Abstract
An advanced photochemical reduction system involving the UV/Fe(III)–oxalate system was developed for the reduction of nitrate (NO3−) to harmless N-gaseous species, primarily nitrogen (N2), by carbon dioxide radical (·CO2−) generated in the presence of dissolved oxygen (DO). Electron paramagnetic resonance (EPR) analyses confirmed the presence of both ·CO2− and ·OH radicals. Systematic investigations were conducted on various operational parameters, such as the initial Fe(III) concentration, oxalate concentration, and pH levels, to assess their impacts on the efficiency and products of NO3− reduction. Notably, solution pH played a significant role in influencing the NO3− reduction efficiency and the final products. At pH 2, approximately 75% of NO3− was converted into N2 with an 80% selectivity. In the pH range of 3 to 5, a remarkable NO3− removal rate of about 90% was achieved. Furthermore, higher concentrations of Fe(III) (2 mM) and oxalate (10 mM) were found to enhance NO3− removal to 91.95% and 88.71%, respectively. The presence of DO increased the oxidative potential in the reaction system, subsequently enhancing the selectivity conversion of NO3− to N2. In summary, the UV/Fe(III)–oxalate system exhibits significant potential for effective removal of NO3− while achieving high selectivity for the production of N2 in water remediation applications.
1. Introduction
Water quality is a critical concern for the global environment, with far-reaching implications for human health and societal development [1,2]. The contamination of water by nitrate (NO3−) is a persistent and widespread issue, primarily stemming from an excessive use of nitrogen fertilizers in agriculture, industrial wastewater discharge, and feedlot runoff [3]. The accumulation of NO3− level not only poses health risks, such as blue baby syndrome, cancer, and other health problems in the human body, but also leads to ecological issues like eutrophication [4,5,6].
To address this challenge, numerous technologies have been explored to remove NO3− from waters, including biological denitrification [7], ion exchange [1], electrochemical methods [8,9], photocatalysis [6,10]), chemical reduction processes [11], and more. Among these, chemical reduction offers distinct advantages in terms of cost-effectiveness, reaction rate, and operational simplicity, making it a promising and widely applicable approach for aqueous NO3− removal [12].
The ·CO2− radical, possessing a low potential of −1.9V [13], is a potent and environmentally friendly reductive species capable of converting NO3− into NO2−, NH4+, and mainly harmless gaseous N-species (N2), with the end product of itself being non-harmful carbon dioxide. Typically, ·CO2− radicals can be generated in the presence of formic acid (HCOOH) or formate (HCOO−) along with hydroxyl radicals (·OH) at a potential of 2.7 V [14,15,16] or sulfate radicals (·SO4−) at a potential of 2.6V [17,18]. Previous research has explored the UV/H2O2/HCOOH system for NO3− removal via ·CO2− radical generation [19]; however, challenges related to superoxide storage and transport have hindered its widespread application in water treatment.
Incorporating dissolved Fe(III) and oxalate can yield stable and photoactive ferrioxalate complexes, which, upon UV irradiation, generate an array of reactive species (such as ·CO2−, ·OH, H2O2, and ·O2−) [20]. While most studies have focused on oxidative degradation of pollutants by ferrioxalate complex photolysis [21,22,23], few have investigated photoreduction processes [24,25,26,27]. Our prior work has demonstrated the feasibility of the UV/Fe(III)–oxalate system for NO3− reduction via ·CO2− radical generation [28]; however, the observed selectivity of NO3− to N2 was only 60%, which is the percentage of the molar concentration of the product over the initial molar concentration of NO3−. Several studies have indicated that the generation of ·OH radicals can elevate the oxidation–reduction potential (ORP) of a reaction system [29,30]. Therefore, we propose introducing a low dissolved oxygen (DO) concentration into the UV/ferrioxalate system through air exposure, augmenting the oxidation environment of the reaction system and thereby enhancing the selectivity of NO3− conversion to N2.
In this study, we established a UV/Fe(III)–oxalate system for NO3− reduction based on the generation of reductive ·CO2− radical in the presence of DO. We investigated the NO3− reduction rate and selectivity, considering factors such as Fe(III) concentration, oxalate concentration, and initial pH level. Our objectives were to confirm the potential enhancement of NO3− to N2 selectivity through the presence of DO, explore the influence of various conditions on NO3− reduction rate and selectivity, and elucidate potential reaction mechanisms and pathways for NO3− reduction.
2. Materials and Methods
2.1. Materials
Potassium nitrate (KNO3, >99%), iron chloride hexahydrate (FeCl3·6H2O, >99%), sodium oxalate (Na2C2O4, >99.8%), ammonium sulfamate (NH4SO3NH2, ≥99%), hydrazine dihydrochloride (N2H4∙2HCl, ≥99%), p-dimethylaminobenzaldehyde (C9H11NO, 98%), hydrochloric acid (HCl, 36%~38%), and sodium hydroxide (NaOH, ≥96%) were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). 1,10-Phenanthroline (C12H8N2·HCl·H2O, >97%) and 5,5-dimethyl-1-pyrroline N-oxide (C6H11NO, DMPO, >97%) were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). All the chemicals were used without any purification in experiments.
2.2. Photochemical Experiments
A series of batch tests of NO3− removal were performed in a GHX-B photoreactor (Shanghai Jiapeng Technology Co., Ltd., Shanghai, China) with 0.1 L quartz tubes and a magnetic stirrer (Figure 1). A 500 W UV lamp (GG2500 from Osram Lighting Ltd., Shanghai, China) corresponding calculated incident UV photon flux were 25.3 mW/cm2 and 772.6 µmol m2 s−1 [13], respectively. The reactor was initially filled with suitable concentrations of Fe(III) and oxalate. Subsequently, the pH of the solution was adjusted to desired levels by adding 1M HCl or NaOH, and the appropriate concentration of KNO3 was then added into the solution before UV irradiation. Next, 10 mL samples were withdrawn from the photoreactor periodically or after 120 min of reaction and then immediately filtered through 0.22 µm membranes of syringe filters to remove precipitates for analysis. The DO concentration was maintained between 2 and 3 mg/L during the reaction processes [31].

Figure 1.
The photochemical reactor.
2.3. Analytical Methods
The sample concentrations of NO3−, NO2−, NH4+/NH3, and oxalate residual were analyzed quantitatively by ion chromatography (925CN from Metrohm Co. Ltd., Shanghai, China). The concentrations of dissolved Fe(II) and N2H4 were determined using spectrophotometer (UV-2600 from Shimadzu Co. Ltd., Shanghai, China) by the 1,10-phenanthroline method (λmax = 510 nm) [32,33] and the p-dimethylaminobenzaldehyde spectrophotometric method (λmax = 458 nm) [34], respectively. In our previous study [13], N-gaseous species could be regarded as mainly N2 with a small amount of other gaseous N-species (i.e., N2O3 and N2O4), and the corresponding selectivity was presented as the difference of removed NO3− concentration and the residual concentrations of byproducts (NO2−, NH4+, and N2H4) based on the mass balance of N-species. In this study, only 0.01 mg N/L (i.e., ~0.07% of total N) N2H4 was found to be under the condition of 1 mM Fe(III), 10 mM oxalate, and 14 mg N/L NO3− at pH 3. As a thermodynamically unstable intermediate compound [35], N2H4 is easy to convert to other N-species in the processes of NO3− reduction. Therefore, NO2−, NH4+, and N2 were considered the main reductive byproducts in the UV/Fe(III)–oxalate system.
Main radicals of ·CO2− and ·OH were examined by electron paramagnetic resonance (EPR) experiment (ELEXSYS E580 from BrukerScientific Technology Co. Ltd., Beijing, China) in the presence of 1 mM Fe(III) and 10 mM oxalate under UV irradiation. The EPR experiment test conditions were set as 9.64 GHz microwave frequency, 0.94 mW microwave power, 100 kHz modulation frequency, and 2.0 G modulation amplitude [36].
3. Results and Discussion
3.1. NO3− Removal in Different Systems
The efficiency of NO3− reduction with UV irradiation was investigated under various conditions, including NO3− alone, Fe(III) alone, oxalate alone, and the Fe(III)–oxalate system. The results are illustrated in Figure 2. When NO3− aqueous solution was exposed to UV light, minimal NO3− removal was observed. The addition of Fe(III) led to only ~5.9% NO3− removal. In contrast, using oxalate for NO3− reduction achieved a removal rate of 40.9%, which is approximately 6.8 times as high as the removal rate of Fe(III) alone. This enhancement is likely due to the generated ·CO2− radical. More specifically, the oxidative ·OH radical generated via photolysis of NO3− or NO2− reacts with oxalate (HC2O4− and C2O42−) to produce ·CO2− radicals [37] (Equations (9) and (10)). In addition, photolysis of oxalate could be neglected because an insignificant absorbance at 365 nm was observed according to our prior study [28]. Clearly, the presence of oxalate enhances the efficiency of NO3− reduction. However, at pH 3 and under UV irradiation, the presence of both Fe(III) and oxalate led to an 88.7% NO3− reduction rate, 2.2 times as high as the rate of oxalate alone. This suggests that much more ·CO2− radicals were generated in the UV/Fe(III)–oxalate system.
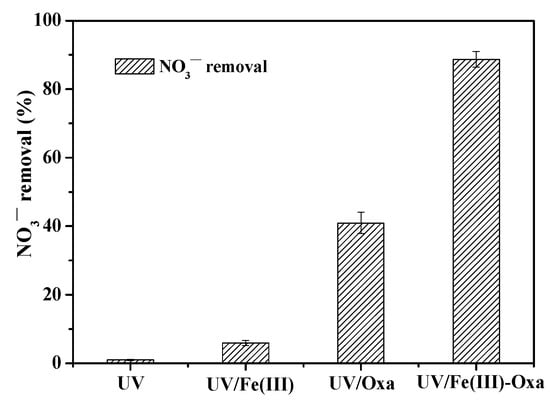
Figure 2.
NO3− reduction rate in various systems of UV/NO3− alone, UV/Fe(III), UV/oxalate, and UV/Fe(III)–oxalate. ([NO3−]0 = 14 mg N/L; [Fe(III)]0 = 1 mM; [Oxalate]0 = 10 mM; pH0 = 3; reaction time = 120 min).
3.2. Identification of Main Radicals
The UV/Fe(III)–oxalate system is known to generate primary ·CO2− and ·OH radicals under UV irradiation in the presence of ferrioxalate complexes [19]. Furthermore, photogenerated ·OH radicals are scavenged by excess oxalate to produce ·CO2− radicals. To identify the main radical species, i.e., ·CO2− and ·OH involved in NO3− removal, the EPR spin trapping technique was utilized [38,39]. As shown in Figure 3, EPR signals corresponding to DMPO-·CO2− (αH = 22.10 G, αβH = 15.25 G, and g = 2.0059) and DMPO-·OH (αN = αH = 14.9 G, and g = 2.0059) spin adducts were observed in the UV/Fe(III)–oxalate system. These spectral patterns are consistent with previous research [40,41] and provide compelling support for the proposed mechanism of ·OH and ·CO2− generation.
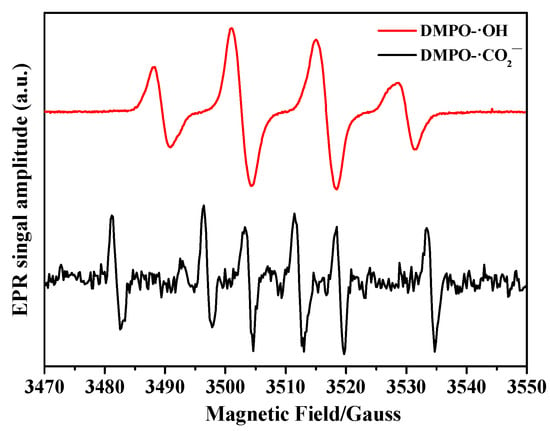
Figure 3.
EPR spectra of DMPO-·CO2− and DMPO-·OH in the UV/Fe(III)–oxalate system ([Fe(III)]0 = 1 mM; [Oxalate]0 = 10 mM; pH0 = 2).
3.3. NO3− Reduction and Selectivity Under Various Factors
3.3.1. Fe(III) Concentration
The effect of Fe(III) concentration on NO3− reduction efficiency and selectivity is illustrated in Figure 4. As Fe(III) concentration increased from 0.5 to 2.0 mM, the NO3− reduction rate remained stable at approximately 90%. Meanwhile, the selectivity toward NH4+ increased, while that toward gaseous N-species (mainly N2) decreased. Notably, a low selectivity for NO2− (~2.9%) was observed at Fe(III) concentrations of 0.5–2.0 mM, likely due to higher Fe(III) levels favoring the formation of more ferric oxalate complexes [28]. A corresponding decrease in oxalate residue to 2.9 mM occurred as Fe(III) concentration increased to 2 mM (Figure S1). Furthermore, the photoreactive nature of Fe(III) increases photon absorbance under UV irradiation [21,42] with rising concentrations, leading to faster ·CO2− radical generation. Thus, a higher Fe(III) concentration accelerates ·CO2− radical generation, resulting in elevated NH4+ accumulation due to over-reduction of NO3− and thus decreased selectivity toward gaseous N-species (mainly N2). It is worth noting that with 0.5 mM Fe(III) and 10 mM oxalate at pH 3, approximately 90% NO3− reduction rate and 63% selectivity toward gaseous N-species (mainly N2) were achieved. This demonstrates the advantages of low Fe(III) concentration, e.g., effective NO3− conversion to gaseous N-species (mainly N2), high NO3− reduction rate (~90%), and cost savings by avoiding excessive Fe(III) addition.
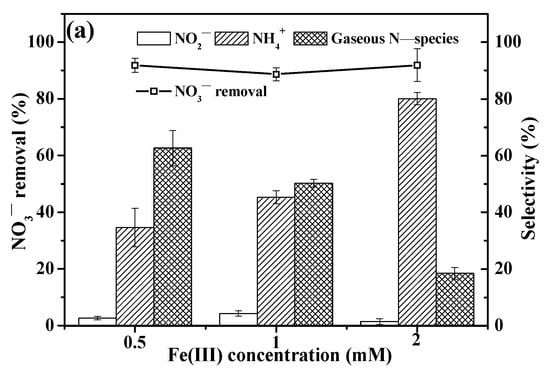
Figure 4.
Effect of Fe(III) concentration on NO3− reduction rate and selectivity. ([NO3−]0 = 14 mg N/L; [Fe(III)]0 = 0.5, 1, and 2 mM; [Oxalate]0 = 10 mM; pH0 = 3; reaction time = 120 min).
3.3.2. Oxalate Concentration
Figure 5 displays the NO3− reduction rate and selectivity at varied oxalate concentrations. With an increase from 1 to 10 mM oxalate, the NO3− reduction rate initially rose and then stabilized at around 90%. At 1 mM oxalate, the predominant form of ferrioxalate complexes was Fe(C2O4)2− (Figure S2). As the concentration was low, there was weak photoactivity and less ·CO2− radical generation, so the NO3− reduction rate was low. However, at 3 mM oxalate, more Fe(C2O4)2− and Fe(C2O4)33− (Figure S2) contributed to greater NO3− removal. However, the NO3− reduction rate remained roughly constant at 10 mM oxalate. Here, increased Fe(C2O4)33− levels (Figure S2) led to stronger photoactivity [28] and greater ·CO2− radical generation, increasing the removal rate of NO3−. The corresponding result was reflected in the selectivity of byproducts as well. As oxalate concentration increased from 1 to 10 mM, NO2− selectivity decreased, while NH4+ selectivity and N-gaseous species (mainly N2) selectivity increased. At 1 mM oxalate, Fe(C2O4)2− was the main ferrioxalate complex form with low concentration and weak photoactivity, generating limited ·CO2− radicals [21,43]. As a result, a less reductive environment was established, resulting in high NO2− (~76.5%) and N2 (~23.5%) selectivity. With an increase in oxalate concentration, more ferrioxalate complexes, particularly Fe(C2O4)33−, were formed, leading to increased ·CO2− radical generation [21,43]. Consequently, NO3− was over-reduced to NH4+, and N2 selectivity thus decreased. Additionally, at 10 mM oxalate, an oxalate residue of about 4.5 mM was observed (Figure S3). When the oxalate concentration was excessive, it also caused the formation of more NH4+. Thus, an appropriate oxalate concentration is important for effective NO3− removal, resulting in both good reduction efficiency and high N2 selectivity.
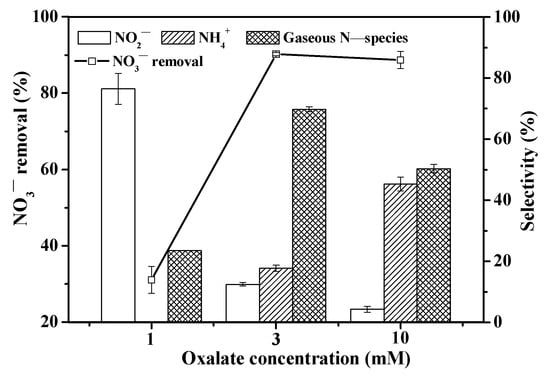
Figure 5.
Effect of oxalate concentration on NO3− reduction rate and selectivity. ([NO3−]0 = 14 mg N/L; [Fe(III)]0 = 1 mM; [Oxalate]0 = 1, 3, and 10 mM; pH0 = 3; reaction time = 120 min).
3.3.3. Initial pH
pH is an important parameter because it determines the speciation of ferrioxalate complexes. The effect of initial pH on NO3− reduction efficiency and selectivity is shown in Figure 6. With pH increasing from 2 to 3, the NO3− removal rate exhibited an upward trend. This is likely due to a greater amount of photoactive Fe(C2O4)33− complex formed [28]. Under UV irradiation, more ·CO2− radical was generated, and this resulted in a higher NO3− removal rate. With a further increase in pH, the NO3− removal rate generally remained at about 90%. A low NO3− removal rate of 75.8% was found at pH 2, which is likely due to a low amount of photoactive Fe(C2O4)33− [28]; the presence of excess H+ also consumed ·CO2− radical and led to poor NO3− removal (Equation (1)).
H+ +·CO2− → 1/2H2 + CO2
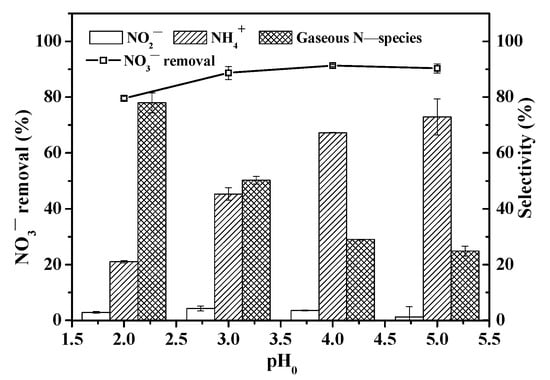
Figure 6.
Effect of initial pH on NO3− reduction rate and selectivity. ([NO3−]0 = 14 mg N/L; [Fe(III)]0 = 1 mM; [Oxalate]0 = 10 mM; pH0 = 2, 3, 4, and 5; reaction time = 120 min).
In addition, because HCl and NaOH were used to adjust pH, a lower pH meant a greater concentration of chloride ions (Cl−). Prior research has demonstrated that under acidic conditions, Cl− can undergo a reaction with ·OH radicals, resulting in a scavenging effect of ·OH radicals and formation of chlorine radicals. This phenomenon is described by the following equations [44]:
·OH + Cl− → ClHO·−
ClHO·− + H+ → Cl·− + H2O
Cl· + Cl− → Cl2·−
The presence of Cl− can potentially hinder the production of ·CO2− radicals due to the rapid reaction between ·OH and Cl− at a second-order reaction rate constant of 4.3 × 109 M−1·s−1 [45,46], which is significantly higher by factors of 558 and 91 than that of C2O42− and HC2O4−, respectively. Additionally, bicarbonate (HCO3−) and carbonate (CO32−) generated from the NO3− reduction process may also act as scavengers for ·OH radicals (Equation 5 k = 8.5 × 106 M−1∙s−1 and Equation (6) k = 3.9 × 108 M−1∙s−1) [45]. In our study, the prevalent forms were HCO3− and H2CO3 due to the final pH values being between 2.2 and 7.7 (see Figure S4), given pKa1 = 6.35 and pKa2 = 10.33 of H2CO3 [47]. The second-order reaction rate constant of ·OH with HCO3− was only 1.1 and 0.2 times that of C2O42− and HC2O4−. This suggests a lesser impact on ·CO2− radical production compared to the influence of Cl−. Additionally, Shi et al. [48] reported similar findings, showing that low NO3− reduction occurred in the presence of Cl− and HCO3−. Specifically, the presence of Cl− or HCO3− can hinder the production of the ·CO2− radical by scavenging the ·OH radical, thereby resulting in reduced NO3− reduction.
·OH + HCO3− → H2O + CO3·−
·OH + CO32− → OH− + CO3·−
The pH 2 condition yielded a notably high oxalate utilization rate (~77%) (Figure S5). This result underscores the favorable impact of an acidic condition on the Fe(II)/Fe(III) cycle in the UV/Fe(III)–oxalate system, facilitating continuous ·CO2− radical generation.
Furthermore, the selectivity toward the reaction product of NH4+ increased substantially from 4.1% to 72.9%, while the selectivity of N2 decreased from 82.8% to 24.8% as the initial pH was raised from 2 to 5 (Figure 6). Reduction of 1 mol of NO3− to N2 and NH4+ stoichiometrically requires 5 mol and 8 mol of ·CO2− respectively, as illustrated in Equations (7)–(10) [13].
NO3− + 2H+ + 2·CO2− → NO2− + H2O + 2CO2
2NO2− + 8H+ + 6·CO2− → N2 + 4H2O + 6CO2
2NO2− + 12H+ +·CO2− → N2H4 + 4H2O + CO2
2NO2− + 8H+ + 6·CO2− → NH4+ + 2H2O + 6CO2
It becomes evident that maintaining a lower level of ·CO2− radicals is more favorable for the formation of N2, whereas an elevated ·CO2− radical concentration is prone to over-reduction of NO3− to NH4+. With the rise in pH, the concentration of high photochemical activity of Fe(C2O4)33− increases [28], leading to heightened ·CO2− radical generation under UV irradiation. In addition, ·OH radicals, generated from UV/ferrioxalate complexes (Equations (13)–(16) and (20)), further enhance the ·CO2− radical generation rate through their reactions with oxalate residues (Equations (22) and (23)). Consequently, the conversion of NO3− to NH4+ is greater.
In summary, as shown in Table 1, the changes in various operational parameters appear to have minimal impact on NO3− removal. However, the selectivity of NO3− reduction products varies significantly. With increasing Fe(III) concentration, oxalate concentration, and pH, the selectivity shifts toward NO3− reduction to NH₄⁺ rather than N2. This is attributed to the generation of a greater amount of reductive ·CO2− radicals under these conditions.

Table 1.
The effects of operational parameters on NO3− removal and selectivity.
3.4. Mechanisms of NO3− Reduction
Figure 7a depicts the evolution of N-species in the UV/Fe(III)–oxalate system at pH 2 with time. With an increase in the UV dose, an incremental conversion of the initial 14 mg N/L NO3− into N-gaseous species (mainly N2, 8.5 mg N/L over 120 min) was observed. The relatively low levels of NO2− (1.4 mg N/L) and NH4+ (0.4 mg N/L) underline the efficacy of the UV/Fe(III)–oxalate system for NO3− reduction. For NO2−, it can be reduced to NH4+ and N2 by reductive radicals, including ·CO2−. Moreover, increasing pH is likely due to the consumption of H+ during NO3− reduction (Figure S6).
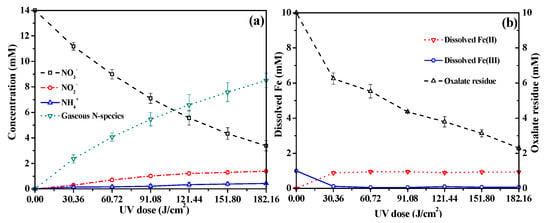
Figure 7.
(a) The N-species conversion process in NO3− reduction; (b) the change in Fe(III), Fe(II) concentrations, and oxalate residue with UV dose. ([NO3−]0 = 14 mg N/L; [Fe(III)]0 = 1 mM; [Oxalate]0 = 10 mM; pH0 = 2; reaction time = 120 min).
The changes in Fe(II), Fe(III), and oxalate concentrations over reaction time are illustrated in Figure 7b. The results revealed a continuous decrease in oxalate concentration with increasing UV dose. Fe(II) levels remained relatively constant at around 1 mM, while only trace amounts of Fe(III) were detected, indicating a weak Fe(II)/Fe(III) cycle and likely precipitation of Fe(III) when the reactions increased the pH level of the solution. The diminishing oxalate concentration with increasing UV dose suggests it was consumed via the reactions in the UV/Fe(III)–oxalate system. Additionally, a rapid reduction in oxalate concentration was observed at a UV dose of 30.36 J/cm2. This might be attributed to the reaction between oxalate and ·OH radicals generated from the photolysis of the Fe(III)-hydroxyl complex (Equations (11) and (12)) [37,49] leading to the production of ·CO2− radicals and the formation of ferrioxalate with Fe(III).
Fe3+ + H2O ↔ Fe(OH)2+ + H+
Fe(OH)2+ + hv → Fe2+ + ·OH
To provide further insight into the selectivity and mechanism of NO3− reduction in the UV/ferrioxalate system, controlled experiments were conducted under DO = 2.77 mg/L and anoxic conditions provided with stripping of N2. Shi et al. [48] had reported that the reduction of NO3− was not significantly affected by DO concentrations between 0.5 and 8.0 mg/L. Considering the DO concentration was around 2.77 mg/L in the open-air system of this study, a higher DO concentration is unnecessary. As depicted in Figure 8, under anoxic conditions, the selectivity toward NO2−, NH4+, and N2 was found to be 2.8%, 54.4%, and 42.8%, respectively. Interestingly, with the presence of DO, the selectivity for N2 increased to 50.3%, while that for NH4+ decreased to 45.4%. Notably, regardless of DO, the generation processes of ·CO2− radicals remain similar (Equations (13) and (14)). Ferrioxalate complexes undergo intramolecular electron transfer from photoexcited oxalate ligands to Fe(III) [20], leading to the generation of Fe(II) and ·CO2− radicals upon UV irradiation.
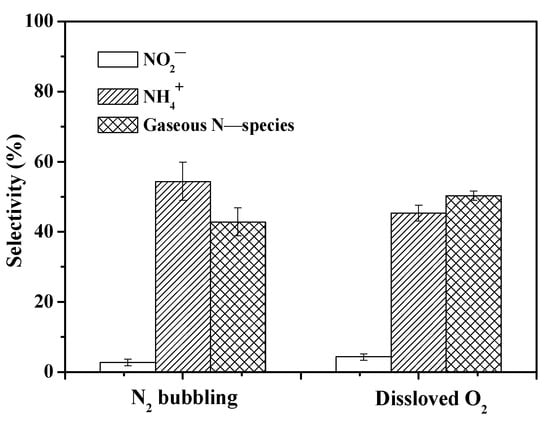
Figure 8.
Effect of N2 and dissolved O2 on NO3− reduction selectivity. ([NO3−]0 = 14 mg N/L; [Fe(III)]0 = 1 mM; [Oxalate]0 = 10 mM; pH0 = 2; reaction time = 120 min).
Under aerobic conditions or with DO, a portion of the ·CO2− radicals reacts with DO to form H2O2 species (Equations (15)–(18)). Subsequently, oxidative ·OH radicals are produced through the reaction between Fe(II) and H2O2, thereby elevating the oxidation level of the reaction system and further enhancing the selectivity toward N-species. The second-order reaction rate constants of H2O2, ·O2−, and HO2· with Fe(II) are 53, 107, and 1.2 × 105 M−1 s−1 [34], respectively. It is evident that the reaction between Fe(II) and·O2− radical occurs at a faster rate than with HO2· radical and H2O2. In addition, DO could directly participate in the oxidation of Fe(II) with the first-order reaction rate constant of 1.1 × 10−6 s−1 [50,51] (Equation (19)). Furthermore, ·OH radicals produced from the reactions between Fe(II) and H2O2 (Equation (20)) and the photolysis of H2O2 (Equation (21)) are captured by oxalate (HC2O4− and C2O42−) and subsequently yield ·CO2− radicals (Equations (22) and (23)). The combined ·CO2− radical generation processes in the UV/Fe(III)–oxalate system in the presence of DO can be summarized as follows (Equations (13)–(23)) [23,52,53]:
Fe(C2O4)n3−2n + hv → Fe2+ + (n − 1) C2O42− + ·C2O4−
·C2O4− → ·CO2− + CO2
·CO2− + O2 → CO2 + ·O2−
·O2− + H+ ↔ HO2·
Fe2+ + HO2· + H+ → Fe3+ + H2O2
Fe2+ + ·O2− + 2H+ → Fe3+ + H2O2
Fe2+ + O2 → Fe3+ + ·O2−
Fe2+ + H2O2 →Fe3+ + ·OH + OH−
H2O2 + hv → 2·OH
·OH + C2O42− → ·CO2− + CO2 + OH−
·OH + HC2O4 → ·CO2− + CO2 + H2O
Based on the aforementioned results and findings, the potential mechanism for NO3− reduction can be summarized as follows (refer to Figure 9). (i) First, ·CO2− radicals and Fe(II) are generated through the photolysis of ferrioxalate complexes triggered by ligand-to-metal charge transfer (LMCT) transitions. (ii) The Fe(II)/Fe(III) cycle is established with the participation of ·O2−, HO2· radicals, and H2O2. This cycle continuously generates ·CO2− radicals. (iii) Subsequently, ·CO2− radicals contribute to the reduction of NO3− to NO2−. (iv) Finally, NO2− is rapidly reduced to N2. These mechanisms and reaction pathways concisely describe the series of reactions leading to the reduction of NO3− to N2 and other nitrogenous products in the UV/Fe(III)–oxalate system.
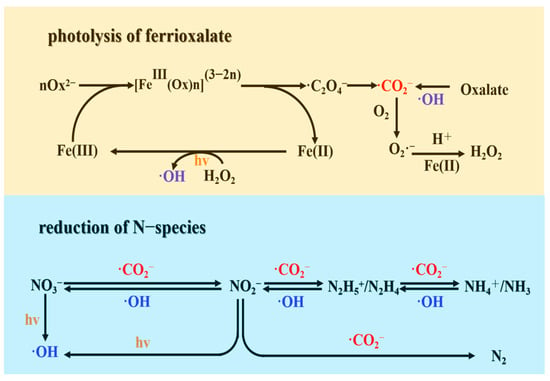
Figure 9.
Mechanism of ·CO2− radical production and NO3− reduction pathway.
4. Conclusions
An advanced photochemical reduction system based on the UV/Fe(III)–oxalate system was established for the selective reduction of NO3− to harmless N2. This system demonstrated high efficiency in NO3− reduction, with enhanced selectivity toward N2, primarily driven by the reductive ·CO2− radicals generated in the presence of DO. The findings suggest that the presence of DO increased the selectivity toward N2 in the system. Moreover, elevated concentrations of Fe(III) and oxalate were found to accelerate NO3− removal rates but also resulted in a high selectivity toward NH4+ because of the over-reduction of NO3−. Notably, a substantial NO3− reduction of over 75% was achieved across a relatively acidic pH range (2–5) by utilizing 1 mM Fe(III) and 10 mM oxalate.
Under conditions of pH 2, the UV/Fe(III)–oxalate system exhibited an approximately 75% removal rate of NO3−, accompanied by a high selectivity toward N2 formation of ~80%. These results collectively suggest that maintaining a controlled level of ·CO2− radicals enhanced the selectivity toward the end product of N2. Conversely, excessive ·CO2− radicals led to the over-reduction of NO3− to NH4+. As a result, the UV/Fe(III)–oxalate system emerges as a sound solution for enhancing selectivity toward N2 while ensuring a high NO3− reduction rate in water and wastewater treatment applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w16243658/s1, Figure S1: The oxalate residue after reaction at various initial Fe(III) concentrations; Figure S2: The calculation of Fe(III) speciation fraction at different concentrations of initial oxalate by MEDUSA software; Figure S3: The oxalate residue after reaction at various initial oxalate concentrations; Figure S4: The change in pH after reaction at different pH0; Figure S5: The oxalate residue after reaction at various pH0; Figure S6: The change in pH with UV dose.
Author Contributions
Investigation, J.C. and J.X.; writing—original draft preparation, J.C.; conceptualization, Y.X.; writing—review and editing, D.C. and X.Z.; supervision, D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
Authors Yi Xie, Jun Xia, and Xiaolin Zhang were employed by the company Shanghai Urban Construction Maintenance Management Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Tugaoen, H.O.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Challenges in photocatalytic reduction of nitrate as a water treatment technology. Sci. Total Environ. 2017, 599–600, 1524–1551. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Meenakshi, S. Enhanced removal of phosphate and nitrate ions by a novel Zn Fe LDHs-activated carbon composite. Sustain. Mater. Technol. 2020, 25, e00154. [Google Scholar] [CrossRef]
- Song, N.; Xu, J.; Cao, Y.; Xia, F.; Zhai, J.; Ai, H.; Shi, D.; Gu, L.; He, Q. Chemical removal and selectivity reduction of nitrate from water by (nano) zero-valent iron/activated carbon micro-electrolysis. Chemosphere 2020, 248, 125986. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Li, Y.; Zhang, C.; Wang, Y.; Zhang, W.; Wang, L.; Niu, L.; Wang, P.; Wang, C. Intimately coupled TiO2/g-C3N4 photocatalysts and in-situ cultivated biofilms enhanced nitrate reduction in water. Appl. Surf. Sci. 2020, 503, 144092. [Google Scholar] [CrossRef]
- Follett, R.F.; Hatfield, J.L. Nitrogen in the environment: Sources, problems, and management. Sci. World J. 2001, 1, 920–926. [Google Scholar] [CrossRef]
- Doudrick, K.; Yang, T.; Hristovski, K.; Westerhoff, P. Photocatalytic nitrate reduction in water: Managing the hole scavenger and reaction by-product selectivity. Appl. Catal. B-Environ. 2013, 136–137, 40–47. [Google Scholar] [CrossRef]
- Shrimali, M.; Singh, K.P. New metods of nitrate removal from water. Environ. Pollut. 2001, 112, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Pulkka, S.; Martikainen, M.; Bhatnagar, A.; Sillanpää, M. Electrochemical methods for the removal of anionic contaminants from water-A review. Sep. Purif. Technol. 2014, 132, 252–271. [Google Scholar] [CrossRef]
- Martínez, J.; Ortiz, A.; Ortiz, I. State-of-the-art and perspectives of the catalytic and electrocatalytic reduction of aqueous nitrates. Appl. Catal. B-Environ. 2017, 207, 42–59. [Google Scholar] [CrossRef]
- Adamu, H.; McCue, A.J.; Taylor, R.S.F.; Manyar, H.G.; Anderson, J.A. Simultaneous photocatalytic removal of nitrate and oxalic acid over Cu2O/TiO2 and Cu2O/TiO2-AC composites. Appl. Catal. B-Environ. 2017, 217, 181–191. [Google Scholar] [CrossRef]
- Fanning, J.C. The chemical reduction of nitrate in aqueous solution. Coord. Chem. Rev. 2000, 199, 159–179. [Google Scholar] [CrossRef]
- Hao, S.Z.; Zhang, H.Z. High catalytic performance of nitrate reductioin by synergistic effect of zero-valent iron and bimetallic composite carrier catalyst. J. Clean. Prod. 2007, 167, 192–200. [Google Scholar] [CrossRef]
- Chen, J.L.; Liu, J.Y.; Zhou, J.S.; Chen, D. Reductive removal of nitrate by carbon dioxide radical with high product selectivity to form N2 in a UV/H2O2/HCOOH system. J. Water Process Eng. 2020, 33, 101097. [Google Scholar] [CrossRef]
- Jiang, W.C.; Tang, P.; Lu, S.G.; Xue, Y.F.; Zhang, X.; Qiu, Z.F.; Sui, Q. Comparative studies of H2O2/Fe(II)/formic acid, sodium percarbonate/Fe(II)/formic acid and calcium peroxide/Fe(II)/formic acid processes for degradation performance of carbon tetrachloride. Chem. Eng. J. 2018, 344, 453–461. [Google Scholar] [CrossRef]
- Jiang, W.; Tang, P.; Lu, S.; Zhang, X.; Qiu, Z.; Su, Q. Enhanced reductive degradation of carbon tetrachloride by carbon dioxide radical anion-based sodium percarbonate/Fe(II)/formic acid system in aqueous solution. Front. Environ. Sci. Eng. 2018, 12, 6. [Google Scholar] [CrossRef]
- Azqandi, M.; Nateq, K.; Golrizkhatami, F.; Nasseh, N.; Seyedi, N.; Sadat, N.; Moghaddam, M.; Fanaei, F. InnovationInnovative RGO-bridged S-scheme CuFe2O4@Ag2S heterojunction for efficient Sun-light-driven photocatalytic disintegration of Ciprofloxacin. Carbon 2025, 231, 119725. [Google Scholar] [CrossRef]
- Ren, H.; Hou, Z.; Han, X.; Zhou, R. Highly reductive radical CO2− deriving from a system with SO4·− and formate anion: Implication for reduction of Cr(VI) from wastewater. Chem. Eng. J. 2017, 309, 638–645. [Google Scholar] [CrossRef]
- Gu, X.; Lu, S.; Fu, X.; Qiu, Z.; Sui, Q.; Guo, X. Carbon dioxide radical anion-based UV/S2O82−/HCOOH reductive process for carbon tetrachloride degradation in aqueous solution. Sep. Purif. Technol. 2017, 172, 211–216. [Google Scholar] [CrossRef]
- Perissinotti, L.L.; Brusa, M.A.; Grela, M.A. Yield of Carboxyl Anion Radicals in the Photocatalytic Degradation of Formate over TiO2 Particles. Langmuir 2001, 17, 8422–8427. [Google Scholar] [CrossRef]
- Mangiante, D.M.; SchPiotr, R.D.; Zarzycki, P.; Banfield, J.F.; Gilbert, B. Mechanism of Ferric oxalate photolysis. Earth Space Chem. 2017, 1, 270–276. [Google Scholar] [CrossRef]
- Qiu, H.; Geng, J.; Shen, C.; Ren, H.; Xu, Z. Aquatic photooxidation of phosphite in the presence of ferric and oxalate ions. Chem. Eng. J. 2015, 269, 408–415. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, F.; Lin, Y.; Deng, N.; Bazhin, N.; Glebov, E. Photodegradation of glyphosate in the ferrioxalate system. J. Hazard Mater. 2007, 148, 360–365. [Google Scholar] [CrossRef]
- Zhou, T.; Lim, T.T.; Wu, X. Sonophotolytic degradation of azo dye reactive black 5 in an ultrasound/UV/ferric system and the roles of different organic ligands. Water Res. 2011, 45, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Hug, S.J.; Laubscher, H.U.; James, B.R. Iron catalyzed photochemical reduction of chromium by oxalate and citrate in aqueous solutions. Environ. Sci. Technol. 1997, 31, 160–170. [Google Scholar] [CrossRef]
- Feng, X.H.; Ding, S.; Ran, H.B. Simultaneous reduction of hexavalent chromium and degradation of bisphenol A induced by the photolysis of ferric-oxalate complexes. Fresen Environ. Bull. 2013, 22, 1845–1851. [Google Scholar]
- Ababneh, F.A.; Scott, S.L.; Al-Reasi, H.A.; Lean, D.R.S. Photochemical reduction and reoxidation of aqueous mercuric chloride in the presence of ferrioxalate and air. Sci. Total Environ. 2006, 367, 831–839. [Google Scholar] [CrossRef]
- Huston, P.L.; Pignatello, J.J. Reduction of Perchloroalkanes by Ferrioxalate-Generated Carboxylate Radical Preceding Mineralization by the Photo-Fenton Reaction. Environ. Sci. Technol. 1996, 30, 3457–3463. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhang, R.N.; Chen, D.; Liu, J.Y.; Chen, S.P. Carbon dioxide radical reducing nitrate to nitrogen gas in a UV/Fe(III)-oxalate system. J. Water Process Eng. 2021, 40, 101934. [Google Scholar] [CrossRef]
- Lin, C.H.; Yu, R.F.; Cheng, W.P.; Liu, C.R. Monitoring and control of UV and UV-TiO2 disinfections for municipal wastewater reclamation using artificial neural networks. J. Hazard. Mater. 2012, 209–210, 348–354. [Google Scholar] [CrossRef]
- Wu, H.; Wang, S. Impacts of operating parameters on oxidation-reduction potential and pretreatment efficacy in the pretreatment of printing and dyeing wastewater by Fenton process. J. Hazard. Mater. 2012, 243, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Molamahmood, H.V.; Geng, W.; Wei, Y.; Miao, J.; Yua, S.Q.; Shahi, A.; Chen, C.; Long, M.C. Catalyzed H2O2 decomposition over iron oxides and oxyhydroxides: Insights from oxygen production and organic degradation. Chemosphere 2022, 292, 133037. [Google Scholar] [CrossRef] [PubMed]
- Adhikamsetty, R.K.; Gollapalli, N.R.; Jonnalagadda, S.B. Complexation kinetics of Fe2+ with 1,10-phenanthroline forming ferroin in acidic solutions. Int. J. Chem. Kinet. 2008, 40, 515–523. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Sakyi, N.Y.; Arif Khan, M. Simultaneous removal of NO and SO2 from flue gas by combined heat and Fe2+ activated aqueous persulfate solutions. Chemosphere 2018, 193, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.W.; Chrisp, J.D. Spectrophotometric Method for Determination of Hydrazine. Anal. Chem. 1952, 24, 2006–2008. [Google Scholar] [CrossRef]
- Pretzer, L.A.; Carlson, P.J.; Boyd, J.E. The effect of Pt oxidation state and concentration on the photocatalytic removal of aqueous ammonia with Pt-modified titania. J. Photoch Photobio A Chem. 2008, 200, 246–253. [Google Scholar] [CrossRef]
- Wang, L.L.; Li, Q.C.; Xu, C.X.; Fu, Y.; Tang, Y.; Wang, P.; Zhang, Z.; Xia, Y.Q.; Liu, X.J.; Cao, J.H.; et al. Phosphate-Mediated Degradation of Organic Pollutants in Water with Peroxymonisulfate Revisited: Radical or Non-radical Oxidation? Water Res. 2024, 255, 12159. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.H.; He, M.C. Mechanisms of Sb(III) Photooxidation by the Excitation of Organic Fe(III) Complexes. Environ. Sci. Technol. 2016, 50, 6974–6982. [Google Scholar] [CrossRef]
- Wang, C.; Yan, R.; Cai, M.; Liu, Y.; Li, S. A novel organic/inorganic S-scheme heterostructure of TCPP/Bi12O17Cl2 for boosting photodegradation of tetracycline hydrochloride: Kinetic, degradation mechanism, and toxic assessment. Appl. Surf. Sci. 2023, 610, 155346. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Y.; Wang, C.; Lin, W.; Li, S. Novel Cd0.5Zn0.5S/Bi2MoO6 S-scheme heterojunction for boosting the photodegradation of antibiotic enrofloxacin: Degradation pathway, mechanism and toxicity assessment. Sep. Purif. Technol. 2023, 304, 122401. [Google Scholar] [CrossRef]
- Liu, X.W.; Zhong, J.; Fang, L.; Wang, L.L.; Ye, M.M.; Shao, Y.; Li, J.; Zhang, T.Q. Trichloroacetic acid reduction by an advanced reduction process based on carboxyl anion radical. Chem. Eng. J. 2016, 303, 56–63. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, D.; Liu, J. Diverse redox chemistry of photo/ferrioxalate system. RSC Adv. 2014, 4, 44654–44658. [Google Scholar] [CrossRef]
- Pozdnyakov, I.; Sherin, P.; Bazhin, N.; Plyusnin, V. [Fe(Ox)3]3- complex as a photodegradation agent at neutral pH: Advances and limitations. Chemosphere 2018, 195, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Dong, Y.C.; Li, B.; Li, F. Comparative study of three solid oxidants as substitutes of H2O2 used in Fe-oxalate complex mediated Fenton system for photocatalytic elimination of reactive azo dye. J. Clean. Prod. 2018, 177, 245–253. [Google Scholar] [CrossRef]
- Dong, Y.; He, L.; Wang, Q.; Yang, M.; Qi, R.; Li, K. Effect of inorganic salts on ferric oxalate-induced decomposition of Cl Acid Black 234 under different weather conditions. Color. Technol. 2008, 124, 19–26. [Google Scholar] [CrossRef]
- Deng, J.; Shao, Y.; Gao, N.; Xia, S.; Tan, C.; Zhou, S.; Hu, X. Degradation of the antiepileptic drug carbamazepine upon different UV-based advanced oxidation processes in water. Chem. Eng. J. 2013, 222, 150–158. [Google Scholar] [CrossRef]
- Liao, C.H.; Kang, S.F.; Wu, F.A. Hydroxyl radical scavenging role of chloride and bicarbonate ions in the H2O2-UV process. Chemosphere 2001, 44, 1193–1200. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Shi, Z.Y.; Wang, F.L.; Xiao, Q.; Yu, S.L.; Ji, X.L. Selectivity and efficient reducition of nitrate to gaseous nitrogen from drinking water source by UV/Oxalic Acid/Ferric Iron Systems: Effectivenes and Mechamisms. Catalysis 2022, 12, 348. [Google Scholar]
- Nogueira, A.A.; Souza, B.M.; Dezotti, M.W.C.; Boaventura, R.A.R.; Vilar, V.J.P. Ferrioxalate complexes as strategy to drive a photo-fenton reaction at mild pH conditions a case study on levofloxacin oxidation. J. Photoch Photobio A. 2017, 345, 109–123. [Google Scholar] [CrossRef]
- Lowson, R.T. Aqueous Oxidation of Pyrite by Molecular Oxygen. Chem. Rev. 1982, 82, 461–497. [Google Scholar] [CrossRef]
- Ardo, S.G.; Nelieu, S.; Ona-Nguema, G.; Delarue, G.; Brest, J.; Pironin, E.; Morin, G. Oxidative degradation of nalidixic acid by nano-magnetite via Fe2+/O2-mediated reactions. Environ. Sci. Technol. 2015, 49, 4506–4514. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Yoon, J. pH effect on OH radical production in photo/ferrioxalate system. Water Res. 2005, 39, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Yoon, J. Dual roles of ·CO2− for degrading synthetic organic chemicals in the photo/ferrioxalate system. Water Res. 2004, 38, 3531–3540. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).