Abstract
Arsenic (As) contamination is a severe problem in drinking-water sources. This study designed and investigated a novel combined electrocoagulation–filtration (ECF) system to investigate As treatment and filtration in drinking water in collaboration with HANDS-Pakistan and Medico International, Germany. Two separate pilot-scale ECF systems were designed and developed with an electrocoagulation (EC) unit and a commercially available PAUL® filter configured with vertical flat-sheet ultra-low-pressure membranes of 0.04 µm pore size for the combined treatment and filtration of different As concentrations. Real drinking water at different As concentrations, i.e., 100, 200, and 300 μg/L were tested on one ECF system with EC electrodes of iron (Fe) and another system with aluminum (Al), at different treatment times (0, 5, 10, 20, 45, 60, 120, 180 min), at a fixed current density (12 mA/cm2) and water flow rate of 1 L/min. The initial results showed 99% As removal within 5 min with the combined ECF treatment for both electrodes of Fe and Al. In addition, the effect of ECF on different water-quality parameters and the ionic interference on ECF performance and As filtration were analyzed. The results showed the promising potential of combined ECF treatment and filtration for treating and purifying As.
1. Introduction
The prevalence of arsenic (As) contamination in drinking-water sources is considered a major problem in developing countries, as it is found in natural water sources (ground and surface water), and trace amounts are a serious problem [1]. As contamination is mainly due to geogenic sources and anthropogenic activities, including the excessive use of fly ash disposal generated from incinerator plants, arsenical pesticides, untreated mine drainage, mining industries, etc., [2]. Due to improper treatment of industrial and domestic wastewater, solid waste effluent is directly discharged into water bodies and releases toxic metals, including As [3]. It was shown that As exists in four different oxidation forms, but that in natural water systems (ground and surface), arsenite (As(III)) and arsenate (As(V)) are the most abundant forms [4]. As (III) is considered more toxic than As(V) [5]. The presence of As traces in drinking water is one of the most serious threats to health, specifically for children [6,7]. Ground and surface water contamination also enters the food chain through irrigation activities due to the toxic effect of As-contaminated water, which may cause serious health issues such as cancer [8]. It is of prime importance to reduce the concentrations of As in potable water below the guideline value set by WHO for drinking water, which is 10 μg/L (ppb) for developed countries and 50 μg/L for developing countries [9]. As contamination in groundwater is a severe problem in Pakistan and other developing countries, especially in remote areas, where groundwater is considered the main source of drinking water and is highly contaminated by As and fluoride [10,11]. Furthermore, in these areas, electricity supply is a major problem, which reducing the capacity to run large drinking-water treatment plants.
Several methods have been introduced to remove As from contaminated water, including [12] ion exchange, coagulation and flocculation techniques, adsorption [13], membrane technologies, nanofiltration and reverse osmosis, and other techniques. However, while the methods can be used for As removal, they require additional costs. The most common method is reverse osmosis (RO) membrane treatment [14], which can remove 90% of As from groundwater. Furthermore, RO treatment produces rejected water, which is considered secondary waste and contains very toxic metals and other pollutants. In recent years, the electrocoagulation (EC) technique has received a great deal of attention for the removal of As from water sources because it is easy to operate, and no harmful chemicals are needed in the EC process [15,16].
The EC technique includes using electrodes, usually aluminum (Al), iron (Fe), carbon graphite etc., but the use of Fe and Al electrodes has been investigated and demonstrated for the efficient removal of metals. The EC process is a simple and low-cost treatment method for removing heavy metals (Ni, Cu, Zn, Cd, etc.) [17] and inorganic pollutants; however, the efficiency of EC is much higher than conventional chemical coagulation and other methods. During the EC process, low direct current (DC) is required to run the system, which can also be provided by solar panels [18,19]. The Fe and Al electrodes dissociate into ions at the anode, which split the hydrogen/hydroxyl ions in water and occur at the cathode side. However, after this, Al3+, Fe3+, H+, and OH− ions perform as electrocoagulants and react with dissolved ions, organics and metals to generate flocs, sediments, sludge, suspended solids, and other types of residues, requiring additional filtration steps. In most research studies on EC, the treatment procedures and handling methods of the generated sludge and suspended solids are scarcely studied.
German scientists (including one co-author, Franz-Bernd Frechen) from the University of Kassel have invented PAUL®, a ‘water backpack’ potable gravity-driven water filter, weighing around 23 Kg, with a height of 0.8 m and a water-filtration capacity of 1200 L/day (http://waterbackpack.org/). The PAUL filter has an outer casing made of high-density polyethylene (HDPE) plastic, while the inner-membrane module includes a cascade of 50 vertical flat sheets of ultra-low-pressure polymeric membranes (ULPM) with a pore size of around 0.04 µm (40 nm) and surface area of 10 m2, for the filtration of bacteria, viruses, turbidity, and suspended solids from water. As per data from the website (http://waterbackpack.org/), around 4218 PAUL filtration units are distributed worldwide in 91 countries, including Pakistan, for safe drinking-water provision to local communities.
Many studies have been conducted on As removal through the EC method, using Fe and Al electrodes [20,21,22]. However, few researchers have studied the combined electrocoagulation–filtration (ECF) system for removing heavy metals and wastewater treatment. Tang et al. [23] studied the EC coupled with a ceramic membrane filtration system for wastewater treatment. Similarly, Gong et al. [24] studied an ECF integrated system for treating polyaromatic hydrocarbon compounds (PAHs) and industrial wastewater that achieved 90% removal of PAH and TOC from wastewater. Furthermore, there are few studies in the literature on combined ECF systems for As removal. McBeath et al. [25] studied and investigated the combined EC with an oxidative media filter to remove manganese and As from groundwater. The combined system obtained a higher removal efficiency, with a final concentration of less than 0.1 μg/L.
The current project aims to investigate the treatment and filtration of As in drinking water through a combined ECF system in collaboration with HANDS-Pakistan and Medico International, Germany. This project introduced a two-stage treatment technology for As-contaminated water treatment, in which the first stage includes two EC units (one configured with an Fe electrode and second with an Al electrode), while the second stage includes the commercially available PAUL® filter (made in Germany and donated by Medico International, Germany) configured with a membrane module of vertical flat sheets of ULPM (for details: http://waterbackpack.org/). Initially, the synthetic As-contaminated water was prepared at 100–300 μg/L concentration in real drinking water and experiments were performed on both EC electrode configurations (Fe and Al). As removal efficiency was tested and evaluated at different concentrations (100, 200, and 300 μg/L); further investigations were made to understand the mechanism and interference between ions and As in real drinking water. Meanwhile, the process parameters of the system were varied for a better understanding of the system’s efficiency and kinetics. The project aimed to investigate the potential of the combined ECF treatment for As water purification in simulating real drinking-water filtration conditions for upscaling and commercialization opportunities to provide safe drinking water to As-contaminated regions.
2. Materials and Methods
2.1. Materials
All the analytical grade chemicals and As standard solutions (As2O3, with 1000 ppm As concentration) were purchased from Sigma Merk (Schnelldorf, Germany). The SPAND solution (for fluoride analysis) and sulfate and nitrate powder pillows (for sulfate and nitrate analysis) were purchased from HACH (Dusseldorf, Germany), respectively. Finally, sulfuric acid (H2SO4), sodium hydroxide (NaOH), nitric acid (HNO3) and (HCl) were purchased from Chemicals and Metals Co., Ltd. Daejung, (Daejung Chemicals & Metals Co., Ltd., Republic of Korea).
2.2. Design and Fabrication of Combined ECF Systems
Two pilot-scale combined ECF systems with 200 L capacity were designed and developed, as shown in a schematic and digital image in Figure 1. Both ECF systems consist of three main components: (a) a feeding tank with the capacity to feed and store 200 L of water; (b) an EC reactor/unit; and (c) a PAUL filter (PAUL, WaterBackPack Company GmbH, Kassel, Germany). The only difference between both ECFs was of different EC electrode configurations in the EC unit. One ECF system is configured with twenty Al (96.3% purity) EC electrodes and another with twenty Fe (93.4% purity) electrodes. Each Al and Fe electrode has dimensions of length (40.6 cm) × width (20.2 cm) × height (40.6 cm), and each was spaced 1 cm apart from the other electrode inside a rectangle acrylic tank with a size of 200 L. During the ECF experiments, all the electrodes were vertically positioned and dipped in the As-contaminated water. All electrodes in both ECF systems were separately connected to a direct current (DC) power supply (model SPS-3010, China) with a controlling system, and the constant current was supplied to the electrode for all experiments. Before starting the experiments, the electrodes were washed with MilliQ water, dried at room temperature, and attached to the filtration system (c).
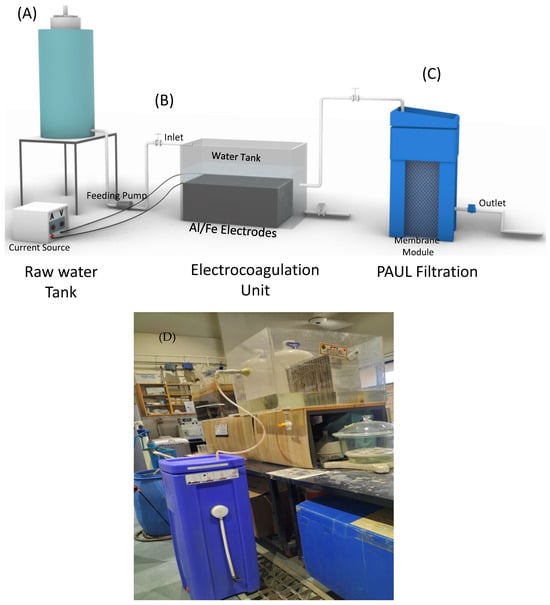
Figure 1.
Schematic and digital image of the combined ECF system: (A) raw feeding tank with the capacity to store 200 L; (B) EC unit consisting of the EC electrodes inside a water tank with a volume of 200 L; (C) PAUL filtration unit; and (D) digital image of the complete pilot ECF system with Fe and Al electrodes EC units and PAUL filters.
2.3. Experimental Set-Up and Procedures
The As filtration experiments were performed on the pilot-scale ECF system using separate electrode configurations, i.e., Al and Fe electrodes. The raw water was initially synthesized by diluting the As standard solution in real drinking water to obtain the required concentrations (100, 200, and 300 μg/L). The drinking water used in the experiments was collected in bulk from a tap available at this location (GPS coordinates: 25.401845 latitude, 68.256627 longitude).
Initially, the EC reactors were washed three times with MilliQ water and dried. Then, the As-contaminated water was pumped through the pump (Peristaltic Pump, High-Quality DC 12V, Shanghai GL Environmental Technology Co., Ltd., Shanghai, China) at a fixed flow rate (1 L/min) into the EC reactor. Meanwhile, the outlet point was connected to the PAUL filter. All the combined ECF experiments were performed with a constant electrode current density of 12 mA/cm2. Finally, the 500 mL samples were collected from the outlet of the PAUL filter and analyzed through physio-chemical parameters.
Real drinking-water ECF experiments were also performed to see the effect of interfering ions (sulfates (SO42−), fluoride (F−), and nitrate (NO3−)) and metals (Fe+, Mg+, Ca+, K+) during the ECF treatment. Furthermore, changes in pH, electrical conductivity (EC), and turbidity were investigated before and after treatment. However, only two operating parameter changes in As concentrations (100, 200, and 300 μg/L) and the type of electrode (Fe and Al) were selected for this study to check the efficiency of the combined ECF system. After each run, the EC reactor and electrode were washed thrice, and then the As concentration was changed in the feed water.
The analyzed drinking-water-quality results are given in Table 1, showing the values of analyzed parameters.

Table 1.
Water-quality analysis of experimented real drinking-water sample.
2.4. Analytical Methods
To analyze the changes in the As concentration, i.e., after the combined ECF treatment, the water samples were collected at the outlet of the PAUL filter at ECF treatment times of 0, 5, 10, 20, 45, 60, 120 and 180 min. In addition, the ECF treatment-induced changes in pH were analyzed through a portable pH meter Hanna H18424 (Hanna Instruments, Vöhringen, Germany), electrical conductivity (EC) was analyzed through a portable meter Hanna Hi99301 (Hanna Instruments), and turbidity was analyzed through a portable meter, Hanna 9844, (Hanna Instruments) [26]. Furthermore, chloride, fluoride, and nitrate concentrations were analyzed through a UV–visible spectrophotometer (Shimadzu, 1800, Tokyo, Japan), followed by standard procedures and methods by the APHA (American Public Health Association).
The As and other elemental (Mg+, Ca+, K+) analyses were performed through an inductively coupled plasma mass spectrometer (ICP-MS NexION 350, by PerkinElmer, Shelton, CT, USA). The samples collected before and after treatment were analyzed in replicates, and plots were generated after taking their average values.
3. Results
The As removal efficiency of the combined ECF in two electrode configurations, As concentrations and treatment times, but at a fixed flow rate and electrode current density. Figure 2A,B shows the removal efficiency of As concentrations of 100, 200 and 300 μg/L on Al and Fe electrodes. A 99% As removal rate was achieved within 5 min of the ECF treatment. Both Fe and Al electrodes’ performance remained consistent, and further maximum As removal was achieved quickly after the 5 min treatment and remained unchanged at longer ECF treatments. Afterward, the experiments were performed at a neutral pH (no addition of acid/base to change pH). Generally, the pH of drinking water (surface and ground) is in the range of 5 to 8.5, depending on the conditions and characteristics of the water [27]. The maximum As removal (99%) was achieved at the solution’s natural/slightly basic pH, as shown in Figure 2. The As removal rate was higher at the beginning of the EC treatment. The sharp reduction in As concentration was observed within 5 min, and then the removal continued at a constant value with time.
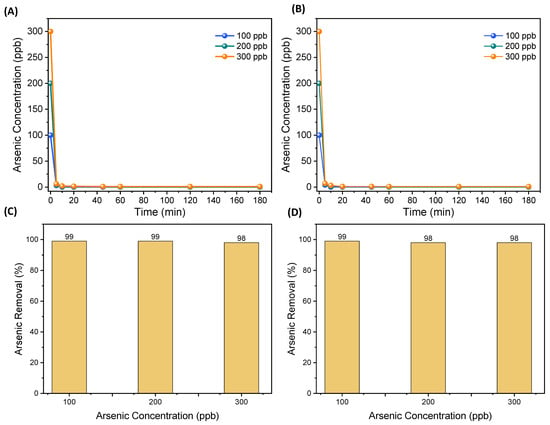
Figure 2.
As removal at different concentrations: (A) removal through Al electrode with fixed flow rate at neutral pH of water samples; (B) removal through Fe electrode with fixed flow rate at neutral pH of water samples; and (C,D) overall removal of As at different concentrations.
3.1. pH, EC and Turbidity Changes after ECF Treatment
Figure 3A,B shows the changes in pH and conductivity after the ECF treatment at different times. A slight increase in pH values was observed after the 5 min treatment, which was stabilized after 60 min. However, the obtained final pH of the water was within the WHO (World Health Organization) permissible limit, i.e., 8.5. This change in pH could be due to the formation of Al and Fe hydroxyl radicals from the electrode, Al (OH)3 and Fe(OH)2 compounds, and other OH− compounds of ionic species present in water. A study by Das et al. [28] investigated the effect of pH changes on As removal, and they observed that lower As reduction was recorded in alkaline conditions and maximum As removal occurred in natural and acidic conditions. However, in alkaline conditions, the solution produced more OH− that may interact with the Fe2+ and Al2+ and form their hydroxides, slowing down the EC process of transforming other ions and the EC of metal species. Kobya et al. [21] studied the effects of pH using Fe and Al electrodes and observed a higher reaction rate with a pH range of 6.5–8.5 using Fe electrodes. Furthermore, most studies suggest the removal rate was better when pH ≥ 6.5 [29]. The pH ranged from 7.1 to 8.2 in our drinking-water sample. However, the pH value slightly increased during treatment and stabilized after 60 min, as shown in Figure 3B. This increase in pH is related to the release of OH− radicals and the resulting alkaline Al (OH)3 and Fe(OH)2 flocs after the EC process in both EC electrode configurations, i.e., Al and Fe.
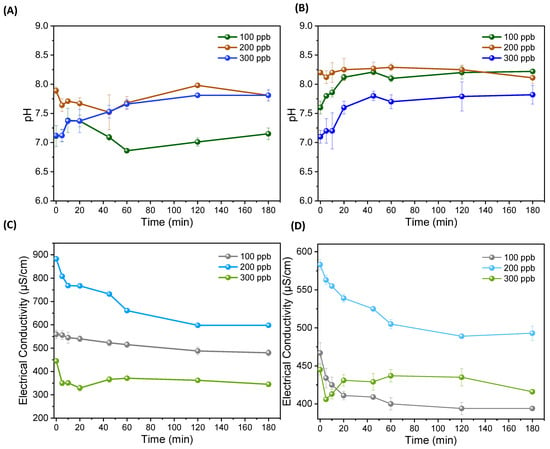
Figure 3.
pH changes after treatment: (A) treatment through Al electrode with fixed flow rate at neutral pH of water samples; (B) treatment through Fe electrode with fixed flow rate at neutral pH of water samples; (C) changes in electrical conductivity after treatment at different treatment time using Al electrode; and (D) changes in electrical conductivity after treatment at different treatment time using Fe electrode.
Furthermore, after the ECF treatment, the electrical conductivity also decreased with both electrode configurations due to chemical conversion and the reaction of dissolved ions, metals organic and inorganic species with the released ions of Fe and Al from the EC electrode and formed radicals of OH− in the water [30]. The results for both electrodes suggested a high decrease (up to 30%) in conductivity values during the initial 20 min treatment and a later slight decrease in conductivity values at longer ECF treatments. An earlier study [31] of electrical conductivity and TDS stated that their concentrations decreased due to precipitation and EC of dissolved ions and metals. Furthermore, EC treatment directly influences the conductivity concentration in water and depends on the quality of water, as the groundwater contains high conductivity and could affect the EC process and require more energy. On the other hand, it could be more beneficial for metals removal due to the higher formation of total suspended solids (TSS) [32] that could adsorb more pollutants.
The results showed turbidity (at the outlet of the PAUL filter) for all concentrations after 10 min for all experiments for both Fe and Al electrodes and different ECF treatment times (Figure 4A,B). The decrease in turbidity was due to the EC effect of the generated Al+ and Fe+ ions and OH− radicals that stabilized the turbid and colloidal particles.
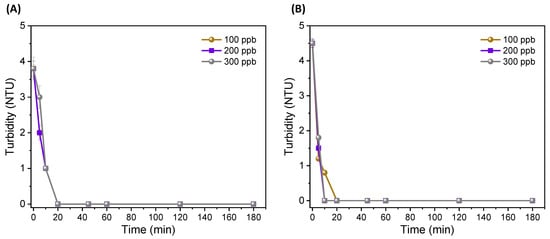
Figure 4.
Turbidity changes after treatment: (A) through Al electrode; and (B) Fe electrode.
Here, the role of the PAUL filter and ULPM sheets are important because their integration with the EC process was specifically aimed to filter and purify any flocs, suspended solids and residues resulting from the EC process. The water samples after the EC process (before the PAUL filter) showed high turbidity values for both EC electrode configurations, i.e., ranging between 8–13 NTU, which confirmed that the EC process may increase the turbidity index in continuous treatment mode. However, after the EC process/treatment, the suspension could result in the settling and sedimentation of large flocs as sludge in the bottom of the EC tank (shown in the scheme and digital images in Figure 1) and low turbidity values in the range of 3–4 NTU (measured after around 1 h of the EC process and settling of suspended flocs and residues). Moreover, the results in Figure 4 show that with combined and continuous ECF treatment, the suspended flocs and residue (resulting from the EC process) filtration could be achieved to 0 NTU turbidity right after the continuous EC process.
3.2. Effect on Sulfate and Fluoride Removal
As WHO suggested, fluoride concentrations are considered important for drinking; the threshold limit in drinking water should be between (0.5–1.5 mg/L) [33]. Generally, in surface water, fluoride concentrations are lower than in groundwater, for various reasons. Hence, treated drinking-water samples must have fluoride concentrations for dental growth. Based on the results, it was observed that both fluoride and sulfate concentrations were reduced after the ECF treatment. Almost 100% fluoride reduction was recorded, and less reduction was observed in sulfate. After 60 min, the values stabilized, as shown in Figure 5A–D. During the EC process, the release of Al and Fe ions and counter production of OH− radicals could have reacted with the sulfate and fluoride ions to produce their precipitates or non-soluble compounds completely filtered by PAUL filtration [34]. Furthermore, few studies [15,16] investigated removal efficacy through the EC process. However, drinking-water samples used in the experiments contained low nitrate concentrations, and due to low concentrations, nitrate removal was observed in 180 min, as shown in Figure 5E,F. However, previous studies investigated nitrate removal from water [35,36].
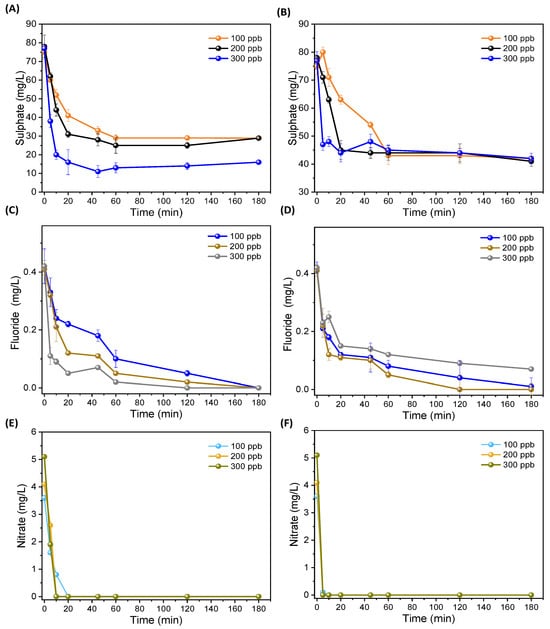
Figure 5.
Fluoride, nitrate, and sulfate concentration changes after treatment at: (A) sulfate changes after treatment at different treatment times through Al electrode; (B) sulfate changes after treatment at different treatment times through Fe electrode; (C) fluoride changes after treatment at different treatment time through Al electrode; (D) fluoride changes after treatment at different treatment times through Fe electrode; (E) nitrate changes after treatment at different treatment times through Al electrode; and (F) nitrate changes after treatment at different treatment times through Fe electrode.
3.3. Effect of ECF Treatment on Interfering Ions
As the As solution was prepared from real tap water, in which the interfering ions naturally existed, and earlier discussed results shown in Figure 2A,B showed effective and rapid treatment and filtration of As, even in the presence of interfering ions, this section includes the results of ECF treatment on the commonly found ions in water, i.e., magnesium, calcium, and potassium ions. Figure 6 shows the change in concentrations in elements/ions for both EC electrode configurations. It was observed that the release of Al and Fe ions from the EC electrode and targeting As was also sufficient to mitigate their capturing and/or bonding with other dissolved ions in the water.
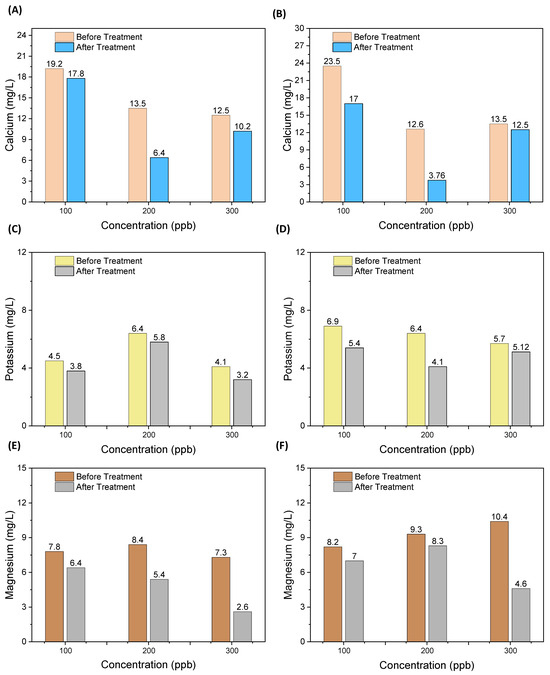
Figure 6.
Ions (calcium, magnesium, and potassium) changing during EC treatment at (A,C,E) after treatment through the Al electrode; and (B,D,F) after treatment through the Fe electrode.
Along with the As removal, the concentrations of all the identified ions, i.e., magnesium, calcium, and potassium ions (Figure 6), were slightly decreased, and there was a minimal effect of these ionic species on the EC process. For all the experimented concentrations of As (100, 200 and 300 μg/L), magnesium, calcium, and potassium ion concentrations decreased between 8 to 70% from the initial concentrations for both EC electrode configurations. The decrease in the concentrations of the identified ions appeared random due to the uncontrolled release of Al and Fe ions and other radicals and their bonding with the dissolved ions and As. The results in Figure 6 show that the calcium and magnesium ion concentrations were observed to decrease, i.e., due to bonding with OH− and other ions, such as SO3−, and converted into flocs, residues and other suspended forms (filtered out from PAUL). Commonly, ionic interference affects the EC process due to the capture of the resulting EC ions in bonding and compounding with the interfering ions, i.e., other than the targeted metals like As in our case.
During the EC process, metallic hydroxide forms are combined with magnesium, calcium, and potassium salts and accumulate at the cathode. In our case, a lower reduction was observed during treatment due to the low current density provided. Medina-Collana et al. [37] investigated the effect of electrical potential (V) on calcium hardness removal (8–13%) over 60 min, and observed that due to higher electrical potential, the OH− flocs increased, resulting in the adsorption of magnesium, calcium, and potassium.
4. Discussion
During the EC process, several reactions are involved in the EC reactor. When DC is applied to the electrode, the dissociation occurs at the anode, which releases coagulant ionic species reacting with the water and dissolved and undissolved species in the water, whereas hydrogen is produced at the cathode [38,39]. The (Al3+) and (Fe2+) metallic ions react with the hydroxide to form Al(OH)3 and Fe(OH)2, and the pH of the water solution increases towards the alkaline side. The possible EC reactions and mechanism of their interaction with As are shown in Figure 7 and Equations (1)–(10). During the EC process, for the treatment of As, the dissolved form of As probably reacted with released ions Fe and Al in both electrode configurations and could have formed arsenate and/or arsenite that could have adsorbed and accumulated on the hydroxides of (Fe) and (Al) and residues [40].
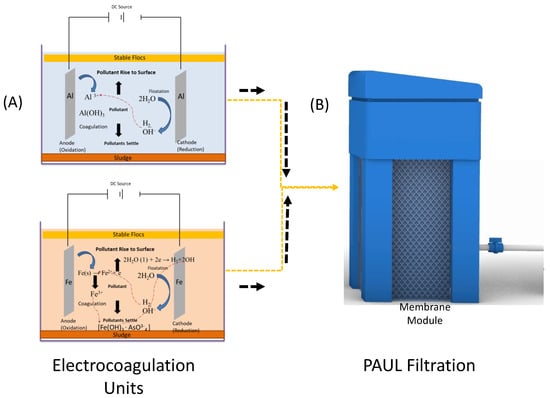
Figure 7.
Mechanism of combined ECF treatment: (A) with Al electrode and Fe electrode; and (B) PAUL filtration unit.
Main anodic reactions with Fe or Al electrode: First, oxidation of Fe to ferrous ion occurs, and, depending on the anode potential, its subsequent oxidation to Fe ion may occur, as shown in the following equations [22,28,41,42]. With the Fe electrode configuration at the anode side of the EC system, the reactions (Equations (1)–(6)) occurred to initiate the EC process and removal of As and other dissolved ionic, organic, inorganic and elemental species [22,43], as follows:
Fe → Fe2+ +2e−
Cathodic reactions:
8H + 8e → 4H2
2H2O + 2e− → H2 + 2OH−
The possible bulk reactions are as follows:
2Fe2+ + 4H2O + O2 → 2Fe(OH)3+ H2
4Fe2+ + 10H2O + O2 → 4Fe(OH)3+ 8H2
Fe2+ + 2OH− → Fe(OH)2
The Fe(OH) flocs formed during the EC process trap the As ions in the water solution by absorption and precipitation, then settle down due to coagulation, as follows:
Fe(OH) + AsO3−4 → [Fe(OH)3·AsO3−4]
Similarly, the following reactions occurring as a result of the Al electrode are as follows:
At the anode:
Al → Al3+ +3e−
At the cathode, the reactions are as follows:
2H2O + 2e− → H2 + 2OH−
Afterwards, the Al3+ combined with (OH−) to form the following:
Al3+ + 3H2O → Al(OH)3 + 3H+
After the EC process, the integrated ULPM sheets inside the PAUL filter control and purify all the flocs, suspended solids, and residues resulting from the EC process. The changes in pH (increased), conductivity (slightly decreased), and TDS (slightly decreased) in the treated and filtered water are mainly related to the effect of the EC process, while the decrease in TSS and turbidity values are mainly associated with ULPM-assisted filtration in continuous mode.
5. Conclusions
This study investigated and revealed the performance of the combined ECF system for the continuous EC treatment and PAUL membrane filtration-assisted removal and purification of As and other ionic species present in real drinking-water sources. Experiments were performed at different As concentrations, two EC electrode configurations (Al and Fe), and a fixed water flow rate and electrode current density. Different As concentrations (100, 200, and 300 μg/L) were almost treated and filtered at the combined ECF treatment of 5 min, and their concentrations remained unchanged for longer ECF treatments, i.e., up to 180 min. The maximum As removal was observed in both electrode configurations and in a very short time of treatment, i.e., 5 min. The effect of ECF treatment on the pH and EC indicated an increase in pH values due to the formation of hydroxides of Al and Fe, while a 30% decrease in EC values and a non-significant change in the turbidity levels of the ECF-treated water, i.e., compared to raw water before ECF treatment. Further, the results of other elemental/ionic species in the experimented water, i.e., F−, SO42−, NO3−, Ca+, Mg+, Na+ and K+, showed a decrease in their values and a minimum effect of their interference on As removal.
The treatment cost of As-contaminated drinking water depends on the electrode types and their sacrificial characteristics (ionic leaching) and EC system power consumption. Using the reported ECF system, the estimated treatment and filtration cost for the Al electrode configuration is around 0.795$/200 L, and for the Fe electrode is around 0.223$/200 L. The difference in the cost is due to the lower cost of Fe electrodes compared to Al. However, these cost estimations need to be validated on longer-run operation and maintenance and field testing of the ECF system, which is the aim of the next phase of this project.
Overall, the results suggested that the combined ECF treatment appeared promising for As-contaminated water, providing rapid and reliable filtration. Moreover, to understand the challenges and mechanism of this combined ECF system and potential challenges observed during the upscaling, more research is required for bulk water treatment and the sludge handling and disposal generated from the ECF treatment.
Author Contributions
Conceptualization, F.-B.F., S.W., R.B.M. and T.A.G.; methodology, T.A.G., R.B.M., F.-B.F., B.B.,T.A.G., I.A. and N.C.; validation, F.-B.F., S.W., R.B.M., B.B. and T.A.G.; formal analysis, T.A.G., N.C., I.A. and S.S.; investigation, F.-B.F., S.W., R.B.M. and T.A.G.; resources, F.-B.F., S.W., R.B.M. and T.A.G.; data curation, T.A.G., N.C., I.A., F.S.F. and S.S.; writing—original draft preparation, T.A.G. and N.C.; writing—review and editing, R.B.M., B.B. and F.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by Medico International, Germany and HANDS-Pakistan, and the electrocoagulation system and PAUL filtration units were donated by Medico International, Germany.
Data Availability Statement
Data are included in the manuscript.
Acknowledgments
The authors acknowledge the technical and administrative support of the HANDS-Pakistan and Medico International, Germany. In addition, the authors acknowledge the support of Shaikh Tanveer Ahmed, HANDS-Pakistan, Naeem Memon, Head of Department, HANDS-Pakistan and Mir Keerio, representative and project collaborator from HANDS-Pakistan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Can, B.Z.; Boncukcuoglu, R.; Yilmaz, A.E.; Fil, B.A. Effect of Some Operational Parameters on the Arsenic Removal by Electrocoagulation Using Iron Electrode. J. Environ. Health Sci. Eng. 2014, 12, 95. [Google Scholar] [CrossRef]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A Review on Decontamination of Arsenic-Contained Water by Electrocoagulation: Reactor Configurations and Operating Cost along with Removal Mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air Soil. Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Shafiquzzaman, M.; Azam, M.S.; Nakajima, J.; Bari, Q.H. Investigation of Arsenic Removal Performance by a Simple Iron Removal Ceramic Filter in Rural Households of Bangladesh. Desalination 2011, 265, 60–66. [Google Scholar] [CrossRef]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H. Effect of Redox Potential and PH on Arsenic Speciation and Solubility in a Contaminated Soil. Environ. Sci. Technol. 1991, 25, 1414–1419. [Google Scholar] [CrossRef]
- Wasserman, G.A.; Liu, X.; LoIacono, N.J.; Kline, J.; Factor-Litvak, P.; Van Geen, A.; Mey, J.L.; Levy, D.; Abramson, R.; Schwartz, A.; et al. A Cross-Sectional Study of Well Water Arsenic and Child IQ in Maine Schoolchildren. Environ. Health 2014, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Asadullah, M.N.; Chaudhury, N. Poisoning the Mind: Arsenic Contamination of Drinking Water Wells and Children’s Educational Achievement in Rural Bangladesh. Econ. Educ. Rev. 2011, 30, 873–888. [Google Scholar] [CrossRef]
- Demissie, S.; Mekonen, S.; Awoke, T.; Teshome, B.; Mengistie, B. Examining Carcinogenic and Noncarcinogenic Health Risks Related to Arsenic Exposure in Ethiopia: A Longitudinal Study. Toxicol. Rep. 2024, 12, 100–110. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A Meta-Analysis of the Distribution, Sources and Health Risks of Arsenic-Contaminated Groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319. [Google Scholar] [CrossRef]
- Uddin, M.M.; Harun-Ar-Rashid, A.K.M.; Hossain, S.M.; Hafiz, M.A.; Nahar, K.; Mubin, S.H. Slow Arsenic Poisoning of the Contaminated Groundwater Users. Int. J. Environ. Sci. Technol. 2006, 3, 447–453. [Google Scholar]
- Chiavola, A.; D’Amato, E.; Gavasci, R.; Sirini, P. Arsenic Removal from Groundwater by Ion Exchange and Adsorption Processes: Comparison of Two Different Materials. Water Sci. Technol. Water Supply 2015, 15, 981–989. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic Removal Fromwater by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef]
- Pezeshki, H.; Hashemi, M.; Rajabi, S. Removal of Arsenic as a Potentially Toxic Element from Drinking Water by Filtration: A Mini Review of Nanofiltration and Reverse Osmosis Techniques. Heliyon 2023, 9, e14246. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Alavi Moghaddam, M.R.; Arami, M. Optimization of Acid Black 172 Decolorization by Electrocoagulation Using Response Surface Methodology. Iran. J. Environ. Health Sci. Eng. 2012, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Shahedi, A.; Darban, A.K.; Jamshidi-Zanjani, A.; Homaee, M. An Overview of the Application of Electrocoagulation for Mine Wastewater Treatment. Environ. Monit. Assess. 2023, 195, 522. [Google Scholar] [CrossRef] [PubMed]
- Mehri, M.; Fallah, N.; Nasernejad, B. Mechanisms of Heavy Metal and Oil Removal from Synthetic Saline Oilfield Produced Water by Electrocoagulation. npj Clean. Water 2021, 4, 45. [Google Scholar] [CrossRef]
- Nguyen, T.T.Q.; Loganathan, P.; Dinh, B.K.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing Arsenate from Water Using Batch and Continuous-Flow Electrocoagulation with Diverse Power Sources. J. Water Process Eng. 2021, 41, 102028. [Google Scholar] [CrossRef]
- Oh, C.; Pak, S.; Han, Y.S.; Ha, N.T.H.; Hong, M.; Ji, S. Field Demonstration of Solar-Powered Electrocoagulation Water Treatment System for Purifying Groundwater Contaminated by Both Total Coliforms and Arsenic. Environ. Technol. 2021, 42, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.R.; Chaudhari, S.; Khilar, K.C.; Mahajan, S.P. Removal of Arsenic from Water by Electrocoagulation. Chemosphere 2004, 55, 1245–1252. [Google Scholar] [CrossRef]
- Kobya, M.; Gebologlu, U.; Ulu, F.; Oncel, S.; Demirbas, E. Removal of Arsenic from Drinking Water by the Electrocoagulation Using Fe and Al Electrode. Electrochim. Acta 2011, 56, 5060–5070. [Google Scholar] [CrossRef]
- Montefalcon, M.F.V.; Chiong, M.R.; Resurreccion, A.C.; Garcia-Segura, S.; Ocon, J.D. Arsenic Removal by Advanced Electrocoagulation Processes: The Role of Oxidants Generated and Kinetic Modeling. Catalysts 2020, 10, 928. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, C.; Quan, B.; Tang, Y.; Zhang, Y.; Su, C.; Zhao, G. Electrocoagulation Coupled with Conductive Ceramic Membrane Filtration for Wastewater Treatment: Toward Membrane Modification, Characterization, and Application. Water Res. 2022, 220, 118612. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Huang, H.; Qian, Y.; Zhang, Z.; Wu, H. Integrated Electrocoagulation and Membrane Filtration for PAH Removal from Realistic Industrial Wastewater: Effectiveness and Mechanisms. RSC Adv. 2017, 7, 52366–52374. [Google Scholar] [CrossRef]
- McBeath, S.T.; Hajimalayeri, A.; Jasim, S.Y.; Mohseni, M. Coupled Electrocoagulation and Oxidative Media Filtration for the Removal of Manganese and Arsenic from a Raw Ground Water Supply. J. Water Process Eng. 2021, 40, 101983. [Google Scholar] [CrossRef]
- Ahmed, J.; Wong, L.P.; Chua, Y.P.; Channa, N. Drinking Water Quality Mapping Using Water Quality Index and Geospatial Analysis in Primary Schools of Pakistan. Water 2020, 12, 3382. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Vo, L.D.; Nguyen, T.X.; Quang, N.X. The Interactive Effects of Natural Factor and Pollution Source on Surface Water Quality in the Lower Mekong River Basin, Southwestern Vietnam. Water Resour. 2020, 47, 865–876. [Google Scholar]
- Das, D.; Nandi, B.K. Arsenic Removal from Tap Water by Electrocoagulation: Investigation of Process Parameters, Kinetic Analysis, and Operating Cost. J. Dispers. Sci. Technol. 2021, 42, 328–337. [Google Scholar] [CrossRef]
- Ansari, K.; Shrikhande, A.; Malik, M.A.; Alahmadi, A.A.; Alwetaishi, M.; Alzaed, A.N.; Elbeltagi, A. Optimization and Operational Analysis of Domestic Greywater Treatment by Electrocoagulation Filtration Using Response Surface Methodology. Sustainability 2022, 14, 15230. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Anji Reddy, M. A Comparative Study of the Efficiency of Chemical Coagulation and Electrocoagulation Methods in the Treatment of Pharmaceutical Effluent. J. Water Process Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Mendez-Ruiz, J.I.; Medina-Toala, A.N.; Gutierrez, L.; Valverde-Armas, P.E. Comparative Evaluation of an Advanced Electrocoagulation Treatment System versus a Conventional Lime Softening Treatment for Removing Ca2+, SO42−, and Mn in Groundwater. Case Stud. Chem. Environ. Eng. 2023, 8, 100448. [Google Scholar] [CrossRef]
- Ahangarnokolaei, M.A.; Ganjidoust, H.; Ayati, B. Optimization of Parameters of Electrocoagulation/Flotation Process for Removal of Acid Red 14 with Mesh Stainless Steel Electrode. J. Water Reuse Desalination 2018, 8, 278–292. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride Contamination, Consequences and Removal Techniques in Water: A Review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Sharma, A.K.; Chopra, A.K. Removal of Nitrate and Sulphate from Biologically Treated Municipal Wastewater by Electrocoagulation. Appl. Water Sci. 2017, 7, 1239–1246. [Google Scholar] [CrossRef]
- Malakootian, M.; Yousefi, N.; Fatehizadeh, A. Survey Efficiency of Electrocoagulation on Nitrate Removal from Aqueous Solution. Int. J. Environ. Sci. Technol. 2011, 8, 107–114. [Google Scholar]
- Amarine, M.; Lekhlif, B.; Sinan, M.; El Rharras, A.; Echaabi, J. Treatment of Nitrate-Rich Groundwater Using Electrocoagulation with Aluminum Anodes. Groundw. Sustain. Dev. 2020, 11, 100371. [Google Scholar] [CrossRef]
- Medina-Collana, J.T.; Reyna-Mendoza, G.E.; Montaño-Pisfil, J.A.; Rosales-Huamani, J.A.; Franco-Gonzales, E.J.; Córdova García, X. Evaluation of the Performance of the Electrocoagulation Process for the Removal of Water Hardness. Sustainability 2022, 15, 590. [Google Scholar] [CrossRef]
- Almukdad, A.; Hawari, A.H.; Hafiz, M. An Enhanced Electrocoagulation Process for the Removal of Fe and Mn from Municipal Wastewater Using Dielectrophoresis (DEP). Water 2021, 13, 485. [Google Scholar] [CrossRef]
- Müller, D.; Nina Stirn, C.; Veit Maier, M. Arsenic Removal from Highly Contaminated Groundwater by Iron Electrocoagulation—Investigation of Process Parameters and Iron Dosage Calculation. Water 2021, 13, 687. [Google Scholar] [CrossRef]
- Khan, S.U.; Farooqi, I.H.; Usman, M.; Basheer, F. Energy Efficient Rapid Removal of Arsenic in an Electrocoagulation Reactor with Hybrid Fe/Al Electrode: Process Optimization Using CCD and Kinetic Modeling. Water 2020, 12, 2876. [Google Scholar] [CrossRef]
- Song, P.; Yang, Z.; Zeng, G.; Yang, X.; Xu, H.; Wang, L.; Xu, R.; Xiong, W.; Ahmad, K. Electrocoagulation Treatment of Arsenic in Wastewaters: A Comprehensive Review. Chem. Eng. J. 2017, 317, 707–725. [Google Scholar] [CrossRef]
- Gilhotra, V.; Das, L.; Sharma, A.; Kang, T.S.; Singh, P.; Dhuria, R.S.; Bhatti, M.S. Electrocoagulation Technology for High Strength Arsenic Wastewater: Process Optimization and Mechanistic Study. J. Clean. Prod. 2018, 198, 693–703. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Singh, T.S.A. Arsenic Removal by Electrocoagulation Process: Recent Trends and Removal Mechanism. Chemosphere 2017, 181, 418–432. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).