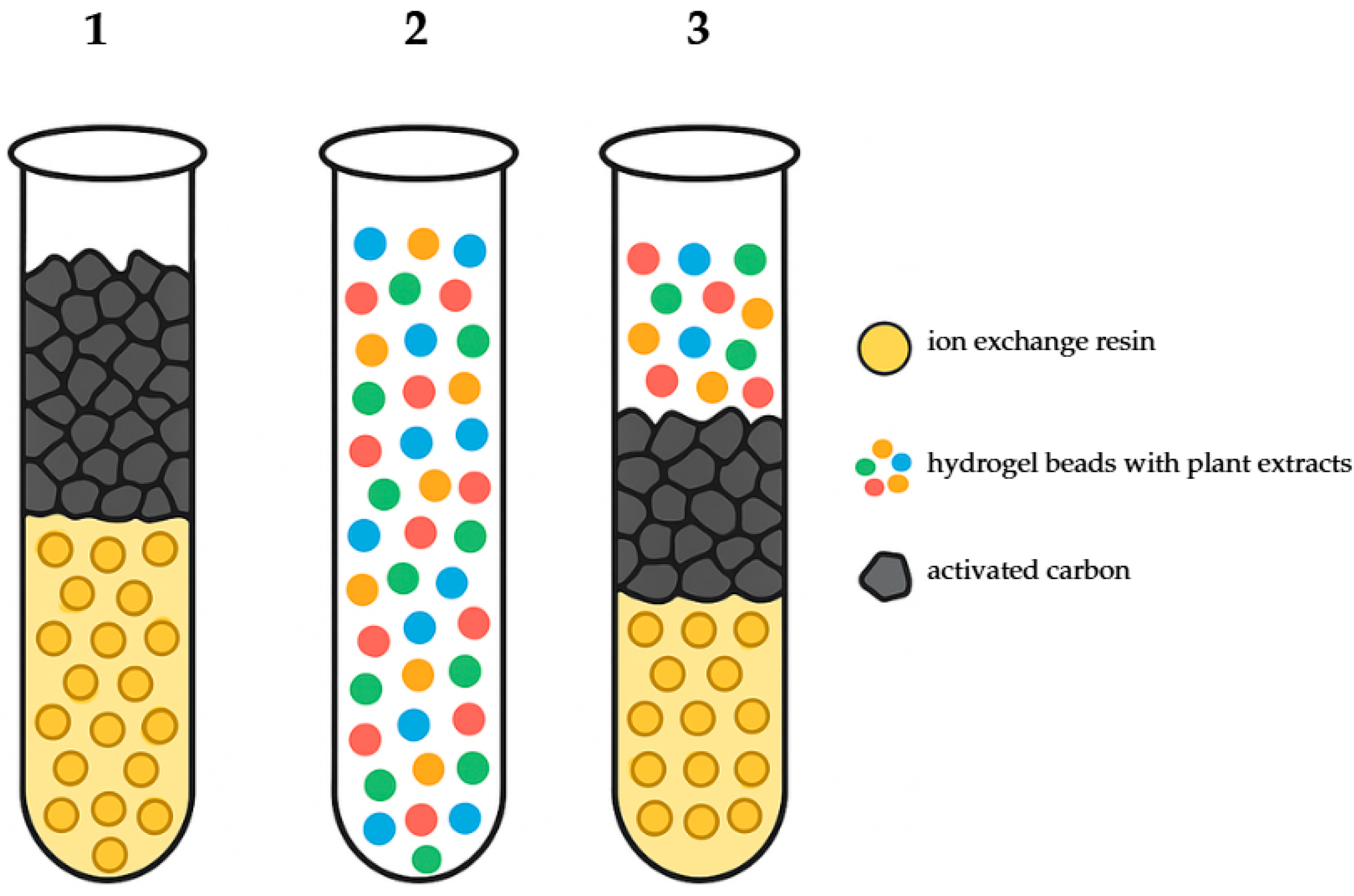
Twenty-five liters of sterile water were inoculated with 1.5 L of bacterial suspensions containing the test strains (6 × 250 mL) and transferred into the preliminary tank. After opening the control valves and activating the pump, the water was passed through filtration columns in one of the following three configurations: (1) a filter filled with activated carbon and ion-exchange resin; (2) a filter containing hydrogel; and (3) a filter composed of a mixture of activated carbon, ion-exchange resin, and hydrogel (
Figure 3).
Water samples were collected and analyzed at six intervals: 1 h, 2 h, 3 h, 6 h, and 12 h. Approximately 4 L of water were circulated through the system during each test. Following filtration, microbiological analyses were performed in duplicate: 1 mL of filtered water was plated on appropriate agar medium and spread using a sterile spreader. The results were compared across the different filter configurations.
2.5. Log Reduction and Statistical Analysis
Filter performance was evaluated by monitoring the decrease in bacterial counts in the outflow over time. Results were described as a log
10 reduction in colony-forming units (CFU), calculated using the following equation:
—initial bacterial concentration in water before filtration (CFU/mL).
—bacterial concentration in the sample collected from the filter outflow at a given time point (CFU/mL)
The concentration of CFU/mL was determined using colony counts from serial dilution plates, in accordance with standard microbiological procedures. When the number of colonies exceeded 300 (designated as “TNTC”—too numerous to count), the result was estimated using data from the next highest dilution that produced countable growth.
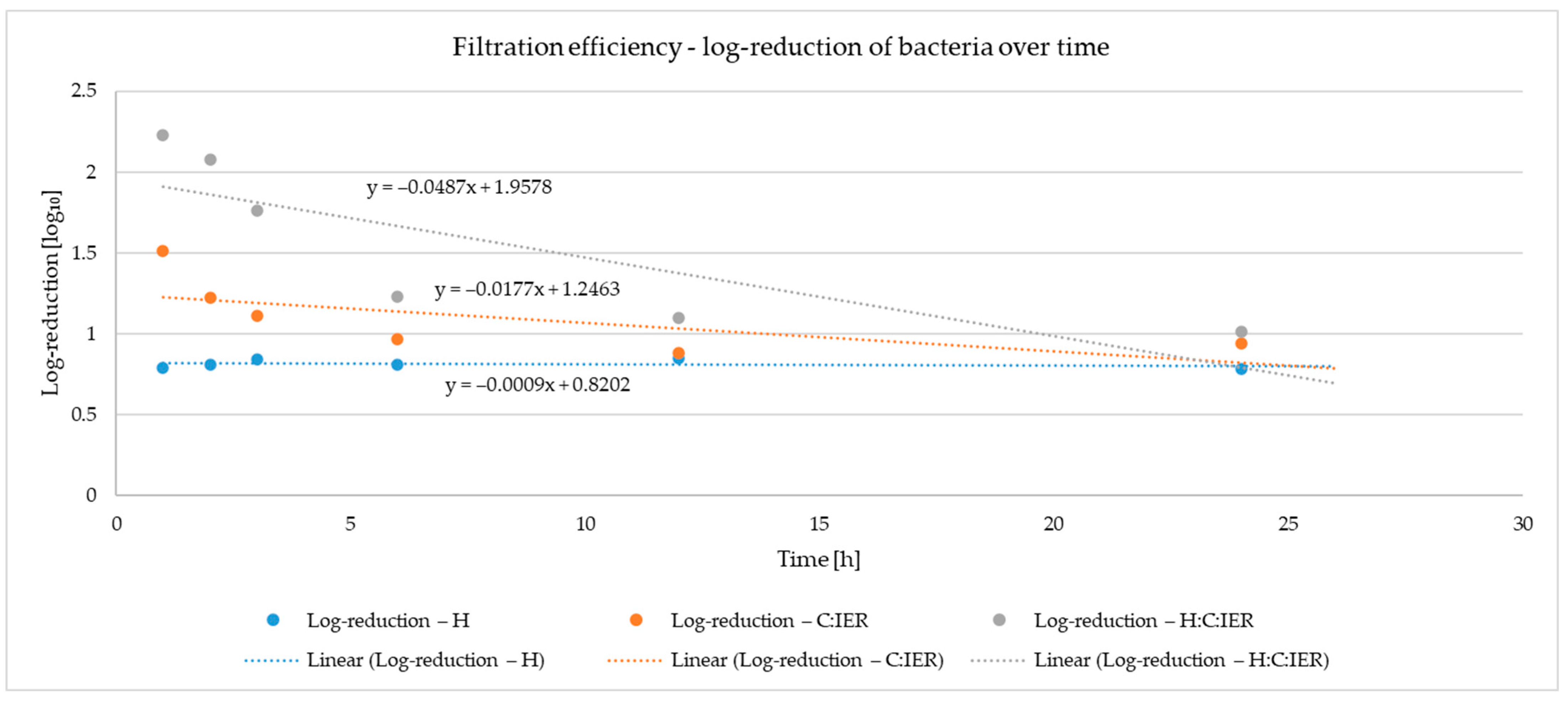
The obtained microbiological data were subjected to statistical analysis using two-way analysis of variance (ANOVA). The first factor (A) corresponded to the type of filter medium: H—hydrogel; C-IER—activated carbon with ion-exchange resin; H-C-IER—a combination of hydrogel, activated carbon, and ion-exchange resin. The second factor represented the sampling time interval (1, 2, 3, 6, 12, and 24 h). To assess the significance of the individual effects and their interaction, Tukey’s post hoc test with a 95% confidence interval was performed, allowing for comparison of the mean values of the analyzed parameters.
2.6. Validation of the System on Water from the Agri-Food Industry
The facility studied operates under an integrated food safety management system, with certification covering all stages of the production chain—from field cultivation to delivery of fruit to the end consumer. Annually, approximately 10,000 metric tons of fruit are produced, mainly apples and pears. Process water samples were collected from the main drainage channel of a fruit processing plant located in the Kuyavian-Pomeranian Voivodeship (northern Poland).
4. Discussion
Increasing efforts to implement a circular economy and to minimize losses—particularly in preserving and conserving valuable water resources—have become a prominent research focus [
36,
37]. The main driver of this trend is increasing anthropogenic pressure, along with progressing climate change, which adversely affects water resources through prolonged droughts and short periods of intense rainfall. These phenomena disrupt the hydrological balance in natural reservoirs and watercourses, as well as in groundwater systems [
38,
39]. In this context, the fresh-cut fruit and vegetable industry is receiving growing attention due to its high water consumption, low water recycling rates, and the associated potential microbiological risks [
40].
According to the FDA Produce Safety Rule, water used during and after harvest must contain no detectable
E. coli in 100 mL of water, reflecting the use of this bacterium as the primary indicator of microbiological safety [
41]. In the European Union, by contrast, water intended for contact with food must meet the criteria of potable water, as defined by Directive (EU) 2020/2184, which requires the absence of
E. coli and
Enterococcus spp. in 100 mL [
42]. However, our earlier field observations indicated that in some processing facilities, the wash water was not always replaced between product batches, which significantly increases the risk of microbial accumulation and highlights the practical relevance of developing effective treatment strategies [
35].
In our study, we designed and evaluated the performance of multilayer filters composed of activated carbon, ion exchange resin, and hydrogel hydrated with natural plant extracts. The system was subsequently validated using industrial process water. To the best of the authors’ knowledge, this is the first report to apply a filtration approach incorporating natural plant extracts immobilized within the filter bed for use in the fresh-cut industry. Conventional disinfection techniques are typically employed, including chlorine-based compounds (which are predominant), ozonation, peracetic acid, ultraviolet (UV) irradiation, hydrogen peroxide, as well as combined or hybrid methods [
11]. In addition, the study was carried out using environmental bacterial isolates obtained from food-processing facilities and real industrial process water, rather than the reference strains or model systems employed in most previous studies [
43,
44,
45]. This choice reflects real operating conditions and provides a preliminary indication of the applicability of the proposed approach in an industrial context.
The study was carried out under several assumptions that informed both its design and the interpretation of the findings. It was assumed that the bacterial isolates obtained from food-processing facilities are representative of the microbial contaminants typically present in wastewater from the fresh-cut industry. The multilayer filtration bed was considered to act through the complementary effects of hydrogel with natural extracts, ion-exchange resin, and activated carbon. The pilot-scale system was regarded as sufficiently representative of industrial conditions, although the experiments were conducted over limited operating intervals. Finally, the industrial process water used for validation was assumed to reflect the composition of effluents commonly generated in agro-food processing environments.
The study by Ignat et al. [
46] on the disinfection of wash water using acidic electrolyzed water (30 mg Cl
2 L
−1) demonstrated a reduction in mesophilic bacteria of approximately 1.0–1.2 log
10 CFU mL
−1 after 10 min of contact. In contrast, the composite filter proposed in the present study reduced the number of inoculated bacteria by 2.2 log
10 CFU mL
−1 in the initial interval, without the addition of chlorine. This suggests that the multilayer system saturated with natural plant extracts may offer higher microbiological efficacy while remaining free of chlorine-based compounds. A similar level of effectiveness was reported by López-Gálvez et al. [
47], who applied 2–3 mg L
−1 ClO
2 in the wash water recirculation system at a tomato-sorting facility, achieving a reduction in mesophilic bacteria, coliforms, and
E. coli in process water by slightly more than 1 log
10 CFU mL
−1. Importantly, the authors also did not detect any disinfection by-products in the final product. This highlights the critical importance of selecting an appropriate dosage when using conventional disinfection methods. Higher levels of microbial reduction were achieved by Banach et al. [
48] in a pilot-scale setup at an iceberg lettuce sorting facility. The study confirmed the high effectiveness of adding a low-chlorine oxidant (5 mg ClO
2 L
−1 or 3 mg ClO
2 L
−1) to the recirculating process water, resulting in a ≥5-log
10 reduction in inoculated
E. coli in less than 1 min.
Another example of disinfectants used in agri-food processing facilities is ozone. Compared to chlorine, its stability in water is significantly lower due to its spontaneous decomposition, which is initiated by reactions with hydroxyl ions or reduced substances [
49]. Nevertheless, studies conducted by Selma et al. [
50] demonstrated that 60 min of exposure to O
3, or its combination with UV-C, resulted in a 5.9–6.6 log
10 reduction in microbial load in wastewater from vegetable processing. It is important to note, however, that ozonation generates a wide range of secondary inorganic and organic compounds. Ozonation by-products can be classified into two main groups: oxidation by-products, formed from naturally occurring constituents of the water itself (e.g., bromide ions), and transformation products, resulting from reactions between ozone and trace contaminants present in the solution [
51].
In contrast, our study was based on the use of non-invasive methods involving natural filter components—namely, activated carbon and natural plant extracts—supported by an ion exchange resin. In tests using inoculated water, this system demonstrated greater stability and a higher level of bacterial reduction. Activated carbon filters are widely used as a medium for water purification. While their primary function is the removal of contaminants through adsorption onto a highly porous surface, they may also exert effects on microbial populations [
52]. Microorganisms can become immobilized within the pores of activated carbon, which consequently leads to a reduction in their abundance in the flowing water [
53]. As noted by Sbardella et al. [
54], over time, colonization of the filter bed occurs, along with the stabilization of interactions between microorganisms and the carbon phase. This leads to the phenomenon of biological activated carbon (BAC) filtration, in which sessile microorganisms degrade immobilized contaminants. While this process can be considered beneficial in the case of natural colonization or the use of microbial inoculants, it may be highly undesirable in the context of our study, due to the use of opportunistic pathogens in the inoculated water. Therefore, a subsequent step in the development of the system should include a detailed analysis of biofilm formation within the filtration unit, together with the implementation of risk management strategies aimed at minimizing potential adverse effects. Many authors highlight one of the major limitations associated with the use of activated carbon filters—their potential to become reservoirs of antibiotic resistance genes and multidrug-resistant bacteria, which may pose a significant risk to public health safety [
55,
56,
57]. Nevertheless, activated carbon remains one of the longest-used and most thoroughly documented sorbents in environmental engineering, with its industrial application dating back to the early 20th century [
58].
Ion exchange resins, traditionally used in water softening and demineralization processes, have in recent years gained increasing attention as components of active antibacterial barriers [
59]. However, in our study, they were employed as a conventional component complementing the composite filter bed, with the aim of enhancing its functionality by improving sorption capacity and potentially contributing to overall water quality improvement.
The final component of the filter bed was a hydrogel hydrated with natural plant extracts—an approach that has been scarcely described in the scientific literature to date. Previous in vitro studies on the extracts [
34] demonstrated their effectiveness under laboratory conditions; however, the present work represents the first report in which this solution has been implemented in the context of process water treatment. Importantly, most available publications concerning hydrogels enriched with plant extracts primarily relate to biomedical applications, particularly wound healing and localized therapy [
60,
61,
62]. Compounds identified in the earlier GC-MS analysis—such as epigallocatechin gallate, shikimic, ellagic, and gallic acids, as well as betulin, salicin, and procyanidins—may be responsible for the observed antimicrobial activity of the extracts.
Filtration is commonly employed in environmental engineering as a final polishing step to lower particle-associated microbial loads and enhance the effectiveness of subsequent disinfection [
63,
64]. An example of effective bacterial removal is the study by Zhang et al., which showed that adding biochar to sand columns enhances the elimination of
E. coli and
B. subtilis under both slow and rapid filtration, mainly due to the material’s higher adsorption capacity [
65]. Other studies have shown that the depth of the sand bed influences both filter performance and flow rate. Deeper beds improve the removal of suspended solids, turbidity and coliform bacteria, while dissolved contaminants are less effectively reduced [
66]. These findings demonstrate the effectiveness of conventional granular media mainly through physical straining and adsorption. By contrast, our multilayer bed integrates these mechanisms with the antimicrobial activity of plant-extract-infused hydrogel, offering an additional mode of bacterial reduction beyond polishing alone.
Sodium polyacrylate-based hydrogels are superabsorbents that undergo extensive swelling in aqueous environments. As a result of this process, a porous structure is formed within the polymer network. This feature enables the gradual release of immobilized compounds into the surrounding medium, both in medical applications and in our own studies with plant extracts [
67,
68]. The release of active substances (e.g., in medicines) from hydrogels can occur through different mechanisms: diffusion of the active substance with the absorbed liquid, erosion of the polymer matrix leading to pore enlargement, or swelling of the matrix due to water uptake [
67]. Although we did not perform a kinetic analysis of the release process in our study, the literature clearly indicates that sodium polyacrylate-based systems typically exhibit non-Fickian diffusion behavior and therefore require models that account for both diffusive transport and polymer network relaxation [
69,
70].
With an initial load of approximately 10
7 CFU mL
−1, the laboratory-inoculated water represented an extremely challenging test scenario, significantly exceeding the level of contamination observed in the wastewater collected from the fruit processing facility. Despite this high microbial burden, the H-C-IER composite filter achieved a reduction in more than 2 log
10 within the first three hours, and after 24 h, it still maintained an effectiveness greater than 1 log
10. The bacterial concentrations used in our study are consistent with common research practice. For example, Faith et al. employed an inoculum concentration of 10
7 CFU of
Salmonella enterica in their experimental design. An alternative approach was presented by Yesil et al. [
71], who applied a broader range of inoculum concentrations—10
8, 10
7, and 10
5 CFU/g—for the inoculation of spinach leaves. In real process water—characterized by a lower bacterial concentration but a higher content of organic matter following apple-washing—the observed log reductions were lower (≈1–1.3 log
10), indicating that such contaminants may partially affect the performance of the composite. Nevertheless, the filter met the minimum efficiency criterion of 90%. As noted in the study by López-Gálvez et al., the physicochemical quality of process water in a fresh produce facility varied between processing lines, which was attributed to differences in the types of raw materials being washed as well as the uneven efficiency of the washing systems themselves [
72]. This, in turn, can lead to differences in the microbial load of the process water itself.
A limitation of the present study lies in the barrier properties of the hydrogel. The observed antibacterial effect is primarily attributable to the diffusion of plant extracts from the hydrogel matrix into the surrounding medium. While this release mechanism is intrinsic to the functioning of the system, it makes it difficult to assess the potential barrier effect of the hydrogel itself. A comprehensive characterization of both the release kinetics and the barrier properties will be required in future studies to optimize the hydrogel formulation and to gain a clearer understanding of its role in microbial inactivation. In this preliminary study, the operational time of the prototype was limited to 24 h, during which a marked decrease in the efficiency of the C and IER components was observed. Although this time frame allowed us to obtain a first evaluation of the system, further investigations are needed to assess its performance over longer operating periods. Future optimization of the filter will focus not only on testing extended operational times but also on increasing the effective filtration surface, with the aim of improving both efficiency and durability. In this context, the H component appears particularly promising, and further studies will be directed toward refining its performance and stability.
An interesting approach aimed at enhancing the efficiency of bacterial inactivation in process water could involve the integration of additional modules into the proposed system—for example, a sedimentation tank, which reduces suspended solids that may serve as a habitat for microorganisms [
73]. Another strategy involves increasing the filtration surface area by adding a greater number of filter tubes, in order to adjust the performance and lifespan of the filtration unit to the specific conditions of a given production facility—such as the type of processed crop, continuous vs. seasonal operation, or geographical location. In addition, future work should include long-term performance tests under continuous operation to better reflect industrial practice. Further studies will also be necessary to characterize the release kinetics of natural extracts from the hydrogel matrix and to optimize the balance between diffusion and barrier properties.
The proposed filter system in our study may serve as an alternative or complementary solution to conventional disinfection methods, which are known to generate undesirable by-products.