The Role of Abundant Organic Macroaggregates in Planktonic Metabolism in a Tropical Bay
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirano, M.; Lessa, G.C. Oceanographic Characteristics of Baía de Todos Os Santos, Brazil. Rev. Bras. Geofísica 2007, 25, 363–387. [Google Scholar] [CrossRef]
- Oliveira, W.P.; Lessa, G.C. Suspended Macro-Aggregates of a Tropical Estuary in Northeast Brazil: Composition and Settling Velocities. Geo-Mar. Lett. 2020, 40, 821–828. [Google Scholar] [CrossRef]
- Passow, U.; Ziervogel, K.; Asper, V.; Diercks, A. Marine Snow Formation in the Aftermath of the Deepwater Horizon Oil Spill in the Gulf of Mexico. Environ. Res. Lett. 2012, 7, 035301. [Google Scholar] [CrossRef]
- Alldredge, A.L.; Passow, U.; Logan, B.E. The Abundance and Significance of a Class of Large, Transparent Organic Particles in the Ocean. Deep Sea Res. Part Oceanogr. Res. Pap. 1993, 40, 1131–1140. [Google Scholar] [CrossRef]
- Kaltenbock, E.; Herndl, G. Ecology of Amorphous Aggregations (Marine Snow) in the Northern Adriatic Sea. IV. Dissolved Nutrients and the Autotrophic Community Associated with Marine Snow. Mar. Ecol. Prog. Ser. 1992, 87, 147–159. [Google Scholar] [CrossRef]
- Aktan, Y.; Dede, A.; Ciftci, P. Mucilage Event Associated with Diatoms and Dinoflagellates in Sea of Marmara, Turkey. Harmful Algae News 2008, 36, 1–3. [Google Scholar]
- Lipsman, V.; Segev, E. Increased Nutrient Levels Enhance Bacterial Exopolysaccharides Production in the Context of Algae. Environ. Microbiol. Rep. 2025, 17, e70071. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Skoog, A. Abiotic Aggregation of Organic Matter in Coastal and Estuarine Waters: Cases in the Eastern Long Island Sound, USA. Water 2021, 13, 3077. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Skoog, A. Effects of Salinity on Abiotic Aggregation of Organic Matter and Subsequent Microbial Responses. Gels 2022, 8, 836. [Google Scholar] [CrossRef]
- Lessa, G.C.; Souza, M.F.L.; Mafalda Jr, P.O.; Gomes, D.F.; Souza, C.S.; Teixeira, C.E.P.; Souza, J.L.B.; Zucchi, M.R. Variabilidade Intra-Anual Da Oceanografia Da Baía de Todos Os Santos: Evidências de Três Anos de Monitoramento. In Baía de Todos os Santos: Avanços nos Estudos de Longo Prazo; EDUFBA: Salvador, Brazil, 2018; Volume 1, pp. 155–192. [Google Scholar]
- Alldredge, A.L.; Silver, M.W. Characteristics, Dynamics and Significance of Marine Snow. Prog. Oceanogr. 1988, 20, 41–82. [Google Scholar] [CrossRef]
- Lyons, M.M.; Dobbs, F.C. Differential Utilization of Carbon Substrates by Aggregate-Associated and Water-Associated Heterotrophic Bacterial Communities. Hydrobiologia 2012, 686, 181–193. [Google Scholar] [CrossRef]
- Alldredge, A.L.; Cohen, Y. Can Microscale Chemical Patches Persist in the Sea? Microelectrode Study of Marine Snow, Fecal Pellets. Science 1987, 235, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Shanks, A.; Reeder, M. Reducing Microzones and Sulfide Production in Marine Snow. Mar. Ecol. Prog. Ser. 1993, 96, 43–47. [Google Scholar] [CrossRef]
- Paerl, H.W.; Pinckney, J.L. A Mini-Review of Microbial Consortia: Their Roles in Aquatic Production and Biogeochemical Cycling. Microb. Ecol. 1996, 31, 225–247. [Google Scholar] [CrossRef]
- Wang, T.; Li, J.; Xu, Y.; Zou, T.; Qin, S. Aggregating Synechococcus Contributes to Particle Organic Carbon Export in Coastal Estuarine Waters: Its Lineage Features and Assembly Processes. Sci. Total Environ. 2024, 917, 170368. [Google Scholar] [CrossRef]
- Lan, J.; Liu, P.; Hu, X.; Zhu, S. Harmful Algal Blooms in Eutrophic Marine Environments: Causes, Monitoring, and Treatment. Water 2024, 16, 2525. [Google Scholar] [CrossRef]
- Affe, H.M.d.J.; Conceição, L.P.; Rocha, D.S.B.; Proença, L.A.d.O.; Nunes, J.M.d.C. Phytoplankton Community in a Tropical Estuarine Gradient after an Exceptional Harmful Bloom of Akashiwo Sanguinea (Dinophyceae) in the Todos Os Santos Bay. Ocean Coast. Res. 2021, 69, e21008. [Google Scholar] [CrossRef]
- Amorim Reis-Filho, J.; da Silva, E.M.; de Anchieta Cintra da Costa Nunes, J.; Barros, F. Effects of a Red Tide on the Structure of Estuarine Fish Assemblages in Northeastern Brazil. Int. Rev. Hydrobiol. 2012, 97, 389–404. [Google Scholar] [CrossRef]
- Zeldis, J.R.; Currie, K.I.; Graham, S.L.; Gall, M.P. Attributing Controlling Factors of Acidification and Hypoxia in a Deep, Nutrient-Enriched Estuarine Embayment. Front. Mar. Sci. 2022, 8, 803439. [Google Scholar] [CrossRef]
- Gobler, C.J.; Baumann, H. Hypoxia and Acidification in Ocean Ecosystems: Coupled Dynamics and Effects on Marine Life. Biol. Lett. 2016, 12, 20150976. [Google Scholar] [CrossRef]
- Cao, L.; Caldeira, K.; Jain, A.K. Effects of Carbon Dioxide and Climate Change on Ocean Acidification and Carbonate Mineral Saturation. Geophys. Res. Lett. 2007, 34, 2006GL028605. [Google Scholar] [CrossRef]
- Üçok, D.; Tosun, Ş.Y.; Erkan, N.; Can Tunçelli, İ.; Doğruyol, H.; Ulusoy, Ş.; Mol, S.; Özden, Ö.; Dagsuyu, E.; Yanardag, R. Microbial Seafood Safety Assessment Following a Marine Mucilage Disaster in the Sea of Marmara. Environ. Microbiol. Rep. 2025, 17, e70050. [Google Scholar] [CrossRef] [PubMed]
- Roth, F.; Lessa, G.C.; Wild, C.; Kikuchi, R.K.P.; Naumann, M.S. Impacts of a High-Discharge Submarine Sewage Outfall on Water Quality in the Coastal Zone of Salvador (Bahia, Brazil). Mar. Pollut. Bull. 2016, 106, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Pelaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Lessa, G.C.; Mariani, R.; Fonseca, L. Variability of the Thermohaline Field in a Large Tropical, Well-Mixed Estuary: The Influence of an Extreme Draught Event. Estuaries Coasts 2019, 42, 2020–2037. [Google Scholar] [CrossRef]
- Guenther, M.; Gonzalez-Rodriguez, E.; Flores-Montes, M.; Araújo, M.; Neumann-Leitão, S. High Bacterial Carbon Demand and Low Growth Efficiency at a Tropical Hypereutrophic Estuary: Importance of Dissolved Organic Matter Remineralization. Braz. J. Oceanogr. 2017, 65, 382–391. [Google Scholar] [CrossRef]
- Agusti, S.; Vigoya, L.; Duarte, C.M. Annual Plankton Community Metabolism in Estuarine and Coastal Waters in Perth (Western Australia). PeerJ 2018, 6, e5081. [Google Scholar] [CrossRef]
- McKinnon, A.D.; Duggan, S.; Logan, M.; Lønborg, C. Plankton Respiration, Production, and Trophic State in Tropical Coastal and Shelf Waters Adjacent to Northern Australia. Front. Mar. Sci. 2017, 4, 346. [Google Scholar] [CrossRef]
- Brandini, F.; Michelazzo, L.S.; Freitas, G.R.; Campos, G.; Chuqui, M.; Jovane, L. Carbon Flow for Plankton Metabolism of Saco Do Mamanguá Ría, Bay of Ilha Grande, a Subtropical Coastal Environment in the South Brazil Bight. Front. Mar. Sci. 2019, 6, 584. [Google Scholar] [CrossRef]
- Prichett, D.; Bonilla Pagan, J.M.; Hodgkins, C.L.S.; Testa, J.M. Controls on Water-Column Respiration Rates in a Coastal Plain Estuary: Insights from Long-Term Time-Series Measurements. Estuaries Coasts 2024, 47, 2542–2551. [Google Scholar] [CrossRef]
- Cloern, J.E.; Foster, S.Q.; Kleckner, A.E. Phytoplankton Primary Production in the World’s Estuarine-Coastal Ecosystems. Biogeosciences 2014, 11, 2477–2501. [Google Scholar] [CrossRef]
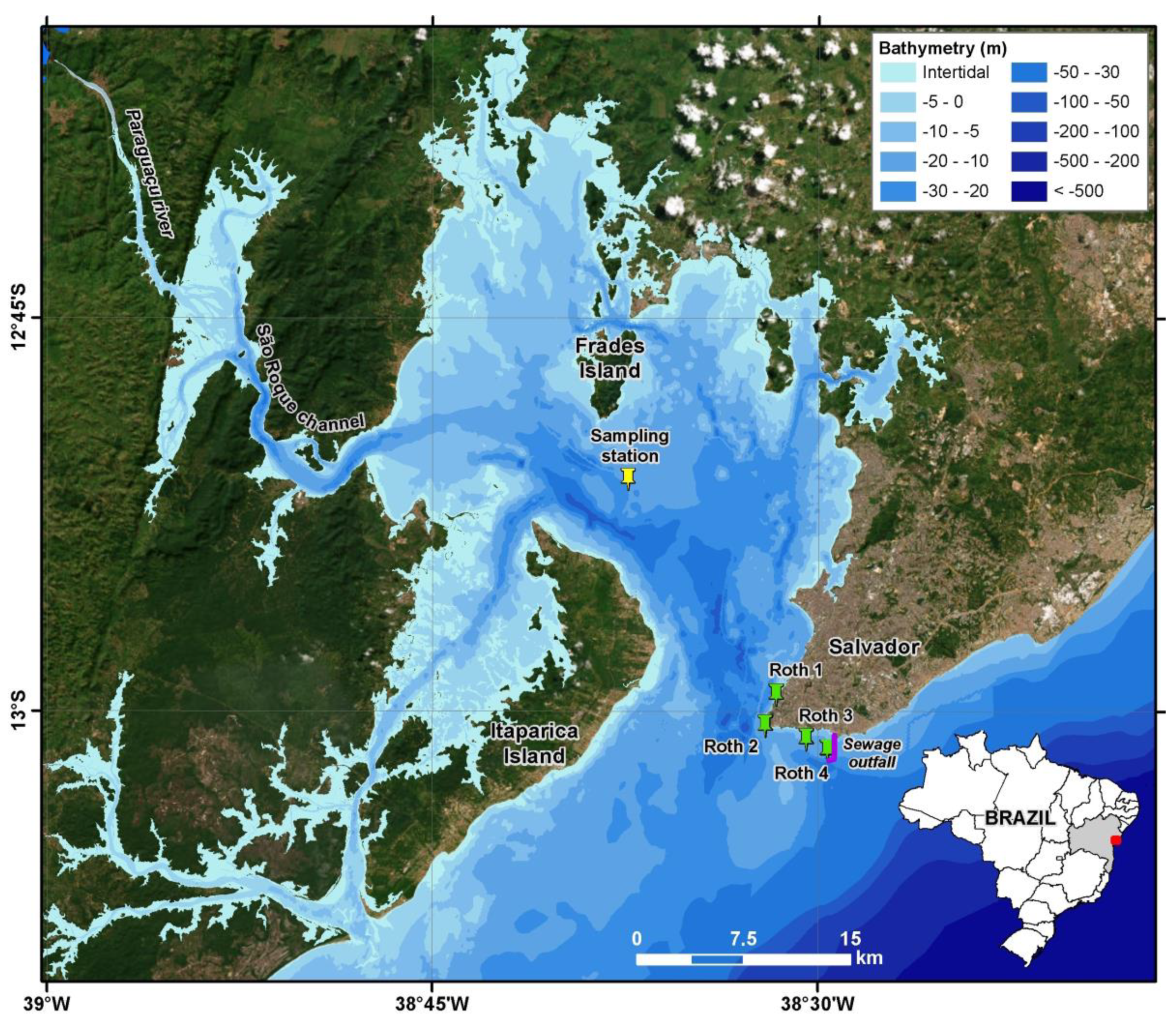
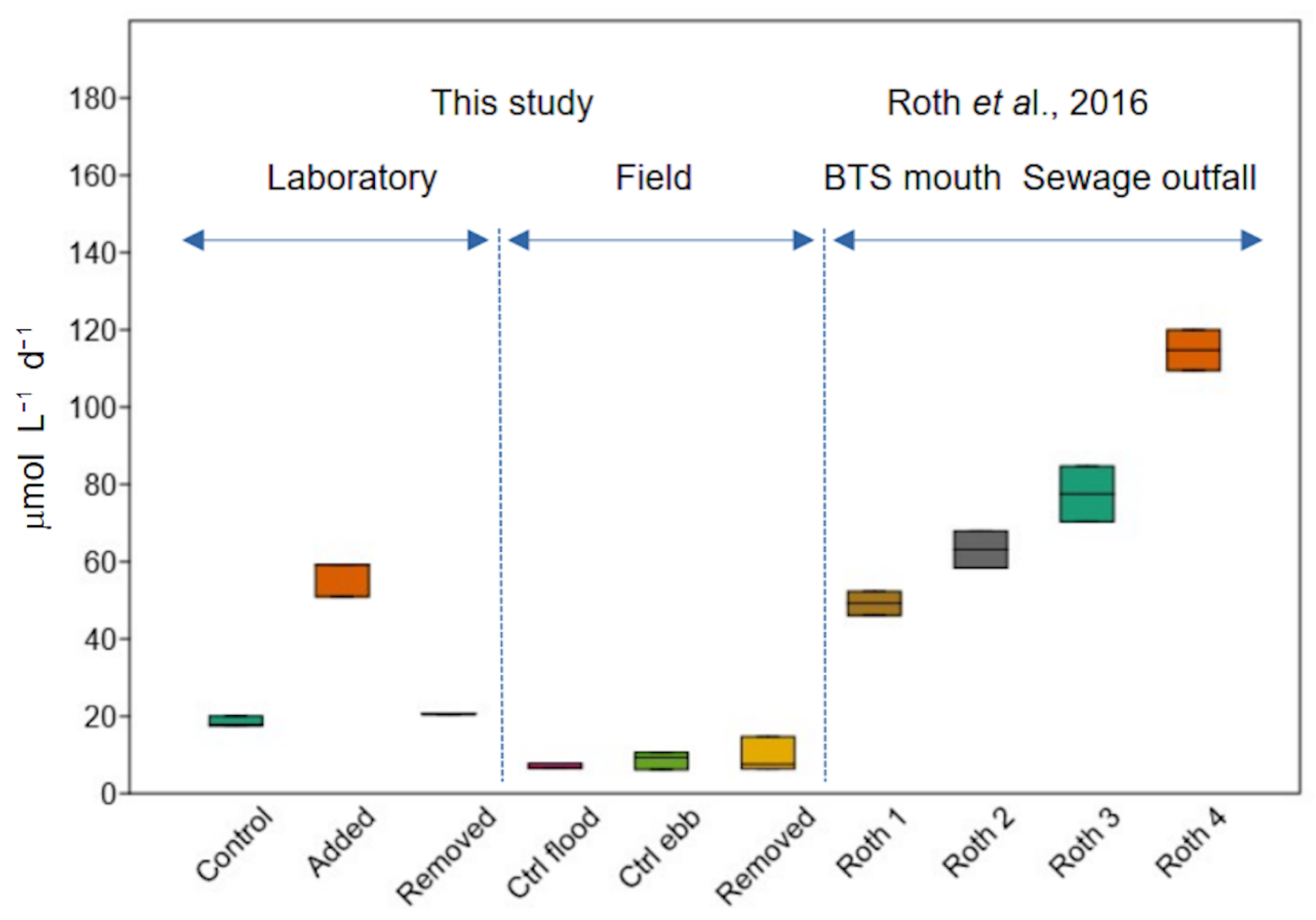
| Laboratory | Field | |||||
|---|---|---|---|---|---|---|
| Variable | Control | Added | Removed | Control Flood | Control Ebb | Ebb Removed |
| S | 31.8 | 31.8 | 31.8 | 36.9 | 34.4 | 34.4 |
| pH | 7.51 | 7.51 | 7.51 | 8.16 | 7.28 | 7.28 |
| CDOMi ppb | 18.2 | 19.9 | 19.9 | 6.2 | 9.7 | 9.7 |
| CDOMf ppb | 18.4 ± 1.1 | 20.4 ± 0.8 | 18.5 ± 0.3 | - | - | - |
| Ti °C | 22.3 ± 0.8 | 22.3 ± 0.8 | 22.3 ± 0.8 | 28.3 | 27.5 | 27.5 |
| Tf °C | 22.5 ± 0.1 | 22.5 ± 0.1 | 22.8 ± 0.5 | 26.4 ± 0.3 | 26.8 ± 0.2 | 26.8 ± 0.3 |
| DOi μM | 216 | 216 | 216 | 196 ± 0.0 | 199 ± 0.5 | 199 ± 0.0 |
| DOf μM | 211 ± 0.4 | 201 ± 1.3 | 211 ± 0.0 | 193 ± 0.6 | 194 ± 2.5 | 194 ± 1.1 |
| DOi% | 97.0 ± 0.0 | 97.0 ± 0.0 | 97.0 ± 0.0 | 97.1 ± 0.0 | 97.8 ± 0.0 | 97.9 ± 0.2 |
| DOf% | 93.6 ± 0.2 | 89.0 ± 0.6 | 93.9 ± 0.8 | 93.6 ± 0.3 | 93.9 ± 0.5 | 93.7 ± 1.6 |
| R | 18.4 ± 1.4 | 56.5 ± 4.8 | 20.6 ± 0.1 | 7.0 ± 0.7 | 8.7 ± 2.3 | 9.5 ± 4.5 |
| NPP | - | - | - | 8.9 ± 4.5 | - | - |
| GPP | - | - | - | 16.0 ± 9.6 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, M.F.L.d.; Lessa, G.C. The Role of Abundant Organic Macroaggregates in Planktonic Metabolism in a Tropical Bay. Water 2025, 17, 1967. https://doi.org/10.3390/w17131967
Souza MFLd, Lessa GC. The Role of Abundant Organic Macroaggregates in Planktonic Metabolism in a Tropical Bay. Water. 2025; 17(13):1967. https://doi.org/10.3390/w17131967
Chicago/Turabian StyleSouza, Marcelo Friederichs Landim de, and Guilherme Camargo Lessa. 2025. "The Role of Abundant Organic Macroaggregates in Planktonic Metabolism in a Tropical Bay" Water 17, no. 13: 1967. https://doi.org/10.3390/w17131967
APA StyleSouza, M. F. L. d., & Lessa, G. C. (2025). The Role of Abundant Organic Macroaggregates in Planktonic Metabolism in a Tropical Bay. Water, 17(13), 1967. https://doi.org/10.3390/w17131967






