Abstract
Increases in poultry industry production have resulted in the generation of more hazardous effluents with high nitrogen and chemical oxygen demand (COD) concentrations. It is necessary to develop more efficient technologies in terms of water purification without the need to increase the volumes of commonly used reactors. This work analyzed the addition of micronutrients (Mo, Zn, Cu, and Mn) for the cultivation of the microalgae Chlorella spp. and Spirulina maxima in poultry wastewater. The effects of micronutrients on the production of biomass and algal cells were also assessed. For the Chlorella species, removal efficiencies of up to 99.14% for COD and 99.33% for nitrogen were achieved; for the Spirulina strain, these efficiencies were 98% for COD and 99% for nitrogen. The modified Gompertz equation was used to analyze the kinetic parameters. For both microalgae, the R2 values were greater than 98%. The results indicated that the dose with the highest algal cell generation rate was dose 2 for Chlorella spp., at 4.35 days, and dose 1 for Spirulina maxima, at 6.26 days. Microalgae are biological alternatives suitable for wastewater treatment, and their pollutant removal efficiency can increase with the addition of micronutrients, which has additional benefits for the production of valuable biomasses for industrial applications.
1. Introduction
The global increase in pollution is exacerbated by the excessive generation of industrial effluents. Furthermore, the increase in pollution can be due to the laxity in laws governing the discharge and processing of industrial wastewater in some developing countries such as Mexico, as well as the lack of interest on the part of companies in complying with environmental legislation. One of the industrial sectors that contributes greatly to environmental pollution is the poultry industry. It is estimated that a farm with a capacity of 13,000 birds per day produces approximately 140 metric tons of wastewater [1]. In addition, the poultry processing industry generates other types of waste, such as feces, litter substrates, hatchery waste, and liquid waste, such as urine, blood, fats, the remains of pesticides used in disinfection, and water used during the entire rearing process, from breeding to slaughtering of birds [2]. All these wastes tend to be discharged into the surrounding aquifers without prior treatment, as the latter leads to more expenses and, therefore, economic losses for companies. For these reasons, it is important to look for alternative uses and treatments for such effluents.
Poultry wastewater is a highly polluting waste since its chemical composition includes blood; complex mixtures of fats, proteins, and fibers; and high chemical oxygen demand (COD), biochemical oxygen demand (BOD), and total suspended solids [3]. The adaptation of microalgae cultures in effluent tanks is a viable and low-cost option for the industry. Additionally, the addition of micronutrients to these crops enhanced the removal efficiency of the evaluated pollutants.
There are different treatment methods for wastewater from the poultry industry, which are divided into biological, physical, and chemical methods; these are also known as conventional methods [4]. Some of the physicochemical methods are coagulation/flocculation, membrane process systems, dissolved air flotation (DAF), electrochemical reactions, ion exchange, membrane filtration, photocatalysis, electrodialysis, adsorption, chemical precipitation, and ultrafiltration, within which adsorption is more economical and becomes more efficient using hydrogels and magnetic nanoparticles [5]. However, these methods do not have great removal efficiency and cannot reach the maximum contaminant limits allowed by national and international regulations [6]. The biological methods used are wetlands and aerobic and anaerobic systems, but they present a disadvantage in terms of the generation of sludge with a complex chemical composition that also represents an environmental problem due to the difficulty in its treatment, degradation, and disposal [7].
The use of microalgae and bacteria as alternative wastewater treatments is viable since they do not have high operating costs and there is no sludge generation [8]. Microalgae can develop in wastewater-based substrates, as the effluents have high levels of nitrogen and phosphorus, which are indispensable for the development of microalgae. Additionally, these living organisms can assimilate heavy metals and absorb the CO2 present in the environment, thus reducing the negative impact that such waste generates in the atmosphere [9]. Once microalgae use waste to nourish and develop, algae can be extracted from the culture medium. This resulting biomass is rich in pigments (chlorophyll, beta-carotene), enzymes (hydroxygenase, carbonic anhydrase, lipoxygenase, nitrilase, nitrogenase, phosphatase, and acetyl-coenzyme A thiolase) [10], sugars, and lipids [11]. Algal biomass has diverse applications, such as in human food supplements, fodder supplements, paper production, nutraceuticals, naturopathic cosmetic products, bioethanol, biodiesel, and biochar [12].
It is crucial to assess the effectiveness of microalgae in the removal of organic compounds from wastewater from the poultry sector following the addition of different micronutrients. Using these eukaryotic organisms as an alternative treatment to wastewater can contribute to the production of biomass with greater nutritional value, which can later be used as a supplement in feed mixtures.
The aim of this study was to assess the impact of micronutrients on the elimination of organic matter and nutrients from poultry wastewater by strains of Spirulina maxima and Chlorella spp., as well as to identify novel kinetic parameters for microalgal growth.
2. Materials and Methods
2.1. Production of the Microalgae Chlorella spp. and Spirulina maxima
The sample of freshwater microalgae of the Chlorella species was obtained from the Centro de Estudios Técnicos del Mar (C.E.T. Mar 07), located in the city of Veracruz, in the state of the same name, while the Spirulina microalgae were obtained from the National Collection of Microbial Strains and Cell Cultures of the Center for Research and Advanced Studies of the National Polytechnic Institute (CINVESTAV). Both species were cultivated in an area called the “humid room”, which is equipped with air conditioning, a temperature of 20 ± 2 °C, and fluorescent light lamps with an intensity of 35 μmoL·m−2·s−1 that provides 12 h of lighting per day.
2.2. Propagation of Microalgal Species
Chlorella spp. algae were grown in Bold Basal triple nitrogen plus vitamins medium, which is composed of the following reagents: NaNO3 (8.82 mM, Meyer®, Mexico City, Mexico, CAS 7631-99-4), CaCl2·2H2O (0.17 mM, Merck®, Mexico City, Mexico, CAS 10035-04-8), MgSO4·3H2O (0.03 mM, Meyer®, CAS 10034-99-8), K2HPO4·3H2O (0.43 mM, Merck®, CAS 16788-57-1), KH2PO4 (1.29 mM, Merck®, CAS 7778-77-0), and NaCl (0.43 mM, Meyer®, CAS 7647-14-5). To this medium was added 6 mL of a trace metal stock solution composed of Na2EDTA (2 mM, Meyer®, CAS 6381-92-6), FeCl3·6H2O (0.36 mM, Meyer®, CAS 10025-77-1), MnCl2·4H2O (0.21 mM, Meyer®, CAS 13446-34-9), ZnCl2·6H2O (0.037 mM, Sigma-Aldrich®, Mexico City, Mexico, CAS 10196-18-6), CoCl2·6H2O (0.0084 mM, Merck®, CAS 7791-13-1), and Na2MoO4·2H2O (0.017 mM, Merck®, CAS 10102-40-6).
On the other hand, the microalga Spirulina maxima was seeded in UTEX media composed of NaHCO3 (Sigma-Aldrich®, CAS 144-55-8), K2HPO4·3H2O (0.43 mM, Merck®, CAS 16788-57-1), NaNO3 (8.82 mM, Meyer®, CAS 7631-99-4), K2SO4 (Sigma-Aldrich®, CAS 7778-80-5), NaCl (0.43 mM, Meyer®, CAS 7647-14-5), MgSO4·7H2O (Merck®, CAS 10034-99-8), CaCl2·2H2O (0.17 mM, Merck®, CAS 10035-04-8), FeSO4·7H2O (Meyer®, CAS 7782-63-0), and Na2EDTA (2 mM, Meyer®, CAS 6381-92-6). In addition, 1 mL of Chu micronutrient solution, prepared with H3BO3 (Merck®, CAS 10043-35-3), MnCl2·4H2O (0.21 mM, Meyer®, CAS 13446-34-9), ZnSO4·7H2O (Meyer®, CAS 7446-20-0), CuSO4·5H2O (Meyer®, CAS 7758-99-8), and Co(NO3)2·6H2O (Meyer®, CAS 10026-22-6), was added to 1 L of solution. Among the components of the Utex medium, 1 mL of trace metal solution was also used in the preparation of BBSM.
Both of the microalgal species were seeded and propagated in laboratory-scale microphotobioreactors with a total volume of 1 L and a useful volume of 500 mL. For both strains, a constant temperature of 25 °C was maintained. A pump was used to apply aeration at a flow rate of 1500 cc·min−1 and a pressure of 3.5 psi. The use of oxygen in aeration provides CO2 from the air as a carbon source and maintains the microphotobioreactors under agitation. For illumination, 20 W cold light LED lamps were used with an intensity of 35 μmoL·m−2·s−1 and light/shade photoperiods of 12 h each.
2.3. Sampling and Physicochemical Characterization of Poultry Wastewater
Poultry wastewater was sampled from a poultry processing plant with coordinates of 18°54′40.6″ N and 97°00′27.6″ W located in the Orizaba–Córdoba region. This company processes more than 200 thousand birds a day, which generates a large amount of contaminated water with high contamination potential. The parameters evaluated in the initial characterization, as well as the regulations followed and the equipment used, are listed in Table 1. All analyses were carried out in accordance with the methodologies established in the official Mexican regulations.

Table 1.
Equipment and references used for initial characterization.
2.4. Cultivation of Microalgae in Poultry Wastewater
The growth kinetics of the two microalgal species were determined in laboratory-scale photobioreactors under previously described maintenance conditions (Figure 1). All bioreactors used 3.36 L of poultry wastewater, corresponding to an organic load of 2879 g/L COD, 1.8 L of microalgae, and 0.84 L of distilled water. The semicontinuous process had a daily input and output of 10 mL of wastewater and sample, respectively. The total residence time was 42 days divided into 2 stages: the first with a duration of 21 days was for the growth of the microalgae in the wastewater, while in the second stage (with the same duration as the first), the addition of the different micronutrients was carried out for each microalgal strain. In addition, the biomass generated in the photobioreactors was cultivated every 15 days.

Figure 1.
Chlorella spp. cultivation in photobioreactors.
2.5. Addition of Micronutrients to Microalgal Cultures in Poultry Wastewater
For the correct development of the microalgae, the following micronutrients were added to the reactors: ZnCl2, MnCl2, NaMoO4, and CuSO4. Three doses were evaluated, which are shown in Table 2. Dose 2 is the maximum sublethal dose indicated by various authors, which also promotes cell growth and lipid content of the microalgae. For the first three micronutrients, the amounts established by Ghafari et al. [24] were used, while for copper, what was indicated by Hamed et al. [25] was used. Doses 1 and 3 were established equidistantly, taking dose 2 as a basis.

Table 2.
Dose to evaluate micronutrients in microalgal strains.
2.6. Mathematical Modeling of Microalgal Growth
The Gompertz mathematical model (Equation (1)) was used to describe the behavior of microalgal strains grown in poultry wastewater supplemented with micronutrients:
where Y is the biomass concentration in cells/mL, A is the asymptote, is the maximum specific growth rate in days, is the dormancy phase, and t is the time in days [26]. The modified equation employed in this work is described in Equation (2):
where Y indicates the cell growth as a function of time, A is the maximum population, b is the result of multiplying the specific growth rate by the latency phase by the maximum population plus one, and the parameter c is the lag rate.
2.7. Experimental Design
The presence of micronutrients has been shown to influence the growth of species with photosynthetic activity. For this reason, the dose of micronutrients was evaluated as the study factor of this work, where the central point of the single-factorial experimental design for the micronutrients Mn, Zn, and Mo was selected based on what was indicated by Ghafari et al. [24], while for Cu, what was mentioned by Hamed et al. [25] was used, varying ±50% of the content of each micronutrient according to Table 2. The process was monitored by evaluating the pH, total and volatile solids, cell density, CODs, and biomass daily for 7 weeks, which were considered the response variables. In addition, heavy metals such as copper (Cu), zinc (Zn), and manganese (Mn) were determined by atomic absorption using the Mexican Standard ECOL 01-1996 (AAnalyst 400 Perkin Elmer, Mexico City, Mexico), and arsenic was determined with an atomic absorption spectrophotometer (Avanta, GBC Scientific Equipment, Mexico City, Mexico).
3. Results and Discussion
3.1. Wastewater Characteristics
The results of the physicochemical and microbiological evaluation are shown in Table 3. The pH is similar to the value of 6.13 reported by Basitere et al. [27]. The sample is slightly acidic, which is caused by the supplementation of poultry feed with various organic acids, which are usually added to drinking water [28]. The effluent outlet temperature is similar to the ambient temperature. NOM-001-SEMARNAT-2021 establishes 35 °C as the maximum permissible limit, so this parameter is within the limits established in Mexican regulations for discharge into bodies of water [29]. The COD results obtained are close to those of Yaakob et al. [3], who reported a value of 5422.25 mg/L. COD indicates the amount of organic matter contained in the sample. Therefore, in the case of poultry wastewater, the presence of fecal matter, blood, and fat can increase this value [30]. Poultry effluent has a high turbidity, as confirmed by the result obtained by Caldera et al. [31] of 1100 NTU. This parameter is a consequence of the presence of the same organic pollutant compounds that increase the COD of the wastewater. The obtained value of dissolved oxygen was 1.5 mg/L, which is slightly greater than that reported by Oyewale et al. [32], who reported a value of 1.2 mg/L. Dissolved oxygen and its saturation indicate the amount of oxygen available to various species of flora and fauna in aquatic environments. However, it should be noted that most such species require at least 5 mg/L or saturation of 80 to 120% O2 to survive [33]. From the above, it can be deduced that dumping wastewater into aquifers would be harmful to the biota of such ecosystems. The amount of phosphorus contained in the sample was lower than the 120 mg/L reported by Pinto et al. [34], and this variation may be caused by the treatments prior to final disposal that poultry processors carry out on their wastewater. Phosphorus in wastewater causes eutrophication or excessive growth of algae and macrophyte plants in the receiving bodies, which, with the density of the roots, subsequently prevents the incidence of sunlight into the water, which ultimately causes no development in the fauna and prevents aquatic plants from carrying out the photosynthesis process, which helps provide oxygen to the aquatic environment [35].

Table 3.
Results of physicochemical and microbiological characterization of poultry wastewater.
Since most of the parameters are outside the maximum allowable values, the effluent must be processed before final discharge. Usually, treatments such as dissolved air flocculation (DAF) are used to remove oil, grease, and small particle contaminants [36], as well as for sedimentation, coagulation, biodegradation, or filtration [7], which, although widely used techniques, do not remove all pathogens or microparticulate contaminants [37]. Therefore, such waste can also be re-evaluated for alternative uses; for example, poultry sludge can be used to manufacture activated carbon for the use of organic matter, as well as for the total elimination of pathogenic microorganisms [38]. Additionally, by applying certain strains of microalgae, bioremediation of wastewater can be carried out, which would contribute to reducing its COD and heavy metal content, generating an algal biomass with high nutrient content [39].
3.2. Effect of Micronutrients on the Removal of Organic Pollutants by Microalgae
Once the microalgal species were propagated in their corresponding culture media, the different response variables were analyzed daily (except for biomass, which was analyzed every five days) for each strain.
3.2.1. Chlorella spp.
The first parameter evaluated in the Chlorella spp. cultures was pH. A constant value of 6.1 was maintained until the addition of micronutrients in the third week, where there was a slight increase to 6.4 in this parameter for the three doses, which subsequently remained stable until the end of the process. Chlorella microalgae grow adequately in media with a pH between 7 and 8 [40], so the addition of micronutrients promoted algal growth.
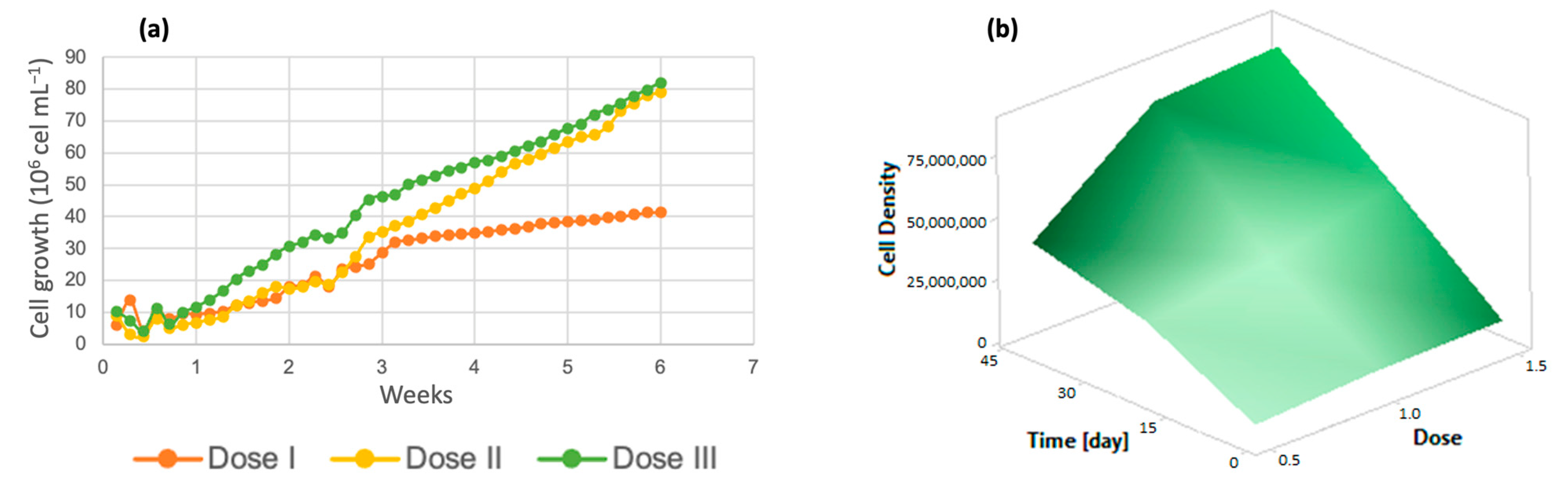
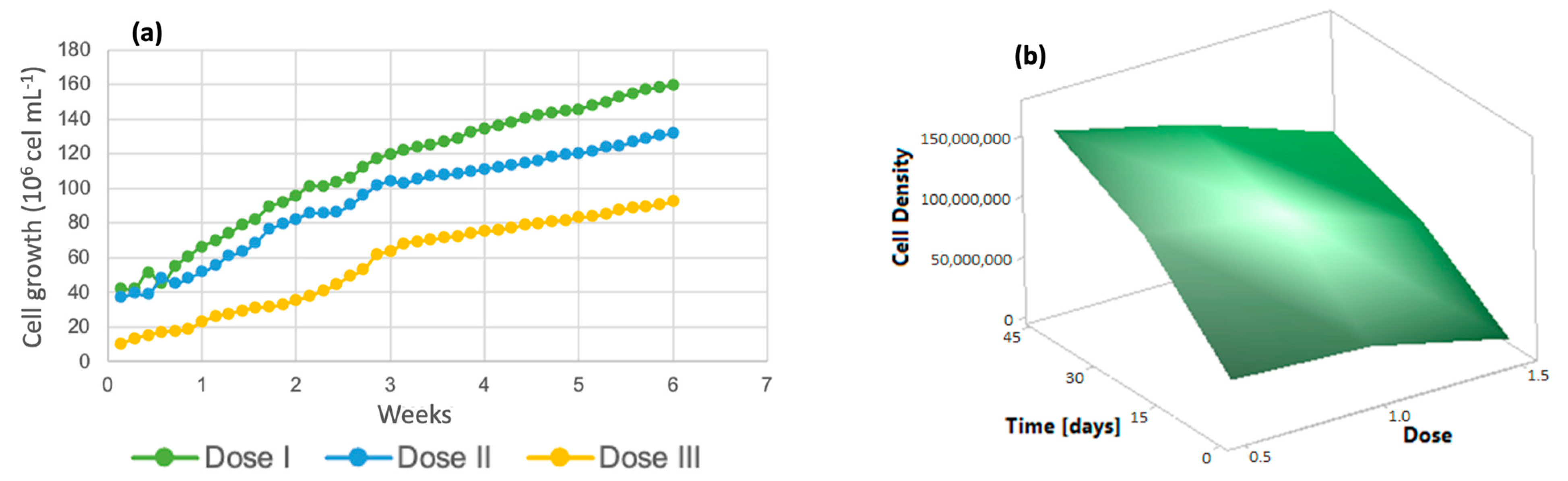
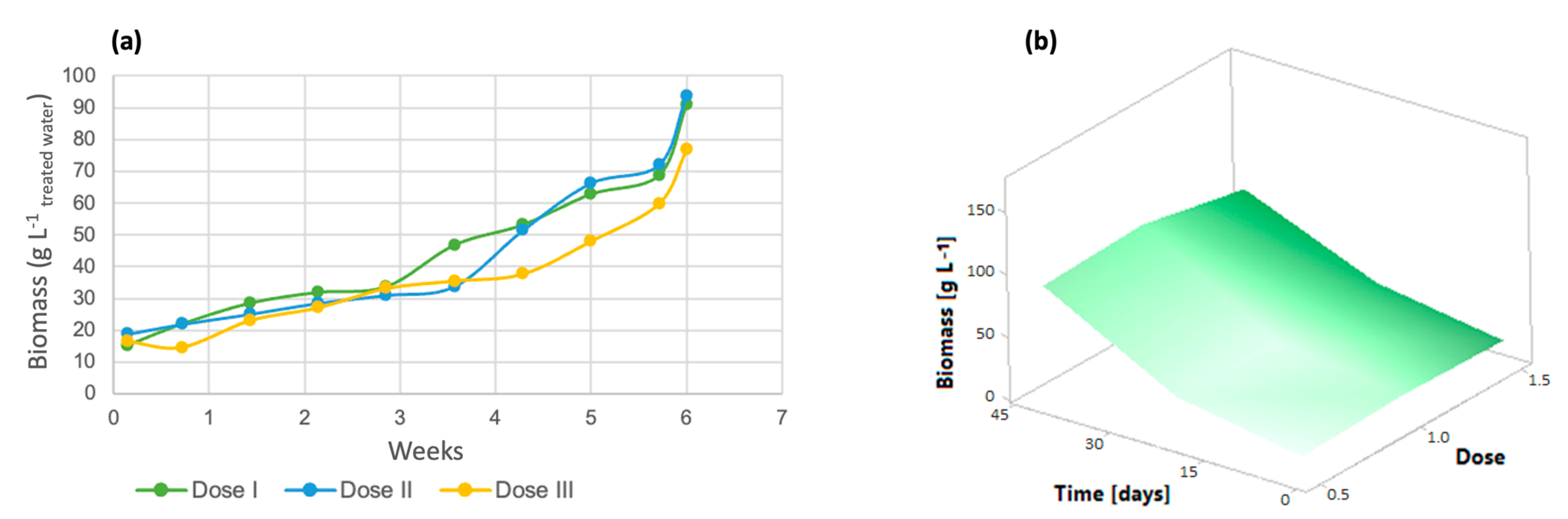
For the analysis of algal growth by quantifying the cell density of the cultures, dose 1 presented a lower number of cells, as shown in Figure 2a, while at dose 3, there was greater cell growth. Solutions of trace metals or trace elements contribute to increasing the yields of metabolic biological processes carried out by photosynthetic organisms [41], increasing cell growth and crop propagation. In addition, it is important to emphasize that the life cycle of the algal species Chlorella lasts approximately 8 days [42] and the reproduction time is 6 days, with an average growth rate of 4.75 million cells/mL [40]. The response surface graph (Figure 2b) shows how the addition of micronutrients caused changes in these variables, which indicates that the cell growth rate reached 75 million algal cells when dose 3 (denoted as 1.5) was applied. On the other hand, dose 2 had a 10% lower performance, and dose 1 reached only 40 million cells; these three doses were evaluated over the same time period of 7 weeks.
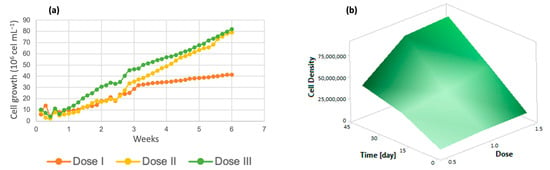
Figure 2.
(a) Cell density (b) Response surface of Chlorella spp.
The total solids in the three treatment groups tended to decrease gradually since they started at a value of 1.86% (w/w), and at the end of the process, only 0.79% of the total solids remained, which represents a removal of 42% in this parameter. In the photobioreactors, the total solids were composed of algal biomass, as well as various solid fragments from wastewater.
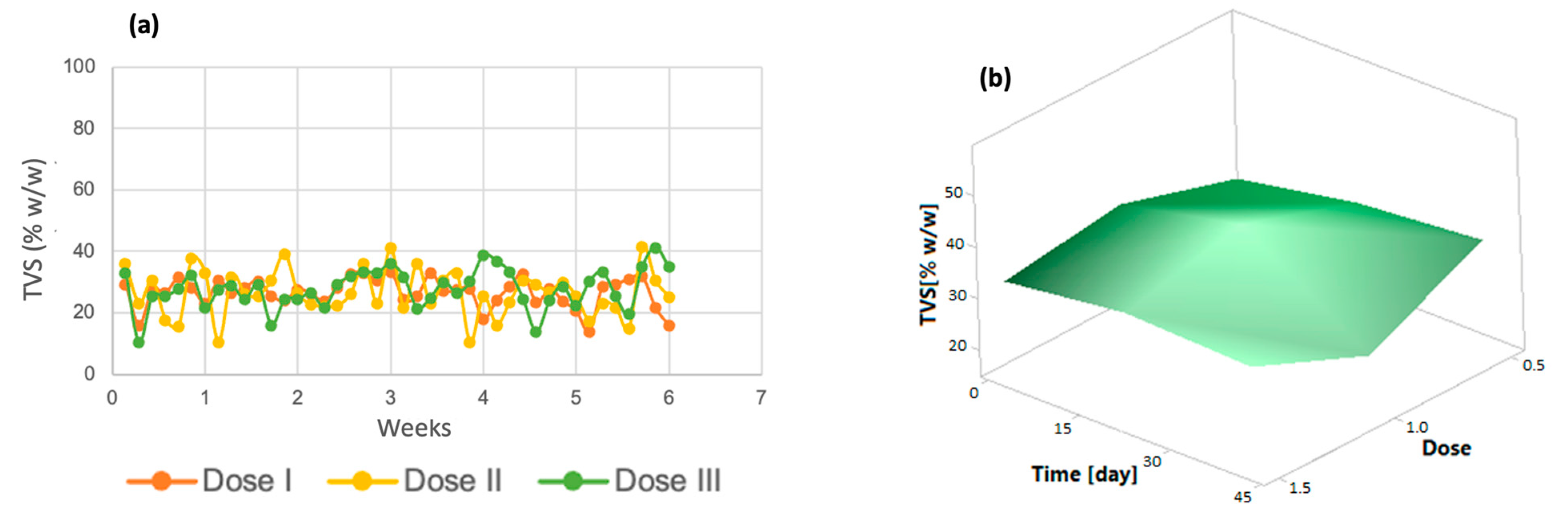
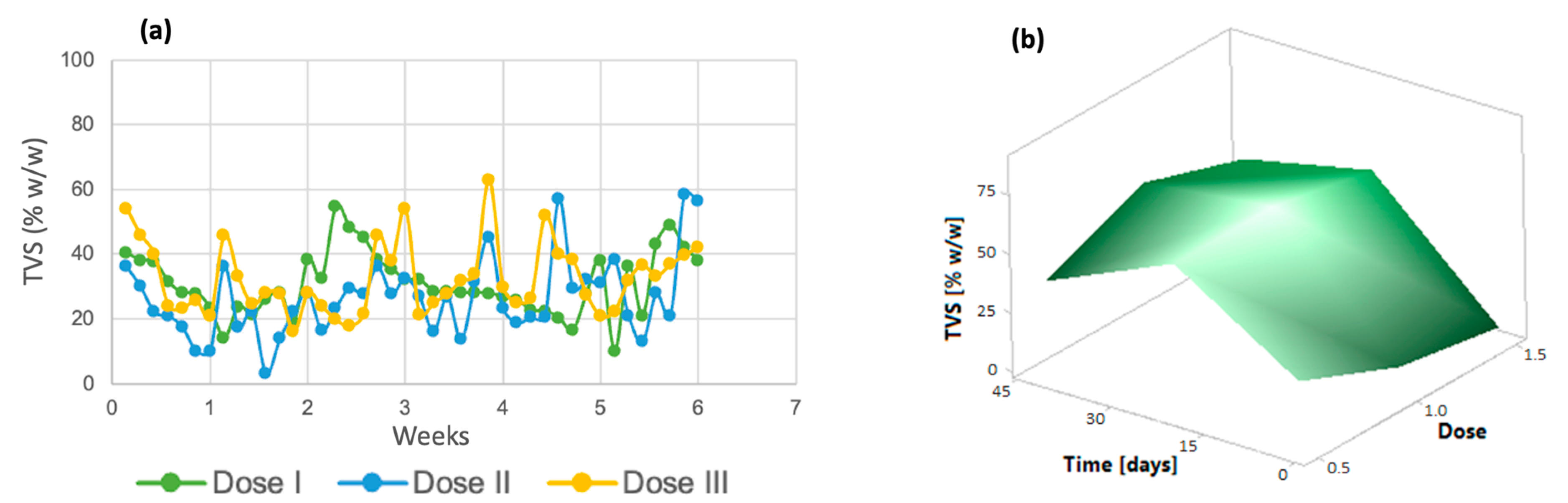
Similarly, volatile solids tended to decrease in the last days of the process (Figure 3a). The same trend was observed in the response surface graph (Figure 3b); only dose 3 increased in the last days of the process. Volatile total solids correspond to a fraction of the organic matter contained in a sample [43]; therefore, the decrease in these indicates that the removal of organic matter derived from the improvement in microalgal metabolism due to the addition of micronutrients is taking place.
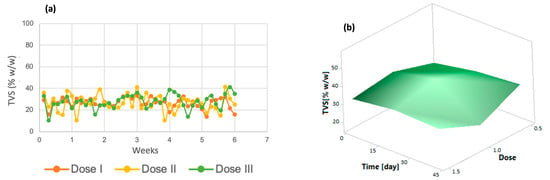
Figure 3.
(a) Total volatile solids (b) Response surface of Chlorella spp.
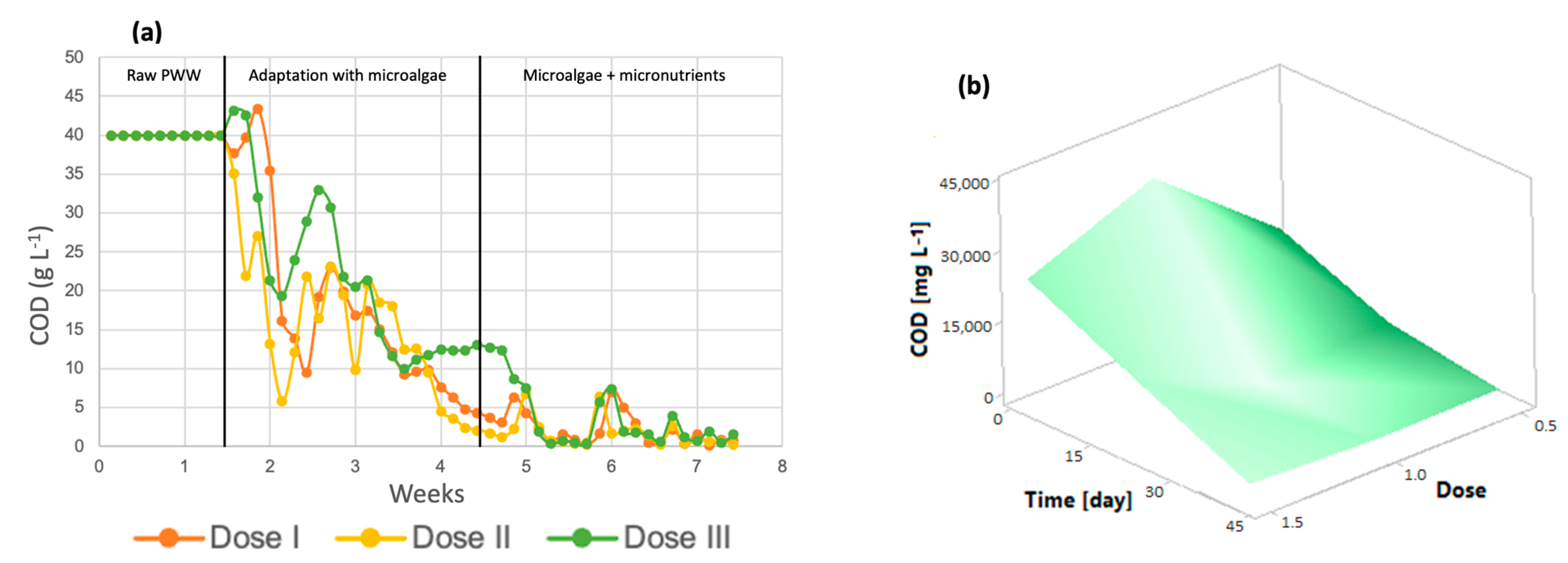
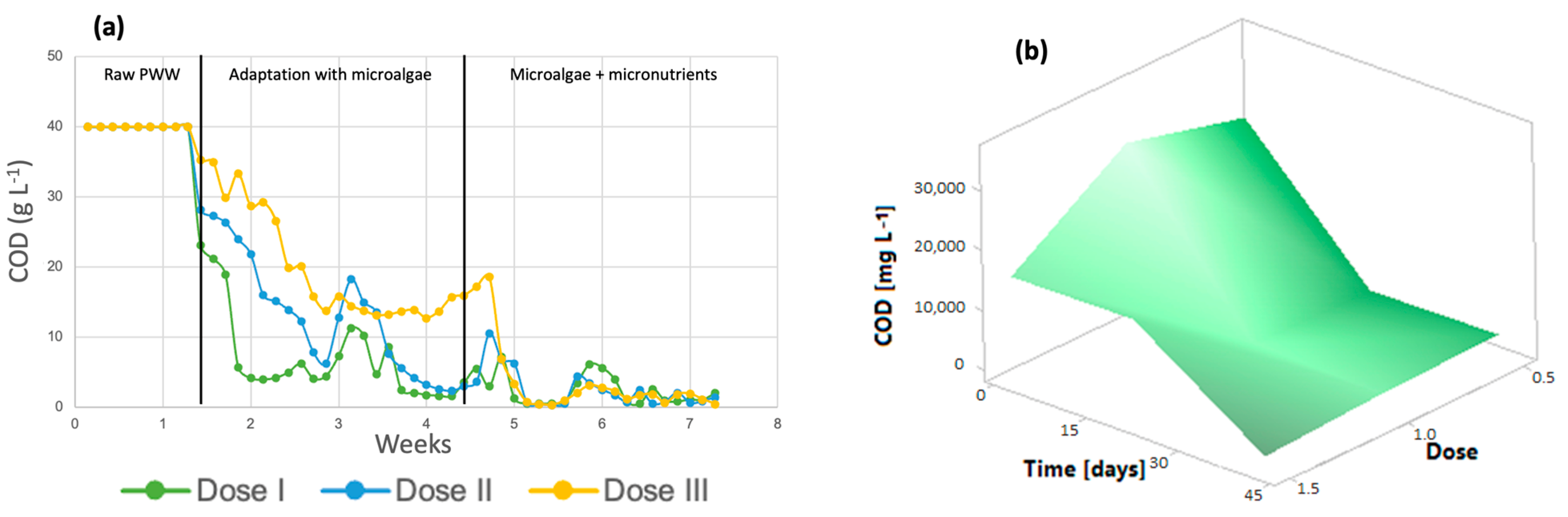
Figure 4a shows that throughout the 7 weeks of the process, the organic load in the microphotobioreactors decreased, reaching very small values after the addition of the micronutrients. According to the results obtained, the COD removal efficiency of the system can be calculated to be at least 96% for dose 3, 99.14% for dose 2, and 98% for dose 1. The initial COD was 39.95 g/L. The graph shows the three steps of the process: photobioreactors containing only wastewater, the stage in which microalgae are added for wastewater treatment, and finally, the addition of micronutrients to the algal cultures, where the greatest removal of organic matter is achieved. It is important to mention that microalgae do not carry out the COD removal process in waste, but the aerobic bacteria present in the effluent generate CO2, which is subsequently used for photosynthesis by the microalgae [44]. The response surface shown in Figure 4b shows a decrease in this parameter throughout the treatment at all three doses, which indicates that any dose is suitable for improving the COD removal efficiency.
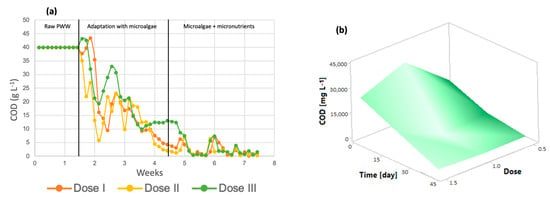
Figure 4.
(a) The chemical oxygen demand (b) Response surface of Chlorella spp.
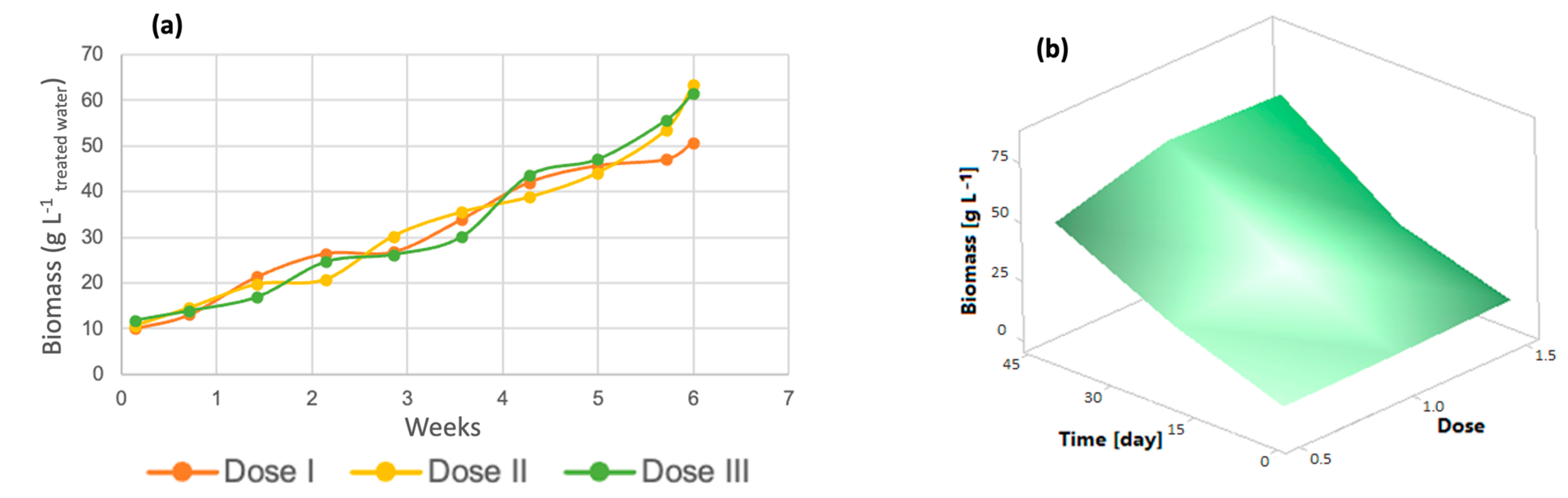
In wastewater treatment with microorganisms such as microscopic algae, it is important to evaluate biomass generation, which is influenced by the cultivation of microalgae in poultry wastewater [45]. As shown in Figure 5a, the three doses of micronutrients contributed equally to algal biomass generation, as no significant differences were observed between the doses (p-value > 0.005). Dose 1 had the lowest amount of biomass, with a total of 50.73 g/L, while dose 2 had 61.53 g/L and dose III had 63.52 g/L, as confirmed by the response surface graph in Figure 5b since it was observed that algal biomass was generated during the course of the experiment and that dose 2 had a better effect on this variable than dose 1, which had a yield almost 20% lower.
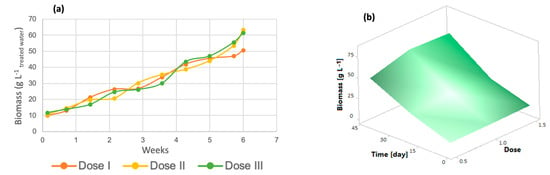
Figure 5.
(a) Biomass generation (b) Response surface of Chlorella spp.
The cultivation of microalgae in wastewater and the use of micronutrients enhance the growth and generation of microalgal biomass [46]. The biomass yields in relation to COD removed are as follows: 1.039 g biomass/g COD removed for dose 1, 1.335 g biomass/g COD removed in dose 2, and 1.296 g biomass/g COD removed for dose 3.
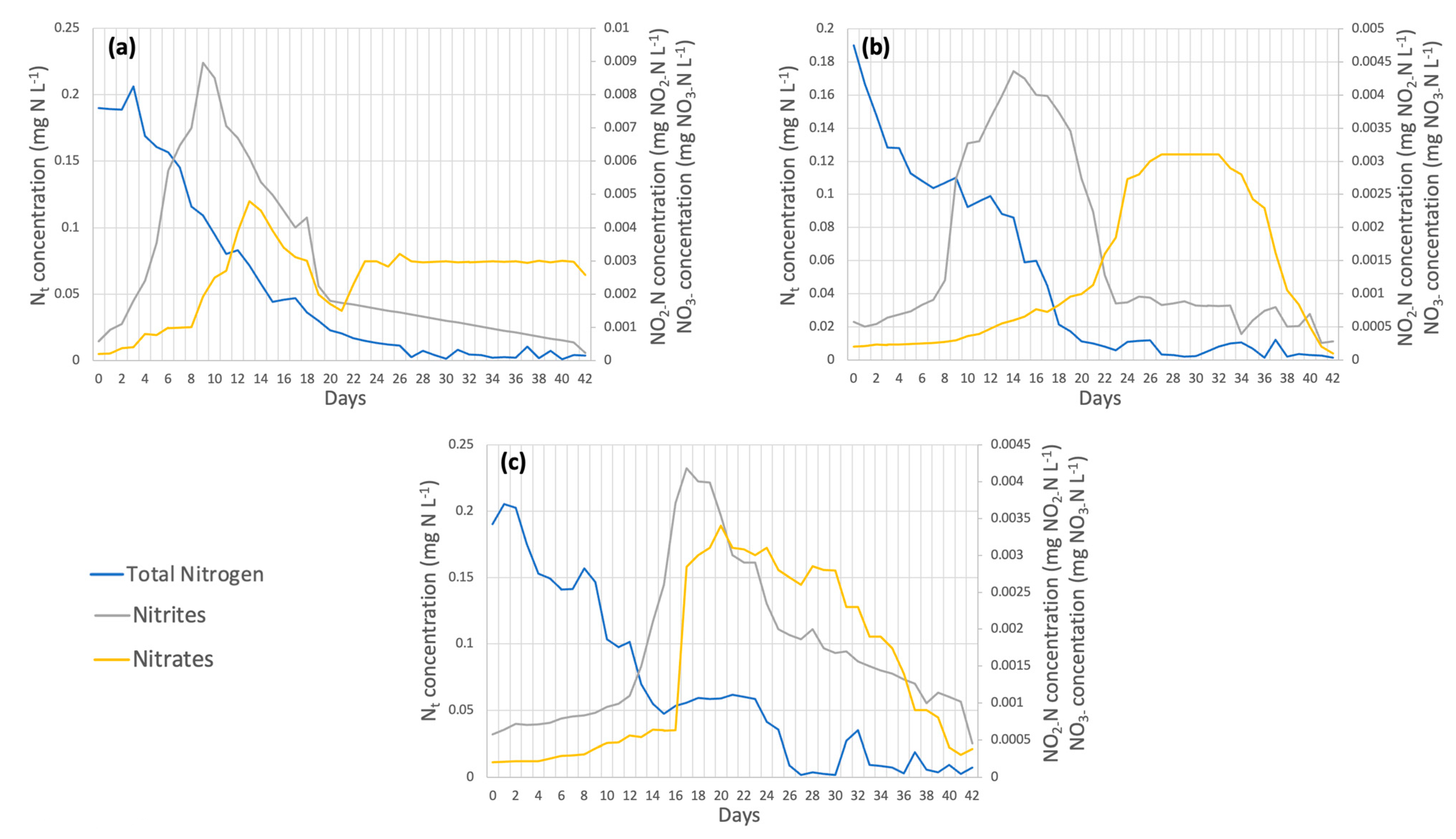
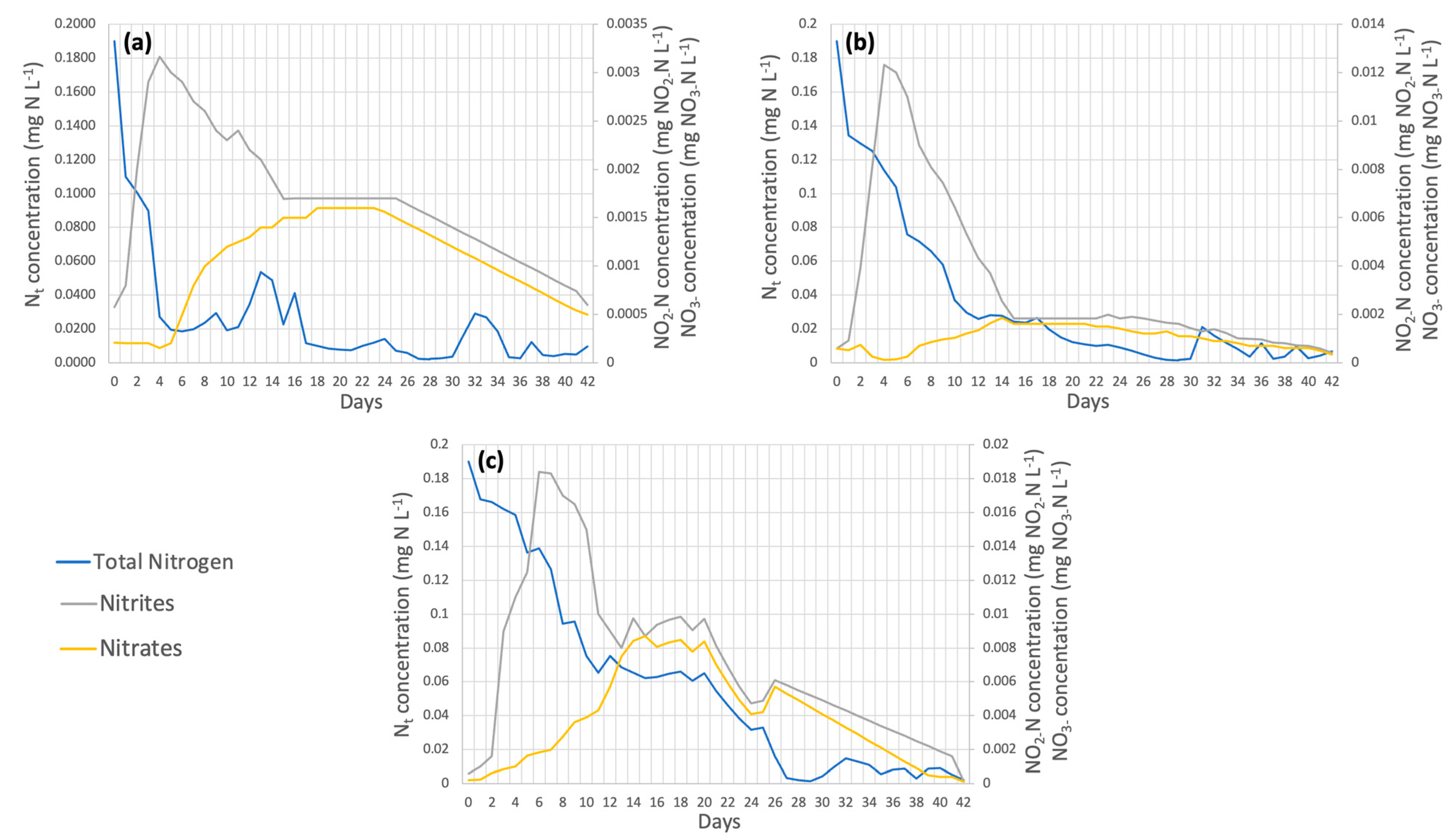
On the other hand, nitrogen removal efficiencies were obtained, as shown in Figure 6. These percentages were 98.08% for dose 1, 99.33% for dose 2, and 96.11% for dose 3, with dose 2 being the best. Dębowski et al. [47] achieved up to 95% removal of nitrogen in the form of NH4+ using the species Chlorella vulgaris; therefore, the increased removal of this element may be due to the application of micronutrients in the cultivation of this strain in the present work. The nitrogen contained in the wastewater is in the ammoniacal form, which is oxidized in two stages: in the first stage, it goes from N-NH4+ to N-NO2−, and later, the second stage goes from N-NO2− to N-NO3− [48]. The first stage occurs faster in dose 1, as it peaks on day 10 (Figure 6a); in dose 2, it occurs on day 14 (Figure 6b); and in dose 3, it occurs until day 18 (Figure 6c). The oxidation of nitrite to nitrate occurred at similar times for the three doses between days 21 and 22, as shown in Figure 6a–c).
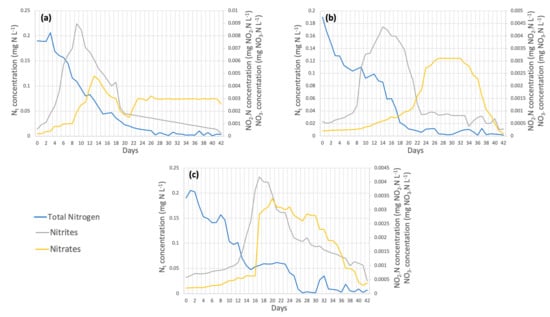
Figure 6.
(a) Dose 1 (b) Dose 2 (c) Dose 3 Nitrogen removal in wastewater by Chlorella spp.
3.2.2. Spirulina maxima
The pH of the Spirulina algal strain decreased after the addition of micronutrients on day 21. This result is similar to that obtained with Chlorella algae, and it was observed that the medium tended to be neutralized by trace elements. The lower pH in the Spirulina culture may be due to the generation of CO2 during the process since, as indicated by Henry’s Law, there is a relationship between these two factors: the greater the amount of carbon dioxide is, the lower the decrease in pH will be [49].
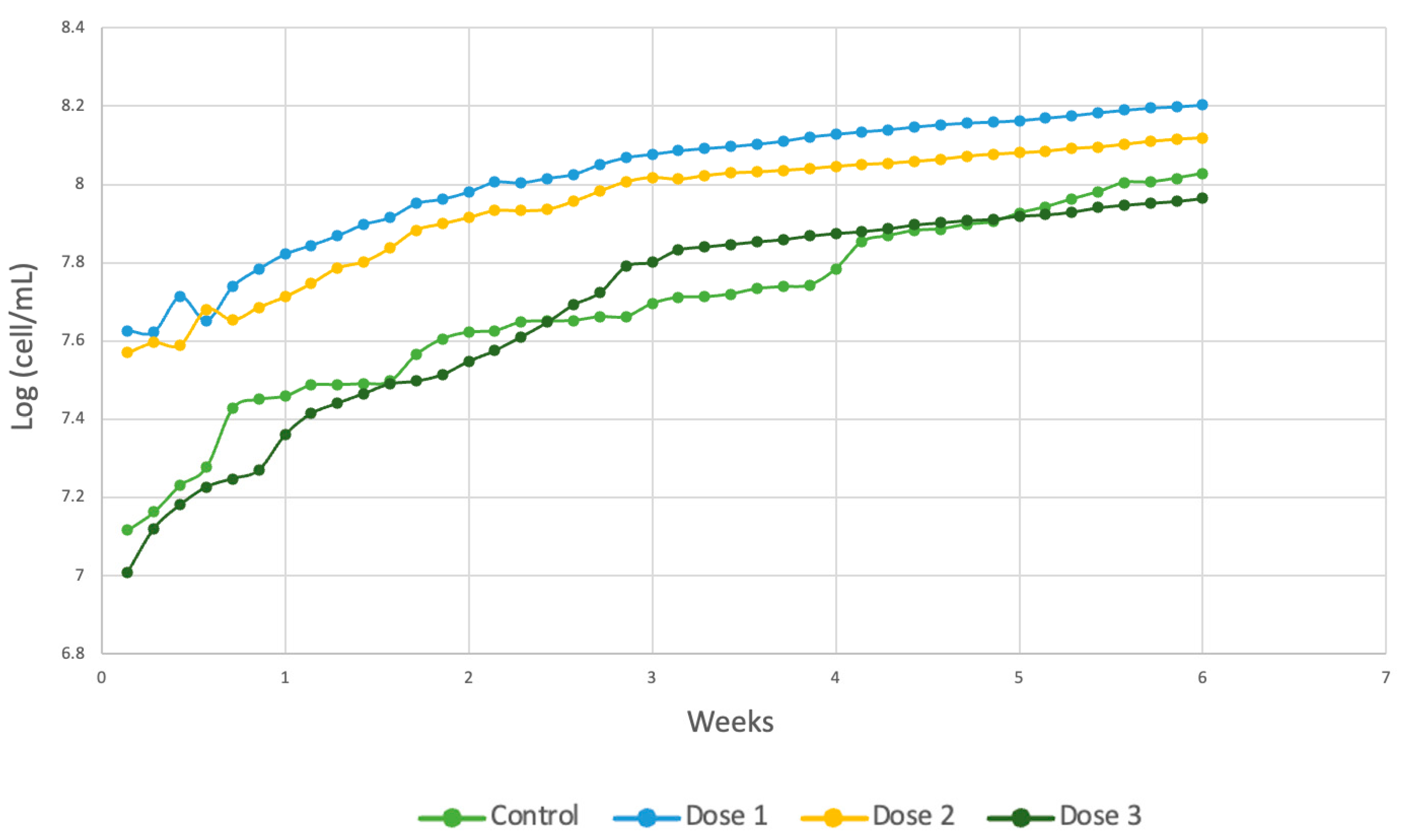
Spirulina maxima presented greater cell growth at dose 1, as shown in Figure 7a. Contrary to what was obtained for the previous strain, in the Spirulina species, dose 3 presented lower efficiency in terms of the generation of algal cells, and dose 2 had an intermediate effect on the two previous strains, as shown in Figure 7b. It is important to mention that this species has a greater uptake of trace metals in media with a pH of 8 [50], and by subjecting the cultures to a higher basicity level, the micronutrients do not favor cell growth.
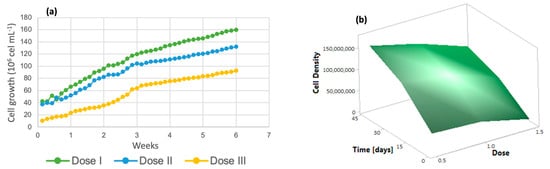
Figure 7.
(a) Cell density (b) Response surface of Spirulina maxima.
During the wastewater treatment, a low concentration of total solids was detected, but with the addition of micronutrients, the amount of solids decreased at the end of the process at the three treatment levels, and this strain presented lower total solids than Chlorella spp. This is due to microalgal development, which contributes to the removal of total volatile solids (constituents of total solids) used as a source of nutrients and the precipitation of solids present in poultry wastewater.
Total volatile solids are greater in the Spirulina strains, as shown in Figure 8a; therefore, it can be inferred that such cultures have a higher concentration of organic matter, unlike the Chlorella spp. reactors. In the third week, the total volatile solids increased and decreased at the end, and according to Figure 8b, the best dose for total volatile solids removal was 1.
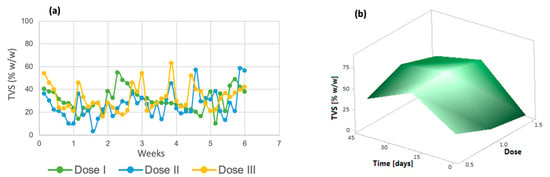
Figure 8.
(a) Total volatile solids (b) Response surface of Spirulina maxima.
For the organic load, this parameter decreased at the three tested doses (Figure 9a). There was an increase in the COD in the third week when the micronutrients were added. COD removal percentages of 88% for dose 1, 94% for dose 2, and 98% for dose 3 were achieved. As with the previous algal species, this strain followed the procedure of first adding the raw wastewater to the photobioreactors and then adding Spirulina to each for effluent treatment, followed by adding various doses of micronutrients to improve algal metabolism and contribute to the removal of the organic load. The response surface plot in Figure 9b shows that all doses promoted the removal of organic matter, expressed as COD.
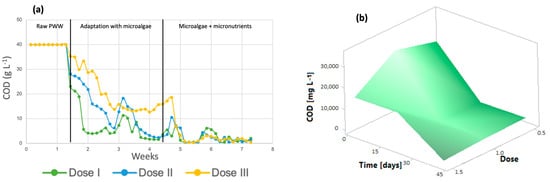
Figure 9.
(a) Chemical oxygen demand (b) Response surface of Spirulina maxima.
The biomass of the Spirulina maxima strain can be seen in Figure 10a. All three doses favored biomass production, although dose 3 had a lower effect, with a total of 76.66 g/L, while doses 1 and 2 had very similar effects, with values of 91.02 g/L and 93.73 g/L, respectively. The addition of inorganic micronutrients (such as Fe, Mg, and Mn) to algal cultures has been shown to aid in cell development, lipid production, and carbohydrate, protein, pigment, and carotenoid storage in microalgae [51]. The response surface (Figure 10b) shows that dose 2 promoted the generation of microalgal biomass in this strain, while the dose with the lowest yield was 3. Regarding the biomass yields in relation to the amount of organic matter removed, the following results were obtained: for dose 1, for each gram of COD removed, 2.001 g of biomass was generated; for dose 2, 1.946 g biomass/g COD removed was produced; and finally, for dose 3, 1.524 g biomass/g COD removed was obtained.
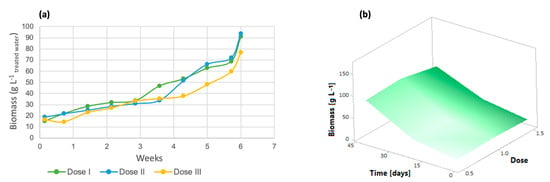
Figure 10.
(a) Biomass generation (b) Response surface of Spirulina maxima.
In terms of nitrogen removal (Figure 11), 98.08%, 99.33%, and 96.11% of the total nitrogen removal efficiency occurred in dose 1, dose 2, and dose 3, respectively, showing that the dose with the highest removal efficiency was dose 1. Samori et al. [52] established that the nitrogen removed is incorporated into the microalgal biomass in the form of proteins, which explains why Spirulina maxima is one of the species richest in protein content [53]. Nitrite oxidation to nitrate occurred on week 2 day 16 for dose 1 (Figure 11a), week 2 day 15 for dose 2 (Figure 11b), and week 2 day 13 for dose 3 (Figure 11c).
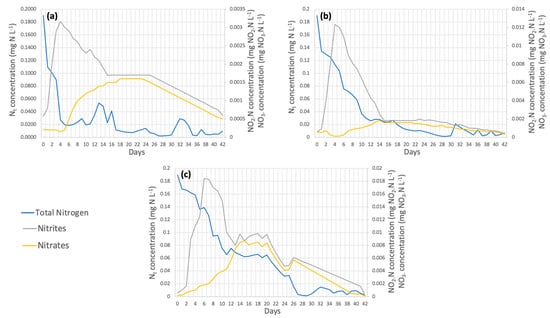
Figure 11.
(a) Dose 1 (b) Dose 2 (c) Dose 3 Nitrogen removal in wastewater by Spirulina maxima.
The microalgae Chlorella spp. and Spirulina maxima present a metal absorption percentage greater than 90% [54], which causes these chemical species to not be detected in the process effluent by atomic absorption spectrometry. In a previous study by Gutierrez-Casiano et al. [44] this effect was demonstrated.
3.3. Kinetic Parameters
The Gompertz equations obtained for each dose applied to Chlorella spp. were as follows:
The kinetic parameters corresponding to the maximum population, lag phase, duration of the latency phase, and generation time were obtained by means of the previous models in addition to their corresponding graphs, which are shown in Table 4 for Chlorella spp.

Table 4.
Kinetic parameters for Chlorella spp.
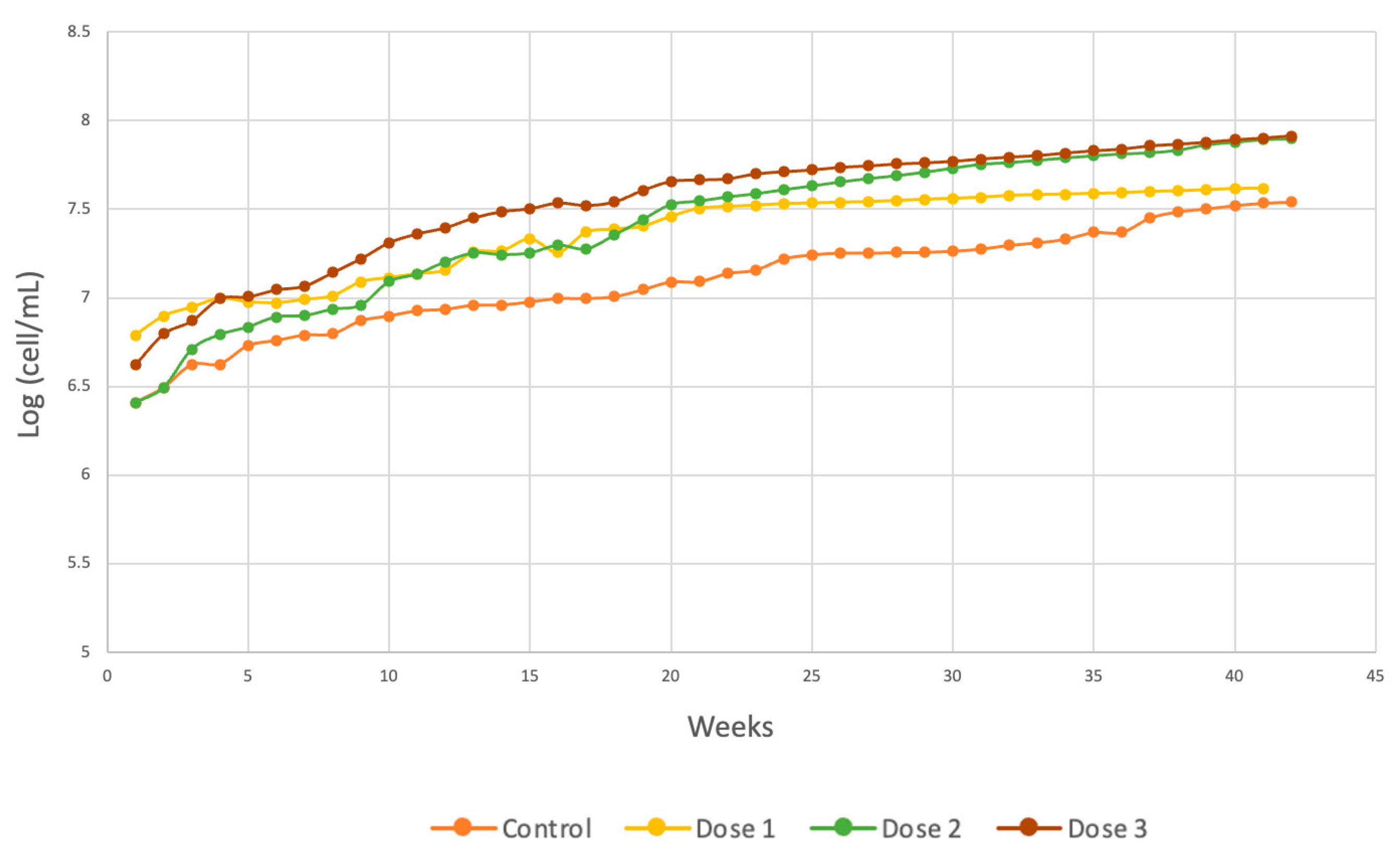
According to Equation (5) and Figure 12, dose 2 reached a greater population than did the other two applied doses, indicating that the interaction of the substrate and the presence of micronutrients produced greater affinity between the species and the substrate, initiating the exponential phase on day 4.
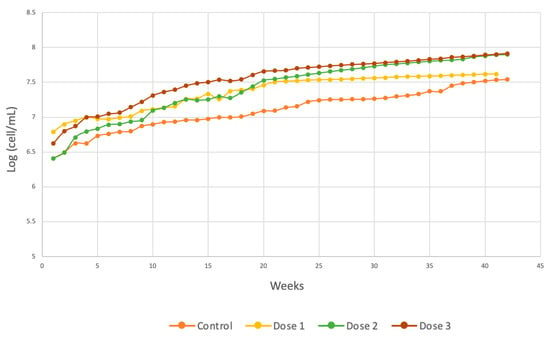
Figure 12.
Comparison of Chlorella spp. cell generation.
Finally, the generation time was shorter in the dose 3 group, reaching a time of 1 day, which was almost 2 days shorter than that in the control group. These results can be compared with those of the control, which did not receive any type of micronutrient, as indicated in Equation (3). It can also be seen from the other kinetic parameters obtained and broken down in Table 3 that μm was lower in the control group and higher at dose 3. The best synergy between the substrate and contaminants of a biodegradable organic nature present in the wastewater and the microalgal species evaluated was achieved with dose 3, increasing algal growth. The degree of fit of the Gompertz model for each of the doses was 97.56% for dose 1, 99.13% for dose 2, and 99.4% for dose 3.
For Spirulina maxima, the mathematical models found for each of the micronutrient doses are as follows:
The various parameters obtained are listed in Table 5, and the growth graphs are shown in Figure 13. Additionally, the graphs corresponding to microbial growth are shown according to each of the doses applied. In this case, the maximum population reached was in the reactors with dose 3 added, as shown in Equation (10), while doses 1 and 2 presented lower maximum populations. The generation time was similar for doses 1 and 3, and the growth rate was lower for dose 2 (Equation (9)), although the longest generation time was obtained for dose 2 at 8 days.

Table 5.
Kinetic parameters for Spirulina maxima.
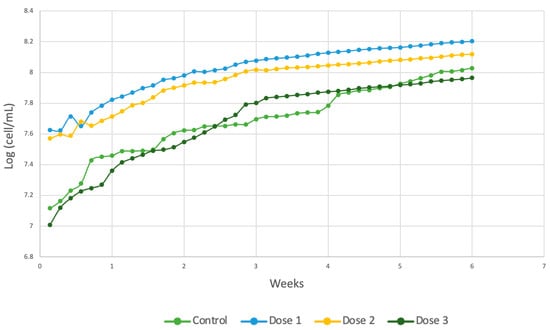
Figure 13.
Comparison of cell generation in Spirulina maxima.
The kinetic parameters for the control group without micronutrients are expressed in Equation (7). The control group had a generation time double that required by the crops with the other three doses of micronutrients. Based on previously reported data, the best dose for improving cell development in Spirulina maxima is dose 3 since it takes 1.3 d to reach the maximum population, 8.048 cells/mL. Therefore, the addition of the minimum dose improves the ability of microalgae to adapt to wastewater [55], which is reflected by the difference between the μm, which is greater in dose 1, at 0.5888, and lower in the control blank, at 0.2816. The correlation coefficients for each of the doses in this strain were 99.32% for dose 1, 99.06% for dose 2, and 99% for dose 3.
Table 6 shows the most relevant parameters per microalgal strain obtained during the experiments.

Table 6.
Summary results obtained by dose and strain.
3.4. Color and Turbidity Analysis
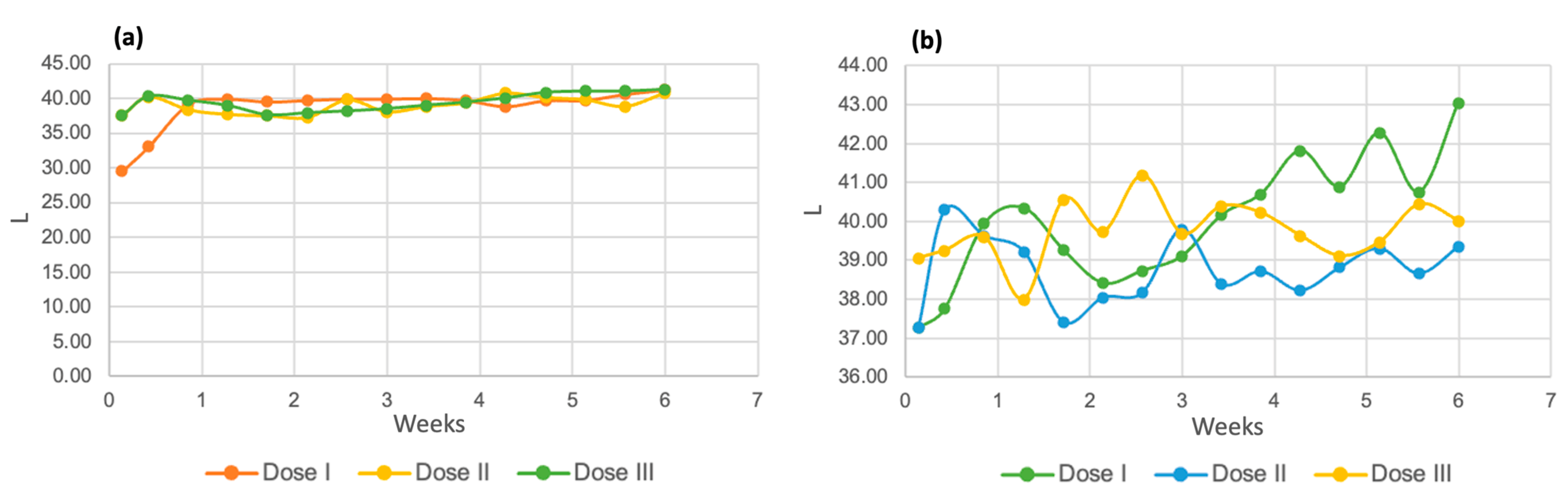
The results of the three-color parameters evaluated for both species of microalgae can be seen in the following images. Figure 14a shows the luminosity of the Chlorella spp. strain. It can be seen that in the first days of the process, this value tends to increase until it remains at a constant value of 40 units for the three tested doses. Regarding the Spirulina maxima strain, dose 2 presented lower overall luminosity values, followed by dose 3. The dose that showed the highest results was dose 1, with a final value of 43.06.
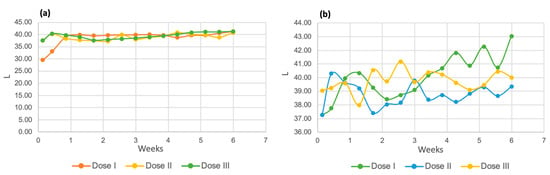
Figure 14.
Luminosity of Chlorella spp. (a) and Spirulina maxima (b).
The luminosity readings and their increasing trend indicate that the samples became lighter as the biological purification process progressed [56].
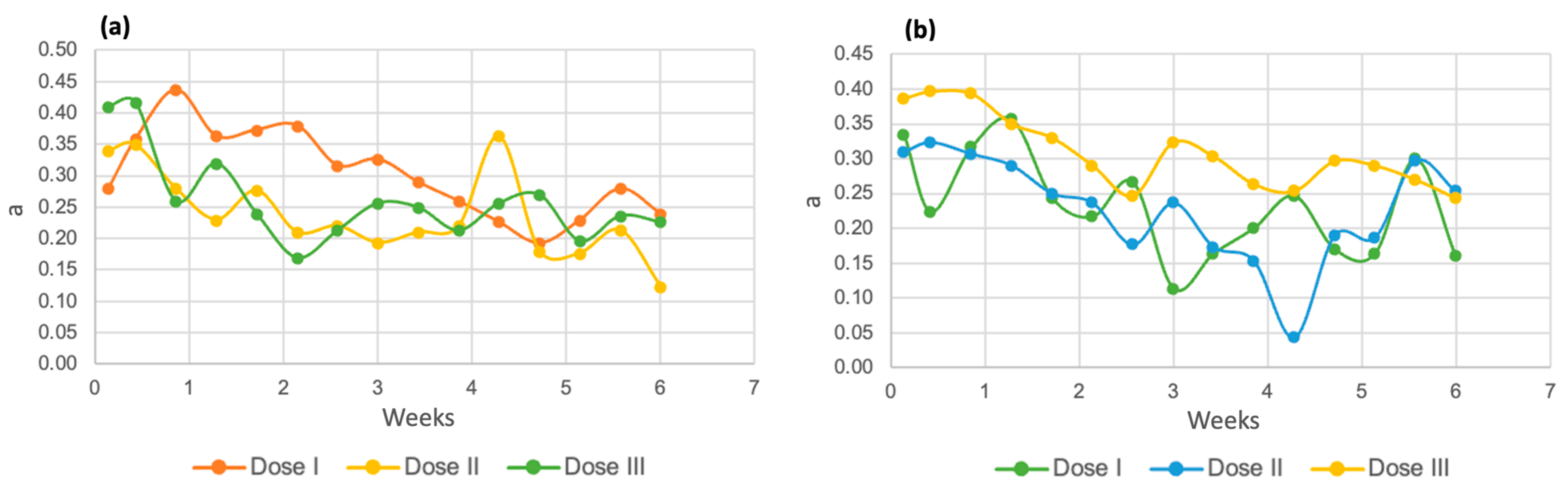
The behavior of the “a” coordinate in Chlorella spp. (Figure 15a) was similar at the three tested doses, with readings in the range of 0.20 to 0.40 units, while in the Spirulina maxima strain (Figure 15b), this coordinate had lower results, between 0.40 and 0.04. Since this axis indicates the hue between red and green, it can be interpreted that both samples have a very slight tendency toward red, which may be because the residual water among its components has blood from processed birds [57]. Subsequently, the values decrease until they almost reach zero, which indicates closeness to the white tone in the samples.
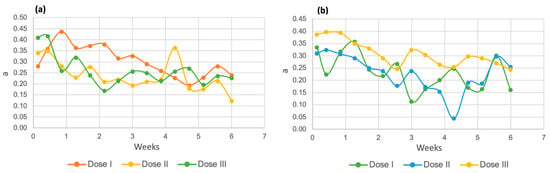
Figure 15.
Coordinate “a” for Chlorella spp. (a) and Spirulina maxima (b).
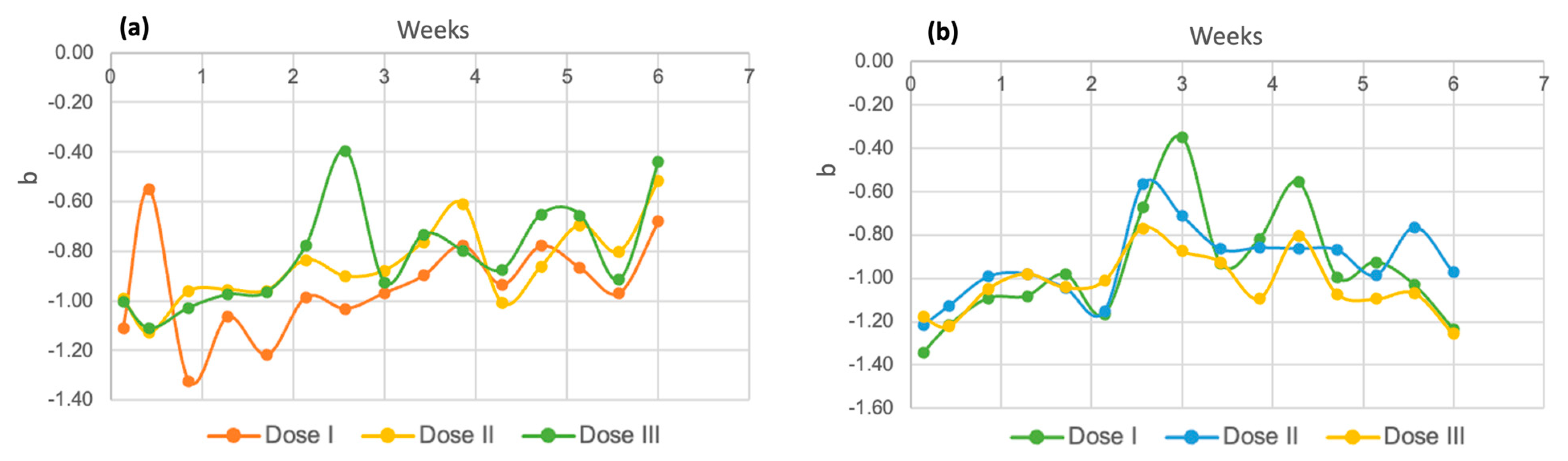
Regarding the “b” axis, in Figure 16, it can be noted that in both species, the results are given in negative numbers, which is an indicator of a tendency toward a blue tone [58]. More negative values were detected for Spirulina maxima (Figure 16b) than for the other Chlorella species (Figure 16a), which is consistent with the fact that the Spirulina variety is considered a blue–green alga, which is why it is called a cyanophyte [59].
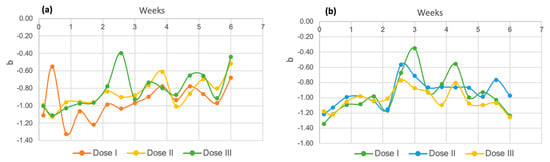
Figure 16.
Coordinate “b” for Chlorella spp. (a) and Spirulina maxima (b).
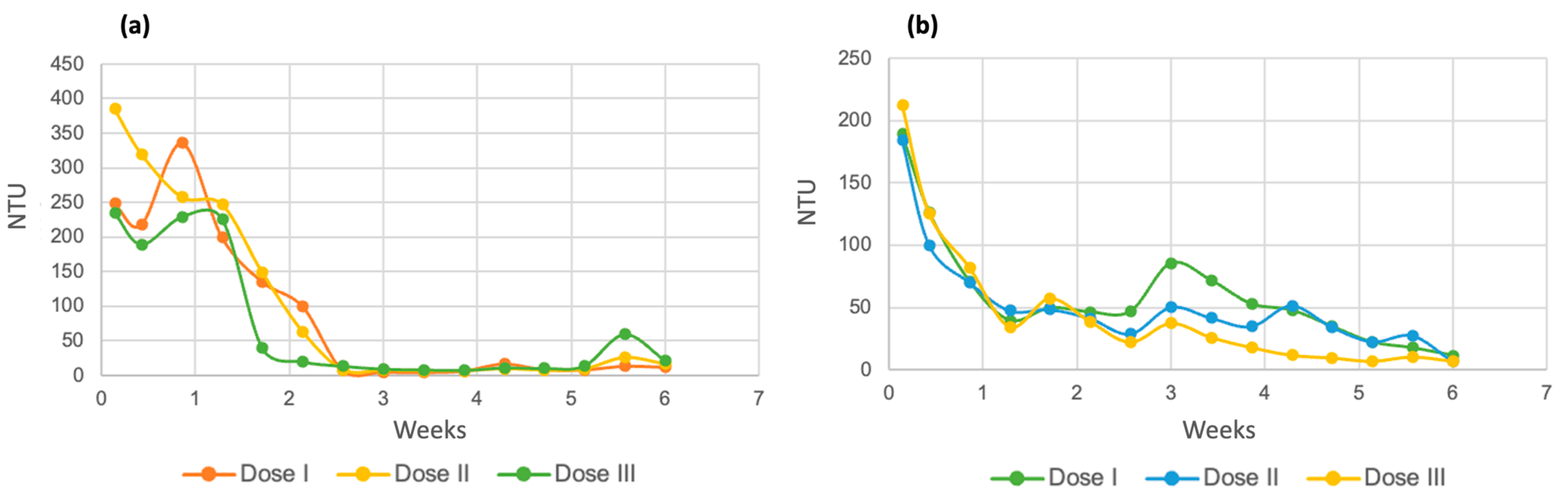
The turbidity results of the evaluation of this parameter for both varieties of microalgae are shown in Figure 17. Part a indicates the values for Chlorella spp., and it can be seen in the three doses that the removal of very evident turbidity was observed. At dose 1, the process began with a value of 248 NTU, ending with 11.82 NTU; at dose 2, it ranged from 385 NTU to 16.17; and at dose 3, the first turbidity reading was 234 NTU, which led to a total of 21.27 NTU. With the previous values, the removal percentages were 95.23% for dose 1, 95.8% for dose 2, and 90.91% for dose 3.
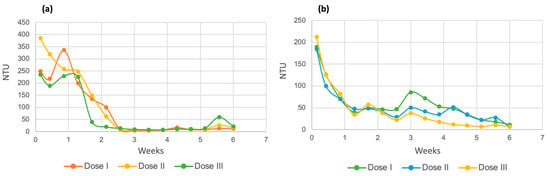
Figure 17.
Turbidity for Chlorella spp. (a) and Spirulina maxima (b).
In the case of Spirulina maxima (Figure 17b), a notable removal of turbidity was similarly observed for all doses and runs. At dose 1, the initial value was 189.33 NTU, and the final value was 11.60 NTU; at dose 2, the initial value was 184.33 NTU, and the final value was 6.89 NTU; while at the third dose, the corresponding values were 212.33 NTU and 7.07 NTU. The removal percentages for each dose were 93.87% for dose 1, 96.26% for dose 2, and 96.67% for the last dose of micronutrients.
The turbidity results are consistent with those of COD since the decrease in turbidity in a sample is due to the degradation of organic matter present in the form of suspended solids [60].
The use of microalgae as an alternative treatment for effluent treatment is viable since it has been demonstrated that the species Chlorella, Scenedesmus, Euglena, Chlamydomonas, Oscillatoria, Ankistrodesmus [61], Hematococcus [62], and Nannochloropsis [63] can grow in residual substrates, taking advantage of the macronutrients they contain for their development, such as nitrogen, which is used in algal metabolism for the synthesis of proteins and is mainly incorporated into ribosomal RNA [64]. Trace elements, such as Cu, Mn, Mo, and Zn, are necessary to carry out enzymatic processes and metabolic reactions within the cellular structure of microalgae [61]. The aforementioned heavy metals are biosorbed in a two-step mechanism: the first is where the adsorbate binds rapidly and reversibly to the active sites on the surface of the microalgae, followed by the slow phase, which refers to positive intracellular diffusion that mainly involves various microalgal metabolic reactions [61].
The use of microalgae for the treatment of wastewater of poultry origin is a technique with considerable potential because it avoids the generation of residual sludge derived from physicochemical treatments, which is highly polluting to the environment. On the other hand, with the proposed biological treatment, valuable nutritional biomasses are obtained that can be applied at an industrial level, in addition to the generation of oxygen from harmful greenhouse gases present in the atmosphere due to the action of microalgae.
4. Conclusions
According to physicochemical and microbiological evaluations, poultry wastewater is an effluent with high contamination potential and the capacity to alter the ecosystem if it is discharged indiscriminately into the environment. Microalgal cultures with these waters were demonstrated to remove organic matter, and these effects were enhanced by the addition of micronutrients. For Chlorella spp., dose 3 increased the cell density, while the three doses decreased the total solids and total volatiles in the treated effluent. The removal of organic matter, expressed as total COD, increased from 88.59% to 98% for dose 1 compared to the control, from 94.01% to 99.14% for dose 2, and from 69.9% to 96% for dose 3.
Kinetic parameters calculated with the Gompertz model revealed a precision of up to 99% for the behavior of the microalgal populations with the addition of three doses of micronutrients, and it was observed that dose 1 was appropriate for Chlorella spp. Additionally, dose 3 improved the efficiency of contaminant removal and therefore the metabolism of the Spirulina maxima species. Therefore, it was concluded that the addition of micronutrients to microalgae can integrate a highly efficient operation strategy for photobioreactors that can be used to treat poultry wastewater to ensure compliance with official regulations in terms of water quality.
Author Contributions
Conceptualization, J.M.M.-C.; methodology, J.M.M.-C., S.M.P.-G., E.H.-A. and A.A.-L.; validation, J.M.M.-C. and S.M.P.-G.; formal analysis, J.M.M.-C., S.M.P.-G., E.H.-A. and A.A.-L.; investigation, S.M.P.-G., E.H.-A. and A.A.-L.; resources, J.M.M.-C.; writing—original draft preparation, S.M.P.-G., E.H.-A. and J.M.M.-C.; writing—review and editing, S.M.P.-G., E.H.-A. and J.M.M.-C.; supervision, J.M.M.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the support of the infrastructure use facilities of the Tecnológico Nacional de México Campus Orizaba. This research was supported by the Tecnológico Nacional de México (TecNM) through project 14368.22-P. Solmaría Mandi Pérez-Guzmán thanks the National Consejo Nacional de Ciencia y Tecnología (CONACyT) for the scholarship awarded for a master’s degree with CVU (scholarship holder) 1103203.
Data Availability Statement
All data are available in the manuscript.
Acknowledgments
The graphical abstract was created with BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdul-Aziz, H.; Ahmad-Puat, N.N.; Alazaiza, M.Y.D.; Hung, Y.T. Poultry Slaughterhouse Wastewater Treatment Using Submerged Fibers in an Attached Growth Sequential Batch Reactor. Int. J. Environ. Res. Public Health 2018, 15, 1734. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mondal, T.; Sharma, R.; Mahalakshmi, N.; Gupta, M. Poultry Waste Management. Int. Curr. Microbiol. Appl. Sci. 2018, 7, 701–712. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Saphira Radin Mohamed, R.M.; Saeed Al-Gheethi, A.A.; Mohd Kassim, A.H. Characteristics of Chicken Slaughterhouse Wastewater. Chem. Eng. Trans. 2018, 63, 637–642. [Google Scholar] [CrossRef]
- Fatima, F.; Du, H.; Kommalapati, R.R. Treatment of poultry Slaughterhouse Wastewater with Membrane Technologies: A Review. Water 2021, 13, 1905. [Google Scholar] [CrossRef]
- Sabando-Fraile, C.; Corral-Bobadilla, M.; Lostado-Lorza, R.; Somovilla-Gomez, F. Multiresponse Performance Evaluation and Life Cycle Assessment for the Optimal Elimination of Pb (II) from Industrial Wastewater by Adsorption Using Vine Shoot Activated Carbon. Sustainability 2023, 15, 11007. [Google Scholar] [CrossRef]
- Baker, B.R.; Mohamed, R.; Al-Gheethi, A.; Aziz, H.A. Advanced technologies for poultry slaughterhouse wastewater treatment: A systematic review. J. Dispers. Sci. Technol. 2020, 42, 880–899. [Google Scholar] [CrossRef]
- Terán-Hilares, R.; Atoche-Garay, D.F.; Pinto-Pagaza, D.A.; Ahmed, M.A.; Colina-Andrade, G.J.; Santos, J.C. Promising physicochemical technologies for poultry slaughterhouse wastewater treatment: A critical review. J. Environ. Chem. Eng. 2021, 9, 105174. [Google Scholar] [CrossRef]
- Menaa, F.Z.; Arbib, Z.; Perales, J.A. Urban wastewater photobiotreatment with microalgae in a continuously operated photobioreactor: Growth, nutrient removal kinetics and biomass coagulation—Flocculation. Environ. Technol. 2019, 40, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Geremia, E.; Rippa, M.; Catone, C.M.; Ulgiati, S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments 2021, 8, 136. [Google Scholar] [CrossRef]
- Hernández, E.; Lobato-Benítez, C.; Hernández, C.A. Algal enzymes, biotechnological potential uses: A review. Cymbella 2017, 3, 1–15. [Google Scholar]
- Emparan, Q.; Harun, R.; Danquah, M.K. Role of phycoremediation for nutrient removal from wastewaters: A review. Appl. Ecol. Environ. Res. 2019, 17, 889–915. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- NMX-AA-008-SCFI-2016; Análisis de Aguas—Medición del pH en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2016.
- NMX-AA-007-SCFI-2013; Análisis de Agua—Medición de la Temperatura en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2013.
- NMX-AA-038-SCFI-2001; Análisis de Agua—Determinación de Turbiedad en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-034-SCFI-2015; Análisis de Agua—Medición de Sólidos y Sales Disueltas en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2015.
- NMX-AA-012-SCFI-2001; Análisis de Agua—Determinación de Oxígeno Disuelto en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-093-SCFI-2000; Análisis de Agua—Determinación de la Conductividad Electrolítica. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2000.
- NMX-AA-029-SCFI-2001; Diario Oficial de la Federación. Análisis de aguas—Determinación de fósforo total en aguas naturales, residuales y residuales tratadas. Método de prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-030-SCFI-2001; Análisis de Agua—Determinación de la Demanda Química de Oxígeno en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-026-SCFI-2010; Análisis de Agua—Medición de Nitrógeno Total Kjeldahl en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2010.
- NMX-AA-113-SCFI-2012; Análisis de Agua—Medición del Número de Huevos de Helminto en Aguas Residuales y Residuales Tratadas por Observación Microscópica. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2012.
- NMX-AA-042-SCFI-2015; Análisis de Agua—Enumeración de Organismos Coliformes Totales, Organismos Coliformes Fecales (Termotolerantes) y Escherichia coli—Método del Número más Probable en Tubos Múltiples. Diario Oficial de la Federación: Mexico City, Mexico, 2015.
- Ghafari, M.; Rashidi, B.; Haznedaroglu, B.Z. Effects of macro and micronutrients on neutral lipid accumulation in oleaginous microalgae. Biofuels 2016, 9, 147–156. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.A.; Sousa, H.; Vale, F.; Simões, M. Microalgae-based bioremediation of wastewaters—Influencing parameters and mathematical growth modeling. Chem. Eng. J. 2021, 425, 131412. [Google Scholar] [CrossRef]
- Basitere, M.; Njoya, M.; Rinquest, Z.; Ntwampe, S.K.O.; Sheldon, M.S. Performance evaluation and kinetic parameter analysis for static granular bed reactor (SGBR) for treating poultry slaughterhouse wastewater at mesophilic condition. Water Pract. Technol. 2019, 14, 259–268. [Google Scholar] [CrossRef]
- Ángel-Isaza, J.; Mesa-Salgado, N.; Narváez-Solarte, W. Ácidos orgánicos, una alternativa en la nutrición avícola: Una revisión. Rev. CES Med. Zootec. 2019, 14, 45–58. [Google Scholar] [CrossRef]
- NOM-001-SEMARNAT-2021; Límites Permisibles de Contaminantes en las Descargas de Aguas Residuales en Cuerpos Receptores Propiedad de la Nación. Diario Oficial de la Federación: Mexico City, Mexico, 2021.
- Meiramkulova, K.; Bazarbayeva, T.; Orynbassar, R.; Tleukulov, A.; Madina, N.; Mashan, T.; Dariya, A.; Apendina, A.; Nurmukhanbetova, N. Assesing the Influence of Electrode Polarity on the Treatment of Poultry Slaughterhouse Wastewater. Molecules 2022, 27, 1014. [Google Scholar] [CrossRef] [PubMed]
- Caldera, Y.; Sánchez, M.; Gutiérrez, E. Calidad física de aguas residuales de una industria avícola en un sistema de flotación por aire disuelto con coagulantes. Rev. Tecnocientífica 2017, 13, 57–66. [Google Scholar]
- Oyewale, A.T.; Adesakin, T.A.; Oyedeji, O.; Aduwo, A.I.; Bakare, M.K. Impact Assessment of Poultry Discharge on the Physico-Chemical and Microbiological Water Quality of Olosuru Stream in Ikire, Southwestern Nigeria. J. Water Resour. Prot. 2018, 10, 1061–1082. [Google Scholar] [CrossRef]
- Bozorg-Haddad, O.; Delpasand, M.; Loáiciga, H.A. 10—Water quality, hygiene, and health. In Economical, Political, and Social Issues in Water Resource; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Pinto, L.A.D.M.; Pinto, M.D.M.; Bovo, J.; Mateus, G.A.P.; Tavres, F.D.O.; Baptista, A.T.A.; Hirata, A.K. Aspectos ambientais do abate de aves: Uma revisão. Uningá Rev. 2015, 22, 44–50. [Google Scholar]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Sánchez, M.; Caldera, Y.; Gutiérrez, E. Eficiencia de coagulantes durante el tratamiento de aguas residuales de la industria avícola en un sistema de flotación. Impacto Cient. 2017, 12, 201–214. [Google Scholar]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Morales, J.L.; Gutiérrez-González, E.C.; Colina-Andrade, G.J. Obtención y caracterización de carbón activado obtenido de lodos de plantas de tratamiento de agua residual de una industria avícola. Ing. Investig. Tecnol. 2016, 17, 453–462. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Barata, A.; Batista, A.P.; Gouveia, L. Scenedesmus obliquus in poultry wastewater bioremediation. Environ. Technol. 2019, 40, 3735–3744. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Pizarro, R. Crecimiento y capacidad de bioremedicación de Chlorella vulgaris (Trebouxiophycea Chlorophyta) cultivada en aguas residuales generadas en el cultivo del pez dorado Seriola lalandi (Perciformes: Carangidae). Rev. Biol. Mar. Oceanogr. 2018, 53, 75–86. [Google Scholar] [CrossRef]
- Rodriguez, I.B.; Ho, T.Y. Trace Metal Requirements and Interactions in Symbiodinium kawagutii. Front. Microbiol. 2018, 9, 142. [Google Scholar] [CrossRef]
- Malagón-Micán, M.L.; Suárez-Chaparro, M.Y. Influencia en la concentración inicial de Chlorella vulgaris y CO2 en la producción de lípidos. Rev. Lasallista Investig. 2020, 17, 56–69. [Google Scholar] [CrossRef]
- Geddes, L.; Kunihiro, K.; Turner, E. Simplified Procedures for Water Examination; AWWA: Denver, CO, USA, 2014. [Google Scholar]
- Gutiérrez-Casiano, N.; Hernández-Aguilar, E.; Alvarado-Lassman, A.; Méndez-Contreras, J.M. Removal of carbon and nitrogen in wastewater from a poultry processing plant in a photobioreactor cultivated with the microalga Chlorella vulgaris. J. Environ. Sci. Health Part A 2022, 57, 620–633. [Google Scholar] [CrossRef]
- Tan, X.B.; Zhao, X.C.; Zhang, Y.L.; Zhou, Y.Y.; Yang, L.B.; Zhang, W.W. Enhanced lipid and biomass production using alcohol wastewater as carbon resource for Chlorella pyrenoidosa cultivation in anaerobically digested starch wastewater in outdoors. Bioresour. Technol. 2018, 247, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Dębowski, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Romanowska-Duda, Z. Biomass Production and Nutrient Removal by Chlorella vulgaris from Anaerobic Digestion Effluents. Energies 2018, 11, 1654. [Google Scholar] [CrossRef]
- Carrasquedo-Ferrer, S.J.; Rincón-Lizardo, N.C.; Díaz-Montiel, A.R.; Pire-Sierra, M.C. Monitoreo de la remoción biológica de nitrógeno en efluentes de tenerías usando un reactor por carga secuencial. Ing. Investig. Tecnol. 2014, 15, 287–298. [Google Scholar] [CrossRef]
- Hudson, R.; De Graaf, R.; Rodin, M.S.; Ohno, A.; Lane, N.; McGlynn, S.E.; Yamada, Y.M.A.; Nakamura, R.; Barge, L.M.; Braun, D.; et al. CO2 reduction driven by a pH gradient. Proc. Natl. Acad. Sci. USA 2020, 117, 22873–22879. [Google Scholar] [CrossRef] [PubMed]
- Al-Homaidan, A.A.; Alabdullatif, J.A.; Al-Hazzani, A.A.; Al-Ghanayem, A.A.; Alabbad, A.F. Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J. Biol. Sci. 2015, 22, 795–800. [Google Scholar] [CrossRef]
- Suastes-Rivas, J.K.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y.; Chairez, I. Simultaneous Optimization of Biomass and Metabolite Production by a Microalgae-Yeast Coculture Under Inorganic Micronutrients. Bioenergy Res. 2020, 13, 974–985. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater: Part I. Water Res. 2013, 2, 791–801. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, S.; Manna, M.S.; Gayen, K.; Bhowmick, T.K. Chapter 12—Extraction of carbohydrates and proteins from algal resources using supercritical and subcritical fluids for high-quality products. In Innovative and Emerging Technologies in the Biomarine Food Sector; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-Mediated Biosorption for Effective Heavy Metals Removal from Wastewater: A Review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- Sandgruber, F.; Gieldsdorf, A.; Baur, A.C.; Schenz, B.; Müller, S.M.; Schwerdtle, T.; Stangl, G.I.; Griehl, C.; Lorkowski, S.; Dawczynski, C. Variability in Macro- and Micronutrients of 15 Commercially Available Microalgae Powders. Mar. Drugs 2021, 19, 310. [Google Scholar] [CrossRef]
- Owens, B.F.; Matthew, D.; Diepenbrock, C.H.; Tiede, T.; Wu, D.; Mateos-Hernández, M.; Gore, M.A.; Rocheford, T. Genome-Wide Association Study and Pathway-Level Analysis of Kernel Color in Maize. G3 2019, 9, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Ngobeni, P.V.; Gutu, L.; Basitere, M.; Harding, T.; Ikumi, D. Poultry Slaughterhouse Wastewater Treatment Using an Integrated Biological and Electrocoagulation Treatment System: Process Optimization Using Response Surface Methodology. Sustainability 2022, 14, 9561. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Martín-Casado, A.M.; Quispe, N.; Gallardo, E.R.; Montero, J. Color Changes of Acetal Resins (CAD-CAM) In Vivo. Appl. Sci. 2023, 13, 181. [Google Scholar] [CrossRef]
- Fernández-Honores, A.M.; Alvítez-Izquierdo, E.; Rodríguez-Izquierdo, E.F. Taxonomía e importancia de “Spirulina” Artrhospira jenneri (Cyanophyceae: Oscillatoriaceae). Arnaldoa 2019, 26, 1091–1104. [Google Scholar]
- Gilpavas, E.; Arbeláez-Castaño, P.E.; Medina-Arroyave, J.D.; Gómez-Atehortua, C.M. Tratamiento de aguas residuales de la industria textil mediante coagulación química acoplada a procesos Fenton intensificados con ultrasonido de baja frecuencia. Rev. Int. Contam. Ambient. 2017, 34, 157–167. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Nishshanka, G.K.S.H.; Premaratne, V.C.L.M.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Kornaros, M. Wastewater-based microalgal biorefineries for the production of astaxanthin and coproducts: Current status, challenges and future perspectives. Bioresour. Technol. 2021, 342, 126018. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Ho, N.; Ogden, K.L.; Arnold, R.G. Cultivation of Nannochloropsis salina in municipal wastewater or digester centrate. Ecotoxicol. Environ. Saf. 2014, 103, 45–53. [Google Scholar] [CrossRef]
- Beuckles, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).