Abstract
The introduction of the first list of priority pathogenic fungi by the World Health Organization stresses the need to research and develop public health actions to mitigate infections caused by fungi. One of those actions involves the water disinfection systems, which comprise classical and alternative methods that have been developed in the last decades. Thereby, this work reviews the disinfection of fungi by classical methods such as chlorination, ozonation, and ultraviolet (UV) treatments and alternative advanced oxidation processes (AOPs) such as photo-Fenton, photocatalysis, or couplings of UV with peroxides. The matrices of aquatic systems (sewage, groundwater, drinking water, among others) were considered. A bibliometric analysis is performed initially, and then some aspects of the resistance to antifungals are presented, and the efficiency of the diverse processes in the reduction in fungal loading is also revised. Herein, it is shown the role of the disinfecting agents (e.g., chlorine, hydroxyl radical, or light) and their effects on fungi structures (e.g., direct DNA damage, or indirect damage due to the action of radicals). Moreover, gaps, such as the treatment of antifungal-resistant fungi and limited information about combinations among AOPs, related to the disinfection of water polluted by fungi, were identified.
1. Introduction
The use of antifungal agents in clinical therapy, agricultural, and industrial sectors has increased significantly in the last decades [1,2,3,4,5] causing a parallel growth in the number of fungal species with intrinsic resistance, dose-dependent susceptibility, and acquired resistance to the antifungals. Nowadays, the aquatic environment is considered a reservoir of antifungal-resistant microorganisms [6,7,8,9]. Consequently, exposure to resistant pathogens such as fungi coming from drinking water sources, river water, or sewage treatment plant sludge is becoming a public health concern. The risk is extremely high for immunocompromised people, such as patients with chronic lung disease, neonates, older adults, or transplanted patients, who may develop invasive fungal diseases (IFDs). More severe is the case of IFDs caused by antimicrobial-resistant fungi. IFDs have high morbidity and mortality between 30–88%, and they are associated with 1.5 million deaths per year [10,11,12]. Then, a strategy to limit the proliferation of IFDs is the application of effective disinfection processes to aqueous systems.
Due to the concerns associated with both susceptible and antifungal-resistant microorganisms in the aqueous medium, diverse classical and alternative disinfection processes have been evaluated. The classical methods most commonly used in aquatic systems for eliminating fungi involve free chlorine and monochloramine [9,13], chlorine dioxide [14], ozone [15], and ultraviolet (UV) radiation [16]. Also, advanced oxidation processes (AOPs) (e.g., Fenton-based systems or UV/peroxy-monosulfate) have been applied [17]. Several previous reviews have dealt with the problem of decontamination of surface water, groundwater, wastewater, and drinking water polluted by microorganisms. Those works are focused on the capacity of different classical and alternative systems to eliminate pathogenic sensible or antimicrobial-resistant bacteria and resistance genes from bacteria [18,19]. In addition, there are some reviews on the decontamination of fungi in aquatic systems (Table 1). However, details about the inactivation kinetics of fungi and bibliometric analyses of the inactivation of fungi in water are still lacking (Table 1). Moreover, in the previous reviews about water disinfection [20,21,22], no information was found on the susceptibility response of fungi to antifungals and anti-fungal resistance mechanisms. Therefore, this work offers a revision of classical and advanced disinfection methods to kill pathogenic fungi. To cover the lacked information, this review shows a bibliometric analysis of fungi inactivation in water, plus the concerns about water pollution by fungi and antifungal resistance. Furthermore, a systematic review of the efficacy of classical and alternative disinfection processes to deal with fungi.

Table 1.
Previous reviews about the inactivation of fungi by classical and alternative treatments.
2. Materials and Methods
This review was conducted according to the PRISMA guide’s identification, screening, choice, and inclusion phases (preferred reporting elements for systematic reviews and meta-analyses) [25]. Multiple approaches to compiling publications were considered. We addressed the objective of methods for the disinfection of fungi and antifungal-resistant genes present in aquatic systems. Data and information associated with these interest topics from each retrieved article were extracted, further described, and analyzed, following the steps detailed below.
2.1. Search Strategy and Selection Criteria
Articles about disinfection methods for the elimination of fungi in water systems (artificial and natural) were consulted in search engines and electronic databases: PubMed, Science Direct, Scopus, WOS, Springer, Scielo, PLOS, Hinari, Redalyc, Taylor. The articles were reviewed without restriction on the year of publication. A strategy was used that incorporated thesauri such as Medical Subject Headings (MeSH) AGROVOC, the descriptors in Health Sciences (Dec’s), UNESCO, and Boolean operators AND, &) (OR,) (NOT, -) (XOR). Different combinations of keywords were made: “Disinfection AND fungi AND Waters”, “Disinfection, Elimination, Antifungal resistance”, “Chlorination, “antifungal resistance genes”, “resistant fungi in the environment”, “water systems”, “wastewater”, “sewage”, “wastewater”, “fungi in water”, “river pollution”, “aquatic environment”, resistance azole antifungals, advanced oxidation process, Ozonation, Fenton/solar Fenton oxidation, Heterogeneous photo-catalysis, Ultraviolet irradiation, Ionizing irradiation. Mendeley® (https://www.mendeley.com/) was used as the reference manager system.
2.2. Eligibility Criteria
Each article included in this systematic review met the following inclusion criteria: (1) The search strategy was restricted to original studies or abstracts published in scientific journals in the English language. We considered the search terms present in the title, abstract, and keywords without restriction of place and time; (2) the search response variable was information describing the different methods of disinfection of fungi in natural and artificial or aquatic environments; (3) systematic reviews, editorials, and policy statements were excluded.
2.3. Search for Articles
For the selection of articles, initially, the keywords were delimited, and the potential articles were identified with the search algorithm (Figure 1). Then, the bibliographic information regarding the main author, date of publication, journal, title, and abstract was tabulated in a spreadsheet. The papers that were relevant and provided useful information for our work (focused on fungi disinfection, antifungal resistance, and water treatment) were selected and subsequently reviewed in their entirety. The articles selected from the previous step were re-read under the inclusion and exclusion criteria. Additionally, a secondary search was conducted in congress abstracts, Colombian and foreign consensus on the subject, and the references of the articles selected for full-text reading (“snowball” strategy).
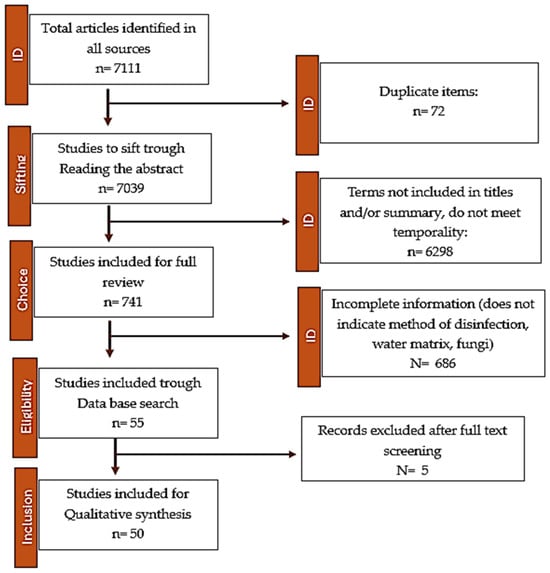
Figure 1.
PRISMA flowchart of the search strategy and selection of articles reporting disinfection methods in aquatic environments.
2.4. Data Extraction
All articles included were reviewed by two people independently using standardized data extraction tools prepared in a Microsoft Excel spreadsheet. Disagreements were resolved by reviewing the entire article. When the two people did not reach a consensus on a specific article, a third person was introduced. The PRISMA flow chart shows the number of articles at each step of the article selection process (Figure 1).
2.5. Bibliometric Analysis
2.5.1. Search for Results
A total of 7111 potentially relevant studies were identified through the database search for the years range: 1983–2022. There were 72 duplicates, and 7061 were excluded based on title and abstract selection. A total of 50 studies [all 50 references] were included in the final review (Figure 2), which were conducted in countries from Asia, Africa, Europe, North America, South America, and Central America, as shown in Figure 2 (see also Table S1 in the Supplementary Material). The largest number of the considered studies came from China (~40.0%). It can be mentioned that studies on aquatic systems (drinking water, hot water systems, groundwater, wastewater, and synthetic wastewater) (see Table S1 in the Supporting Information) using classical disinfection methods (13 studies) were conducted in Asia (China, Iran), Europe (Portugal), North America (United States), and Central America (Cuba). Meanwhile, for alternative disinfection processes (37 studies), the study places were distributed in Asia (China, Iran, Vietnam), Europe (Spain, Portugal, Ireland, Ukraine), Africa (Algeria, Egypt), and South America (Brazil, Colombia).
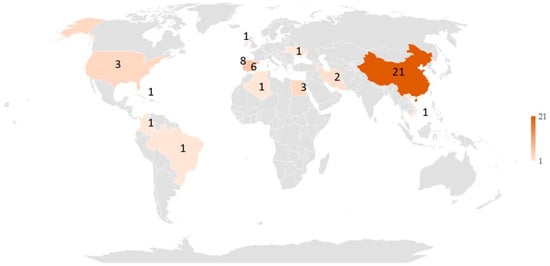
Figure 2.
Worldwide distribution of studies about classical and alternative disinfection methods for fungi inactivation in aquatic systems. Articles per country: China (21), Iran (2), Spain (6), Portugal (8), Ukraine (1), Ireland (1), Vietnam (1), Algeria (1) Egypt (3), United States (3), Havana (1), Brazil (1), and Colombia (1).
Figure 3 shows the annual number of papers about fungal control in the field of disinfection from the first article published in 1983 until 31 December 2022. Figure 3 reveals that the number of publications in this field has increased significantly over the last 5 years. Meanwhile, the cumulative annual number of publications is growing slowly. This indicates that research in the field of disinfection is expanding, and it is of great interest due to the importance of the control of pathogenic fungi from a public health and environmental point of view.

Figure 3.
Annual distribution of the number of publications on disinfection processes for fungi inactivation.
2.5.2. Characteristics of the Included Studies
Chlorination and ultraviolet (UV) light are the most common classical disinfection methods in aquatic systems, accounting for 63.6% (14/22) of these tools used (Figure 4A). Meanwhile, for alternative disinfection methods, advanced oxidation processes such as the combination of UVC light with peroxymonosulfate (PMS), peroxydisulfate (PDS), hydrogen peroxide (H2O2) are the most studied techniques (Figure 4B). Studies from Asian countries reported both classical disinfection methods (45.8%) and alternative methods (54.1%). In European countries, works about advanced oxidation processes were higher (68.8%) than classical disinfection methods (31.2%).
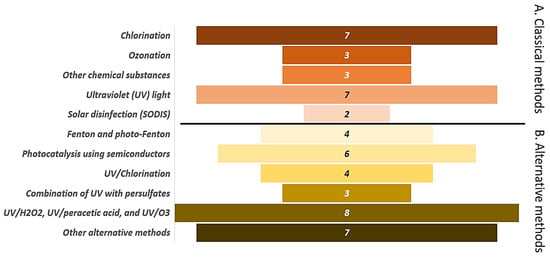
Figure 4.
Disinfection methods for the treatment of fungi in aquatic systems. (A) Classical methods. (B) Alternative methods.
In the Americas, papers from the United States and Cuba described the use of classical treatments (4/6, 66.7%), while works in Brazil and Colombia reported the application of alternative methods (2/6, 33.3%) such as photo-Fenton, TiO2-photocatalysis, and plasma activation. In turn, studies from African countries only considered alternative disinfection methods. Regarding the fungal strains, the most commonly studied are A. niger (16.7% classical vs. 16.4% alternative); P. polonicum (13.0% classical vs. 10.9% alternative); T. harzianum (9.3% classical vs. 10.9% alternative); and A. flavus (5.6% classical vs. 1.8% alternative). In the cases of C. albicans (14.5%) and F. solani (9.1%), they were treated with conventional disinfection methods.
Figure 5 shows the log removal rate of fungal species with the different alternative and classical disinfection methods. The classical methods show a higher number of analyzed species, with 77.8%; the fungi Aspergillus fumigatus (4.6 log) and Penicillium polonicum (5.0 log) are the ones with the highest inhibitions. On the other hand, in the alternative methods, Trichoderma sp. (4.5 log) and Acremonium sp. (4.0 log) are outstanding microorganisms.
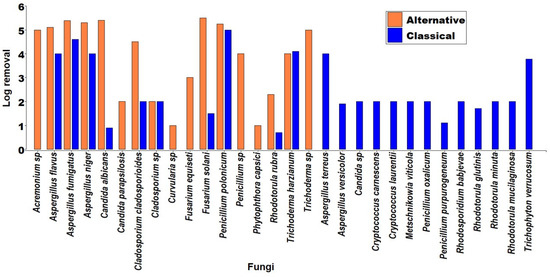
Figure 5.
Fungal strains that have been used in the classical and alternative methods for disinfection of aquatic systems.
3. Medical Impact of Fungi and Pollution of Aquatic Media by Fungi
3.1. Health Problems Related to Fungi
Fungi are eukaryotic, achlorophyllous, and saprophytic microorganisms. They are considered to be the second most abundant group of eukaryotic organisms in nature after insects. Fungi are ubiquitous, and they are both exogenous and endogenous organisms in plants, animals, and humans [26]. Despite the multiple benefits that fungi provide to some industries (e.g., fermentative processes and production of enzymes or metabolites [27]), they have also been associated with health problems (~1.5 million deaths and 1.7 billion superficial infections per year [26]). For instance, Candida, Cryptococcus, Aspergillus, Pneumocystis, and Fusarium genera are the most involved in invasive fungal infections (IFIs) [26,28]. IFIs are a pathology with a high mortality rate, which can range from 30 to 88%. It can be mentioned that among IFIs, candidemia, and invasive candidiasis are very frequent. The epidemiology of candidemia and invasive candidiasis varies according to geographic location, with C. albicans and C. parapsilosis prevalent in South America in Brazil, Argentina, and Colombia [29].
3.2. Fungi in Water Samples
Yeasts and filamentous fungi are common components of aquatic microbial communities. They are present in diverse aqueous matrices such as sewage water [6,30], freshwater, brackish, marine [28,31,32] and hospital wastewater [33], springs, lakes, reservoirs [34], rivers, and surface and groundwater [35]. Fungi have been found in the water supply in countries such as Germany, Greece, and the United Kingdom, causing problems of turbidity, bad taste, and the presence of mycotoxins [28,35]. Additionally, the presence of fungi in showers in hospitals or health centers has been reported, which endangers immunosuppressed people or patients in intensive care units [28]. Besides this, fungi such as Aspergillus, Penicillium, and Fusarium are recognized as sources of waterborne issues. Such issues are considered a public health problem due to their association with invasive fungal diseases [36].
On the other hand, the most-reported genus of microorganisms in diverse water matrices (i.e., rivers, lakes, and effluents of wastewater treatment plants) comprises Candida, Cryptococcus, Kodamea, Rhodotorula, Trichosporon, Aureobasidium, Debaryomyces, Pichia, Saccharomyces, Aspergillus, and Fusarium [7,37,38]. Particularly, Fusarium spp., Penicillium spp., Scedosporium spp., Rhinocladiella spp., Mucor spp., Rhinocladiella spp., Emmonsia spp., Scopulariopsis spp., Geotrichum spp., Trichoderma spp., Pithomyces spp., Phoma spp., Paecilomyces spp., and Candida spp. are reported in hospital water systems [39,40,41,42] (see Table S2 in the Supplementary Materials).
4. Antifungals in the Environment and Antifungal Susceptibility/Resistance
4.1. Antifungals in Aquatic Systems
Antifungal agents are determinants in the prevention and treatment of superficial and systemic mycoses in both humans and animals [43]. However, knowledge about their effects on the environment is still incipient. Antifungal compounds reach aquatic systems through wastewater from domestic, hospital, industrial, and livestock sources, even rainwater runoff (in the case of some antifungals used in agricultural/farming activities) [4,5,44,45]. When antifungals interact with environmental mycobiota (i.e., the microorganisms that play a significant role as decomposers of organic matter), these microorganisms are altered. In the environment, antifungal compounds can contribute to the stress and deterioration of native fungi, also affecting the quality of life of other aquatic populations [46,47].
It is important to mention that the demand for antifungals for human and animal uses has increased in recent decades. Hence, the high prescription in medical institutions and the over-the-counter sales in pharmacies or supermarkets inevitably produce wastes of non-consumed antifungals [1]. These wastes are finally disposed of in landfills, where non-treated leachates containing antifungals are produced and discharged into the environment [3,7,46,48] On the other hand, diverse farming antifungals are used to increase production in agriculture on high-value crops, such as fruit trees, corn, tomato, sugar cane, rice, and banana.
In agricultural activities, not all of the initial amount of the fungicide reaches the plant; a portion of the antifungal ends up in the soil, groundwater, or surface water [4]. Also, agricultural fungicides are released by the rain and then reach the environment [49,50,51]. Furthermore, in the animal production sector, antifungals are utilized for treating superficial and deep infections at individual or collective levels [52]. Antifungals are also added to water in aquaculture [53]. Additionally, if insufficient care is taken, they can reach the soil, waste dumps, leachates, sewage, and environmental surface water [54].
Many personal care products (e.g., paints, cosmetics, food preservatives, papers, soaps, shampoos, and creams) on the market have antifungal substances. They find their way into sewage or wastewater systems after being used in households [3,55]. In addition, not all initial amounts of antifungals consumed or applied to the skin, hair, and mucous membranes are assimilated/metabolized by our bodies during health treatments. A part of antifungals is expelled through urine or feces, or released during bathing and washing. Subsequently, antifungals reach sewers, sewage canals, and municipal wastewater treatment plants [7,56]. As the typical physical and biological processes in municipal sewage plants are not able to degrade antifungals, they end up in the environment [46,48,57].
4.2. Susceptibility, Resistance to Antifungals, and Resistant Fungi in the Aquatic Systems
In medical applications, three groups of antifungals (polyenes, azoles, and echinocandins) are available for the treatment of IFIs [58,59]. Some of these compounds are useful for various etiological agents. For instance, azoles and echinocandins have been used for Candida as well as for Aspergillus [60,61]. In turn, polyene antifungals (such as amphotericin B) are employed to deal with strong species (e.g., Cryptococcus) [62]. As previously mentioned, antifungals are useful to control diseases such as IFIs; however, the proliferation of antifungal-resistant microorganisms is a currently increasing and warning situation [43,63,64,65,66,67,68]. Before the massive use of antifungals in agricultural and household activities, almost all filamentous fungi and yeasts were susceptible to these agents. Nevertheless, uncontrolled use of antifungals has altered the epidemiology of IFIs, leading to resistant fungi to one or several groups of antimycotics, which are responsible for high mortality and therapeutic failure cases [69,70].
Studies of the epidemiology of IFIs and antifungal susceptibility have been performed, especially in developed countries, thanks to data collected and published by various sentinel and population-based surveillance programs [71,72,73]. Such studies represent an advancement to face antifungal resistance. Indeed, in the United States, the Antifungal Surveillance Program-ARTEMIS is raising alarms about the increase in cases involving multidrug-resistant yeast strains. Each region must consider the use of these different antifungal agents, the appearance frequency of the different fungi species, and their drug-resistant phenotype [74].
To determine antifungal resistance, susceptibility tests are carried out. Indeed, tests using amphotericin B, itraconazole, fluconazole, econazole, ketoconazole, and micafungin are commonly reported in the literature [75,76,77,78]. Some works have informed the study of fungal species present in aquatic systems and their susceptibility to distinct groups of antifungal agents [42,79,80,81]. For instance, Milanezi et al. tested 104 samples and found that 95 yeast isolates and 63 were resistant to at least one antifungal of the azole class. There was a predominance of resistance to the antifungal itraconazole (57.9%), followed by fluconazole (44.2%), voriconazole (24.2%) and ketoconazole (5.3%) [8].
Other studies have revealed that in the last two decades, antifungal resistance in Aspergillus and Candida species has increased worldwide. This resistance is not only in environmental isolates (aquatic systems, air, soil) but also in hospital settings, with C. albicans and A. fumigatus being the species most associated with IFI [82,83,84,85]. A. fumigatus [86,87] and different Candida species resistant to azoles and multidrug-resistant (MDR) Candida auris have been reported [87,88]. Additionally, there are fungi with intrinsic resistance, such as C. krusei (resistant to fluconazole) and Aspergillus terreus (resistant to amphotericin B) [89,90,91].
The most common resistance mechanisms are molecular mutations of the antifungal enzyme target, alteration of enzymes related to ergosterol synthesis, and alterations in efflux pumps, ATP binding cassette (ABC), or major facilitators (MF) [92,93,94,95] For instance, azole resistance of Candida and Aspergillus species generally occurs by two main mechanisms: Alteration of genes in the metabolic pathways of ergosterol synthesis and changes in drug efflux [95]. Overexpression of the ERG11 gene has been observed in several Candida species, such as C. albicans, C. parapsilosis, and C. tropicalis [96]. Mutations in this gene can lead to amino acid substitution, altering the target protein of the azole antifungals. More than 140 substitutions have been found in Candida spp. Furthermore, in A. fumigatus, there is overexpression of the CYP51A gene, and mutations of this gene with more than 30 substitutions are known [52,97,98].
We should remark that resistance is not only associated with the utilization of medical antifungals. Several fungicides used in agriculture on fruit trees can also promote antifungal resistance in crops [53,99,100,101]. Moreover, some publications have suggested a possible relationship between the use of azole fungicides in agrochemicals. The presence of resistance genes TR34/L98H and TR46/Y121F/T289A mutations in the CYP51A gene (and its promoter region) of Aspergillus fumigatus among people who had never been treated with antifungal agents is an indicator of this phenomenon [4]. Such resistance genes have been reported in clinical and environmental isolates from Europe, Asia, and Africa, but not from the 22 states in the United States [44] or Latin America, where no fungicide use-related resistance is shown [5,45,102,103].
Le Pape et al. conducted a soil survey of greenhouse flower fields and found 38/60 (63%) Aspergillus fumigatus strains resistant to two azole antifungals (itraconazole and voriconazole). The results also showed the presence of mutations TR46/Y121F/T289A (n = 17), TR34/L98H (n = 1), and TR53 (n = 1) and one isolate had a wild-type CYP51 sequence. Thus, there was evidence of the need to implement agricultural surveillance and monitoring of antifungal resistance in isolates from animals and humans [104], in addition to the research for resistant environmental strains, and the evaluation of pesticide uses in floriculture, agriculture, and livestock systems [74,97,98,105]. In the specific case of aqueous media, some studies have reported that filamentous fungi and yeasts, after passing through municipal treatment plants, can acquire resistance to one or several antifungals [58,99,101]. Also, rivers receiving effluents from municipal wastewater treatment plants and water runoff from agricultural areas contain more resistant microorganisms than areas upstream of the sources of pollution [106,107,108].
It is important to note that due to the same classes of antifungals being used in humans and animals and a few new antimycotics being developed to replace those that have become resistant. Thus, there is a need to formulate management strategies to prevent or contain resistance [109,110]. These control strategies should include the prudent use of antimicrobials, surveillance of their use in food-producing animals, surveillance for emerging resistance in both the human health and veterinary sectors [111,112], and appropriate education and training of farmers and prescribers. Also, the application of proper and effective physical-chemical disinfection treatments to fungi in water is relevant to facing the antifungal resistance problem (see Table S1). Indeed, diverse research works using conventional and alternative methods to inactivate fungi in water samples have been published. Therefore, this work aims to review the topic of the treatment of water polluted by fungi using alternative and conventional processes, as detailed in the following sections.
5. The Methods for Fungi Elimination in Aquatic Systems
Conventional and alternative methods, which comprise physical, chemical, and physicochemical systems, have been applied to disinfect aquatic systems polluted with fungi. Some physical methods transfer the fungi from one phase to another. For instance, coagulation removes both particles and microorganisms from the liquid phase to the solid phase [113]. Meanwhile, in the chemical processes, strong oxidants are used, and they can react with the basic components of the fungus cell or alter the metabolism of fungi and ultimately inactivate it. However, oxidative reactions do not affect one substrate specifically, and other organic and inorganic components in the water matrix may be involved in the process [114,115,116,117]. Furthermore, only one method of treatment can have drawbacks (such as the tolerance of microorganisms to the process action, or unsafe/limited operational procedures), so the combination of two or more processes has been proposed, resulting in novel and alternative systems [9,17,118]. Below is reviewed the utilization of classical processes (e.g., chlorination, ozonation, and ultraviolet irradiation (UV)) and alternative methods (e.g., UV/chlorine, UV/ozone, UV/persulfates, photo-Fenton, and heterogenous photocatalysis) for the fungi inactivation in aqueous matrices.
5.1. Classical Processes for Fungi Inactivation
5.1.1. Chlorination
Water treatment by chlorination is very well known. Chlorination disinfection is widely used in drinking water treatment plants because of its high efficiency, safe use, and low economic costs. Among the disinfectant species involved in the chlorination methods, molecular chlorine (Cl2, E° = 1.36 V), hypochlorous acid (HOCl, E° = 1.2–1.3 V), and hypochlorite ion (OCl−, E° = 0.65–0.85 V), are mainly distinguished, as a function of the pH (Equations (1) and (2)). It has been established that the chlorine species (i.e., Cl2, HOCl, and OCl−) in aqueous samples can oxidize organic and inorganic matter and inactivate pathogens (e.g., fungi and bacteria). In the case of fungi, chlorine species can induce damage at extracellular sites such as the cell wall and membrane. At the intracellular level, chlorine species attack sites such as nucleic acids, enzymes, and coenzymes [119,120]. However, the biocidal mechanism of the chlorine species is not completely understood, and a multiple-impact approach has been considered [118,121].
The efficacy of chlorination disinfection is dependent on the pH, fungal species, and water matrix [13]. For example, Rosenzweig et al. evaluated the chlorine demand of filamentous fungal conidia and yeast vegetative cells at three different levels [122]. The fungi were isolated from tap water and then scraped off agar slants and washed three times with chlorine demand-free water (~7 mg L−1) at pH 5, 7, or 8 and measured chlorine demand at multiple incubation times (10, 30, and 60 min). The fungal conidia and yeast showed free chlorine demand, with the former exhibiting the highest resistance. The inactivation occurred in the order pH 5 > 7 > 8, and molds were more resistant than yeasts due to differences in cell permeability [1] (see Table 2).

Table 2.
Removal of fungi by chlorination.
Pereira et al. (Table 2) tested the effectiveness of free chlorine in inactivating distinct species of filamentous fungi (i.e., Cladosporium tenuissimum, C. cladosporioides, Phoma glomerata, Aspergillus terreus, A. fumigatus, Penicillium griseofulvum, and P. citrinum) in surface water, spring water, and groundwater, considering the effects of pH and temperature. At acid pH (6.0) and elevated temperature (25 °C), the disinfection was more effective due to the higher proportion of hypochlorous acid (Equation (2)) and higher rates of reaction. Species showed varied recalcitrance to chlorine; Cladosporium, Penicillium, and Trichoderma were the most resilient in a real water matrix. The resistance was dependent on cell structure, cell wall composition, and cell size [13].
Another type of disinfection process that involves chlorine-based species is the use of chloramines (R2N-Cl). Ma X. et al. (2015) studied the in-situ disinfection of fungi using monochloramine in a hospital hot water system. No considerable changes in fungal community structure were observed before and after the treatment [123]. In another study, Ma X. et al. (2017) [124] showed that A. versicolor and Penicillium species exhibited the highest resistance to disinfection using free chlorine and monochloramine, compared to Aspergillus fumigatus type (ATCC 1022) and other clinical isolates (Table 2).
In addition to the use of chloramines, chlorine dioxide is also applied in chlorination systems. Wen, G. et al. [14] have evaluated the effects of chlorine dioxide concentration, pH, temperature, and the presence of humic acid on the treatment of three fungal spore species (Cladosporium cladosporioides, Trichoderma harzianum, and Penicillium polonicum; see Table 2). The authors reported that the inactivation rate constant for chlorine dioxide was significantly higher than that obtained for free chlorine. Additionally, chlorine dioxide concentration and temperature positively influenced the removal of microorganisms, whereas humic acid and water matrices retarded fungal inactivation due to competing effects.
Comparisons in the degree of disinfection between chlorine, chlorine dioxide, and chloramine have been reported. Luo, Z. et al. [125] observed that at low concentrations of disinfectant, chlorine dioxide avoided biofilm formation by A. niger, P. polonicum, and T. harzianum fungi. They reported a higher efficacy of chlorine dioxide than chlorine and chloramine. Also, Zhang, H. et al. [126] stated that the inactivation rate constants of Aspergillus fumigatus using chloramines were up to 4 times lower than those rate constants for monodisperse spores. This is explained considering that among the factors affecting the inactivation efficiency are the higher strength of the outer coat and the aggregation capacity of the spore.
Concerning the inactivation kinetics of fungi by chlorine-based processes, we can say that they fit the Chick, Chick–Watson, and Hom models [14], or the retarded Chick–Watson model [124]. The Chick, Chick–Watson, and Hom models are summarized by Equations (3)–(5):
where Nt represents the number of fungal spores (in CFU mL−1) at time t; N0 means the number of fungal spores at time zero (CFU mL−1); k inactivation rate constant (L mg−1 for the Chick model, and L mg−1 min−1 for the Chick–Watson model and Hom model); C denotes the concentration of disinfectant (mg L−1); t is reaction time (min); n and m are the kinetic parameters. The Chick–Watson model is used when the microorganisms are assumed to be genetically similar or of the same strain, and the disinfectant action is considered under an irreversible bimolecular reaction. Adjustments to this model consider the complexity of the structures of the microorganisms, the transport mechanisms, and the different chemical reactions that take place to inactivate them.
In turn, the retarded Chick–Watson model is represented by Equations (6)–(8):
where N is the number of viable cells, N0 is the initial number of viable cells at time zero, is the random error with a mean of 0 and variance σ2, is the inactivation rate constant, is the integrated product of disinfectant concentration and contact time over defined time intervals, and is the lag coefficient (Equations (7) and (8) describe an initial lag phase followed by pseudo-first-order inactivation); if the equals zero, the delayed Chick–Watson model is in the form of the classical Chick–Watson model [124].
The inactivation kinetics by chlorine dioxide action have been fitted to the Chick (Equation (3)), Chick–Watson (Equation (4)), and Hom (Equation (5)) models, with the Chick–Watson model having the best fit, with a correlation coefficient (R2) of 0.99, followed by the Hom model with an R2 of 0.92 and the Chick model with an R2 value of 0.62. Meanwhile, fungi inactivation using monochloramine has shown a good fit to the retarded Chick–Watson model (Table 2).
On the other hand, according to Table S3, which summarizes the removal efficiency for fungi, it could be established that the chlorination methods are strongly dependent on the structural characteristics of each fungi species, having low disinfection for some yeasts (e.g., Rhodotorula mucilaginosa) and pigmented molds (e.g., Cladosporium sp.).
5.1.2. Ozonation
Ozone (O3) has been used for more than a century as a disinfectant against waterborne pathogens. It is a chemical compound with a dipole that can dislocate its electrons. O3 has a high redox potential (E° = 2.07 V), which provides it with good disinfection efficiency toward pathogenic microorganisms [127]. Ozone is currently used in wastewater treatment plants as a disinfectant before discharge into environmental water and for drinking water purification [127].
Ozone can destroy cell walls and membranes with the loss of intracellular components. O3 can also induce internal oxidative stress and changes in esterase activity in fungal spores. In addition, ozone is more effective than chlorine and chlorine dioxide in reducing fungal contamination in drinking water. We should mention that to determine the effectiveness of ozone mechanisms in the inactivation of fungi, different methodologies have been used. Such methodologies comprise the determination of proteins and total extracellular nitrogen; flow cytometry to measure changes in spore activity indices; dihydroethidium dye (DHE) to determine intracellular reactive oxygen species (ROS), and scanning electron microscopy (SEM) observations. Moreover, the heterotrophic colony plate count per mL (CFU mL−1) method is also used [15].
Ozone inactivates viruses and bacteria in the treatment of drinking water and wastewater [128,129], in addition to the inactivation of yeasts and filamentous fungi in natural and artificial aquatic systems [15,130,131]. Aspergillus, Penicillium, and Trichoderma harzianum are the most treated fungi species by the ozonation processes (Table 3). Besides these fungi, the dermatophyte fungus Trichophyton verrucosum (a common etiologic agent of bovine ringworm) has also been inactivated by ozonation. This is used as an alternative to the thermal sterilization of water in vaccine production. In turn, Perez-Rey et al. established that the dermatophyte fungus Trichophyton and some bacterial spores are more resistant to treatment than viruses; i.e., there is a direct relationship between the structure of the microorganism and recalcitrance to the ozone action [130].

Table 3.
Removal of fungi by ozonation.
Beyond the nature of the microorganisms, the operational parameters also play an important role in the ozonation process, as demonstrated by Wen et al. [15]. They showed that fungicidal activity and inactivation kinetics by ozone action on Aspergillus niger, Penicillium polonicum, and Trichoderma harzianum isolated from groundwater were higher when increasing the initial ozone concentration. Meanwhile, the changes in pH and temperature did not show significant differences. On the other hand, in such a work, the authors determined changes in morphology, intracellular compound leakage, membrane integrity, ROS level variation, and spore esterase activity. The reported results indicated that the fungicidal efficacy of ozone was superior to chlorine and chlorine dioxide, destroying cell walls and membranes first, and causing the release of intracellular compounds.
Some differences in the responses of the three fungi toward O3 were observed. The differences among the three fungi (in spore forms) were ascribed in part to the size of the spores. The diameter of the A. niger spore (2.67 ± 0.12 μm) was larger than those of T. harzianum (2.25 ± 0.22 μm) and P. polonicum (2.16 ± 0.48 μm). The lowest specific surface area of A. niger spores made it more difficult to be inactivated by ozone. In addition to size, cellular structures also play a relevant role during the inactivation by O3. In fact, fungi have higher resistance to ozone than bacteria and viruses, which can be ascribed to their more complex structures, such as eukaryotic cells.
Another strategy applied in the ozonation process is the combination with chlorination. Liang, Zhiting, et al. [131] (Table 3) investigated the efficacy and synergistic effects of combined inactivation of fungal spores using ozone and chlorine in water (Table 2). The combined effects on Aspergillus niger and Trichoderma harzianum spores led to 0.47 and 0.55 log in 10 min, respectively. The inactivation efficacy of both ozone and chlorine on fungal spores increased while decreasing pH and increasing temperature. The results of intracellular leakage of total nitrogen were superior to those caused by individual inactivation by ozone or chlorine, due to high ROS production in the combination [132].
On the other hand, the kinetics of inactivation generally follow the first-order Chick–Watson model (Equation (9)), whereby the number of viable microorganisms decreases exponentially with exposure to the disinfectant [133].
where N is the number or concentration of the microorganism, N0 is the initial number of fungi, kwc is the inactivation or lethality constant, C is the disinfectant (O3) concentration initial, and t is the contact time.
In some cases, because of repair mechanisms in the damaged microorganisms, disinfection does not occur at low concentrations and treatment times. Then, the model that describes this situation is the following (Equations (10) and (11)):
This model is characterized by a time, t0, corresponding to an exposure (C t)0, during which there is no inactivation and which is followed by a first-order loss of viability [133].
We should remark that ozone-based disinfections show higher fungi removal compared to chlorination methods, independent of the treated fungal species. However, it is a costly method that requires an important infrastructure to generate ozone in situ to inactivate fungi, but possible leakage of O3 into the environment must be limited.
5.1.3. Other Disinfection Methods Using Chemical Substances
In addition to chlorine or ozone, other substances have been used to inactivate fungi. This is the case with peracetic acid, 1-bromo-3-chloro-5,5-dimethyl hydantoin (BCDMH), aluminum sulfate, and ferric chloride. Peracetic acid (PAA, CH3COOOH) is a weak acid (pKa:8.2) and a strong oxidizing agent. The standard redox potentials of PAA are 1.75 V and 1.01 V versus standard hydrogen electrodes (SHE) at pH 0 and pH 14, respectively. PAA has a high disinfection capacity, which is 40 times higher than H2O2. It is used as a broad-spectrum antimicrobial, sanitizer, and disinfectant compound in multiple industrial applications, and it is considered a good substitute for chlorine disinfection [134].
Zuo et al. developed a study on the inactivation of A. niger and A. flavus spores in wastewater using PAA. They showed that the inactivation of fungal spores fitted well with the lagged Chick–Watson model (R2 = 0.99). The k values were 0.038 and 0.033 L mg−1 min−1 for A. niger and A. flavus with 10.0 mg L−1 PAA in 45 min, respectively. It can be mentioned that the inactivation of the target fungi was attributed to oxidizing action after cell membrane penetration by PAA rather than cell membrane damage [135].
In the case of the BCDMH reagent, it has been used to deal with A. niger and P. polonicum in water. In a work performed by Wen et al., the inactivation efficiency of BCDMH on A. niger and P. polonicum collected from groundwater was evaluated. In addition, the effect of the addition of iodide or bromide ions was tested. The inactivation kinetics of fungal spores fitted to the Chick–Watson model have an inactivation rate constant of 0.011 and 0.034 L mg−1 min−1 for A. niger and P. polonicum, respectively.
Slow hydrolysis of BCDMH produces hypochlorous acid and hypobromous acid (which can also act as a disinfectant) in aqueous media (Figure 6). Nevertheless, hypobromous acid can generate brominated by-products that exhibit higher genotoxicity and cytotoxicity compared to chlorinated by-products. Furthermore, the addition of 40 μM Br− or I− induced an inactivation efficiency enhanced up to 36-fold for A. niger and up to 15-fold for P. polonicum [136]. The enhanced inactivation efficiency by BCDMH/I− was mainly due to the rate constants that favored the formation of hypoiodous acid. HOI has a higher fungicidal capacity; although its redox potential (0.99 V) is lower than that of HOCl and HOBr, the reactions of HOI with some organic compounds (such as phenols) are very fast [137].
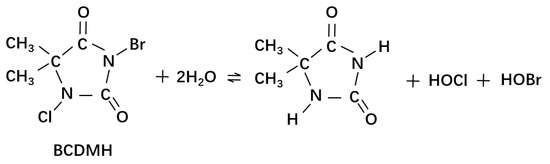
Figure 6.
Hydrolysis of BCDMH reaction.
On the other hand, Al-Gabr et al. determined the efficacy of two coagulants (aluminum sulfate and ferric chloride) at different concentrations to treat drinking water polluted by A. flavus. Aluminum sulfate and ferric chloride at concentrations of 30–50 mg L−1 were effective in removing the fungus. The lower rate of 10 mg L−1 of each coagulant concentration reduced the fungi less than the highest rate of 50 mg L−1. Also, the results revealed that both coagulants were effective in removing fungi and decreasing the turbidity of drinking water, and at the highest concentration of the coagulants, A. flavus was decreased by 99.6% in the treated water [113].
5.1.4. Ultraviolet (UV) Light for Fungi Inactivation
Disinfection based on UV irradiation is a classical process. It has long been used for cleaning aquatic systems, utilizing low-pressure (LP) and medium-pressure (MP) mercury vapor lamps, which emit germicidal UVC light. The LP lamp emits monochromatic emission at a wavelength of 254 nm, and a medium-pressure mercury lamp (MP) emits polychromatic light over a wide range of wavelengths, 200–600 nm [138]. Lamps emitting other UV wavelengths (UV-B: 280–315 nm) and light-emitting diodes (LED) with wavelengths in the UVA range (315–400 nm) are also used for disinfecting water [139]. It must be mentioned that LEDs are solid-state semiconductor devices that emit a narrow spectrum of light, convert direct current (DC) to light via electroluminescence, and can be used over a wide range of wavelengths.
Some UV LEDs can also emit monochromatic light between 210 and 400 nm, use low voltages/currents and battery/solar cell energy supply, and exhibit good germicidal activity. Recently, the use of LEDs has been gaining attention in the disinfection process compared with conventional lamps. LEDs have the advantages of no use of mercury, instantaneous warm-up time, intermittent flux, a smaller lamp size (~1 mm2), and easy integration into well-established reactors [10,140].
The UV irradiation interacts with cellular components, inducing the inactivation of microorganisms. For instance, UV can inactivate microorganisms, involving internal photoreactions and the formation of reactive oxygen species (e.g., H2O2, O2*−, or HO*) [141,142]. Some of the biological effects of UV are growth reduction, denaturation of proteins, formation of DNA pyrimidine dimers, and destruction of the cell membranes of microorganisms [143,144]. Particularly, UVC light can act on DNA molecules, which absorb UV photons between 200 and 300 nm. UVC causes deterioration by altering the pairing of nucleotide bases, which has been related to genomic damage [145].
UV light has been applied for the inactivation of fungi to decontaminate aquatic systems [146,147,148]. For example, Nourmoradi et al. [149] used a continuous flow UV reactor that could be applied as a point-of-use (POU) system to study the inactivation of Aspergillus fumigatus, A. flavus, and A. niger by UV irradiation. The results determined a significant difference between the inactivation rate of A. fumigatus compared to A. niger and A. flavus (p < 0.05) (Table 4). This is related to the characteristics of the microorganism itself. Besides, the efficiency of fungal spore removal by UV irradiation had a significant decrease with increasing turbidity and iron concentration in the water matrix, due to the competence by the UV photons.

Table 4.
Removal of fungi by UV radiation.
The efficacy of low-pressure ultraviolet light (LP) lamps to inactivate different yeast species isolated from drinking water sources, such as Candida sp. Cryptococcus carnescens Metschnikowia viticola/Candida kofuensis, Rhodosporidium babjevae, Rhodotorula minuta, and Rhodotorula mucilaginosa, has been evaluated by Pereira et al. [150]. It can be remarked that UV fluences below 32 mJ cm−2 were sufficient to achieve 99% inactivation levels for Cryptococcus, Candida, and Metschnikowia species (Table 4). Rhodotorula and Rhodosporidium species were more resistant than the other yeast species because the fungi species coming from surface water can acquire photoprotective resistance as a defense mechanism against the permanent solar conditions to which they are subjected. In turn, Rhodotorula mucilaginosa isolated from groundwater was more susceptible to UV radiation than the same species isolated from surface water.
Wen and co-workers [93] have determined the effects of temperature (5–35 °C) on photoreactivation (i.e., exposure to UVA light that induces the microorganism recovery) and dark repair of Trichoderma harzianum, Aspergillus niger, and Penicillium polonicum in phosphate buffer solution (PBS) and real groundwater after inactivation by UVC light. The resistance of the three fungal spore genera to ultraviolet radiation was indistinguishable in real groundwater. The order of resistance determined was: T. harzianum < P. polonicum < A. niger. The photoreactivation of fungal spores was promoted at higher temperatures, and the long-term dark delay can attenuate the photoreactivation of A. niger and T. harzianum, but not P. polonicum. However, the percentage of photoreactivation of the three fungal spores in real groundwater was significantly higher than in PBS (p < 0.05). Furthermore, the dark repair of fungal spores in real groundwater was not significant.
Wan et al. [151] compared the inactivation efficiency of Aspergillus niger, Penicillium polonicum, and Trichoderma harzianum of ultraviolet light-emitting diodes (UV-LED at 265 nm or 280 nm) with LP of 254 nm [151]. The monitoring of fungal growth, viability, and metabolic activity by flow cytometry allowed the authors to determine the membrane permeability and the level of intracellular ROS of the fungal spores. The authors found that the efficiency of UV inactivation (UV-LED and LP) was not affected by the incubation time of fungal spores. The UV-LED inactivation rate constants were significantly higher, with more severe damage to the fungal spore membrane than LP irradiation. The UV-LED irradiation presented higher inactivation efficiency attributable to higher ROS production, which could promote the permeabilization of fungal spores, thus inhibiting photoreactivation more efficiently.
Oliveira et al. have also employed UV-LED to inactivate fungi. Moreover, they have applied flow cytometry monitoring with fluorescence-based viability staining and scanning electron microscopy (SEM) to evaluate the phenotypic changes of the fungal spores of Aspergillus, A. fumigatus, A. niger, and A. terreus after the UV-LED disinfection process [152]. Oliveira et al. used UV-LED radiation at 255 nm and 265 nm in real water (Table 3), establishing that at 265 nm, the inactivation of fungal species was more effective (due to greater damage and inhibition of cell division and growth).
In subsequent studies, Oliveira et al. [153,154] establish that exposure to LEDs emitting light at 265 nm generates mitochondrial dysfunctions such as ROS production, excessive melanin deposition in the cell wall, the production of heat shock proteins, and DNA repair proteins. After exposure to UV-LED irradiation, no new proteins were detected in A. niger. However, in A. fumigatus and A. niger, the formation of cyclobutane pyrimidine dimers was observed after 60 min of exposure to LEDs emitting at 265 nm (Table 4).
On the other hand, the UV inactivation kinetics is determined from the fluence-response curves using a multi-objective model [155], described by Equations (12) and (13).
where N0 and Nt are the microbial concentration (CFU mL−1) at time zero and t, respectively, k is the inactivation rate constant (cm2 mJ−1), F is the UV fluence at exposure time t, and nc is the number of critical targets to cause inactivation. The values of k and nc are the slope and the intersection of the linear region of the fluence–response curve. The multi-target model (Equation (14)) can be reduced to a single-target model, if nc is equal to 1, as follows:
Another inactivation kinetic model used was the “Geeraerd model” [156], which considers shoulder and tail effects for comparison with the multi-objective model, described by (Equation (15)).
where Nres is the microbial concentration of a specific sub-population, either more resistant or appearing as a result of experimental artifacts (CFU or PFU mL−1), k is the maximum inactivation rate constant of critical components, given as the slope of the linear part (cm2 mJ−1), and tl is the shoulder length (mJ cm−2), which can be obtained by dividing the value of the y-intercept of the linear part with the k [157].
Regarding the UV method, it can be mentioned that this method is more effective on filamentous hyaline fungi such as Aspergillus, Penicillium, and Trichoderma species, compared to some yeasts such as Candida sp. and Rhodotorula sp. Additionally, the UV disinfection method does not have concerns related to chlorinated byproducts that form during disinfection by chlorination.
5.1.5. Solar Disinfection (SODIS) for Fungi Inactivation
Apart from the use of mercury lamps or LEDs for fungi inactivation, solar light can also be used for water disinfection. Solar disinfection (SODIS) is a method that uses sunlight to improve drinking water quality and reduce the health risks caused by microorganisms in drinking water. Solar light includes UVB radiation (300–320 nm; radiation below 300 nm is filtered by the atmosphere), UVA (320–400 nm), and even visible light (>400 nm). In SODIS, water to be treated is stored in a PET or glass bottle, and then it is exposed for 6 h to sunlight. This is a low-cost method, it is low-energy consuming, and it has no known side effects on human health, which makes it an important alternative in developing countries and remote areas [158].
SODIS is very effective in inactivating bacteria (e.g., E. coli, Shigella flexneri) and viruses [159,160], and more recently, it has been applied to deal with fungi [161]. Xia et al. (2022) evaluated the kinetics, influencing factors, and disinfection mechanisms for fungal spores treated by SODIS [162]. They found that the solar inactivation of A. niger and P. Polonicum followed the log-linear model (Equation (15)), with P. Polonicum being the most sensitive to the sunlight. Under SODIS action, DNA and respiratory chains were damaged, esterase activity was induced, and spores lost their ability to grow (Table 4).
The log-linear with a shoulder’ model for the Fungi inactivation by SODIS is given by (Equation (16)):
where N0 and N are the numbers of colonies (CFUmL−1) before and after SODIS, Nres is the microbial concentration of a specific sub-population, either more resistant or appearing as a result of experimental artifacts (CFUmL−1), kmax is the maximum inactivation rate constant (min−1), t is the exposure time (min), and SL is the shoulder length (min).
Applying Equation (15) to fungi in water, Xia et al. found that the SL value of A. niger (64.30 min) was higher than that of P. polonicum (32.27 min) (p < 0.05), and the kmax value of A. niger (0.033 min−1) was lower than that of P. polonicum (0.062 min−1) (p < 0.05). P. Polonicum took 110 min to achieve a 2 log inactivation, whereas A. niger required 220 min to achieve the same log inactivation under the same conditions. The lowest inactivation efficiency was obtained at a temperature close to the optimal growth temperature (30–40 °C for A. niger, 20–30 °C for P. polonicum); pH between 5.0 and 9.0. Low concentrations of humic acids (<3.0 mg L−1) did not affect the solar inactivation of fungal spores, but concentrations above 5.0 mg L−1 of humic acids may decrease the disinfection (Table 5).

Table 5.
Removal of fungi by SODIS.
The disinfection kinetics of SODIS is lower compared to the other classical methods for fungi inactivation. No further conclusions can be drawn regarding the different fungal species due to the low number of published articles on this topic. However, SODIS does not require the addition of external reactants, which is a particular advantage that allows for its easy application in low-income countries.
5.2. Advanced Oxidation Process as Alternative Methods for Fungi Inactivation
Advanced oxidation processes (AOPs) are environmentally friendly techniques with a great capacity to treat contaminants of emerging concern (CECs, which are recalcitrant to conventional systems), such as bacteria [163], fungi [164], parasites [165], and antimicrobial resistance genes [18]. AOPs involve the formation of hydroxyl radicals (HO*) that can attack microorganisms and convert organic substances into CO2, H2O, and harmless mineral ions [166,167], or induce the formation of intermediate compounds that are easier to degrade by other methods [168]. Furthermore, some AOPs can also generate sulfate radicals (SO4*−), known as SR-AOPs [169].
Among AOPs, Fenton and photo-Fenton, photocatalysis using semiconductors, and the combination of UVC light with inorganic peroxides or chlorine species have been widely used to inactivate fungi in aqueous samples. These topics are developed below.
5.2.1. Fenton and Photo-Fenton Processes
The Fenton reaction encompasses reactions of H2O2 with iron ions in acidic conditions to form highly reactive hydroxyl radicals (Equation (17)), one of the most powerful oxidants (E° = 2.73 V). The produced radicals are capable of oxidizing organic or inorganic compounds available in the external walls and membranes of cells [170]. In addition to external attacks on microorganisms by radicals, H2O2 can enhance cell membrane permeability, and it also reacts with iron ions inside, leading to cell damage and death [171].
The pH is a critical parameter, and the Fenton reaction is preferred to be carried out in acidic conditions (pH = 2.8–4.0) to avoid its precipitation as ferric hydroxide. Under acidic conditions, the Fenton process can be progressed by the catalytic properties of Fe3+/Fe2+ (Equation (18)) [171]; however, the Fe (II) regeneration is slow [170].
An enhancement of the catalytic cycle of iron is achieved by the introduction of light to the Fenton reaction (forming the photo-Fenton system). The incorporation of UV–visible light (which can take advantage of solar light) provides a significantly faster Fe2+ regeneration. The light effect is attributable to the photoreduction of Fe3+ in aqua-complexes, which are dissociated into Fe2+ and an oxidized ligand through the ligand-to-metal charge transfer (LMTC) mechanism (Equation (19)); thus giving faster degradation/inactivation rates and a higher degree than the “dark” reaction [172]. It can be remarked that HO* production is highly increased by irradiation up to a wavelength of 600 nm [173].
Regarding fungi inactivation by photo-Fenton, it can be mentioned that Polo-Lopez et al. investigated the inactivation of spores of a Fusarium solani strain isolated from samples of the Andarax River in Almeria, Spain [174]. The efficiency of solar photo-Fenton and solar light/H2O2 systems to disinfect simulated municipal effluents was shown. In addition, the authors studied the implications of spore germination in water exposed to the treatments. They found that the fungi inactivation by sunlight irradiation in the presence of relatively low H2O2 concentrations is associated with peroxide diffusion into the cell and the initiation of internal Fenton (Equations (17) and (18)) and Haber–Weiss reactions, as well as photogenerated ROS (Equation (19)) that also attack the cell internally. The solar irradiation in the presence of 10 mg L−1 peroxide produced fungi inactivation below the detection limit of 2 CFU mL−1. In turn, in the photo-Fenton process, the generated radicals also attack the fungi externally. Moreover, the photo-Fenton process (using Fe2+, Fe3+) at low reagent concentrations (1–5 mg L−1) and photo-assisted solar treatment with H2O2 enabled the removal of Fusarium spores in distilled water, simulated municipal wastewater effluents, and real municipal wastewater effluents [175] (Table 6).

Table 6.
Removal of fungi by Fenton-based processes.
The disinfection of distilled water loaded with a strain of Phytophthora capsici using photo-Fenton at low concentrations of reagents (i.e., 1 and 5 mg L−1) has been evaluated, and different control systems: H2O2, Fe2+, Fe3+, and Fe2+/H2O2 or Fe3+/H2O2 with and without natural sunlight have also been applied. For all solar processes evaluated, the following order of inactivation was observed for P. capsici zoospores: Fe3+/H2O2/solar > H2O2/solar > Fe2+/H2O2/solar ≥ Fe3+/solar > Fe2+/solar > solar photoinactivation (Table 6).
The differentiation of the individual kinetics of H2O2 and iron ions in the presence of sunlight establishes that the fast kinetic mechanisms of the photo-Fenton reaction do not allow the diffusion processes of the reactants in the cell to predominate. The addition of Fe3+ evidenced about 50% more H2O2 consumption compared to the addition of Fe2+, plus a higher rate of zoospore inactivation. Although both sources of iron ions lead to the generation of hydroxyl radicals, the use of Fe3+ imparts specific adsorption sites on the cell wall of zoospores, favoring inactivation.
On the other hand, Aguas, et al. [176] evaluated the disinfection of agricultural pathogenic fungi (Curvularia sp.) in distilled water and real secondary effluents from an urban wastewater treatment plant, and they found the best disinfection results using photo-Fenton at 10 mg L−1 of Fe2+ and 20 mg L−1 of H2O2 at pH 3 when 17 kJ L−1 of cumulative solar UV dose was received in the vessel reactor and 19 kJ L−1 for composite parabolic collectors (CPCs) (Table 6).
Due to the few published studies, it still cannot be concluded about the relevance of the nature of the fungi in the Fenton reaction-based processes because only three species have been reported. Also, it can be noted that a main limitation of these methods would be the costs related to Fenton reagents and pH control.
5.2.2. Photocatalysis Using Semiconductors
Photocatalysis with semiconductors (SC) uses a combination of light and catalyst to promote the elimination of water pollutants. Photocatalysts are materials that modify the rate of a chemical reaction when exposed to light. As the semiconductor and the contaminant are in different phases, i.e., solid and liquid, respectively, the process is called heterogeneous photocatalysis [168]. In the photocatalytic system, the mechanisms of the process primarily include light harvesting, separation, and transfer of photogenerated electron-hole (e−-h+) pairs to the catalytic surface, as well as reaction with the pollutants [177] (Equation (20)).
The interaction of light of proper wavelength (having higher energy than the band gap) with the semiconductor promotes an electron from the valence band to the conduction band (Equation (18)), generating an electron–hole pair. The electron has reductive properties, and it can react with dissolved oxygen, forming the superoxide radical anion (Equation (21)). Meanwhile, if the hole has enough redox potential, it can produce a hydroxyl radical from the oxidation of water or a hydroxyl anion (Equation (22)). Then, the formed HO*, O2*−, or h+ in the photocatalytic system can promote water disinfection.
Titanium dioxide (TiO2) is the semiconductor mainly used in photocatalysis that is stable over a wide pH range and is an inexpensive photocatalytic material. TiO2 can be activated under ultraviolet (UV) light of wavelength < 390 nm due to its large band gap (3.2 eV) [178]. In addition, this semiconductor-based photocatalysis has been widely used as an effective method under UV-light irradiation for the removal of pathogenic microorganisms. The most commonly used microorganism for disinfection using photocatalysis is Escherichia coli, followed by Staphylococcus aureus. On a few occasions, this process has been evaluated with fungi [177].
In 2005, Lonnen et al. (Table 7) tested the inactivation efficiency of solar disinfection and solar TiO2-photocatalytic disinfection (SPC-DIS) against fungi and compared it with the inactivation efficiency of SODIS [161]. SPC-DIS recorded lower inactivation times for the same log reduction. Two species were tested, C. albicans and F. solani, using SPC-DIS. After two hours, 4.1 log inactivation of C. albicans and 1.75 log inactivation of F. solani conidia were achieved. Total inactivation (C. albicans: 5.4 log; F. solani: 5.5 log) was recorded after 4 h; the same log reduction required 6 h and 8 h, respectively, with the SODIS technique. The better performance of the SPC-DIS system is associated with the disinfecting action of the produced ROS.

Table 7.
Removal of fungi by photocatalysis with semiconductors.
Polo-López et al. determined the resistance of Fusarium sp. spores to TiO2-photocatalysis (Table 7). They found that the TiO2-photocatalytic treatment caused a 3-log decrease in spore viability compared with the solar light alone, which only achieved a decrease of 0.3 log after 5 h of treatment. Among the tested fungi, Chlamydospores were the most resistant, followed by macroconidia, and finally, microconidia were the most sensitive [179]. The order of inactivation of microorganisms indicates that the species with the thickest and most dense walls are the most capable of resisting the attack of hydroxyl radicals.
Rodrigues-Silva et al. [180] evaluated the efficacy of photocatalytic oxidation in killing bacteria and fungi, including ATCC strains and isolates from wastewater treatment plants. The activity of commercial titanium dioxide in the inactivation of Aspergillus fumigatus and Penicillium spp. in the liquid phase was tested. Fungi were the most resistant of the microorganisms submitted to photocatalysis, demanding nine times more radiation to achieve complete inactivation than bacteria. It is proposed that the inactivation efficiency is determined by the way the cell wall is attacked and the cell integrity is disrupted, thus leading to cell death.
As mentioned above, TiO2 can be activated under ultraviolet (UV) light of wavelength < 390 nm due to its large band gap (3.2 eV) [178]. In turn, UV rays can also promote the Fe3+/Fe2+ redox cycle. Additionally, Fe3+ enters the water, and it can be photo-reduced to Fe2+, generating HO* (Equation (17)). Interestingly, TiO2 and Fe3+ can be combined, and in this system, the electron of the conduction band can promote the regeneration of ferrous ions (Equation (23)) and limit the recombination of electrons and holes. Makoday et al. evaluated the inactivation of Candida albicans by the UV/TiO2/Fe3+ system (using light of λ = 289–365 nm, Table 6) and found that the disinfection efficiency was dependent on the concentration of iron in the solution. The degree of culture inactivation increased from 0.3 to 2.7 orders by changing the Fe3+ concentration from 1 × 10−4 to 1 × 10−2 mol L−1 [181].
Despite the interest in TiO2-photocatalysis to eliminate pollutants in aqueous media, a recent review has evidenced the limitations of this system for massive water treatment. Moreover, kinetically, there is a competition between the recombination processes (10−12 s) and interfacial charge transfer (10−10–10−7 s), which causes limitations in the production of ROS in TiO2-photocatalysis to eliminate pollutants. Although attempts have been made to improve the production of ROS (e.g., heterojunctions), the degradation rates towards biological and chemical targets concerning individual photocatalysts maintain the kinetics in the range of hours [182], in addition to the need to remove the catalyst from water after the treatment. All these aspects represent a strong drawback for practical applications of the TiO2-photocatalysis and competition with other AOPs for fungi inactivation in aqueous matrices.
5.2.3. UV/Chlorination
The combination of UV irradiation and chlorination (UV/chlorination) is an effective disinfection process, which can produce hydroxyl radicals (HO*) and radical-reactive chlorine species (e.g., Cl*) [172]. The results of some research have revealed that individual UV and chlorination processes are less effective than the combination of these processes. UV/Cl2 provides a better removal rate and synergistic effects due to the formation (Equations (24) and (25)) and the action of the radicals to inactivate different microorganisms [183].
Wan et al. [118,184] tested the enhancement of the inactivation of fungal spores by combining UV-LED and Cl2 (using UV-LEDs at 265, 280, and 265/280 nm) and LP/Cl2 (using UVC at 254 nm) for three fungal species (Table 8). For P. polonicum, UV265 and UV280 (fluence: 40 mJ cm−2) pretreatments caused a log count reduction (LCR) of 1.05 log and 0.95 log, and UV265/Cl2 and UV280/Cl2 treatments at the same UV fluence caused a further log count reduction (LCR) of 1.80 log and 2.00 log.

Table 8.
Removal of fungi by UV/chlorination.
The higher inactivation efficiency of LP/Cl2 and UV-LED/Cl2 (especially UV280/Cl2 and UV265/280/Cl2) may be due to the higher formation of intracellular ROS and the reaction of extracellular free radicals. This could cause the physiological dysfunction of fungal spores. The joint effect of DNA damage and other cell damage resulted in a higher inhibition efficiency of reactivation inhibition of the treated fungal spores. In addition to the works of Wan et al., Al-Gabr et al. proved that with a short contact time and a low dose of UV irradiation followed by Cl2 dosing, 4 log inactivation of A. flavus can be achieved [185].
Ali et al. showed the occurrence of fungi in drinking water sources, and their treatment by chlorination and UV irradiation allows disinfection with a very short exposure time and a very low chlorine concentration [186]. Meanwhile, Wu et al. tested the replacement of chlorine by NH2Cl (Table 8). They find that the UV/NH2Cl process could more effectively inactivate three Aspergillus species (A. flavus, A. niger, and A. fumigatus) than UV alone. The higher efficiency is due to an increase in membrane damage and intracellular ROS levels by the UV/NH2Cl combination [187] (Table 8).
We can highlight that advanced oxidation processes such as UVC plus chlorine or chloramine have been used on fungal species of the genera Aspergillus and Penicillium. UV/chloramine was found to have better log removal with Aspergillus spp. compared to UV/chlorine on Penicillium polonicum. Nevertheless, studies with potentially pathogenic fungal strains present in aquatic systems are scarce, and they should be performed in future research.
5.2.4. Combination of UV with Persulfates
The combination of UV irradiation with persulfates leads to the production of sulfate radicals (SO4*−). Sulfate radicals have a high redox potential (2.5–3.1 V). Furthermore, sulfate radicals have high efficiency in the oxidation of contaminants and can be used over a wide pH range and in different aqueous matrices. Persulfates, i.e., peroxydisulfate (PS, S2O82−) and peroxymonosulfate (PMS, HSO5−), are the most commonly used chemical species for the generation of sulfate radicals [171].
The O–O bond cleavage of PS and PMS requires a large amount of energy, which can be supplied by UV radiation having more energetic wavelengths (typically 254 nm, (Equations (26)–(30)) [188]. In persulfate-based processes, the formation of other highly oxidizing radicals such as HO* can also occur from the interaction of sulfate radicals with water (Equation (28)) or hydroxide anion (Equation (29); this reaction is mainly favored at basic pH. Moreover, PS and PMS can generate small amounts of sulfate radicals at room temperature (Δ) from homolytic cleavage of their peroxide bond (Equation (30)), but this process is more effective at temperatures above 30 °C [188].
Regarding the inactivation of fungi by persulfates combined with ultraviolet light, we can mention the work performed by Wen et al. [17], which presents the inactivation study of four dominant fungal spore genera in groundwater using the UV/PMS system (Table 8). In such a work, it is shown that the larger the fungal spore size, the lower the inactivation constant. UV light alone causes slight changes in spore shape and surface structure, plus damage to DNA without destroying the cell wall, while UV/PMS treatment can cause the release of intracellular materials and drastically change the shape and surface integrity of spores by damaging the cell membrane and cell wall.
Other research that evaluates the photoreactivation of fungal spores in water after disinfection (Table 9) reports that the three genera of fungal spores treated with UV/PMS exhibit different photoreactivation tendencies, as those treated with UV are probably attributed to the produced reactive radicals resulting in damage to the cell wall and cell membrane and then the release of intracellular contents, which cannot be repaired by the UVA irradiation (i.e., photoreactivation did not occur) [189].

Table 9.
Removal of fungi by UV/PS or UV/PMS systems.
Wan et al. [16] also showed the efficacy of ultraviolet light-emitting diode (UV-LED)-based advanced disinfection processes, including UV-LED/PS and UV-LED/PMS, for the inactivation of fungal spores in water. The reduction in the cultivability of the three fungal species inactivated by UV-LED was not improved by the addition of PMS or PS, according to the results of log count reduction (LCR) and reaction rate constants (k) (Table 9). However, the combination of UV-LEDs with persulfate increased membrane damage with a lower photoreactivation level, thus improving inactivation. The enhancement was related to the attacks of free radicals on the fungi.
5.2.5. UV/H2O2, UV/Peracetic Acid, and UV/O3 for Fungi Inactivation in Water
UV irradiation combined with hydrogen peroxide (H2O2) has a remarkable ability to remove diverse contaminants from aquatic media. UVC or even UVB irradiation (this last coming from solar light) can break the -O–O- bond in H2O2 and create hydroxyl radicals (Equation (31)) [190]. Those radicals are disinfecting agents. For instance, Sichel et al. demonstrated the lethal effect of sunlight and H2O2 on the spores of a wild-type strain of Fusarium solani (Table 9). The combination of H2O2 with solar irradiation allowed H2O2 to have better disinfection results than hydrogen peroxide in the dark [191]. Similarly, Polo-López et al. evaluated the solar disinfection of Fusarium equiseti chlamydospores in water assisted by low concentrations of hydrogen peroxide in a 60 L photoreactor, and the authors found that the spores were inactivated with 10 mg L−1 H2O2 [192] (Table 10).

Table 10.
Removal of fungi by UV/H2O2, UV/PAA, and UV/O3.
On the other hand, peracetic acid (PAA, which has an O–O bond too) can also be activated by adding external energy (e.g., UV light and ultrasound (US)) to generate hydroxyl (HO*) and organic radicals (RO*, such as CH3CO2* or CH3CO3*) [194], which are useful for inactivating microorganisms in water. For example, Xu et al. evaluated the fungal spore inactivation by the UV/PAA combination and found that the inactivation of fungal spores was more effective than by UV and PAA alone, because of the strong action of the formed radicals [146] (Table 10).
UV light can also be combined with ozone (UV/O3). This last system is a good method to deal with recalcitrant water pollutants. The inactivation capability of the UV/ozone combination is not only due to the high oxidizing power of O3 but also to the formation of strong species such as hydroxyl radicals (Equations (32)–(34)) [195,196]. Sousa et al. tested the potential of ozonation and UV254nm radiation to inactivate culturable fungal populations in urban wastewater. They found that a contact time of 30 min was sufficient to achieve log reductions of 3.3 ± 0.2 for two fungi (Rhodotorula rubra and Aspergillus niger) [193] (Table 10).
After revision of the published works, we should remark that advanced oxidation processes of UV/peroxides show a high inactivation efficiency toward molds and yeasts. Moreover, hyaline molds are more susceptible to these methods compared to black molds (which contain melanin in their cell walls).
5.2.6. Other Alternative Methods of Fungi Disinfection
In addition to the processes mentioned above, other alternative methods have been utilized for the inactivation of fungi in water. According to the research performed by Wen et al., pipe corrosion products (PCPs) used in transporting groundwater to treatment plants are generally MnO2, α-FeOOH, γ-FeOOH, and CuO, and these compounds can activate peroxymonosulfate (PMS) for water disinfection. Those authors considered the inactivation ability of the four PCPs (CuO, FeOOH, γ-FeOOH, and MnO2) toward Trichoderma harzianum A. niger, P. polonicum, and Cladosporium cladosporioides. T. arzianum. Their investigation revealed that fungi are effectively inactivated through the reactive radicals produced by the reaction between the added PMS and the PCPs, and the PMS/CuO system showed the best performance [197].
On the other side, Rodríguez-Chueca et al. evaluated the performance of PMS-based systems (PMS/UV-A LED plus Fe2+ or Co2+) in the inactivation of C. albicans. The total inactivation of C. albicans was accelerated by the metallic activation of PMS using the transition metals (Fe2+ or Co2+), due to the high production of radicals by the interaction of the metals with PMS. This acceleration was observed mainly in the first minute of contact time, reaching inactivation values ranging from 1 to 3 log units. Moreover, the combination of PMS with Co2+ obtained higher inactivation values than Fe2+, due to its higher toxicity against eukaryotic cells [198].
Besides the interaction of PMS with metals or metal oxides for fungi treatment, a new magnetic disinfectant has been proposed. Such material is obtained by polymerization of thiourea and formaldehyde in the presence of magnetite nanoparticles-MTUF, which are subsequently loaded with Ag(I) ions. MTUF-Ag has been tested for disinfection of C. albicans in wastewater, achieving three log reductions at 2.5 mg L−1 of the material. Thus, the MTUF-Ag material could be a good candidate for water disinfection [199].
In turn, Le et al. studied a nanoarchitecture (using Cleistocalyx operculatus leaf extract in the preparation of multifunctional graphene oxide/Fe3O4/Ag nanomaterials). The objective beyond the preparation of the materials was the systematic study of water disinfection as an application of the ability of graphene oxide to generate ROS. They showed that in this system, a reduction of more than 99% of fungi in wastewater was achieved after 60 min of treatment [200].
Meanwhile, Dehghani et al. investigated and evaluated the application of ultrasonic reactor technology (USRT) as a disinfectant for the reduction of fungi in wastewater effluents. This study showed that USRT leads to a reduction of more than 99% of the fungi in the wastewater after 1 h of the process action, and the disinfection mechanism is associated with the generation of shock waves and radicals resulting from cavitation, which contribute to the physical alteration of microbial structures that lead to their inactivation [201].
It should be mentioned that photo-electrocatalysis plus ozonation (PEC + O3) has been used to treat both simulated and real fungi-contaminated pool water [59]. The authors found that treatment based on photo-electrocatalysis plus ozone allowed the removal of 82% of total organic carbon, with 100% removal of benzophenone-3 (as a commercial sunscreen model) in 20 min. The addition of 106 CFU mL−1 of Candida parapsilosis did not interfere with mineralization and was completely inactivated after 45 min of treatment.
Finally, El-Sayyad et al. [202] evaluated the efficacy of gamma-irradiated CoxNi1−x/Fe2O4/SiO2/TiO2(x = 0.9) nanocomposites for the disinfection of Candida tropicalis and C. albicans pathogens in wastewater. The gamma-irradiated nanocomposite showed higher antimicrobial capacity (50.85% Candida albicans inactivation) than the non-irradiated composite (30.10% of disinfection), and this capacity increased proportionally with increasing radiation dose. The improvement in the reactivity of the nanocomposite in the presence of UV radiation suggests the presence of ROS species that can decompose the cell walls of microorganisms [180].
6. Conclusions and Outlooks
The use and research of classical and alternative methods of disinfection in aquatic systems (sewage, groundwater, and drinking water, among others) have become assertive actions for the reduction in fungal loading and, consequently, the decrease in the risk for human and animal health, as well as the contribution to limiting the development of drug-resistant fungi.
From the revised works, it was evidenced that the structure/species of the fungi play a relevant role in disinfection kinetics, mainly in the application of the classical methods. Furthermore, in classical treatments, such as chlorination or ozonation, the concentration of the oxidant is of great relevance for disinfection. The coupling of methodologies allows for improved disinfection rates. On the other hand, in the AOPs, fungi inactivation occurs, either by direct DNA damage or indirect damage due to very reactive radicals, which can promote strong damage to external and internal cell components. Also, it is important to note that solar disinfection techniques are booming and are a good alternative for low-income sites.
Research focused on advanced oxidation processes (UV/hydrogen peroxide, UV/ozone, photo-Fenton, or UV/persulfates) needs to be optimized and scaled up for applications toward the inactivation of fungi in wastewater or drinking water. In addition, advances in the synthesis or use of new materials for disinfection, in homogeneous or heterogeneous systems (e.g., PMS/metal oxides, MTUF-Ag, graphene oxide/Fe3O4/Ag, CoxNi1−x/Fe2O4/SiO2/TiO2), are being discussed. However, in-depth studies using these materials must be developed to clearly understand their environmental impacts, reuse and recovery from aqueous samples, and feasibility for massive applications. Besides, the literature about the coupling/merging of diverse AOPs for fungi inactivation in water is still scarce. Thus, future works should consider all these lacking topics. Also, more studies on SODIS for the inactivation of fungi in water must be performed. This method is promising to be widely applied in low-income countries.
We should also mention that several reports establish the application of a wide variety of methods for the inactivation of fungi, considering mainly commercial strains or strains isolated from surface water. Further works should consider strains from hospital wastewater. Also, it is very relevant to remark that in 2022, the World Health Organization (WHO) introduced the first list of priority fungal pathogens to drive research and development of public policies to mitigate invasive fungal infections, especially those caused by drug-resistant fungi. Several pathogenic fungi are considered potential environmental contaminants in aquatic systems and pose a risk to human health, mainly among immunocompromised populations.
From the elaboration of this review, it was found that despite the use of classical and alternative methods as effective disinfection processes for fungi inactivation, there is a lack of information on antifungal susceptibility after treatments, pathogenicity, and resistance genes in fungi circulating in water. It must be remarked that the key role of wastewater in the evolution and propagation of antibiotic resistance is becoming increasingly clear. In addition, the ability of disinfection processes to inactivate antibiotic-resistant bacteria and eliminate their resistance genes has been demonstrated. However, this is not the case with fungal studies. It has only been possible to demonstrate the degree of inactivation, damage to the cell wall and membrane, attack on DNA, and the respiratory chain of fungal spores. Then, this review has also shown that although resistant fungi can be found in aquatic systems, there are gaps in knowledge concerning the inactivation of antifungal-resistant microorganisms in water that need to be addressed. Thereby, a future research question would be: are the disinfection methods capable of eliminating anti-fungal-resistant pathogenic fungi and decreasing the genetic determinants of antifungal resistance?
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16070936/s1, Table S1. Results of the bibliometric study. Table S2. Relation fungi and resistance antimycotics. Table S3. Fungi and kinetic efficiency inactivation [203].
Author Contributions
Conceptualization, L.D.C.-B. and E.A.S.-G.; methodology, all authors; software, L.D.C.-B. and S.P.C.-N.; validation, L.D.C.-B., A.M.-C. and E.A.S.-G.; formal analysis, all authors; data curation, all authors; writing—original draft preparation, L.D.C.-B., A.M.-C. and E.A.S.-G.; writing—review and editing, L.D.C.-B., S.P.C.-N. and E.A.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by Dirección General de Investigaciones of Universidad Santiago de Cali; Call No. 10-2023 and grant number 939-621122-3489. The APC was funded by Dirección General de Investigaciones of Universidad Santiago de Cali under call No. 01-2024.
Data Availability Statement
All data used for the analyses carried out in this review are included in the Supplementary Materials. Any other data will be available on request to the authors.
Acknowledgments
This research has been funded by Dirección General de Investigaciones of Universidad Santiago de Cali under call No. 01-2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gonzalez-Jimenez, I.; Lucio, J.; Menéndez-Fraga, M.D.; Mellado, E.; Peláez, T. Hospital environment as a source of azole-resistant aspergillus fumigatus strains with TR34/L98H and g448s cyp51a mutations. J. Fungi 2021, 7, 22. [Google Scholar] [CrossRef]
- Lemaire, B.; Normand, A.-C.; Forel, J.-M.; Cassir, N.; Piarroux, R.; Ranque, S. Hospitalized Patient as Source of Aspergillus fumigatus, 2015. Emerg. Infect. Dis. 2018, 24, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.F.; Ying, G.G. Occurrence, fate and ecological risk of five typical azole fungicides as therapeutic and personal care products in the environment: A review. Environ. Int. 2015, 84, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.E.; Riedel, J.; Sae-Ong, T.; Kang, K.; Brabetz, W.; Panagiotou, G.; Deising, H.B.; Kurzai, O. Effects of agricultural fungicide use on Aspergillus fumigatus Abundance, Antifungal Susceptibility, and Population Structure. Appl. Environ. Sci. 2020, 11, e02213-20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jimenez, L.L.; Snelders, E.; Debets, A.J.M.; Rietveld, A.G.; Verweij, P.E.; Schoustra, S.E.; Zwaan, B.J. Dynamics of Aspergillus fumigatus in Azole Fungicide- Containing Plant Waste in the Netherlands (2016–2017) Jianhua. Appl. Environ. Microbiol. 2021, 87, e02295-20. [Google Scholar] [CrossRef] [PubMed]
- Maza-Márquez, P.; Vílchez-Vargas, R.; González-Martínez, A.; González-López, J.; Rodelas, B. Assessing the abundance of fungal populations in a full-scale membrane bioreactor (MBR) treating urban wastewater by using quantitative PCR (qPCR). J. Environ. Manag. 2018, 223, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Ogola, H.J.O.; Mamba, B.B.; Msagati, T.A.M. Azole antifungal resistance in fungal isolates from wastewater treatment plant effluents. Environ. Sci. Pollut. Res. 2021, 28, 3217–3229. [Google Scholar] [CrossRef] [PubMed]
- Milanezi, A.C.M.; Witusk, J.P.D.; van der Sand, S.T. Antifungal susceptibility of yeasts isolated from anthropogenic watershed. An. Acad. Bras. Ciências 2019, 91, e20170369. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Cao, R.; Wen, G.; Xu, X.; Xia, Y.; Wu, G. Sequential use of UV-LEDs irradiation and chlorine to disinfect waterborne fungal spores: Efficiency, mechanism and photoreactivation. J. Hazard. Mater. 2022, 423, 127102. [Google Scholar] [CrossRef]
- Chen, J.; Loeb, S.; Kim, J.-H. LED revolution: Fundamentals and prospects for UV disinfection applications. Environ. Sci. Water Res. Technol. 2017, 3, 188–202. [Google Scholar] [CrossRef]
- Kim, J.Y. Human fungal pathogens: Why should we learn? J. Microbiol. 2016, 54, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.N.M.; Cseresny, Z.; Hartung, S.; Blickensdorf, M.; Saffer, C.; Rennert, K. Invasive aspergillosis-on-chip: A quantitative treatment study of human Aspergillus fumigatus infection. Biomaterials 2022, 283, 121420. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.J.; Marques, R.; Marques, M.; Benoliel, M.J.; Barreto Crespo, M.T. Free chlorine inactivation of fungi in drinking water sources. Water Res. 2013, 47, 517–523. [Google Scholar] [CrossRef]
- Wen, G.; Xu, X.; Huang, T.; Zhu, H.; Ma, J. Inactivation of three genera of dominant fungal spores in groundwater using chlorine dioxide: Effectiveness, influencing factors, and mechanisms. Water Res. 2017, 125, 132–140. [Google Scholar] [CrossRef]
- Wen, G.; Liang, Z.; Xu, X.; Cao, R.; Wan, Q.; Ji, G.; Lin, W.; Wang, J.; Yang, J.; Huang, T. Inactivation of fungal spores in water using ozone: Kinetics, influencing factors and mechanisms. Water Res. 2020, 185, 116218. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Cao, R.; Wen, G.; Xu, X.; Xia, Y.; Wu, G.; Li, Y.; Wang, J.; Xu, H.; Lin, Y.; et al. Efficacy of UV-LED based advanced disinfection processes in the inactivation of waterborne fungal spores: Kinetics, photoreactivation, mechanism and energy requirements. Sci. Total Environ. 2022, 803, 150107. [Google Scholar] [CrossRef]
- Wen, G.; Xu, X.; Zhu, H.; Huang, T.; Ma, J. Inactivation of four genera of dominant fungal spores in groundwater using UV and UV/PMS: Efficiency and mechanisms. Chem. Eng. J. 2017, 328, 619–628. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. Removal of antibiotic resistance genes (ARGs) in various wastewater treatment processes: An overview. Crit. Rev. Environ. Sci. Technol. 2022, 52, 571–630. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, B.; Cui, X.; Ren, Q.; Ren, T.; Zhou, Y. Distribution and dynamics of antibiotic resistance genes in a three-dimensional multifunctional biofilm during greywater treatment. Environ. Pollut. 2023, 327, 121533. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cui, Y.; Cao, R.; Xu, X.; Wu, G.; Wang, J.; Huang, T. Occurrence and control of fungi in water: New challenges in biological risk and safety assurance. Sci. Total Environ. 2023, 860, 160536. [Google Scholar] [CrossRef]
- Zhao, H.X.; Zhang, T.Y.; Wang, H.; Hu, C.Y.; Tang, Y.L.; Xu, B. Occurrence of fungal spores in drinking water: A review of pathogenicity, odor, chlorine resistance and control strategies. Sci. Total Environ. 2022, 853, 158626. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ren, X.; Chen, Y.; Feng, H.; Yu, J.; Peng, K.; Zhang, Y.; Chen, W.; Tang, J.; Wang, J.; et al. Bacteria inactivation by sulfate radical: Progress and non-negligible disinfection by-products. Front. Environ. Sci. Eng. 2023, 17, 29. [Google Scholar] [CrossRef]
- Garvey, M.; Rowan, N.J. Pathogenic Drug Resistant Fungi: A Review of Mitigation Strategies. Int. J. Mol. Sci. 2023, 24, 1584. [Google Scholar] [CrossRef] [PubMed]
- Visconti, V.; Coton, E.; Rigalma, K.; Dantigny, P. Effects of disinfectants on inactivation of mold spores relevant to the food industry: A review. Fungal Biol. Rev. 2021, 38, 44–66. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. Ann. Intern. Med. 2009, 151, W-65–W-94. [Google Scholar] [CrossRef]
- Siscar-Lewin, S.; Hube, B.; Brunke, S. Emergence and evolution of virulence in human pathogenic fungi. Trends Microbiol. 2022, 30, 693–704. [Google Scholar] [CrossRef]
- Paul, S.; Joshi, S.R. Industrial Perspectives of Fungi. In Industrial Microbiology and Biotechnology; Springer: Singapore, 2022; pp. 81–105. [Google Scholar]
- Babič, M.N.; Gunde-Cimerman, N.; Vargha, M.; Tischner, Z.; Magyar, D.; Veríssimo, C.; Sabino, R.; Viegas, C.; Meyer, W.; Brandão, J. Fungal contaminants in drinking water regulation? A tale of ecology, exposure, purification and clinical relevance. Int. J. Environ. Res. Public Health 2017, 14, 636. [Google Scholar] [CrossRef]
- Bohner, F.; Gacser, A. Epidemiological Attributes of Candida Species in Tropical Regions. Curr. Trop. Med. Rep. 2021, 8, 59–68. [Google Scholar] [CrossRef]
- Maza-Márquez, P.; Vilchez-Vargas, R.; Kerckhof, F.; Aranda, E.; González-López, J.; Rodelas, B. Community structure, population dynamics and diversity of fungi in a full-scale membrane bioreactor (MBR) for urban wastewater treatment. Water Res. 2016, 105, 507–519. [Google Scholar] [CrossRef]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanová, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Krauss, G.J.; Solé, M.; Krauss, G.; Schlosser, D.; Wesenberg, D.; Bärlocher, F. Fungi in freshwaters: Ecology, physiology and biochemical potential. FEMS Microbiol. Rev. 2011, 35, 620–651. [Google Scholar] [CrossRef]
- Arvanitidou, M.; Kanellou, K.; Constantinides, T.C.; Katsouyannopoulos, V. The occurrence of fungi in hospital and community potable waters. Lett. Appl. Microbiol. 1999, 29, 81–84. [Google Scholar] [CrossRef]
- Wurzbacher, C.M.; Bärlocher, F.; Grossart, H.P. Fungi in lake ecosystems. Aquat. Microb. Ecol. 2010, 59, 125–149. [Google Scholar] [CrossRef]
- Oliveira, H.M.B.; Santos, C.; Paterson, R.R.M.; Gusmão, N.B.; Lima, N. Fungi from a groundwater-fed drinkingwater supply system in Brazil. Int. J. Environ. Res. Public Health 2016, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wang, Z.; Li, T.; Lu, F.; Sheng, D.; Huang, W. Immunosuppressed Patients with Clinically Diagnosed Invasive Fungal Infections: The Fungal Species Distribution, Antifungal Sensitivity and Associated Risk Factors in a Tertiary Hospital of Anhui Province. Infect. Drug Resist. 2022, 15, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.O.; Kohler, L.M.; Hamdan, J.S.; Missagia, B.S.; Barbosa, F.A.R.; Rosa, C.A. Diversity and antifungal susceptibility of yeasts from tropical freshwater environments in Southeastern Brazil. Water Res. 2008, 42, 3921–3929. [Google Scholar] [CrossRef] [PubMed]
- Monapathi, M.E.; Bezuidenhout, C.C.; Rhode, O.H.J. Water quality and antifungal susceptibility of opportunistic yeast pathogens from rivers. Water Sci. Technol. 2017, 75, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Laurent, J.; Edel-Hermann, V.; Barbezant, M.; Sixt, N.; Dalle, F.; Aho, S.; Bonnin, A.; Hartemann, P.; Sautour, M. Adaptation of Fusarium oxysporum and Fusarium dimerum to the specific aquatic environment provided by the water systems of hospitals. Water Res. 2015, 76, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Anversa, L.; Lara, B.R.; Romani, C.D.; Saeki, E.K.; Nascentes, G.A.N.; Bonfietti, L.X.; de Souza Carvalho Melhem, M.; da Silva Ruiz, L.; Camargo, C.H.; Pereira, V.B.R. Fungi in dialysis water and dialysate: Occurrence, susceptibility to antifungal agents and biofilm production capacity. J. Water Health 2021, 19, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Mataraci-Kara, E.; Ataman, M.; Yilmaz, G.; Ozbek-Celik, B. Evaluation of antifungal and disinfectant-resistant Candida species isolated from hospital wastewater. Arch. Microbiol. 2020, 202, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Montanari, L.B.; Sartori, F.G.; Ribeiro, D.B.M.; Leandro, L.F.; Pires, R.H.; Melhem, M.d.S.C.; de Mello, C.A.; Martins, C.H.G. Yeast isolation and identification in water used in a Brazilian hemodialysis unit by classic microbiological techniques and Raman spectroscopy. J. Water Health 2018, 16, 311–320. [Google Scholar] [CrossRef]
- Ivanov, M.; Ćirić, A.; Stojković, D. Emerging Antifungal Targets and Strategies. Int. J. Mol. Sci. 2022, 23, 2756. [Google Scholar] [CrossRef]
- Doughty, K.J.; Sierotzki, H.; Semar, M.; Goertz, A. Selection and amplification of fungicide resistance in aspergillus fumigatus in relation to DMI fungicide use in agronomic settings: Hotspots versus coldspots. Microorganisms 2021, 9, 2439. [Google Scholar] [CrossRef]
- Snelders, E.; Van Der Lee, H.A.L.; Kuijpers, J.; Rijs, A.J.M.M.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.T.; Melchers, W.J.G.; Verweij, P.E. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 2008, 5, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Selvarajan, R.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Antifungal Azoles and Azole Resistance in the Environment: Current Status and Future Perspectives—A Review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 1011–1041. [Google Scholar] [CrossRef]
- Stevenson, E.M.; Gaze, W.H.; Gow, N.A.R.; Hart, A.; Schmidt, W.; Usher, J.; Warris, A.; Wilkinson, H.; Murray, A.K. Antifungal Exposure and Resistance Development: Defining Minimal Selective Antifungal Concentrations and Testing Methodologies. Front. Fungal Biol. 2022, 3, 918717. [Google Scholar] [CrossRef] [PubMed]
- Assress, H.A.; Nyoni, H.; Mamba, B.B.; Msagati, T.A.M. Occurrence and risk assessment of azole antifungal drugs in water and wastewater. Ecotoxicol. Environ. Saf. 2020, 187, 109868. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, e383–e392. [Google Scholar] [CrossRef]
- Vermeulen, E.; Lagrou, K.; Verweij, P.E. Azole resistance in Aspergillus fumigatus: A growing public health concern. Curr. Opin. Infect. Dis. 2013, 26, 493–500. [Google Scholar] [CrossRef]
- Kang, S.E.; Sumabat, L.G.; Melie, T.; Mangum, B.; Momany, M.; Brewer, M.T. Evidence for the agricultural origin of resistance to multiple antimicrobials in Aspergillus fumigatus, a fungal pathogen of humans. G3 Genes Genomes Genet. 2022, 12, jkab427. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Health and Food Security-TCLocal. Science 2018, 742, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-Y.; Lee, D.-C. Comparative Efficacy of Antifungal Agents for Aquaculture Fish and their Eggs. Korean J. Fish. Aquat. Sci. 2009, 42, 34–40. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, J.L.; Liu, Y.S.; Zhang, Q.Q.; Jiang, Y.X.; Liu, S.; Liu, W.R.; Yang, Y.Y.; Ying, G.G. Personal care products in wild fish in two main Chinese rivers: Bioaccumulation potential and human health risks. Sci. Total Environ. 2018, 621, 1093–1102. [Google Scholar] [CrossRef]
- Kacprzak, M.; Warchol, M.; Widawska, U. Microfungal species composition in raw and treated wastewater from selected wastewater treatment plants. In Environmental Engineering Studies; Pawlowski, L., Dudzinska, M.R., Pawlowski, A., Eds.; Springer: New York, NY, USA, 2003; pp. 167–173. [Google Scholar] [CrossRef]
- Monapathi, M.E.; Oguegbulu, J.C.; Adogo, L.; Klink, M.; Okoli, B.; Mtunzi, F.; Modise, J.S. Pharmaceutical pollution: Azole antifungal drugs and resistance of opportunistic pathogenic yeasts in wastewater and environmental water. Appl. Environ. Soil Sci. 2021, 2021, 9985398. [Google Scholar] [CrossRef]
- Reddy, G.K.K.; Padmavathi, A.R.; Nancharaiah, Y.V. Fungal infections: Pathogenesis, antifungals and alternate treatment approaches. Curr. Res. Microb. Sci. 2022, 3, 100137. [Google Scholar] [CrossRef]
- YujiKim, J.Y.U.; Bessegato, G.G.; de Souza, B.C.; da Silva, J.J.; Zanoni, M.V.B. Efficient treatment of swimming pool water by photoelectrocatalytic ozonation: Inactivation of Candida parapsilosis and mineralization of Benzophenone-3 and urea. Chem. Eng. J. 2019, 378, 122094. [Google Scholar] [CrossRef]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins–structure, mechanism of action and use in antifungal therapy. J. Enzyme Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin b and other polyenes—Discovery, clinical use, mode of action and drug resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-resistant fungi: An emerging challenge threatening our limited antifungal armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Bavaro, D.F.; Balena, F.; Ronga, L.; Signorile, F.; Romanelli, F.; Stolfa, S.; Sparapano, E.; De Carlo, C.; Mosca, A.; Monno, L.; et al. Emerging issue of fluconazole-resistant candidemia in a tertiary care hospital of southern italy: Time for antifungal stewardship program. J. Med. Mycol. 2022, 32, 101206. [Google Scholar] [CrossRef] [PubMed]
- Buil, J.B.; Snelders, E.; Denardi, L.B.; Melchers, W.J.G.; Verweij, P.E. Trends in Azole Resistance in the Netherlands 1994–2016. Emerg. Infect. Dis. 2019, 25, 176–178. [Google Scholar] [CrossRef]
- Friedman, D.Z.P.; Schwartz, I.S. Emerging fungal infections: New patients, new patterns, and new pathogens. J. Fungi 2019, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.R.; Verweij, P.E.; Castanheira, M.; Dannaoui, E.; White, P.L.; Arendrup, M.C. Molecular mechanisms of acquired antifungal drug resistance in principal fungal pathogens and EUCAST guidance for their laboratory detection and clinical implications. J. Antimicrob. Chemother. 2022, 77, 2053–2073. [Google Scholar] [CrossRef] [PubMed]
- Ener, B.; Ergin, Ç.; Gülmez, D.; Aǧca, H.; Tikveşli, M.; Aksoy, S.A.; Otkun, M.; Siǧ, A.K.; Öǧünç, D.; Özhak, B.; et al. Frequency of azole resistance in clinical and environmental strains of Aspergillus fumigatus in Turkey: A multicentre study. J. Antimicrob. Chemother. 2022, 77, 1894–1898. [Google Scholar] [CrossRef]
- Enoch, D.A.; Ludlam, H.A.; Brown, N.M. Invasive fungal infections: A review of epidemiology and management options. J. Med. Microbiol. 2006, 55, 809–818. [Google Scholar] [CrossRef]
- Kluge, S.; Strauß, R.; Kochanek, M.; Weigand, M.A.; Rohde, H.; Lahmer, T. Aspergillosis: Emerging risk groups in critically ill patients. Med. Mycol. 2021, 60, myab064. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, 16–18. [Google Scholar] [CrossRef]
- Rivelli Zea, S.M.; Toyotome, T. Azole-resistant Aspergillus fumigatus as an emerging worldwide pathogen. Microbiol. Immunol. 2022, 66, 135–144. [Google Scholar] [CrossRef]
- Castorena-Montoya, M. A Translational Approach to Improve Clinical Outcomes of Invasive Fungal Infections through Disease Surveillance and Antifungal Drug Discovery; University of Rochester: New York, NY, USA, 2020; 233p. [Google Scholar]
- Lockhart, S.R.; Frade, J.P.; Etienne, K.A.; Pfaller, M.A.; Diekema, D.J.; Balajee, S.A. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob. Agents Chemother. 2011, 55, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gamboa, R.A. New Tools in laboratory diagnosis of invasive fungal infections. In Tha Impact of Climate Change of Fungal Diseases. Fungal Biology; Springer: Cham, Switzerland, 2022; pp. 257–276. [Google Scholar] [CrossRef]
- Kone, A.K.; Thera, M.A. Resistance of Candida isolates to antifungals using VITEK system at the Rodolphe Mérieux Centre, Bamako, Mali. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Ira, V.B.; Roger, F.; Noell, J. Identification, Prevalence and Susceptibility Profile of Candida Isolates at the Pasteur Institute in Côte D ’ ivoire From 2017 to 2019. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Jacobs, J.L.; Dennis, E.K.; Taimur, S.; Rana, M.; Patel, D.; Gitman, M.; Patel, G.; Schaefer, S.; Iyer, K.; et al. Candida auris Pan-Drug-Resistant to Four Classes of Antifungal Agents. Antimicrob. Agents Chemother. 2022, 66, e0005322. [Google Scholar] [CrossRef]
- Brandão, L.R.; Medeiros, A.O.; Duarte, M.C.; Barbosa, A.C.; Rosa, C.A. Diversity and antifungal susceptibility of yeasts isolated by multiple-tube fermentation from three freshwater lakes in Brazil. J. Water Health 2010, 8, 279–289. [Google Scholar] [CrossRef]
- Steffen, H.; Bosch, C.; Wolfaardt, G.; Botha, A. Rising environmental temperatures and polluted surface waters: The prelude to the rise of mycoses in South Africa. Water SA 2022, 48, 199–216. [Google Scholar] [CrossRef]
- Cupozak-Pinheiro, W.J.; Araújo de Almeida-Apolonio, A.; Sasaki, M.H.; Maran, N.H.; Pires de Araújo, R.; Beraldo dos Santos Silva, D.; de Andrade dos Santos, J.V.; Barufatti, A.; Rodrigues Chang, M.; Pires de Oliveira, K.M. Candida species contamination in drinking groundwater from residence wells in three municipalities of midwestern Brazil and the potential human health risks. Microb. Pathog. 2022, 169, 105660. [Google Scholar] [CrossRef]
- Pérez-García, A.; Abad, R.F.; Pestaña, M. Infecciones en el paciente inmunocomprometido (II). Pacientes con trasplante de órgano sólido. Medicine 2022, 13, 3288–3297. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.O. Micosis en pacientes inmunocomprometidos. Med.-Programa Form. Médica Contin. Acreditado 2022, 13, 3415–3425. [Google Scholar] [CrossRef]
- González, L.; Sarabia, D. Prevalencia de Infecciones Fúngicas Oportunistas en Pacientes Hospitalizados en la Unidad de Cuidados Intensivos del Hospital Teodoro Maldonado Carbo en el Periodo Comprendido Entre Enero 2016 a Enero 2021; Universidad Católica de Santiago de Guayaquil: Guayaquil, Ecuador, 2022. [Google Scholar]
- Araya-Rojas, F.; Lasso-Barreto, M. Aspergilosis pulmonar asociada a COVID-19 en pacientes críticos: Experiencia de un hospital público chileno. Rev. Chil. Infectología 2021, 38, 754–760. [Google Scholar] [CrossRef]
- Sánchez Martín, C.; Madrid Martínez, E.; González Pellicer, R.; Armero Ibáñez, R.; Martínez González, E.; Llau Pitarch, J.V. Invasive pulmonary aspergillosis in patients with acute respiratory syndrome by COVID-19. Rev. Española Anestesiol. Reanim. (Engl. Ed.) 2022, 69, 48–53. [Google Scholar] [CrossRef]
- Vinayagamoorthy, K.; Pentapati, K.C.; Prakash, H. Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in Coronavirus disease (COVID-19) patients: A systematic review. Mycoses 2022, 65, 613–624. [Google Scholar] [CrossRef]
- Rybak, J.M.; Fortwendel, J.R.; Rogers, P.D. Emerging threat of triazole-resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 2019, 74, 835–842. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Hubka, V.; Watanabe, A.; Nagi, M.; Miyazaki, Y.; Yaguchi, T.; Kamei, K. Prevalence of Antifungal Resistance, Genetic Basis of Acquired Azole and Echinocandin Resistance, and Genotyping of Candida krusei Recovered from an International Collection. Antimicrob. Agents Chemother. 2022, 66, e01856-21. [Google Scholar] [CrossRef] [PubMed]
- Domán, M.; Makrai, L.; Bányai, K. Molecular Phylogenetic Analysis of Candida krusei. Mycopathologia 2022, 187, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Vahedi-Shahandashti, R.; Dietl, A.M.; Binder, U.; Nagl, M.; Wurzner, R.; Lass-Florl, C. Aspergillus terreus and the Interplay with Amphotericin B: From Resistance to Tolerance? Antimicrob. Agents Chemother. 2022, 66, e0227421. [Google Scholar] [CrossRef] [PubMed]
- Angiolella, L. Virulence Regulation and Drug-Resistance Mechanism of Fungal Infection. Microorganisms 2022, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Wan, Q.; Deng, X.; Cao, R.; Xu, X.; Chen, Z.; Wang, J.; Huang, T. Reactivation of fungal spores in water following UV disinfection: Effect of temperature, dark delay, and real water matrices. Chemosphere 2019, 237, 124490. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Cai, H.Q.; Qu, S.Y.; Lin, W.H.; Liang, C.C.; Liu, H.; Xie, Z.X.; Yuan, Y.J. Genomic Variation-Mediating Fluconazole Resistance in Yeast. Biomolecules 2022, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Niimi, K.; Tanabe, K.; Cannon, R.D.; Lamping, E. Inhibitor-Resistant Mutants Give Important Insights into Candida albicans ABC Transporter Cdr1 Substrate Specificity and Help Elucidate Efflux Pump Inhibition. Antimicrob. Agents Chemother. 2022, 66, e01748-21. [Google Scholar] [CrossRef]
- Maheronnaghsh, M.; Teimoori, A.; Dehghan, P.; Fatahinia, M. The evaluation of the overexpression of the ERG-11, MDR-1, CDR-1, and CDR-2 genes in fluconazole-resistant Candida albicans isolated from Ahvazian cancer patients with oral candidiasis. J. Clin. Lab. Anal. 2022, 36, e24208. [Google Scholar] [CrossRef]
- Gonçalves, P.; Melo, A.; Dias, M.; Almeida, B.; Caetano, L.A.; Veríssimo, C.; Viegas, C.; Sabino, R. Azole-resistant aspergillus fumigatus harboring the tr34/l98h mutation: First report in portugal in environmental samples. Microorganisms 2021, 9, 57. [Google Scholar] [CrossRef]
- Alvarez-Moreno, C.; Lavergne, R.A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Fungicide-driven alterations in azole-resistant Aspergillus fumigatus are related to vegetable crops in Colombia, South America. Mycologia 2019, 111, 217–224. [Google Scholar] [CrossRef]
- Brizzotti-Mazuchi, N.S.; Cunha, K.C.; Siqueira, J.P.Z.; Almeida, B.G.; Lemes, T.H.; Maschio-Lima, T.; Caetano, M.H.; Ribeiro, M.D.; Rodrigues, C.R.; Castilho, E.M.; et al. Diversity, seasonality, and antifungal susceptibility of yeasts in the public drinking water supply in a municipality of southeastern Brazil. Ecohydrol. Hydrobiol. 2020, 20, 450–455. [Google Scholar] [CrossRef]
- Hollomon, D. Does agricultural use of azole fungicides contribute to resistance in the human pathogen Aspergillus fumigatus? Pest Manag. Sci. 2017, 73, 1987–1993. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Flores, D.; Lana, D.; Kelly, G.; Carneiro, M. Resistance of filamental fungi iResistance of filamental fungi in opportunistic mycoses: Literature review. Res. Soc. Dev. 2022, 11, e2011426198. [Google Scholar] [CrossRef]
- Pham, C.D.; Reiss, E.; Hagen, F.; Meis, J.F.; Lockhart, S.R. Passive surveillance for azole-resistant Aspergillus fumigatus, United States, 2011–2013. Emerg. Infect. Dis. 2014, 20, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.W.M.; Arendrup, M.C.; Warris, A.; Lagrou, K.; Pelloux, H.; Hauser, P.M.; Chryssanthou, E.; Mellado, E.; Kidd, S.E.; Tortorano, A.M.; et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 2015, 21, 1041–1044. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.W.M.; Arendrup, M.C.; Melchers, W.J.G.; Verweij, P.E. Multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus, Colombia, 2015. Emerg. Infect. Dis. 2016, 22, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.S.; Whitley, R.D.; Plummer, C.E.; Richardson, R.L.; Hamor, R.E.; Wellehan, J.F.X. In vitro antifungal susceptibility of Fusarium species and Aspergillus fumigatus cultured from eleven horses with fungal keratitis. Vet. Ophthalmol. 2022, 25, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Eric, C.; Guorui, L.; Balaram, V.; Rita, A.; Ribeiro, L. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Oke, L.; Maseko, O.B. Occurrence of antibiotics in wastewater from hospital and convectional wastewater treatment plants and their impact on the effluent receiving rivers: Current knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 2–25. [Google Scholar] [CrossRef]
- Castelo-Branco, D.; Lockhart, S.R.; Chen, Y.-C.; Santos, D.A.; Hagen, F.; Hawkins, N.J.; Lavergne, R.-A.; Meis, J.F.; Le Pape, P.; Rocha, M.F.G.; et al. Collateral consequences of agricultural fungicides on pathogenic yeasts: A One Health perspective to tackle azole resistance. Mycoses 2022, 65, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Alastruey-izquierdo, A.; Berman, J.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; Kuijper, E.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Lota, M.M.M.; Chua, A.Q.; Azupardo, K.; Lumangaya, C.; Reyes, K.A.V.; Yvette, S.; Villanueva, A.M.; Legido-Quigley, H.; Roxas, E.A. A Qualitative Study on the Design and Implementation of the National Action Plan on Antimicrobial Resistance in the Philippines. Antibiotics 2022, 11, 820. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Z.; Vidović, J.; Erdeljan, M.; Cincović, M.; Ružić, Z.; Galić, I.; Kukurić, T.; Stojanac, N.; Horvat, O. Veterinary Practitioners ’ Standpoints and Comprehension towards Antimicrobial Use-Are There Opportunities for Antimicrobial Stewardship Improvement? Antibiotics 2022, 11, 867. [Google Scholar] [CrossRef]
- Al-gabr, H.M.; Zheng, T.; Yu, X. Efficacy of two chemical coagulants and three different filtration media on removal of Aspergillus flavus from surface water. J. Environ. Sci. 2014, 26, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Albolafio, S.; Marín, A.; Allende, A.; García, F.; Sim, P.J.; Abell, M.; Gil, M.I. Strategies for mitigating chlorinated disinfection byproducts in wastewater treatment plants. Chemosphere 2022, 288, 132583. [Google Scholar] [CrossRef]
- Foschi, J.; Bianchi, G.F.; Turolla, A.; Antonelli, M. Disinfection efficiency prediction under dynamic conditions: Application to peracetic acid disinfection of wastewater. Water Res. 2022, 222, 118879. [Google Scholar] [CrossRef]
- Ocampo-Rodríguez, D.B.; Vázquez-Rodríguez, G.A.; Martínez-Hernández, S.; Iturbe-Acosta, U.; Coronel-Olivares, C. Water disinfection: A review of conventional and advanced treatments with chlorine and peracetic acid. Ing. Agua 2022, 26, 185–204. [Google Scholar] [CrossRef]
- Venkobachar, C.; Iyengar, L.; Prabhakara Rao, A.V.S. Mechanism of disinfection: Effect of chlorine on cell membrane functions. Water Res. 1977, 11, 727–729. [Google Scholar] [CrossRef]
- Wan, Q.; Xia, Y.; Li, Y.; Wu, G.; Wang, J.; Huang, T.; Wen, G. Enhanced solar inactivation of fungal spores by addition of low-dose chlorine: Efficiency and mechanism. Water Res. 2022, 222, 118964. [Google Scholar] [CrossRef] [PubMed]
- Adefisoye, M.A.; Olaniran, A.O. Does Chlorination Promote Antimicrobial Resistance in Waterborne Pathogens? Mechanistic Insight into Co-Resistance and Its Implication for Public Health. Antibiotics 2022, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wan, Q.; Tan, L.; Xu, X.; Wu, G.; Wang, J.; Xu, H.; Huang, T.; Wen, G. Evaluation of the vital viability and their application in fungal spores’ disinfection with flow cytometry. Chemosphere 2021, 269, 128700. [Google Scholar] [CrossRef] [PubMed]
- Deborde, M.; von Gunten, U. Reactions of chlorine with inorganic and organic compounds during water treatment—Kinetics and mechanisms: A critical review. Water Res. 2008, 42, 13–51. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, W.D.; Minnigh, H.A.; Pipes, W.O. Chlorine demand and inactivation of fungal propagules. Appl. Environ. Microbiol. 1983, 45, 182–186. [Google Scholar] [CrossRef]
- Ma, X.; Baron, J.L.; Vikram, A.; Stout, J.E.; Bibby, K. Fungal diversity and presence of potentially pathogenic fungi in a hospital hot water system treated with on-site monochloramine. Water Res. 2015, 71, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Bibby, K. Free chlorine and monochloramine inactivation kinetics of Aspergillus and Penicillium in drinking water. Water Res. 2017, 120, 265–271. [Google Scholar] [CrossRef]
- Luo, X.; Xu, X.; Cao, R.; Wan, Q.; Wang, J.; Xu, H.; Lin, Y.; Wen, G.; Huang, T. The formation kinetics and control of biofilms by three dominant fungi species isolated from groundwater. J. Environ. Sci. 2021, 109, 148–160. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, X.; Tan, L.; Liang, Z.; Cao, R.; Wan, Q. The aggregation of Aspergillus spores and the impact on their inactivation by chlorine-based disinfectants. Water Res. 2021, 204, 117629. [Google Scholar] [CrossRef]
- Lim, S.; Lily, J.; von Gunten, U.; Mccurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Wolf, C.; von Gunten, U.; Kohn, T. Kinetics of Inactivation of Waterborne Enteric Viruses by Ozone. Environ. Sci. Technol. 2018, 52, 2170–2177. [Google Scholar] [CrossRef]
- Costa, L.R.d.C.; Féris, L.A. Use of ozonation technology to combat viruses and bacteria in aquatic environments: Problems and application perspectives for SARS-CoV-2. Environ. Technol. 2022, 44, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rey, R.; Cháez, H.; Baluja, C. Ozone Inactivation of Biologically-Risky Wastewaters. Ozone Sci. Eng. 1995, 17, 499–509. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, X.; Cao, R.; Wan, Q.; Xu, H.; Wang, J.; Lin, Y.; Huang, T.; Wen, G. Synergistic effect of ozone and chlorine on inactivating fungal spores: Influencing factors and mechanisms. J. Hazard. Mater. 2021, 420, 126610. [Google Scholar] [CrossRef]
- Huang, N.; Wang, W.-L.; Xu, Z.-B.; Lee, M.-Y.; Wu, Q.-Y.; Hu, H.-Y. A study of synergistic oxidation between ozone and chlorine on benzalkonium chloride degradation: Reactive species and degradation pathway. Chem. Eng. J. 2020, 382, 122856. [Google Scholar] [CrossRef]
- Diaz Fernandez, J.O.S.E. Ecuaciones y Cálculos para el Tratamiento de Aguas; Ediciones Paraninfo S.A.: Madrid, Spain, 2019; ISBN 8428341524. [Google Scholar]
- Zhang, C.; Brown, P.J.B.; Hu, Z. Thermodynamic properties of an emerging chemical disinfectant, peracetic acid. Sci. Total Environ. 2018, 621, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Xu, X.; Wan, Q.; Cao, R.; Liang, Z.; Xu, H.; Li, K.; Huang, T.; Wen, G.; Ma, J. Inactivation of fungal spores in water with peracetic acid: Efficiency and mechanism. Chem. Eng. J. 2022, 427, 131753. [Google Scholar] [CrossRef]
- Wen, G.; Tan, L.; Cao, R.; Wan, Q.; Xu, X.; Wu, G. Inactivation of waterborne fungal spores by 1-bromo-3-chloro-5, 5- dimethylhydantoin: Kinetics, in fl uencing factors and mechanisms. Chemosphere 2021, 274, 129764. [Google Scholar] [CrossRef]
- Xu, X.; Ran, Z.; Wen, G.; Liang, Z.; Wan, Q.; Chen, Z.; Lin, Y.; Li, K.; Wang, J.; Huang, T. Efficient inactivation of bacteria in ballast water by adding potassium peroxymonosulfate alone: Role of halide ions. Chemosphere 2020, 253, 126656. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Li, W.; Qiang, Z. A review of the fluence determination methods for UV reactors: Ensuring the reliability of UV disinfection. Chemosphere 2022, 286, 131488. [Google Scholar] [CrossRef]
- Khan, M.; Mcdonald, M.; Mundada, K. Efficacy of Ultraviolet Radiations against Coronavirus, Bacteria, Fungi, Fungal Spores and Biofilm. Hygiene 2022, 2, 120–131. [Google Scholar] [CrossRef]
- Mukunda, D.C.; Joshi, V.K.; Mahato, K.K. Light emitting diodes (LEDs) in fluorescence-based analytical applications: A review. Appl. Spectrosc. Rev. 2022, 57, 1–38. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J. Oxidatively Generated Damage to Cellular DNA by UVB and UVA Radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.Y.; Murray, V. The influence of DNA methylation on the sequence specificity of UVB- and UVC-induced DNA damage. J. Photochem. Photobiol. B Biol. 2021, 221, 112225. [Google Scholar] [CrossRef] [PubMed]
- Braga, G.U.L.; Rangel, D.E.N.; Fernandes, É.K.K.; Flint, S.D.; Roberts, D.W. Molecular and physiological effects of environmental UV radiation on fungal conidia. Curr. Genet. 2015, 61, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Goldman, G.H.; Kafer, E. Aspergillus nidulans as a model system to characterize the DNA damage response in eukaryotes. Fungal Genet. Biol. 2004, 41, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, S.; Rosado, D.; Moreno-andrés, J.; Cartuche, L.; Cruz, D.; Acevedo-merino, A.; Nebot, E. Inactivation of a wild isolated Klebsiella pneumoniae by photo-chemical processes: UV-C, UV-C/H2O2 and UV-C/H2O2/Fe3+. Catal. Today 2018, 313, 94–99. [Google Scholar] [CrossRef]
- Xu, X.; Zuo, J.; Wan, Q.; Cao, R.; Xu, H.; Li, K. Effective inactivation of fungal spores by the combined UV/PAA: Synergistic effect and mechanisms. J. Hazard. Mater. 2022, 430, 128515. [Google Scholar] [CrossRef]
- França, M.; Deborah, D.; Freitas, L.; Cristina, E.; Leticia, M.; Dutra, C.; Gabriel, L.; Juliana, F.; Araújo, C. De Effects of activated sludge and UV disinfection processes on the bacterial community and antibiotic resistance profile in a municipal wastewater treatment plant. Environ. Sci. Pollut. Res. 2022, 29, 36088–36099. [Google Scholar] [CrossRef]
- Rodríguez, R.A.; Navar, C.; Sangsanont, J.; Linden, K.G. UV inactivation of sewage isolated human adenovirus. Water Res. 2022, 218, 118496. [Google Scholar] [CrossRef] [PubMed]
- Nourmoradi, H.; Nikaeen, M.; Stensvold, C.R.; Mirhendi, H. Ultraviolet irradiation: An effective inactivation method of Aspergillus spp. in water for the control of waterborne nosocomial aspergillosis. Water Res. 2012, 46, 5935–5940. [Google Scholar] [CrossRef]
- Pereira, V.J.; Ricardo, J.; Galinha, R.; Benoliel, M.J.; Crespo, M.T.B. Occurrence and low pressure ultraviolet inactivation of yeasts in real water sources. Photochem. Photobiol. Sci. 2013, 12, 626–630. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cao, R.; Xu, X.; Zhao, H.; Li, K.; Wang, J.; Huang, T. Comparison of UV-LEDs and LPUV on inactivation and subsequent reactivation of waterborne fungal spores. Water Res. 2020, 173, 115553. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.R.; Crespo, M.T.B.; Pereira, V.J. Small but powerful: Light-emitting diodes for inactivation of Aspergillus species in real water matrices. Water Res. 2020, 168, 115108. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Marques, A.P.; Asif, M.; Crespo, M.T.B.; Pereira, V.J. Light-emitting diodes effect on Aspergillus species in filtered surface water: DNA damage, proteome response and potential reactivation. Environ. Pollut. 2021, 287, 117553. [Google Scholar] [CrossRef]
- Moreira, C.J.S.; Pereira, C.S.; Oliveira, B.R.; Marques, A.P.; Ressurreiç, M.; Crespo, T.B.; Pereira, V.J. Inactivation of Aspergillus species in real water matrices using medium pressure mercury lamps. J. Photochem. Photobiol. B Biol. 2021, 221, 112242. [Google Scholar] [CrossRef]
- Severin, B.F. Kinetic modeling of UV disinfetion of water. Water Res. 1983, 17, 1669–1678. [Google Scholar] [CrossRef]
- Geeraerd, A.H.; Herremans, C.H.; Van Impe, J.F. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 2000, 59, 185–209. [Google Scholar] [CrossRef]
- Rattanakul, S.; Oguma, K. Inactivation kinetics and ef fi ciencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms. Water Res. 2018, 130, 31–37. [Google Scholar] [CrossRef]
- Mcguigan, K.G.; Conroy, R.M.; Mosler, H.; Ubomba-jaswa, E.; Fernandez-iba, P. Solar water disinfection (SODIS): A review from bench-top to roof-top. J. Hazard. Mater. 2012, 235–236, 29–46. [Google Scholar] [CrossRef]
- Berney, M.; Weilenmann, H.; Egli, T. Flow-Cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS) Flow-cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS). Microbiology 2006, 152, 1719–1729. [Google Scholar] [CrossRef]
- Kadir, K.; Nelson, K.L. Sunlight mediated inactivation mechanisms of Enterococcus faecalis and Escherichia coli in clear water versus waste stabilization pond water. Water Res. 2013, 50, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; McGuigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005, 39, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wan, Q.; Xu, X.; Cao, R.; Li, Y.; Wang, J. Solar disinfection of fungal spores in water: Kinetics, influencing factors, mechanisms and regrowth. Chem. Eng. J. 2022, 428, 132065. [Google Scholar] [CrossRef]
- Isaac, S.; Salmer, I. Solar-driven free chlorine advanced oxidation process for simultaneous removal of microcontaminants and microorganisms in natural water at pilot-scale. Chemosphere 2022, 288, 132493. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Wan, Q.; Xu, X.; Cao, R.; Wang, J. Inactivation of fungal spores in water by CuO-activated peracetic acid: Kinetics, mechanism and regrowth. J. Hazard. Mater. 2022, 439, 129611. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.R.; Maura, R.; Franco, B.; Guadagnini, R.A.; Urbano, L. Giardia duodenalis: Number and Fluorescence Reduction Caused by the Advanced Oxidation Process (H2O2/UV). Int. Sch. Res. Not. 2014, 2014, 525719. [Google Scholar] [CrossRef]
- Augugliaro, V.; Litter, M.; Palmisano, L.; Soria, J. The combination of heterogeneous photocatalysis with chemical and physical operations: A tool for improving the photoprocess performance. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 127–144. [Google Scholar] [CrossRef]
- Alarco, D.C.; Maldonado, M.I.; Malato, S.; Gernjak, W. Photocatalytic decontamination and disinfection of water with solar collectors. Catal. Today 2007, 122, 137–149. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Surbhi, B.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Wastewater Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, UK, 2018; pp. 135–175. [Google Scholar]
- Guerra-Rodriguez, S.; Rodríguez-Chueca, J.; Peres, J.A.; Lucas, M.S. Inactivation of Pathogenic Microorganisms with Sulfate Radical-based Advanced Oxidation Processes. In Persulfate-Based Oxidation Processes in Environmental Remediation; Zhu, M., Bian, Z., Zhao, C., Eds.; The Royal Society of Chemistry: London, UK, 2022; pp. 229–251. ISBN 978-1-83916-308-1. [Google Scholar]
- Khan, J.A.; Sayed, M.; Khan, S.; Shah, N.S.; Dionysiou, D.D.; Boczkaj, G. Advanced oxidation processes for the treatment of contaminants of emerging concern. In Contaminants of Emerging Concern in Water and Wastewater; Hernández-Maldonado, A.J., Blaney, L., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 299–365. [Google Scholar]
- Ioannou-ttofa, L.; Raj, S.; Prakash, H.; Fatta-kassinos, D. Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effl uents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Carbajo, J.; Silveira, J.E.; Pliego, G.; Zazo, J.A.; Casas, J.A. Increasing Photo-Fenton process Efficiency: The effect of high temperatures. Sep. Purif. Technol. 2021, 271, 118876. [Google Scholar] [CrossRef]
- Polo-López, M.I.; García-Fernández, I.; Velegraki, T.; Katsoni, A.; Oller, I.; Mantzavinos, D.; Fernández-Ibáñez, P. Mild solar photo-Fenton: An effective tool for the removal of Fusarium from simulated municipal effluents. Appl. Catal. B Environ. 2012, 111–112, 545–554. [Google Scholar] [CrossRef]
- Polo-López, M.I.; Castro-Alférez, M.; Oller, I.; Fernández-Ibáñez, P. Assessment of solar photo-Fenton, photocatalysis, and H2O2 for removal of phytopathogen fungi spores in synthetic and real effluents of urban wastewater. Chem. Eng. J. 2014, 257, 122–130. [Google Scholar] [CrossRef]
- Oller, I.; Fernández-ibá, P. Benefits of photo-Fenton at low concentrations for solar disinfection of distilled water. A case study: Phytophthora capsici. Catal. Today 2013, 209, 181–187. [Google Scholar] [CrossRef]
- Aguas, Y.; Hincapie, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Solar photocatalytic disinfection of agricultural pathogenic fungi (Curvularia sp.) in real urban wastewater. Sci. Total Environ. 2017, 607–608, 1213–1224. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Naeem, K.; Ouyang, F. Preparation of Fe3+-doped TiO2 nanoparticles and its photocatalytic activity under UV light. Phys. B Phys. Condens. Matter 2010, 405, 221–226. [Google Scholar] [CrossRef]
- Polo-López, M.I.; Fernández-Ibáñez, P.; García-Fernández, I.; Oller, I.; Salgado-Tránsito, I.; Sichel, C. Resistance of Fusarium sp. spores to solar TiO2 photocatalysis: Influence of spore type and water (scaling-up results). J. Chem. Technol. Biotechnol. 2010, 85, 1038–1048. [Google Scholar] [CrossRef]
- Makoday, N.M.; Saprykina, M.N.; Soboleva, N.M.; Savluk, O.S.; Goncharuk, V.V. Inactivation of Candida Albicans in the UV/TiO2/Fe3+ system. J. Water Chem. Technol. 2015, 37, 140–144. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Miranda, S.M.; Lopes, F.V.S.; Silva, M.; Dezotti, M.; Silva, A.M.T.; Faria, J.L.; Boaventura, R.A.R. Bacteria and fungi inactivation by photocatalysis under UVA irradiation: Liquid and gas phase. Environ. Sci. Pollut. Res. 2017, 24, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
- Rengifo-Herrera, J.A.; Pulgarin, C. Why five decades of massive research on heterogeneous photocatalysis, especially on TiO2, has not yet driven to water disinfection and detoxification applications? Critical review of drawbacks and challenges. Chem. Eng. J. 2023, 477, 146875. [Google Scholar] [CrossRef]
- Yeom, Y.; Han, J.; Zhang, X.; Shang, C.; Zhang, T.; Li, X.; Duan, X.; Dionysiou, D.D. A review on the degradation efficiency, DBP formation, and toxicity variation in the UV/chlorine treatment of micropollutants. Chem. Eng. J. 2021, 424, 130053. [Google Scholar] [CrossRef]
- Wan, Q.; Wen, G.; Cao, R.; Zhao, H.; Xu, X. Simultaneously enhance the inactivation and inhibit the photoreactivation of fungal spores by the combination of UV-LEDs and chlorine: Kinetics and mechanisms. Water Res. 2020, 184, 116143. [Google Scholar] [CrossRef] [PubMed]
- Al-gabr, H.M.; Zheng, T.; Yu, X. Inactivation of Aspergillus flavus in drinking water after treatment with UV irradiation followed by chlorination. Sci. Total Environ. 2013, 463–464, 525–529. [Google Scholar] [CrossRef]
- Ali, E.; Abdelrahman, T.; Sayed, M.; Abd Al Khalek, S. Occurrence of Fungi in Drinking Water Sources and Their Treatment by Chlorination and UV-Irradiation. Egypt. J. Bot. 2017, 57, 621–632. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, H.; Wan, Q.; Xu, X.; Cao, R.; Li, K.; Wang, J.; Huang, T.; Lu, J.; Wen, G. Inactivation and subsequent reactivation of Aspergillus species by the combination of UV and monochloramine: Comparisons with UV/chlorine. J. Environ. Sci. 2022, 117, 105–118. [Google Scholar] [CrossRef]
- Rehman, F.; Sayed, M.; Khan, J.A.; Shah, N.S.; Khan, H.M.; Dionysiou, D.D. Oxidative removal of brilliant green by UV/S2O8 2null, UV/HSO5 null and UV/H2O2 processes in aqueous media: A comparative study. J. Hazard. Mater. 2018, 357, 506–514. [Google Scholar] [CrossRef]
- Wen, G.; Deng, X.; Wan, Q.; Xu, X.; Huang, T. Photoreactivation of fungal spores in water following UV disinfection and their control using UV-based advanced oxidation processes. Water Res. 2019, 148, 1–9. [Google Scholar] [CrossRef]
- Ashrafivala, M.; Borhan, S.; Zeinali, S.; Heidari, M.; Mohammadpourfard, M.; Aslani, H. Investigation of H2O2/UV advanced oxidation process on the removal rate of coliforms from the industrial effluent: A pilot-scale study. Int. J. Hydrogen Energy 2022, 47, 33530–33540. [Google Scholar] [CrossRef]
- Sichel, C.; Fernández-Ibáñez, P.; de Cara, M.; Tello, J. Lethal synergy of solar UV-radiation and H2O2 on wild Fusarium solani spores in distilled and natural well water. Water Res. 2009, 43, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Polo-López, M.I.; Garcia-Fernández, I.; Oller, I.; Fernández-Ibáñez, P. Solar disinfection of fungal spores in water aided by low concentrations of hydrogen peroxide. Photochem. Photobiol. Sci. 2011, 2, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254 nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Liu, B. Mechanism study on the effect of peracetic acid (PAA), UV/PAA and ultrasonic/PAA oxidation on ultrafiltration performance during algae-laden water treatment. Water Res. 2022, 220, 118705. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Komasa, S.; Morimoto, Y.; Sekino, T.; Kawazoe, T.; Okazaki, J. UV/ozone irradiation manipulates immune response for antibacterial activity and bone regeneration on titanium. Mater. Sci. Eng. C 2021, 129, 112377. [Google Scholar] [CrossRef] [PubMed]
- Oliva, G.; Comia, J.R.; Senatore, V.; Zarra, T.; Ballestreros, F.; Belgiorno, V.; Naddeo, V. Degradation of gaseous volatile organic compounds (VOCs) by a novel UV-ozone technology. Sci. Rep. 2022, 12, 11112. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Chen, Z.; Wan, Q.; Zhao, D.; Xu, X.; Wang, J.; Li, K.; Huang, T. Activation of PMS by pipe corrosion products for fungi disinfection in water: Performance and mechanisms. Chem. Eng. J. 2020, 382, 123003. [Google Scholar] [CrossRef]
- Rodriguez-Chueca, J.; Moreira, I.; Lucas, M.S.; Tavares, P.B.; Sampaio, A. Disinfection of simulated and real winery wastewater using sulphate radicals: Peroxymonosulphate/transition metal/UV-A LED oxidation. J. Clean. Prod. 2017, 149, 805–817. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-liethy, M.A.; Ahmed, M.S.; Ezzat, S.M.; Kamel, M.M. Facile synthesis of magnetic disinfectant immobilized with silver ions for water pathogenic microorganism’s deactivation. Environ. Sci. Pollut. Res. 2018, 25, 22797–22809. [Google Scholar] [CrossRef]
- Le, T.T.H.; Ngo, T.T.; Nguyen, T.H.H.; Pham, T.D.; Vu, T.X.H.; Tran, Q.V. Green Nanoarchitectonics Using Cleistocalyx Operculatus Leaf Extract in the Preparation of Multifunctional Graphene Oxide/Fe3O4/Ag Nanomaterials for Water Decontamination and Disinfection. J. Inorg. Organomet. Polym. Mater. 2022, 32, 547–559. [Google Scholar] [CrossRef]
- Hadi, D.M.; Hossein, M.A.; Reza, J.G.; Razieh, S. Investigation and evaluation of ultrasound reactor for reduction of fungi from sewage. J. Zhejiang Univ. Sci. B 2007, 8, 493–497. [Google Scholar] [CrossRef]
- El-sayyad, G.S.; Elkodous, M.A.; El-khawaga, A.M.; Elsayed, M.A.; El-batal, A.I.; Gobara, M. Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1−xFe2O4/SiO2/TiO2; x = 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection: Implication for wastewater treatment. R. Soc. Chem. 2020, 10, 5241–5259. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Paiva, M.A.N.; Sampaio, C.M.S.; Castelo-Branco, D.S.C.M.; Teixeira, C.E.C.; de Alencar, L.P.; Bandeira, T.J.P.G.; Monteiro, A.J.; Cordeiro, R.A.; Pereira-Neto, W.A.; et al. Azole Resistance in Candida Spp. Isolated from Catú Lake, Ceará, Brazil: An Efflux-Pump-Mediated Mechanism. Braz. J. Microbiol. 2016, 47, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).