Characteristics of Natural Ti-Bearing Nanoparticles in Groundwater within Karst Areas of Northern China
Abstract
1. Introduction
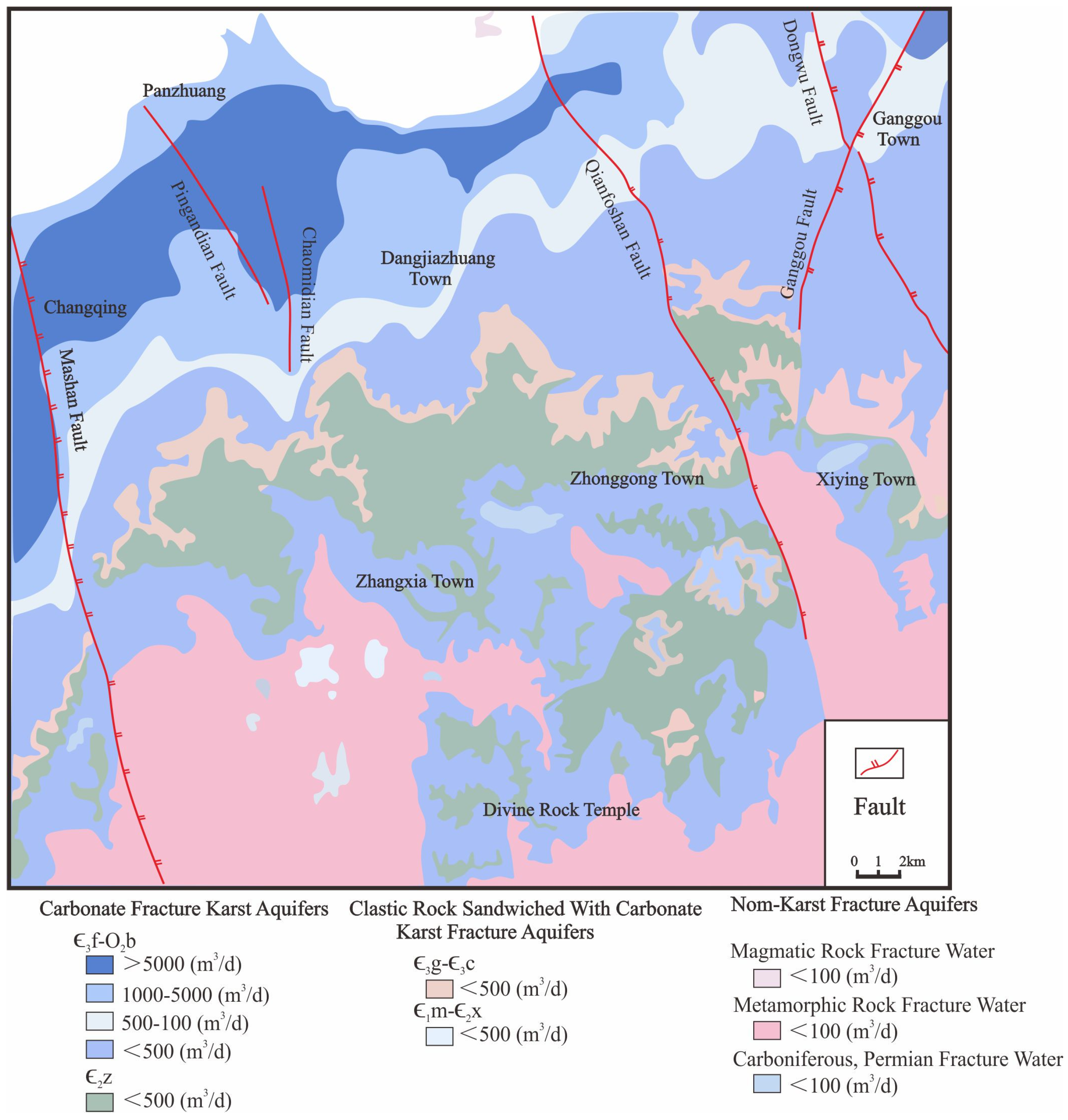
2. Geological Setting
3. Sampling and Analytical Methods
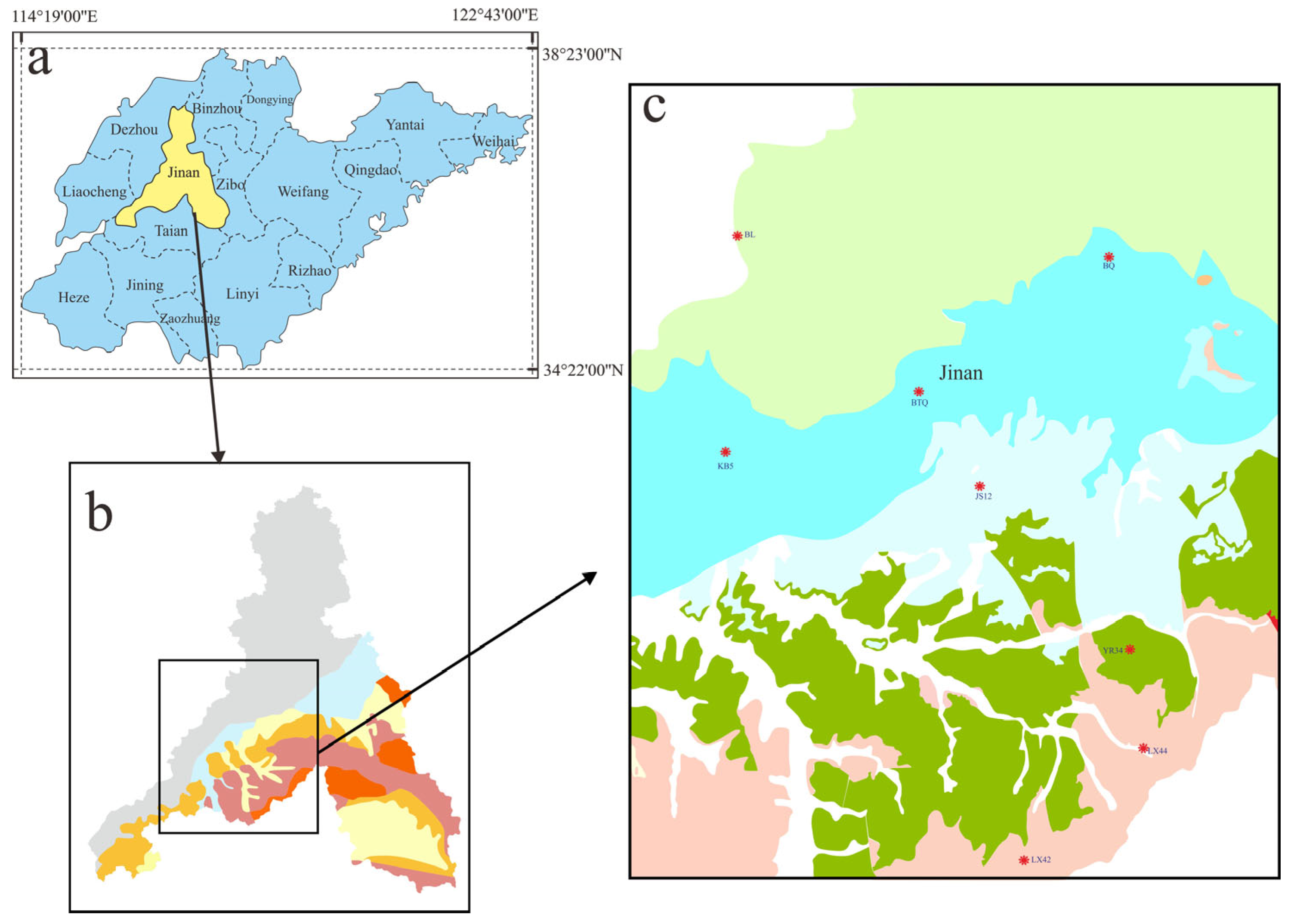
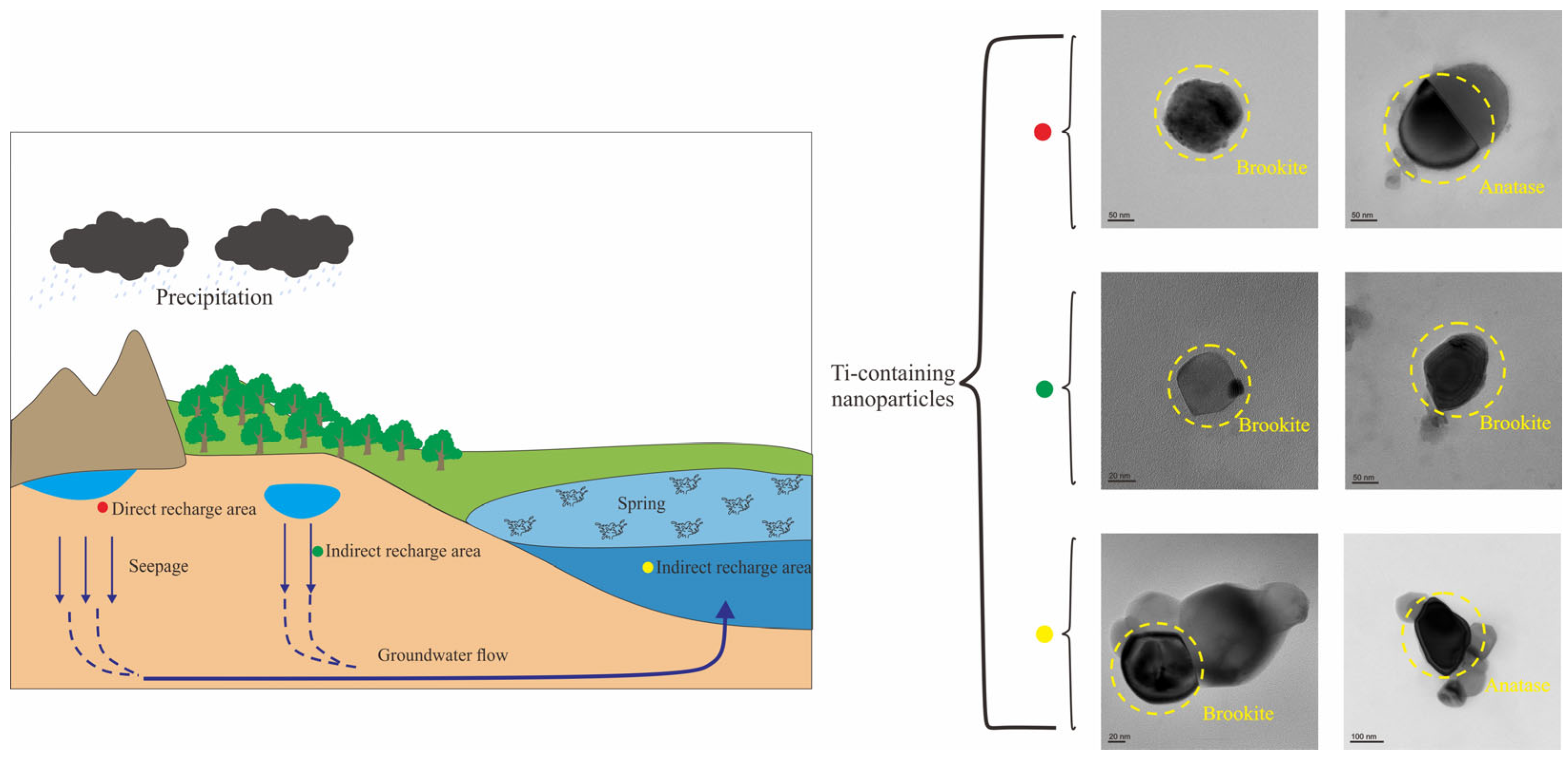
3.1. Sampling Sites
3.2. Analytical Methods
4. Results
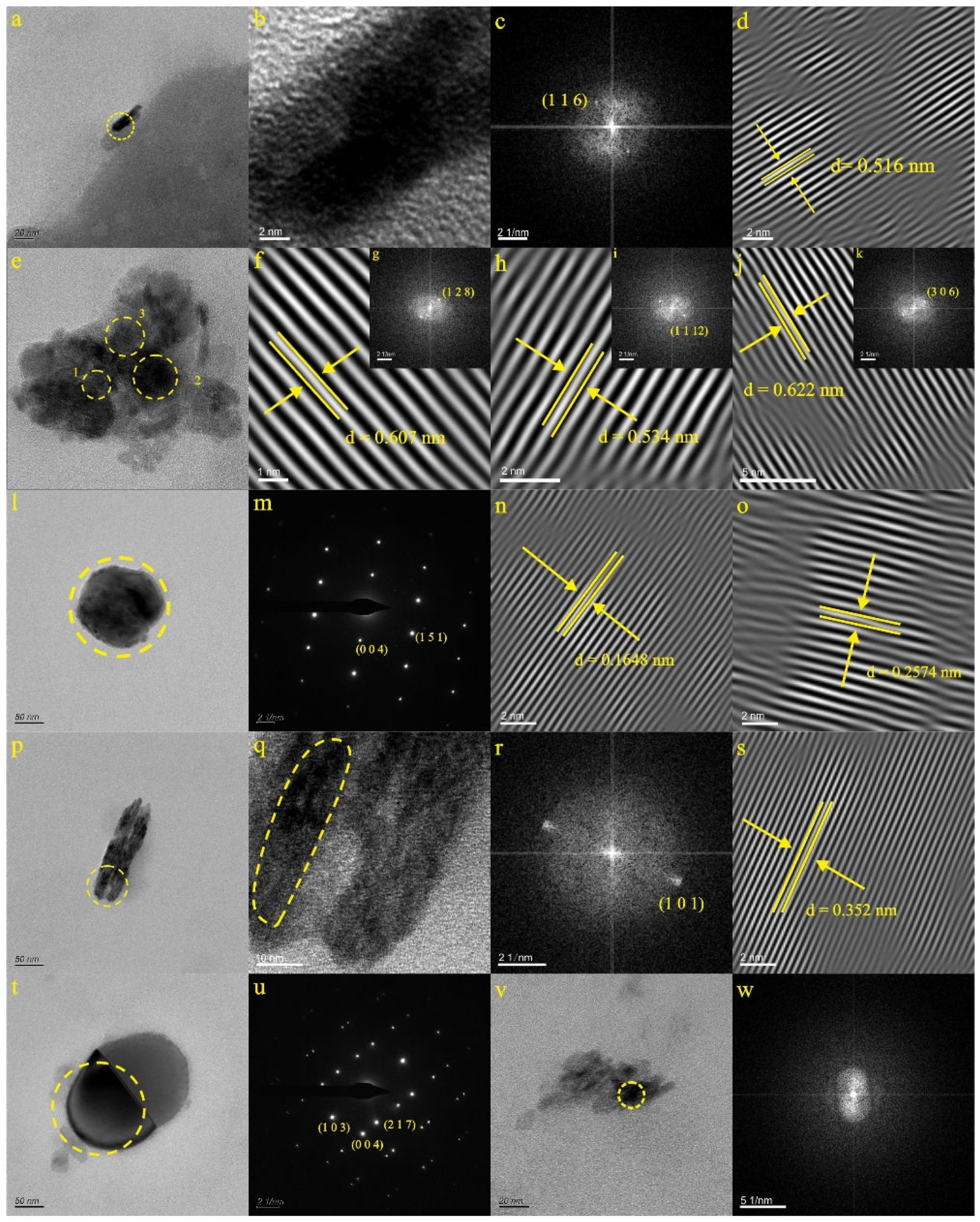
4.1. Characteristics of Natural Nanoparticles in Spring Water
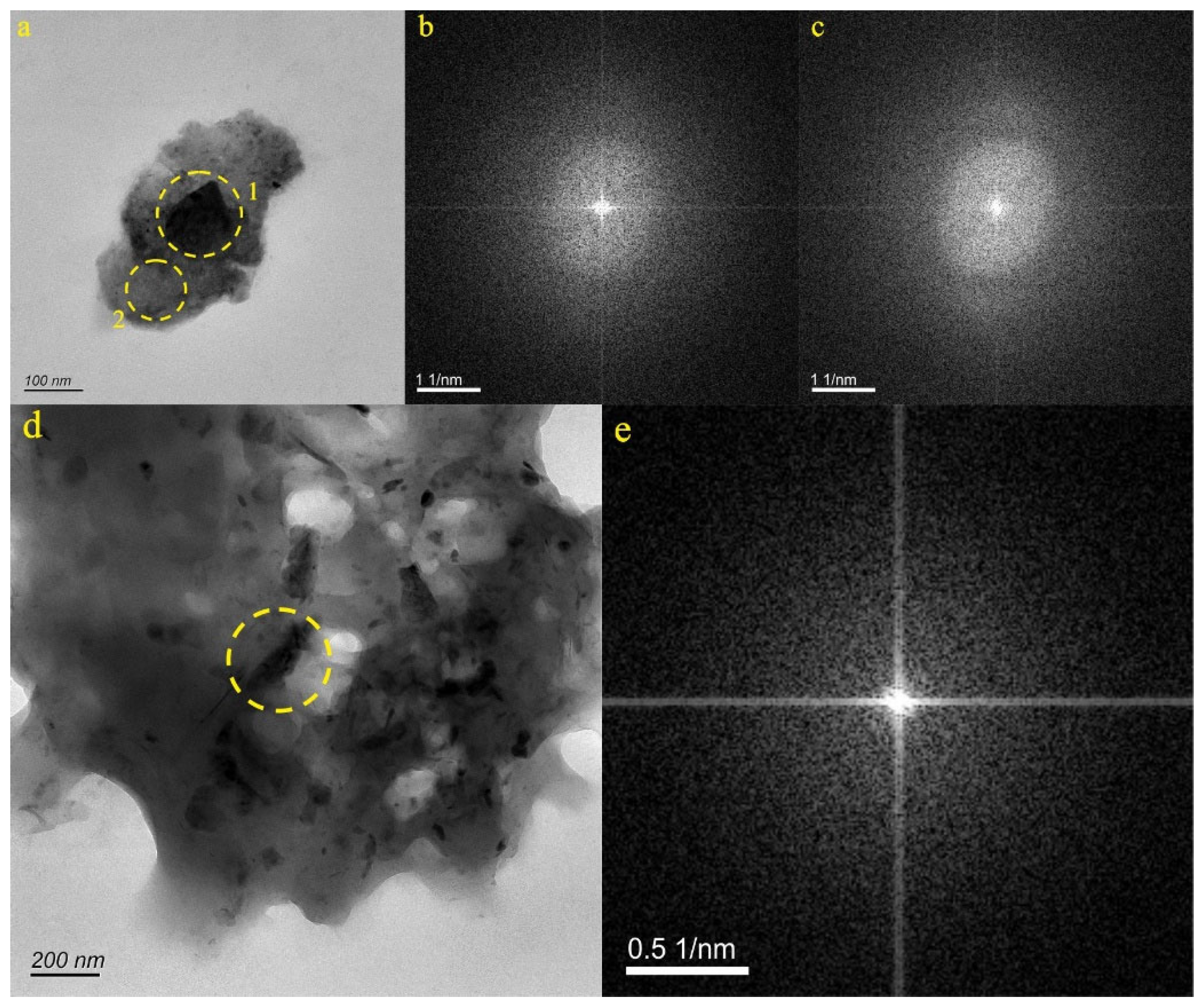
4.2. Characteristics of Natural Nanoparticles in Karst Water
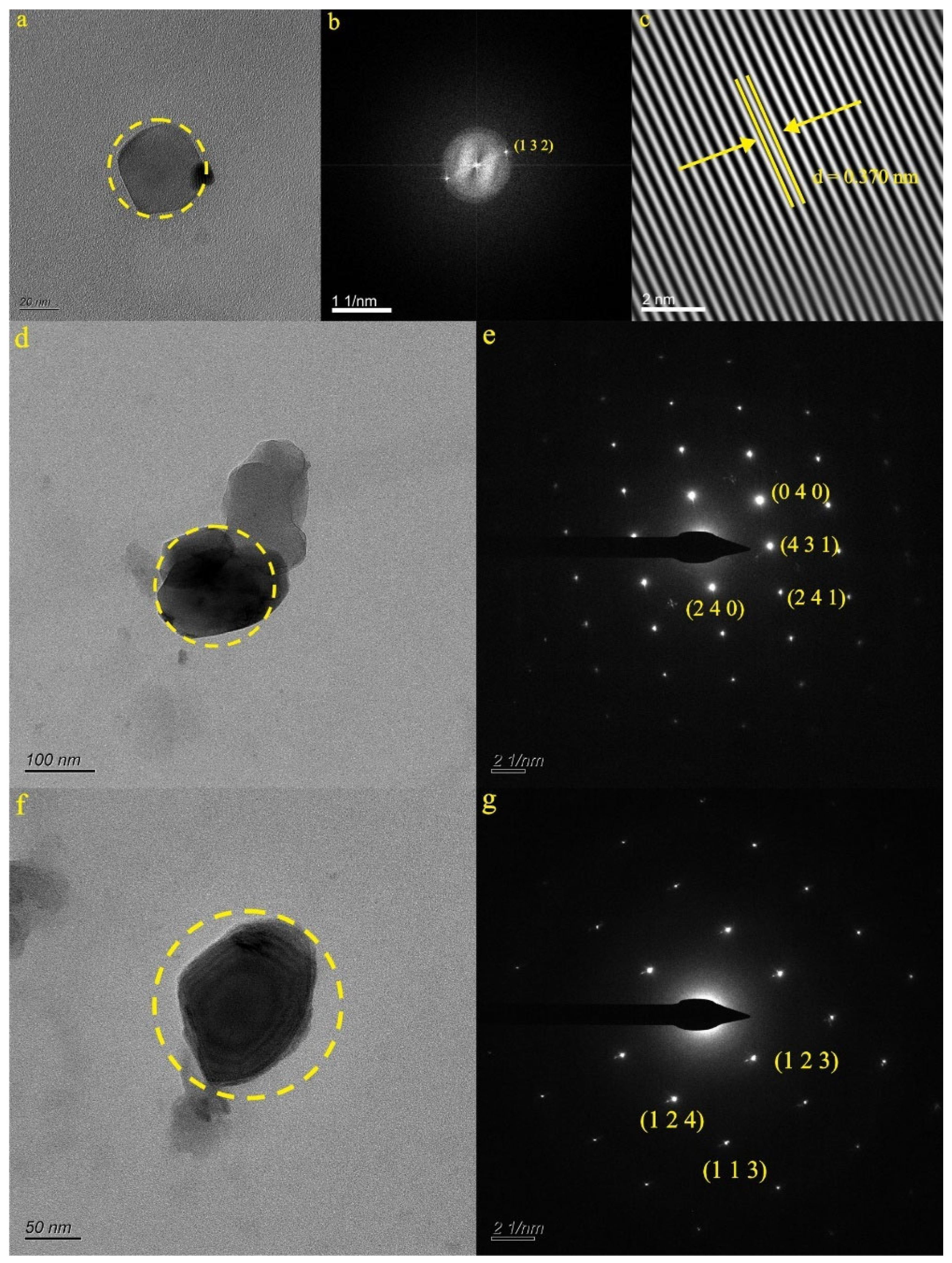
4.2.1. Characteristics of Natural Ti-Bearing Nanoparticles in LX42 and LX44 Samples
4.2.2. Characteristics of Natural Ti-Bearing Nanoparticles in JS12 Sample
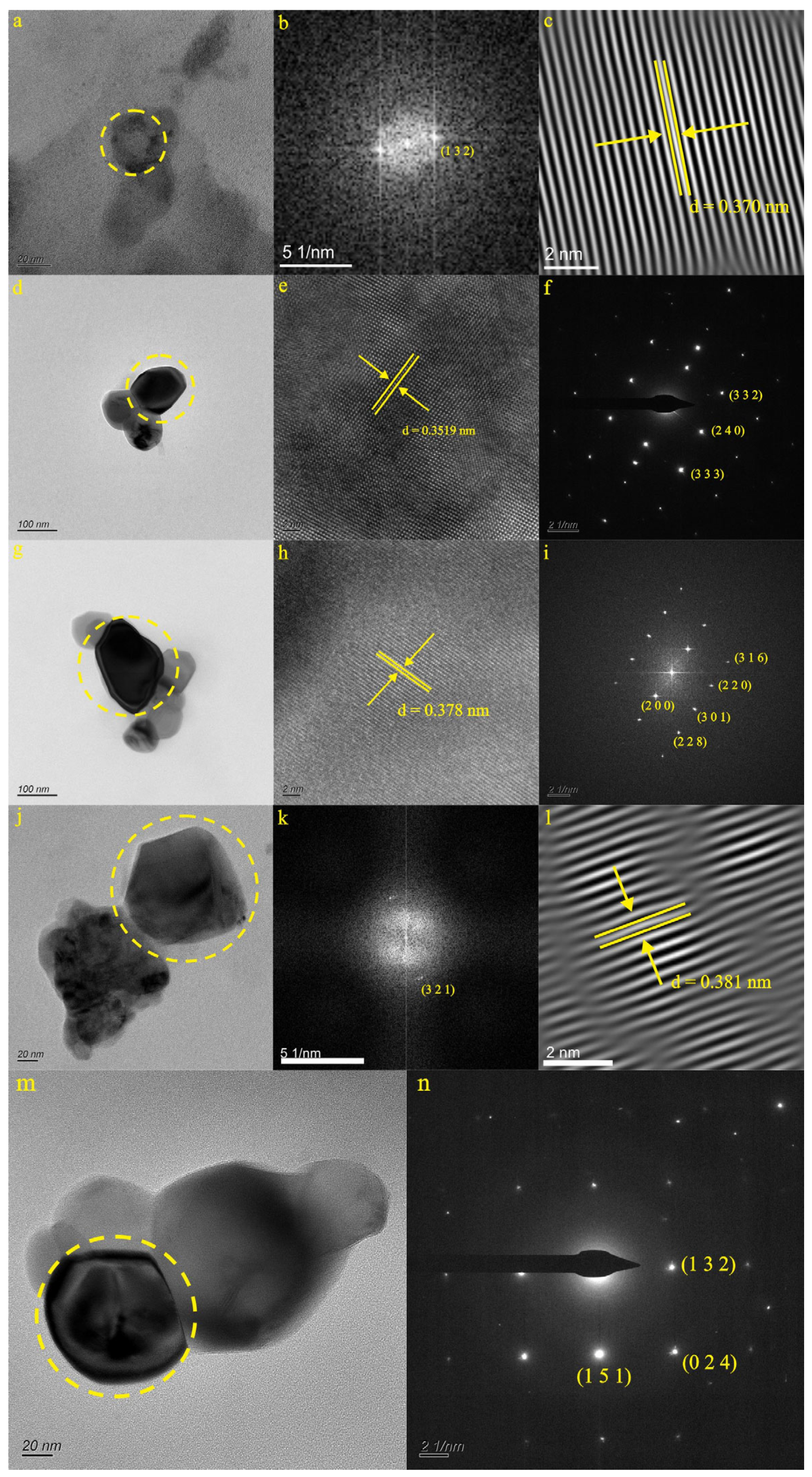
4.2.3. Characteristics of Natural Ti-Bearing Nanoparticles in KB5 and YR34 Samples
4.3. Characteristics of Natural Nanoparticles in Deep Geothermal Water
5. Discussion
5.1. Occurrence State of Ti in Groundwater
5.2. Growth Characteristics of Natural Ti-Bearing Nanoparticles
5.3. Environmental Significance of Natural TiO2 Nanoparticles in Karst Groundwater
5.4. Hydrogeological Significance of Natural Ti-Bearing Nanoparticles in Karst Groundwater
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bakshi, S.; He, Z.L.; Harris, W.G. Natural nanoparticles: Implications for environment and human health. Crit. Rev. Environ. Sci. Technol. 2015, 45, 861–904. [Google Scholar] [CrossRef]
- Frimmel, F.H.; Niessner, R. Nanoparticles in the Water Cycle: Properties, Analysis and Environmental Relevance; Springer Science and Business Media LLC.: Dordrecht, The Netherlands, 2010; pp. 1–239. [Google Scholar] [CrossRef]
- Wigginton, N.S.; Haus, K.L.; Hochella, M.F. Aquatic environmental nanoparticles. J. Environ. Monit. 2007, 9, 1306–1316. [Google Scholar] [CrossRef]
- Dibyanshu, K.; Chhaya, T.; Raychoudhury, T. A review on the fate and transport behavior of engineered nanoparticles: Possibility of becoming an emerging contaminant in the groundwater. Int. J. Environ. Sci. Technol. 2023, 20, 4649–4672. [Google Scholar] [CrossRef]
- Banfield, J.F.; Zhang, H. Nanoparticles in the environment. Rev. Miner. Geochem. 2001, 44, 1–58. [Google Scholar] [CrossRef]
- Ju, Y.; Li, X.; Ju, L.; Feng, H.; Tan, F.; Cui, Y.; Yang, Y.; Wang, X.; Cao, J.; Qiao, P.; et al. Nanoparticles in the Earth surface systems and their effects on the environment and resource. Gondwana Res. 2022, 110, 370–392. [Google Scholar] [CrossRef]
- Consani, S.; Carbone, C.; Dinelli, E.; Balić-Žunić, T.; Cutroneo, L.; Capello, M.; Salviulo, G.; Lucchetti, G. Metal transport and remobilisation in a basin affected by acid mine drainage: The role of ochreous amorphous precipitates. Environ. Sci. Pollut. Res. 2017, 24, 15735–15747. [Google Scholar] [CrossRef] [PubMed]
- Konrad-Schmolke, M.; Halama, R.; Wirth, R.; Thomen, A.; Klitscher, N.; Morales, L.; Schreiber, A.; Wilke, F.D. Mineral dissolution and reprecipitation mediated by an amorphous phase. Nat. Commun. 2018, 9, 1637. [Google Scholar] [CrossRef]
- Giuffre, A.J.; Hamm, L.M.; Han, N.; De Yoreo, J.J.; Dove, P.M. Polysaccharide chemistry regulates kinetics of calcite nucleation through competition of interfacial energies. Proc. Natl. Acad. Sci. USA 2013, 110, 9261–9266. [Google Scholar] [CrossRef]
- Faulstich, L.; Griffin, S.; Nasim, M.J.; Masood, M.I.; Ali, W.; Alhamound, S.; Omran, Y.; Kim, H.; Kharma, A.; Schäfer, K.H.; et al. Nature’s Hat-trick: Can we use sulfur springs as ecological source for materials with agricultural and medical applications? Int. Biodeterior. Biodegrad. 2017, 119, 678–686. [Google Scholar] [CrossRef]
- Nazari, A.; Nakhaei, M.; Yari, A.R. Arsenic Adsorption by TiO2 Nanoparticles Under Conditions Similar to Groundwater: Batch and Column Studies. Int. J. Environ. Res. 2021, 15, 79–91. [Google Scholar] [CrossRef]
- Eljamal, O.; Sasaki, K.; Tsuruyama, S.; Hirajima, T. Kinetic Model of Arsenic Sorption onto Zero-Valent Iron (ZVI). Water Qual. Expo. Health 2011, 2, 125–132. [Google Scholar] [CrossRef]
- Ken, D.S.; Sinha, A. Recent developments in surface modification of nano zero-valent iron (nZVI): Remediation, toxicity and environmental impacts. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100344. [Google Scholar] [CrossRef]
- Aredes, S.; Klein, B.; Pawlik, M. The removal of arsenic from water using natural iron oxide minerals. J. Clean. Prod. 2013, 60, 71–76. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Eng. J. 2012, 180, 81–90. [Google Scholar] [CrossRef]
- Chen, L.; He, B.Y.; He, S.; Wang, T.J.; Su, C.L.; Jin, Y. Fe-Ti oxide nano-adsorbent synthesized by co-precipitation for fluoride removal from drinking water and its adsorption mechanism. Powder Technol. 2012, 227, 3–8. [Google Scholar] [CrossRef]
- Xu, M.; Mao, B.Y.; Zhang, Q. Evolvement and expectation of research on the modern deep karst. Adv. Earth Sci. 2008, 25, 495–500, (In Chinese with English Abstract). [Google Scholar]
- Yue, F.J.; Li, S.L.; Waldron, S.; Wang, Z.J.; Oliver, D.M.; Chen, X.; Liu, C.Q. Rainfall and conduit drainage combine to accelerate nitrate loss from a karst agroecosystem: Insights from stable isotope tracing and high-frequency nitrate sensing. Water Res. 2020, 186, 116388. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, Z. Karst Aquifers-Characterization and Engineering; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Di, C.B. Study on Comprehensive Technology of Baoquan in Jinan. Master’s Thesis, Shandong University, Jinan, China, 2007. (In Chinese with English Abstract). [Google Scholar]
- Li, C.M. Analysis on karst resources and preservation of famous springs in Jinan. Carsologica Sin. 1985, Z1, 37–745, (In Chinese with English Abstract). [Google Scholar]
- Wang, J.Q.; Wu, Y.F.; Qian, J.Z.; Li, F.L. Scheme of groundwater exploited to keep spring spurting and water supply in Jinan Spring zone. J. Agro-Environ. Sci. 2004, 23, 1228–1231, (In Chinese with English Abstract). [Google Scholar]
- Wu, X.P. Experimental Research on Artificial Compensation for Groundwater to Protect Springs in Jinan. Master’s Thesis, Wuhan University, Wuhan, China, 2004. (In Chinese with English Abstract). [Google Scholar]
- Xu, J.X.; Xing, L.T.; Tong, G.Y.; Fan, L.Q. Groundwater environment evolution and its conservation in Jinan spring catchment. Hydrogeol. Eng. Geol. 2004, 6, 69–73, (In Chinese with English Abstract). [Google Scholar]
- Zou, S.Z.; Zhang, W.H.; Liang, B.; Chen, H.F.; Liang, X.P. A discussion of the assessment of ground water vulnerability in epi karst zone of the karst area, Southwest China. Earth Sci. Front. 2005, 12, 152–158, (In Chinese with English Abstract). [Google Scholar]
- Raman, K.V.; Jackson, M.L. Rutile and anatase determination in soils and sediments. Am. Mineral. J. Earth Planet. Mater. 1965, 50, 1086–1092. [Google Scholar]
- Sayin, M.; Jackson, M.L. Anatase and rutile determination in kaolinite deposits. Clays Clay Miner. 1975, 23, 437–443. [Google Scholar] [CrossRef]
- Baioumy, H.M. Ti-bearing minerals in sedimentary kaolin deposits of Egypt. Appl. Clay Sci. 2014, 101, 345–353. [Google Scholar] [CrossRef]
- Mankin, C.J. Proposed Reference Illite from the Ouachita Mountains of Southeastern Oklahoma1. Clays Clay Miner. 1961, 10, 372–379. [Google Scholar] [CrossRef]
- Weiss, A.; Range, K.J. On titanium in the kaolinite lattice: Int. In Proceedings of the Clay Conference, Jerusalem, Israel, 20–24 June 1966; pp. 53–66. [Google Scholar]
- Plavsa, D.; Reddy, S.M.; Agangi, A.; Clark, C.; Kylander-Clark, A.; Tiddy, C.J. Microstructural, trace element and geochronological characterization of TiO2 polymorphs and implications for mineral exploration. Chem. Geol. 2018, 476, 130–149. [Google Scholar] [CrossRef]
- Hu, G.; Cao, J.; Wang, C.; Lu, M.; Lin, Z.X. Study on the characteristics of naturally formed TiO2 nanoparticles in various surficial media from China. Chem. Geol. 2020, 550, 119703. [Google Scholar] [CrossRef]
- Liang, P.; Shi, T.; Jing, L.I. Nanometer-size titanium dioxide separation/preconcentration and FAAS determination of trace Zn and Cd in water sample. Int. J. Environ. Anal. Chem. 2004, 84, 315–321. [Google Scholar] [CrossRef]
- Engates, K.E.; Shipley, H.J. Adsorption of Pb, Cd, Cu, Zn, and Ni to titanium dioxide nanoparticles: Effect of particle size, solid concentration, and exhaustion. Environ. Sci. Pollut. Res. 2011, 18, 386–395. [Google Scholar] [CrossRef]
- Petsev, D.N.; Chen, K.; Gliko, O.; Vekilov, P.G. Diffusion-limited kinetics of the solution-solid phase transition of molecular substances. Proc. Natl. Acad. Sci. USA 2003, 100, 792–796. [Google Scholar] [CrossRef]
- Penna, D.; Hopp, L.; Scandellari, F.; Allen, S.T.; Benettin, P.; Beyer, M.; Geris, J.; Klaus, J.; Marshall, J.D.; Schwendenmann, L.; et al. Ideas and perspectives: Tracing terrestrial ecosystem water fluxes using hydrogen and oxygen stable isotopes—Challenges and opportunities from an interdisciplinary perspective. Biogeosciences 2018, 15, 6399–6415. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Story, S.D.; Walker, S.L.; Huang, Q.; Liang, W.; Cai, P. Role of pH and ionic strength in the aggregation of TiO2 nanoparticles in the presence of extracellular polymeric substances from Bacillus subtilis. Environ. Pollut. 2017, 228, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Le Coustumer, P.; Stoll, S. TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids. pH and concentration effects on nanoparticle stability. Water Res. 2013, 47, 6052–6063. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.L.; Hall, C.E.; Mesmer, R.E. The system dipotassium hydrogen phosphate-water at high temperatures (100–400 °C); Liquid-liquid immiscibility and concentrated solutions. J. Inorg. Nucl. Chem. 1981, 43, 449–455. [Google Scholar] [CrossRef]
- Zhang, H.; Gilbert, B.; Huang, F.; Banfield, J.F. Water-driven structure transformation in nanoparticles at room temperature. Nature 2003, 424, 1025–1029. [Google Scholar] [CrossRef]
- Guo, H.; Barnard, A.S. Naturally occurring iron oxide nanoparticles: Morphology, surface chemistry and environmental stability. J. Mater. Chem. A 2013, 1, 27–42. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, J.; Sang, Y.; Cabot, A.; Liu, H. Structure, Synthesis, and Applications of TiO2 Nanobelts. Adv. Mater. 2015, 27, 2557–2582. [Google Scholar] [CrossRef]
- Adesina, A.A. Industrial exploitation of photocatalysis: Progress, perspectives and prospects. Catal. Surv. Asia 2004, 8, 265–273. [Google Scholar] [CrossRef]
- Chitose, N.; Ueta, S.; Seino, S.; Yamamoto, T.A. Radiolysis of aqueous phenol solutions with nanoparticles. 1. Phenol degradation and TOC removal in solutions containing TiO2 induced by UV, γ-ray and electron beams. Chemosphere 2003, 50, 1007–1013. [Google Scholar] [CrossRef]
- Kabra, K.; Chaudhary, R.; Sawhney, R.L. Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: A review. Ind. Eng. Chem. Res. 2004, 43, 7683–7696. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Ma, L.; Tu, S. Arsenic removal from water using a modified rutile ore and the preliminary mechanisms. Desalination Water Treat. 2011, 32, 445–452. [Google Scholar] [CrossRef]
- Kabra Clark, I.D.; Fritz, P. Environmental Isotopes in Hydrogeology; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Jasechko, S. Global Isotope Hydrogeology―Review. Rev. Geophys. 2019, 57, 835–965. [Google Scholar] [CrossRef]
- Sappa, G.; Barbieri, M.; Ergul, S.; Ferranti, F. Hydrogeological Conceptual Model of Groundwater from Carbonate Aquifers Using Environmental Isotopes (18O, 2H) and Chemical Tracers: A Case Study in Southern Latium Region, Central Italy. J. Water Resour. Prot. 2012, 4, 695–716. [Google Scholar] [CrossRef]
- Edmunds, W.M. Groundwater nitrate as a palaeoenvironmental indicator. In Geochemistry of the Earth’s Surface; Springer: Berlin/Heidelberg, Germany, 1999; pp. 35–39. [Google Scholar]
- Edmunds, W.M.; Smedley, P.L. Residence time indicators in groundwater: The East Midlands Triassic sandstone aquifer. Appl. Geochem. 2000, 15, 737–752. [Google Scholar] [CrossRef]
- Glynn, P.D.; Plummer, L.N. Geochemistry and the understanding of ground-water systems. Hydrogeol. J. 2005, 13, 263–287. [Google Scholar] [CrossRef]
- Han, D.; Kohfahl, C.; Song, X.; Xiao, G.; Yang, J. Geochemical and isotopic evidence for palaeo-seawater intrusion into the south coast aquifer of Laizhou Bay, China. Appl. Geochem. 2011, 26, 863–883. [Google Scholar] [CrossRef]
- Stimson, J.; Frape, S.; Drimmie, R.; Rudolph, D. Isotopic and geochemical evidence of regional-scale anisotropy and interconnectivity of an alluvial fan system, Cochabamba Valley, Bolivia. Appl. Geochem. 2001, 16, 1097–1114. [Google Scholar] [CrossRef]
- Pan, G.F.; Li, X.Q.; Zhang, J.; Liu, Y.D.; Liang, H. Groundwater-flow-system characterization with hydrogeochemistry: A case in the lakes discharge area of the Ordos Plateau, China. Hydrogeol. J. 2019, 27, 669–683. [Google Scholar] [CrossRef]

| Samples | Size (nm) | Concentration (Particles/mL) | Groundwater Types | Area |
|---|---|---|---|---|
| BQ | 155.9–352.0 | 2.5–4.1 × 105 | Spring | Discharge area |
| BTQ | 151.5–355.6 | 0.95–1.9 × 105 | ||
| LX42 | 149.3–410.6 | 5.8–6.9 × 104 | Karst water | Indirect recharge area |
| LX44 | 152.9–408.6 | 0.82–1.8 × 105 | Indirect recharge area | |
| JS12 | 183.1–299.4 | 1.2–1.5 × 106 | Direct recharge area | |
| KB5 | 155.0–418.5 | 1.6–5.3 × 105 | Discharge area | |
| YR34 | 194.8–513.2 | 3.7–8.1 × 104 | Indirect recharge area | |
| BL | 152.6–469.5 | 1.6–3.9 × 105 | Geothermal water | Northern geothermal heat |
| Sample | N | O | Ti | Mg | Al | Si | S | K | Ca | Mn | Fe | V | P | Zn | Ar | Cr | Co | Mo | Ni | Cl | Zr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BQ-1 | - | 61.84 | 34.11 | - | 1.16 | 1.13 | 0.52 | - | 0.58 | - | 0.66 | - | - | - | - | - | - | - | - | - | - |
| BTQ-1 | - | 50.44 | 49.56 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| BTQ-2 | 17.07 | 43.47 | 39.45 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| BQT-3 | 12.06 | 51.64 | 36.31 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| BTQ-4 | 11.53 | 47.67 | 40.79 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| LX42-1 | 18.16 | 42.17 | 31.20 | - | 0.65 | 1.38 | - | - | - | - | - | - | - | - | - | - | 1.80 | - | 1.34 | - | 3.32 |
| LX44-1 | 17.01 | 47.08 | 35.91 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| LX44-2 | 18.51 | 49.10 | 31.03 | - | 0.43 | 0.94 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| JS12-1 | 14.60 | 53.85 | 7.00 | 0.89 | 3.94 | 4.27 | 0.53 | 1.12 | 1.83 | 0.86 | 11.09 | - | - | - | - | - | - | - | - | - | - |
| JS12-2 | 6.87 | 43.57 | 20.98 | - | 1.68 | 2.16 | - | - | 1.06 | - | 23.67 | - | - | - | - | - | - | - | - | - | - |
| JS12-3 | 12.87 | 38.89 | 39.01 | - | 0.42 | 0.38 | - | - | - | - | 2.95 | 5.48 | - | - | - | - | - | - | - | - | - |
| JS12-4 | 16.09 | 39.73 | 39.87 | - | 2.37 | 0.10 | 0.39 | - | 1.12 | - | - | - | 0.33 | - | - | - | - | - | - | - | - |
| JS12-5 | 16.37 | 44.60 | 38.28 | - | 0.52 | 0.24 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| JS12-6 | 11.99 | 48.22 | 34.67 | - | 1.75 | 0.80 | 0.24 | - | 0.48 | - | - | - | - | 1.85 | - | - | - | - | - | - | - |
| KB5-1 | - | 50.32 | 6.12 | - | 3.89 | 0.87 | - | - | - | 1.41 | 5.24 | 0.94 | - | - | 2.77 | 8.12 | 8.37 | 2.56 | 9.38 | - | - |
| KB5-2 | - | 52.47 | 13.55 | 0.79 | 6.64 | 1.13 | 6.10 | - | - | 1.87 | 3.96 | 2.08 | - | - | 4.38 | 1.60 | - | 3.83 | 1.59 | - | - |
| YR34-1 | 14.93 | 29.20 | 32.41 | 1.80 | 0.99 | 3.24 | 0.96 | 0.23 | 3.32 | - | - | - | 0.39 | - | - | - | - | - | - | 12.53 | - |
| BL-1 | - | 37.13 | 5.59 | - | 0.95 | 1.83 | - | - | 2.01 | - | 52.50 | - | - | - | - | - | - | - | - | - | - |
| BL-2 | - | 33.42 | 5.77 | - | 0.69 | 1.40 | - | - | 1.23 | - | 56.81 | - | 0.69 | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, L.; Li, C.; Zhang, P.; Wang, Y.; Gao, S.; Sun, B.; Liu, R. Characteristics of Natural Ti-Bearing Nanoparticles in Groundwater within Karst Areas of Northern China. Water 2024, 16, 650. https://doi.org/10.3390/w16050650
Zuo L, Li C, Zhang P, Wang Y, Gao S, Sun B, Liu R. Characteristics of Natural Ti-Bearing Nanoparticles in Groundwater within Karst Areas of Northern China. Water. 2024; 16(5):650. https://doi.org/10.3390/w16050650
Chicago/Turabian StyleZuo, Lei, Changsuo Li, Peng Zhang, Yaqin Wang, Shuai Gao, Bin Sun, and Rui Liu. 2024. "Characteristics of Natural Ti-Bearing Nanoparticles in Groundwater within Karst Areas of Northern China" Water 16, no. 5: 650. https://doi.org/10.3390/w16050650
APA StyleZuo, L., Li, C., Zhang, P., Wang, Y., Gao, S., Sun, B., & Liu, R. (2024). Characteristics of Natural Ti-Bearing Nanoparticles in Groundwater within Karst Areas of Northern China. Water, 16(5), 650. https://doi.org/10.3390/w16050650






