Ultrasonic-Cavitation-Enhanced Biodegradation of Ciprofloxacin: Mechanisms and Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
2.2.1. Biodegradation
2.2.2. Ultrasonic Degradation
2.2.3. Ultrasound-Assisted Biological Degradation
2.3. CIP Concentration and TOC Analysis
2.4. Degradation Product Analysis
2.5. Toxicity Assessment
3. Results and Discussion
3.1. Effects of Different Factors on the Biodegradation Efficiency of CIP
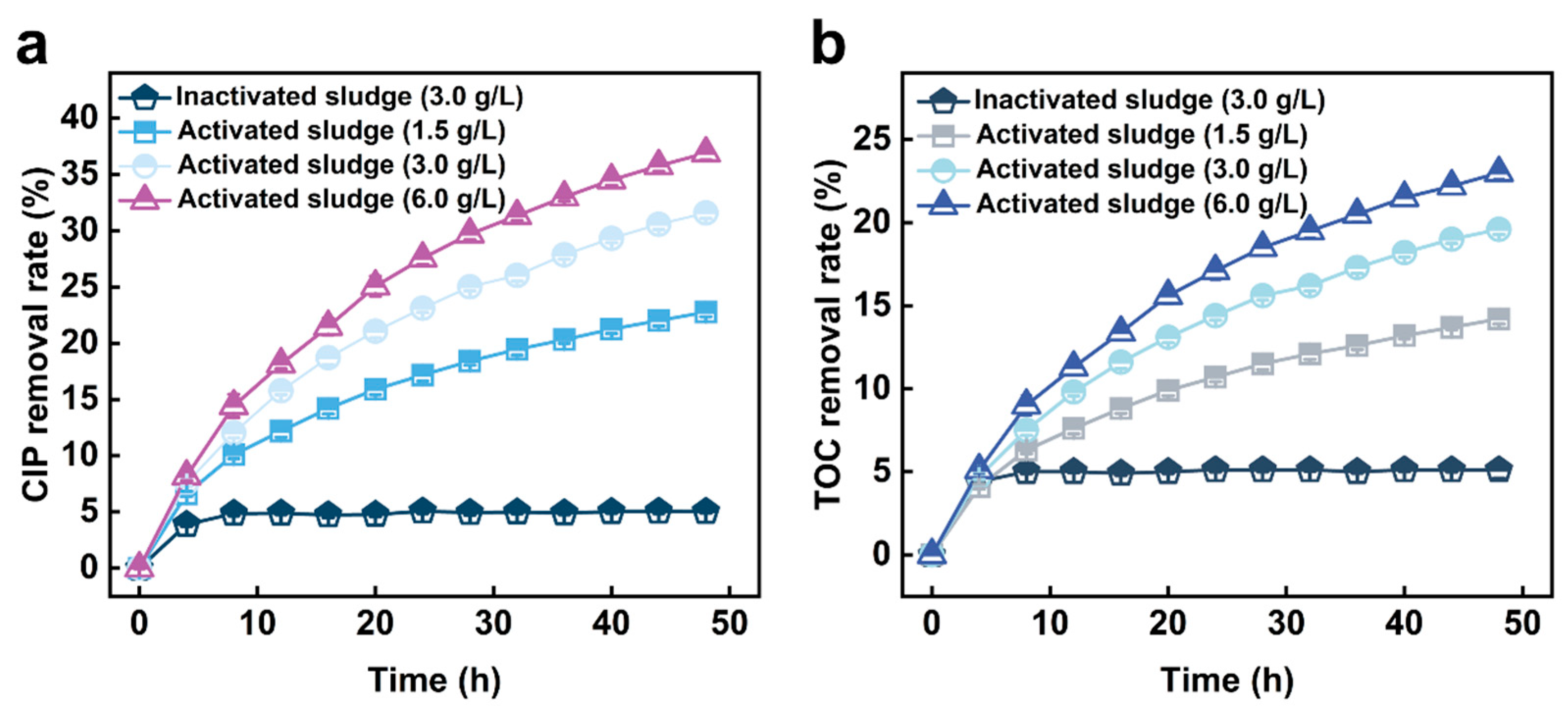
3.1.1. Effect of Sludge Concentration
3.1.2. Effect of pH
3.2. Effect of Different Factors on Ultrasonic Cavitation Degradation of CIP
3.2.1. Effect of Frequency
3.2.2. Effect of pH
3.3. Ultrasonic Pretreatment Enhances CIP Biodegradation Efficiency
3.4. Molecular Mechanisms by Which Ultrasonic Pretreatment Enhances CIP Biodegradation Efficiency
3.4.1. Transformation Pathways of Ultrasonic Degradation Products
3.4.2. Toxicity Reduction of Ultrasonic Degradation Products
4. Conclusions
- Optimal ultrasonic conditions were identified as 15 kHz frequency and pH 9, under which 58.9% of CIP degradation and 35.2% of total organic carbon (TOC) removal were achieved within 30 min.
- The combined treatment process achieved removal rates of 96.3% for CIP and 90.4% for TOC after 24 h.
- LC-MS analysis revealed that ultrasonic cavitation induced ring cleavage, hydroxylation, defluorination, and the formation of low-molecular-weight organic acids in CIP.
- Toxicity assessments indicated significant reductions in acute toxicity, bioaccumulation potential, and developmental toxicity for CIP and its degradation products.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.; Garg, A. Degradation of ciprofloxacin using Fenton’s oxidation: Effect of operating parameters, identification of oxidized by-products and toxicity assessment. Chemosphere 2018, 193, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, T.; Zhao, L.; Shi, X.; Li, R.; Ma, L.; Huang, Y.; Wang, Y.; Lee, S.-C. Manganese oxide on activated carbon with peroxymonosulfate activation for enhanced ciprofloxacin degradation: Activation mechanism and degradation pathway. Appl. Surf. Sci. 2024, 645, 158835. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, L.; Wang, W.; Zhu, Y.; Wei, G.; Lan, W. Strongly coupled bentonite-based Co3O4 quantum dot composites as efficient peroxymonosulfate activator for ciprofloxacin degradation: Fabrication, application and catalytic mechanism. Sep. Purif. Technol. 2025, 362, 131869. [Google Scholar] [CrossRef]
- Zhu, G.; Gao, M.; Bi, K.; Zhao, S. Pyrolysis of wheat straw for preparation of magnetic biochar for ciprofloxacin removal from wastewater and bio-oil/bio-gas analysis. J. Mol. Struct. 2025, 1342, 142696. [Google Scholar] [CrossRef]
- Zou, M.; Tian, W.; Zhao, J.; Chu, M.; Song, T. Quinolone antibiotics in sewage treatment plants with activated sludge treatment processes: A review on source, concentration and removal. Process Saf. Environ. Prot. 2022, 160, 116–129. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Oba, S.N.; Aniagor, C.O.; Adeniyi, A.G.; Ighalo, J.O. Adsorption of ciprofloxacin from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 93, 57–77. [Google Scholar] [CrossRef]
- Song, C.; Li, L.; Zhang, C.; Qiu, L.; Fan, L.; Wu, W.; Meng, S.; Hu, G.; Chen, J.; Liu, Y.; et al. Dietary risk ranking for residual antibiotics in cultured aquatic products around Tai Lake, China. Ecotoxicol. Environ. Saf. 2017, 144, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wei, Y.; Cui, J.; Liu, X.; Zhao, F.; Zheng, L.; Wang, P.; Liu, D. Toxic effects of chlorantraniliprole on zebrafish (Danio rerio) at different developmental stages under antibiotic pressure. Environ. Pollut. 2025, 367, 125590. [Google Scholar] [CrossRef]
- Nadgir, C.A.; Biswas, D.A. Antibiotic Resistance and Its Impact on Disease Management. Cureus 2023, 15, e38251. [Google Scholar] [CrossRef]
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The spread of antibiotic resistance to humans and potential protection strategies. Ecotoxicol. Environ. Saf. 2023, 254, 114734. [Google Scholar] [CrossRef] [PubMed]
- Kamenická, B.; Weidlich, T. A comparison of different reagents applicable for destroying halogenated anionic textile dye mordant blue 9 in polluted aqueous streams. Catalysts 2023, 13, 460. [Google Scholar] [CrossRef]
- Hegedus, M.; Lacina, P.; Plotěný, M.; Lev, J.; Kamenická, B.; Weidlich, T. Fast and efficient hydrodehalogenation of chlorinated benzenes in real wastewaters using Raney alloy. J. Water Process Eng. 2020, 38, 101645. [Google Scholar] [CrossRef]
- Zhao, K.; Si, T.; Liu, S.; Liu, G.; Li, D.; Li, F. Co-metabolism of microorganisms: A study revealing the mechanism of antibiotic removal, progress of biodegradation transformation pathways. Sci. Total Environ. 2024, 954, 176561. [Google Scholar] [CrossRef]
- Rusch, M.; Spielmeyer, A.; Zorn, H.; Hamscher, G. Degradation and transformation of fluoroquinolones by microorganisms with special emphasis on ciprofloxacin. Appl. Microbiol. Biotechnol. 2019, 103, 6933–6948. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Liang, C.; Wang, N.; Song, S.; Peng, L. Enhanced biodegradation of ciprofloxacin by enrich nitrifying sludge: Assessment of removal pathways and microbial responses. Water Sci. Technol. 2022, 85, 409–419. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Dai, M.; Wang, L.; Shi, X.; Guo, R. Accelerated ciprofloxacin biodegradation in the presence of magnetite nanoparticles. Chemosphere 2017, 188, 168–173. [Google Scholar] [CrossRef]
- Wang, L.; Qiang, Z.; Li, Y.; Ben, W. An insight into the removal of fluoroquinolones in activated sludge process: Sorption and biodegradation characteristics. J. Environ. Sci. 2017, 56, 263–271. [Google Scholar] [CrossRef]
- Sun, Y.; Jiao, Y.; Wang, M.; Zhao, Y.; Han, Y.; Wu, Q.; Toshiyuki, S.; Wang, C. Effect of sludge retention time on the removal of fluoroquinolone antibiotics in wastewater treatment of activated sludge. J. Water Process Eng. 2024, 59, 104941. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of ciprofloxacin using photo, sono, and sonophotocatalytic oxidation with visible light and low-frequency ultrasound: Degradation kinetics and pathways. Chem. Eng. J. 2020, 392, 124853. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Maria Botero-Coy, A.; Martinez-Pachon, D.; Moncayo-Lasso, A.; Ibanez, M.; Hernandez, F.; Torres-Palma, R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef]
- Zeng, T.; Yang, T.; Yun, X.; Luo, X.; Liu, Y.; Ren, H.; Wang, B. Intensification of ozone mass transfer and hydroxyl radical generation in HC-UC/RF coupled system: Promotion and application. J. Environ. Chem. Eng. 2025, 13, 117215. [Google Scholar] [CrossRef]
- Jia, X.; Wu, T.; Zhao, X.; Huang, J.; He, H.; Wang, C. Application of CuFeLDH-NCNT in ultrasound-assisted-Fenton-like process for removing ciprofloxacin from aqueous solutions. Water Sci. Technol. 2024, 89, 1401–1417. [Google Scholar] [CrossRef]
- Cui, Y.; Yan, X.; Han, G.; Lin, B.; Wu, Q.; Kang, W.; Ma, K. Generation mechanisms of active free radicals during ciprofloxacin degradation in the ultrasonic/K2S2O8 system. Water Sci. Technol. 2021, 83, 2051–2062. [Google Scholar] [CrossRef]
- Song, K.; Liu, Y.; Umar, A.; Ma, H.; Wang, H. Ultrasonic cavitation: Tackling organic pollutants in wastewater. Chemosphere 2024, 350, 141024. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Y.; Chen, C.; Zhou, X.; Liu, Y.; Yang, J.; Geng, Q.; Chen, G.; Ding, Y.; Yang, F. Current Progress in Natural Degradation and Enhanced Removal Techniques of Antibiotics in the Environment: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10919. [Google Scholar] [CrossRef]
- Yang, C.; Wu, T. A comprehensive review on quinolone contamination in environments: Current research progress. Environ. Sci. Pollut. Res. 2023, 30, 48778–48792. [Google Scholar] [CrossRef] [PubMed]
- De Bel, E.; Janssen, C.; De Smet, S.; Van Langenhove, H.; Dewulf, J. Sonolysis of ciprofloxacin in aqueous solution: Influence of operational parameters. Ultrason. Sonochem. 2011, 18, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Labrada, K.; Alcorta Cuello, D.R.; Saborit Sanchez, I.; Garcia Batle, M.; Manero, M.-H.; Barthe, L.; Javier Jauregui-Haza, U. Optimization of ciprofloxacin degradation in wastewater by homogeneous sono-Fenton process at high frequency. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2018, 53, 1139–1148. [Google Scholar] [CrossRef]
- Qi, H.; Chen, Y.; Li, L.; Li, X.; Li, Y. Degradation of Levofloxacin via Fenton Oxidation Combined with Ultrasonic Treatment in Water. Pol. J. Environ. Stud. 2024, 33, 6305–6321. [Google Scholar] [CrossRef]
- Hasani, K.; Peyghami, A.; Moharrami, A.; Vosoughi, M.; Dargahi, A. The efficacy of sono-electro-Fenton process for removal of Cefixime antibiotic from aqueous solutions by response surface methodology (RSM) and evaluation of toxicity of effluent by microorganisms. Arab. J. Chem. 2020, 13, 6122–6139. [Google Scholar] [CrossRef]
- Xu, S.; Zong, Y.; Feng, Y.; Liu, R.; Liu, X.; Hu, Y.; Han, S.; Wan, M. Dependence of pulsed focused ultrasound induced thrombolysis on duty cycle and cavitation bubble size distribution. Ultrason. Sonochem. 2015, 22, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Jawale, R.H.; Gogate, P.R.; Pandit, A.B. Treatment of cyanide containing wastewater using cavitation based approach. Ultrason. Sonochem. 2014, 21, 1392–1399. [Google Scholar] [CrossRef]
- Kidak, R.; Ince, N.H. Catalysis of advanced oxidation reactions by ultrasound: A case study with phenol. J. Hazard. Mater. 2007, 146, 630–635. [Google Scholar] [CrossRef]
- Befenzi, H.; Ezzariai, A.; Baghor, J.; Arrach, H.; Armengaud, J.; Kielbasa, M.; Doan, A.; Lambert, J.; Lomascolo, A.; Albert, Q.; et al. Bjerkandera adusta TM11 for the bioremediation of fluoroquinolone antibiotics spiked in wastewater: A sustainable approach to pharmaceutical contaminant biotransformation. Ecotoxicol. Environ. Saf. 2025, 291, 117898. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Silva-Agredo, J.; Hernandez, F.; Botero-Coy, A.M.; Torres-Palma, R.A. Methods involved in the treatment of four representative pharmaceuticals in hospital wastewater using sonochemical and biological processes. MethodsX 2023, 10, 102128. [Google Scholar] [CrossRef]
- Gupta, S.P.; Samanta, S.K. Thermal activation of persulfate for degradation of ciprofloxacin in water: Mechanism, influencing factors and toxicity assessment. J. Water Process Eng. 2025, 72, 107646. [Google Scholar] [CrossRef]
- Dorival-García, N.; Zafra-Gómez, A.; Navalón, A.; González-López, J.; Hontoria, E.; Vílchez, J.L. Removal and degradation characteristics of quinolone antibiotics in laboratory-scale activated sludge reactors under aerobic, nitrifying and anoxic conditions. J. Environ. Manag. 2013, 120, 75–83. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, Y.; Guo, L.; Xiao, Y.; Li, H.; Yang, P.; Xia, L.; Liu, X.; Chen, Z.; Li, L.; et al. Microbial Degradation of Tetracycline Antibiotics: Mechanisms and Environmental Implications. J. Agric. Food Chem. 2024, 72, 13523–13536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, A.; Zhang, Y.; Bao, R.; Tian, X.; Li, J. Electro-Fenton degradation of antibiotic ciprofloxacin (CIP): Formation of Fe3+-CIP chelate and its effect on catalytic behavior of Fe2+/Fe3+ and CIP mineralization. Electrochim. Acta 2017, 256, 185–195. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Jafari, A.J.; Farzadkia, M.; Kermani, M.; Sheikhmohammadi, A.; Pasalari, H.; Esrafili, A. Photodegradation and mineralization of ciprofloxacin by consecutive application UV/iodide process and biological treatment. Desalination Water Treat. 2023, 293, 170–181. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Biodegradation and metabolic pathway of sulfamethoxazole by a novel strain Acinetobacter sp. Appl. Microbiol. Biotechnol. 2018, 102, 425–432. [Google Scholar] [CrossRef]
- Wang, L.; Ben, W.; Li, Y.; Liu, C.; Qiang, Z. Behavior of tetracycline and macrolide antibiotics in activated sludge process and their subsequent removal during sludge reduction by ozone. Chemosphere 2018, 206, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, Y.; Zhao, X.; Du, Q.; Wang, Z.; Xia, Y.; Xia, L. Biosorption Behavior of Ciprofloxacin onto Enteromorpha prolifera: Isotherm and Kinetic Studies. Int. J. Phytoremediat. 2015, 17, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Zhu, G.; Yang, Z.; Lu, Y.; Lang, Y.; Gong, L.; Shan, H. Effect of the C/N Ratio on Biodegradation of Ciprofloxacin and Denitrification from Low C/N Wastewater as Assessed by a Novel 3D-BER System. Sustainability 2020, 12, 7611. [Google Scholar] [CrossRef]
- Zhang, C.-C.; Guo, J.-S.; Chen, Y.-P.; Ji, F.-Y.; Wang, J.; Yan, P.; Bai, Y. Thermodynamics of the interaction between antibiotics and extracellular polymeric substances within activated sludge. Environ. Technol. 2019, 40, 1525–1533. [Google Scholar] [CrossRef]
- Thomas, S.; Rayaroth, M.P.; Menacherry, S.P.M.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical degradation of benzenesulfonic acid in aqueous medium. Chemosphere 2020, 252, 126485. [Google Scholar] [CrossRef]
- Villaroel, E.; Silva-Agredo, J.; Petrier, C.; Taborda, G.; Torres-Palma, R.A. Ultrasonic degradation of acetaminophen in water: Effect of sonochemical parameters and water matrix. Ultrason. Sonochem. 2014, 21, 1763–1769. [Google Scholar] [CrossRef]
- Munoz-Calderon, A.; Zuniga-Benitez, H.; Valencia, S.H.; Rubio-Clemente, A.; Upegui, S.A.; Penuela, G.A. Use of low frequency ultrasound for water treatment: Data on azithromycin removal. Data Brief 2020, 31, 105947. [Google Scholar] [CrossRef]
- Karim, A.V.; Shriwastav, A. Degradation of amoxicillin with sono, photo, and sonophotocatalytic oxidation under low-frequency ultrasound and visible light. Environ. Res. 2021, 200, 111515. [Google Scholar] [CrossRef]
- Petkovsek, M.; Dular, M. Cavitation dynamics in water at elevated temperatures and in liquid nitrogen at an ultrasonic horn tip. Ultrason. Sonochem. 2019, 58, 104652. [Google Scholar] [CrossRef]
- Wu, Z.; Yuste-Cordoba, F.J.; Cintas, P.; Wu, Z.; Boffa, L.; Mantegna, S.; Cravotto, G. Effects of ultrasonic and hydrodynamic cavitation on the treatment of cork wastewater by flocculation and Fenton processes. Ultrason. Sonochem. 2018, 40, 3–8. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, J.; Zhang, J.; Feng, F.; Mu, B.; Zhang, N. Carbon-dot/attapulgite composite sonocatalytic towards efficient indigo-containing wastewater degradation based on the cavitation mechanism. J. Water Process Eng. 2024, 63, 105551. [Google Scholar] [CrossRef]
- De Bel, E.; Dewulf, J.; De Witte, B.; Van Langenhove, H.; Janssen, C. Influence of pH on the sonolysis of ciprofloxacin: Biodegradability, ecotoxicity and antibiotic activity of its degradation products. Chemosphere 2009, 77, 291–295. [Google Scholar] [CrossRef]
- Wei, Z.; Spinney, R.; Ke, R.; Yang, Z.; Xiao, R. Effect of pH on the sonochemical degradation of organic pollutants. Environ. Chem. Lett. 2016, 14, 163–182. [Google Scholar] [CrossRef]
- Estrada-Florez, S.E.; Serna-Galvis, E.A.; Torres-Palma, R.A. Photocatalytic vs. sonochemical removal of antibiotics in water: Structure-degradability relationship, mineralization, antimicrobial activity, and matrix effects. J. Environ. Chem. Eng. 2020, 8, 104359. [Google Scholar] [CrossRef]
- Wen, H.; Cheng, D.; Chen, Y.; Yue, W.; Zhang, Z. Review on ultrasonic technology enhanced biological treatment of wastewater. Sci. Total Environ. 2024, 925, 171260. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, D.; Hamdaoui, O.; Vasseghian, Y.; Momotko, M.; Boczkaj, G.; Kyzas, G.Z.; Wang, C. Bibliometric analysis and literature review of ultrasound-assisted degradation of organic pollutants. Sci. Total Environ. 2023, 876, 162551. [Google Scholar] [CrossRef] [PubMed]
- Movahed, S.M.A.; Calgaro, L.; Marcomini, A. Trends and characteristics of employing cavitation technology for water and wastewater treatment with a focus on hydrodynamic and ultrasonic cavitation over the past two decades: A Scientometric analysis. Sci. Total Environ. 2023, 858, 159802. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Thanekar, P.D.; Oke, A.P. Strategies to improve biological oxidation of real wastewater using cavitation based pre-treatment approaches. Ultrason. Sonochem. 2020, 64, 105016. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, S.; Li, S.; Zhang, L. Synergistic mechanism of ultrasonic cavitation and advanced oxidation: Free radical path optimization and advanced treatment of industrial wastewater. J. Environ. Chem. Eng. 2025, 13, 117232. [Google Scholar] [CrossRef]
- Yan, M.; Lo, J.C.; Edwards, J.T.; Baran, P.S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Degradation of 2,4-dinitrophenol using a combination of hydrodynamic cavitation, chemical and advanced oxidation processes. Ultrason. Sonochem. 2013, 20, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Alfonso-Muniozguren, P.; Bussemaker, M.J.; Sears, P.; Serna-Galvis, E.A.; Torres-Palma, R.A.; Lee, J. A fundamental study on the degradation of paracetamol under single- and dual-frequency ultrasound. Ultrason. Sonochem. 2023, 94, 106320. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Karri, R.R.; Koduru, J.R.; Manickam, S.; Tyagi, I.; Mubarak, N.M.; Suhas. Recent trends in the applications of sonochemical reactors as an advanced oxidation process for the remediation of microbial hazards associated with water and wastewater: A critical review. Ultrason. Sonochem. 2023, 94, 106302. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, L.; Ji, B.; Ren, Y.; Xu, X.; He, J.; Xue, Y.; Sun, H. Ultrasonic cavitation treatment of o-cresol wastewater and long-term pilot-scale study. J. Environ. Manag. 2025, 375, 124208. [Google Scholar] [CrossRef]
- Wang, J.; Chang, X.; Zhao, Y.; Xu, H.; He, G.; Chen, H. A novel Bi2WO6/BiOBr/RGO photocatalyst for enhanced degradation of ciprofloxacin under visible light irradiation: Performance, mechanism and toxicity evaluation. Diam. Relat. Mater. 2022, 128, 109274. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Isaza-Pineda, L.; Moncayo-Lasso, A.; Hernandez, F.; Ibanez, M.; Torres-Palma, R.A. Comparative degradation of two highly consumed antihypertensives in water by sonochemical process. Determination of the reaction zone, primary degradation products and theoretical calculations on the oxidative process. Ultrason. Sonochem. 2019, 58, 104635. [Google Scholar] [CrossRef]
- Yuan, S.; Li, C.; Zhang, Y.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W. Degradation of parathion methyl in bovine milk by high-intensity ultrasound: Degradation kinetics, products and their corresponding toxicity. Food Chem. 2020, 327, 127103. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Montoya-Rodriguez, D.; Isaza-Pineda, L.; Ibanez, M.; Hernandez, F.; Moncayo-Lasso, A.; Torres-Palma, R.A. Sonochemical degradation of antibiotics from representative classes-Considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason. Sonochem. 2019, 50, 157–165. [Google Scholar] [CrossRef] [PubMed]






| Method | Mass Removed (g/m3) | Cost (in USD) per g of CIP Removal | ||
|---|---|---|---|---|
| Chemical Cost | Energy Cost | Total Cost | ||
| U+B | 9.63 | 0.208 | 0.011 | 0.219 |
| Fenton | 9.00 | 0.310 | 0.013 | 0.324 |
| Compound | Retention (min) | Experimental Mass (m/z) | Chemical Formula | Molecular Structure |
|---|---|---|---|---|
| P1 | 1.046 | 237 | C13H17FN2O |  |
| P2 | 4.934 | 163 | C10H14N2 |  |
| P3 | 4.527 | 195 | C10H14N2O2 |  |
| P4 | 6.767 | 319 | C17H19FN2O3 |  |
| P5 | 6.508 | 275 | C16H19FN2O |  |
| P6 | 3.823 | 307 | C15H15FN2O4 |  |
| P7 | 6.471 | 243 | C14H14N2O2 |  |
| P8 | 9.415 | 230 | C13H11NO3 |  |
| P9 | 8.212 | 208 | C10H6FNO3 |  |
| P10 | 5.101 | 114 | C5H7NO2 |  |
| P11 | 9.711 | 102 | C4H7NO2 |  |
| P12 | 1.416 | 73 | C3H4O2 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Q.; Peng, Q.; Pham, T.; He, X. Ultrasonic-Cavitation-Enhanced Biodegradation of Ciprofloxacin: Mechanisms and Efficiency. Water 2025, 17, 2495. https://doi.org/10.3390/w17162495
Wen Q, Peng Q, Pham T, He X. Ultrasonic-Cavitation-Enhanced Biodegradation of Ciprofloxacin: Mechanisms and Efficiency. Water. 2025; 17(16):2495. https://doi.org/10.3390/w17162495
Chicago/Turabian StyleWen, Qianheng, Qiwei Peng, ThuThi Pham, and Xiwei He. 2025. "Ultrasonic-Cavitation-Enhanced Biodegradation of Ciprofloxacin: Mechanisms and Efficiency" Water 17, no. 16: 2495. https://doi.org/10.3390/w17162495
APA StyleWen, Q., Peng, Q., Pham, T., & He, X. (2025). Ultrasonic-Cavitation-Enhanced Biodegradation of Ciprofloxacin: Mechanisms and Efficiency. Water, 17(16), 2495. https://doi.org/10.3390/w17162495






