Investigating Algal Sensor Utilization Methods for Three-Dimensional Algal Control Technology Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Control Assessment Analysis in Relation to Pigments
2.2. Field Sampling
2.3. Laboratory Experiments
2.4. Three-Dimensional Algal Concentration Analysis along a Depth Gradient
2.5. Statistical Analysis
3. Results
3.1. Examination of Sensor Utilization Possibilities
3.2. Analysis of the Spatial Effects of the Algal Fences
3.3. Estimation of Algae Distribution by Water Depth
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graham, L.E.; Graham, J.E.; Wilcox, L.W. Algae, 2nd ed.; Pearson Education: London, UK, 2009. [Google Scholar]
- Brient, L.; Lengronne, M.; Bertrand, E.; Rolland, D.; Sipel, A.; Steinmann, D.; Baudin, I.; Legeas, M.; Le Rouzic, B.; Bormans, M.; et al. A phycocyanin probe as a tool for monitoring cyanobacteria in freshwater bodies. J. Environ. Monit. 2008, 10, 248–255. [Google Scholar] [CrossRef]
- Mohamed, H.I.; El-Beltagi, H.E.-D.S.; Abd-Elsalam, K.A. Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Kang, J.-J.; Min, J.-O.; Kim, Y.; Lee, C.-H.; Yoo, H.; Jang, H.-K.; Kim, M.-J.; Oh, H.-J.; Lee, S.-H.; Oh, H.-J.; et al. Vertical distribution of phytoplankton community and pigment production in the Yellow Sea and the East China sea during the late summer season. Water 2021, 13, 3321. [Google Scholar] [CrossRef]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef]
- Xu, H.; Tan, X.; Liang, J.; Cui, Y.; Gao, Q. Impact of agricultural non-point source pollution on river water quality: Evidence from China. Front. Ecol. Evol. 2022, 10, 858822. [Google Scholar] [CrossRef]
- Bonsdorff, E. Eutrophication: Early warning signals, ecosystem-level and societal responses, and ways forward: This article belongs to Ambio’s 50th Anniversary Collection. Theme: Eutrophication. Ambio 2021, 50, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Cirés, S.; de Pedro, Z.M.; Colina, J.Á.; Velásquez-Figueroa, Y.; Carmona-Jiménez, J.; Caro-Borrero, A.; Salazar, A.; Santa María Fuster, M.C.; Contreras, D.; et al. Overview of toxic cyanobacteria and cyanotoxins in Ibero-American freshwaters: Challenges for risk management and opportunities for removal by advanced technologies. Sci. Total Environ. 2021, 761, 143197. [Google Scholar] [CrossRef]
- Wiley, D.Y.; McPherson, R.A. The role of climate change in the proliferation of freshwater harmful algal blooms in inland water bodies of the United States. Earth Interact. 2024, 28, e230008. [Google Scholar] [CrossRef]
- Hamilton, D.P.; Wood, S.A.; Dietrich, D.R.; Puddick, J. Costs of Harmful Blooms of Freshwater Cyanobacteria. Cyanobacteria: An Economic Perspective; Wiley-Blankwell: Hoboken, NJ, USA, 2014; pp. 245–256. [Google Scholar]
- Almuhtaram, H.; Kibuye, F.A.; Ajjampur, S.; Glover, C.M.; Hofmann, R.; Gaget, V.; Owen, C.; Wert, E.C.; Zamyadi, A.; Wert, E.C.; et al. State of knowledge on early warning tools for cyanobacteria detection. Ecol. Indic. 2021, 133, 108442. [Google Scholar] [CrossRef]
- Catherine, A.; Escoffier, N.; Belhocine, A.; Nasri, A.B.; Hamlaoui, S.; Yéprémian, C.; Bernard, C.; Troussellier, M.; Troussellier, M. On the use of the FluoroProbe®, a phytoplankton quantification method based on fluorescence excitation spectra for large-scale surveys of lakes and reservoirs. Water Res. 2012, 46, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Pilon, S.; Zastepa, A.; Taranu, Z.E.; Gregory-Eaves, I.; Racine, M.; Blais, J.M.; Poulain, A.J.; Pick, F.R.; Pick, F.R. Contrasting histories of microcystin-producing cyanobacteria in two temperate lakes as inferred from quantitative sediment DNA analyses. Lake Reserv. Manag. 2019, 35, 102–117. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Naseef, H.A.; Karaman, D.; Bufo, S.A.; Scrano, L.; Karaman, R. Understanding the risks of diffusion of cyanobacteria toxins in Rivers, lakes, and potable water. Toxins 2023, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Trainer, V.L.; Hardy, F.J. Integrative monitoring of marine and freshwater harmful algae in Washington State for public health protection. Toxins 2015, 7, 1206–1234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Shang, M.; Wang, G.; Feng, L.; Shan, K.; Liu, X.; Wu, L.; Zhang, X.; Zhang, X. Remote estimation of cyanobacterial blooms using the risky grade index (RGI) and coverage area index (CAI): A case study in the Three Gorges Reservoir, China. Environ. Sci. Pollut. Res. Int. 2017, 24, 19044–19056. [Google Scholar] [CrossRef] [PubMed]
- GAO. Water Quality: Agencies Should Take More Actions to Manage Risks from Harmful Algal Blooms and Hypoxia. 2022. Available online: https://www.gao.gov/products/gao-22-104449 (accessed on 11 March 2024).
- Newton, A.R.; Melaram, R. Harmful algal blooms in agricultural irrigation: Risks, benefits, and management. Front. Water 2023, 5, 1325300. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G.; Kudela, R. Mitigating the Expansion of Harmful Algal Blooms across the Freshwater-to-Marine Continuum; ACS Publications: Washington, DC, USA, 2018. [Google Scholar]
- Bowling, L.; Ryan, D.; Holliday, J.; Honeyman, G. Evaluation of in situ fluorometry to determine cyanobacterial abundance in the Murray and Lower Darling Rivers, Australia. River Res. Appl. 2013, 29, 1059–1071. [Google Scholar] [CrossRef]
- Directive, W.F. Common Implementation Strategy for the Water Framework Directive (2000/60/EC); Guidance document 7; European Communities: Brussels, Belgium, 2003. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Abingdon, UK, 2021. [Google Scholar]
- Song, L.; Jia, Y.; Qin, B.; Li, R.; Carmichael, W.W.; Gan, N.; Xu, H.; Shan, K.; Sukenik, A.; Shan, K.; et al. Harmful cyanobacterial blooms: Biological traits, mechanisms, risks, and control strategies. Annu. Rev. Environ. Resour. 2023, 48, 123–147. [Google Scholar] [CrossRef]
- Dos Santos Severiano, J.; dos Santos Almeida-Melo, V.L.; Bittencourt-Oliveira, M.D.C.; Chia, M.A.; do Nascimento Moura, A. Effects of increased zooplankton biomass on phytoplankton and cyanotoxins: A tropical mesocosm study. Harmful Algae 2018, 71, 10–18. [Google Scholar] [CrossRef]
- Neetu Shahi, S.R.; Richa Pathak, S.K.M.; Veena Pande, B.S. Biocontrol of toxin producing cyanobacterium Microcystis aeruginosa by algicidal bacterium Exiguobacterium acetylicum Strain TM2 isolated from mid-altitudinal Himalayan lake of Northern India. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 170–187. [Google Scholar] [CrossRef]
- Oh, K.-H.; Jeong, D.-H.; Yang, S.-Y.; Jeon, T.-W.; Cho, Y.-C. Effects of submerged aerator on the growth of algae in Daechung reservoir. J. Korean Soc. Environ. Eng. 2013, 35, 268–275. [Google Scholar] [CrossRef]
- Hasan, K.; Alam, K.; Chowdhury, M.S.A. The use of an aeration system to prevent thermal stratification of water bodies: Pond, lake, and water supply reservoir. Appl. Ecol. Environ. Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Jang, M.-H.; Park, S.-B.; Jung, J.-M.; Roh, J.-S.; Joo, G.-J. The application of an algal fence for the reduction of algal intake into the water intake facility. Korean J. Ecol. Environ. 2003, 36, 467–472. [Google Scholar]
- Mejica, B.N.; Ebert, D.A.; Tanaka, S.K.; Deas, M.L. Managing cyanobacteria with a water quality control curtain in Iron Gate Reservoir, California. Lake Reserv. Manag. 2023, 39, 291–310. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, B.; Yue, Q. Coagulation performance and residual aluminum speciation of Al2(SO4)3 and polyaluminum chloride (PAC) in Yellow River water treatment. Chem. Eng. J. 2010, 165, 122–132. [Google Scholar] [CrossRef]
- Sengco, M.R.; Anderson, D.M. Controlling harmful algal blooms through clay flocculation. J. Eukaryot. Microbiol. 2004, 51, 169–172. [Google Scholar] [CrossRef]
- Lee, C.S.; Ahn, C.-Y.; La, H.-J.; Lee, S.; Oh, H.-M. Technical and strategic approach for the control of cyanobacterial bloom in fresh waters. Korean J. Environ. Biol. 2013, 31, 233–242. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jeong, G.-W.; Choi, C.; Ahn, G.; Nah, J.-W. Flocculation and algicidal effect of mixture of red-soil or clay and chitosan against harmful green-tide. J. Chitin Chitosan 2017, 22, 47–53. [Google Scholar] [CrossRef]
- Chow, C.W.K.; Drikas, M.; House, J.; Burch, M.D.; Velzeboer, R.M.A. The impact of conventional water treatment processes on cells of the cyanobacterium Microcystis aeruginosa. Water Res. 1999, 33, 3253–3262. [Google Scholar] [CrossRef]
- Oh, H.-S.; Kang, S.-H.; Yang, S.-C.; Nam, S.-H.; Kim, E.-J.; Hwang, T.-M. Comparison of different type coagulants for the removal of harmful algae in Pilot scale cyclonic-DAF system. KSWST J. Water Treat. 2018, 26, 69–80. [Google Scholar] [CrossRef]
- Chen, J.-J.; Yeh, H.-H.; Tseng, I.-C. Effect of ozone and permanganate on algae coagulation removal–Pilot and bench scale tests. Chemosphere 2009, 74, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Castro-Jiménez, C.C.; Grueso-Domínguez, M.C.; Correa-Ochoa, M.A.; Saldarriaga-Molina, J.C.; García, E.F. A coagulation process combined with a multi-stage filtration system for drinking water treatment: An alternative for small communities. Water 2022, 14, 3256. [Google Scholar] [CrossRef]
- Ho, L.; Lambling, P.; Bustamante, H.; Duker, P.; Newcombe, G. Application of powdered activated carbon for the adsorption of cylindrospermopsin and microcystin toxins from drinking water supplies. Water Res. 2011, 45, 2954–2964. [Google Scholar] [CrossRef] [PubMed]
- Bosse, K.R.; Sayers, M.J.; Shuchman, R.A.; Fahnenstiel, G.L.; Ruberg, S.A.; Fanslow, D.L.; Stuart, D.G.; Johengen, T.H.; Burtner, A.M.; Johengen, T.H.; et al. Spatial-temporal variability of in situ cyanobacteria vertical structure in western Lake Erie: Implications for remote sensing observations. J. Great Lakes Res. 2019, 45, 480–489. [Google Scholar] [CrossRef]
- Bertone, E.; O’halloran, K. Analysis and modelling of taste and odour events in a shallow subtropical reservoir. Environments 2016, 3, 22. [Google Scholar] [CrossRef]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef]
- Leboulanger, C.; Dorigo, U.; Jacquet, S.; Le Berre, B.; Paolini, G.; Humbert, J.F. Application of a submersible spectrofluorometer for rapid monitoring of freshwater cyanobacterial blooms: A case study. Aquat. Microb. Ecol. 2002, 30, 83–89. [Google Scholar] [CrossRef]
- Znachor, P.; Zapomělová, E.; Řeháková, K.; Nedoma, J.; Šimek, K. The effect of extreme rainfall on summer succession and vertical distribution of phytoplankton in a lacustrine part of a eutrophic reservoir. Aquat. Sci. 2008, 70, 77–86. [Google Scholar] [CrossRef]
- Hart, R.C.; Wragg, P.D. Recent blooms of the dinoflagellate Ceratium in Albert Falls Dam (KZN): History, causes, spatial features and impacts on a reservoir ecosystem and its zooplankton. Water S A 2009, 35, 455–468. [Google Scholar] [CrossRef]
- Ziemińska-Stolarska, A.; Imbierowicz, M.; Jaskulski, M.; Szmidt, A.; Zbiciński, I. Continuous and periodic monitoring system of surface water quality of an impounding reservoir: Sulejow Reservoir, Poland. Int. J. Environ. Res. Public Health 2019, 16, 301. [Google Scholar] [CrossRef]
- Heo, W.-M.; Kim, B. The effect of artificial destratification on phytoplankton in a reservoir. Hydrobiologia 2004, 524, 229–239. [Google Scholar] [CrossRef]
- Gao, S.; Du, M.; Tian, J.; Yang, J.; Yang, J.; Ma, F.; Nan, J. Effects of chloride ions on electro-coagulation-flotation process with aluminum electrodes for algae removal. J. Hazard. Mater. 2010, 182, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.; Ibelings, B.; Van Der Veer, B.; Koedood, J.; Mur, R. Artificial mixing prevents nuisance blooms of the cyanobacterium Microcystis in Lake Nieuwe Meer, the Netherlands. Freshw. Biol. 1996, 36, 435–450. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jeong, G.-W.; Choi, C.; Nah, J.-W. Flocculation and algicidal effect of harmful green-tide according to molecular-weight of chitosan. Polymer-Korea 2017, 41, 561–568. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Jung, J.-H.; Lim, H.-M.; Yoon, Y.H.; Ahn, K.-H.; Chang, H.-Y.; Kim, W.-J. Decision algorithm of natural algae coagulant dose to control algae from the influent of water works. J. Korea Soc. Environ. Eng. 2016, 38, 482–496. [Google Scholar] [CrossRef]
- Lee, B.; Oh, H.-C.; Ahn, J.-H.; Kim, Y.; Kang, H.; Kim, S.-K. Algae and nutrient control by using the mineralized coagulant. KSWST J. Water Treat. 2018, 26, 45–52. [Google Scholar] [CrossRef]
- Jeong, K.; Kim, D.-G.; Kim, S.-K.; Kim, W.-J.; Ko, S.-O. Evaluation of operation parameters for the removal of algae by electro-coagulation. J. Korean Soc. Water Environ. 2015, 31, 94–102. [Google Scholar] [CrossRef]
- Lürling, M.; van Oosterhout, F. Case study on the efficacy of a lanthanum-enriched clay (Phoslock®) in controlling eutrophication in Lake Het Groene Eiland (The Netherlands). Hydrobiologia 2013, 710, 253–263. [Google Scholar] [CrossRef]
- Schaus, M.H.; Vanni, M.J. Effects of gizzard shad on phytoplankton and nutrient dynamics: Role of sediment feeding and fish size. Ecology 2000, 81, 1701–1719. [Google Scholar] [CrossRef]
- Robb, M.; Greenop, B.; Goss, Z.; Douglas, G.; Adeney, J. Application of Phoslock TM, an innovative phosphorus binding clay, to two Western Australian waterways: Preliminary findings. In Interact between Sediments Water; Springer: Berlin/Heidelberg, Germany, 2003; pp. 237–243. [Google Scholar]
- Zeng, G.; Wang, P.; Wang, Y. Algicidal efficiency and mechanism of Phanerochaete chrysosporium against harmful algal bloom species. Algal Res. 2015, 12, 182–190. [Google Scholar] [CrossRef]
- Tongman, I.; Poungcharean, S.; Jitchum, P.; Chaichana, R. A field experiment on restoration of a hyper-eutrophic urban shallow pool using polyaluminium chloride in Thailand. Pol. J. Environ. Stud. 2023, 33, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Dong, M.M.; Rosario-Ortiz, F.L. Using digital flow cytometry to assess the degradation of three cyanobacteria species after oxidation processes. Water Res. 2013, 47, 3752–3761. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-K.; Kim, H.; Kim, S.W.; Chong, S.A.; Moon, B.; Lee, S.; Choi, J.W. A practical new technology of removing algal bloom: K-water GATe water combine. Korean J. Ecol. Environ. 2014, 47, 214–218. [Google Scholar] [CrossRef]
- Park, M.-H.; Lee, S.J.; Yoon, B.-D.; Oh, H.-M. Effects of CellCaSi and bioflocculant on the control of algal bloom. Korean Environ. Biol. 2001, 19, 129–135. [Google Scholar]
- Son, H.-J.; Jung, J.-M.; Kim, S.-G.; Jang, S.-H. Using CuSO4 for preventing algae attachment on the sedimentation basin of industrial water treatment plant. J. Korean Soc. Environ. Eng. 2012, 34, 780–785. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Ritika, B.; Sneha, B.; Chandrasekaran, N.; Mukherjee, A. A temporal study on fate of Al2O3 nanoparticles in a fresh water microcosm at environmentally relevant low concentrations. Ecotoxicol. Environ. Saf. 2012, 84, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ziegmann, M.; Abert, M.; Müller, M.; Frimmel, F.H. Use of fluorescence fingerprints for the estimation of bloom formation and toxin production of Microcystis aeruginosa. Water Res. 2010, 44, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Schaap, A.; Rohrlack, T.; Bellouard, Y. Lab on a chip technologies for algae detection: A review. J. Biophotonics 2012, 5, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.; Cardin, R.; Veilleux, E.; Deblois, C.; Warren, A.; Laurion, I. Performance evaluation of phycocyanin probes for the monitoring of cyanobacteria. J. Environ. Monit. 2011, 13, 110–118. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, Y.; Wu, H.; Kutser, T.; Han, Y.; Ma, R.; Yao, Z.; Zhao, H.; Xu, P.; Jiang, C.; et al. Potential of Mie–fluorescence–Raman lidar to profile chlorophyll a concentration in inland waters. Environ. Sci. Technol. 2023, 57, 14226–14236. [Google Scholar] [CrossRef]
- Beutler, M.; Wiltshire, K.H.; Meyer, B.; Moldaenke, C.; Lüring, C.; Meyerhöfer, M.; Hansen, U.P.; Dau, H.; Dau, H. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth. Res. 2002, 72, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Ministry of the Environment. Water Pollution Standard Method; SK: Moe; National Institute of Environmental Research. 2023. Available online: https://law.go.kr/행정규칙/수질오염공정시험기준 (accessed on 22 March 2024).
- Hodges, C.M.; Wood, S.A.; Puddick, J.; McBride, C.G.; Hamilton, D.P. Sensor manufacturer, temperature, and cyanobacteria morphology affect phycocyanin fluorescence measurements. Environ. Sci. Pollut. Res. Int. 2018, 25, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Lou, I.; Zhang, Y.; Lou, C.U.; Mok, K.M. Using an online phycocyanin fluorescence probe for rapid monitoring of cyanobacteria in Macau freshwater reservoir. Hydrobiologia 2014, 741, 33–49. [Google Scholar] [CrossRef]
- Hodges, C.M. A Validation Study of Phycocyanin Sensors for Monitoring Cyanobacteria in Cultures and Field Samples. Master’s Thesis, University of Waikato, Waikato, New Zealand, 2016. [Google Scholar]
- McQuaid, N.; Zamyadi, A.; Prévost, M.; Bird, D.F.; Dorner, S. Use of in vivo phycocyanin fluorescence to monitor potential microcystin-producing cyanobacterial biovolume in a drinking water source. J. Environ. Monit. 2011, 13, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Zamyadi, A. The Value of In Vivo Monitoring and Chlorination for the Control of Toxic Cyanobacteria in Drinking Water Production. Ph.D. Thesis, University of École Polytechnique de Montréal, Montreal, Canada, 2011. [Google Scholar]
- Zamyadi, A.; Henderson, R.K.; Stuetz, R.; Newcombe, G.; Newtown, K.; Gladman, B. Cyanobacterial management in full-scale water treatment and recycling processes: Reactive dosing following intensive monitoring. Environ. Sci. Water Res. Technol. 2016, 2, 362–375. [Google Scholar] [CrossRef]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence probes for real-time remote cyanobacteria monitoring: A review of challenges and opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, J.; Ylöstalo, P.; Kaitala, S.; Hällfors, S.; Raateoja, M.; Maunula, P. Ship-of-opportunity based phycocyanin fluorescence monitoring of the filamentous cyanobacteria bloom dynamics in the Baltic Sea. Estuar. Coast. Shelf Sci. 2007, 73, 489–500. [Google Scholar] [CrossRef]
- Zamyadi, A.; Choo, F.; Newcombe, G.; Stuetz, R.; Henderson, R.K. A review of monitoring technologies for real-time management of cyanobacteria: Recent advances and future direction. TrAC Trends Anal. Chem. 2016, 85, 83–96. [Google Scholar] [CrossRef]
- Gregor, J.; Maršálek, B.; Šípková, H. Detection and estimation of potentially toxic cyanobacteria in raw water at the drinking water treatment plant by in vivo fluorescence method. Water Res. 2007, 41, 228–234. [Google Scholar] [CrossRef]
- Lee, T.-y.; Tsuzuki, M.; Takeuchi, T.; Yokoyama, K.; Karube, I. Quantitative determination of cyanobacteria in mixed phytoplankton assemblages by an in vivo fluorimetric method. Anal. Chim. Acta 1995, 302, 81–87. [Google Scholar] [CrossRef]
- Bowling, L.C.; Zamyadi, A.; Henderson, R.K. Assessment of in situ fluorometry to measure cyanobacterial presence in water bodies with diverse cyanobacterial populations. Water Res. 2016, 105, 22–33. [Google Scholar] [CrossRef] [PubMed]
- GRDA(Grand River Dam Authority). Ecosystems Explorations: Research, Conservation, and Protection. Available online: https://grda.com/wp-content/uploads/2023/08/2021-Algae-Special-Spreads-1.pdf (accessed on 9 March 2024).
- Sukenik, A.; Kaplan, A. Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Hoaoashi, Y.; Aguilar, L.L.P.; Postigo-Malaga, M.; Garcia-Bravo, J.M.; Min, B.C. A low-cost and small USV platform for water quality monitoring. HardwareX 2019, 6, e00076. [Google Scholar] [CrossRef]
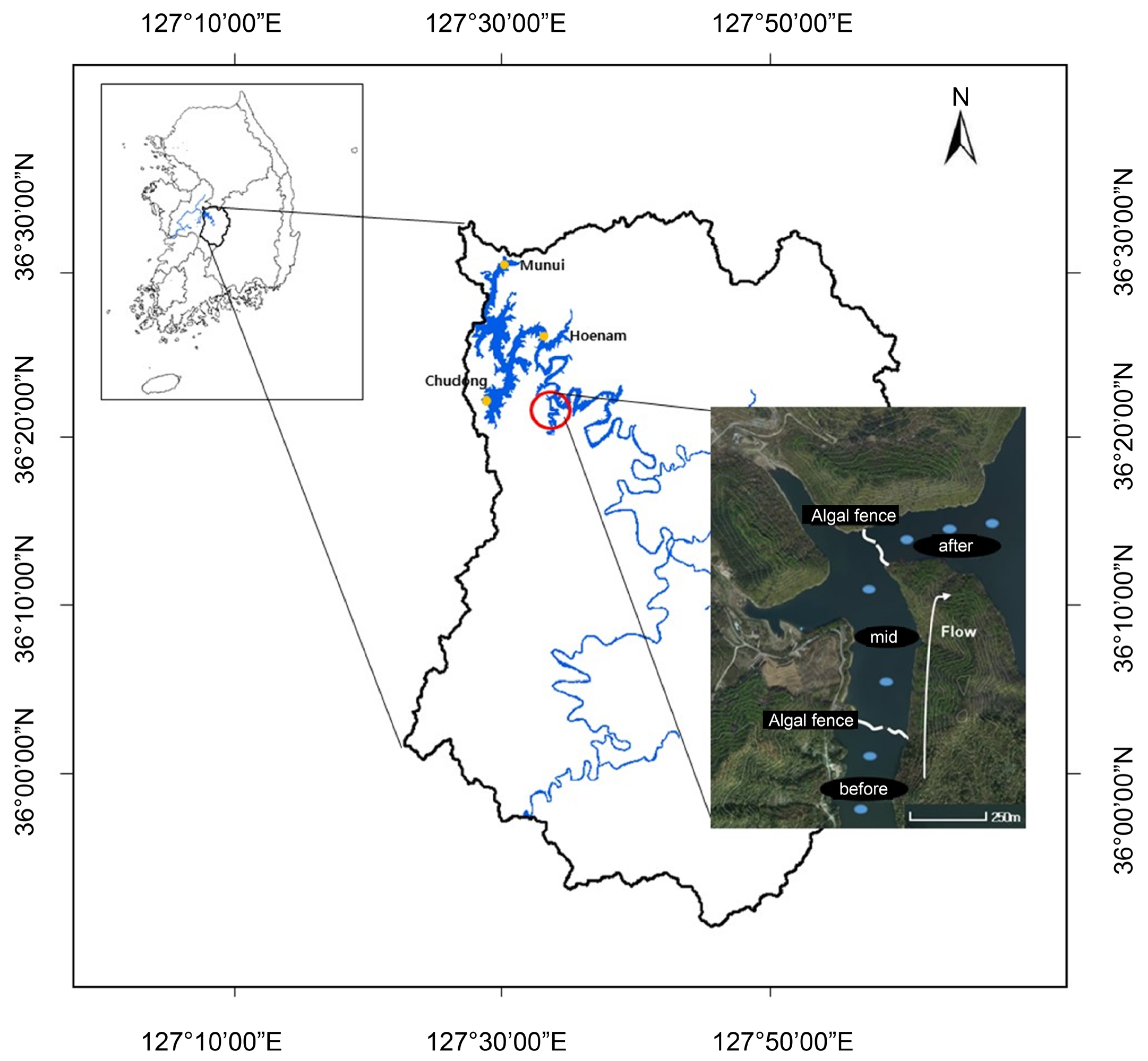

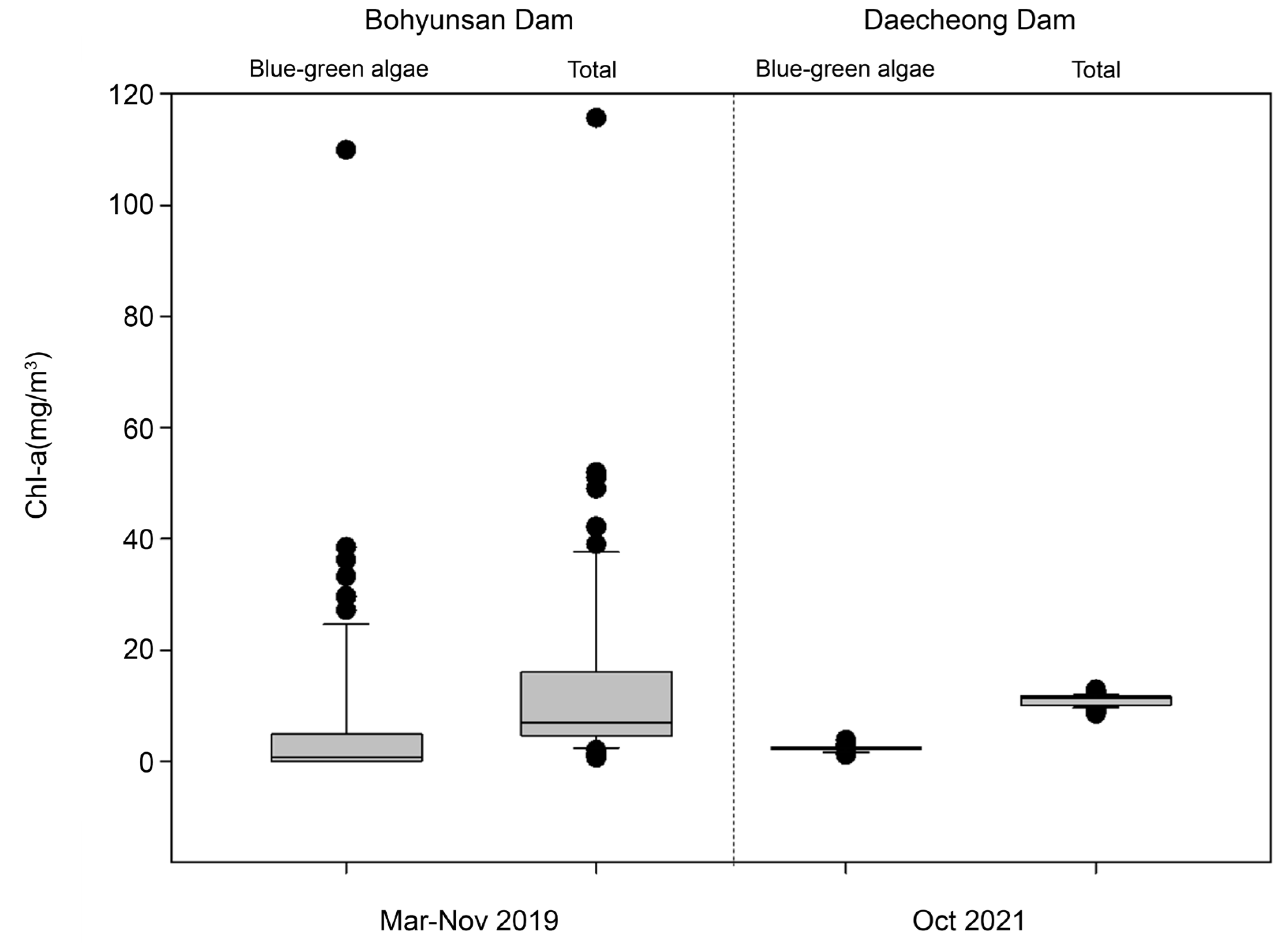
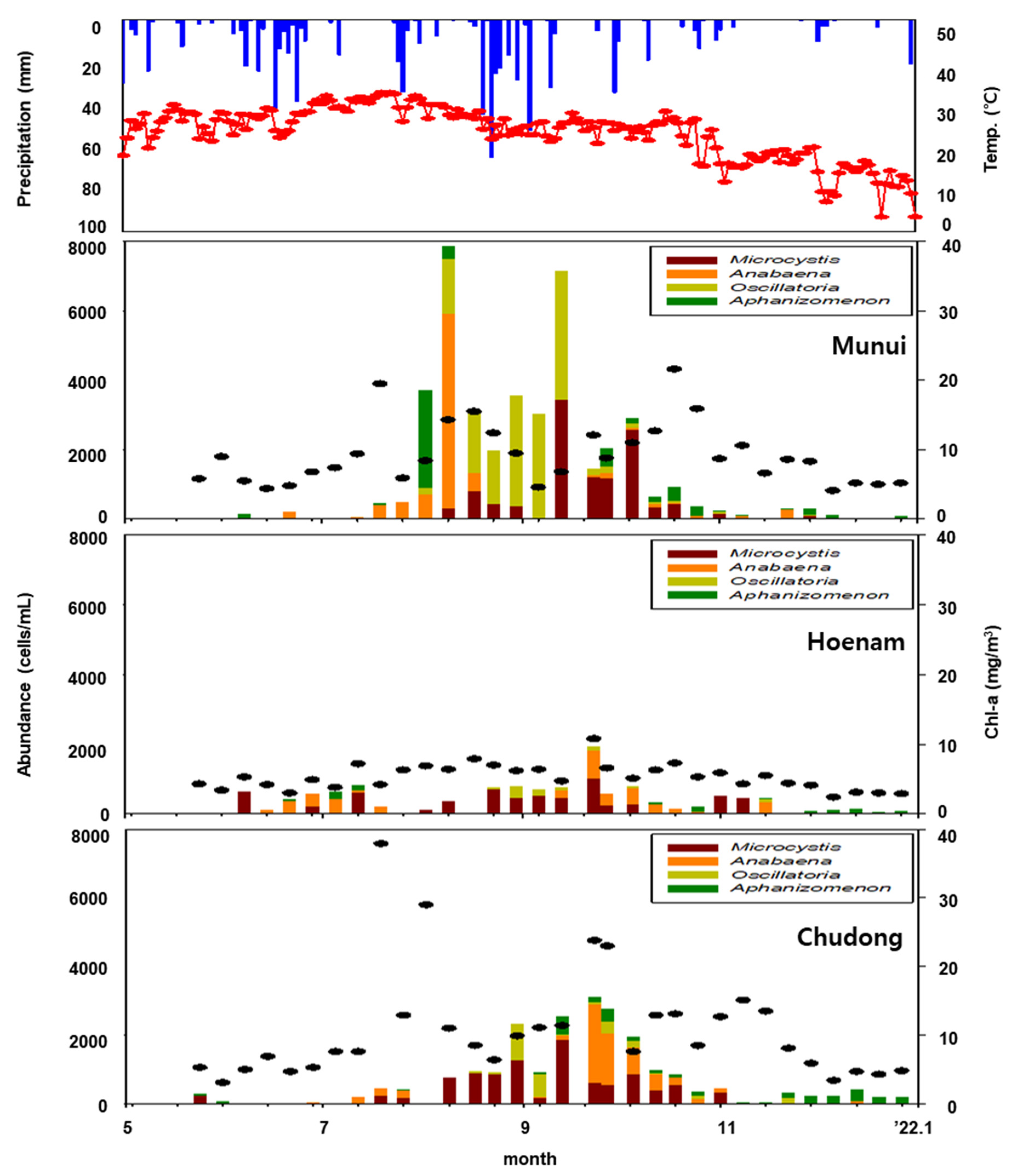
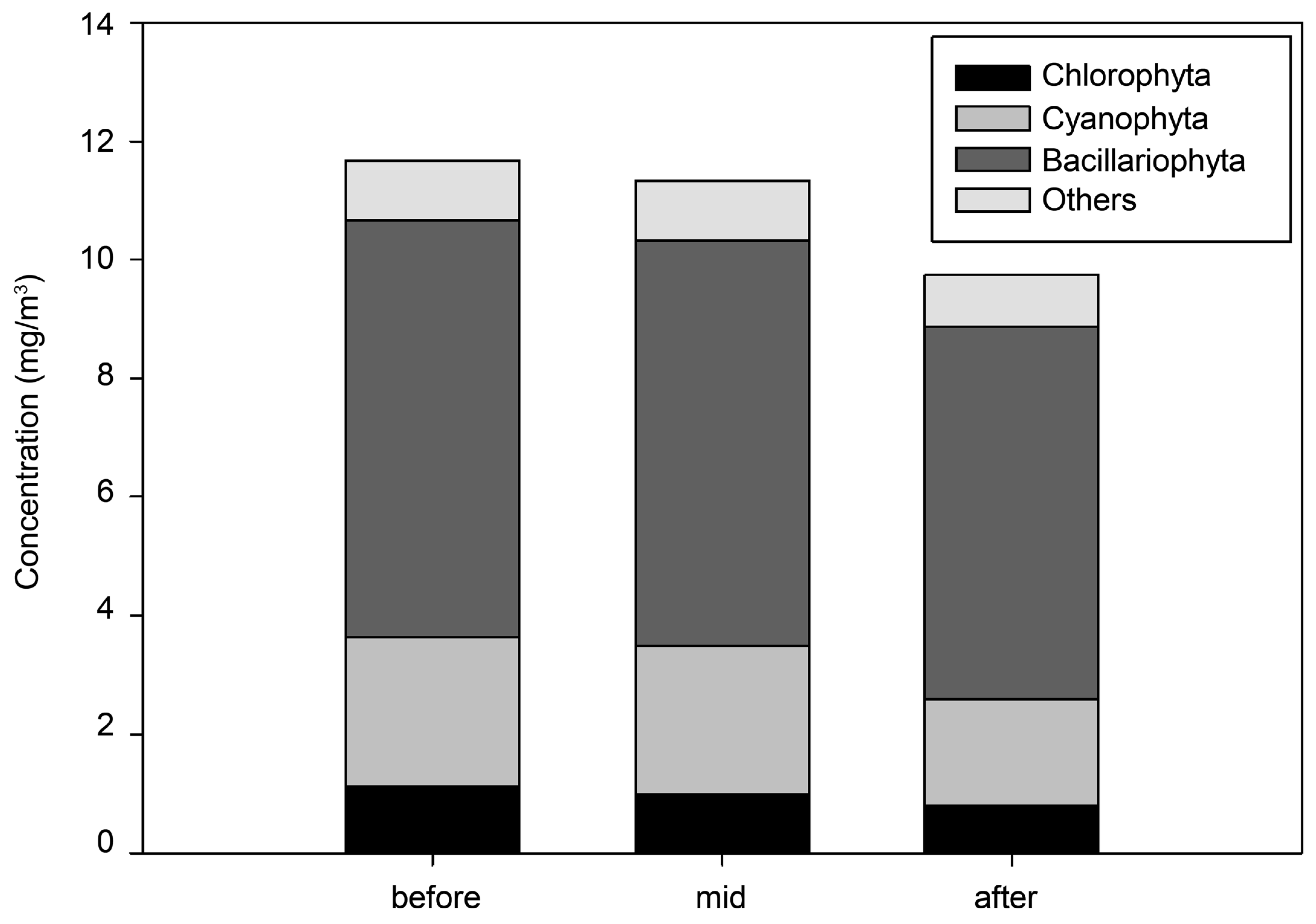


| Stage | Criteria | |
|---|---|---|
| Water supply source section | Caution | Harmful blue-green algal cell counts greater than 1000 cells/mL and less than 10,000 cells/mL in two consecutive collections 1 |
| Warning | Harmful blue-green algal cell counts greater than 10,000 cells/mL and less than 1,000,000 cells/mL in two consecutive collections 2 | |
| Outbreak | Harmful blue-green algal cell counts of 1,000,000 cells/mL or more in two consecutive collections 2 | |
| Clear | Harmful blue-green algal cell counts of less than 1000 cells/mL in two consecutive collections | |
| Algal Control | References | ||
|---|---|---|---|
| Chl-a_UV | Physical | Submerged aerator ECF (electro-coagulation-flotation) Artificial mixing | [28] [48] [49] [50] [51] |
| Chemical and biological | Chitosan, red soil/clay Biological predators Coagulant Electro-coagulation Algicidal substance Phosphorus insolubilization Oxidation Phanerochaete chrysosporium | [52] [53] [54] [35] [36] [55] [56] [57] [58] [59] [60] | |
| Multiple | Cyclonic-DAF system GATe water combination | [37] [61] | |
| Chl-a_sensor, fluorescence measurement | Chemical and biological | Bioflocculant Al2O3 nanoparticle Algicidal substance | [62] [63] [64] |
| Phycocyanin_UV | Chemical and biological | Copper sulfate, aluminum, sulfate | [36] |
| Pigment_HPLC | Coagulant | [40] | |
| Location | Study Dates | Microscopy | Sensor | Algal Control |
|---|---|---|---|---|
| Total Chl-a/Cyanobacterial Chl-a | ||||
| Bohyunsan Dam | 3 November 2019 | 75 | 75 | - |
| Daecheong Dam | October 2021 | - | 266 | Algal fence |
| Species No. | Ratio of Abundance | ||
|---|---|---|---|
| Taxa | Chlorophyta | 24 | 7.5% |
| Bacillariophyta | 32 | 19.7% | |
| Cyanophyta | 7 | 71.7% | |
| Others | 6 | 1.1% |
| FluoroProbe sensor | ||||
|---|---|---|---|---|
| log_Cyano | log_Total | |||
| Total | log_Total | Pearson correlation coefficient | 0.627 ** | 0.629 ** |
| N | 75 | 75 | ||
| log_Hcyano | Pearson correlation coefficient | 0.618 ** | 0.411 ** | |
| N | 75 | 75 | ||
| Depth/Site | Before | In between | After | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 3 | ||
| 0–2 m | 2.2 | 2.1 | 1.9 | 2.2 | 1.4 | 1.7 | 1.7 | |
| 3–7 m | 2.6 | 2.6 | 2.6 | 2.6 | 1.6 | 1.7 | 1.7 | |
| 8–14 m | 2.5 | 2.2 | 2.1 | 2.2 | 2.1 | 1.8 | 1.9 | |
| ≥15 m | 1.7 | 1.5 | 1.8 | 1.5 | 1.1 | 0.9 | 1.3 | |
| Average | 0–2 m | 2.2 ± 0.3 | 2.1 ± 0.1 | 1.6 ± 0.2 | ||||
| 3–7 m | 2.6 ± 0.1 | 2.6 ± 0.2 | 1.7 ± 0.2 | |||||
| 8–14 m | 2.4 ± 0.4 | 2.2 ± 0.2 | 1.9 ± 0.2 | |||||
| ≥15 m | 1.6 ± 0.7 | 1.7 ± 0.8 | 1.1 ± 0.8 | |||||
| Algal Fence | Measuring Area (m2) | Algal Weight of Total Measuring Area (mg) | Algal Concentration per Volume Depending on Water Depth (mg/m3) |
|---|---|---|---|
| Before | 3822 | 205,912 | 53.9 |
| In between | 6304 | 371,481 | 58.9 |
| After | 1634 | 70,727 | 43.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-J.; Yi, H.-S.; Youn, S.-J.; Lee, S.-J.; Jin, D.-H.; Lee, H.-S.; Kim, H.-S. Investigating Algal Sensor Utilization Methods for Three-Dimensional Algal Control Technology Evaluation. Water 2024, 16, 1679. https://doi.org/10.3390/w16121679
Park Y-J, Yi H-S, Youn S-J, Lee S-J, Jin D-H, Lee H-S, Kim H-S. Investigating Algal Sensor Utilization Methods for Three-Dimensional Algal Control Technology Evaluation. Water. 2024; 16(12):1679. https://doi.org/10.3390/w16121679
Chicago/Turabian StylePark, Yeon-Jeong, Hye-Suk Yi, Seok-Jea Youn, Seung-Jae Lee, Deok-Hyeon Jin, Hee-Suk Lee, and Han-Soon Kim. 2024. "Investigating Algal Sensor Utilization Methods for Three-Dimensional Algal Control Technology Evaluation" Water 16, no. 12: 1679. https://doi.org/10.3390/w16121679
APA StylePark, Y.-J., Yi, H.-S., Youn, S.-J., Lee, S.-J., Jin, D.-H., Lee, H.-S., & Kim, H.-S. (2024). Investigating Algal Sensor Utilization Methods for Three-Dimensional Algal Control Technology Evaluation. Water, 16(12), 1679. https://doi.org/10.3390/w16121679






