Feasibility of Groundwater Extraction in Nitrate-Impacted Groundwater Source in Serbia: Hydrodynamic Modeling and Nitrate Tracing
Abstract
1. Introduction
2. Materials and Methods
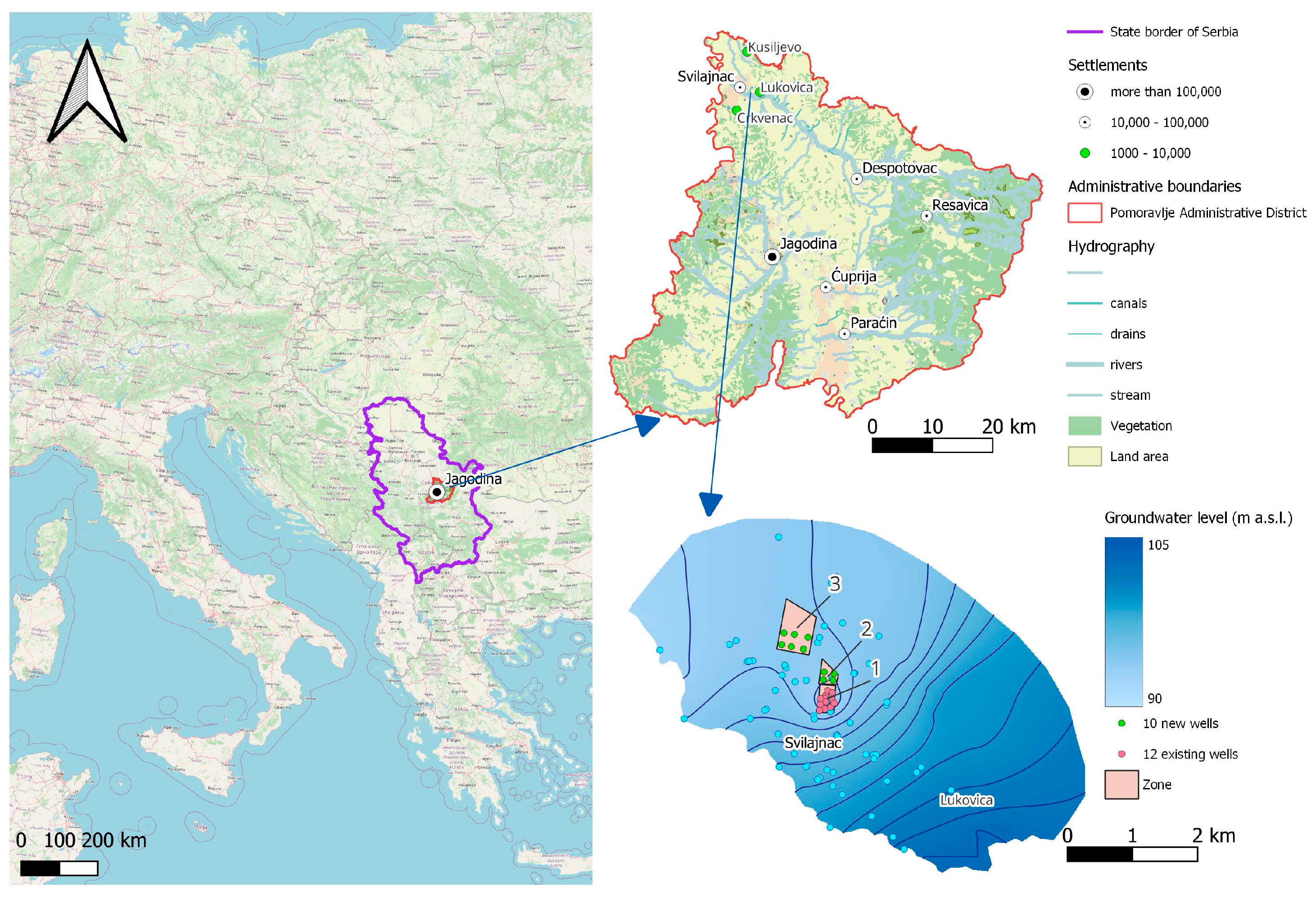
2.1. Investigated Site
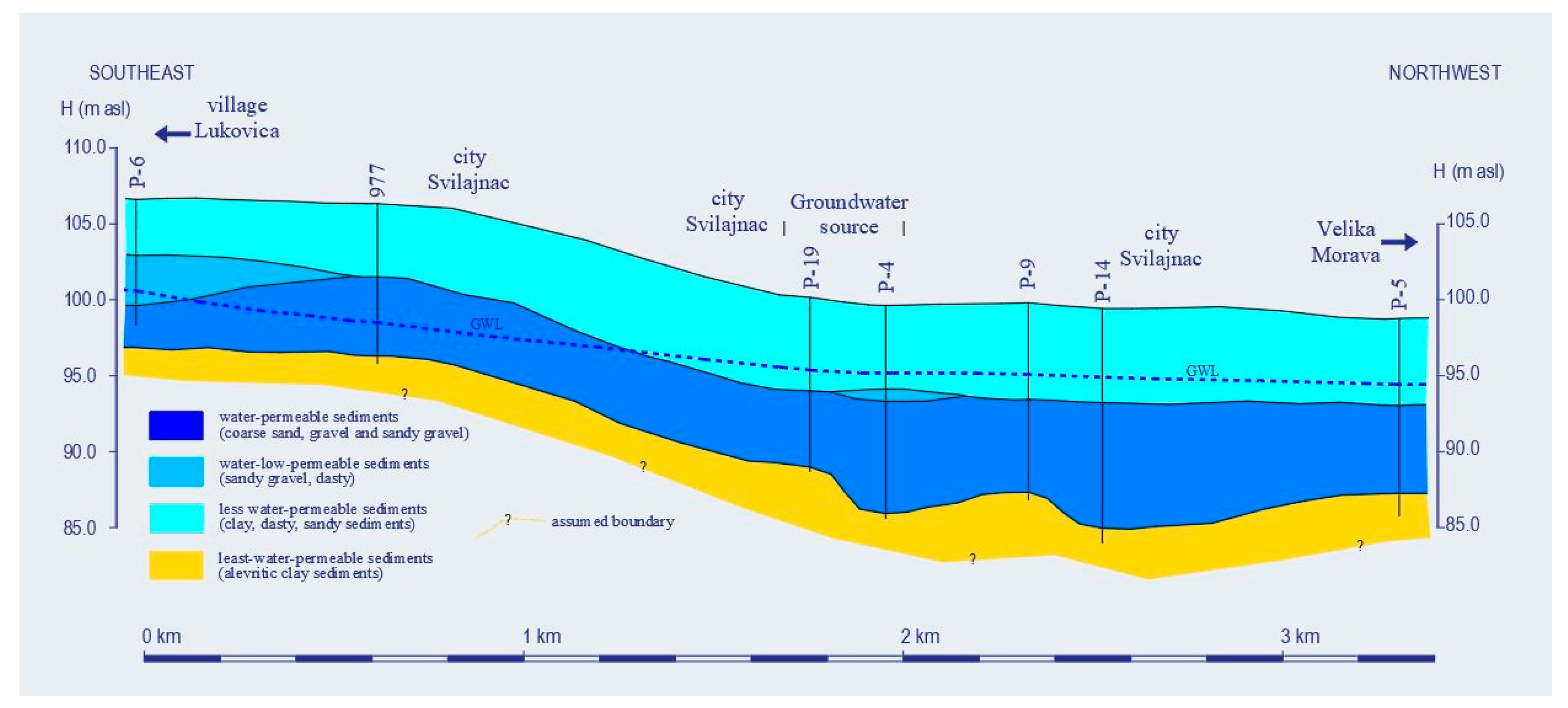
Hydrogeological Characteristics
2.2. Hydrogeological Modeling
Development and Calibration of a Hydrodynamic Model
2.3. Water Quality
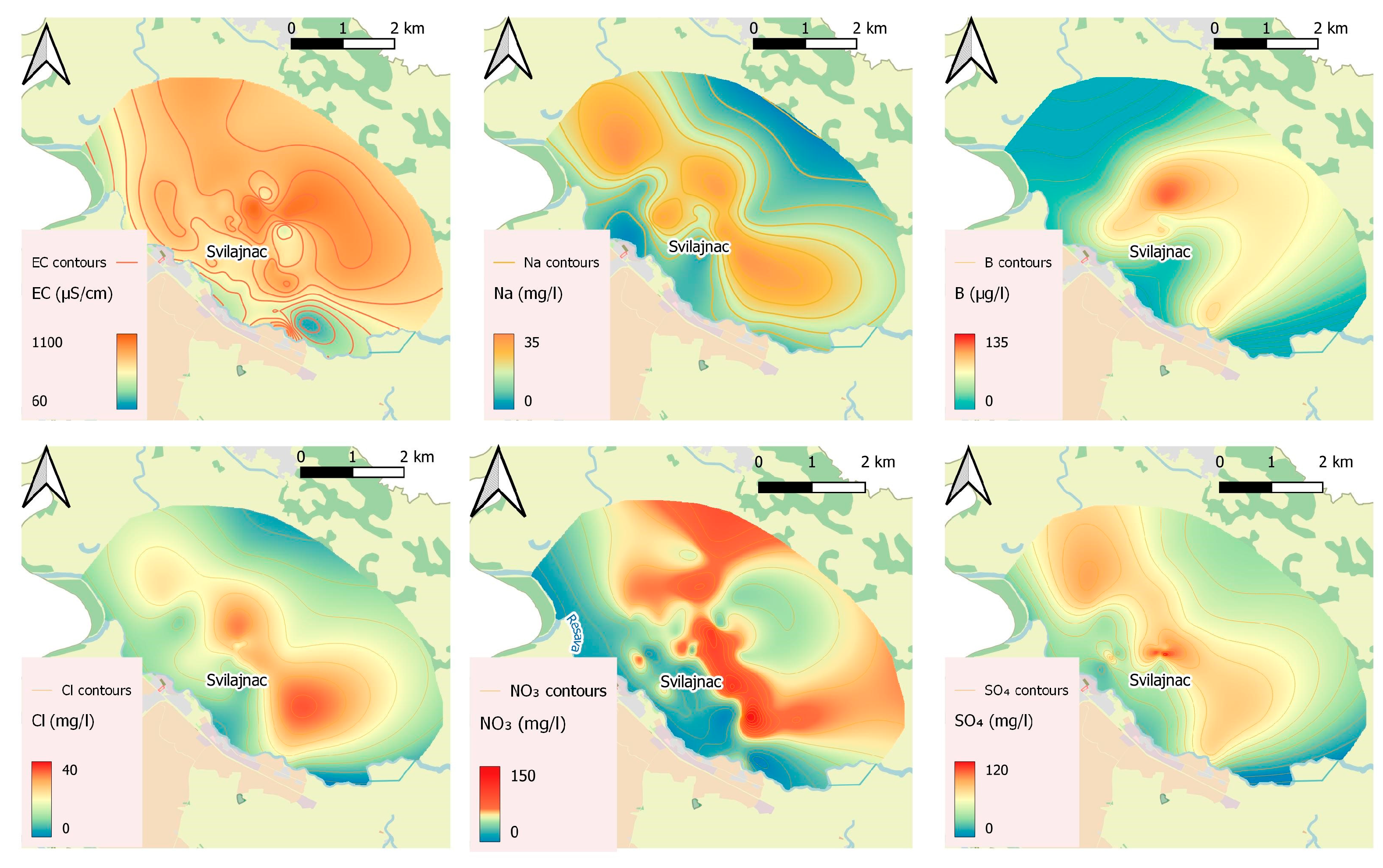
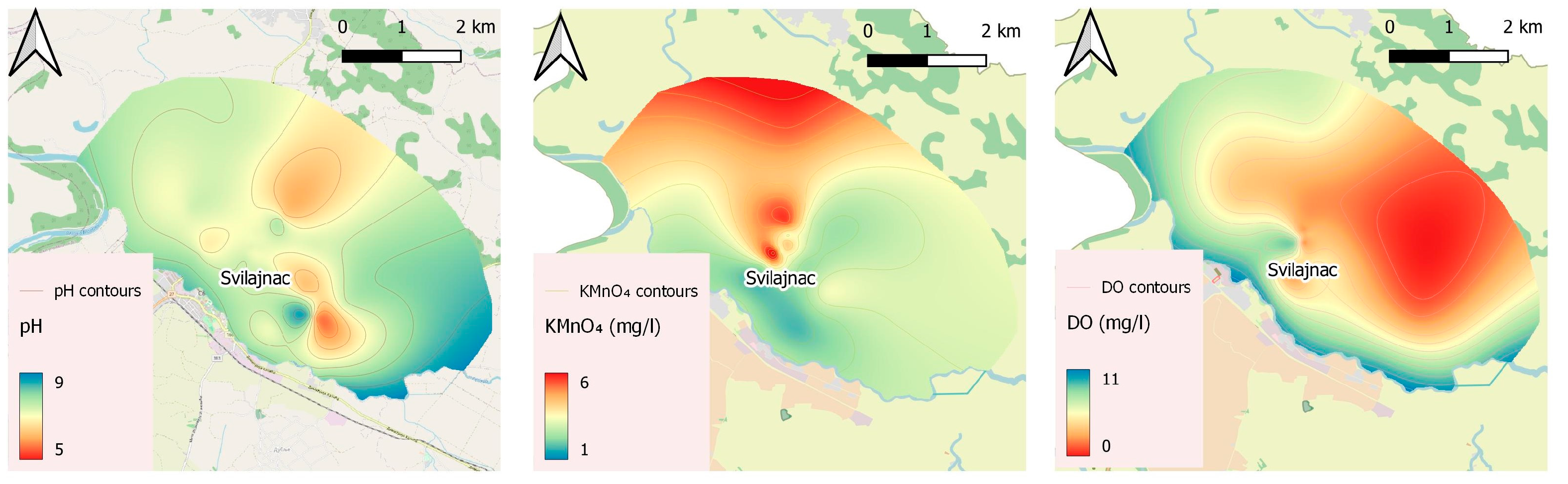
2.3.1. Groundwater Quality
2.3.2. Surface Water Quality
3. Results
3.1. Modeling Results
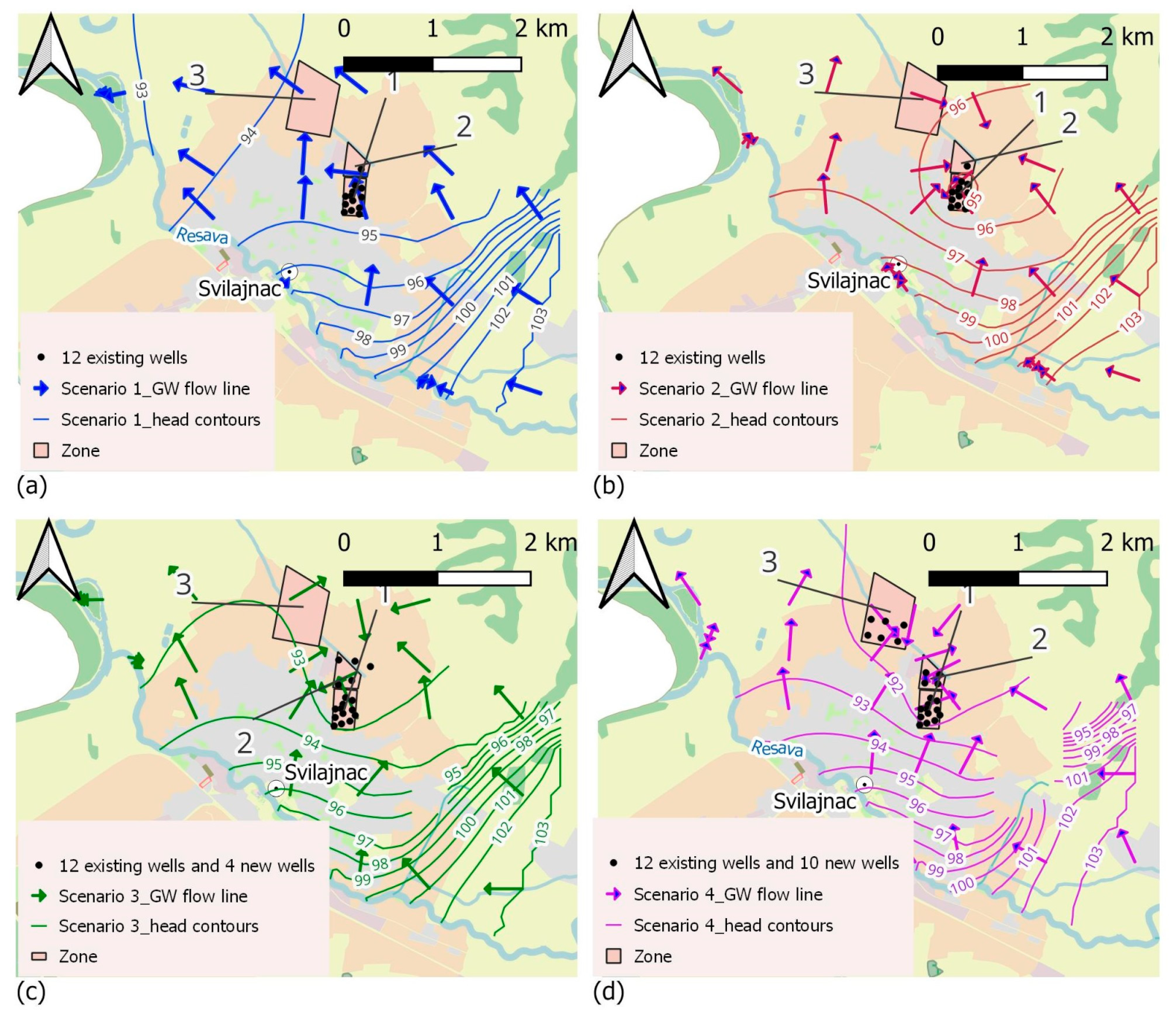
3.1.1. Scenario 1—Existing State, Average Annual Exploitation Rate of Q = 55 L/s, with 12 Existing Wells (in Zone 1)
3.1.2. Scenario 2—Existing State with Maximum Exploitation Rate of Q = 80 L/s for a Duration of Three Months during the Dry Period, with 12 Existing Wells (in Zone 1)
3.1.3. Scenario 3—Average Annual Exploitation Rate of Q = 75 L/s, with 12 Existing Wells and 4 New Wells in Zone 2 (Recommended Source Expansion—Future State)
3.1.4. Scenario 4—Expansion of the Water Source to Zones 2 and 3—Average Annual Exploitation Rate of Q = 105 L/s, with 12 Existing Wells, 4 New Wells in Zone 2, and 6 New Wells in Zone 3 (Potential Source Expansion—Future State)
3.2. Physicochemical Results of Water Quality
3.2.1. Groundwater
3.2.2. Surface Water
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins; (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 94.) 6, Evaluation and Rationale; International Agency for Research on Cancer: Lyon, France, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326535/ (accessed on 21 June 2023).
- Dimkić, M.A.; Brauch, H.J.; Kavanaugh, M. Groundwater Management in Large River Basins; IWA Publishing: London, UK, 2008; ISBN 9781843391906. [Google Scholar]
- Pastén-Zapata, E.; Ledesma-Ruiz, R.; Harter, T.; Ramírez, A.I.; Mahlknecht, J. Assessment of sources and fate of nitrate in shallow groundwater of an agricultural area by using a multi-tracer approach. Sci. Total Environ. 2014, 470–471, 855–864. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for Drinking Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- Arıman, S.; Soydan-Oksal, N.G.; Beden, N.; Ahmadzai, H. Assessment of Groundwater Quality through Hydrochemistry Using Principal Components Analysis (PCA) and Water Quality Index (WQI) in Kızılırmak Delta, Turkey. Water 2024, 16, 1570. [Google Scholar] [CrossRef]
- Banda, L.C.; Kalin, R.M.; Phoenix, V. Isotope Hydrology and Hydrogeochemical Signatures in the Lake Malawi Basin: A Multi-Tracer Approach for Groundwater Resource Conceptualisation. Water 2024, 16, 1587. [Google Scholar] [CrossRef]
- Ghannem, S.; Paredes-Arquiola, J.; Bergillos, R.J.; Solera, A.; Andreu, J. Assessing the Effects of Environmental Flows on Water Quality for Urban Supply. Water 2024, 16, 1509. [Google Scholar] [CrossRef]
- Perovic, M.; Obradovic, V.; Zuber-Radenković, V.; Obrovski, B.; Vojinovic-Miloradov, M.; Dimkić, M. Comprehensive Biogeochemical Analysis of Nitrogen Transformation Parameters. Water Resour. 2020, 47, 156–170. [Google Scholar] [CrossRef]
- Perović, M.; Dimkić, M. Transformation of Nitrogen Compounds in Groundwater; Chapter 8 in Alluvial Aquifer Processes; IWA Publishing: London, UK, 2021; ISBN 9781789060904. [Google Scholar]
- Weng, T.N.; Liu, C.W.; Kao, Y.H.; Hsiao, S.S.Y. Isotopic evidence of nitrogen sources and nitrogen transformation in arse-nic-contaminated groundwater. Sci. Total Environ. 2017, 578, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Nikolenko, O.; Jurado, A.; Borges, A.V.; Knöller, K.; Brouyre, S. Isotopic composition of nitrogen species in groundwater under agricultural areas: A review. Sci. Total Environ. 2018, 621, 1415–1432. [Google Scholar] [CrossRef]
- Dimkić, M. Importance of the Aerobic State of Alluvial Aquifers for Groundwater use, Nakdong River International Water Week. In Proceedings of the 7th World Water Forum, September, Andong, Republic of Korea, 69–96, Conference “Contemporary Issues of Adaptive Water Management”, Belgrade, Serbia, 31 October 2012 ; pp. 29–37. [Google Scholar]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.N.; Bemment, C.D. Nitrate Attenuation in Groundwater: A Review of Bioge-ochemical Controlling Processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef]
- Perović, M.; Obradović, V.; Zuber-Radenković, V.; Knoeller, K.; Mitrinović, D.; Čepić, Z. The comprehensive evaluation of nitrate origin and transformation pathways in the oxic alluvial aquifer in Serbia. Environ. Sci. Pollut. Res. 2024, 31, 33030–33046. [Google Scholar] [CrossRef]
- Xue, D.; Botte, J.; De Baets, B.; Accoe, F.; Nestler, A.; Taylor, P.; Van Cleemput, O.; Berglund, M.; Boeckx, P. Present limitations and future prospects of stable isotope methods for nitrate source identification in surface-and groundwater. Water Res. 2009, 43, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Gasser, C.A.; Ammann, E.M.; Shahgaldian, P.; Corvini, P.F.-X. Laccases to take on the challenge of emerging organic contaminants in wastewater. Appl. Microbiol. Biotechnol. 2014, 98, 9931–9952. [Google Scholar] [CrossRef]
- Ellis, J.B.; Bertrand-Krajewski, J. Assessing Exfiltration and Infiltration on the Performance of Urban Sewer Systems; Water Intelligence Online; IWA Publishing: London, UK, 2010; Volume 9. [Google Scholar]
- Lindebaum, J. Identification of Sources of Ammonium in Groundwater Using Stable Nitrogen and Boron Isotopes in Nam Du, Hanoi. Ph.D. Thesis, Lund University, Lund, Sweden, 2012. [Google Scholar]
- Kalagasidis Krušić, M. Organic Industrial Synthesis; Internal Script of the Department of Organic Chemical Technology, Faculty of Technology and Metallurgy, University of Belgrade: Belgrade, Serbia, 2017. (In Serbian) [Google Scholar]
- Widory, D.; Kloppmann, W.; Chery, L.; Bonnin, J.; Rochdi, H.; Guinamant, J.L. Nitrate in groundwater: An isotopic multi-tracer approach. J. Contam. Hydrol. 2004, 72, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Bohlke, J.K.; Smith, R.L.; Miller, D.N. Ammonium transport and reaction in contaminated groundwater: Application of isotope tracers and isotope fractionation studies. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef]
- Drinking Water Directive, Council Directive 98/83/EC of 3 November 1998 on the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083 (accessed on 21 June 2023).
- Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources, Nitrate Directive. Available online: https://eur-lex.europa.eu/eli/dir/1991/676/oj (accessed on 21 June 2023).
- Zhang, M.; Huang, G.; Liu, C.; Zhang, Y.; Chen, Z.; Wang, J. Distributions and origins of nitrate, nitrite, and ammonium in various aquifers in an urbanized coastal area, south China. J. Hydrol. 2020, 582, 124528. [Google Scholar] [CrossRef]
- Ledoux, E.; Gomez, E.; Monget, J.M.; Viavattene, C.; Viennot, P.; Ducharne, A.; Benoit, M.; Mignolet, C.; Schott, C.; Mary, B. Agriculture and Groundwater Nitrate Contamination in the Seine Basin. The STICS-MODCOU modelling chain. Sci. Total Environ. 2007, 375, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Burow, K.R.; Nolan, B.T.; Rupert, M.G.; Dubrovsky, N.M. Nitrate in groundwater of the United States, 1991–2003. Environ. Sci. Technol. 2010, 44, 4988–4997. [Google Scholar] [CrossRef]
- Rivett, M.O.; Smith, J.W.N.; Buss, S.R.; Morgan, P. Nitrate occurrence and attenuation in the major aquifers of England and Wales. Q. J. Eng. Geol. Hydrogeol. 2007, 40, 335–352. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, J.; Liu, J.; Huang, G.; Lu, C.; Zhang, Y. Driving mechanism and sources of groundwater nitrate contamination in the rapidly urbanized region of south China. J. Contam. Hydrol. 2015, 182, 221–230. [Google Scholar] [CrossRef]
- Huang, G.; Liu, C.; Zhang, Y.; Chen, Z. Groundwater is important for the geochemical cycling of phosphorus in rapidly urbanized areas: A case study in the Pearl River Delta. Environ. Pollut. 2020, 260, 114079. [Google Scholar] [CrossRef]
- Reed, E.M.; Wang, D.; Duranceau, S.J. Evaluating Nitrate Management in the Volusia Blue Springshed. J. Environ. -Ment. Eng. 2018, 144, 3. [Google Scholar] [CrossRef]
- Reed, E.M.; Wang, D.; Duranceau, S.J. Modeling anthropogenic boron in groundwater flow and discharge at Volusia Blue Spring (FL, USA). Hydrogeol. J. 2017, 25, 91–101. [Google Scholar] [CrossRef]
- Correa-González, A.; Hernández-Bedolla, J.; Martínez-Cinco, M.A.; Sánchez-Quispe, S.T.; Hernández-Hernández, M.A. As-sessment of Nitrate in Groundwater from Diffuse Sources Considering Spatiotemporal Patterns of Hydrological Systems Using a Coupled SWAT/MODFLOW/MT3DMS Model. Hydrology 2023, 10, 209. [Google Scholar] [CrossRef]
- Kazakis, N.; Karakatsanis, D.; Ntona, M.M.; Polydoropoulos, K.; Zavridou, E.; Voudouri, K.A.; Busico, G.; Kalaitzidou, K.; Patsialis, T.; Perdikaki, M.; et al. Groundwater Depletion. Are Environmentally Friendly Energy Recharge Dams a Solution? Water 2024, 16, 1541. [Google Scholar] [CrossRef]
- Shakeri, R.; Nassery, H.R.; Ebadi, T. Numerical modeling of groundwater flow and nitrate transport using MODFLOW and MT3DMS in the Karaj alluvial aquifer, Iran. Environ. Monit. Assess. 2023, 195, 242. [Google Scholar] [CrossRef] [PubMed]
- Hafen, K.C.; Wheaton, J.M.; Roper, B.B.; Bailey, P.; Macfarlane, W.W.; Neilson, B.T.; Tennant, C.J. Estimating Increased Tran-sient Water Storage with Increases in Beaver Dam Activity. Water 2024, 16, 1515. [Google Scholar] [CrossRef]
- Ncibi, K.; Mastrocicco, M.; Colombani, N.; Busico, G.; Hadji, R.; Hamed, Y.; Shuhab, K. Differentiating Nitrate Origins and Fate in a Semi-Arid Basin (Tunisia) via Geostatistical Analyses and Groundwater Modelling. Water 2022, 14, 4124. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General requirements for the competence of testing and calibration laboratories. International Organization for Standardization: Geneva, Switzerland, 2017.
- Regulation (EC) No 1774/2002 of the European Parliament and of the Council of 3 October 2002 Laying Down Health Rules Concerning Animal by-Products not Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32002R1774 (accessed on 21 June 2023).
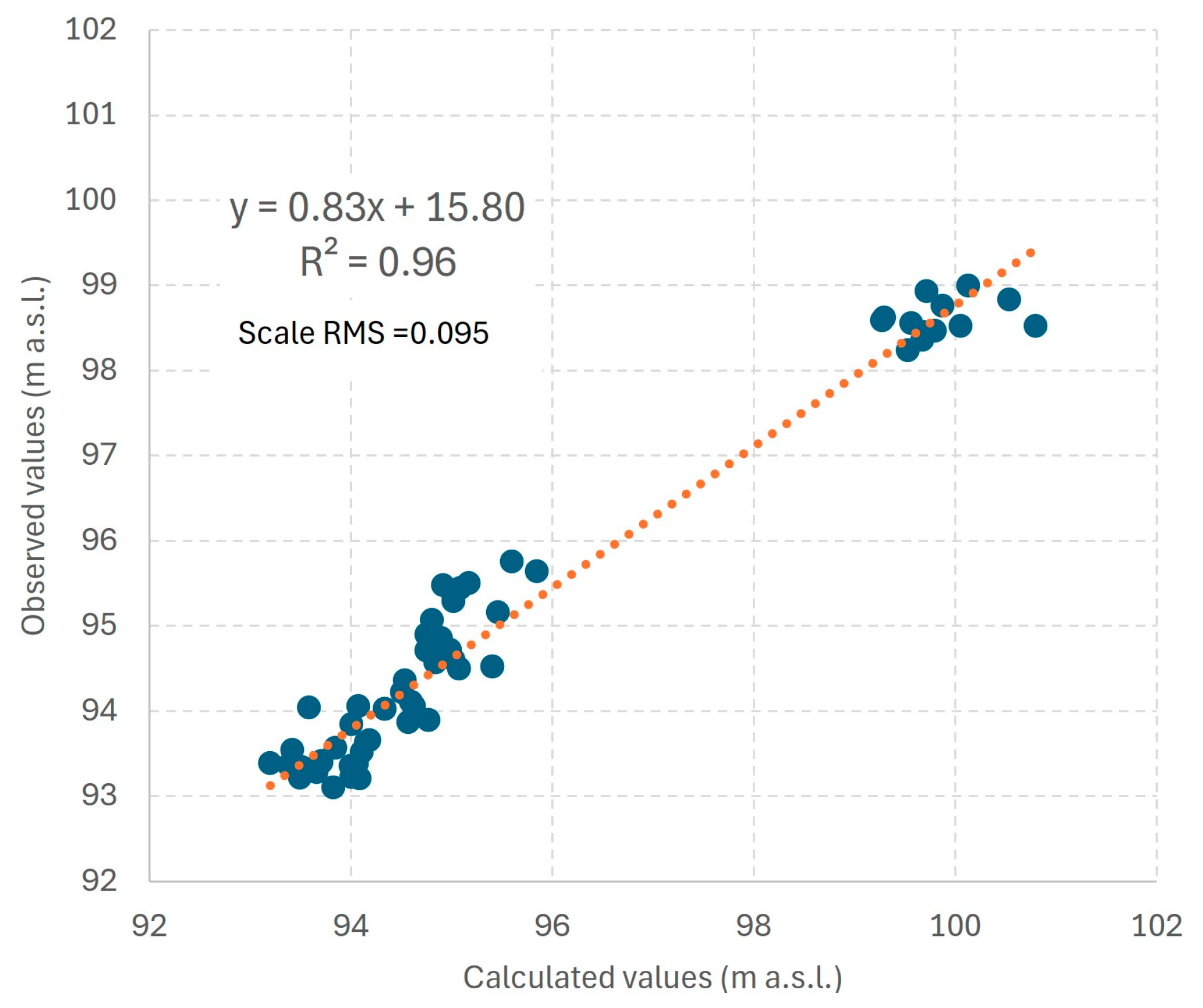

| Observed Value (m a.s.l.) | Model Value (m a.s.l.) | Observed Value (m a.s.l.) | Model Value (m a.s.l.) | Observed Value (m a.s.l.) | Model Value (m a.s.l.) |
|---|---|---|---|---|---|
| 93.66 | 93.28 | 100.80 | 98.52 | 94.08 | 94.06 |
| 93.83 | 93.10 | 99.69 | 98.45 | 93.71 | 93.41 |
| 93.39 | 93.36 | 99.68 | 98.36 | 94.00 | 93.36 |
| 94.11 | 93.52 | 100.06 | 98.52 | 94.19 | 93.65 |
| 93.54 | 93.27 | 95.09 | 95.44 | 94.75 | 94.71 |
| 93.50 | 93.21 | 94.92 | 95.48 | 94.54 | 94.36 |
| 93.71 | 93.39 | 95.60 | 95.75 | 94.84 | 94.57 |
| 94.90 | 94.86 | 95.85 | 95.64 | 94.63 | 94.06 |
| 95.02 | 94.60 | 95.02 | 95.35 | 94.34 | 94.02 |
| 94.75 | 94.90 | 95.02 | 95.28 | 94.60 | 94.10 |
| 95.46 | 95.16 | 95.17 | 95.50 | 94.77 | 93.89 |
| 94.81 | 95.07 | 93.67 | 93.35 | 94.51 | 94.22 |
| 94.79 | 94.77 | 94.09 | 93.21 | 95.08 | 94.50 |
| 94.99 | 94.71 | 93.42 | 93.54 | 95.41 | 94.52 |
| 99.72 | 98.93 | 94.01 | 93.84 | 94.84 | 94.57 |
| 99.88 | 98.75 | 93.59 | 94.04 | 94.63 | 94.06 |
| 100.13 | 98.99 | 93.20 | 93.38 | 94.34 | 94.02 |
| 99.80 | 98.46 | 93.52 | 93.34 | 94.60 | 94.10 |
| 99.30 | 98.62 | 94.15 | 93.63 | 94.77 | 93.89 |
| 99.28 | 98.59 | 94.06 | 93.37 | 94.51 | 94.22 |
| 99.53 | 98.23 | 94.01 | 93.22 | 95.08 | 94.50 |
| 99.57 | 98.55 | 93.85 | 93.56 | 95.41 | 94.52 |
| 100.54 | 98.83 | 94.57 | 93.87 |
| Parameter | Unit | No. * | Mean | Median | Std. Dev. | Min | Max | Percentiles | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||||||
| pH | 113 | 7.22 | 7.20 | 0.23 | 6.67 | 7.68 | 7.10 | 7.20 | 7.40 | |
| KMnO4 consumption | mg/L | 79 | 4.08 | 3.47 | 1.95 | 1.73 | 15.80 | 3.16 | 3.47 | 4.43 |
| NH4+ | mg/L | 102 | 0.04 | 0.03 | 0.21 | <0.02 | 2.10 | <0.02 | 0.03 | 0.03 |
| Cl− | mg/L | 86 | 24.35 | 22.25 | 8.36 | 8.04 | 42.00 | 18.93 | 22.25 | 30.05 |
| NO2− | mg/L | 86 | 0.01 | 0.002 | 0.04 | <0.002 | 0.34 | <0.002 | 0.002 | 0.003 |
| NO3− | mg/L | 199 | 53.72 | 48.00 | 28.16 | <0.05 | 128.10 | 33.20 | 48.00 | 72.00 |
| Ec | µS/cm | 168 | 780.42 | 781.00 | 139.81 | 254.00 | 1120.00 | 692.50 | 781.00 | 875.00 |
| Fetot | mg/L | 57 | 0.15 | 0.03 | 0.28 | <0.01 | 1.22 | 0.01 | 0.03 | 0.20 |
| Mntot | mg/L | 57 | 0.01 | <0.01 | 0.04 | <0.01 | 0.32 | <0.01 | <0.01 | 0.01 |
| SO42− | mg/L | 46 | 80.04 | 73.24 | 36.39 | 35.01 | 247.70 | 57.85 | 73.24 | 92.45 |
| Na+ | mg/L | 28 | 19.06 | 17.75 | 7.16 | 6.95 | 32.45 | 14.32 | 17.75 | 25.21 |
| B | µg/L | 17 | 64.26 | 71.60 | 23.35 | 23.45 | 110.84 | 49.75 | 71.60 | 78.65 |
| DO ** | mg/L | 18 | 3.90 | 3.15 | 1.70 | 1.87 | 7.62 | 2.68 | 3.15 | 5.31 |
| PCs | Factor Loadings | Initial Eigenvalues | Rotation Sums of Squared Loadings | |||||
|---|---|---|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | Cumulative % | |||
| PC1 | Cl | 0.991 | 5.67 | 63.01 | 63.01 | 5.64 | 62.66 | 62.66 |
| NO3− | 0.979 | |||||||
| Ec | 0.991 | |||||||
| SO42− | 0.975 | |||||||
| Na | 0.994 | |||||||
| B | 0.837 | |||||||
| PC2 | pH | 0.860 | 2.51 | 27.88 | 90.89 | 2.54 | 28.23 | 90.89 |
| KMnO4 | 0.934 | |||||||
| DO | 0.950 | |||||||
| Velika Morava—Velika Plana | Unit | No. * | Mean | Median | Std. Dev. | Min | Max | Percentiles | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 50 | 75 | ||||||||
| pH | 21 | 8.01 | 8.00 | 0.32 | 7.40 | 8.50 | 7.80 | 8.00 | 8.30 | |
| Ec | µS/cm | 34 | 441.50 | 447.00 | 55.63 | 315.00 | 596.00 | 400.50 | 447.00 | 468.75 |
| KMnO4 consumption | mg/L | 42 | 3.70 | 3.60 | 1.17 | 1.70 | 6.20 | 2.90 | 3.60 | 4.40 |
| NH4+ | mg/L | 37 | 0.09 | 0.08 | 0.05 | <0.001 | 0.20 | 0.07 | 0.08 | 0.11 |
| NO3− | mg/L | 42 | 6.71 | 6.65 | 2.85 | 1.28 | 13.16 | 4.42 | 6.65 | 8.86 |
| NO2− | mg/L | 32 | 0.005 | 0.000 | 0.011 | <0.003 | 0.039 | <0.003 | <0.003 | 0.003 |
| SO42− | mg/L | 16 | 23.69 | 23.00 | 4.96 | 14.00 | 34.00 | 20.25 | 23.00 | 26.00 |
| Cl− | mg/L | 16 | 9.00 | 9.05 | 2.69 | 4.10 | 15.00 | 8.00 | 9.05 | 10.75 |
| Na+ | mg/L | 16 | 11.02 | 11.00 | 2.06 | 7.40 | 15.60 | 9.50 | 11.00 | 12.28 |
| Fetot | µg/L | 14 | 159.67 | 188.50 | 126.82 | 0.05 | 406.00 | 0.19 | 188.50 | 227.75 |
| Mntot | µg/L | 14 | 44.86 | 40.50 | 50.36 | 0.01 | 200.00 | 0.04 | 40.50 | 53.75 |
| TN | mgN/L | 8 | 2.98 | 2.50 | 1.78 | 1.50 | 7.20 | 2.03 | 2.50 | 3.05 |
| DO | mg/L | 42 | 9.41 | 9.55 | 2.08 | 3.00 | 12.50 | 8.00 | 9.55 | 11.00 |
| Resava—Svilajnac | Unit | No. | Mean | Median | Std. Dev. | Min | Max | Percentiles | ||
| 25 | 50 | 75 | ||||||||
| pH | 58 | 8.17 | 8.30 | 0.27 | 7.60 | 8.50 | 7.98 | 8.30 | 8.33 | |
| Ec | µS/cm | 95 | 486.25 | 493.00 | 60.90 | 314.00 | 644.00 | 452.00 | 493.00 | 519.00 |
| KMnO4 consumption | mg/L | 100 | 2.60 | 2.30 | 1.03 | 0.80 | 5.80 | 1.90 | 2.30 | 3.00 |
| NH4+ | mg/L | 98 | 0.10 | 0.01 | 0.32 | 0.01 | 3.00 | 0.01 | 0.01 | 0.08 |
| NO3− | mg/L | 99 | 9.50 | 7.80 | 6.89 | 0.89 | 44.74 | 6.11 | 7.80 | 11.08 |
| NO2− | mg/L | 98 | 0.03 | 0.01 | 0.08 | <0.003 | 0.53 | 0.01 | 0.01 | 0.01 |
| SO42− | mg/L | 68 | 26.16 | 27.00 | 8.22 | 11.00 | 48.00 | 20.00 | 27.00 | 30.00 |
| Cl− | mg/L | 68 | 7.94 | 7.00 | 5.29 | 2.00 | 34.60 | 5.00 | 7.00 | 9.00 |
| Na+ | mg/L | 35 | 8.50 | 8.30 | 2.51 | 2.80 | 15.30 | 6.60 | 8.30 | 10.50 |
| Fetot | µg/L | 31 | 177.03 | 140.00 | 120.98 | 30.00 | 540.00 | 80.00 | 140.00 | 250.00 |
| Mntot | µg/L | 31 | 54.26 | 35.00 | 82.67 | 10.00 | 445.00 | 10.00 | 35.00 | 56.00 |
| TN | mgN/L | 23 | 3.61 | 2.60 | 2.38 | 1.20 | 10.30 | 2.10 | 2.60 | 4.60 |
| DO | mg/L | 100 | 10.42 | 10.10 | 2.00 | 4.80 | 15.10 | 9.13 | 10.10 | 12.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perović, M.; Zuber-Radenković, V.; Zorić, M. Feasibility of Groundwater Extraction in Nitrate-Impacted Groundwater Source in Serbia: Hydrodynamic Modeling and Nitrate Tracing. Water 2024, 16, 2105. https://doi.org/10.3390/w16152105
Perović M, Zuber-Radenković V, Zorić M. Feasibility of Groundwater Extraction in Nitrate-Impacted Groundwater Source in Serbia: Hydrodynamic Modeling and Nitrate Tracing. Water. 2024; 16(15):2105. https://doi.org/10.3390/w16152105
Chicago/Turabian StylePerović, Marija, Vesna Zuber-Radenković, and Miloš Zorić. 2024. "Feasibility of Groundwater Extraction in Nitrate-Impacted Groundwater Source in Serbia: Hydrodynamic Modeling and Nitrate Tracing" Water 16, no. 15: 2105. https://doi.org/10.3390/w16152105
APA StylePerović, M., Zuber-Radenković, V., & Zorić, M. (2024). Feasibility of Groundwater Extraction in Nitrate-Impacted Groundwater Source in Serbia: Hydrodynamic Modeling and Nitrate Tracing. Water, 16(15), 2105. https://doi.org/10.3390/w16152105







