Recent Advances in Organic Pollutant Removal Technologies for High-Salinity Wastewater
Abstract
1. Introduction
2. Sources and Characteristics of High-Salinity Wastewater
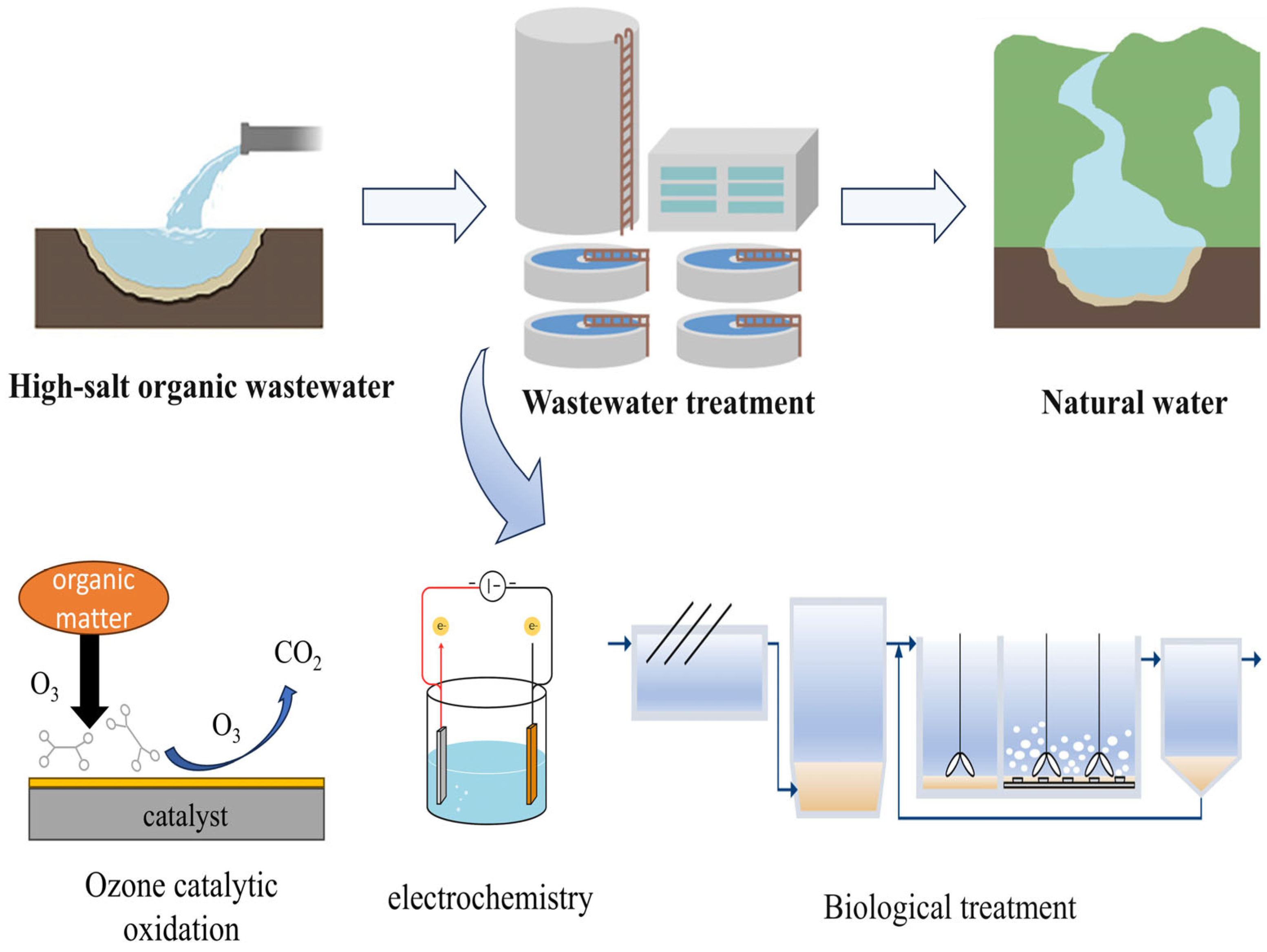
3. Physicochemical Methods
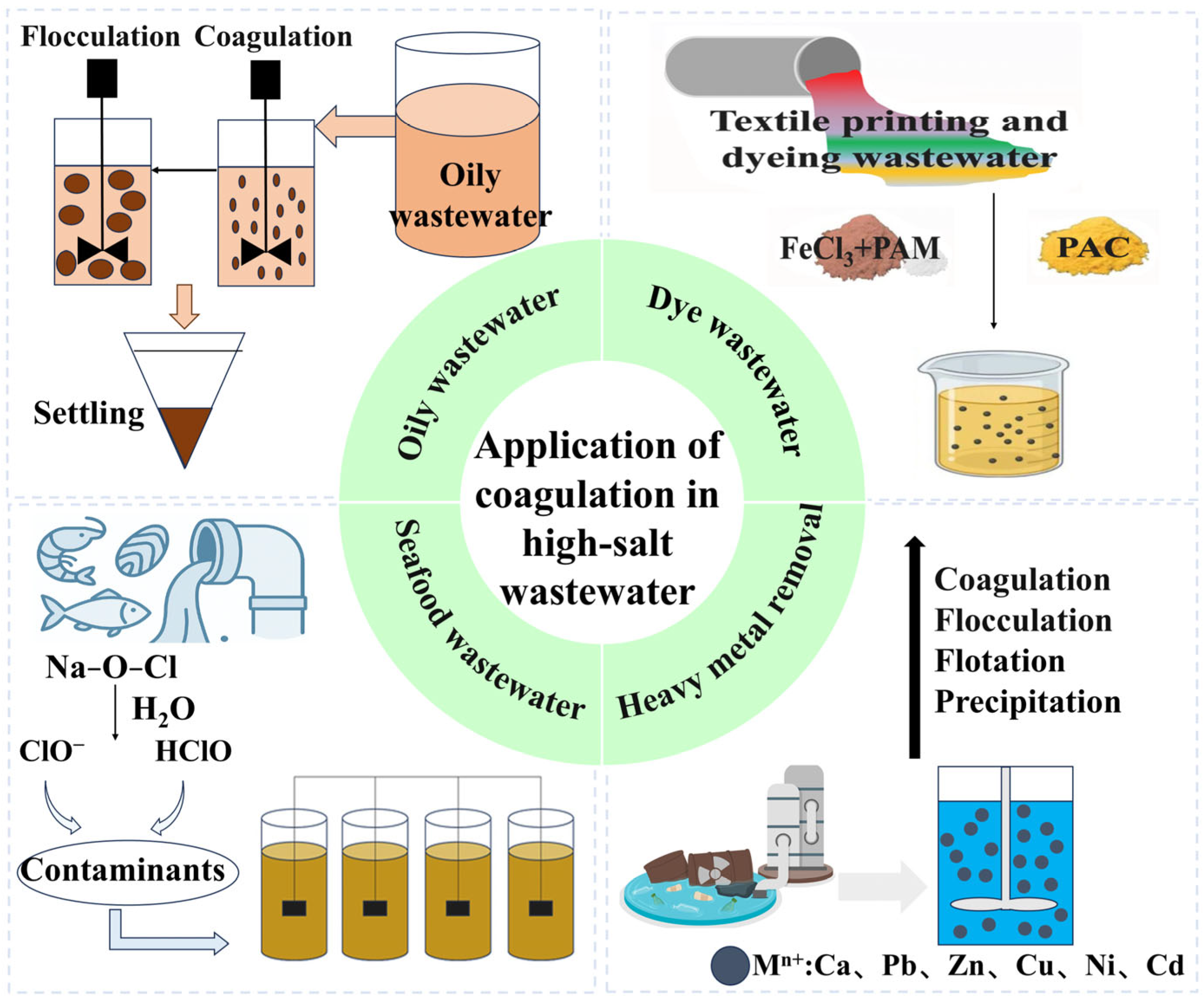
3.1. Coagulation–Flocculation Technology
3.2. Membrane Technology
3.3. Advanced Oxidation Techniques
4. Biological Methods
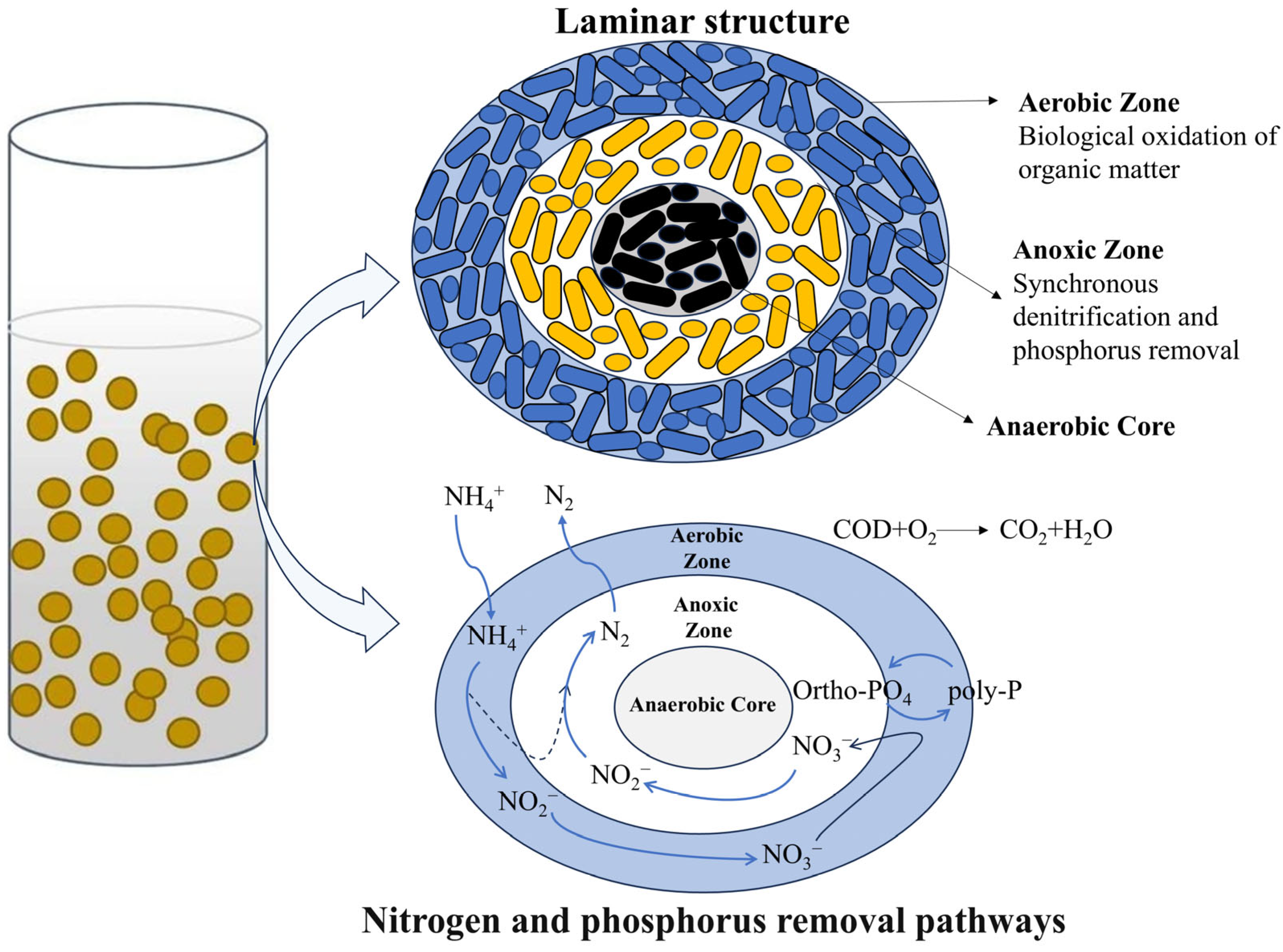
4.1. Aerobic Biotechnology
4.2. Anaerobic Biotechnology
5. Combined Processes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Y.; Zhuang, X.; Ahmad, S.; Sung, S.; Ni, S.-Q. Biotreatment of high-salinity wastewater: Current methods and future directions. World J. Microbiol. Biotechnol. 2020, 36, 37. [Google Scholar] [CrossRef]
- Maharaja, P.; Boopathy, R.; Anushree, V.; Mahesh, M.; Swarnalatha, S.; Ravindran, B.; Chang, S.W.; Sekaran, G. Bio removal of proteins, lipids and mucopolysaccharides in tannery hyper saline wastewater using halophilic bacteria. J. Water Process Eng. 2020, 38, 101674. [Google Scholar] [CrossRef]
- Guo, L.; Xie, Y.; Sun, W.; Xu, Y.; Sun, Y. Research Progress of High-Salinity Wastewater Treatment Technology. Water 2023, 15, 684. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Song, Q.; Chen, X.; Hua, Y.; Chen, S.; Ren, L.; Dai, X. Biological treatment processes for saline organic wastewater and related inhibition mechanisms and facilitation techniques: A comprehensive review. Environ. Res. 2023, 239, 117404. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, O.; Vasudevan, N.; Torrijos, M.; Thanasekaran, K.; Moletta, R. Anaerobic digestion of tannery soak liquor with an aerobic post-treatment. Water Res. 2006, 40, 1492–1500. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current advances in membrane technologies for saline wastewater treatment: A comprehensive review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Z.; Guo, S.; Liu, J.; Hao, X.; Xing, X.; Xian, G.; Wei, Y. Practical optimization of scale removal in circulating cooling water: Electrochemical descaling-filtration crystallization coupled system. Sep. Purif. Technol. 2022, 284, 120268. [Google Scholar] [CrossRef]
- Mseddi, S.; Chakchouk, I.; Aloui, F.; Sayadi, S.; Kallel, M. Development of a process for the treatment of fish processing saline wastewater. Desalination Water Treat. 2014, 52, 2301–2308. [Google Scholar] [CrossRef]
- Younker, J.M.; Walsh, M.E. Impact of salinity on coagulation and dissolved air flotation treatment for oil and gas produced water. Water Qual. Res. J. Can. 2014, 49, 135–143. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Cheng, S.; Li, C.; Liu, F.; Wang, P.; Sun, L.; Huang, J.; Zhang, W.; Zhang, X. Enhanced Microcystis Aeruginosa removal and novel flocculation mechanisms using a novel continuous co-coagulation flotation (CCF). Sci. Total Environ. 2023, 857, 159532. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Yang, L.; Wang, Y.; Li, T.; Yang, L. Research on synchronous coagulation-ozonation coupled with electrodialysis for treatment of high-salinity organic wastewater. J. Environ. Chem. Eng. 2025, 13, 118109. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Wang, Y.; Cui, C.; Yan, X.; Dai, W.; Zhang, A.; Zhang, Z.; Tang, D.; Zhou, Y.; et al. In-situ formation of green rust during Fe(II) coagulation: Dual reductive and adsorptive pathways for dyeing wastewater treatment. Water Res. 2025, 285, 124174. [Google Scholar] [CrossRef] [PubMed]
- Odabaşı, Ç.; Dologlu, P.; Gülmez, F.; Kuşoğlu, G.; Çağlar, Ö. Investigation of the factors affecting reverse osmosis membrane performance using machine-learning techniques. Comput. Chem. Eng. 2022, 159, 107669. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Iborra-Clar, M.-I.; Alcaina-Miranda, M.-I.; Mendoza-Roca, J.-A.; Pastor-Alcañiz, L. Treatment of table olive processing wastewaters using novel photomodified ultrafiltration membranes as first step for recovering phenolic compounds. J. Hazard. Mater. 2015, 290, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, X.; Li, Y.; Dai, R.; Wang, Z. Tuning of nanofiltration membrane by multifunctionalized nanovesicles to enable an ultrahigh dye/salt separation at high salinity. J. Membr. Sci. 2022, 644, 120094. [Google Scholar] [CrossRef]
- Liu, M.; Lü, Z.; Chen, Z.; Yu, S.; Gao, C. Comparison of reverse osmosis and nanofiltration membranes in the treatment of biologically treated textile effluent for water reuse. Desalination 2011, 281, 372–378. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, S.; Liao, J.; Qiu, Y.; Dong, J.; Liu, C.; Ruan, H.; Shen, J. An integrated adsorption-and membrane-based system for high-salinity aniline wastewater treatment with zero liquid discharge. Desalination 2022, 527, 115537. [Google Scholar] [CrossRef]
- Pei, B.; Chen, J.; Liu, P.; He, T.; Li, X.; Zhang, L. Hyperbranched poly (amidoamine)/TMC reverse osmosis membrane for oily saline water treatment. Environ. Technol. 2019, 40, 2779–2788. [Google Scholar] [CrossRef]
- Zhang, Y.; Bu, X.; Dong, X.; Wang, Y.; Chen, Z. Nanofiltration combined with membrane capacitive deionization for efficient classification and recovery salts from simulated coal chemical industrial wastewater. Sep. Purif. Technol. 2023, 322, 124156. [Google Scholar] [CrossRef]
- Adiba, N.; Wang, X.; Chang, C.; Xu, X.; Liu, Y.; Ji, C.; Wang, Q.; Ren, Y.; Wang, J.; Liu, Z.; et al. Multi-stage membrane integrated system to achieving low-mixed-salt-discharge of high-salinity mining wastewater: System design and experimental validation. Chem. Eng. Res. Des. 2024, 202, 12–22. [Google Scholar] [CrossRef]
- Kamel, A.H.; Al-Juboori, R.A.; Al-shaeli, M.; Ladewig, B.; Ibrahim, S.S.; Alsalhy, Q.F. Potential application of hybrid forward osmosis-Membrane distillation (FO-MD) system for various water treatment processes. Process. Saf. Environ. Prot. 2023, 180, 1023–1052. [Google Scholar] [CrossRef]
- Suleman, M.; Al-Rudainy, B.; Lipnizki, F. Overcoming the Limitations of Forward Osmosis and Membrane Distillation in Sustainable Hybrid Processes Managing the Water-Energy Nexus. Membranes 2025, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhou, L.; Liu, Y.; Zhang, J.; Lei, J. Efficient degradation and adsorption of roxarsone by FeOOH quantum decorated resorcinol-formaldehyde resins via Fenton-like process. Res. Chem. Intermed. 2023, 49, 2569–2582. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Yu, H.; Yao, N.; Shen, J.; Li, Y.; Zhang, H.; Bai, X. Efficient degradation of N-nitrosopyrrolidine using CoFe-LDH/AC particle electrode via heterogeneous Fenton-like reaction. Chemosphere 2023, 313, 137446. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, M.; Xie, S.; Xiao, Z.; Sun, W.; Xu, Y.; Zhou, J.; Sun, Y. Catalytic ozonation of high-salinity wastewater using salt-resistant catalyst Fe-Bi@ γ-Al2O3. J. Water Process Eng. 2022, 49, 103160. [Google Scholar] [CrossRef]
- Mejri, A.; Soriano-Molina, P.; Miralles-Cuevas, S.; Trabelsi, I.; Sánchez Pérez, J.A. Effect of liquid depth on microcontaminant removal by solar photo-Fenton with Fe (III): EDDS at neutral pH in high salinity wastewater. Environ. Sci. Pollut. Res. 2019, 26, 28071–28079. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Wang, H.; Bian, Z. Hybrid electrocatalytic ozonation treatment of high-salinity organic wastewater using Ni–Ce/OMC particle electrodes. Sci. Total. Environ. 2020, 724, 138170. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Su, R.; Lv, G.; Wang, Z.; Gao, B.; Zhou, W. Enhanced degradation of bisphenol F in a porphyrin-MOF based visible-light system under high salinity conditions. Chem. Eng. J. 2022, 428, 132106. [Google Scholar] [CrossRef]
- An, W.; Xiao, S.; Wang, Y.; Zhan, J.; Ma, L. Enhanced biodegradability and ammonia nitrogen removal of high-salinity pharmaceutical wastewater by ozonation with iron-based monolithic catalyst packing. Chem. Eng. J. 2024, 479, 147843. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Qin, L.; Wu, W.; Meng, Q.; Shen, C.; Zhang, G. Membrane photo-bioreactor coupled with heterogeneous Fenton fluidized bed for high salinity wastewater treatment: Pollutant removal, photosynthetic bacteria harvest and membrane anti-fouling analysis. Sci. Total. Environ. 2019, 696, 133953. [Google Scholar] [CrossRef]
- Liu, Z.; Teng, Y.; Xu, Y.; Zheng, Y.; Zhang, Y.; Zhu, M.; Sun, Y. Ozone catalytic oxidation of biologically pretreated semi-coking wastewater (BPSCW) by spinel-type MnFe2O4 magnetic nanoparticles. Sep. Purif. Technol. 2021, 278, 118277. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; He, C.; Yuan, R.; Wu, X.; Guo, S.; He, X.; Van Hulle, S.W. Effect of pre-coagulation on catalytic ozonation in the tertiary treatment of coking wastewater: Kinetic and ozone consumption analysis. J. Water Process Eng. 2022, 48, 102856. [Google Scholar] [CrossRef]
- Qin, Y.; Yuan, R.; Wang, S.; Zhang, X.; Luo, S.; He, X. Catalytic Ozonation Treatment of Coal Chemical Reverse Osmosis Concentrate: Water Quality Analysis, Parameter Optimization, and Catalyst Deactivation Investigation. Toxics 2024, 12, 681. [Google Scholar] [CrossRef]
- Wang, B.; Shi, W.; Zhang, H.; Ren, H.; Xiong, M. Promoting the ozone-liquid mass transfer through external physical fields and their applications in wastewater treatment: A review. J. Environ. Chem. Eng. 2021, 9, 106115. [Google Scholar] [CrossRef]
- Yildirim, R.; Eskikaya, O.; Keskinler, B.; Karagunduz, A.; Dizge, N.; Balakrishnan, D. Fabric dyeing wastewater treatment and salt recovery using a pilot scale system consisted of graphite electrodes based on electrooxidation and nanofiltration. Environ. Res. 2023, 234, 116283. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, T.; Li, K.; Liu, H.; Zhao, Z.; Wu, X.; Wang, J. Development of expanded polytetrafluoroethylene hollow fiber membranes for membrane Fenton oxidation in wastewater treatment. J. Hazard. Mater. 2025, 492, 138247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, S.; Xu, M.; Jiang, S.; Ou, X.; Wang, C.; Yu, Y.; Wu, C. Bio-electro-Fenton systems in wastewater treatment: Research progress and prospects. J. Electroanal. Chem. 2025, 981, 118959. [Google Scholar] [CrossRef]
- Rong, H.; Zhang, C.; Sun, Y.; Wu, L.; Lian, B.; Wang, Y.; Chen, Y.; Tu, Y.; Waite, T.D. Electrochemical degradation of Ni-EDTA complexes in electroless plating wastewater using PbO2-Bi electrodes. Chem. Eng. J. 2022, 431, 133230. [Google Scholar] [CrossRef]
- Fong, S.T.; Suzaimi, N.D.; Hairom, N.H.; Ghani, R.A.; Khayet, M.; Almanassra, I.W.; Harun, M.H.C.; Mohammad, A.W.; Hamzah, S. Influence of reaction parameters on titanium anode electro-oxidation coupled with filtration system for leachate treatment. Colloids Surf. A Physicochem. Eng. Asp. 2025, 725, 137693. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, J.; Bai, S.; Mo, R.; Yang, Y.; Liu, W.; Tang, X.; Yu, H.; Zhu, Y. Preparation of novel Ti-based MnOx electrodes by spraying method for electrochemical oxidation of Acid Red B. Water Sci. Technol. 2019, 80, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, E.A.; Nabi, A.; Kamli, M.R.; Albukhari, S.M.; Althabaiti, S.A.; Al-Harbi, S.A.; Khan, I.; Malik, M.A. Facile Green Synthesis of ZnO NPs and Plasmonic Ag-Supported ZnO Nanocomposite for Photocatalytic Degradation of Methylene Blue. Water 2023, 15, 384. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Miao, Q.; Wu, P.; He, J.; Liu, C.; Jiang, W. Rapid green degradation of ethylene glycol-based antifreeze wastewater via a coupled photolytic and photocatalytic double-pathway mechanism. J. Water Process Eng. 2025, 71, 107191. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Gao, Y.; Yue, Q.; Gao, B.; Liu, B.; Guo, K.; Xu, X. Pilot-scale advanced treatment of actual high-salt textile wastewater by a UV/O3 pressurization process: Evaluation of removal kinetics and reverse osmosis desalination process. Sci. Total Environ. 2023, 857, 159725. [Google Scholar] [CrossRef]
- Corsino, S.F.; Capodici, M.; Morici, C.; Torregrossa, M.; Viviani, G. Simultaneous nitritation–denitritation for the treatment of high-strength nitrogen in hypersaline wastewater by aerobic granular sludge. Water Res. 2016, 88, 329–336. [Google Scholar] [CrossRef]
- Hou, M.; Li, W.; Li, H.; Li, C.; Wu, X.; Liu, Y.-D. Performance and bacterial characteristics of aerobic granular sludge in response to alternating salinity. Int. Biodeterior. Biodegrad. 2019, 142, 211–217. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, H.-D.; Park, J.-H.; Zhang, F.; Chen, C.; Li, X.; Zhao, D.; Zhao, F. Effect of different salinity adaptation on the performance and microbial community in a sequencing batch reactor. Bioresour. Technol. 2016, 216, 808–816. [Google Scholar] [CrossRef]
- Woolard, C.R.; Irvine, R.L. Biological treatment of hypersaline wastewater by a biofilm of halophilic bacteria. Water Environ. Res. 1994, 66, 230–235. [Google Scholar] [CrossRef]
- Sun, R.; Jin, Y. Pilot Scale Application of a Ceramic Membrane Bioreactor for Treating High-Salinity Oil Production Wastewater. Membranes 2022, 12, 473. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Y.; Chen, H.; Lv, Y. Biological nitrogen removal characteristics and mechanisms of a novel salt-tolerant strain Vibrio sp. LV-Q1 and its application capacity for high-salinity nitrogen-containing wastewater treatment. J. Water Process Eng. 2024, 59, 105098. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, X.; Ng, H.Y. Aerobic granular sludge systems for treating hypersaline pharmaceutical wastewater: Start-up, long-term performances and metabolic function. J. Hazard. Mater. 2021, 412, 125229. [Google Scholar] [CrossRef]
- Carrera, P.; Campo, R.; Méndez, R.; Di Bella, G.; Campos, J.; Mosquera-Corral, A.; Del Rio, A.V. Does the feeding strategy enhance the aerobic granular sludge stability treating saline effluents? Chemosphere 2019, 226, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.D.M.; Oosterkamp, M.J.; Wang, W.; Spanjers, H.; van Lier, J.B. Comparative performance of upflow anaerobic sludge blanket reactor and anaerobic membrane bioreactor treating phenolic wastewater: Overcoming high salinity. Chem. Eng. J. 2019, 366, 480–490. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Boylan, B. Batch treatment of saline wastewater by Halanaerobium lacusrosei in an anaerobic packed bed reactor. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 34–38. [Google Scholar] [CrossRef]
- Song, Q.; Chen, X.; Zhou, W.; Xie, X. Application of a Spiral Symmetric Stream Anaerobic Bioreactor for treating saline heparin sodium pharmaceutical wastewater: Reactor operating characteristics, organics degradation pathway and salt tolerance mechanism. Water Res. 2021, 205, 117671. [Google Scholar] [CrossRef]
- Hülsen, T.; Hsieh, K.; Batstone, D.J. Saline wastewater treatment with purple phototrophic bacteria. Water Res. 2019, 160, 259–267. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, C.; Liu, X.; Sun, D.; Li, P.; Qiu, B.; Dang, Y.; Karpinski, N.A.; Smith, J.A.; Holmes, D.E. Magnetite enhances anaerobic digestion of high salinity organic wastewater. Environ. Res. 2020, 189, 109884. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Li, W.; Wang, H.; Wang, Y. Enhancing bioelectrochemical processes in anaerobic membrane bioreactors for municipal wastewater treatment: A comprehensive review. Chem. Eng. J. 2024, 484, 149420. [Google Scholar] [CrossRef]
- Huang, Z.; He, X.; Nye, C.; Bagley, D.; Urynowicz, M.; Fan, M. Effective anaerobic treatment of produced water from petroleum production using an anaerobic digestion inoculum from a brewery wastewater treatment facility. J. Hazard. Mater. 2021, 407, 124348. [Google Scholar] [CrossRef]
- Dorji, U.; Dorji, P.; Shon, H.; Badeti, U.; Dorji, C.; Wangmo, C.; Tijing, L.; Kandasamy, J.; Vigneswaran, S.; Chanan, A.; et al. On-site domestic wastewater treatment system using shredded waste plastic bottles as biofilter media: Pilot-scale study on effluent standards in Bhutan. Chemosphere 2022, 286, 131729. [Google Scholar] [CrossRef]
- Pollice, A.; Laera, G.; Cassano, D.; Diomede, S.; Pinto, A.; Lopez, A.; Mascolo, G. Removal of nalidixic acid and its degradation products by an integrated MBR-ozonation system. J. Hazard. Mater. 2012, 203, 46–52. [Google Scholar] [CrossRef]
- Guo, L.; Xie, Y.; Xu, Y.; Zhou, J.; Sun, W.; Sun, Y. Pilot-scale study and biochemical verification of salt-tolerant catalyst Fe-Bi@γ-Al2O3 for catalytic ozonation of high-salinity wastewater. J. Environ. Chem. Eng. 2023, 11, 110031. [Google Scholar] [CrossRef]
- Chai, Y.; Qin, P.; Wu, Z.; Bai, M.; Li, W.; Pan, J.; Cao, R.; Chen, A.; Jin, D.; Peng, C. A coupled system of flow-through electro-Fenton and electrosorption processes for the efficient treatment of high-salinity organic wastewater. Sep. Purif. Technol. 2021, 267, 118683. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Wu, J.; Wang, H. Hybrid electrocatalytic ozonation treatment of simulated high-salinity carbamazepine wastewater with Ni0.2–Ce0.2/OMC particle electrodes. Int. J. Electrochem. Sci. 2020, 15, 5698–5711. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Hu, T.; Li, L.; Huang, X.; Zhang, J. Flexible Solar Interfacial Evaporators with Photocatalytic Function for Purification of High-Salinity Organic Wastewater. Nanomaterials 2025, 15, 632. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Xu, H.; Yao, C.; Ma, J.; Wang, J.; Hu, T.; Zong, D.; Lin, T.; Ding, M. Membrane fouling alleviation by thermal activation of hydrogen peroxide-membrane distillation hybrid system: Insights into interfacial phenomena and NOM-ion interactions. Desalination 2025, 604, 118701. [Google Scholar] [CrossRef]
- Hsieh, I.M.; Lin, B.; Mahbub, H.; Carter, Z.; Jebur, M.; Cao, Y.; Brownlow, J.; Wickramasinghe, R.; Malmali, M. Field demonstration of intensified membrane distillation for treating oilfield produced waters from unconventional wells. Desalination 2023, 564, 116771. [Google Scholar] [CrossRef]
- Shu, J.; Li, Z.; Ge, R.; Ni, T.; Zhang, J.; Zhao, X.; Qi, M.; Zhang, J.; Xu, D. Characterization of underwater discharge of microporous air plasma jet and its effect on degradation of liquid-phase formaldehyde. Phys. Scr. 2025, 100, 055607. [Google Scholar] [CrossRef]
- Fan, G.; Huang, J.; Jiang, X.; Meng, W.; Yang, R.; Guo, J.; Fang, F.; Yang, J. Microalgae biofilm photobioreactor and its combined process for long-term stable treatment of high-saline wastewater achieved high pollutant removal efficiency. J. Environ. Chem. Eng. 2023, 11, 111473. [Google Scholar] [CrossRef]
| Wastewater Type | Total Dissolved Solids (TDS) | COD Range (mg/L) | pH Range | Key Organic Pollutants |
|---|---|---|---|---|
| Textile | 2767–11,745 mg/L | 500–2000 | 8–10 | Dyes, additives, surfactants |
| Tannery | 1000–3500 mg/L | 2000–4000 | 7–9 | Proteins, lipids, hair and skin fragments |
| Pharmaceutical | 18,521–52,477 mg/L | 2000–6000 | 5–9 | Drug residues, organic solvents |
| Petrochemical | 6629–63,423 mg/L | 1000–4000 | 6–9 | Oils, benzenes |
| Circulating Cooling Water | >35,000 mg/L | 500–1000 | >9 | Corrosion inhibitors, biocides |
| Food Processing (Seafood) | 10,000–25,000 mg/L | 3000–10,000 | 6–8 | Proteins, fats, residual organic acids |
| Dyeing and Printing | 5000–15,000 mg/L | 1000–3000 | 9–11 | Reactive dyes, surfactants |
| Fertilizer/Pesticide Industry | 20,000–40,000 mg/L | 2000–5000 | 4–8 | Organic pesticide intermediates, phenols, acids |
| Wastewater Type | Wastewater Characteristics | Membrane Technology | Removal Performance | Reference |
|---|---|---|---|---|
| Fresh Olive Processing Wastewater | pH = 4.75, COD = 7250 mg/L, Cl− = 41,120 mg/L, Conductivity = 80.7 mS/cm | UF (Ultrafiltration) | COD removal rate of 66.0% | [15] |
| Textile Wastewater | Salinity = 60 g/L, Pressure = 600 kPa, T = 25 °C, CFV = 0.2 m/s | NF (Nanofiltration) | Organic removal up to 99.8% | [16] |
| Biologically Treated Textile WW | pH = 6.5–6.8, COD = 96–108 mg/L, Conductivity = 1850–2050 μs/cm | RO (Reverse Osmosis) | COD removal rate of 94.5% | [17] |
| Aniline Wastewater | Acid conc. = 1.25 mol/L, Base conc. = 0.85 mol/L | ED (Electrodialysis, Bipolar) | COD removal rate of 93.3% | [18] |
| Stable Saline Emulsion | Oil particle size = 300 nm, RO flux = 18.42 L/(m2·h) | RO (PAMAM-TMC Modified Membrane) | Oil and NaCl rejection rates above 98% and 88%, respectively | [19] |
| Coal Chemical Industrial Wastewater | NaCl up to 300 mM (TDS ≈ 18,000 mg/L) | RO + NF | Desalination rate > 70% for both NaCl and MgSO4 | [20] |
| Technology Type | Organic Removal Efficiency | Actual Wastewater Type | Salinity (mg/L) | Catalyst/Core Conditions | Reference |
|---|---|---|---|---|---|
| Catalytic Ozonation (O3/Cat) | COD removal rate: 83.9% | High-salinity organic wastewater (simulated) | 9110 | Fe-Bi@γ-Al2O3, pH = 11, O3 flow rate = 0.2 L/min | [26] |
| Photo-Fenton Process | SMZ removal >80% | High-salinity agricultural wastewater | 5000 | Fe(III)/EDDS = 1:1, pH = 7, solar irradiation | [27] |
| Electrochemical Oxidation | COD removal rate: 93.7% | High-salinity petrochemical wastewater | 1490 | Ni-Ce/OMC/GAC particle electrode, Cl− = 6000 mg/L | [28] |
| Photocatalytic Oxidation | BPF removal >78% | BPF-containing brine (NaCl = 500 mM) | 29,200 | Porphyrin Zr-MOF (PCN-223), visible light system | [29] |
| Ozone Oxidation | COD removal rate ≈ 70% | High-salinity pharmaceutical wastewater | 17,900 | Iron-based monolithic catalytic filler | [30] |
| Fenton Oxidation | COD removal rate >85% | High-salinity seafood processing wastewater | 25,000 | Heterogeneous Fenton bed | [31] |
| Wastewater Type | Process Type | Salinity (g/L) | COD Removal Rate (%) | Reference |
|---|---|---|---|---|
| Canned Fish Wastewater | SBR + Anaerobic Granular Sludge (AGS) | 20 | 80–90 | [52] |
| Tannery Wastewater | Upflow Anaerobic Sludge Bed (UASB) | 71 | 78 | [53] |
| Phenol-containing Wastewater | UASB | ≤26 | 47 | [54] |
| Synthetic High-Salinity Wastewater | Halanaerobium lacusrosei Reactor | 30 | 84 | [55] |
| Heparin Sodium Pharmaceutical Wastewater | Spiral Symmetry Stream Anaerobic Bioreactor (SSSAB) | 35.7 | 82 | [56] |
| High-Salinity Domestic Wastewater | Purple Phototrophic Bacteria (under seawater conditions) | Seawater | 86 | [57] |
| Simulated High-Salinity Wastewater | UASB + Magnetite Conductive Material | 23.4 | 78.2 | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Gao, Y.; Shah, K.J.; Sun, Y. Recent Advances in Organic Pollutant Removal Technologies for High-Salinity Wastewater. Water 2025, 17, 2494. https://doi.org/10.3390/w17162494
Dai J, Gao Y, Shah KJ, Sun Y. Recent Advances in Organic Pollutant Removal Technologies for High-Salinity Wastewater. Water. 2025; 17(16):2494. https://doi.org/10.3390/w17162494
Chicago/Turabian StyleDai, Jun, Yun Gao, Kinjal J. Shah, and Yongjun Sun. 2025. "Recent Advances in Organic Pollutant Removal Technologies for High-Salinity Wastewater" Water 17, no. 16: 2494. https://doi.org/10.3390/w17162494
APA StyleDai, J., Gao, Y., Shah, K. J., & Sun, Y. (2025). Recent Advances in Organic Pollutant Removal Technologies for High-Salinity Wastewater. Water, 17(16), 2494. https://doi.org/10.3390/w17162494








