Isotope Hydrology and Hydrogeochemical Signatures in the Lake Malawi Basin: A Multi-Tracer Approach for Groundwater Resource Conceptualisation
Abstract
1. Introduction
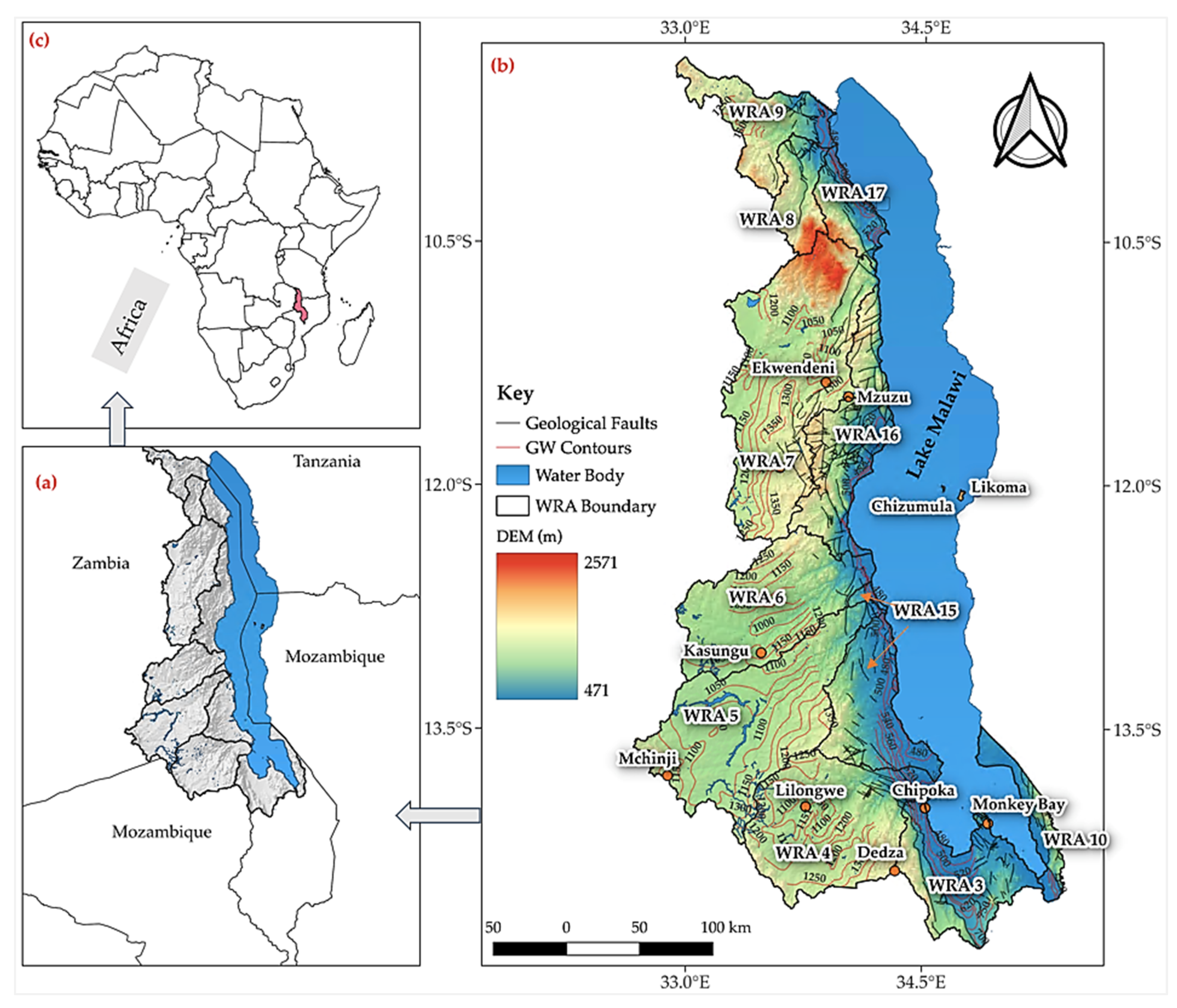
2. Study Area
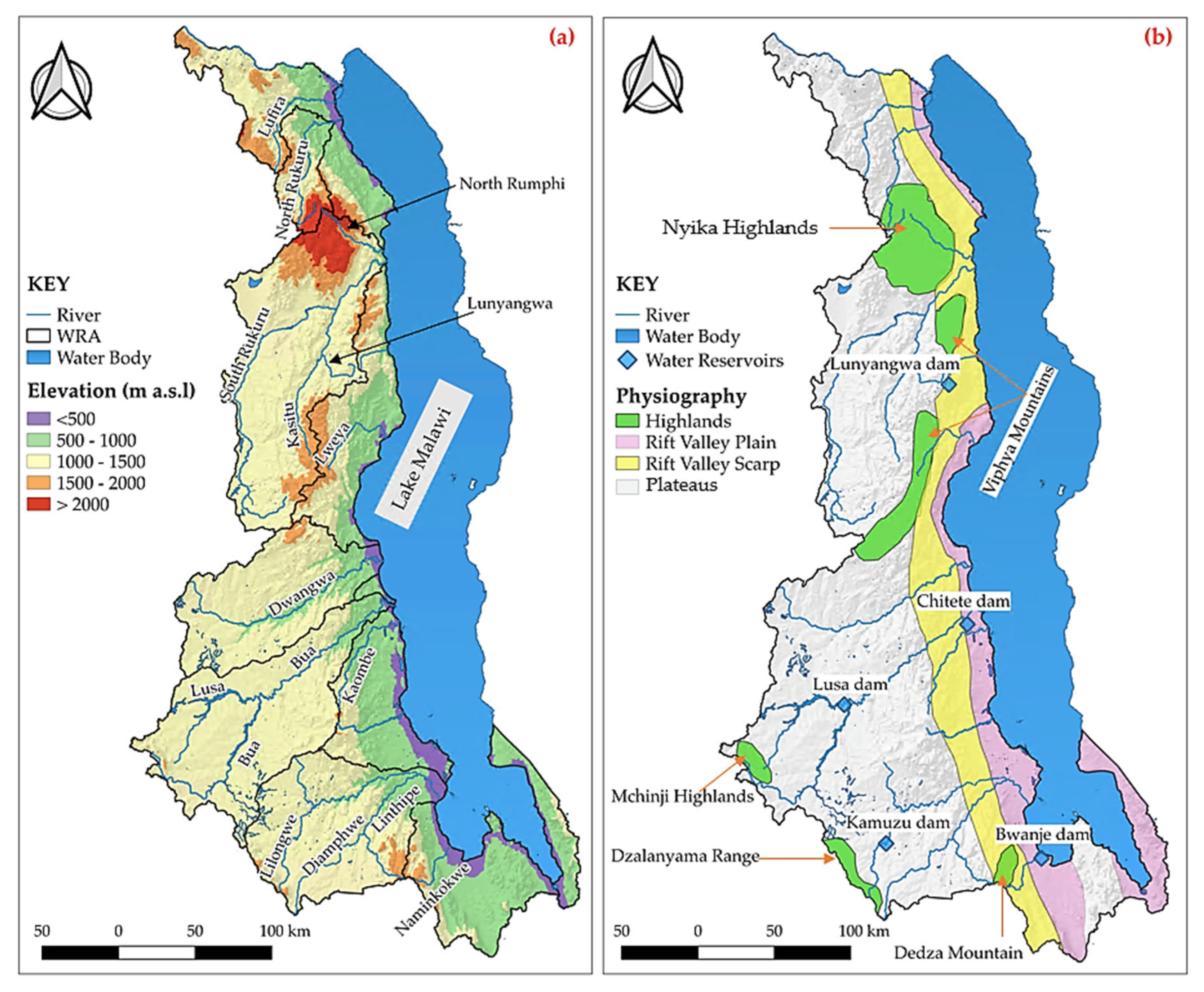
2.1. Water Resources
2.2. Topography
2.3. Geological Setting
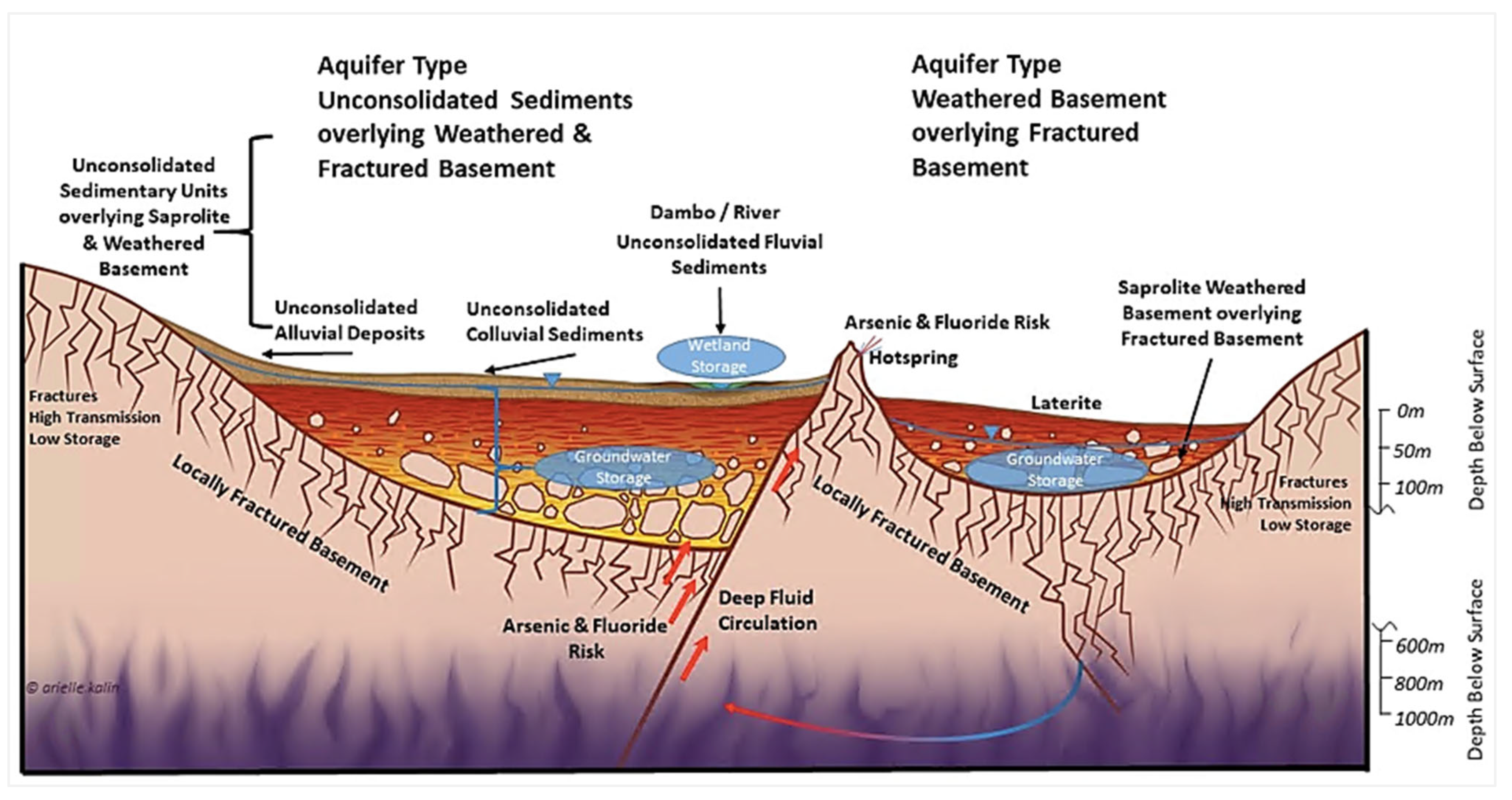
2.4. Hydrogeological Setting
2.5. Climate
3. Materials and Methods
3.1. Sampling and Analytical Techniques
3.2. Computation of Gibbs Diagram, Chloro-Alkaline Indices, and Saturation Index (SI)
4. Results
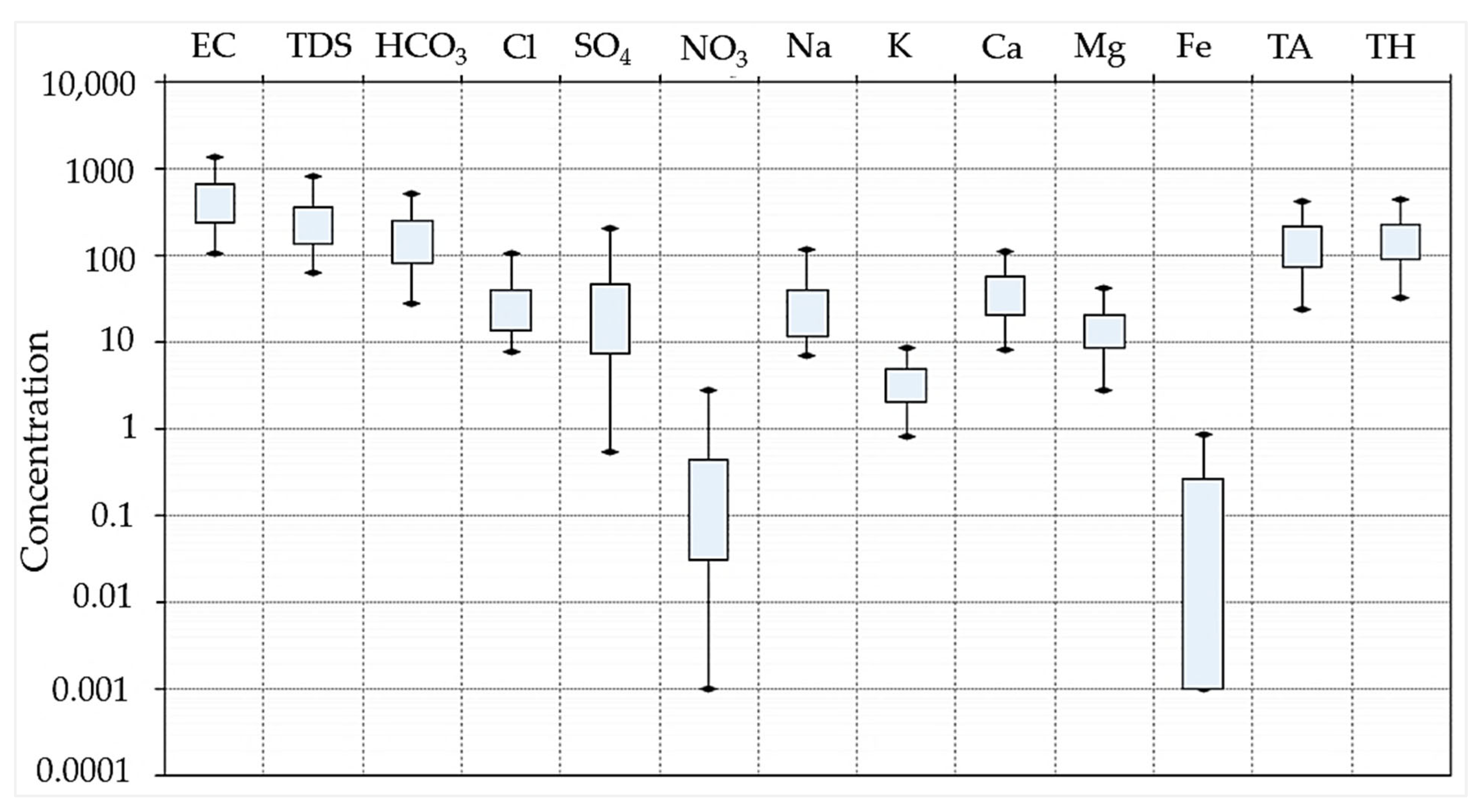
4.1. Hydrogeochemical Characteristics
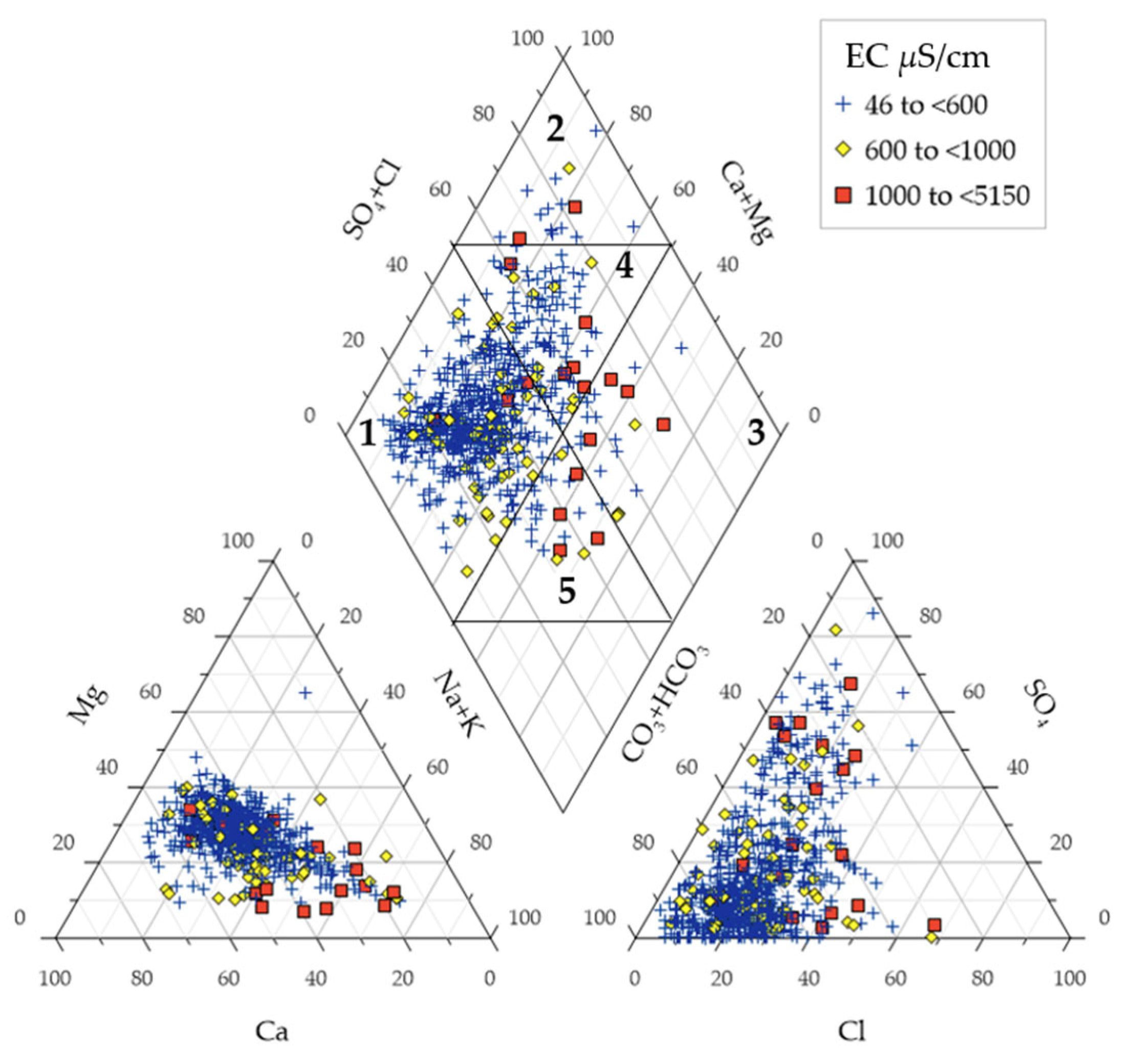
4.2. Hydrogeochemical Facies
4.3. Mechanisms Controlling Mineral Enrichment
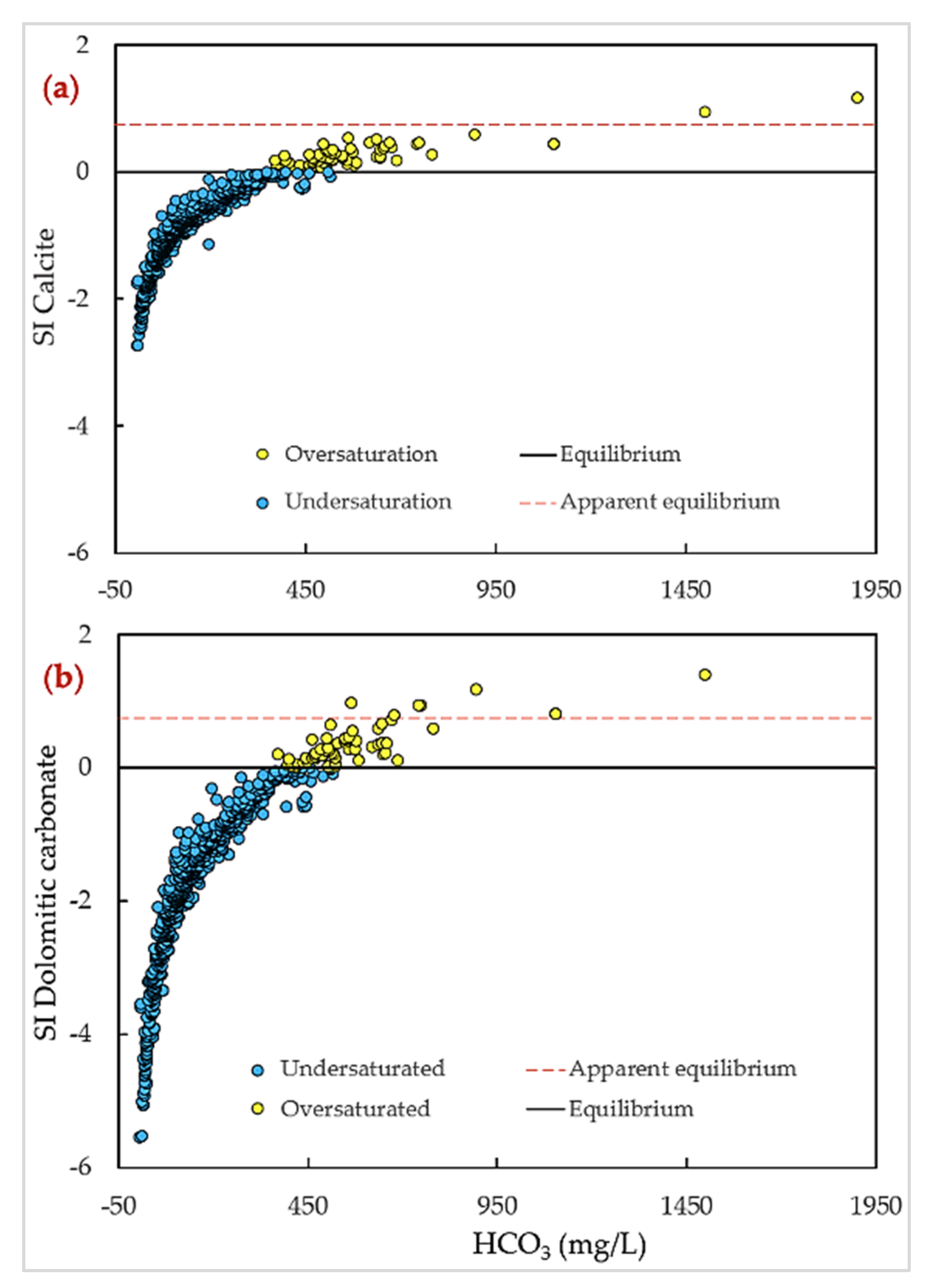
4.4. Geochemical Modelling
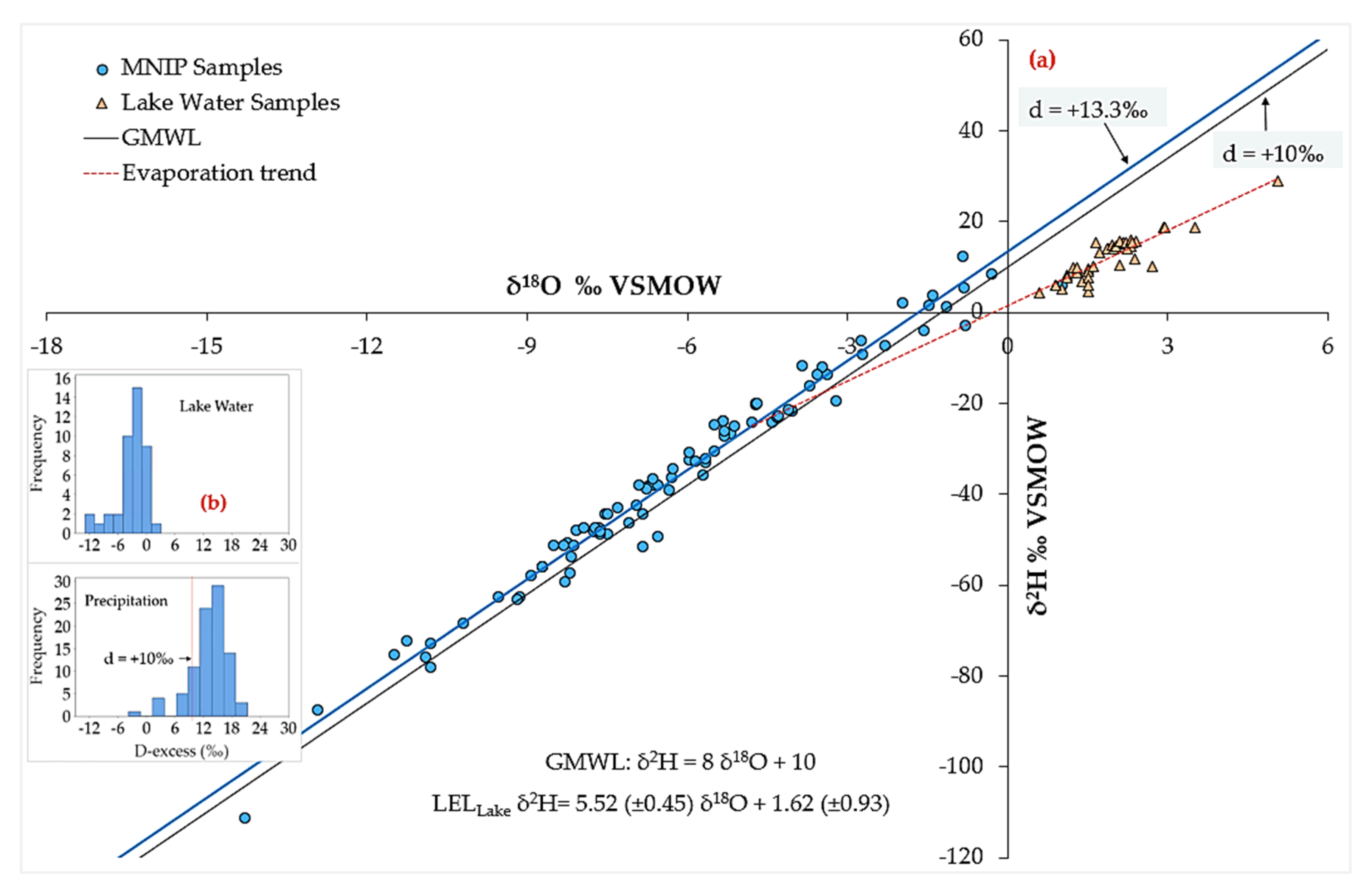
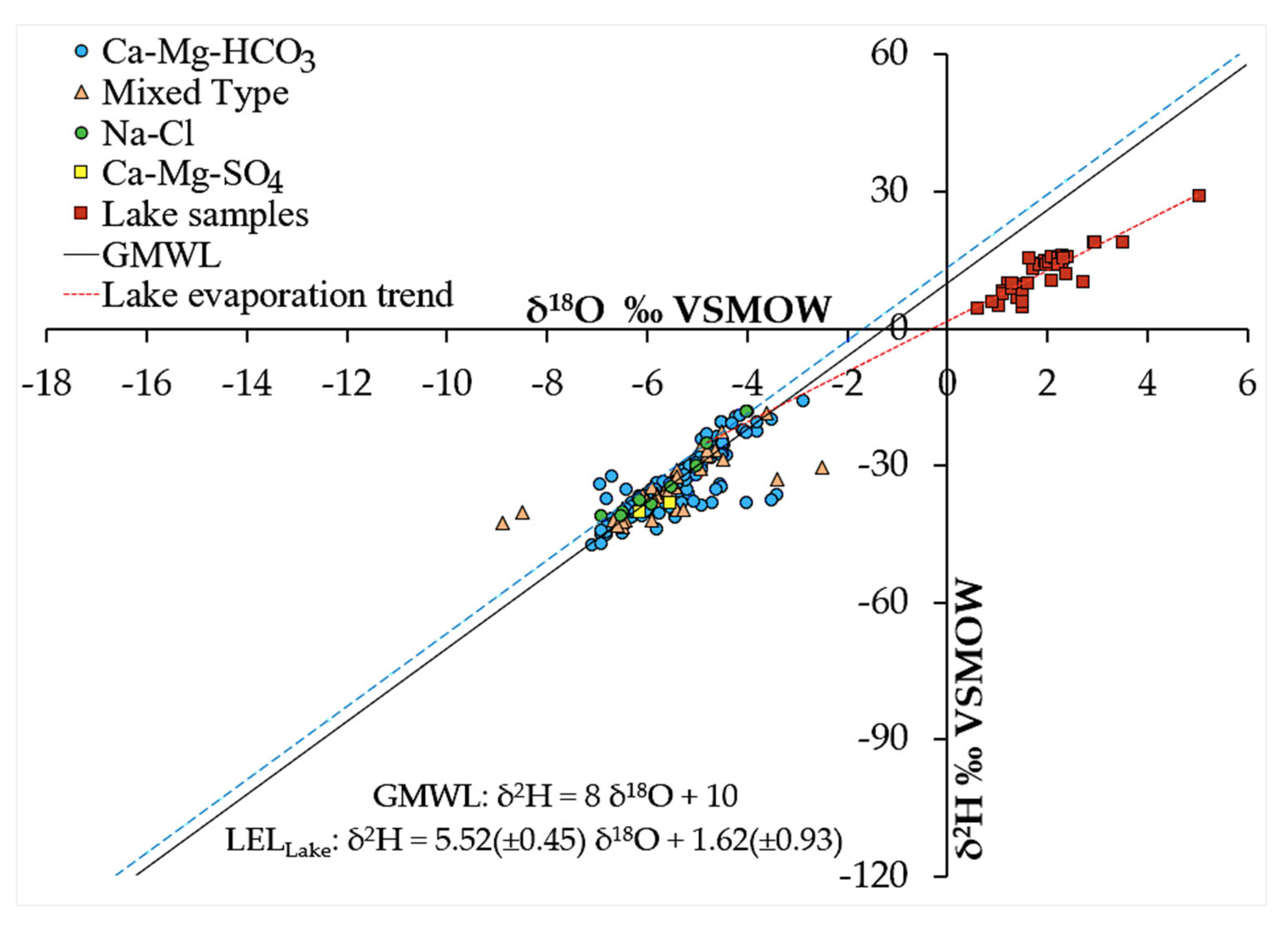
4.5. Isotope Hydrology Characteristics
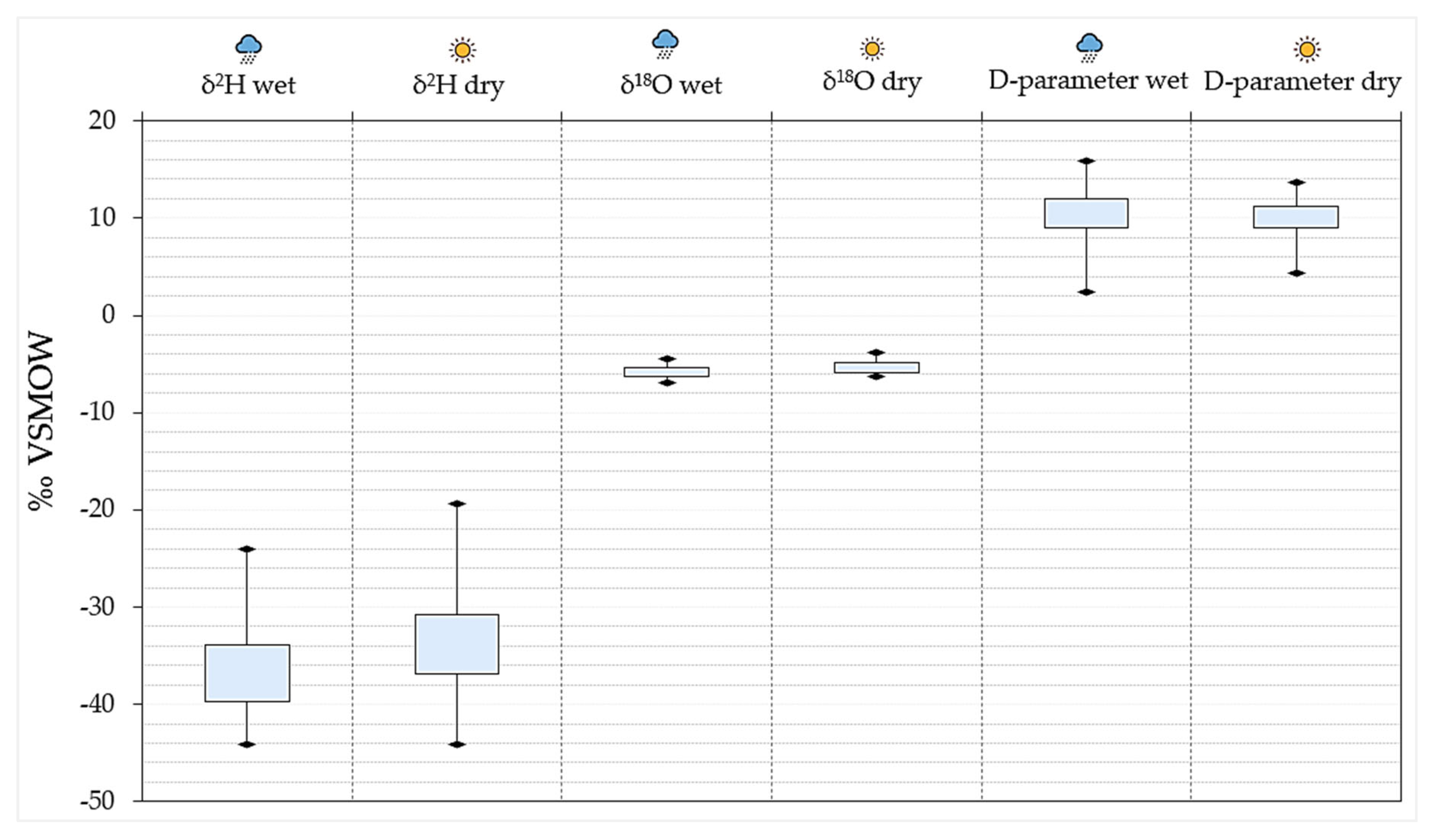
4.5.1. Precipitation Stable Isotope Signatures
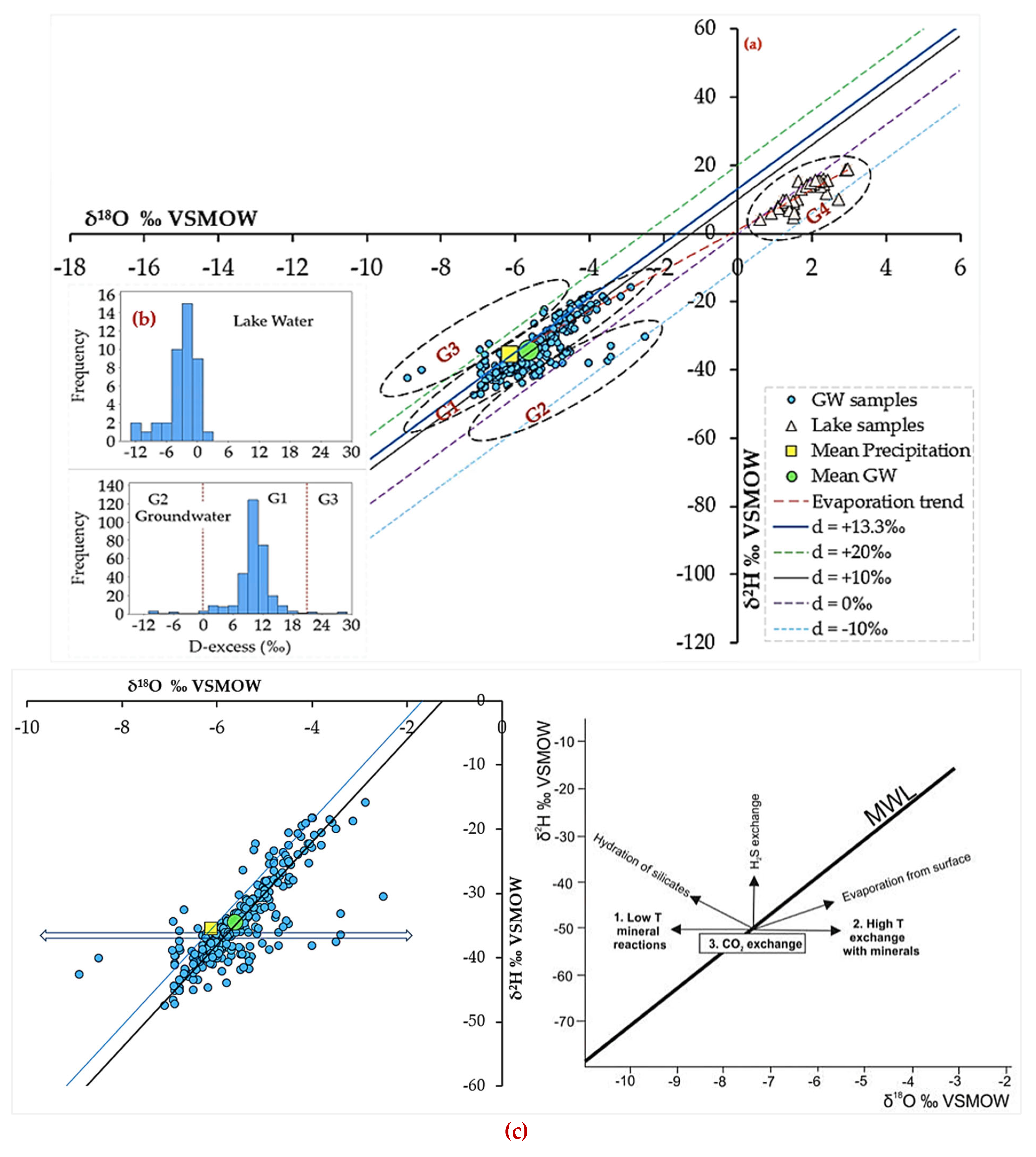
4.5.2. Groundwater Stable Isotope Signatures
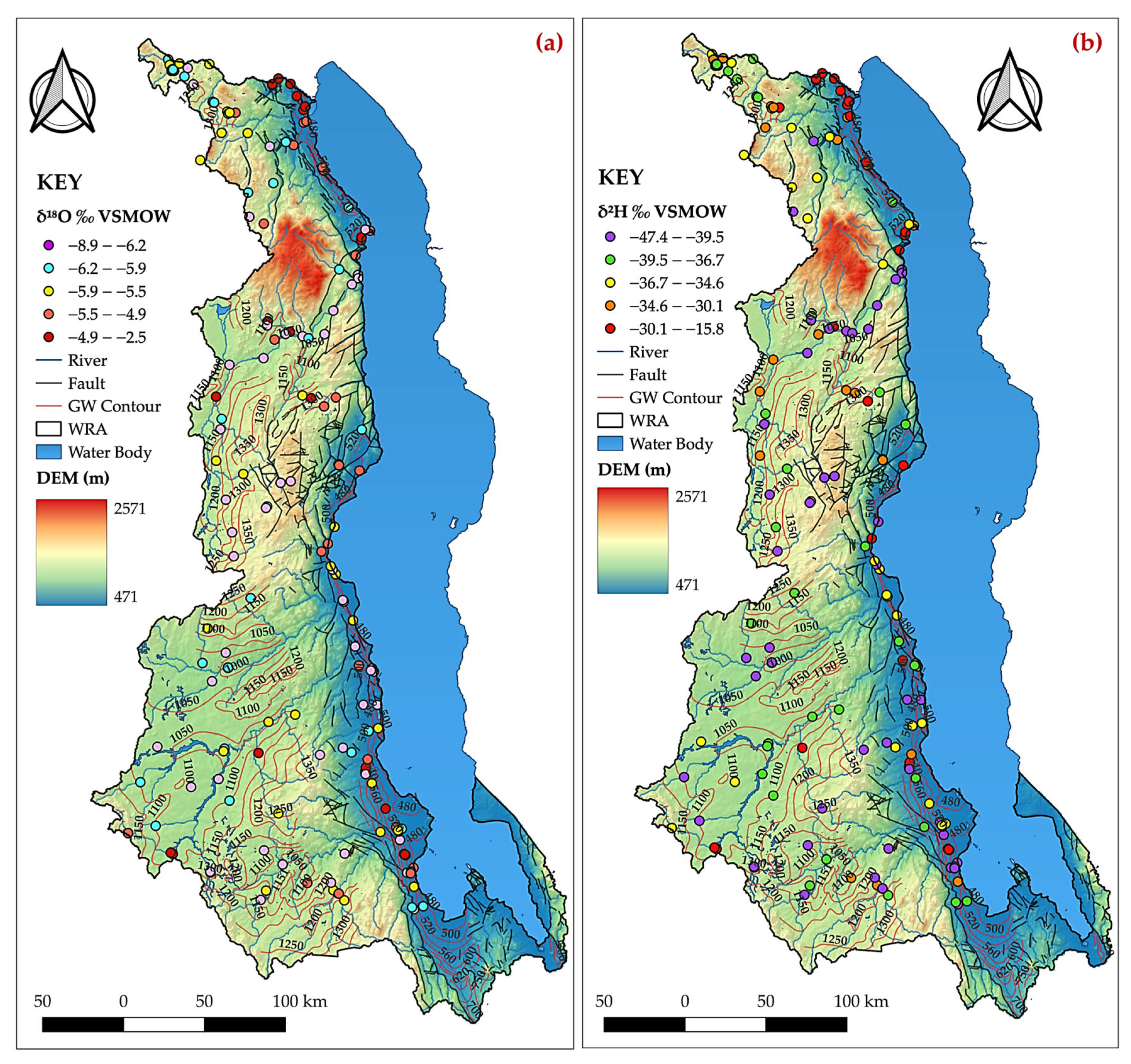
4.5.3. Dynamics: Spatial Distribution and Seasonal Effects
4.6. Integrated Isotopic-Hydrogeochemical and Geospatial Insights
5. Discussion
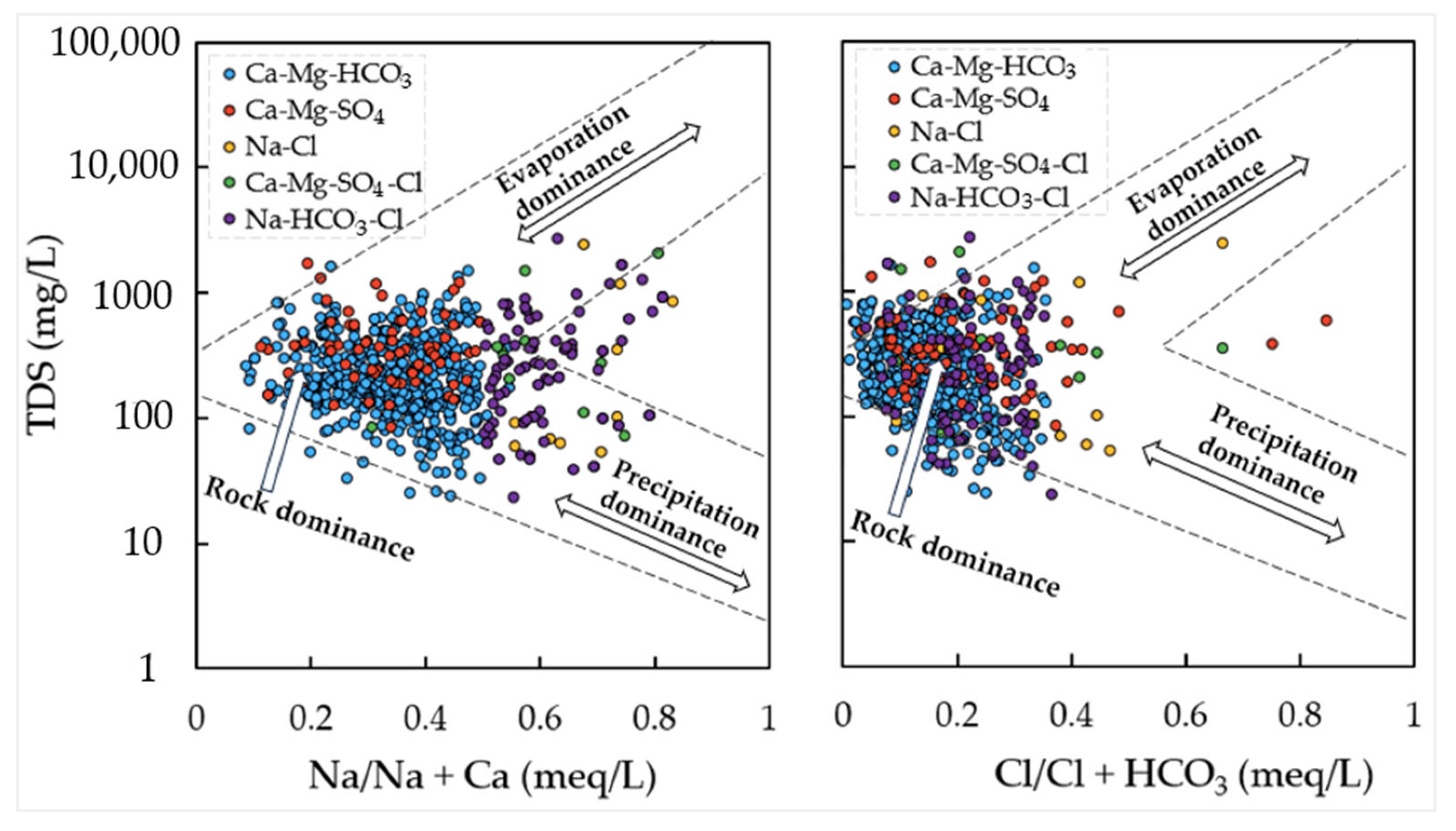
5.1. Origin of Ionic Species in Groundwater
5.2. Groundwater Evolution
5.3. Implications for Water Resource Management
- Understanding the origins and influences impacting groundwater recharge can guide the development and implementation of sustainable water resource management policies. These policies can focus on safeguarding and maintaining groundwater quality, especially in areas at risk of pollution or contamination, thereby underpinning efforts towards SDG 6 targets related to water and sanitation;
- Utilising knowledge about different groundwater types and their unique characteristics can shape decisions related to land-use planning. Areas with sensitive groundwater recharge zones may necessitate stricter regulations to preserve water quality and promote responsible usage;
- Policy initiatives should emphasise the importance of the ongoing monitoring of groundwater quality and quantity. Regulations could be implemented to govern land practices near recharge zones and protected groundwater areas based on identified hydrochemical features and isotopic markers;
- Recognising the potential impact of evaporation on groundwater quality can spur the development of strategies to mitigate these effects. Policies targeting the reduction of evaporation-related factors, such as managing surface–water interactions or regulating land use activities, may be essential;
- Given the intricate nature of groundwater recharge processes highlighted in this study, policies promoting collaboration among various stakeholders, including governmental bodies, researchers, and local communities, can be instrumental. This collaborative approach can facilitate a comprehensive strategy for groundwater management and conservation;
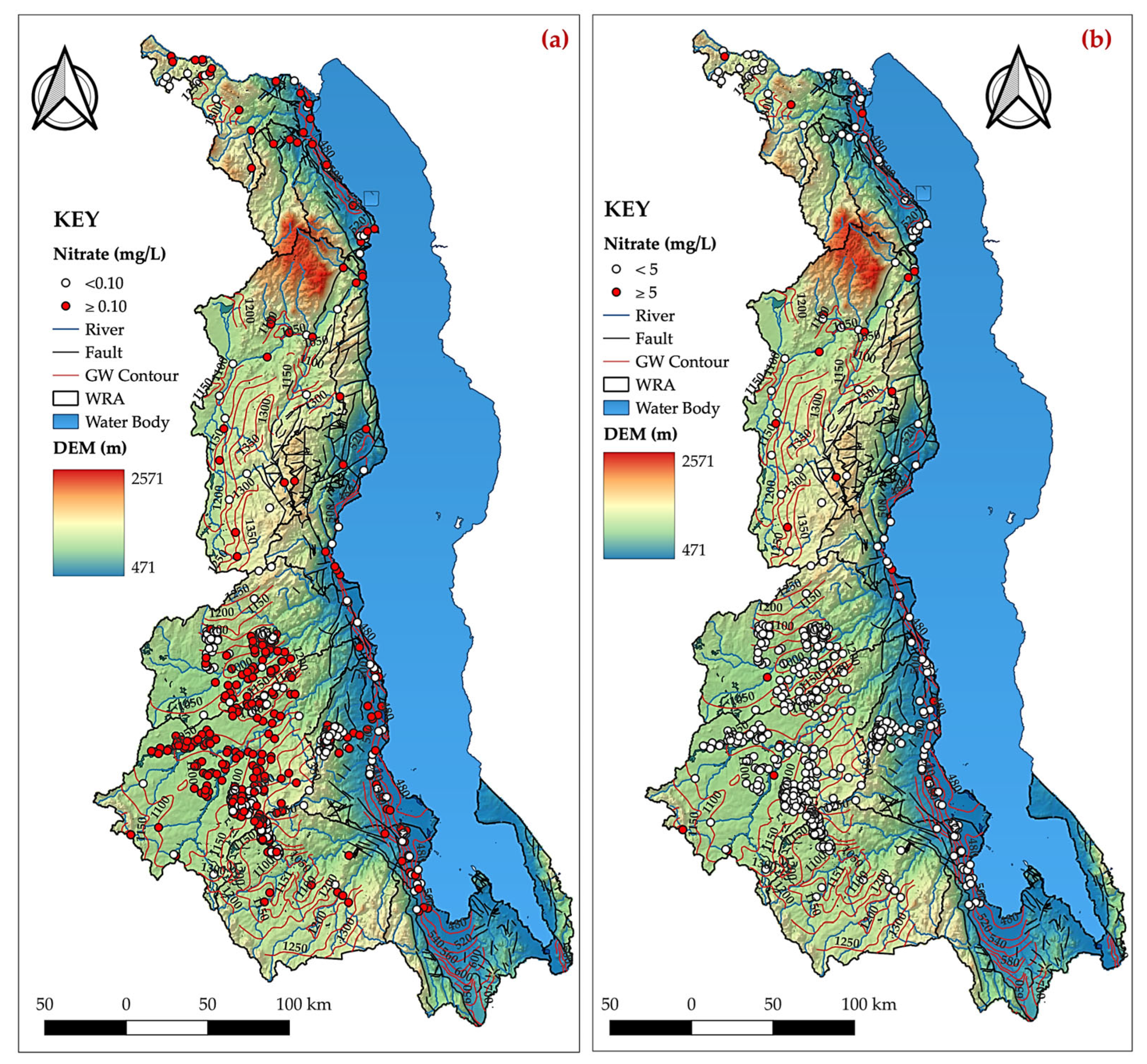
- The nitrate concentration in the groundwater aligns with the recommended guidelines by the World Health Organisation for safe drinking water, as illustrated in Figure 5. Despite this compliance, there is a pressing necessity to develop a detailed model that specifically addresses groundwater contamination associated with nitrates, particularly in light of the upsurge in fertiliser use, pit latrines, and septic systems arising from the rapid population growth. By creating a more comprehensive and focused analysis of nitrate-related pollution, we can better understand and mitigate the potential risks posed by these sanitation systems to ensure the continued safety of our drinking water sources;
- Future isotope hydrology studies should include δ15N−δ18O (NO3), δ13C (dissolved and mineral phases), and δ34S−δ18O (SO4) along with methods to study groundwater ages;
- In essence, this study’s outcomes provide valuable insights to guide policymakers in crafting regulations and actions that support sustainable groundwater management, ensure water availability, and safeguard water resources for future generations.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations General Assembly Resolution 64/292. The Human Right to Water and Sanitation, A/RES/64/292. Available online: http://daccess-ods.un.org/access.nsf/Get?Open&DS=A/RES/64/292&Lang=E (accessed on 5 February 2024).
- United Nations: The Sustainable Development Goals Report. 2021. Available online: https://unstats.un.org/sdgs/report/2021/ (accessed on 5 February 2024).
- United Nations Water. Groundwater Overview: Making the Invisible Visible. Int. Groundw. Resour. Assess. Cent. 2018, 1–60. [Google Scholar]
- Jasechko, S.; Seybold, H.; Perrone, D.; Fan, Y.; Shamsudduha, M.; Taylor, R.G.; Fallatah, O.; Kirchner, J.W. Rapid groundwater decline and some cases of recovery in aquifers globally. Nature 2024, 625, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Chidya, R.C.G.; Matamula, S.; Nakoma, O. Evaluation of groundwater quality in rural-areas of northern Malawi: Case of Zombwe extension planning area in Mzimba. Phys. Chem. Earth 2016, 93, 55–62. [Google Scholar] [CrossRef]
- Kalin, R.M.; Mleta, P.; Addison, M.J.; Banda, L.C.; Butao, Z.; Nkhata, M.; Rivett, M.O.; Mlomba, P.; Phiri, O.; Mambulu, J. Hydrogeology and Groundwater Quality Atlas of Malawi, Bulletin; Ministry of Water and Sanitation, Government of Malawi: Lilongwe, Malawi, 2022; p. 151. ISBN 978-1-915509-00-0.
- Chavula, G.M.S. Malawi Country Report on the Water, Energy and Food (WEF) Nexus; A Malawi Country Report submitted to SADC as part of the SADC—EU Project on “Fostering Water, Energy and Food Security Nexus Dialogue and Multi-Sector Investment in the SADC Region”; Malawi Ministry of Forestry and Natural Resources: Lilongwe, Malawi, 2018.
- Kelly, L.; Bertram, D.; Kalin, R.; Ngongondo, C.; Sibande, H. A National Scale Assessment of Temporal Variations in Groundwater Discharge to Rivers: Malawi. Am. J. Water Sci. Eng. 2020, 6, 39–49. [Google Scholar] [CrossRef]
- Kelly, L.; Bertram, D.; Kalin, R.; Ngongondo, C. Characterization of Groundwater Discharge to Rivers in the Shire River Basin, Malawi. Am. J. Water Sci. Eng. 2019, 5, 127–138. [Google Scholar] [CrossRef]
- Monjerezi, M.; Vogt, R.D.; Aagaard, P.; Saka, J.D.K. Using δ87Sr/δ86Sr, δ18O and δ2H isotope data along with major chemistry composition to assess groundwater salinization in lower Shire River Valley, Malawi. Appl. Geochem. 2011, 26, 2201–2214. [Google Scholar] [CrossRef]
- Rivett, M.O.; Robinson, H.; Wild, L.; Melville, J.; McGrath, L.; Phiri, P.; Flink, J.; Wanangwa, G.; Mleta, P.; MacLeod, S.; et al. Arsenic occurrence in Malawi groundwater. J. Appl. Sci. Environ. Manag. 2018, 22, 1807–1816. [Google Scholar] [CrossRef][Green Version]
- Addison, M.J.; Rivett, M.O.; Robinson, H.; Fraser, A.; Miller, A.M.; Phiri, P.; Mleta, P.; Kalin, R.M. Fluoride occurrence in the lower East African Rift System, Southern Malawi. Sci. Total. Environ. 2019, 712, 136260. [Google Scholar] [CrossRef] [PubMed]
- Smith-Carrington, A.K.; Chilton, P.J. Groundwater Resources of Malawi; Overseas Development Administration Institute of Geological Sciences: London, UK, 1983; Available online: http://resources.bgs.ac.uk/sadcreports/malawi1983smithcarringtonmalawigwresources.pdf (accessed on 8 December 2023).
- BGS. WaterAid. Groundwater Quality: Malawi. In British Geological Survey/WaterAid; Natural Environment Research Council: Swindon, UK, 2008. [Google Scholar]
- Chavula, G.M.S. Malawi. In Groundwater Availability and Use in Sub-Saharan Africa: A Review of Fifteen Countries; Pavelic, P., Giordano, M., Keraita, B., Ramesh, V., Rao, T., Eds.; International Water Management Institute: Colombo, Sri Lanka, 2012; Available online: http://www.iwmi.cgiar.org/Publications/Books/PDF/groundwater_availability_and_use_in_sub-saharan_africa_a_review_of_15_countries.pdf (accessed on 15 December 2023).
- Kalin, R.M.; Mwanamveka, J.; Coulson, A.B.; Robertson, D.J.C.; Clark, H.; Rathjen, J.; Rivett, M.O. Stranded Assets as a Key Concept to Guide Investment Strategies for Sustainable Development Goal 6. Water 2019, 11, 702. [Google Scholar] [CrossRef]
- Banda, L.C.; Rivett, M.O.; Kalin, R.M.; Zavison, A.S.K.; Phiri, P.; Chavula, G.; Kapachika, C.; Kamtukule, S.; Fraser, C.; Nhlema, M. Seasonally Variant Stable Isotope Baseline Characterisation of Malawi’s Shire River Basin to Support Integrated Water Resources Management. Water 2020, 12, 1410. [Google Scholar] [CrossRef]
- United Nations. The Sustainable Development Goals Report. 2017. Available online: https://www.unwater.org/publications/un-water-annual-report-2017 (accessed on 5 February 2024).
- UNDP. UN World Water Development Report. 2020. Available online: http://Unwater.org (accessed on 5 February 2024).
- Hinton, R.G.K.; Macleod, C.J.A.; Troldborg, M.; Wanangwa, G.; Kanjaye, M.; Mbalame, E.; Mleta, P.; Harawa, K.; Kumwenda, S.; Kalin, R.M. Factors Influencing the Awareness and Adoption of Borehole-Garden Permaculture in Malawi: Lessons for the Promotion of Sustainable Practices. Sustainability 2021, 13, 12196. [Google Scholar] [CrossRef]
- Rivett, M.O.; Halcrow, A.W.; Schmalfuss, J.; Stark, J.A.; Truslove, J.P.; Kumwenda, S.; Harawa, K.A.; Nhlema, M.; Songola, C.; Wanangwa, G.J.; et al. Local scale water-food nexus: Use of borehole-garden permaculture to realise the full potential of rural water supplies in Malawi. J. Environ. Manag. 2018, 209, 354–370. [Google Scholar] [CrossRef]
- Wassenaar, L.I.; Van Wilgenburg, S.L.; Larson, K.; Hobson, K.A. A groundwater isoscapes (δD, δ18O) for Mexico. J. Geochem. Explor. 2009, 102, 123–136. [Google Scholar] [CrossRef]
- Han, Z.; Shi, X.; Jia, K.; Sun, B.; Zhao, S.; Fu, C. Determining the Discharge and Recharge Relationships between Lake and Groundwater in Lake Hulun Using Hydrogen and Oxygen Isotopes and Chloride Ions. Water 2019, 11, 264. [Google Scholar] [CrossRef]
- Attandoh, N.; Yidana, S.M.; Abdul-Samed, A.; Sakyi, P.A.; Banoeng-Yakubo, B.; Nude, P.M. Conceptualization of the hydro-geological system of some sedimentary aquifers in Savelugu-Nanton and surrounding areas, Northern Ghana. Hydrol. Process. 2013, 27, 1664–1676. [Google Scholar] [CrossRef]
- Rivett, M.O.; Symon, S.; Jacobs, L.; Banda, L.C.; Wanangwa, G.J.; Robertson, D.J.C.; Hassan, I.; Miller, A.V.M.; Chavula, G.M.S.; Songola, C.E.; et al. Paleo-Geohydrology of Lake Chilwa, Malawi is the Source of Localised Groundwater Salinity and Rural Water Supply Challenges. Appl. Sci. 2020, 10, 6909. [Google Scholar] [CrossRef]
- Abdou Babaye, M.S.; Orban, P.; Ousmane, B.; Favreau, G.; Brouyère, S.; Dassargues, A. Characterization of recharge mechanisms in a pre-cambrian basement aquifer in semi-arid south-west Niger. Hydrogeol. J. 2019, 27, 475–491. [Google Scholar] [CrossRef]
- Cartwright, I.; Cendon, D.; Currell, M.; Meredith, K. A review of radioactive isotopes and other residence time tracers in Hydrogeology Journal understanding groundwater recharge: Possibilities, challenges, and limitations. J. Hydrol. 2017, 555, 797–811. [Google Scholar] [CrossRef]
- Balagizi, C.M.; Kasereka, M.M.; Cuoco, E.; Liotta, M. Influence of moisture source dynamics and weather patterns on stable isotopes ratios of precipitation in Central-Eastern Africa. Sci. Total. Environ. 2018, 628, 1058–1078. [Google Scholar] [CrossRef]
- Kalin, R.M. Basic concepts and formulations for isotope-geochemical process investigations, procedures, and methodologies of geochemical modelling of groundwater systems. In Manual on Mathematical Models in Isotope Hydrology; Yurtsever, Y., Ed.; IAEA: Vienna, Austria, 1995; Volume 910, p. 155. Available online: http://wwwnaweb.iaea.org/napc/ih/documents/TECDOCS/TECDOC%200910%20Mathematical%models%201996.PDF (accessed on 10 February 2023).
- Banda, L.C.; Rivett, M.O.; Kalin, R.M.; Zavison, A.S.; Phiri, P.; Kelly, L.; Chavula, G.; Kapachika, C.C.; Nkhata, M.; Kamtukule, S.; et al. Water-Isotope Capacity Building and Demonstration in a Developing World Context: Isotopic Baseline and Conceptualization of a Lake Malawi Catchment. Water 2019, 11, 2600. [Google Scholar] [CrossRef]
- Kalin, R.M.; Long, A. Application of hydrogeochemical modelling for validation of hydrologic flow modelling in the Tucson Basin Aquifer, Arizona, USA. In Mathematical Models and Their Applications to Isotope Studies in Groundwater Hydrology; IAEA: Vienna, Austria, 1994; pp. 209–254. [Google Scholar]
- International Atomic Energy Agency (IAEA). Isotope Hydrology and Integrated Water Resources Management, C&S Papers Series (CD-ROM) 23, IAEA, Vienna. 2004. Available online: https://www.iaea.org/publications/7184/isotope-hydrology-and-integrated-water-resources-management (accessed on 16 July 2023).
- Government of the Republic of Malawi, Ministry of Agriculture, Irrigation and Water Development. Project for National Water Resources Master Plan in the Republic of Malawi—Final Report; Volume II: Main Report; Ministry of Agriculture, Irrigation and Water Development: Lilongwe, Malawi, 2014; Available online: http://open_jicareport.jica.go.jp/pdf/12184537_01.pdf (accessed on 21 September 2023).
- GoM (NWRB). Environmental and Social Impact Assessment for Lunyangwa Dam Raising, Mzuzu: European Investment Bank. 2019. Available online: https://www.eib.org/en/registers/all/123952467 (accessed on 21 September 2023).
- Ebinger, C.J.; Crow, M.J.; Rosendahl, B.R.; Lefournier, J.; Livingstone, D.A. Structural evolution of Lake Malawi, Africa. Nature 1984, 308, 627–629. [Google Scholar] [CrossRef]
- GoM. Atlas for the hydrogeological and water quality Maps. In National Water Development Programme; Ministry of Agriculture, Irrigation and Water Development: Lilongwe, Malawi, 2015. [Google Scholar]
- Carter, G.S.; Bennett, J.D. The Geology and Mineral Resources of Malawi. Malawi Geol. Surv. Dep. Bull. 1973, 6, 1–62. [Google Scholar]
- Chorowicz, J. The East African rift system. J. Afr. Earth Sci. 2005, 43, 379–410. [Google Scholar] [CrossRef]
- Bath, A.H. Hydrochemistry in Groundwater Development: Report on an Advisory Visit to Malawi; Report WD/OS/80/20; British Geological Survey: London, UK, 1980. [Google Scholar]
- Chilton, P.J.; Smith-Carington, A.K. Characteristics of the Weathered Basement Aquifer in Malawi in Relation to Rural Water Supplies; IAHS Press: Wallingford, UK, 1984; pp. 57–65. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Ngongondo, C.; Xu, C.-Y.; Gottschalk, L.; Alemaw, B. Evaluation of spatial and temporal characteristics of rainfall in Malawi: A case of data scarce region. Theor. Appl. Clim. 2011, 106, 79–93. [Google Scholar] [CrossRef]
- Nicholson, S.E.; Klotter, D.; Chavula, G. A detailed rainfall climatology for Malawi, Southern Africa. Int. J. Clim. 2013, 34, 315–325. [Google Scholar] [CrossRef]
- Sene, K.; Piper, B.; Wykeham, D.; McSweeney, R.T.; Tych, W.; Beven, K. Long-term variations in the net inflow record for Lake Malawi. Hydrol. Res. 2016, 48, 851–866. [Google Scholar] [CrossRef]
- Vincent, K.; Dougill, A.J.; Mkwambisi, D.D.; Cull, T.; Stringer, L.C.; Chanika, D. Analysis of Existing Weather and Climate In-formation for Malawi; Kulima Integrated Development Solutions: Pietermaritzburg, South Africa, 2014. [Google Scholar]
- Department of Climate Change and Meteorological Services. Available online: http://www.rvatlas.org (accessed on 20 September 2021).
- Coplen, T.B.; Wassenaar, L.I. LIMS for lasers 2015 for achieving long-term accuracy and precision of δ2H, δ17O, and δ18O of waters using laser absorption spectrometry: LIMS for lasers 2015. Rapid Commun. Mass Spectrom. 2015, 29, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- IAEA (International Atomic Energy Agency). Laser Spectroscopic Analysis of Liquid Water Samples for Stable Hydrogen and Oxygen Isotopes; Training Course Series No. 35; International Atomic Energy Agency: Vienna, Austria, 2009; Available online: https://www.iaea.org/publications/8195/laser-spectroscopic-analysis-of-liquid-water-samples-for-stable-hydrogen-and-oxygen-isotopes (accessed on 10 February 2021).
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative evaluation of groundwater resources. In Methods and Techniques of Groundwater Investigations and Development; UNESCO: Paris, France, 1965; pp. 54–83. [Google Scholar]
- Singh, C.K.; Kumar, A.; Shashtri, S.; Kumar, A.; Kumar, P.; Mallick, J. Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J. Geochem. Explor. 2017, 175, 59–71. [Google Scholar] [CrossRef]
- Srinivasamoorthy, K.; Chidambaram, M.; Prasanna, M.V.; Vasanthavigar, M.; John Peter, A.; Anandhan, P. Identification of major sources controlling groundwater chemistry from a hard rock terrain—A case study from Mettur taluk, Salem District, Tamil Nadu, India. J. Earth Syst. Sci. 2008, 117, 49–58. [Google Scholar] [CrossRef]
- Merkel, B.J.; Planer-Friedrich, B. Groundwater Geochemistry: A Practical Guide to Modelling of Natural and Contaminated Aquatic Systems, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; 2328-7055; U.S. Geological Survey: Reston, VA, USA, 2013. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. [Google Scholar]
- Piper, A.M. A graphical procedure in the geochemical interpretation of water analysis. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar]
- Monjerezi, M.; Vogt, R.D.; Aagaard, P.; Saka, J.D. The hydro-geochemistry of groundwater resources in an area with prevailing saline groundwater, lower Shire Valley, Malawi. J. Afr. Earth Sci. 2012, 68, 67–81. [Google Scholar] [CrossRef]
- Addison, M.J.; Rivett, M.O.; Phiri, P.; Mleta, P.; Mbalame, E.; Banda, M.; Phiri, O.; Lakudzala, W.; Kalin, R.M. Identifying Groundwater Fluoride Source in a Weathered Basement Aquifer in Central Malawi: Human Health and Policy Implications. Appl. Sci. 2020, 10, 5006. [Google Scholar] [CrossRef]
- Vystavna, Y.; Harjung, A.; Monteiro, L.R.; Matiatos, I.; Wassenaar, L. Stable isotopes in global lakes integrate catchment and climatic controls on evaporation. Nat. Commun. 2021, 12, 7224. [Google Scholar] [CrossRef]
- Tuinenburg, O.A.; Theeuwen, J.J.E.; Staal, A. High-resolution global atmospheric moisture connections from evaporation to precipitation. Earth Syst. Sci. Data 2020, 12, 3177–3188. [Google Scholar] [CrossRef]
- Terzer-Wassmuth, S.; Araguás-Araguás, L.J.; Wassenaar, L.I.; Stumpp, C. Global and local meteoric water lines for δ17O/δ18O and the spatiotemporal distribution of Δ′17O in Earth’s precipitation. Sci. Rep. 2023, 13, 19056. [Google Scholar] [CrossRef]



| Mineral/Ion Species Source | Chemical Formulas | Ionic Species | Reaction Type |
|---|---|---|---|
| Carbon Dioxide (CO2) | CO2 (+H2O) | H+, HCO3− | Congruent |
| Plagioclase | (Na,Ca)(Al,Si)4O8 | Ca2+, Na+ | Incongruent |
| Anorthite | CaAl2Si2O8 | Ca2+, HCO3− | Incongruent |
| Biotite | K(Mg,Fe)3(AlSi3O10)(F,OH)2 | K+, Mg2+, F− | Incongruent |
| Calcite | CaCO3 | Ca2+, CO3−, HCO3− | Congruent |
| Albite | NaAlSi3O8 | Na+ | Incongruent |
| Muscovite | KAl2(AlSi3O10)(F,OH)2 | K+, F− | Incongruent |
| Olivine | Mg2SiO4 | Mg2+ | Incongruent |
| Amphiboles | Ca2(MgFeAl)5(AlSi)8O22 | Mg2+, Ca2+ | Incongruent |
| Fluorapatite | Ca5(PO4)3F | Ca2+, F−, PO4 | Incongruent |
| Fluorite Pyrite | CaF2 FeS2 | Ca2+, F− SO42− | Congruent Incongruent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banda, L.C.; Kalin, R.M.; Phoenix, V. Isotope Hydrology and Hydrogeochemical Signatures in the Lake Malawi Basin: A Multi-Tracer Approach for Groundwater Resource Conceptualisation. Water 2024, 16, 1587. https://doi.org/10.3390/w16111587
Banda LC, Kalin RM, Phoenix V. Isotope Hydrology and Hydrogeochemical Signatures in the Lake Malawi Basin: A Multi-Tracer Approach for Groundwater Resource Conceptualisation. Water. 2024; 16(11):1587. https://doi.org/10.3390/w16111587
Chicago/Turabian StyleBanda, Limbikani C., Robert M. Kalin, and Vernon Phoenix. 2024. "Isotope Hydrology and Hydrogeochemical Signatures in the Lake Malawi Basin: A Multi-Tracer Approach for Groundwater Resource Conceptualisation" Water 16, no. 11: 1587. https://doi.org/10.3390/w16111587
APA StyleBanda, L. C., Kalin, R. M., & Phoenix, V. (2024). Isotope Hydrology and Hydrogeochemical Signatures in the Lake Malawi Basin: A Multi-Tracer Approach for Groundwater Resource Conceptualisation. Water, 16(11), 1587. https://doi.org/10.3390/w16111587









