Hydrogeochemical Characterization and Determination of Arsenic Sources in the Groundwater of the Alluvial Plain of the Lower Sakarya River Basin, Turkey
Abstract
1. Introduction
2. Study Area
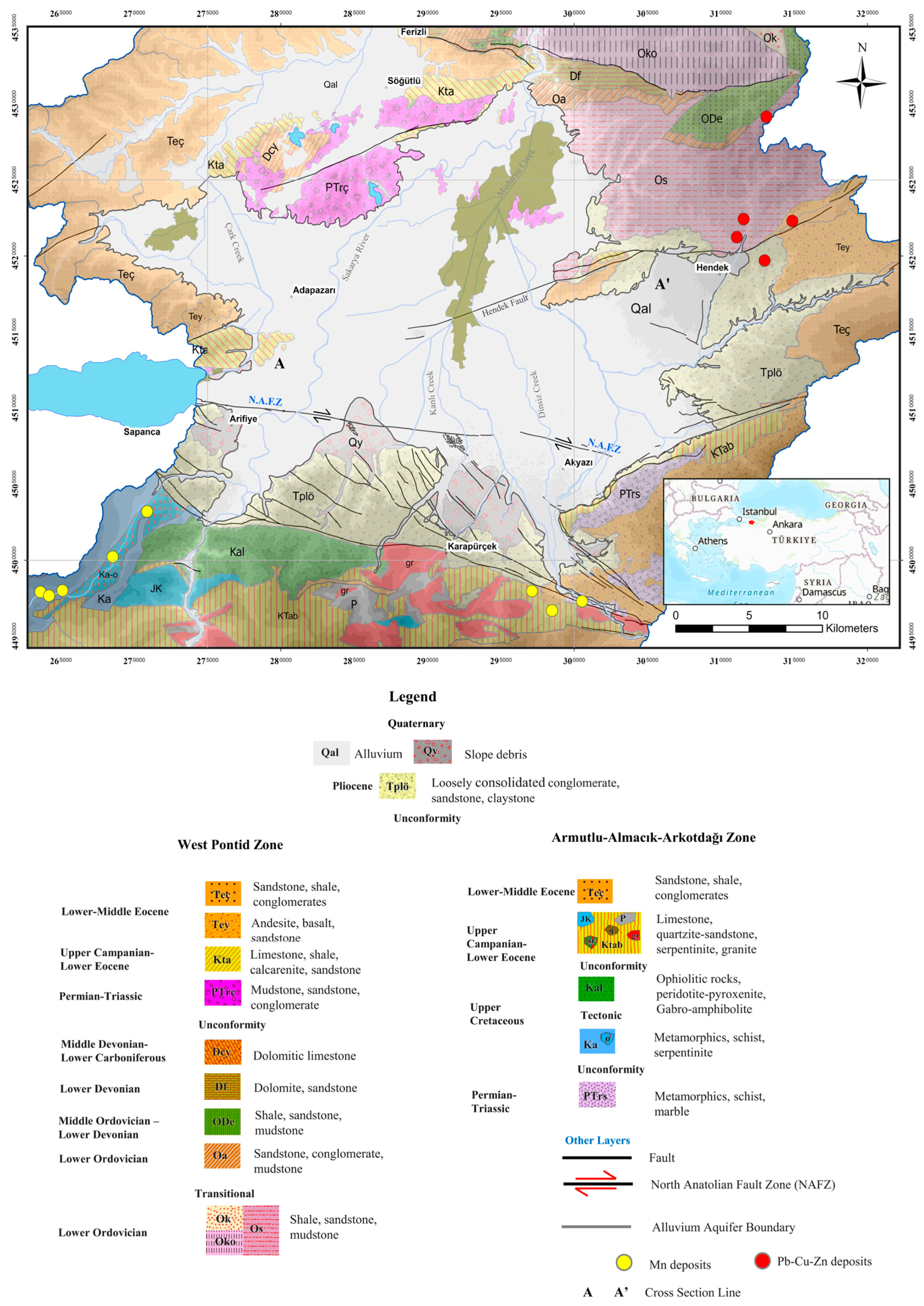
3. Geological Settings
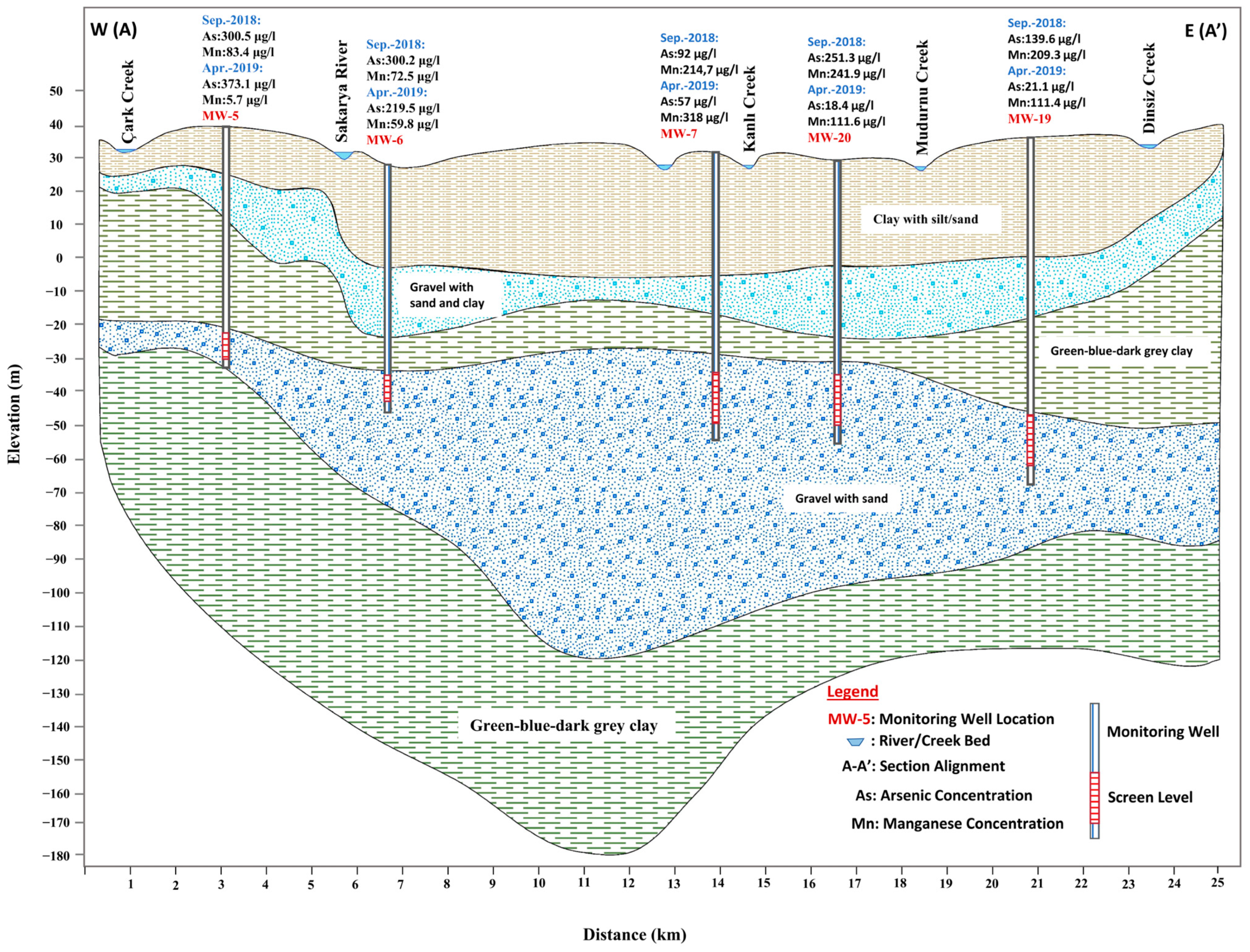
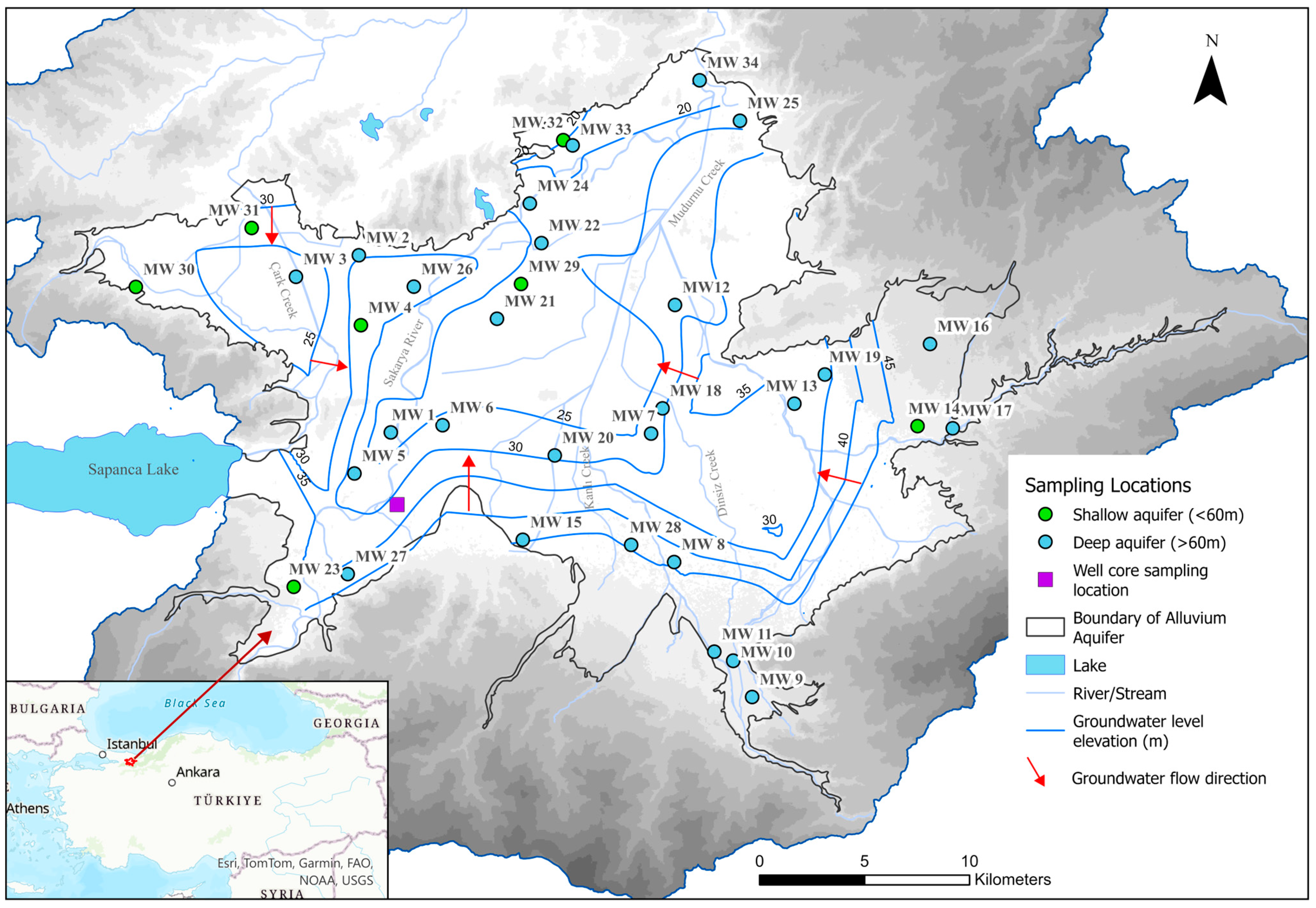
4. Hydrology and Hydrogeology
5. Materials and Methods
6. Results and Discussion
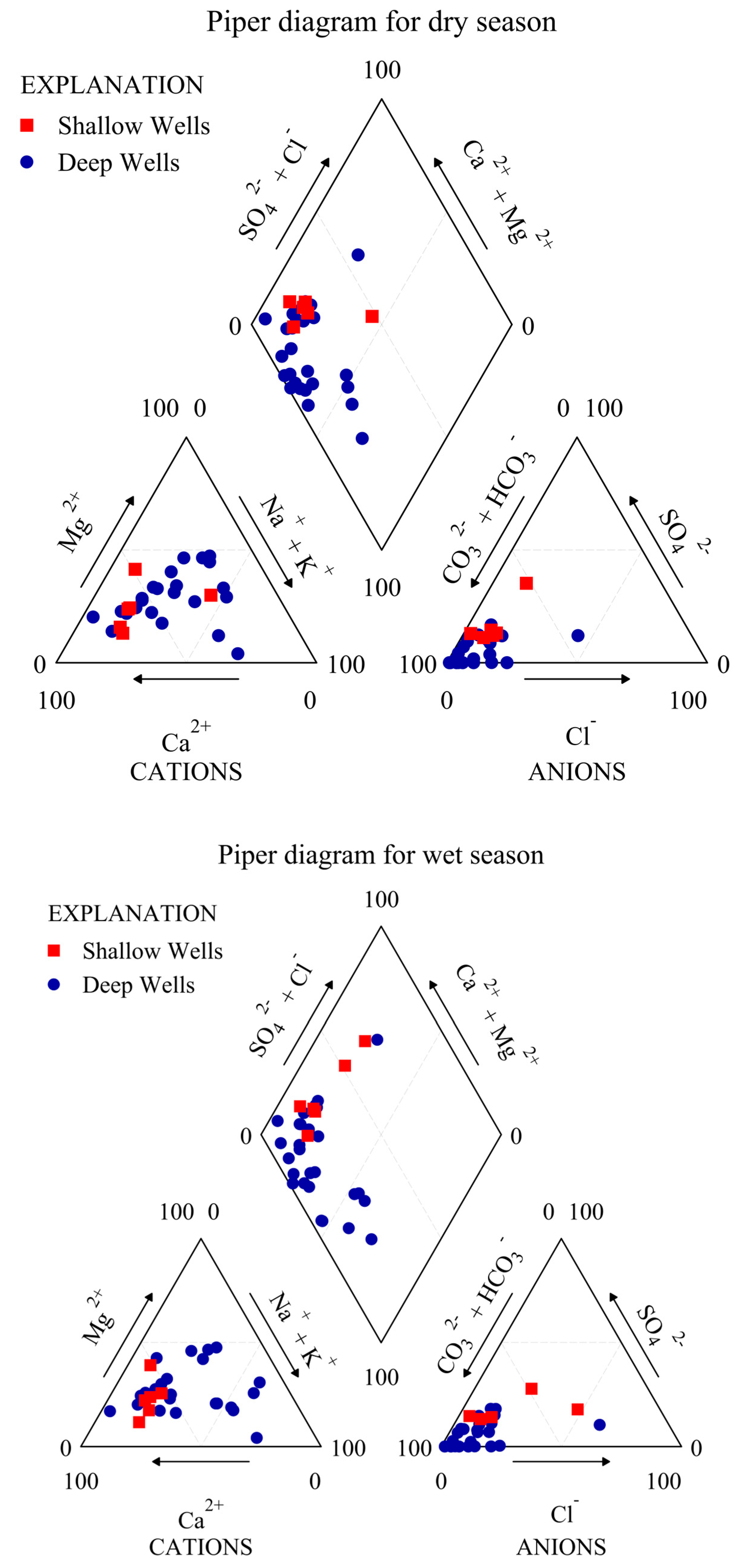
6.1. Assessment of Hydrogeochemical Characteristics and Groundwater Quality of Alluvium Aquifer
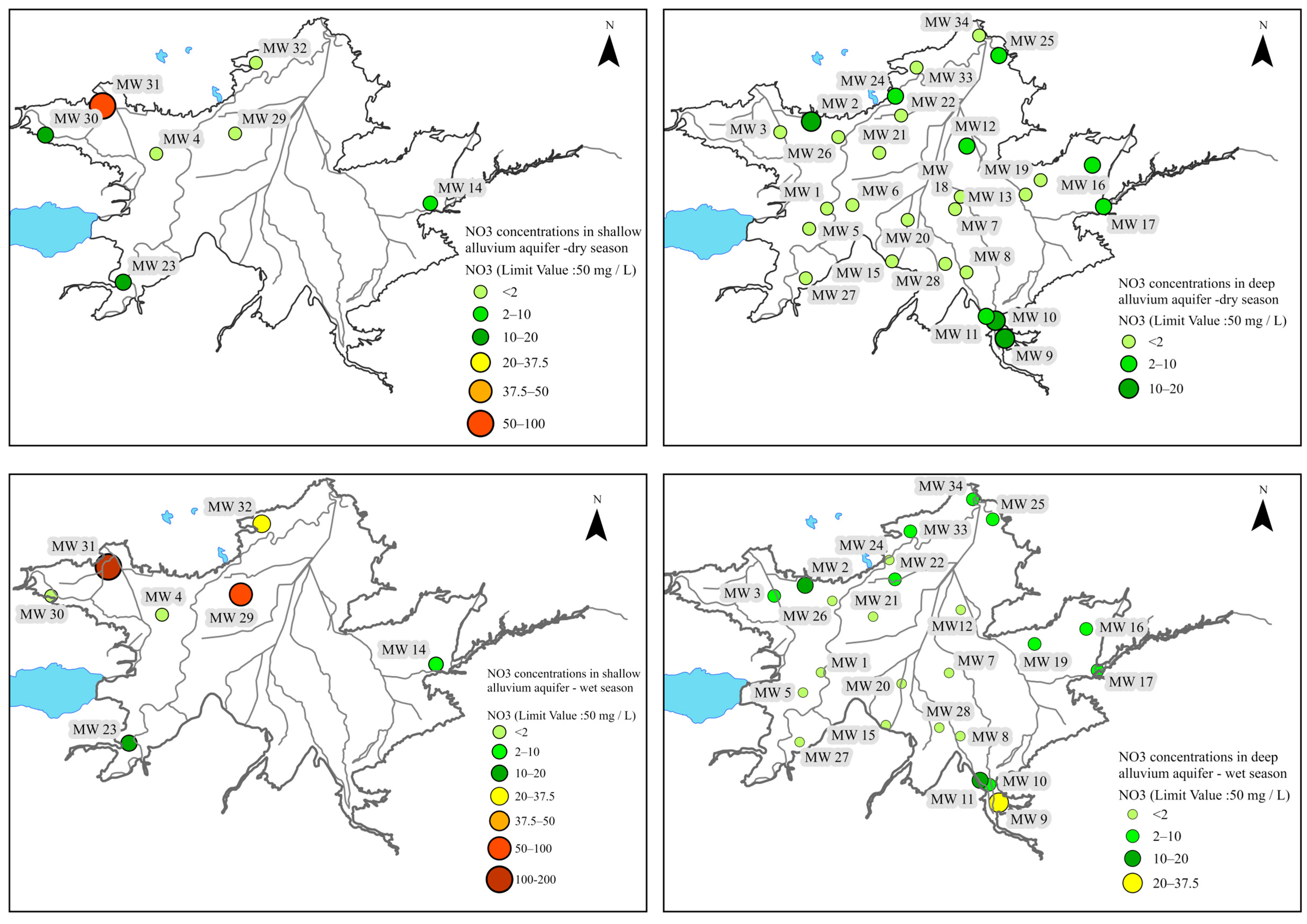
6.1.1. Shallow Alluvium Aquifer
6.1.2. Deep Alluvium Aquifer
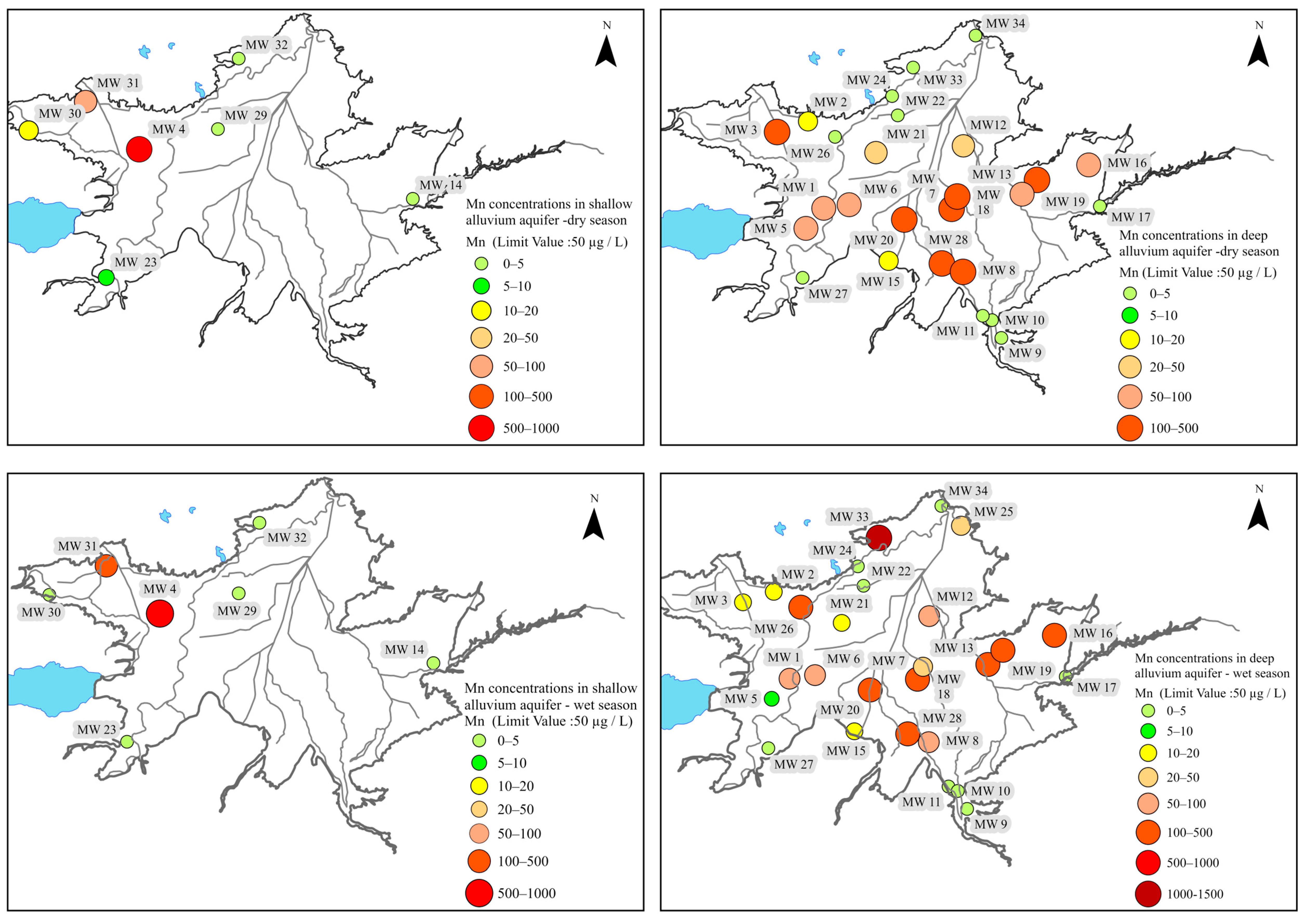
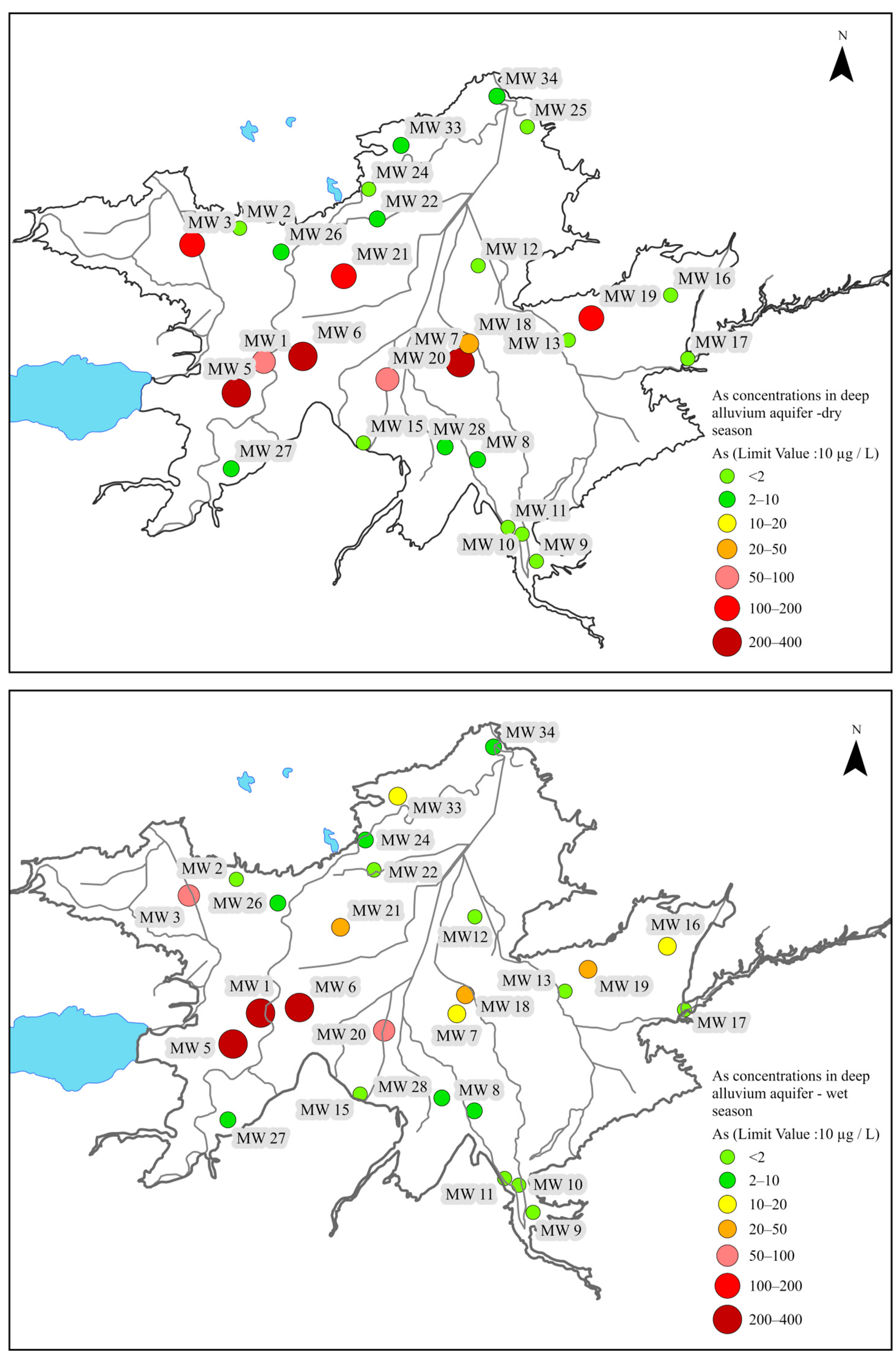
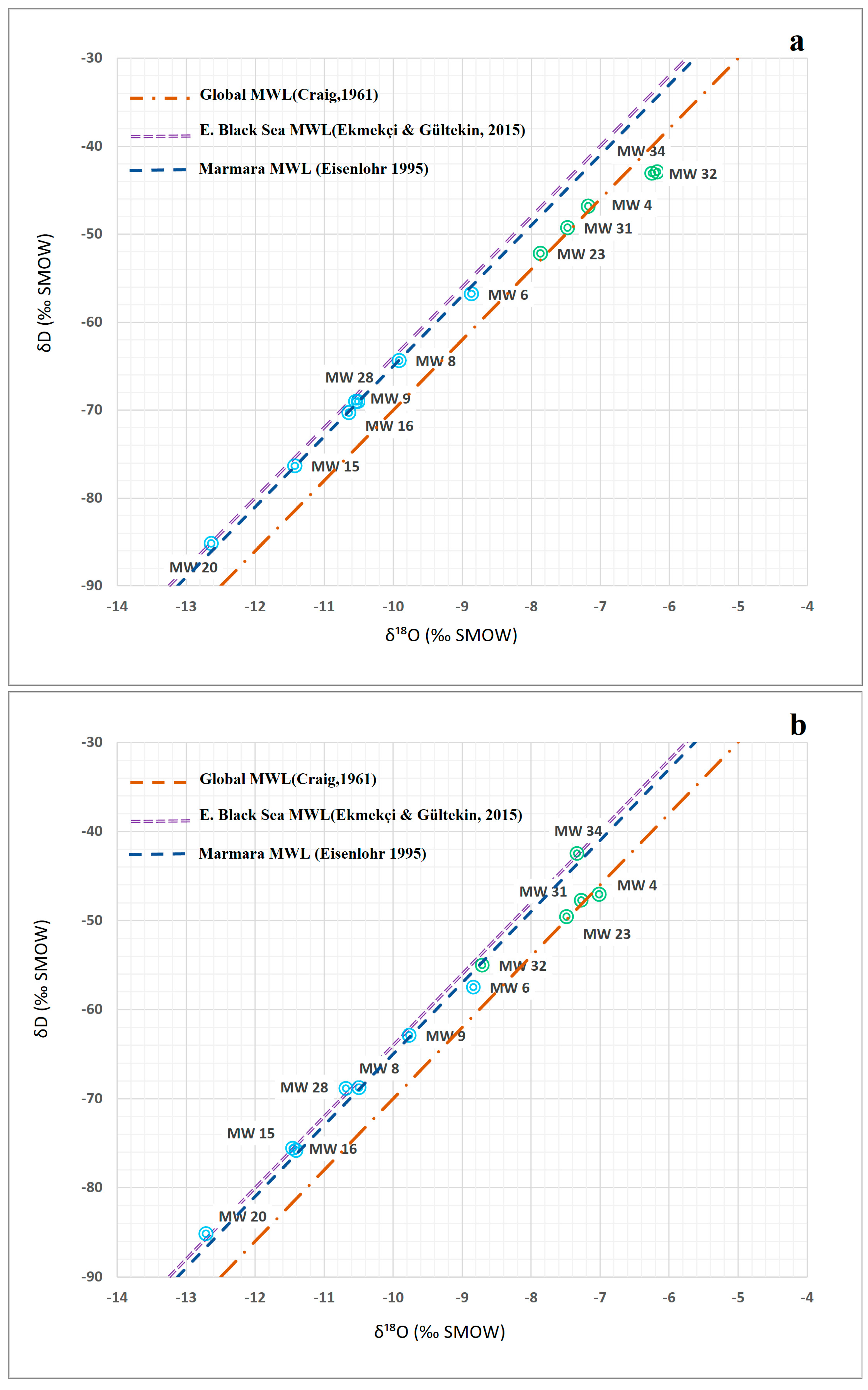
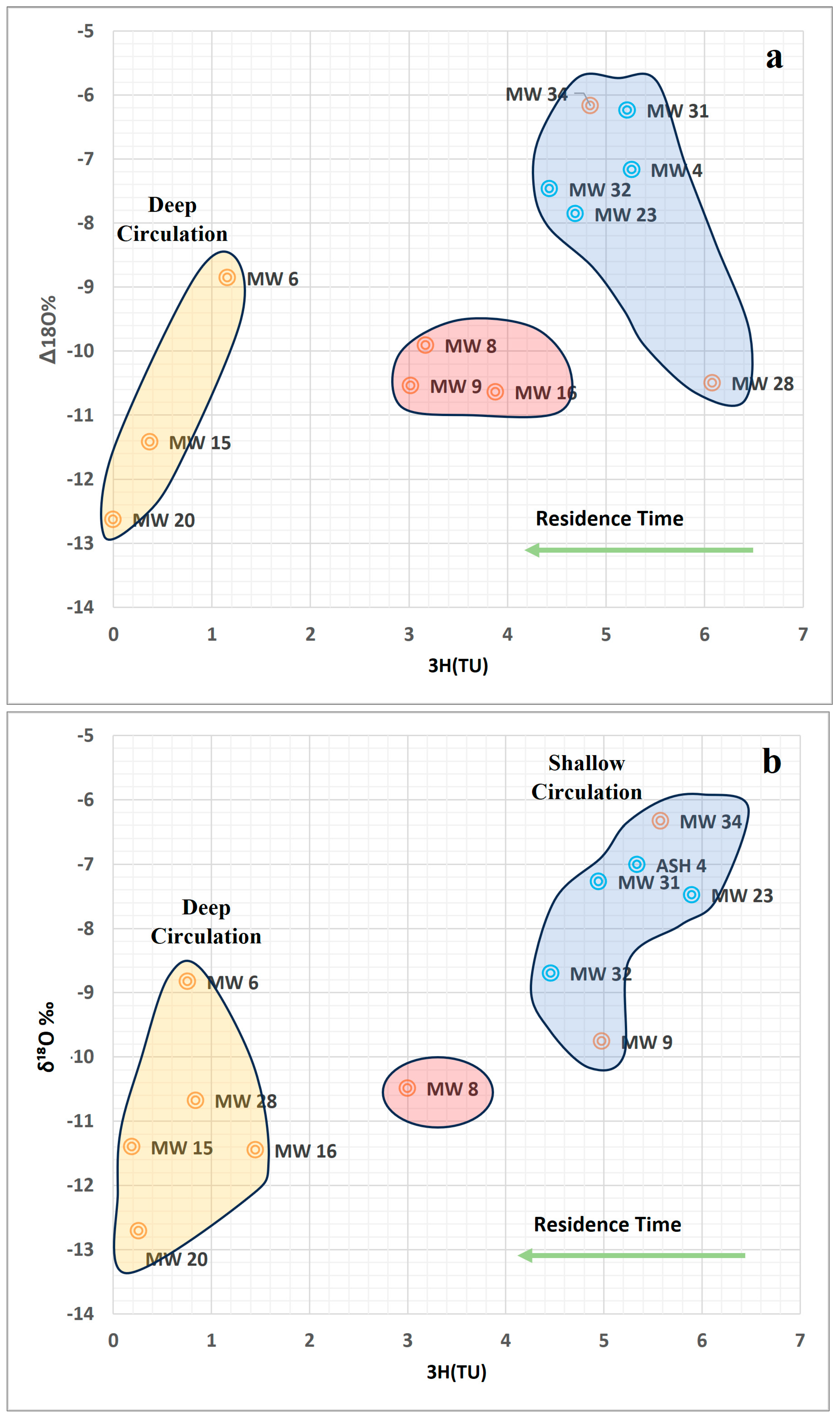
6.2. Assessment of Isotopic Characteristics of Alluvium Aquifer
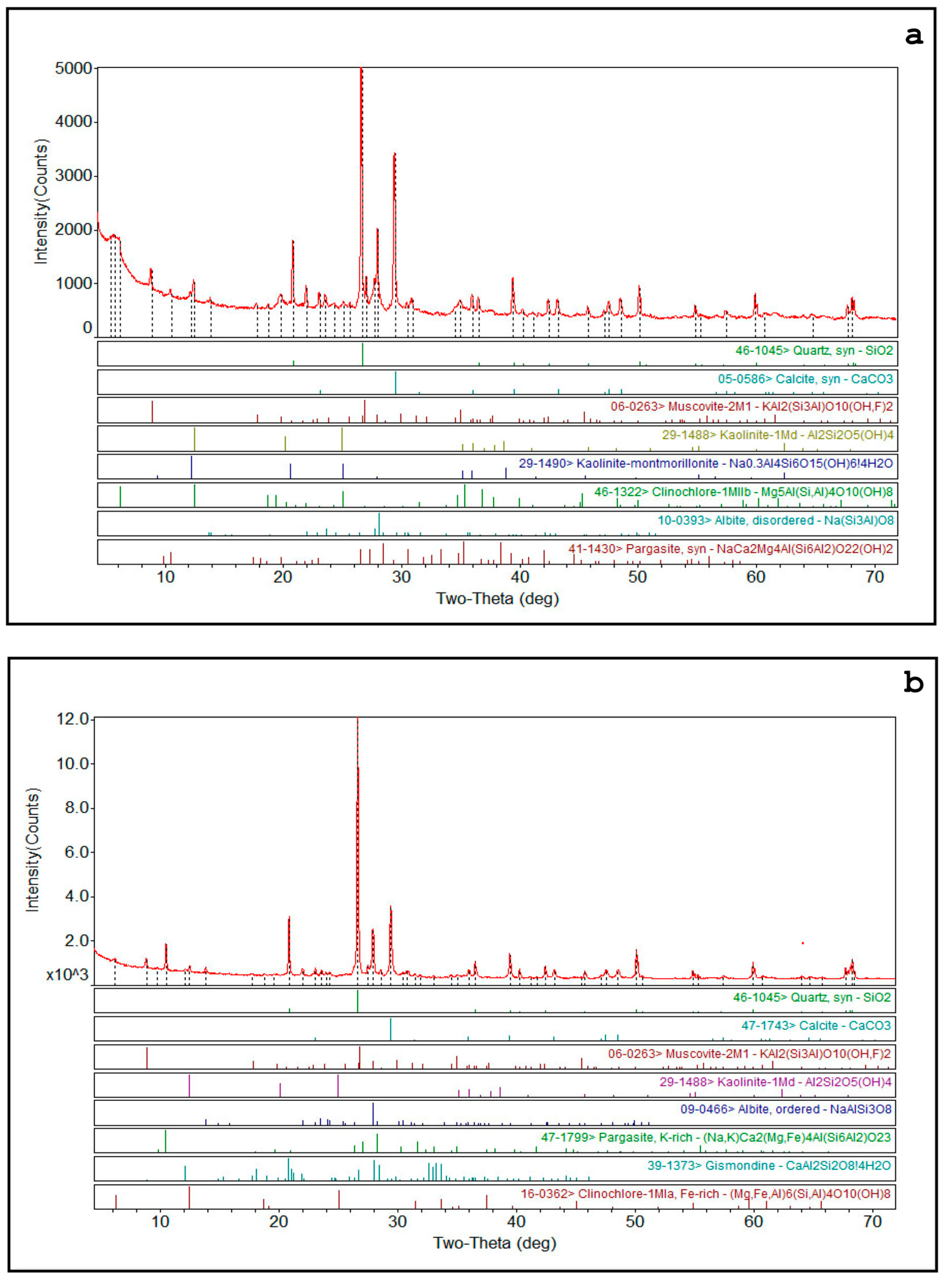
6.3. Mineralogical and Geochemical Characterization of Alluvial Sediments
6.4. Statistical Analysis of Groundwater Chemistry Data
6.5. As and Mn Sources and Release Mechanism in Alluvium Aquifer
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample No | pH | DO | EC | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | F− | NO3− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | µS/cm at 25 °C | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/l | mg/L | mg/L | ||
| MW 4 | 7.17 | 1.29 | 1590.40 | 48.71 | 19.24 | 104.38 | 46.59 | 30.76 | 81.32 | 456.21 | 0.37 | 0.11 |
| MW 14 | 8.20 | 10.43 | 173.74 | 5.81 | 0.16 | 20.80 | 2.95 | 1.39 | 9.17 | 73.86 | <0.15 | 2.20 |
| MW 23 | 7.30 | 7.68 | 924.00 | 17.48 | 2.98 | 85.01 | 43.67 | 25.05 | 44.13 | 401.55 | 0.18 | 10.17 |
| MW 29 | 8.09 | 10.39 | 246.40 | 8.63 | 1.37 | 31.67 | 7.56 | 10.95 | 15.70 | 110.63 | 0.15 | 0.31 |
| MW 30 | 7.89 | 5.02 | 772.70 | 26.91 | 1.80 | 86.83 | 10.16 | 22.00 | 44.50 | 294.29 | 0.16 | 15.01 |
| MW 31 | 7.22 | 4.67 | 2258.00 | 189.28 | 0.31 | 94.89 | 67.41 | 73.09 | 270.10 | 503.63 | 1.01 | 77.40 |
| MW 32 | 7.16 | 1.40 | 460.00 | 9.02 | 1.44 | 31.69 | 7.86 | 10.44 | 14.90 | 116.46 | 0.12 | 0.41 |
| Min | 7.16 | 1.29 | 173.74 | 5.81 | 0.16 | 20.80 | 2.95 | 1.39 | 9.17 | 73.86 | 0.12 | 0.11 |
| Max | 8.20 | 10.43 | 2258.00 | 189.28 | 19.24 | 104.38 | 67.41 | 73.09 | 270.10 | 503.63 | 1.01 | 77.43 |
| Mean | 7.58 | 7.13 | 917.89 | 43.69 | 3.90 | 65.04 | 26.60 | 24.81 | 68.55 | 279.52 | 0.33 | 15.09 |
| STDV | 0.43 | 3.32 | 705.45 | 61.01 | 6.32 | 32.72 | 23.60 | 21.78 | 85.49 | 166.46 | 0.31 | 26.02 |
| Sample No | pH | DO | EC | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | F− | NO3− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | µS/cm at 25 °C | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/l | mg/L | mg/L | ||
| MW 4 | 7.21 | 2.56 | 1285.20 | 33.53 | 15.75 | 107.79 | 33.32 | 43.61 | 59.65 | 559.80 | 0.34 | 0.56 |
| MW 14 | 8.36 | 11.58 | 227.36 | 4.93 | 0.13 | 13.96 | 2.35 | 2.49 | 11.26 | 76.80 | 0.13 | 3.42 |
| MW 23 | 7.57 | 2.86 | 952.00 | 14.16 | 2.38 | 74.14 | 34.17 | 29.20 | 53.62 | 393.57 | 0.10 | 11.34 |
| MW 29 | 8.03 | 9.08 | 435.30 | 6.94 | 1.27 | 26.76 | 5.80 | 13.51 | 18.75 | 118.89 | 0.18 | 83.93 |
| MW 30 | 8.15 | 10.71 | 323.12 | 7.06 | 1.26 | 23.67 | 5.76 | 13.59 | 18.78 | 119.09 | 0.20 | 1.01 |
| MW 31 | 6.78 | 4.44 | 3290.22 | 106.79 | 12.79 | 256.71 | 74.32 | 580.84 | 294.12 | 714.00 | 0.74 | 196.71 |
| MW 32 | 8.05 | 1.88 | 871.60 | 28.50 | 1.19 | 96.78 | 9.85 | 46.36 | 73.85 | 161.42 | 0.25 | 32.53 |
| Min | 6.78 | 1.88 | 227.36 | 4.93 | 0.13 | 13.96 | 2.35 | 2.49 | 11.26 | 76.80 | 0.10 | 0.56 |
| Max | 8.36 | 11.58 | 3290.22 | 106.79 | 15.75 | 256.71 | 74.32 | 580.84 | 294.12 | 714.00 | 0.74 | 196.71 |
| Mean | 7.74 | 6.16 | 1054.97 | 28.84 | 4.97 | 85.69 | 23.65 | 104.23 | 75.72 | 306.22 | 0.28 | 47.07 |
| STDV | 0.53 | 3.85 | 978.20 | 33.46 | 5.97 | 77.88 | 24.07 | 195.16 | 91.88 | 233.58 | 0.20 | 67.00 |
| Sample No | Al | As | Ba | Cd | Co | Cr | Cu | Fe | Mn | Mo | Ni | Pb | Sb | Se | Sn | U | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | |
| MW 4 | 5.84 | 1.08 | 238.56 | <0.01 | 0.49 | 4.71 | 0.11 | <5 | 783.27 | 4.37 | 9.91 | <0.01 | 0.10 | 0.44 | <0.01 | 3.20 | 1.20 | 3.28 |
| MW 14 | 2.23 | 0.54 | 19.97 | <0.01 | 0.04 | 1.21 | <0.1 | <5 | 0.05 | 0.38 | 0.84 | 0.09 | 0.09 | 0.30 | <0.01 | 0.11 | 5.38 | 18.51 |
| MW 23 | 6.95 | 0.84 | 29.46 | <0.01 | 0.27 | 2.63 | 2.32 | <5 | 5.50 | 0.29 | 23.69 | 0.17 | 0.49 | 1.08 | <0.01 | 0.35 | 1.01 | 73.26 |
| MW 29 | 11.40 | 3.80 | 8.47 | 0.05 | 0.12 | 0.53 | 0.45 | <5 | 1.53 | 0.30 | 3.03 | 0.66 | 0.31 | 0.55 | <0.01 | 0.42 | 0.05 | 6.27 |
| MW 30 | 2.87 | 0.30 | 36.91 | 0.04 | 0.43 | 1.94 | 1.73 | <5 | 11.23 | 0.20 | 5.62 | 0.23 | 0.46 | 0.80 | 0.10 | 0.89 | 0.08 | 441.58 |
| MW 31 | 5.55 | 0.84 | 40.05 | <0.01 | 0.25 | 1.81 | 1.20 | <5 | 51.14 | 0.26 | 5.76 | 0.04 | 0.25 | 2.53 | 0.07 | 7.18 | 1.51 | 6.66 |
| MW 32 | 9.20 | 3.95 | 9.29 | 0.11 | 0.09 | 1.21 | 9.69 | <5 | 0.22 | 0.31 | 56.88 | 0.76 | 0.35 | 0.48 | 1.30 | 0.37 | 0.09 | 153.69 |
| Min | 2.23 | 0.30 | 8.47 | <0.01 | 0.04 | 0.53 | <0.1 | <5 | 0.05 | 0.20 | 0.84 | <0.01 | 0.09 | 0.30 | <0.01 | 0.11 | 0.05 | 3.28 |
| Max | 11.40 | 3.95 | 238.56 | 0.11 | 0.49 | 4.71 | 9.69 | <5 | 783.27 | 4.37 | 56.88 | 0.76 | 0.49 | 2.53 | 1.30 | 7.18 | 5.38 | 441.58 |
| Mean | 6.29 | 1.62 | 54.67 | 0.07 | 0.24 | 2.01 | 2.58 | - | 121.85 | 0.87 | 15.10 | 0.33 | 0.29 | 0.88 | 0.49 | 1.79 | 1.33 | 100.47 |
| STDV | 3.02 | 1.44 | 75.95 | 0.03 | 0.16 | 1.27 | 3.26 | - | 270.54 | 1.43 | 18.41 | 0.28 | 0.15 | 0.72 | 0.57 | 2.41 | 1.74 | 148.21 |
| Sample No | Al | As | Ba | Cd | Co | Cr | Cu | Fe | Mn | Mo | Ni | Pb | Sb | Se | Sn | U | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | |
| MW 4 | <0.1 | 1.03 | 218.31 | 0.05 | 0.54 | 2.69 | 1.66 | <1 | 588.59 | 3.03 | 9.97 | 0.05 | 0.72 | 0.21 | 0.40 | 2.76 | 1.06 | 19.95 |
| MW 14 | <0.1 | 0.53 | 18.18 | <0.001 | 0.05 | 1.81 | 0.40 | <1 | 0.01 | 0.35 | 1.90 | 0.04 | 0.75 | 0.19 | 0.02 | 0.09 | 4.74 | 8.83 |
| MW 23 | <0.1 | 0.91 | 26.58 | 0.01 | 0.24 | 7.17 | 0.96 | <1 | 1.81 | 0.22 | 25.26 | <0.01 | 0.85 | 0.98 | 0.16 | 0.27 | 1.40 | 132.18 |
| MW 29 | 19.27 | 2.90 | 9.85 | 0.51 | 0.28 | 2.20 | 1.10 | <1 | 0.46 | 0.27 | 2.90 | 7.06 | 0.52 | 0.48 | 0.11 | 0.32 | 0.18 | 13.70 |
| MW 30 | 43.81 | 3.16 | 7.48 | 0.01 | 0.09 | 2.02 | 1.14 | <1 | 0.14 | 0.29 | 2.71 | 0.03 | 0.85 | 0.71 | 0.05 | 0.41 | 0.18 | 4.03 |
| MW 31 | 0.88 | 9.83 | 110.14 | 0.16 | 1.10 | 23.65 | 4.07 | <1 | 471.03 | 0.71 | 28.66 | 0.02 | 0.85 | 4.23 | 0.18 | 16.14 | 7.58 | 24.08 |
| MW 32 | <0.1 | 0.47 | 46.94 | 0.01 | 0.30 | 2.06 | 0.33 | <1 | 0.97 | 0.11 | 8.12 | <0.01 | 0.43 | 1.04 | 0.03 | 0.96 | 0.86 | 64.56 |
| Min | <0.1 | 0.47 | 7.48 | <0.001 | 0.05 | 1.81 | 0.33 | <1 | 0.01 | 0.11 | 1.90 | <0.01 | 0.43 | 0.19 | 0.02 | 0.09 | 0.18 | 4.03 |
| Max | 43.81 | 9.83 | 218.31 | 0.51 | 1.10 | 23.65 | 4.07 | <1 | 588.59 | 3.03 | 28.66 | 7.06 | 0.85 | 4.23 | 0.40 | 16.14 | 7.58 | 132.18 |
| Mean | 21.32 | 2.69 | 62.50 | 0.12 | 0.37 | 5.94 | 1.38 | - | 151.86 | 0.71 | 11.36 | 1.44 | 0.71 | 1.12 | 0.13 | 2.99 | 2.29 | 38.19 |
| STDV | 17.59 | 3.09 | 71.56 | 0.18 | 0.33 | 7.44 | 1.18 | - | 241.09 | 0.96 | 10.29 | 2.81 | 0.16 | 1.31 | 0.12 | 5.44 | 2.60 | 42.58 |
| Sample No | pH | DO | EC | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | F− | NO3− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | µS/cm at 25 °C | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/l | mg/L | mg/L | ||
| MW 1 | 7.82 | 1.45 | 1292.20 | 126.09 | 3.91 | 43.88 | 38.89 | 78.87 | 0.20 | 450.25 | 0.36 | 0.21 |
| MW 2 | 6.27 | 4.68 | 647.50 | 26.15 | 1.49 | 48.16 | 12.48 | 70.89 | 26.09 | 118.07 | 0.25 | 13.25 |
| MW 3 | 7.03 | 1.46 | 1523.20 | 160.21 | 11.62 | 58.60 | 61.38 | 62.30 | 83.13 | 629.45 | 0.45 | 0.23 |
| MW 5 | 7.54 | 1.76 | 1705.20 | 142.86 | 5.18 | 64.87 | 94.23 | 59.44 | 0.20 | 891.41 | 0.40 | 0.29 |
| MW 6 | 7.95 | 9.31 | 1535.80 | 100.48 | 5.22 | 97.12 | 98.67 | 18.36 | 0.20 | 809.56 | 0.34 | 0.11 |
| MW 7 | 7.57 | 1.34 | 1212.40 | 95.88 | 1.29 | 70.48 | 34.87 | 9.51 | 6.53 | 607.13 | 0.28 | 0.10 |
| MW 8 | 7.54 | 2.26 | 487.76 | 17.62 | 1.19 | 35.57 | 15.73 | 4.15 | 14.01 | 224.92 | 0.25 | 0.57 |
| MW 9 | 6.89 | 7.15 | 833.00 | 26.01 | 2.76 | 92.52 | 19.82 | 37.46 | 12.82 | 357.18 | 0.21 | 16.62 |
| MW 10 | 7.37 | 6.87 | 749.00 | 23.93 | 1.66 | 67.25 | 17.37 | 32.78 | 34.90 | 268.62 | 0.21 | 12.14 |
| MW 11 | 7.75 | 8.00 | 564.20 | 13.54 | 1.18 | 58.06 | 12.62 | 9.70 | 27.15 | 226.38 | 0.23 | 7.58 |
| MW 12 | 7.42 | 6.34 | 580.58 | 21.26 | 1.47 | 53.45 | 16.92 | 18.29 | 24.63 | 238.73 | 0.23 | 5.66 |
| MW 13 | 8.16 | 6.80 | 661.08 | 39.81 | 0.76 | 55.61 | 11.70 | 18.90 | 4.72 | 305.21 | 0.31 | 0.60 |
| MW 15 | 8.14 | 3.09 | 576.80 | 77.11 | 0.78 | 28.12 | 2.44 | 28.32 | 0.20 | 232.85 | 0.16 | 0.10 |
| MW 16 | 7.16 | 4.82 | 491.12 | 53.27 | 0.61 | 26.25 | 6.06 | 16.94 | 16.35 | 186.39 | 0.27 | 3.90 |
| MW 17 | 7.82 | 12.49 | 327.36 | 1.98 | 0.36 | 35.40 | 5.75 | 1.96 | 4.86 | 134.41 | 0.17 | 2.26 |
| MW 18 | 6.71 | 6.01 | 2296.60 | 128.90 | 6.28 | 171.30 | 117.49 | 9.76 | 0.18 | 1213.28 | 0.29 | 1.18 |
| MW 19 | 7.62 | 3.65 | 992.60 | 58.12 | 2.38 | 68.08 | 32.88 | 12.21 | 0.20 | 538.82 | 0.33 | 0.47 |
| MW 20 | 7.39 | 4.39 | 1926.40 | 128.20 | 1.63 | 141.73 | 79.96 | 5.74 | 0.20 | 1015.59 | 0.38 | 0.25 |
| MW 21 | 7.87 | 8.04 | 1160.60 | 86.19 | 4.65 | 48.25 | 66.35 | 17.42 | 0.34 | 642.44 | 0.59 | 0.08 |
| MW 22 | 8.10 | 9.44 | 289.38 | 9.08 | 1.52 | 33.43 | 7.85 | 10.53 | 15.11 | 119.52 | 0.20 | 1.38 |
| MW 24 | 6.44 | 10.60 | 924.00 | 5.71 | 0.16 | 21.07 | 2.76 | 1.45 | 9.07 | 78.06 | 0.17 | 2.25 |
| MW 25 | 6.24 | 6.24 | 168.00 | 4.29 | 0.08 | 18.61 | 2.20 | 1.36 | 5.57 | 63.06 | <0.15 | 2.55 |
| MW 26 | 8.44 | 11.10 | 1242.80 | 103.99 | 5.54 | 46.03 | 76.05 | 23.67 | 14.95 | 626.26 | 0.26 | 0.68 |
| MW 27 | 8.04 | 9.52 | 908.60 | 8.53 | 1.36 | 30.92 | 7.39 | 10.56 | 0.20 | 108.73 | 0.14 | 0.72 |
| MW 28 | 7.56 | 3.33 | 554.80 | 15.16 | 1.49 | 39.39 | 12.94 | 6.87 | 15.70 | 247.86 | 0.16 | 0.08 |
| MW 33 | 8.61 | 2.29 | 270.90 | 8.78 | 1.42 | 32.60 | 7.60 | 11.22 | 14.95 | 108.19 | 0.12 | 0.46 |
| MW 34 | 7.92 | 10.10 | 267.80 | 8.86 | 1.44 | 33.00 | 7.77 | 10.73 | 15.05 | 116.03 | 0.13 | 0.52 |
| Min | 6.24 | 1.34 | 168.00 | 1.98 | 0.08 | 18.61 | 2.20 | 1.36 | 0.18 | 63.06 | 0.12 | 0.08 |
| Max | 8.61 | 12.49 | 2296.60 | 160.21 | 11.62 | 171.30 | 117.49 | 78.87 | 83.13 | 1213.28 | 0.59 | 16.62 |
| Mean | 7.53 | 6.02 | 895.91 | 55.26 | 2.50 | 56.29 | 32.23 | 21.83 | 17.39 | 391.05 | 0.27 | 2.96 |
| STDV | 0.61 | 3.27 | 539.08 | 49.94 | 2.49 | 34.55 | 33.72 | 21.30 | 16.84 | 309.29 | 0.11 | 4.40 |
| Sample No | pH | DO | EC | Na+ | K+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | F− | NO3− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | µS/cm at 25 °C | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/l | mg/L | mg/L | ||
| MW 1 | 7.47 | 1.73 | 1464.40 | 74.91 | 4.84 | 63.98 | 58.03 | 49.03 | 0.20 | 661.97 | 0.46 | 0.60 |
| MW 2 | 6.09 | 7.73 | 524.40 | 22.92 | 1.46 | 41.62 | 11.35 | 103.30 | 24.10 | 82.34 | 0.19 | 19.60 |
| MW 3 | 7.98 | 4.54 | 1209.60 | 118.42 | 15.50 | 28.53 | 29.34 | 53.11 | 78.16 | 508.27 | 0.33 | 4.04 |
| MW 5 | 8.04 | 7.49 | 2828.00 | 310.54 | 8.64 | 47.28 | 87.20 | 134.69 | 1.41 | 1429.38 | 0.74 | 0.88 |
| MW 6 | 7.73 | 7.73 | 1541.40 | 78.43 | 4.50 | 95.18 | 85.61 | 21.53 | 0.20 | 875.64 | 0.31 | 0.48 |
| MW 7 | 8.20 | 2.12 | 1288.00 | 78.92 | 1.24 | 50.73 | 19.05 | 11.53 | 4.01 | 533.69 | 0.31 | 0.62 |
| MW 8 | 8.32 | 1.94 | 483.28 | 15.28 | 1.11 | 33.94 | 14.01 | 5.65 | 16.64 | 213.78 | 0.25 | 0.87 |
| MW 9 | 7.55 | 6.8 | 666.26 | 14.69 | 1.65 | 66.53 | 12.28 | 23.41 | 18.89 | 279.00 | 0.17 | 26.99 |
| MW 10 | 8.07 | 10.06 | 547.40 | 11.09 | 1.07 | 50.63 | 11.94 | 13.74 | 33.08 | 216.44 | 0.25 | 9.96 |
| MW 11 | 7.90 | 9.66 | 1027.60 | 42.13 | 2.47 | 91.79 | 16.41 | 49.36 | 28.68 | 395.55 | 0.18 | 16.83 |
| MW 12 | 8.08 | 4.45 | 436.80 | 14.65 | 1.07 | 37.06 | 13.13 | 7.93 | 17.69 | 229.28 | 0.27 | 0.80 |
| MW 13 | 7.37 | 3.85 | 650.02 | 35.64 | 0.65 | 52.26 | 9.80 | 22.77 | 6.20 | 301.36 | 0.32 | 0.10 |
| MW 15 | 8.34 | 2 | 588.98 | 63.74 | 0.65 | 19.40 | 2.00 | 35.64 | 0.20 | 232.56 | 0.10 | 1.01 |
| MW 16 | 7.19 | 2.96 | 487.06 | 53.72 | 0.94 | 23.98 | 9.13 | 22.42 | 22.23 | 182.72 | 0.31 | 3.88 |
| MW 17 | 7.86 | 7.11 | 380.80 | 1.67 | 0.30 | 35.20 | 4.57 | 3.11 | 8.00 | 134.81 | 0.13 | 2.89 |
| MW 18 | 7.97 | 2.16 | 656.32 | 55.79 | 1.09 | 25.88 | 10.54 | 48.05 | 1.11 | 256.24 | 0.35 | 0.10 |
| MW 19 | 8.18 | 4.8 | 1192.80 | 82.80 | 1.22 | 51.77 | 19.76 | 11.81 | 4.11 | 509.07 | 0.30 | 3.08 |
| MW 20 | 7.23 | 1.15 | 1887.20 | 82.09 | 1.82 | 145.03 | 44.04 | 8.55 | 0.20 | 979.82 | 0.37 | 0.56 |
| MW 21 | 8.42 | 7.66 | 833.00 | 45.06 | 3.67 | 32.85 | 39.22 | 16.02 | 2.18 | 449.55 | 0.66 | 0.70 |
| MW 22 | 8.40 | 7.15 | 232.80 | 4.79 | 1.11 | 19.07 | 3.98 | 7.97 | 17.04 | 81.64 | 0.14 | 2.13 |
| MW 24 | 7.30 | 11.6 | 252.00 | 5.32 | 0.99 | 20.41 | 4.56 | 9.66 | 17.42 | 81.21 | 0.18 | 1.60 |
| MW 25 | 7.34 | 5.09 | 388.00 | 7.62 | 2.26 | 33.64 | 8.78 | 11.41 | 11.01 | 142.31 | 0.16 | 2.27 |
| MW 26 | 7.60 | 3.3 | 1513.10 | 91.58 | 5.39 | 50.04 | 73.17 | 31.05 | 0.20 | 664.44 | 0.34 | 0.65 |
| MW 27 | 8.32 | 10.39 | 253.00 | 7.01 | 1.28 | 24.12 | 5.79 | 13.68 | 19.16 | 110.65 | 0.19 | 1.32 |
| MW 28 | 7.87 | 2.06 | 612.20 | 13.08 | 1.49 | 38.93 | 11.82 | 8.79 | 0.20 | 245.47 | 0.24 | 0.83 |
| MW 33 | 7.43 | 2.42 | 1143.75 | 21.59 | 2.35 | 91.90 | 50.34 | 12.51 | 13.04 | 576.72 | 0.44 | 5.48 |
| MW 34 | 8.35 | 9.36 | 369.60 | 6.51 | 1.24 | 24.39 | 5.50 | 13.67 | 18.82 | 125.53 | 0.20 | 2.82 |
| Min | 6.09 | 1.15 | 232.80 | 1.67 | 0.30 | 19.07 | 2.00 | 3.11 | 0.20 | 81.21 | 0.10 | 0.10 |
| Max | 8.42 | 11.60 | 2828.00 | 310.54 | 15.50 | 145.03 | 87.20 | 134.69 | 78.16 | 1429.38 | 0.74 | 26.99 |
| Mean | 7.80 | 5.46 | 868.81 | 50.37 | 2.59 | 48.01 | 24.49 | 27.79 | 13.49 | 388.87 | 0.29 | 4.11 |
| STDV | 0.51 | 3.09 | 591.94 | 60.62 | 3.11 | 28.65 | 24.92 | 29.81 | 15.97 | 314.69 | 0.15 | 6.51 |
| Sample No | Al | As | Ba | Cd | Co | Cr | Cu | Fe | Mn | Mo | Ni | Pb | Sb | Se | Sn | U | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | |
| MW 1 | 2.32 | 72.26 | 218.33 | 0.02 | 0.41 | 4.89 | 0.53 | <5 | 72.60 | 6.27 | 3.01 | 0.02 | 2.80 | 0.07 | 1.63 | 0.004 | 1.17 | 2.47 |
| MW 2 | 4.42 | 0.83 | 84.02 | 0.03 | 0.17 | 1.64 | 0.12 | <5 | 15.60 | 0.02 | 4.18 | 0.07 | 2.52 | 0.46 | 0.03 | 0.38 | 0.99 | 28.84 |
| MW 3 | 8.38 | 178.87 | 144.49 | 0.03 | 0.79 | 7.19 | 1.44 | 25.20 | 179.50 | 10.23 | 7.97 | 0.28 | 5.11 | 0.19 | <0.01 | 1.14 | 4.24 | 1.39 |
| MW 5 | 0.32 | 300.52 | 736.89 | 0.04 | 1.53 | 15.96 | 0.57 | <5 | 83.40 | 13.52 | 13.00 | <0.01 | 6.11 | 0.12 | 0.07 | 0.01 | 0.69 | 8.21 |
| MW 6 | 4.80 | 300.18 | 214.42 | 0.03 | 0.86 | 12.22 | 2.81 | <5 | 72.49 | 0.59 | 8.26 | 0.39 | 1.80 | 0.13 | <0.01 | 0.01 | 0.27 | 17.21 |
| MW 7 | 2.77 | 251.34 | 77.46 | <0.01 | 0.47 | 5.37 | 1.30 | <5 | 241.95 | 1.98 | 4.25 | 0.19 | 2.15 | 0.08 | <0.01 | 0.02 | 0.38 | 2.32 |
| MW 8 | 3.34 | 3.76 | 32.23 | <0.01 | 0.12 | 2.14 | <0.1 | <5 | 103.16 | 1.44 | 1.76 | 0.11 | 0.25 | 0.06 | <0.01 | 0.41 | 0.48 | 3.38 |
| MW 9 | 1.90 | 0.71 | 25.88 | <0.01 | 0.22 | 4.78 | 3.16 | <5 | 1.55 | 0.13 | 5.97 | 0.02 | 0.76 | 0.05 | <0.01 | 0.77 | 1.52 | 6.38 |
| MW 10 | 3.54 | 0.64 | 71.46 | <0.01 | 0.17 | 3.61 | 0.47 | <5 | 0.55 | 0.33 | 3.78 | 0.31 | 0.62 | 0.30 | <0.01 | 0.71 | 0.95 | 16.20 |
| MW 11 | 1.56 | 0.37 | 45.40 | <0.01 | 0.14 | 2.70 | <0.1 | <5 | 0.11 | 0.45 | 3.07 | 0.24 | 0.46 | 0.07 | <0.01 | 0.54 | 0.74 | 1.53 |
| MW 12 | 1.25 | 1.84 | 53.32 | <0.01 | 0.14 | 2.28 | 0.27 | <5 | 49.25 | 0.88 | 2.70 | <0.01 | 0.37 | 0.13 | <0.01 | 0.54 | 0.76 | 3.63 |
| MW 13 | 1.54 | 1.72 | 29.57 | <0.01 | 0.14 | 3.49 | 5.16 | <5 | 91.40 | 3.58 | 2.85 | 1.05 | 0.72 | 0.27 | <0.01 | 0.88 | 2.23 | 13.31 |
| MW 15 | 4.19 | 1.41 | 42.20 | 0.02 | 0.10 | 2.54 | 1.00 | <5 | 18.99 | 1.83 | 1.77 | 0.36 | 0.38 | 0.25 | <0.01 | 0.01 | 0.41 | 2.65 |
| MW 16 | 4.80 | 0.78 | 25.08 | 0.03 | 0.22 | 2.28 | 1.68 | <5 | 51.27 | 9.92 | 2.43 | 0.08 | 0.90 | 0.08 | <0.01 | 0.68 | 0.70 | 340.96 |
| MW 17 | 16.46 | 0.26 | 9.89 | <0.01 | 0.09 | 1.86 | 24.34 | <5 | 1.27 | 0.22 | 4.15 | 0.84 | 0.30 | 0.07 | <0.01 | 0.35 | 0.57 | 85.05 |
| MW 18 | <0.1 | 39.49 | 328.63 | <0.01 | 1.05 | 65.24 | 1.08 | <5 | 153.89 | 0.18 | 11.29 | 0.33 | 4.11 | 0.05 | <0.01 | 0.02 | 0.15 | 14.99 |
| MW 19 | 27.54 | 139.60 | 52.54 | 0.02 | 0.51 | 3.97 | 0.52 | <5 | 209.26 | 5.22 | 6.36 | 0.03 | 1.26 | 0.26 | <0.01 | 0.01 | 0.08 | 27.43 |
| MW 20 | 5.03 | 91.91 | 207.84 | <0.01 | 0.95 | 11.73 | 2.49 | <5 | 214.73 | 1.41 | 17.43 | 0.09 | 4.05 | 0.14 | <0.01 | 0.05 | 0.91 | 64.64 |
| MW 21 | 8.96 | 133.32 | 354.48 | 0.08 | 0.40 | 4.74 | 3.20 | <5 | 26.25 | 23.15 | 7.95 | 0.03 | 2.07 | 0.30 | 0.17 | 0.05 | 0.23 | 15.14 |
| MW 22 | 28.78 | 3.89 | 10.50 | <0.01 | 0.08 | 0.79 | 3.99 | <5 | 0.22 | 0.33 | 3.04 | 0.12 | 0.34 | 0.13 | <0.01 | 0.42 | 0.07 | 9.49 |
| MW 24 | 7.41 | 0.58 | 17.79 | <0.01 | 0.05 | 1.12 | 0.24 | <5 | 0.10 | 0.37 | 0.84 | 0.14 | 0.48 | 0.10 | <0.01 | 0.09 | 5.27 | 6.09 |
| MW 25 | 4.45 | 0.26 | 0.79 | <0.01 | 0.06 | 1.34 | <0.1 | <5 | 0.02 | 0.20 | 0.63 | 0.21 | 0.40 | 0.08 | <0.01 | 0.04 | 11.11 | 2.44 |
| MW 26 | 6.78 | 5.43 | 152.04 | <0.01 | 0.17 | 7.81 | 13.03 | <5 | 1.08 | 1.87 | 4.88 | 0.04 | 3.58 | 0.72 | 0.04 | 0.03 | 0.24 | 7.87 |
| MW 27 | 7.90 | 3.00 | 9.98 | <0.01 | 0.10 | 1.43 | 1.26 | <5 | 1.74 | 0.33 | 2.17 | 0.07 | 0.43 | 0.30 | <0.01 | 0.33 | 0.02 | 109.16 |
| MW 28 | 3.93 | 6.79 | 37.39 | <0.01 | 0.12 | 2.27 | 0.10 | <5 | 147.70 | 0.77 | 1.80 | 0.12 | 0.25 | 0.20 | <0.01 | 0.004 | 0.01 | 57.11 |
| MW 33 | 17.15 | 3.58 | 9.06 | <0.01 | 0.09 | 1.09 | 2.61 | <5 | 0.59 | 0.28 | 2.06 | <0.01 | 0.57 | 0.34 | 0.09 | 0.34 | 0.08 | 8.74 |
| MW 34 | 12.08 | 3.76 | 9.17 | <0.01 | 0.09 | 0.93 | 4.21 | <5 | 1.19 | 0.29 | 4.30 | 0.35 | 0.65 | 0.31 | 0.08 | 0.35 | 0.06 | 49.97 |
| Min | <0.1 | 0.26 | 0.79 | <0.01 | 0.05 | 0.79 | 0.10 | <5 | 0.02 | 0.02 | 0.63 | <0.01 | 0.25 | 0.05 | <0.01 | 0.004 | 0.01 | 1.39 |
| Max | 28.78 | 300.52 | 736.89 | 0.08 | 1.53 | 65.24 | 24.34 | 25.19 | 241.95 | 23.15 | 17.43 | 1.05 | 6.11 | 0.72 | 1.63 | 0.004 | 11.11 | 340.96 |
| Mean | 7.37 | 57.30 | 111.14 | 0.03 | 0.34 | 6.50 | 3.15 | - | 64.44 | 3.18 | 4.89 | 0.23 | 1.61 | 0.19 | 0.30 | 0.30 | 1.27 | 33.58 |
| STDV | 7.31 | 94.20 | 155.99 | 0.02 | 0.37 | 12.11 | 5.15 | - | 76.22 | 5.26 | 3.87 | 0.25 | 1.63 | 0.15 | 0.54 | 0.32 | 2.28 | 66.14 |
| Sample No | Al | As | Ba | Cd | Co | Cr | Cu | Fe | Mn | Mo | Ni | Pb | Sb | Se | Sn | U | V | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | µg/L | |
| MW 1 | <0.1 | 250.07 | 503.03 | 0.05 | 1.02 | 0.82 | 1.04 | <1 | 63.09 | 8.89 | 8.06 | 0.11 | 0.86 | 4.27 | 0.11 | 0.002 | 0.56 | 24.63 |
| MW 2 | <0.1 | 0.74 | 103.26 | 0.07 | 0.12 | 0.69 | 0.30 | <1 | 10.11 | 0.04 | 5.03 | <0.01 | 0.76 | 2.38 | 0.09 | 0.11 | 1.14 | 49.25 |
| MW 3 | <0.1 | 77.59 | 100.93 | 0.04 | 0.64 | 44.83 | 1.87 | <1 | 15.92 | 10.59 | 4.62 | 0.03 | 3.19 | 5.45 | 0.26 | 0.39 | 10.24 | 30.70 |
| MW 5 | 2.53 | 373.09 | 479.80 | 0.10 | 0.99 | 26.04 | 2.36 | <1 | 5.65 | 20.25 | 15.01 | 0.52 | 0.83 | 9.19 | 0.42 | 0.01 | 1.51 | 19.46 |
| MW 6 | <0.1 | 219.49 | 210.05 | 0.02 | 0.85 | 4.61 | 0.95 | <1 | 59.85 | 0.61 | 8.35 | 0.01 | 0.94 | 1.85 | 0.27 | 0.02 | 0.36 | 13.21 |
| MW 7 | <0.1 | 18.37 | 184.82 | 0.01 | 0.50 | 6.33 | 0.40 | <1 | 111.55 | 1.83 | 4.88 | <0.01 | 1.15 | 2.48 | 2.33 | 0.01 | 0.14 | 7.89 |
| MW 8 | <0.1 | 3.12 | 29.28 | 0.01 | 0.12 | 4.30 | 0.76 | <1 | 93.86 | 1.34 | 2.72 | 0.18 | 0.65 | 0.13 | 0.08 | 0.39 | 0.51 | 2.15 |
| MW 9 | <0.1 | 0.61 | 20.54 | <0.01 | 0.20 | 2.88 | 0.32 | <1 | 0.29 | 0.13 | 5.44 | 0.09 | 0.74 | 0.33 | 0.03 | 0.62 | 1.42 | 1.90 |
| MW 10 | <0.1 | 0.35 | 36.97 | 0.01 | 0.14 | 2.25 | 0.31 | <1 | 0.11 | 0.35 | 4.02 | 0.04 | 0.62 | 0.29 | 0.01 | 0.55 | 0.95 | 1.81 |
| MW 11 | <0.1 | 0.56 | 82.92 | 0.01 | 0.25 | 7.42 | 0.39 | <1 | 4.52 | 0.38 | 6.38 | 0.02 | 0.80 | 0.42 | 0.01 | 0.62 | 0.94 | 3.60 |
| MW 12 | <0.1 | 1.71 | 29.99 | 0.01 | 0.13 | 0.21 | 0.34 | <1 | 65.61 | 1.28 | 2.67 | 0.04 | 0.77 | 0.03 | 0.06 | 0.39 | 0.48 | 10.61 |
| MW 13 | <0.1 | 1.50 | 28.76 | 0.04 | 0.20 | 0.20 | 0.26 | <1 | 189.36 | 3.36 | 3.97 | 0.06 | 0.13 | 0.55 | 0.04 | 0.73 | 2.34 | 3.11 |
| MW 15 | <0.1 | 1.22 | 36.78 | 0.01 | 0.08 | 5.88 | 0.22 | 18.39 | 16.73 | 1.77 | 1.71 | <0.01 | 0.52 | 0.38 | <0.01 | 0.002 | 0.52 | 1.27 |
| MW 16 | <0.1 | 11.28 | 50.38 | 0.06 | 1.05 | 1.06 | 0.86 | <1 | 161.12 | 8.10 | 5.02 | 0.02 | 0.23 | 0.87 | 0.34 | 0.57 | 0.60 | 359.51 |
| MW 17 | <0.1 | 0.26 | 12.41 | <0.01 | 0.09 | 3.57 | 0.95 | <1 | 0.00 | 0.23 | 2.38 | 0.37 | 0.70 | 0.12 | 0.01 | 0.32 | 0.50 | 5.30 |
| MW 18 | <0.1 | 30.55 | 57.69 | 0.02 | 0.23 | 4.22 | 0.17 | <1 | 36.16 | 2.29 | 2.15 | 0.03 | 0.63 | 1.03 | 0.06 | 0.01 | 0.73 | 5.14 |
| MW 19 | <0.1 | 21.14 | 179.37 | 0.01 | 0.50 | 15.18 | 0.60 | <1 | 111.40 | 1.83 | 4.78 | <0.01 | 1.08 | 2.06 | 2.32 | 0.01 | 0.13 | 11.03 |
| MW 20 | <0.1 | 56.92 | 214.61 | 0.02 | 0.59 | 7.75 | 0.65 | <1 | 317.90 | 2.51 | 11.53 | 0.02 | 0.63 | 2.39 | 0.08 | 0.01 | 0.11 | 7.97 |
| MW 21 | <0.1 | 35.62 | 262.16 | 0.06 | 0.49 | 8.63 | 1.53 | <1 | 18.33 | 17.53 | 5.32 | 0.16 | 0.76 | 1.21 | 0.12 | 0.01 | 0.24 | 2057.47 |
| MW 22 | 13.64 | 1.41 | 7.19 | 0.02 | 0.06 | 2.14 | 1.13 | <1 | 0.36 | 0.50 | 1.90 | 0.05 | 0.63 | 0.32 | 0.18 | 0.19 | 1.96 | 8.07 |
| MW 24 | 23.10 | 2.25 | 7.04 | <0.01 | 0.07 | 0.46 | 0.76 | <1 | 0.19 | 0.39 | 1.94 | 0.03 | 0.75 | 0.14 | 0.03 | 0.29 | 1.19 | 5.56 |
| MW 25 | <0.1 | 0.17 | 75.05 | <0.01 | 0.34 | 0.43 | 0.41 | <1 | 47.55 | 0.20 | 2.92 | <0.01 | 0.72 | 0.15 | 0.03 | 0.11 | 0.11 | 5.33 |
| MW 26 | <0.1 | 7.31 | 279.32 | 0.01 | 0.26 | 2.72 | 2.37 | <1 | 173.17 | 1.76 | 4.72 | 0.04 | 0.93 | 3.29 | 0.18 | 0.02 | 0.29 | 12.10 |
| MW 27 | 2.91 | 3.75 | 8.58 | <0.01 | 0.08 | 1.92 | 0.88 | <1 | 0.57 | 0.27 | 2.64 | 0.07 | 0.76 | 0.61 | 0.12 | 0.41 | 0.19 | 9.48 |
| MW 28 | <0.1 | 6.68 | 36.95 | 0.01 | 0.13 | 6.36 | 0.55 | <1 | 140.49 | 0.76 | 3.31 | <0.01 | 0.74 | 0.17 | <0.01 | 0.001 | 0.12 | 35.31 |
| MW 33 | <0.1 | 10.38 | 132.61 | 0.02 | 2.11 | 16.00 | 0.27 | <1 | 1228.79 | 4.27 | 9.34 | 0.02 | 0.74 | 0.50 | 0.21 | 0.90 | 0.28 | 120.53 |
| MW 34 | 26.47 | 3.11 | 8.68 | 0.01 | 0.09 | 1.18 | 9.39 | <1 | 0.26 | 0.29 | 2.67 | 0.59 | 0.85 | 0.36 | 0.45 | 0.35 | 0.26 | 10.44 |
| Min | <0.1 | 0.17 | 7.04 | <0.01 | 0.06 | 0.20 | 0.17 | <1 | 0.00 | 0.04 | 1.71 | <0.01 | 0.13 | 0.03 | <0.01 | 0.001 | 0.11 | 1.27 |
| Max | 26.47 | 373.09 | 503.03 | 0.10 | 2.11 | 44.83 | 9.39 | 18.39 | 1228.79 | 20.25 | 15.01 | 0.59 | 3.19 | 9.19 | 2.33 | 0.90 | 10.24 | 2057.47 |
| Mean | 13.73 | 42.19 | 117.75 | 0.023 | 0.42 | 6.60 | 1.11 | - | 106.41 | 3.40 | 4.94 | 0.12 | 0.82 | 1.52 | 0.31 | 0.26 | 1.03 | 104.55 |
| STDV | 9.93 | 89.12 | 132.84 | 0.024 | 0.45 | 9.44 | 1.73 | - | 233.09 | 5.17 | 3.10 | 0.16 | 0.51 | 2.03 | 0.61 | 0.26 | 1.90 | 389.14 |
| Sample No | X Easting | Y Northing | Elevation (m) | Well Depth (m) | Deuterium (δD, ‰) | Oxygen-18 (δ18O, ‰) | Tritium (TU) | D-Excess | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sep-18 | Apr-19 | Sep-18 | Apr-19 | Sep-18 | Apr-19 | Sep-18 | Apr-19 | |||||
| MW 4 | 280558 | 4518352 | 32 | 36.0 | −46.84 | −47.04 | −7.17 | −7.01 | 5.26 | 5.34 | 10.52 | 9.04 |
| MW 6 | 284453 | 4513591 | 30 | 102.0 | −56.83 | −57.5 | −8.86 | −8.83 | 1.16 | 0.76 | 14.05 | 13.14 |
| MW 8 | 295482 | 4507074 | 4 | 94.0 | −64.39 | −68.79 | −9.91 | −10.49 | 3.17 | 3.00 | 14.89 | 15.13 |
| MW 9 | 299200 | 4500659 | 42 | 107.00 | −69.05 | −62.94 | −10.54 | −9.76 | 3.02 | 4.98 | 15.27 | 15.14 |
| MW 15 | 287913 | 4512153 | 40 | 102.00 | −76.36 | −75.87 | −11.42 | −11.4 | 0.37 | 0.19 | 15.00 | 15.33 |
| MW 16 | 307667 | 4517462 | 14 | 106.00 | −70.33 | −75.61 | −10.64 | −11.45 | 3.88 | 1.45 | 14.79 | 15.99 |
| MW 20 | 289797 | 4512153 | 28 | 112.00 | −85.2 | −85.2 | −12.63 | −12.71 | 0 | 0.26 | 15.84 | 16.48 |
| MW 23 | 277363 | 4505896 | 42 | 24.00 | −52.23 | −49.62 | −7.86 | −7.48 | 4.69 | 5.9 | 10.65 | 10.22 |
| MW 28 | 293438 | 4507889 | 45 | 72.00 | −69.07 | −68.88 | −10.5 | −10.68 | 6.08 | 0.84 | 14.93 | 16.56 |
| MW 31 | 274752 | 4523782 | 22 | 28.00 | −49.3 | −47.74 | −7.47 | −7.27 | 4.43 | 4.95 | 10.46 | 10.42 |
| MW 32 | 290203 | 4527168 | 25 | 22.00 | −43.1 | −55.04 | −6.24 | −8.7 | 5.22 | 4.46 | 6.82 | 14.56 |
| MW 34 | 296679 | 4531796 | 33 | 65.00 | −42.94 | −42.52 | −6.17 | −7.33 | 4.84 | 5.58 | 6.42 | 16.12 |
| Sampling Depths (m) | As | Mn | Cd | V | Al | Fe | Cr | Co | Ni | Cu | Zn | Mo | Pb | Tl | Sn | Sb | Se |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | µg/kg | µg/kg | µg/kg | µg/kg | |
| ALANCUMA (0–50 m) | 8.2 | 577 | 0.1 | 16.3 | 5118 | 10,667 | 48.7 | 9.1 | 65.4 | 42.6 | 69.7 | 0.7 | 11.4 | 87.2 | 121 | 10.2 | <1 |
| ALANCUMA (50–110 m) | 3.1 | 313 | 0.1 | 7.0 | 1940 | 4463 | 50.5 | 4.0 | 56.6 | 9.4 | 10.1 | 3.3 | 2.7 | 27.0 | 48.4 | 6.5 | <1 |
| Variables | Na | K | Ca | Mg | HCO3 | Al | As | Ba | Co | Mn | Mo | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | 1 | |||||||||||
| K | 0.765 ** | 1 | ||||||||||
| Ca | 0.547 ** | 0.392 * | 1 | |||||||||
| Mg | 0.832 ** | 0.731 ** | 0.743 ** | 1 | ||||||||
| HCO3 | 0.861 ** | 0.647 ** | 0.835 ** | 0.957 ** | 1 | |||||||
| Al | −0.273 | −0.094 | −0.263 | −0.234 | −0.250 | 1 | ||||||
| As | 0.686 ** | 0.532 ** | 0.344 | 0.663 ** | 0.655 ** | −0.066 | 1 | |||||
| Ba | 0.719 ** | 0.550 ** | 0.423 * | 0.790 ** | 0.739 ** | −0.293 | 0.664 ** | 1 | ||||
| Co | 0.820 ** | 0.628 ** | 0.680 ** | 0.866 ** | 0.883 ** | −0.218 | 0.783 ** | 0.858 ** | 1 | |||
| Mn | 0.579 ** | 0.323 | 0.532 ** | 0.450 * | 0.624 ** | −0.063 | 0.544 ** | 0.241 | 0.565 ** | 1 | ||
| Mo | 0.489 ** | 0.443 * | −0.068 | 0.349 | 0.330 | −0.025 | 0.456 * | 0.601 ** | 0.396 * | 0.150 | 1 | |
| Ni | 0.713 ** | 0.515 ** | 0.791 ** | 0.826 ** | 0.871 ** | −0.107 | 0.574 ** | 0.713 ** | 0.869 ** | 0.499 ** | 0.319 | 1 |
| Variables | Na | K | Ca | Mg | HCO3 | As | Ba | Co | Mn | Mo | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | 1 | ||||||||||
| K | 0.631 ** | 1 | |||||||||
| Ca | 0.168 | 0.041 | 1 | ||||||||
| Mg | 0.691 ** | 0.551 ** | 0.508 ** | 1 | |||||||
| HCO3 | 0.854 ** | 0.532 ** | 0.594 ** | 0.890 ** | 1 | ||||||
| As | 0.800 ** | 0.547 ** | 0.244 | 0.777 ** | 0.800 ** | 1 | |||||
| Ba | 0.737 ** | 0.482 * | 0.360 | 0.836 ** | 0.826 ** | 0.813 ** | 1 | ||||
| Co | 0.385 * | 0.332 | 0.439 * | 0.600 ** | 0.563 ** | 0.458 * | 0.520 ** | 1 | |||
| Mn | −0.055 | −0.051 | 0.448 * | 0.262 | 0.218 | −0.084 | 0.080 | 0.752 ** | 1 | ||
| Mo | 0.716 ** | 0.601 ** | −0.054 | 0.521 ** | 0.584 ** | 0.638 ** | 0.665 ** | 0.473 * | 0.018 | 1 | |
| Ni | 0.728 ** | 0.412 * | 0.693 ** | 0.794 ** | 0.905 ** | 0.742 ** | 0.755 ** | 0.681 ** | 0.352 | 0.565 ** | 1 |
References
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Muzaffar, S.; Khan, J.; Srivastava, R.; Gorbatyuk, M.S.; Athar, M. Mechanistic understanding of the toxic effects of arsenic and warfare arsenicals on human health and environment. Cell Biol. Toxicol. 2023, 39, 85–110. [Google Scholar] [CrossRef]
- UNICEF; World Health Organization. Arsenic Primer: Guidance on the Investigation and Mitigation of Arsenic contamination; UNICEF: New York, NY, USA, 2018. [Google Scholar]
- Akter, K.F.; Chen, Z.; Smith, L.; Davey, D.; Naidu, R. Speciation of arsenic in ground water samples: A comparative study of CE-UV, HG-AAS and LC-ICP-MS. Talanta 2005, 68, 406–415. [Google Scholar] [CrossRef]
- Apello, T. Arsenic in Groundwater: A World Problem; Netherlands National Committee for the IAH: Utrecht, The Netherlands, 2008; Volume 5. [Google Scholar]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Sadiq, M. Arsenic chemistry in soils: An overview of thermodynamic predictions and field observations. Water Air Soil. Pollut. 1997, 93, 117–136. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Chatterjee, D.; Jacks, G. Occurrence of arsenic-contaminated groundwater in alluvial aquifers from delta plains, eastern India: Options for safe drinking water supply. Int. J. Water Resour. Dev. 1997, 13, 79–92. [Google Scholar] [CrossRef]
- Harvey, C.F.; Swartz, C.H.; Badruzzaman, A.B.M.; Keon-Blute, N.; Yu, W.; Ali, M.A.; Jay, J.; Beckie, R.; Niedan, V.; Brabander, D. Arsenic mobility and groundwater extraction in Bangladesh. Science 2002, 298, 1602–1606. [Google Scholar] [CrossRef]
- Polizzotto, M.L.; Harvey, C.F.; Li, G.; Badruzzman, B.; Ali, A.; Newville, M.; Sutton, S.; Fendorf, S. Solid-phases and desorption processes of arsenic within Bangladesh sediments. Chem. Geol. 2006, 228, 97–111. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and management of arsenic pollution in groundwater: A comprehensive appraisal of recent global scenario, human health impacts, sustainable field-scale treatment technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- Zahid, A.; Hassan, M.Q.; Breit, G.N.; Balke, K.D.; Flegr, M. Accumulation of iron and arsenic in the Chandina alluvium of the lower delta plain, Southeastern Bangladesh. Environ. Geochem. Health 2009, 31 (Suppl. S1), S69–S84. [Google Scholar] [CrossRef]
- Akbulut, N.E.; Tuncer, A.M. Accumulation of heavy metals with water quality parameters in Kızılırmak River Basin (Delice River) in Turkey. Environ. Monit. Assess. 2010, 173, 387–395. [Google Scholar] [CrossRef]
- Alptekin, C.; Yuce, G. Observation of excess heavy metal concentrations in water resources to infer surface water influences on shallow groundwater: A typical example of the Porsuk River (Eskisehir-Turkey). Water Resour. 2016, 43, 184–199. [Google Scholar] [CrossRef]
- Davraz, A.; Batur, B. Hydrogeochemistry characteristics of groundwater and health risk assessment in Yalvaç–Gelendost basin (Turkey). Appl. Water Sci. 2021, 11, 67. [Google Scholar] [CrossRef]
- Küçüksümbül, A.; Akar, A.T.; Tarcan, G. Source, degree and potential health risk of metal(loid)s contamination on the water and soil in the Söke Basin, Western Anatolia, Turkey. Environ. Monit. Assess. 2021, 194, 6. [Google Scholar] [CrossRef]
- Simsek, C. Assessment of naturally occurring arsenic contamination in the groundwater of Sarkisla Plain (Sivas/Turkey). Environ. Earth Sci. 2012, 68, 691–702. [Google Scholar] [CrossRef]
- Yolcubal, İ.; Ataş Gündüz, Ö.C.; Kurtuluş, N. Origin of salinization and pollution sources and geochemical processes in urban coastal aquifer (Kocaeli, NW Turkey). Environ. Earth Sci. 2019, 78, 181. [Google Scholar] [CrossRef]
- Baba, A.; Sözbilir, H. Source of arsenic based on geological and hydrogeochemical properties of geothermal systems in Western Turkey. Chem. Geol. 2012, 334, 364–377. [Google Scholar] [CrossRef]
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Gedik, İ.; Aksay, A. 1:100,000 Scale Geological Maps of Turkey, No. 32, Adapazarı-G25 Sheet; General Directorate of Mineral Research and Exploration (MTA): Ankara, Turkey, 2002. [Google Scholar]
- Timur, E.; Aksay, A. 1:100,000 Scale Geological Maps of Turkey, No. 31, Adapazarı-G24 Sheet; General Directorate of Mineral Research and Exploration (MTA): Ankara, Turkey, 2002. [Google Scholar]
- Okay, H.; Boydaș, T.; Giritlioğlu, T. Lower Sakarya Basin Groundwater Reserve Report; Republic of Turkey Ministry of Energy and Natural Resources, General Directorate of State Hydraulic Works, Department of Groundwater: Ankara, Turkey, 1966. [Google Scholar]
- Sarı, İ.; Kurt, M. Prospection Report of Iron and Manganese Based Metallic Mines of Adapazarı-Sapanca-Geyve-Akyazı Surroundings; General Directorate of Mineral Research and Exploration: Ankara, Turkey, 1983. [Google Scholar]
- Wijkerslooth, P. Manganese Prospection Between Bilecik and Adapazarı (MTA Compilation Report No: 1143); General Directorate of Mineral Research and Exploration (MTA): Ankara, Turkey, 1939. [Google Scholar]
- APHA Method 2320; Standard Methods for the Examination of Water and Wastewater. American Public Health Association: Washington, DC, USA, 2005.
- Ramachandran, M.; Schwabe, K.A.; Ying, S.C. Shallow Groundwater Manganese Merits Deeper Consideration. Environ. Sci. Technol. 2021, 55, 3465–3466. [Google Scholar] [CrossRef]
- Ollivier, P.; Surdyk, N.; Azaroual, M.; Besnard, K.; Casanova, J.; Rampnoux, N. Linking water quality changes to geochemical processes occurring in a reactive soil column during treated wastewater infiltration using a large-scale pilot experiment: Insights into Mn behavior. Chem. Geol. 2013, 356, 109–125. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Van Der Weiden, M.J.J.; Tournassat, C.; Charlet, L. Surface complexation of ferrous iron and carbonate on ferrihydrite and the mobilization of arsenic. Environ. Sci. Technol. 2002, 36, 3096–3103. [Google Scholar] [CrossRef]
- Ekmekçi, M.; Gültekin, F. Evaluation of Environmental Stable Isotope Content of Eastern Black Sea Section Waters; National Engineering Geology Symposium: Trabzon, Turkey, 2015; pp. 459–466. [Google Scholar]
- Eisenlohr, T.; Pfister, M.; Balderer, W. Environmental isotope study and 2-D modelling of cold and thermal karst within the Gemlik (Bursa) area of Northwestern Turkey. In Karst Waters & Environmental Impacts; Günay, G., Johnson, A.I., Eds.; Balkema: Rotterdam, The Netherlands, 1997; ISBN 90-5410-656-4. [Google Scholar]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef]
- Boyle, R.W.; Jonasson, I.R. The geochemistry of arsenic and its use as an indicator element in geochemical prospecting. J. Geochem. Explor. 1973, 2, 251–296. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Sanchez-Valle, C.; Guillot, S.; Borisova, A.Y.; Muñoz, M.; Auzende, A.-L.; Proux, O.; Roux, J.; Hazemann, J.-L.; Testemale, D.; et al. Redox dynamics of subduction revealed by arsenic in serpentinite. Geochem. Perspect. Lett. 2022, 22, 36–41. [Google Scholar] [CrossRef]
- Faust, G.T. The hydrous nickel-magnesium silicates-the garnierite group. Am. Mineral. J. Earth Planet. Mater. 1966, 51, 279–298. [Google Scholar]
- Deuel, L.E.; Swoboda, A.R. Arsenic Solubility in a Reduced Environment. Soil. Sci. Soc. Am. J. 1972, 36, 276–278. [Google Scholar] [CrossRef]
- De Vitre, R.; Belzile, N.; Tessier, A. Speciation and adsorption of arsenic on diagenetic iron oxyhydroxides. Limnol. Oceanogr. 1991, 36, 1480–1485. [Google Scholar] [CrossRef]
- Widerlund, A.; Ingri, J. Early diagenesis of arsenic in sediments of the Kalix River estuary, northern Sweden. Chem. Geol. 1995, 125, 185–196. [Google Scholar] [CrossRef]
- Golterman, H.; Stumm, W.; Morgan, J.J. Aquatic Chemistry. An Introduction Emphasizing Chemical Equilibria in Natural Waters. J. Ecol. 1982, 70, 924–925. [Google Scholar] [CrossRef]
- Postma, D.; Larsen, F.; Thai, N.T.; Trang, P.T.K.; Jakobsen, R.; Nhan, P.Q.; Long, T.V.; Viet, P.H.; Murray, A.S. Groundwater arsenic concentrations in Vietnam controlled by sediment age. Nat. Geosci. 2012, 5, 656–661. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talay, N.; Yolcubal, İ. Hydrogeochemical Characterization and Determination of Arsenic Sources in the Groundwater of the Alluvial Plain of the Lower Sakarya River Basin, Turkey. Water 2025, 17, 1931. https://doi.org/10.3390/w17131931
Talay N, Yolcubal İ. Hydrogeochemical Characterization and Determination of Arsenic Sources in the Groundwater of the Alluvial Plain of the Lower Sakarya River Basin, Turkey. Water. 2025; 17(13):1931. https://doi.org/10.3390/w17131931
Chicago/Turabian StyleTalay, Nisa, and İrfan Yolcubal. 2025. "Hydrogeochemical Characterization and Determination of Arsenic Sources in the Groundwater of the Alluvial Plain of the Lower Sakarya River Basin, Turkey" Water 17, no. 13: 1931. https://doi.org/10.3390/w17131931
APA StyleTalay, N., & Yolcubal, İ. (2025). Hydrogeochemical Characterization and Determination of Arsenic Sources in the Groundwater of the Alluvial Plain of the Lower Sakarya River Basin, Turkey. Water, 17(13), 1931. https://doi.org/10.3390/w17131931






