Phosphorus Fraction in Hydrochar from Co-Hydrothermal Carbonization of Swine Manure and Rice Straw: An Optimization Analysis Based on Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Swine Manure and Rice Straw
2.2. Experiments and Measurements
2.3. Analysis of Basic Parameters
- (1)
- pH measurement: A 0.50 g sample of dried hydrochar was mixed with 10.0 mL of deionized water and agitated at 25 °C and 1500 r/min for 24 h. The glass electrode was implemented to measure the pH of the sample suspension [36].
- (2)
- Electrical conductivity (EC) determination: A 0.50 g sample of dried hydrochar was combined with 5.00 mL of deionized water and agitated at 25 °C and 1500 r/min for 24 h. The sample suspension was then used to determine the EC value using an EC meter [37].
- (3)
- The solid yield calculation: Calculating the solid yield (SY) is crucial for understanding the efficiency of the carbon production process. SY is defined as the ratio of the mass of the obtained hydrochar to the mass of the raw material. The following equation was employed to calculate the SY.
2.4. Analysis of P Fraction
- (1)
- For determining the available phosphorus, 0.25 g of each hydrochar sample was mixed with 30 mL of 0.5 mol/L NaHCO3 solution. The mixture was agitated for 30 min at 25 °C and 150 r/min, followed by the separation of liquid and solid phases and an analysis of the filtrate to determine the available phosphorus concentration.
- (2)
- Sequential fractionation: For H2O-P determination, 0.25 g from each hydrochar sample was mixed with 30 mL of deionized water. The mixture was agitated for 16 h at 25 °C and 150 r/min, then centrifuged at 4500 r/min for 10 min to separate the supernatant. Two tests were conducted, i.e., the filtrate was mixed with 2,4-dinitrophenol and 4 mol/L NaOH until it turned yellow; then, 0.9 mol/L sulfuric acid was added until the yellow faded to measure the concentration of H2O-Pi fractions, and in the second test, 5 mL of the filtrate was mixed with 0.5 g of ammonium persulfate and 10 mL of 0.9 mol/L sulfuric acid. This was then boiled at 121 °C for 1 h. After boiling, 1 drop of 2,4-dinitrophenol was added to the filtrate; then, 4 mol/L NaOH was added until it turned yellow, followed by 0.9 mol/L sulfuric acid until the disappearing of the yellow color, and ultimately, the concentration of H2O-Pt fractions was measured.
3. Results and Discussion
3.1. Basic Parameters
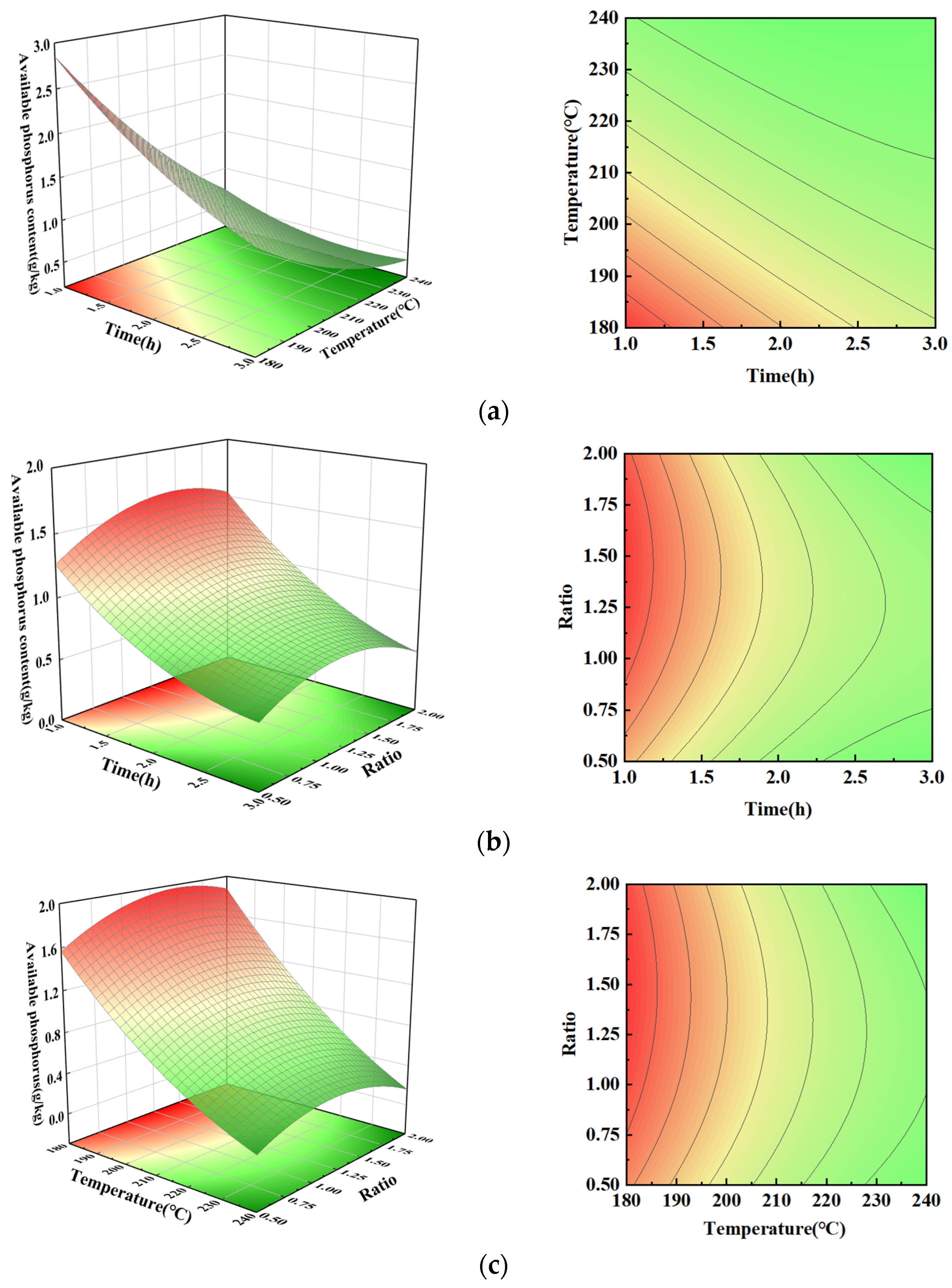
3.2. Available Phosphorus
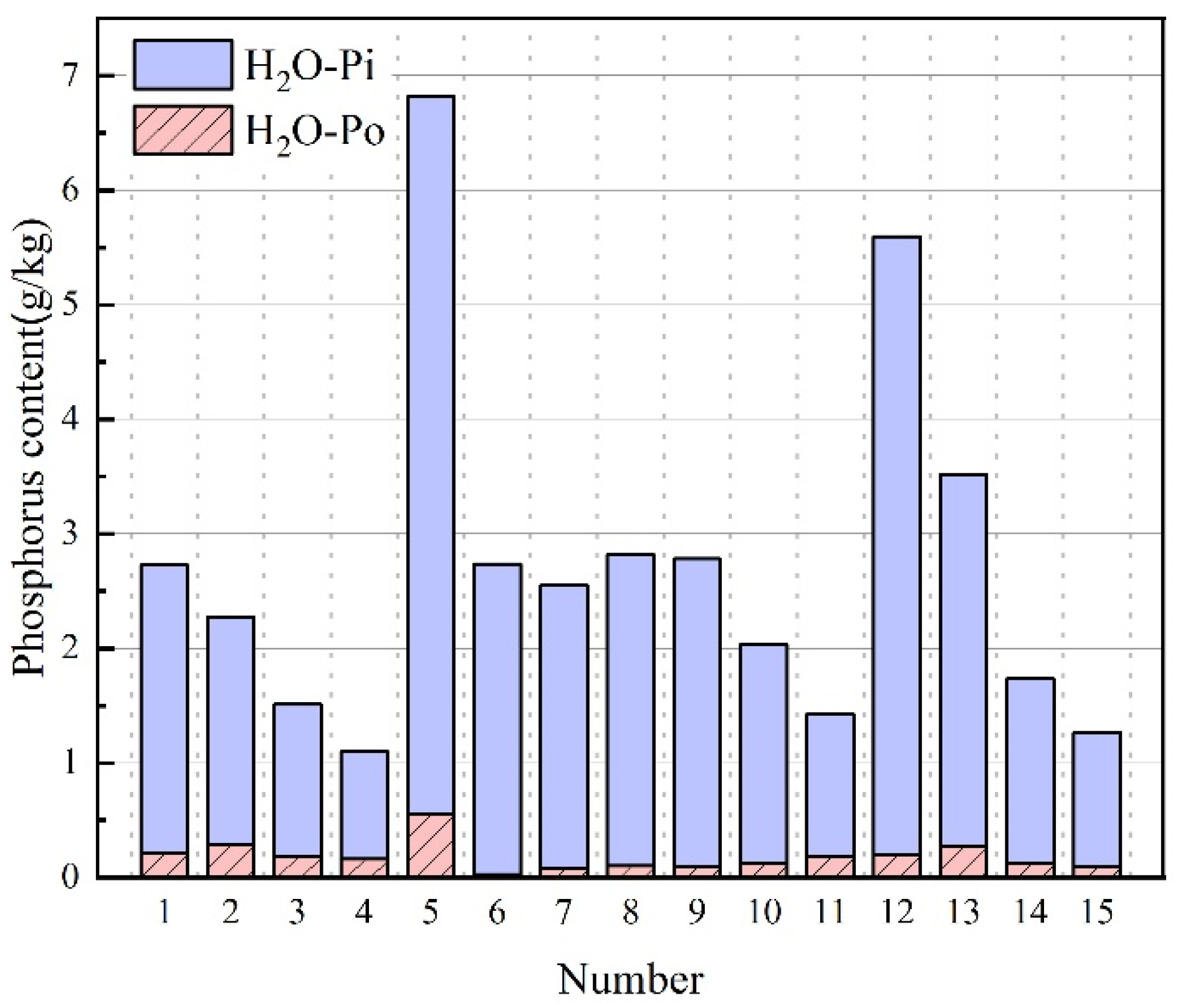
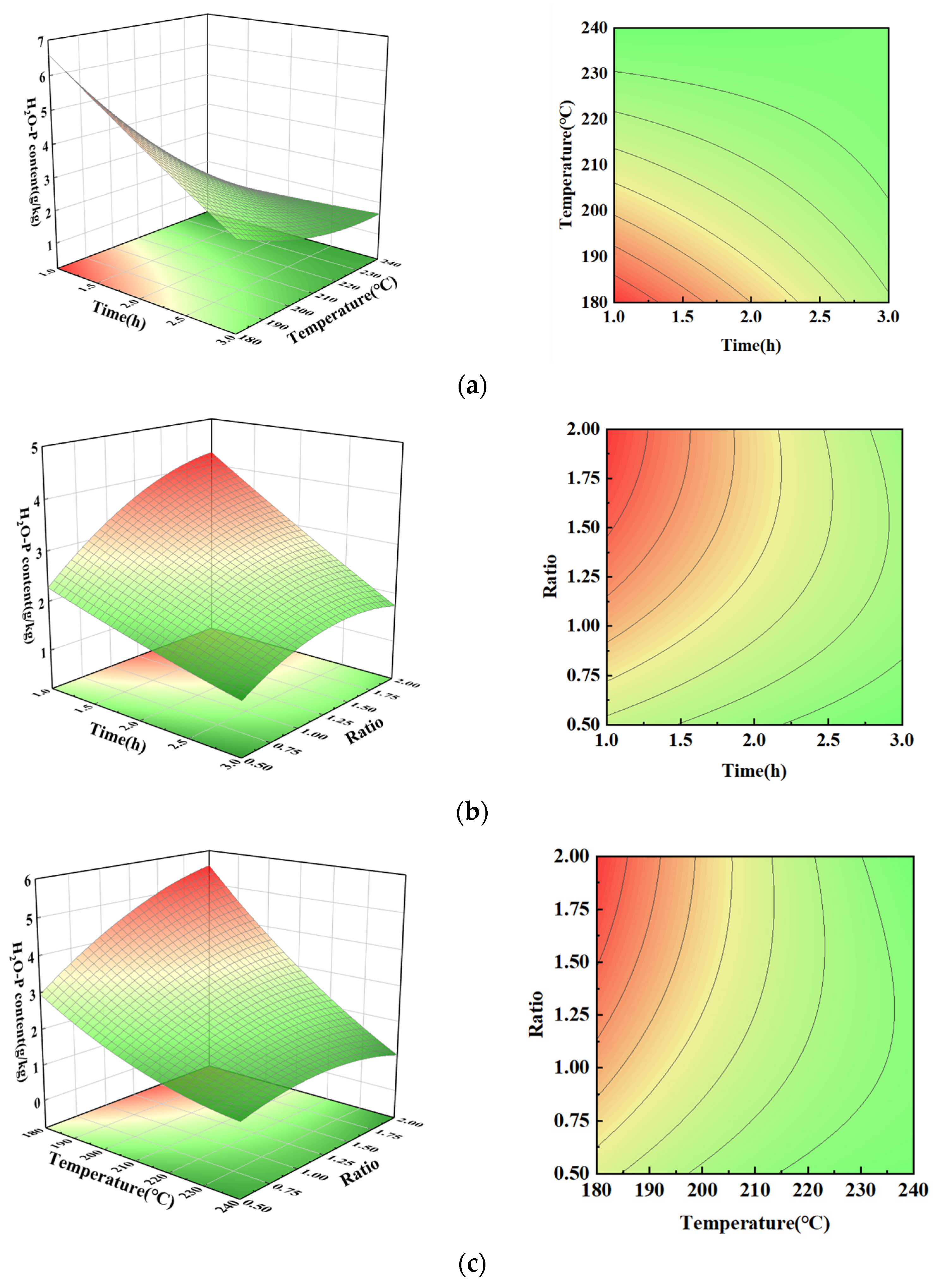
3.3. H2O-P
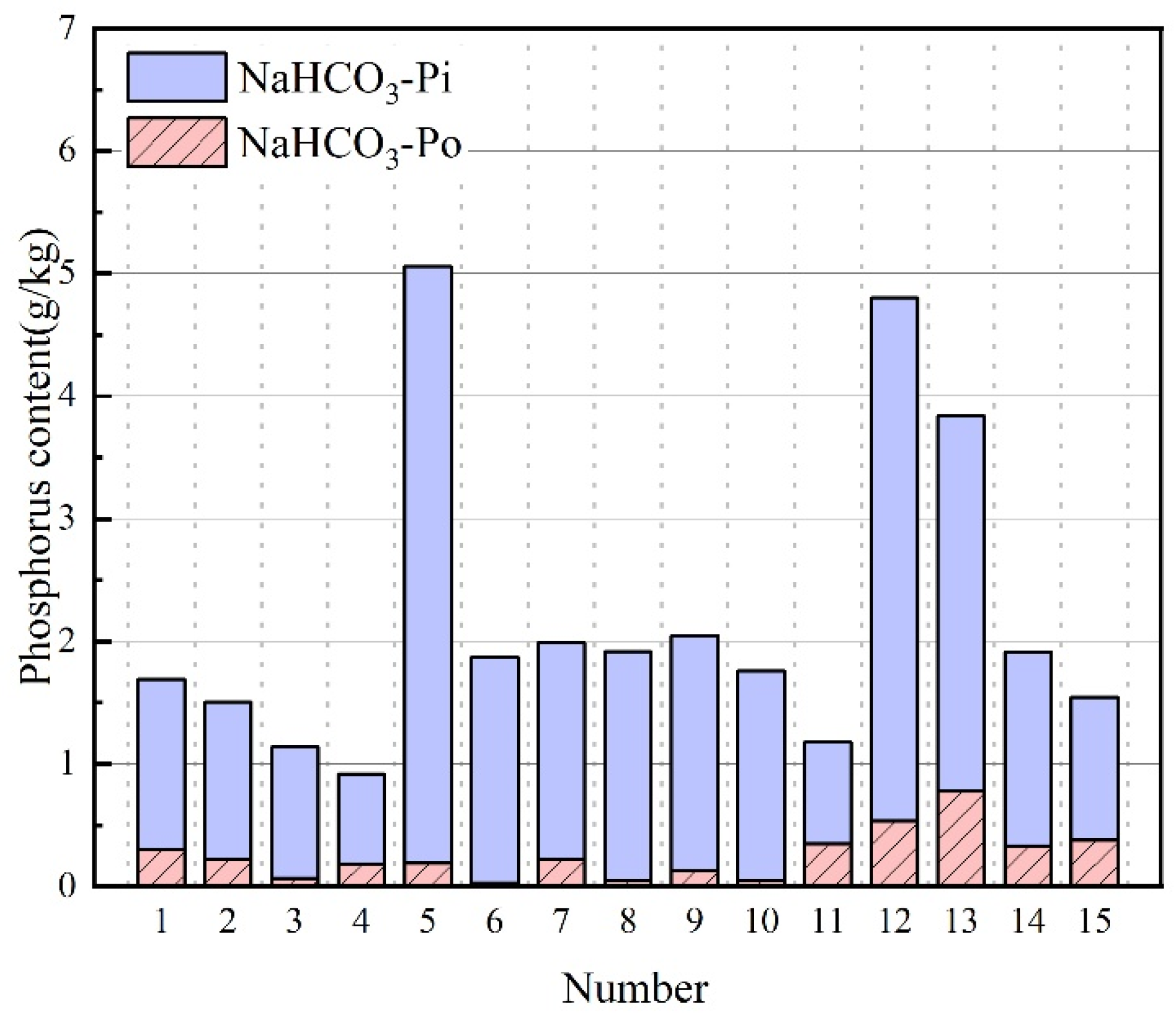
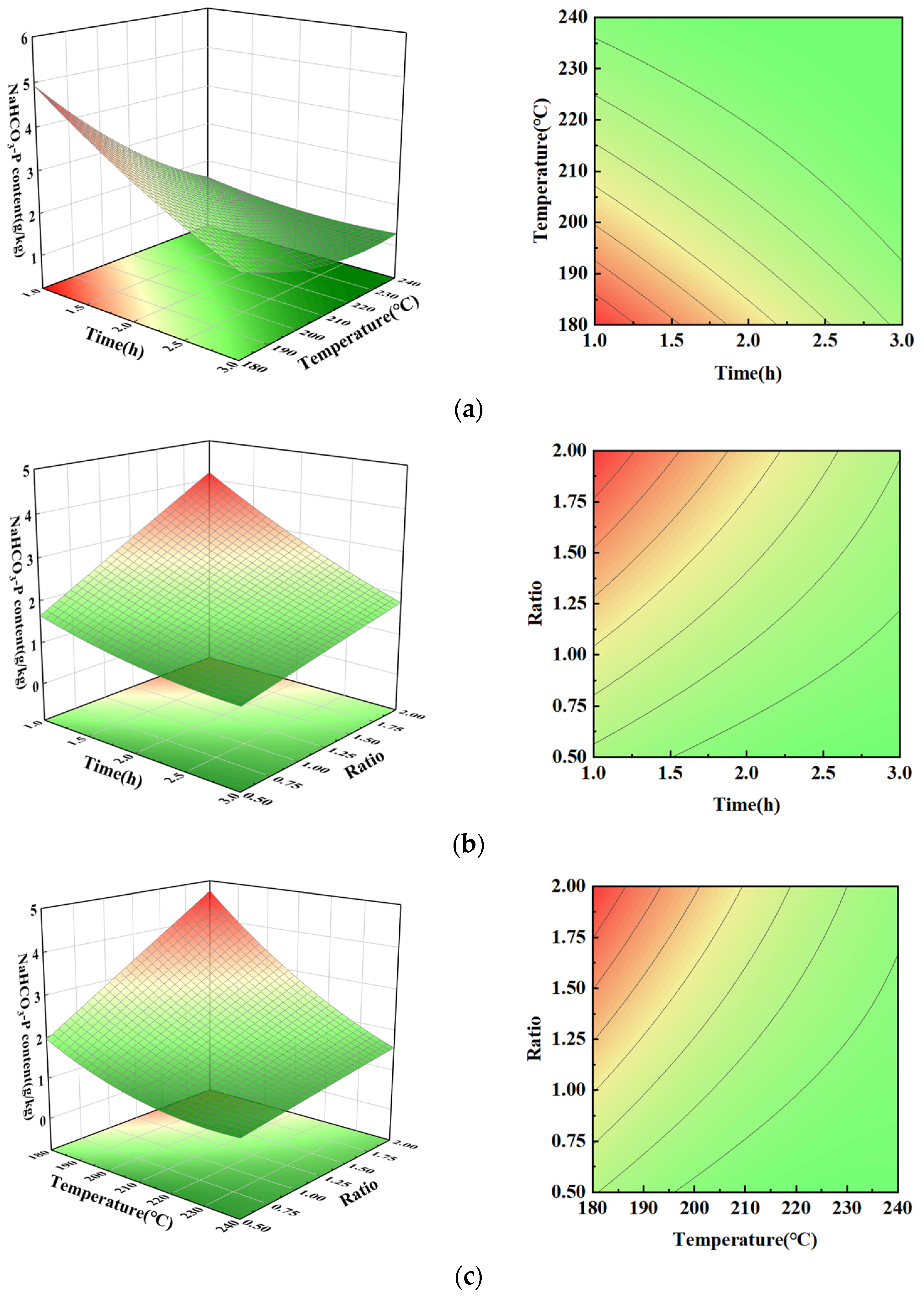
3.4. NaHCO3-P
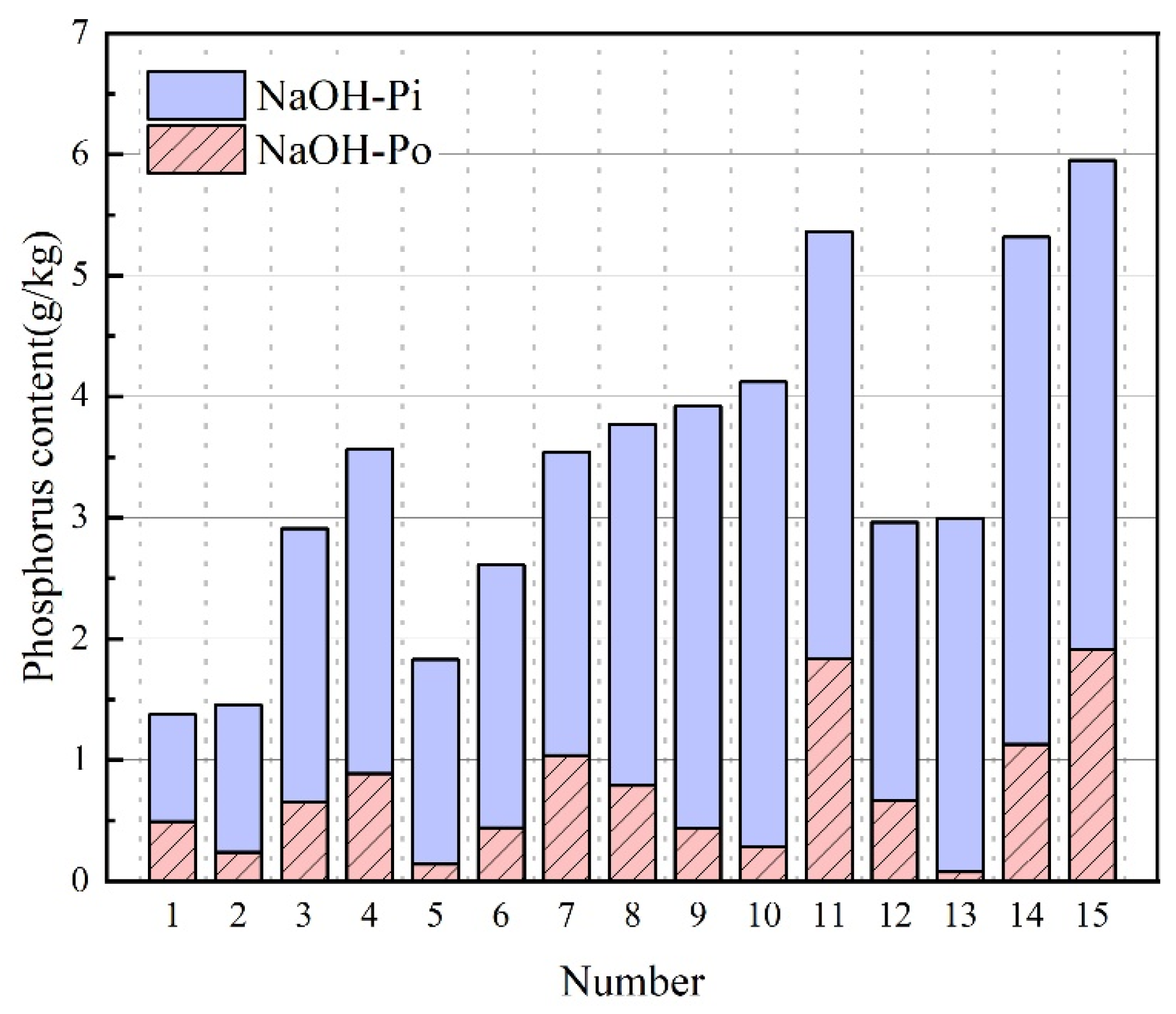
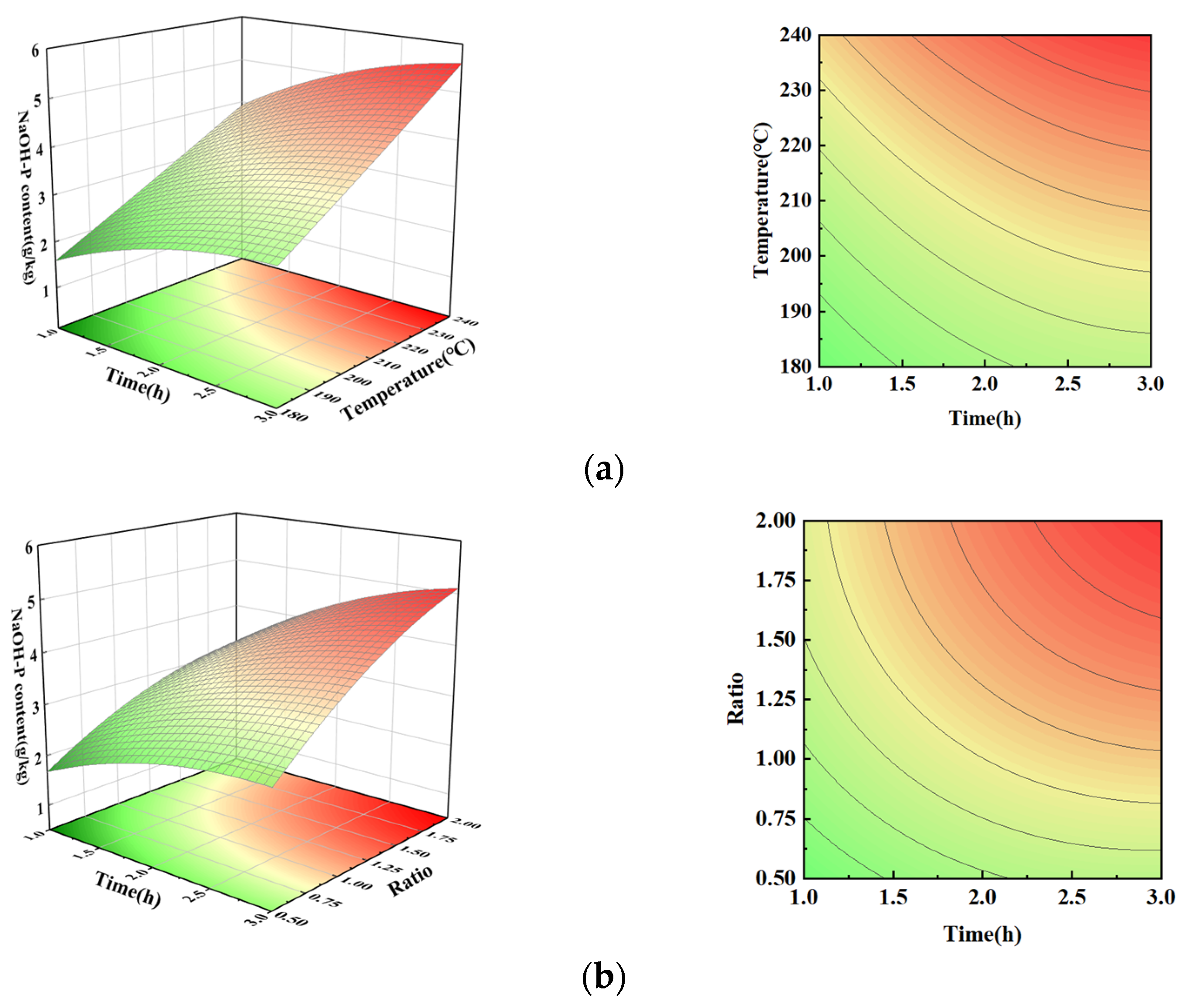
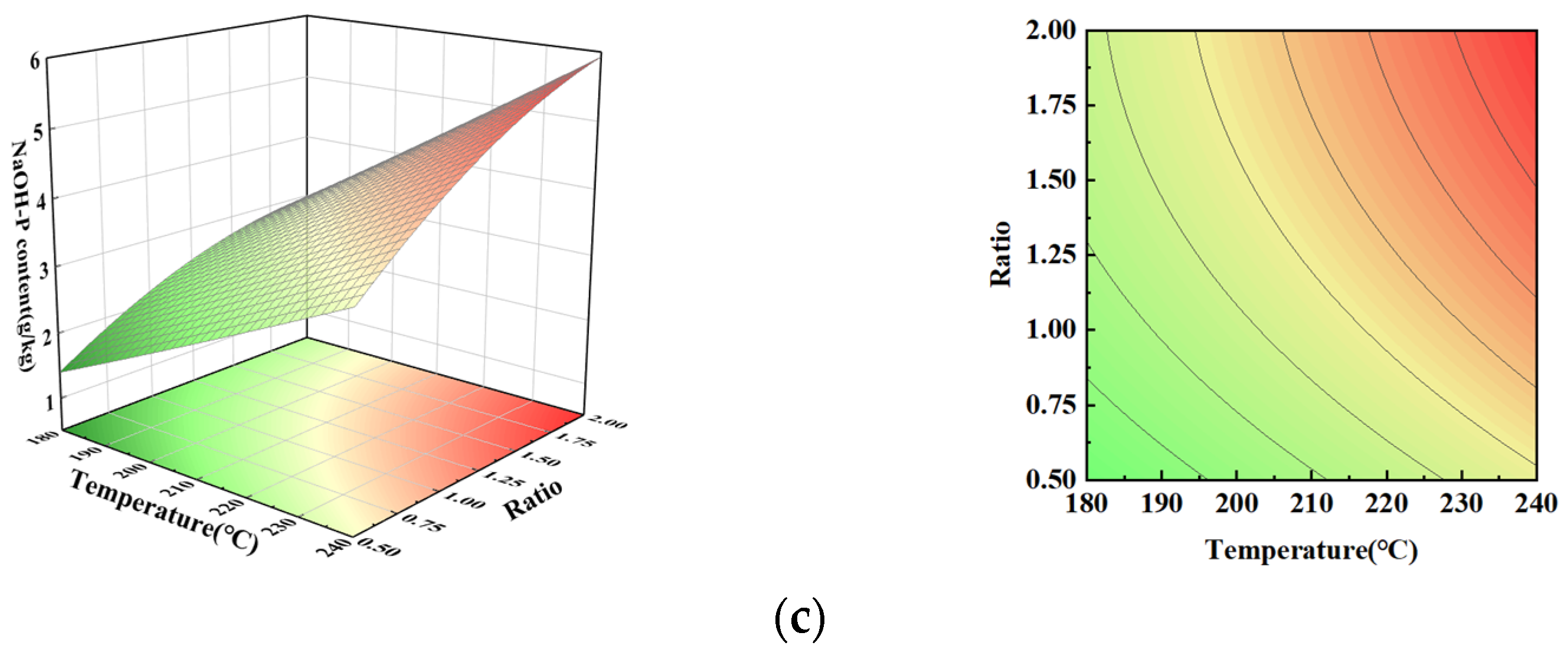
3.5. NaOH-P
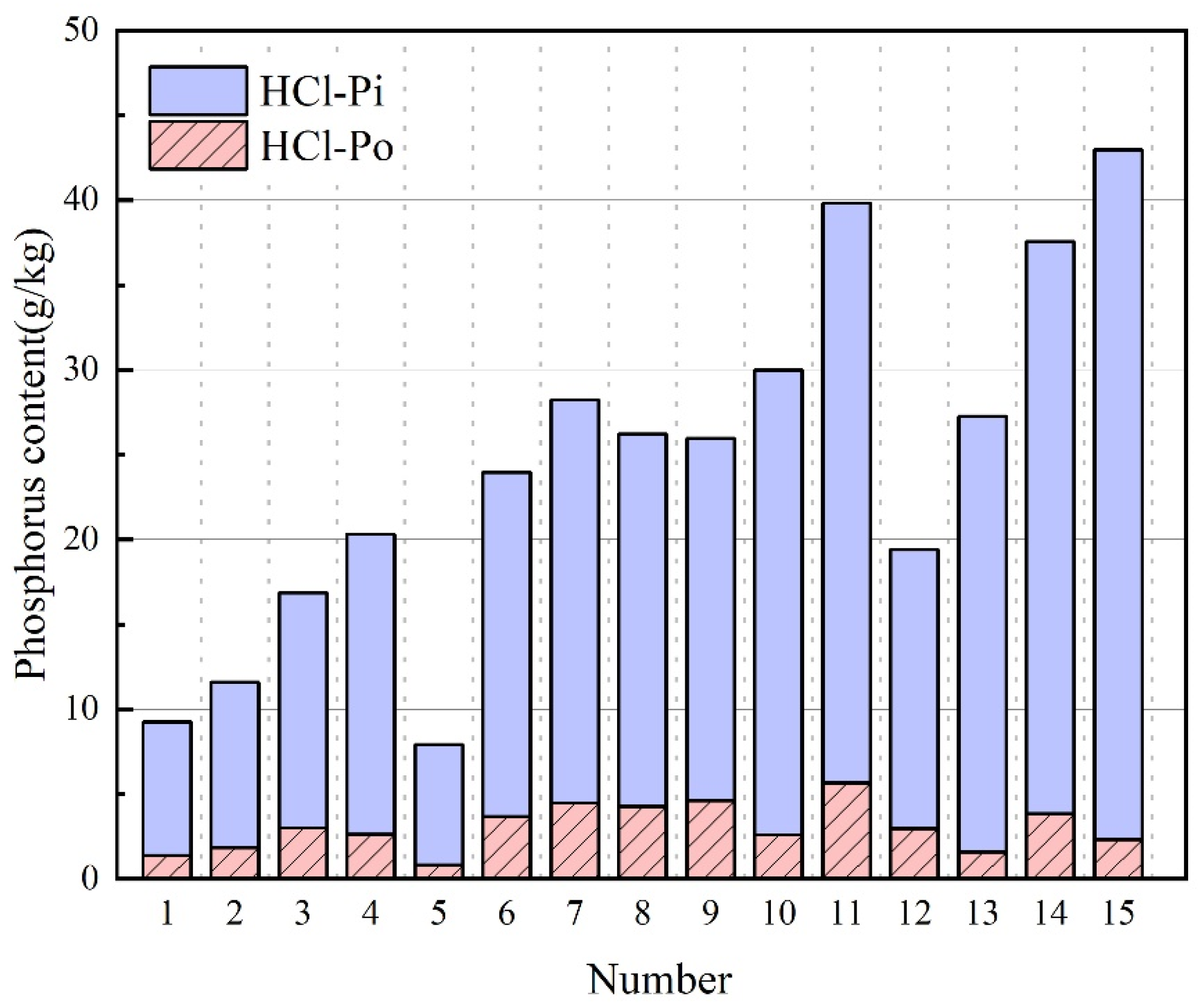
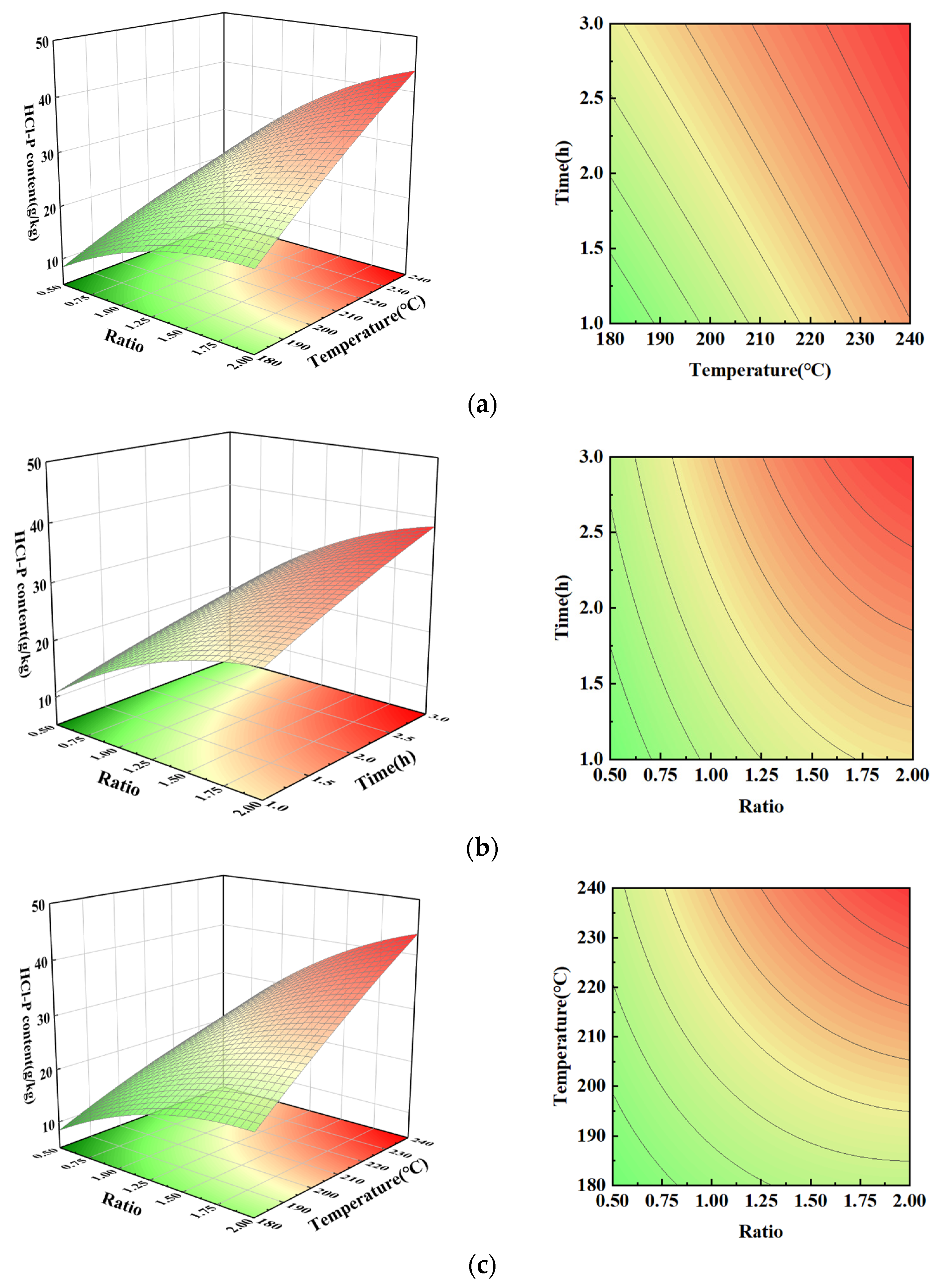
3.6. HCl-P
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Q.; Zhang, T.; Niu, Y.Q.; Mukherjee, S.; Abou-Elwafa, S.F.; Nguyen, N.S.H.; Al Aboud, N.M.; Wang, Y.K.; Pu, M.J.; Zhang, Y.R.; et al. A comprehensive review on agricultural waste utilization through sustainable conversion techniques, with a focus on the additives effect on the fate of phosphorus and toxic elements during composting process. Sci. Total Environ. 2024, 942, 173567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, H.Y.; Li, H.H.; He, X.Y.; Shi, Y.J.; Kruse, A. Microwave digestion-assisted HFO/biochar adsorption to recover phosphorus from swine manure. Sci. Total Environ. 2018, 621, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yang, H.; Wang, X.; Zhu, H.; Wang, Z.; Zhao, C.; Li, B.; Liu, Z. State-of-the-art on animal manure pollution control and resource utilization. J. Environ. Chem. Eng. 2023, 11, 110462. [Google Scholar] [CrossRef]
- Song, C.; Yuan, W.; Shan, S.; Ma, Q.; Zhang, H.; Wang, X.; Niazi, N.K.; Wang, H.L. Changes of nutrients and potentially toxic elements during hydrothermal carbonization of pig manure. Chemosphere 2020, 243, 125331. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, H.; Xu, M.; Lu, Z.; Zhang, Y.; Qu, J.; Luo, L.; Sun, Y. Development of modified maize stover for enhancing soil nutrients and functional microbes: Application of NPK-enriched techniques. Ind. Crop. Prod. 2024, 209, 118016. [Google Scholar] [CrossRef]
- Li, H.H.; Zhang, T.; Shaheen, S.M.; Abdelrahman, H.; Ali, E.F.; Bolan, N.S.; Li, G.X.; Rinklebe, J. Microbial inoculants and struvite improved organic matter humification and stabilized phosphorus during swine manure composting: Multivariate and multiscale investigations. Bioresour. Technol. 2022, 351, 126976. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Li, F.B.; Zhao, X.L.; Bolan, N.S.; Fu, P.; Lam, S.S.; Masek, O.; Ong, H.C.; Pan, B.; Qiu, X.; et al. Meet the challenges in the “Carbon Age”. Carbon Res. 2022, 1, 1. [Google Scholar] [CrossRef]
- He, X.M.; Zhang, T.; Ren, H.Q.; Li, G.X.; Ding, L.L.; Pawlowski, L. Phosphorus recovery from biogas slurry by ultrasound/H2O2 digestion coupled with HFO/biochar adsorption process. Waste Manag. 2017, 60, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dai, Z.; Liu, X.; Dahlgren, R.A.; Xu, J.M. Modification of agricultural wastes to improve sorption capacities for pollutant removal from water—A review. Carbon Res. 2022, 1, 24. [Google Scholar] [CrossRef]
- Tran, H.T.; Lin, C.; Lam, S.S.; Le, T.H.; Hoang, H.G.; Bui, X.T.; Rene, E.R.; Chen, P.H. Biodegradation of high di-(2-Ethylhexyl) phthalate (DEHP) concentration by food waste composting and its toxicity assessment using seed germination test. Environ. Pollut. 2023, 316, 120640. [Google Scholar] [CrossRef]
- Liu, G.; Xu, Q.; Abou-Elwafa, S.F.; Ali Alshehri, M.; Zhang, T. Hydrothermal carbonization technology for wastewater treatment under the “Dual Carbon” goals: Current status, trends, and challenges. Water 2024, 16, 1749. [Google Scholar] [CrossRef]
- Zhao, C.; Yin, X.; Chen, J.; Cao, F.; Abou-Elwafa, S.F.; Huang, M. Effect of rapeseed straw-derived biochar on soil bacterial community structure at tillering stage of Oryza sativa. Can. J. Microbiol. 2022, 68, 483–492. [Google Scholar] [CrossRef]
- He, X.Y.; Zhang, T.; Xue, Q.; Zhou, Y.; Wang, H.; Bolan, N.S.; Jiang, R.; Tsang, D.C.W. Enhanced adsorption of Cu(II) and Zn(II) from aqueous solution by polyethyleneimine modified straw hydrochar. Sci. Total Environ. 2021, 778, 146116. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal carbonization for energy-efficient processing of sewage sludge: A review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Jian, X.; Zhuang, X.; Li, B.; Xu, X.; Wei, Z.; Song, Y.; Jiang, E. Comparison of characterization and adsorption of biochars produced from hydrothermal carbonization and pyrolysis. Environ. Technol. Innov. 2018, 10, 27–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T. Biowaste valorization to produce advance carbon material-hydrochar for potential application of Cr (VI) and Cd (II) adsorption in wastewater: A review. Water 2022, 14, 3675. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, T.; Clark, J.; Aminabhavi, T.; Kruse, A.; Tsang, D.C.W.; Sharma, B.K.; Zhang, F.S.; Ren, H.Q. Mechanisms and modelling of phosphorus solid–liquid transformation during the hydrothermal processing of swine manure. Green Chem. 2020, 22, 5628–5638. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Li, A.Y.; Deng, H.; Ye, C.H.; Wu, Y.Q.; Linmu, Y.D.; Hang, H.L. Characteristics of nitrogen and phosphorus adsorption by Mg-loaded biochar from different feedstocks. Bioresour. Technol. 2019, 276, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, Z.; Lu, Y.; Shan, S.; Zhuang, H.; Gong, C.; Cui, X.; Zhang, F.; Li, P. Facilitating mitigation of agricultural non-point source pollution and improving soil nutrient conditions: The role of low temperature co-pyrolysis biochar in nitrogen and phosphorus distribution. Bioresour. Technol. 2024, 394, 130179. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Deng, Y.; Uppuluri, N.S.T.; Li, B.; Zheng, Y.; Chen, P.; Dong, R.; Muller, J.; Guo, J.; Oechsner, H. Hotspots and future trends of phosphorus recycling from livestock manure: A bibliometric review. Sci. Total Environ. 2023, 892, 164346. [Google Scholar] [CrossRef]
- Zhang, T.; Li, P.; Fang, C.; Jiang, R.F. Phosphate recovery from animal manure wastewater by struvite crystallization and CO2 degasification reactor. Ecol. Chem. Eng. S 2014, 21, 89–99. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Jiang, R.F.; Ohtake, H. Phosphate enhance recovery from wastewater by mechanism analysis and optimization of struvite settleability in fluidized bed reactor. Sci. Rep. 2016, 6, 32215. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Zhang, T.; Niu, Y.; Xue, Q.; Ali, E.F.; Shaheen, S.M.; Tsang, D.C.W.; Rinklebe, J. Impact of catalytic hydrothermal treatment and Ca/Al-modified hydrochar on lability, sorption, and speciation of phosphorus in swine manure: Microscopic and spectroscopic investigations. Environ. Pollut. 2022, 299, 118877. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Zheng, H.; Liu, Q.; Hashemi, M.; Tang, Y.; Xing, B. Production and characterization of hydrochars and their application in soil improvement and environmental remediation. Chem. Eng. J. 2022, 430, 133142. [Google Scholar] [CrossRef]
- Fu, H.; Wang, B.; Wang, H.; Liu, H.; Xie, H.; Han, L.; Wang, N.; Sun, X.; Feng, Y.; Xue, L. Assessment of livestock manure-derived hydrochar as cleaner products: Insights into basic properties, nutrient composition, and heavy metal content. J. Clean. Prod. 2022, 330, 129820. [Google Scholar] [CrossRef]
- Huang, R.; Fang, C.; Zhang, B.; Tang, Y. Transformations of phosphorus speciation during (hydro)thermal treatments of animal manures. Environ. Sci. Technol. 2018, 52, 3016–3026. [Google Scholar] [CrossRef]
- Lang, Q.; Zhang, B.; Liu, Z.; Chen, Z.; Xia, Y.; Li, D.; Ma, J.; Gai, C. Co-hydrothermal carbonization of corn stalk and swine manure: Combustion behavior of hydrochar by thermogravimetric analysis. Bioresour. Technol. 2019, 271, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Guo, Y.; Zheng, Q.; Liu, Z.; Gai, C. Co-hydrothermal carbonization of lignocellulosic biomass and swine manure: Hydrochar properties and heavy metal transformation behavior. Bioresour. Technol. 2018, 266, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Alhnidi, M.J.; Wust, D.; Funke, A.; Hang, L.; Kruse, A. Fate of nitrogen, phosphate, and potassium during hydrothermal carbonization and the potential for nutrient recovery. ACS Sustain. Chem. Eng. 2020, 8, 15507–15516. [Google Scholar] [CrossRef]
- Khalaf, N.; Leahy, J.J.; Kwapinski, W. Phosphorus recovery from hydrothermal carbonization of organic waste: A review. J. Chem. Technol. Biot. 2023, 98, 2365–2377. [Google Scholar] [CrossRef]
- Shettigondahalli Ekanthalu, V.; Narra, S.; Ender, T.; Antwi, E.; Nelles, M. Influence of post-and pre-acid treatment during hydrothermal carbonization of sewage sludge on P-transformation and the characteristics of hydrochar. Processes 2022, 10, 151. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Ab Halim, A.Z.; Mohamed, A.H.; Daud, M.A.A.; Dabwan, A.H.A. Biogas Dry Reforming: Development of Ni/Na Zeolite Catalyst Using Response Surface Methodology Approach with Reaction Mechanism Evaluation. Water Conserv. Manag. 2023, 7, 60–76. [Google Scholar] [CrossRef]
- Prasad, R.; Yadav, K.D. Use of response surface methodology and artificial neural network approach for methylene blue removal by adsorption onto water hyacinth. Water Conserv. Manag. 2020, 4, 83–89. [Google Scholar] [CrossRef]
- Bullo, T.A.; Bayisa, Y.M. Optimizing the removal efficiency of chromium from tanning plant effluent by adsorption method with activated carbon chat stems (catha edulis) using response surface methodology. Water Conserv. Manag. 2022, 6, 15–21. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.C.; Ding, L.L.; Ren, H.Q.; Xu, K.; Wu, Y.G.; Sheng, D. Modeling assessment for ammonium nitrogen recovery from wastewater by chemical precipitation. J. Environ. Sci. 2011, 23, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA; New Delhi, India, 1973. [Google Scholar]
- Hesse, P.R. A Textbook of Soil Chemical Analysis; CBS Publishers & Distributors: Delhi, India, 1998. [Google Scholar]
- Olsen, S.R.; Dean, L.A. Phosphorus, Methods of Soil Analysis Part 2, Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1965. [Google Scholar]
- Hedley, M.J.; Steward, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Gasco, G.; Paz-Ferreiro, J.; Alvarez, M.L.; Saa, A.; Mendez, A. Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure. Waste Manag. 2018, 79, 395–403. [Google Scholar] [CrossRef]
- Yu, J.X.; Xie, S.Y.; Zhang, T. Influences of hydrothermal carbonization on phosphorus availability of swine manure-derived hydrochar: Insights into reaction time and temperature. Mater. Sci. Energy Technol. 2022, 5, 416–423. [Google Scholar] [CrossRef]
- Xiong, J.; Pan, Z.; Xiao, X.; Huang, H.; Lai, F.; Wang, J.; Chen, S. Study on the hydrothermal carbonization of swine manure: The effect of process parameters on the yield/properties of hydrochar and process water. J. Anal. Appl. Pyrolysis 2019, 144, 104692. [Google Scholar] [CrossRef]
- Lang, Q.; Zhang, B.; Liu, Z.; Jiao, W.T.; Xia, Y.; Chen, Z.; Li, D.; Ma, J.; Gai, C. Properties of hydrochars derived from swine manure by CaO assisted hydrothermal carbonization. J. Environ. Manag. 2019, 233, 440–446. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, L.; Trakal, L.; Wang, S.; Shaheen, S.M.; Rinklebe, J.; Chen, Q. Pyrolytic and hydrothermal carbonization affect the transformation of phosphorus fractions in the biochar and hydrochar derived from organic materials: A meta-analysis study. Sci. Total Environ. 2024, 906, 167418. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikova, E.; Arauzo, P.J.; Becker, G.C.; Kruse, A. Experimental and thermodynamic studies of phosphate behavior during the hydrothermal carbonization of sewage sludge. Sci. Total Environ. 2019, 692, 147–156. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, S.; Wang, J.; Wang, Y.; Fang, X.; Huang, H. Speciation of main nutrients (N/P/K) in hydrochars produced from the hydrothermal carbonization of swine manure under different reaction temperatures. Materials 2021, 14, 4114. [Google Scholar] [CrossRef]
- Mekmene, O.; Quillard, S.; Rouillon, T.; Bouler, J.M.; Piot, M.; Gaucheron, F. Effects of pH and Ca/P molar ratio on the quantity and crystalline structure of calcium phosphates obtained from aqueous solutions. Dairy Sci. Technol. 2009, 89, 301–316. [Google Scholar] [CrossRef]
- Fang, Z.; Zhuang, X.; Zhang, X.; Li, Y.; Li, R.; Ma, L. Influence of paraments on the transformation behaviors and directional adjustment strategies of phosphorus forms during different thermochemical treatments of sludge. Fuel 2023, 333, 126544. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal carbonization of wet biomass from nitrogen and phosphorus approach: A review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Zhang, Y.; Wang, C.; Yu, J.; Ohtake, H.; Zhang, T. The potential for livestock manure valorization and phosphorus recovery by hydrothermal technology-a critical review. Mater. Sci. Energy Technol. 2023, 6, 94–104. [Google Scholar] [CrossRef]
- Li, C.; Cai, R.; Hasan, A.; Lu, X.; Yang, X.; Zhang, Y. Fertility assessment and nutrient conversion of hydrochars derived from co-hydrothermal carbonization between livestock manure and corn cob. J. Environ. Chem. Eng. 2023, 11, 109166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Zhang, T.; Zhao, J.; Mukherjee, S.; Alotaibi, N.M.; Abou-Elwafa, S.F.; Tran, H.-T.; Bolan, N.S. Phosphorus Fraction in Hydrochar from Co-Hydrothermal Carbonization of Swine Manure and Rice Straw: An Optimization Analysis Based on Response Surface Methodology. Water 2024, 16, 2208. https://doi.org/10.3390/w16152208
Su X, Zhang T, Zhao J, Mukherjee S, Alotaibi NM, Abou-Elwafa SF, Tran H-T, Bolan NS. Phosphorus Fraction in Hydrochar from Co-Hydrothermal Carbonization of Swine Manure and Rice Straw: An Optimization Analysis Based on Response Surface Methodology. Water. 2024; 16(15):2208. https://doi.org/10.3390/w16152208
Chicago/Turabian StyleSu, Xiaohua, Tao Zhang, Jingyang Zhao, Santanu Mukherjee, Nahaa M. Alotaibi, Salah F. Abou-Elwafa, Huu-Tuan Tran, and Nanthi S. Bolan. 2024. "Phosphorus Fraction in Hydrochar from Co-Hydrothermal Carbonization of Swine Manure and Rice Straw: An Optimization Analysis Based on Response Surface Methodology" Water 16, no. 15: 2208. https://doi.org/10.3390/w16152208
APA StyleSu, X., Zhang, T., Zhao, J., Mukherjee, S., Alotaibi, N. M., Abou-Elwafa, S. F., Tran, H.-T., & Bolan, N. S. (2024). Phosphorus Fraction in Hydrochar from Co-Hydrothermal Carbonization of Swine Manure and Rice Straw: An Optimization Analysis Based on Response Surface Methodology. Water, 16(15), 2208. https://doi.org/10.3390/w16152208






