Hydrochemical Characteristics of Groundwater and Their Significance in Arid Inland Hydrology
Abstract
:1. Introduction
2. Materials and Methods
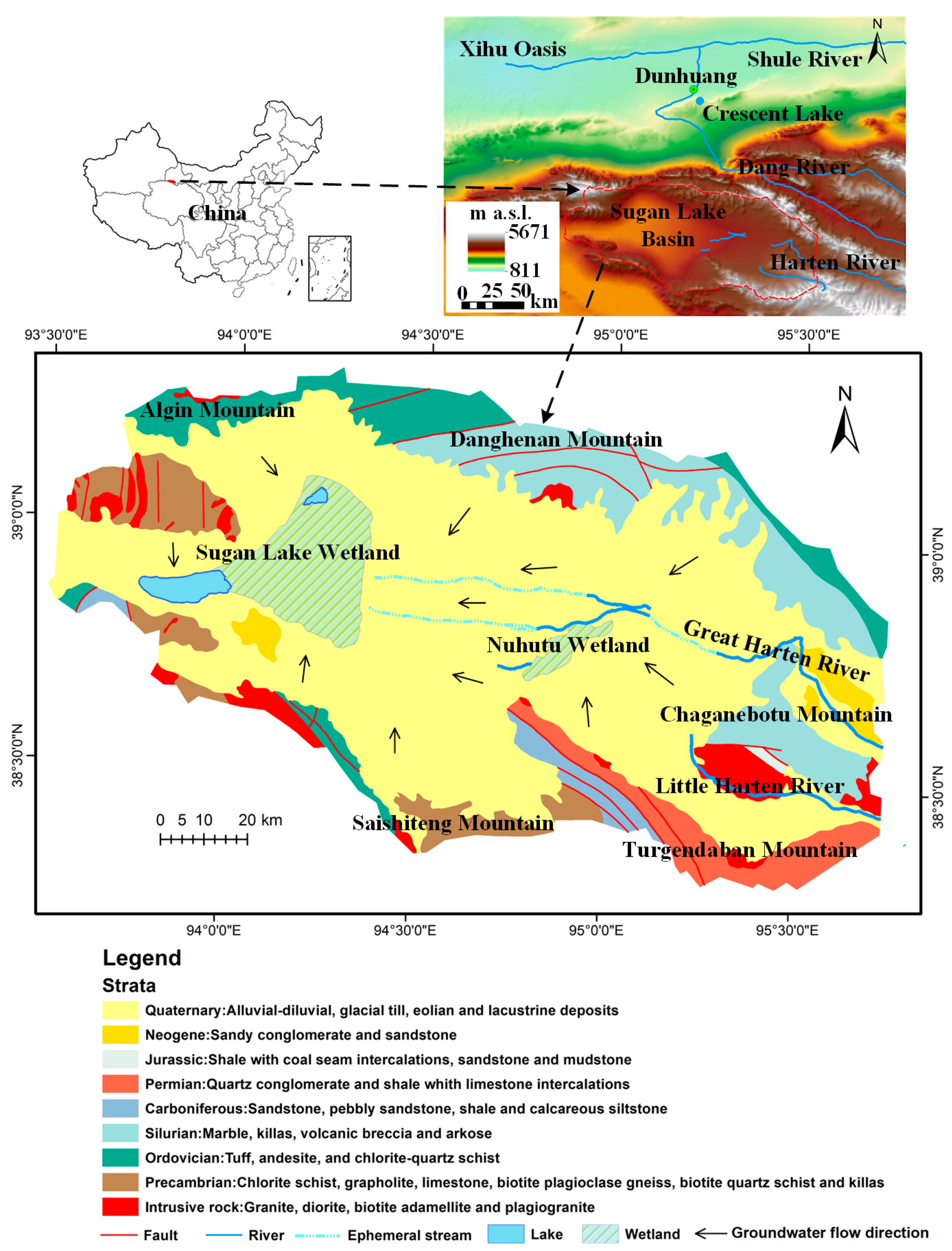
2.1. Study Area
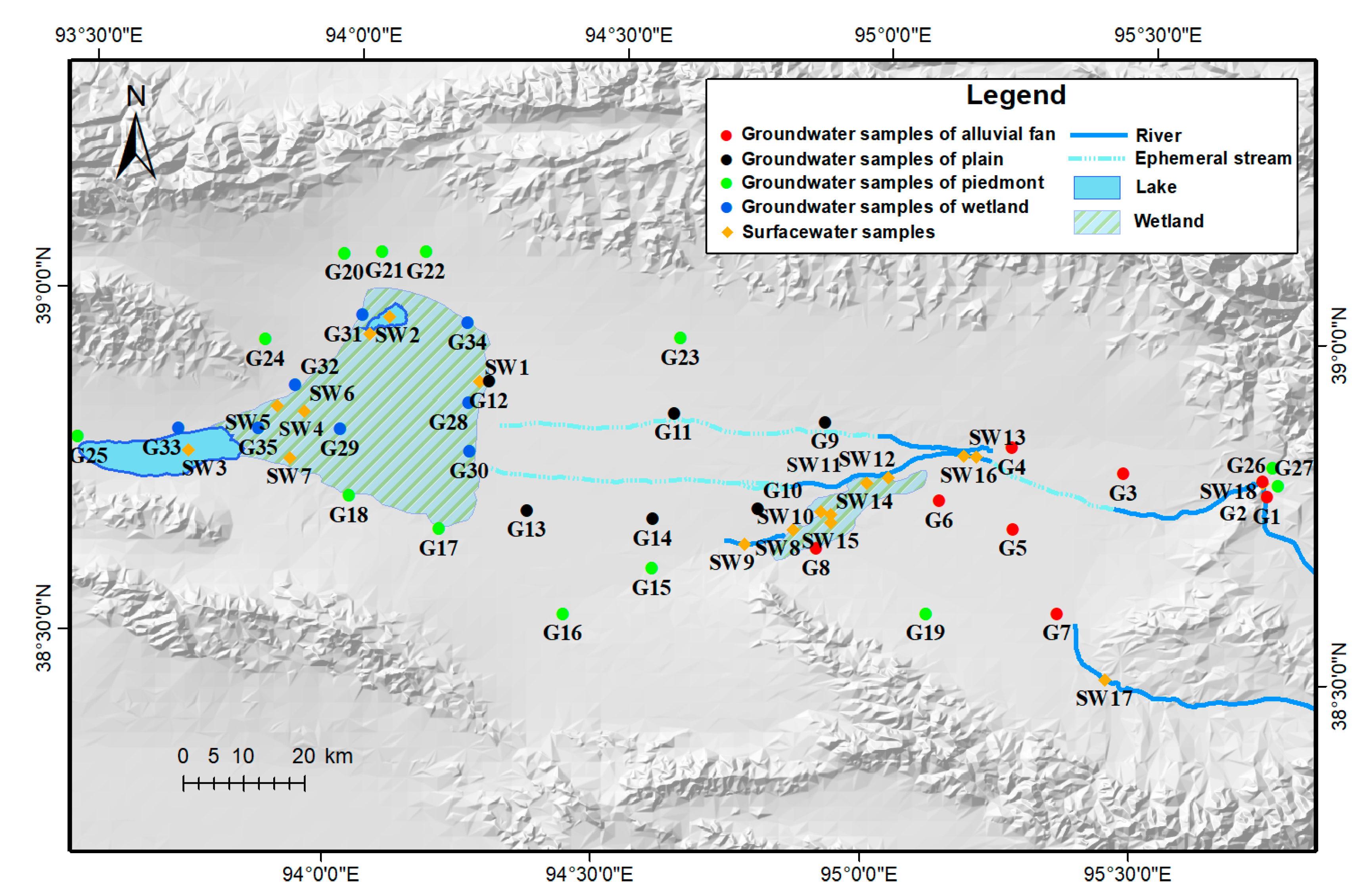
2.2. Sampling
2.3. Analytical Methods
3. Results
3.1. Hydrochemistry of Surface Water and Groundwater
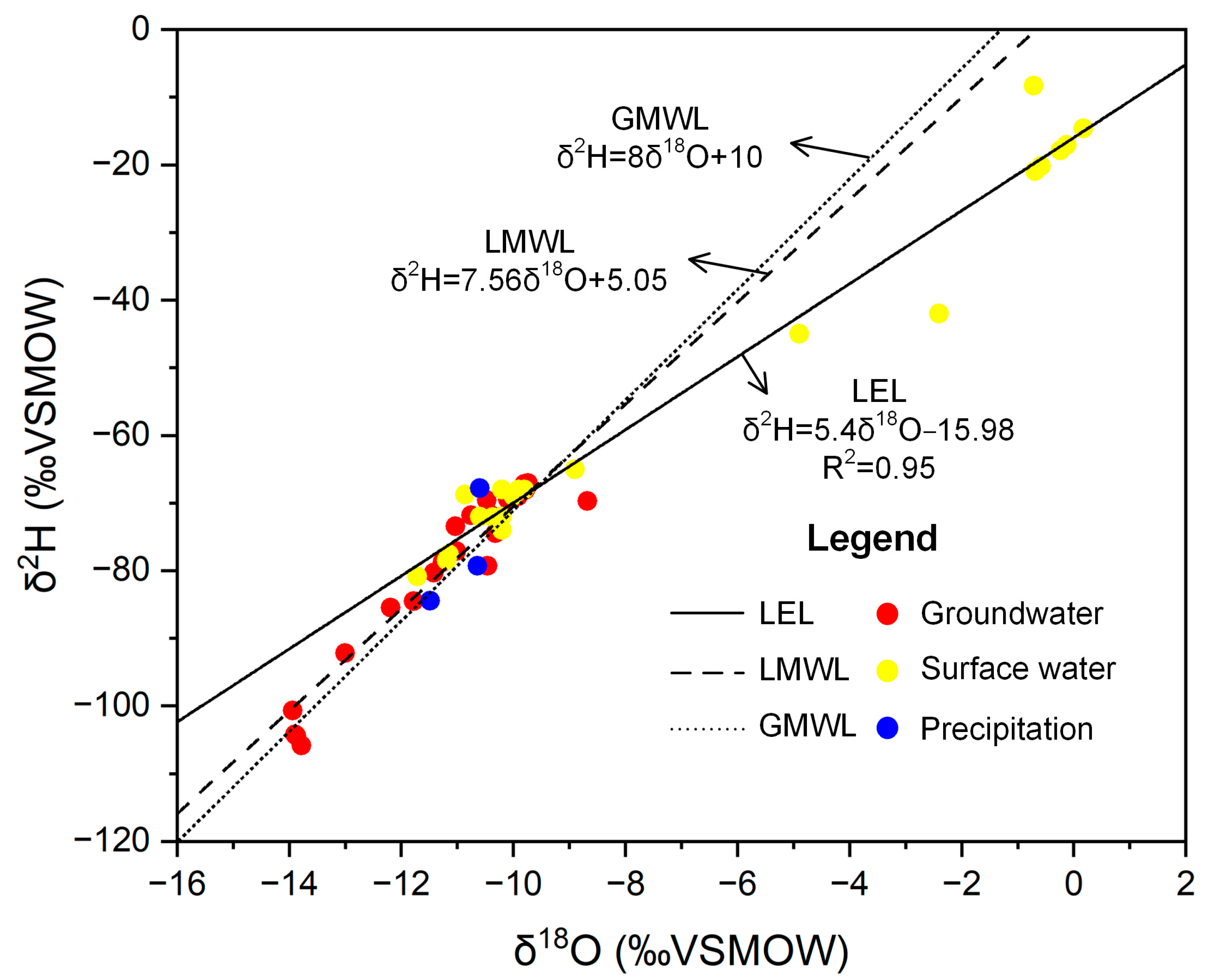
3.2. Ratios of Stable Oxygen and Hydrogen Isotope
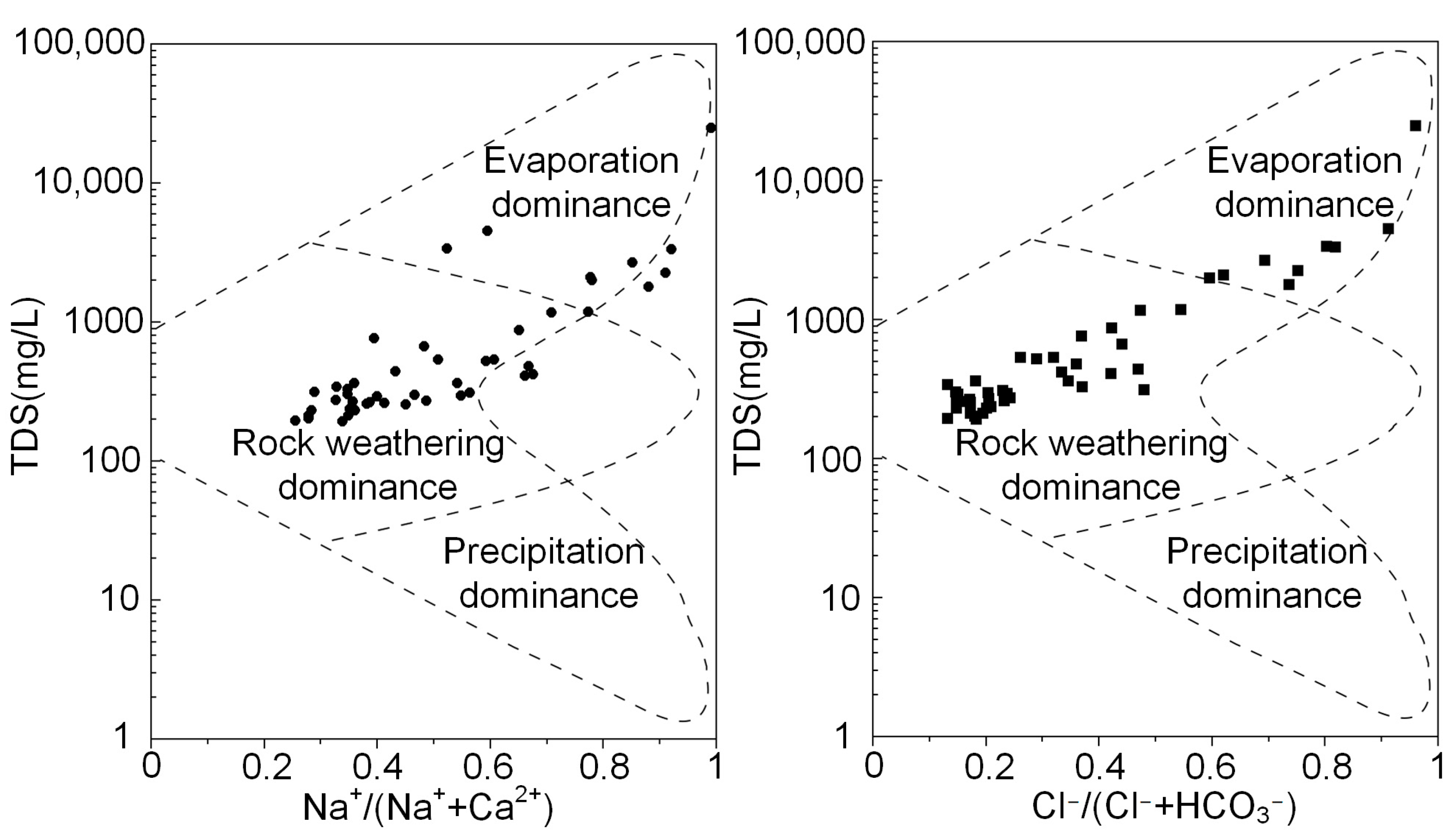
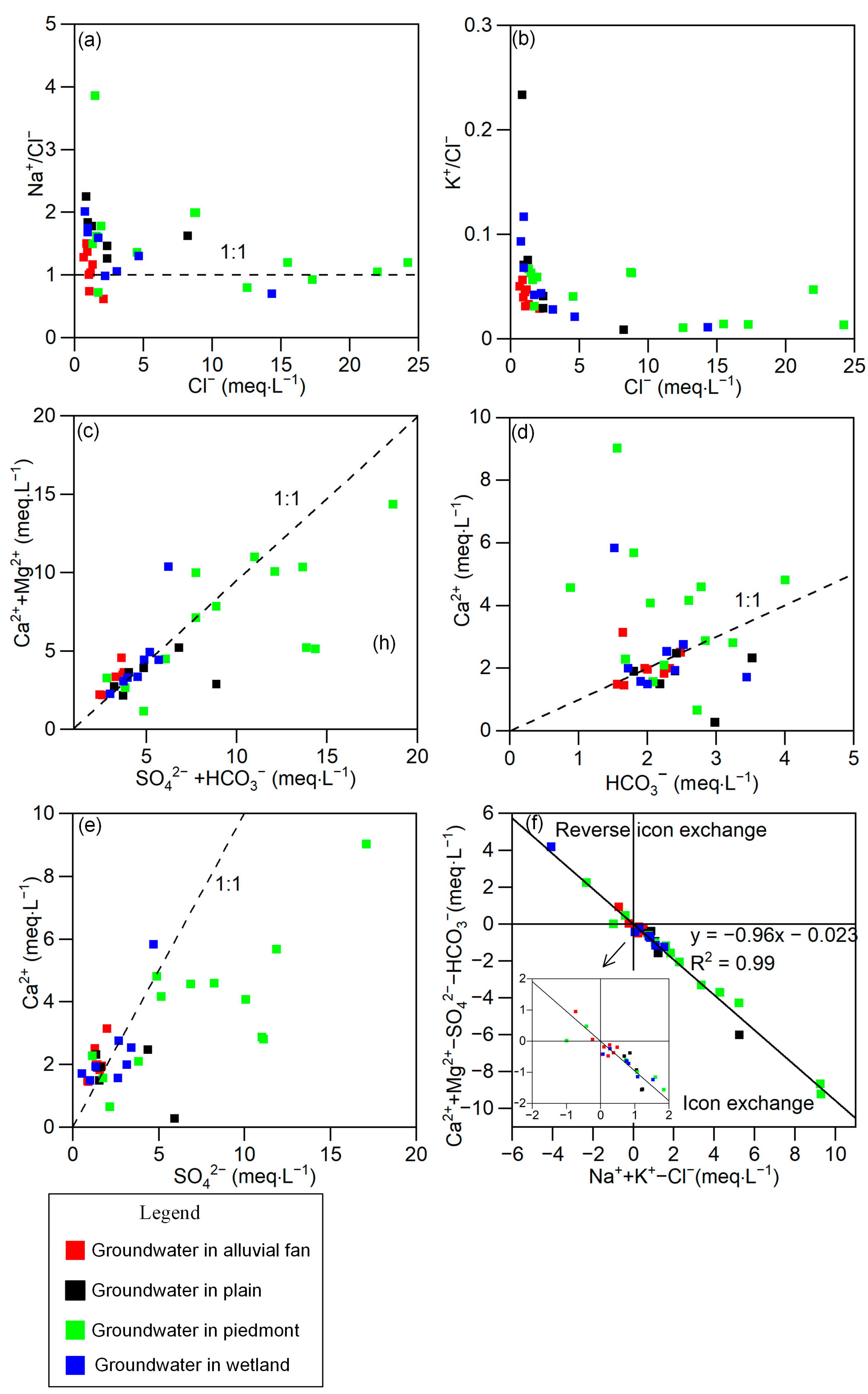
3.3. Hydrogeochemical Processes Based on Ionic Ratios
3.4. Multivariate Statistical Analyses
3.4.1. Principle Component Analysis
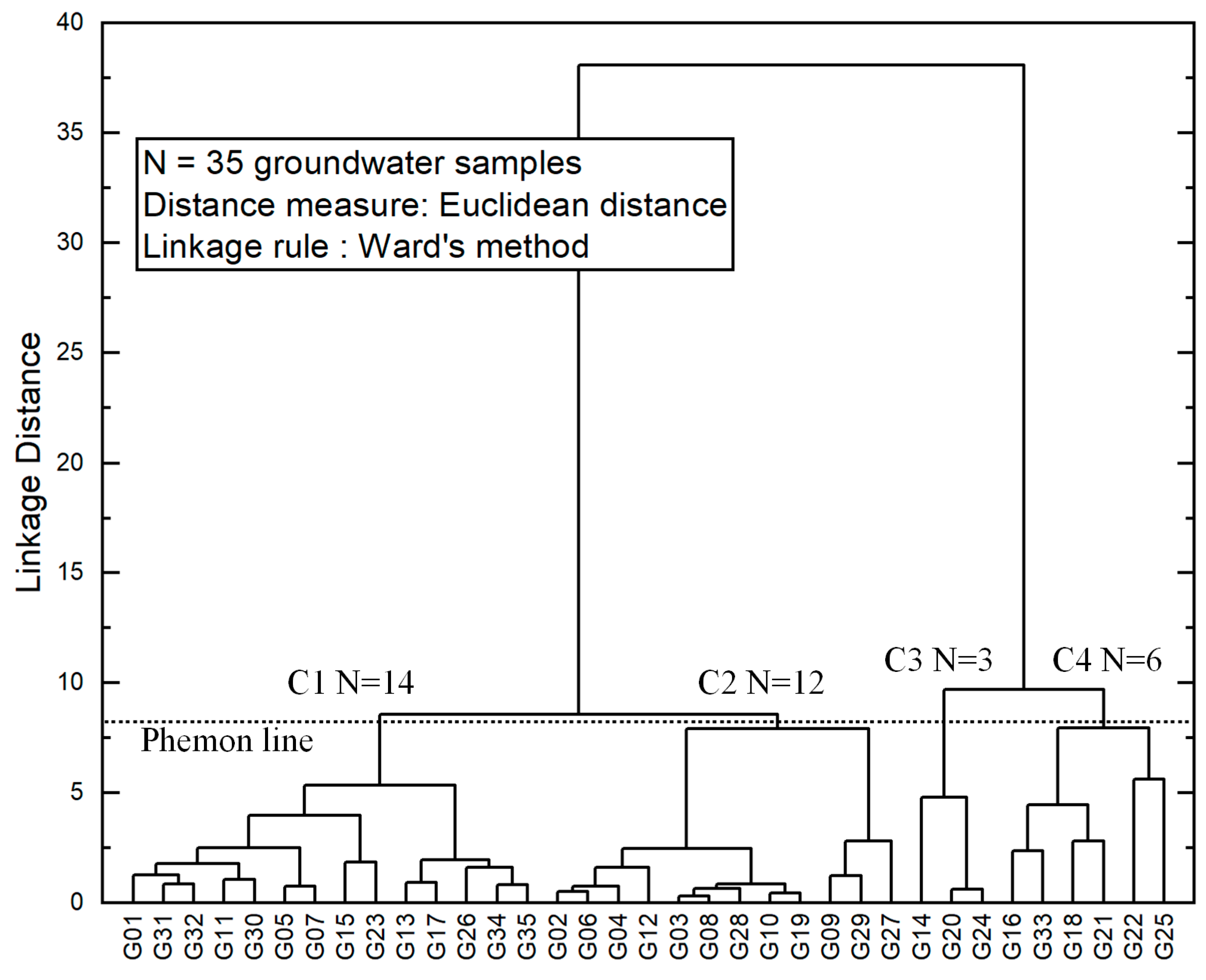
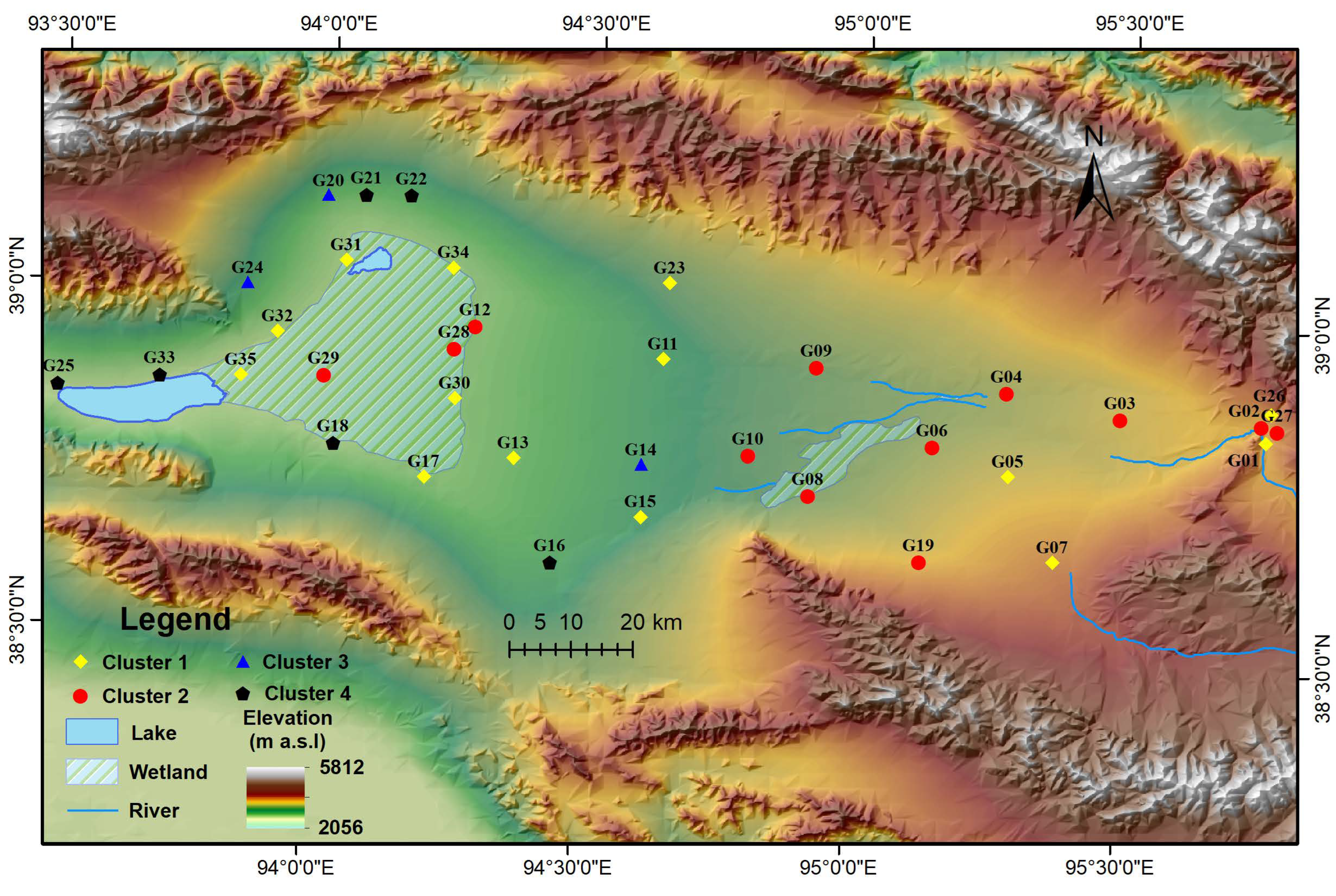
3.4.2. Hierarchical Cluster Analysis
4. Discussion
4.1. Hydrochemistry Evolution of Surface Water
4.2. Mechanisms Controlling Hydrochemistry of Groundwater
4.3. Groundwater Quality and Water Resource Development Potential
5. Conclusions
- The groundwater in the alluvial fan and plain zone and river water originates from the eastern mountains of the Sugan Lake Basin, which are mainly recharged by modern meteoric precipitation. During river flow from east to west, groundwater is closely connected to the river. The stable isotopes and chemical compositions of the groundwater near the river channel were the same as those of the river water, even in the lowest part of the basin. In this flow pattern, the surface water–groundwater interaction is the control mechanism for the surface water and groundwater hydrochemistry.
- Groundwater in the piedmont is mainly recharged by ancient meteoric precipitation that formed in a colder environment. The groundwater flow rate in this region is relatively low. Water–rock interaction is the control mechanism of the chemical composition of groundwater, including mineral weathering and cation exchange processes. Although the results showed that silicate and evaporite minerals, such as mirabilite and gypsum, are the sources of ions in groundwater, the carbonate in groundwater is in equilibrium, whereas sulfate and chloride are unsaturated in all groundwater samples. Thus, carbonate is the dominant mineral in regional rock weathering processes.
- Compared to the nearby arid inland watershed, the evolution of the hydrochemical characteristics and salinity of the surface and groundwater are atypical. The salinity of shallow groundwater in the Sugan Lake Basin is relatively low, especially in the endorheic lake wetlands. The spatial variation in the chemical characteristics of surface water was greater than that of groundwater. A difference exists in the high-salinity stream and closed inland lakes in the wetland zone caused by intense evaporation, whereas the variation in river water samples is inconspicuous. In general, it can be concluded that natural river flooding in summer causes the water circulation rate in the Sugan Lake Basin to be faster than that in other basins with intensive human activities. Controlling the hydrochemical regime and contours of water salinity in the basin is of great significance.
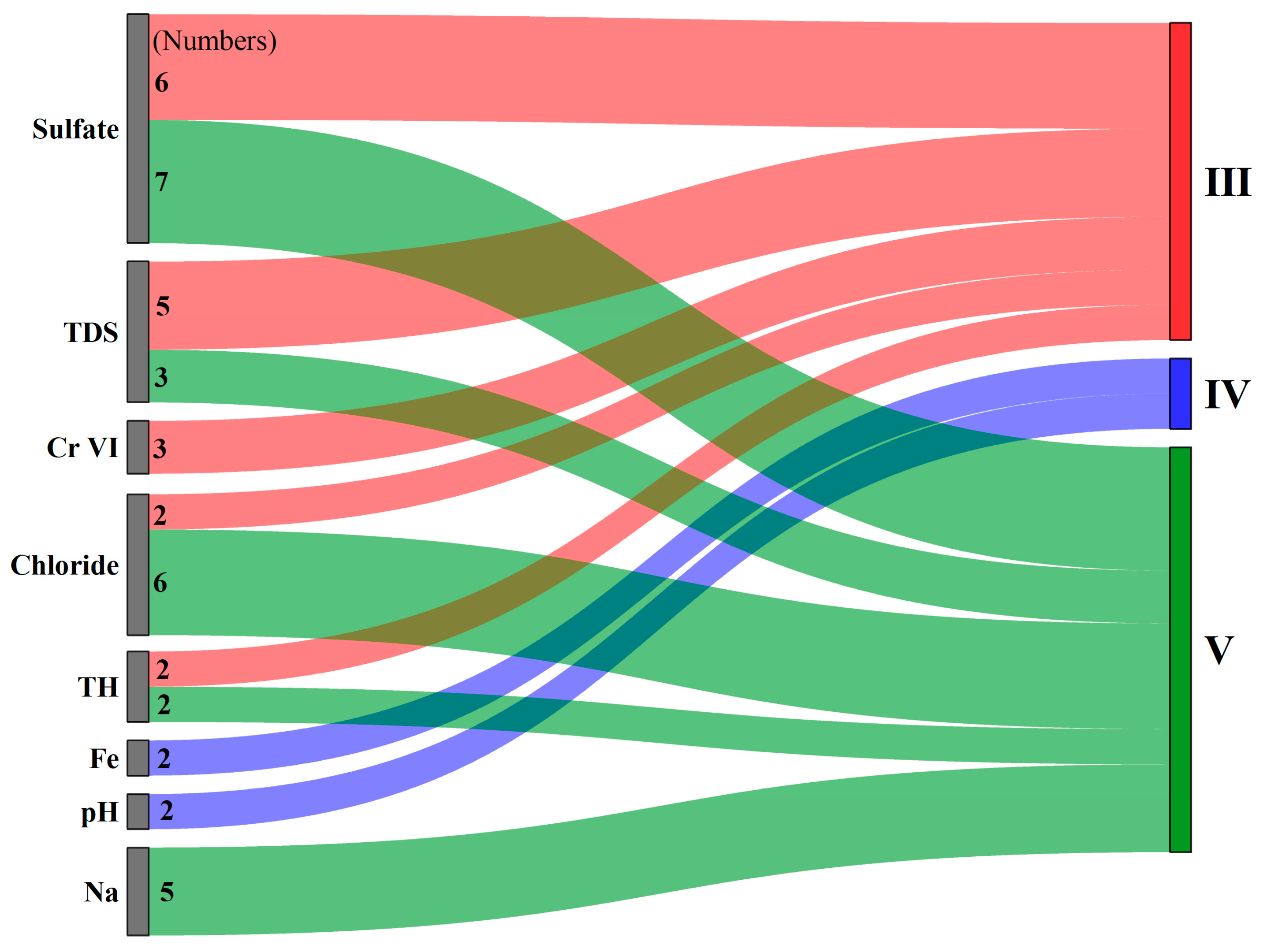
- Class I to Class V groundwater samples accounted for 2.86%, 25.71%, 34.29%, 14.29%, and 22.86%, respectively. Class I and Class II groundwater were basically the same as the samples of C2 in the HCA, which were distributed near river channels. The poorest-quality groundwater is in C3 and C4, which are located in the Piedmont zone. Sulfate, TH, and nitrite in most of the groundwater samples exceeded the upper limit, leading to very little Class II groundwater, while sulfate, chloride, and Na were dominant indicators in poor-quality groundwater. Only a few heavy metals and trace elements concentrations of samples exceeded the upper limit of Class II standards; they are Fe, Hg, and Cr VI.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wada, Y.; van Beek, L.P.H.; van Kempen, C.M.; Reckman, J.; Vasak, S.; Bierkens, M.F.P. Global depletion of groundwater resources. Geophys. Res. Lett. 2010, 37, L20402. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Gosling, S.N.; Kummu, M.; Flörke, M.; Pfister, S.; Hanasaki, N.; Wada, Y.; Zhang, X.; Zheng, C. Water scarcity assessments in the past, present, and future. Earth’s Future 2017, 5, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, Q.; Zheng, X.; Wu, X.; Zhu, M.; Sun, F.; Li, Y. Assessing the impacts of irrigated agriculture on hydrological regimes in an oasis-desert system. J. Hydrol. 2021, 594, 125976. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, Z.; Hou, X.; Zhitong, M.; Chen, B. River-groundwater interaction affected species composition and diversity perpendicular to a regulated river in an arid riparian zone. Glob. Ecol. Conserv. 2021, 27, e01595. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Zhang, Z.; Hou, X.; Duan, L.; Yao, D. Assessment of the effect of water-table depth on riparian vegetation along the middle and lower reaches of the manasi river, northwest China. Hydrogeol. J. 2021, 29, 579–589. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; He, X.; Yang, G.; Du, Y.; Li, X. Groundwater dynamic characteristics with the ecological threshold in the northwest China oasis. Sustainability 2022, 14, 5390. [Google Scholar] [CrossRef]
- Arthington, A.H.; Balcombe, S.R. Extreme flow variability and the ‘boom and bust’ ecology of fish in arid-zone floodplain rivers: A case history with implications for environmental flows, conservation and management. Ecohydrology 2011, 4, 708–720. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, G.; Qiu, D.; Liu, Y.; Sang, L.; Lin, X.; Ma, H.; Zhao, K.; Xu, Y. Effects of agricultural activities on hydrochemistry in the shiyang river basin, CHINA. Environ. Sci. Pollut. Res. 2022, 30, 12269–12282. [Google Scholar] [CrossRef]
- Liu, Z.; Feng, S.; Zhang, D.; Han, Y.; Cao, R. Effects of precipitation, irrigation, and exploitation on groundwater geochemical evolution in the people’s victory canal irrigation area, China. Appl. Water Sci. 2022, 13, 1. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, X.; Wang, X.; Wan, L.; Wang, J. Why mixed groundwater at the outlet of open flowing wells in unconfined-aquifer basins can represent deep groundwater: Implications for sampling in long-screen wells. Hydrogeol. J. 2019, 27, 409–421. [Google Scholar] [CrossRef]
- Khanoranga; Khalid, S. An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of balochistan province, pakistan, through water quality index and multivariate statistical approaches. J. Geochem. Explor. 2019, 197, 14–26. [Google Scholar] [CrossRef]
- Bouchez, C.; Cook, P.G.; Partington, D.; Simmons, C.T. Comparison of surface water-groundwater exchange fluxes derived from hydraulic and geochemical methods and a regional groundwater model. Water Resour. Res. 2021, 57, e2020WR029137. [Google Scholar] [CrossRef]
- Bussi, G.; Whitehead, P.G.; Gutiérrez-Cánovas, C.; Ledesma, J.L.J.; Ormerod, S.J.; Couture, R.-M. Modelling the effects of climate and land-use change on the hydrochemistry and ecology of the river wye (wales). Sci. Total Environ. 2018, 627, 733–743. [Google Scholar] [CrossRef]
- Xiao, Y.; Shao, J.; Frape, S.K.; Cui, Y.; Dang, X.; Wang, S.; Ji, Y. Groundwater origin, flow regime and geochemical evolution in arid endorheic watersheds: A case study from the qaidam basin, northwestern China. Hydrol. Earth Syst. Sci. 2018, 22, 4381–4400. [Google Scholar] [CrossRef]
- Lowenstein, T.K.; Risacher, F. Closed basin brine evolution and the influence of ca–cl inflow waters: Death valley and bristol dry lake california, qaidam basin, China, and salar de atacama, chile. Aquat. Geochem. 2009, 15, 71–94. [Google Scholar] [CrossRef]
- Cartwright, I.; Weaver, T.R.; Simmons, C.T.; Fifield, L.K.; Lawrence, C.R.; Chisari, R.; Varley, S. Physical hydrogeology and environmental isotopes to constrain the age, origins, and stability of a low-salinity groundwater lens formed by periodic river recharge: Murray basin, australia. J. Hydrol. 2010, 380, 203–221. [Google Scholar] [CrossRef]
- Munk, L.A.; Boutt, D.F.; Hynek, S.A.; Moran, B.J. Hydrogeochemical fluxes and processes contributing to the formation of lithium-enriched brines in a hyper-arid continental basin. Chem. Geol. 2018, 493, 37–57. [Google Scholar] [CrossRef]
- Petts, D.C.; Saso, J.K.; Diamond, L.W.; Aschwanden, L.; Al, T.A.; Jensen, M. The source and evolution of paleofluids responsible for secondary minerals in low-permeability ordovician limestones of the michigan basin. Appl. Geochem. 2017, 86, 121–137. [Google Scholar] [CrossRef]
- Guo, X.; Feng, Q.; Liu, W.; Li, Z.; Wen, X.; Si, J.; Xi, H.; Guo, R.; Jia, B. Stable isotopic and geochemical identification of groundwater evolution and recharge sources in the arid shule river basin of northwestern China. Hydrol. Process. 2015, 29, 4703–4718. [Google Scholar] [CrossRef]
- Wang, J.; Liang, X.; Liu, Y.; Jin, M.; Knappett, P.S.K.; Liu, Y. Hydrogeochemical evolution along groundwater flow paths in the manas river basin, northwest China. Groundwater 2019, 57, 575–589. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Hou, R.; Guan, L.; Dang, Y.; Zhang, Z.; Wang, H.; Duan, L.; Wang, Z.F. Modes, hydrodynamic processes and ecological impacts exerted by river–groundwater transformation in junggar basin, China. Hydrogeol. J. 2018, 26, 1547–1557. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Jiang, C. Run-off analyses using isotopes and hydrochemistry in yushugou river basin, eastern tianshan mountains. J. Earth Syst. Sci. 2017, 126, 86. [Google Scholar] [CrossRef]
- Pan, Q.; Zhang, C.; Guo, S.; Sun, H.; Du, J.; Guo, P. An interval multi-objective fuzzy-interval credibility-constrained nonlinear programming model for balancing agricultural and ecological water management. J. Contam. Hydrol. 2022, 245, 103958. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Ochoa, C.G.; Chen, X. Assessing environmental water requirement for groundwater-dependent vegetation in arid inland basins by combining the copula joint distribution function and the dual objective optimization: An application to the turpan basin, China. Sci. Total Environ. 2021, 799, 149323. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hu, L.; Sun, K. The potential impacts of a water transfer project on the groundwater system in the sugan lake basin of China. Hydrogeol. J. 2021, 29, 1485–1499. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Feng, W.; Zhu, R.; Chen, Z.; Zhao, K.; Zhang, B. Geometry and control factors of distributive fluvial system around the sugan lake basin. Acta Geol. Sin. 2019, 93, 2947–2959. [Google Scholar]
- Xiang, J.; Zhou, J.; Yang, J.; Huang, M.; Feng, W.; Li, Q.; Xue, D.; Zhao, Y.; Zhu, G. Applying multivariate statistics for identification of groundwater chemistry and qualities in the sugan lake basin, northern qinghai-tibet plateau, China. J. Mt. Sci. 2020, 17, 448–463. [Google Scholar] [CrossRef]
- Huang, T.; Ma, B.; Pang, Z.; Li, Z.; Li, Z.; Long, Y. How does precipitation recharge groundwater in loess aquifers? Evidence from multiple environmental tracers. J. Hydrol. 2020, 583, 124532. [Google Scholar] [CrossRef]
- Vrzel, J.; Solomon, D.K.; Blažeka, Ž.; Ogrinc, N. The study of the interactions between groundwater and sava river water in the ljubljansko polje aquifer system (slovenia). J. Hydrol. 2018, 556, 384–396. [Google Scholar] [CrossRef]
- Yang, Q.; Mu, H.; Wang, H.; Ye, X.; Ma, H.; Martín, J.D. Quantitative evaluation of groundwater recharge and evaporation intensity with stable oxygen and hydrogen isotopes in a semi-arid region, northwest China. Hydrol. Process. 2018, 32, 1130–1136. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ding, Z.; Edmunds, W.M.; Gates, J.B.; Huang, T. Limits to recharge of groundwater from tibetan plateau to the gobi desert, implications for water management in the mountain front. J. Hydrol. 2009, 364, 128–141. [Google Scholar] [CrossRef]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liang, X.; Ma, B.; Liu, Y.; Jin, M.; Knappett, P.S.K.; Liu, Y. Using isotopes and hydrogeochemistry to characterize groundwater flow systems within intensively pumped aquifers in an arid inland basin, northwest China. J. Hydrol. 2021, 595, 126048. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Ma, H.; Wang, L.; Martín, J.D. Identification of the hydrogeochemical processes and assessment of groundwater quality using classic integrated geochemical methods in the southeastern part of ordos basin, China. Environ. Pollut. 2016, 218, 879–888. [Google Scholar] [CrossRef]
- Pant, R.R.; Zhang, F.; Rehman, F.U.; Wang, G.; Ye, M.; Zeng, C.; Tang, H. Spatiotemporal variations of hydrogeochemistry and its controlling factors in the gandaki river basin, central himalaya nepal. Sci. Total Environ. 2018, 622–623, 770–782. [Google Scholar] [CrossRef]
- Castillo, J.L.U.; Leal, J.A.R.; Cruz, D.A.M.; Martínez, A.C.; Celestino, A.E.M. Identification of the dominant factors in groundwater recharge process, using multivariate statistical approaches in a semi-arid region. Sustainability 2021, 13, 11543. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and the gibbs diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Cheng, D.; Yang, N.; Hou, X.; Li, H.; Zhang, X.; Ayyamperumal, R. Hydrochemical characteristics and paleoclimate changes recorded from sugan lake on the northern boundary of tibetan plateau since mid-holocene. Catena 2022, 217, 106527. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, M.; Wang, J. Insights into groundwater salinization from hydrogeochemical and isotopic evidence in an arid inland basin. Hydrol. Process. 2018, 32, 3108–3127. [Google Scholar] [CrossRef]
- Hussin, N.H.; Yusoff, I.; Tahir, W.Z.W.M.; Mohamed, I.; Ibrahim, A.I.N.; Rambli, A. Multivariate statistical analysis for identifying water quality and hydrogeochemical evolution of shallow groundwater in quaternary deposits in the lower kelantan river basin, malaysian peninsula. Environ. Earth Sci. 2016, 75, 1081. [Google Scholar] [CrossRef]
- Dogramaci, S.; Skrzypek, G.; Dodson, W.; Grierson, P.F. Stable isotope and hydrochemical evolution of groundwater in the semi-arid hamersley basin of subtropical northwest australia. J. Hydrol. 2012, 475, 281–293. [Google Scholar] [CrossRef]
- Ma, B.; Jin, M.; Liang, X.; Li, J. Groundwater mixing and mineralization processes in a mountain–oasis–desert basin, northwest China: Hydrogeochemistry and environmental tracer indicators. Hydrogeol. J. 2018, 26, 233–250. [Google Scholar] [CrossRef]
- Krishnaraj, S.; Murugesan, V.; Vijayaraghavan, K.; Sabarathinam, C.; Paluchamy, A.; Ramachandran, M. Use of hydrochemistry and stable isotopes as tools for groundwater evolution and contamination investigations. Geosciences 2011, 1, 16–25. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, S.; Li, H.; Fu, K.; Xu, Y. Groundwater pollution source identification and apportionment using pmf and pca-apca-mlr receptor models in a typical mixed land-use area in southwestern China. Sci. Total Environ. 2020, 741, 140383. [Google Scholar] [CrossRef]
- Güler, C.; Thyne, G.D.; McCray, J.E.; Turner, K.A. Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeol. J. 2002, 10, 455–474. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, R.; Wang, Y.; Chang, Q.; Wang, S.; Ge, M.; Bu, J.; Sun, Z. Using hydrogeochemical data to trace groundwater flow paths in a cold alpine catchment. Hydrol. Process. 2019, 33, 1942–1960. [Google Scholar] [CrossRef]
- Ma, J.; He, J.; Qi, S.; Zhu, G.; Zhao, W.; Edmunds, W.M.; Zhao, Y. Groundwater recharge and evolution in the dunhuang basin, northwestern China. Appl. Geochem. 2013, 28, 19–31. [Google Scholar] [CrossRef]
- Sahib, L.Y.; Marandi, A.; Schüth, C. Strontium isotopes as an indicator for groundwater salinity sources in the kirkuk region, Iraq. Sci. Total Environ. 2016, 562, 935–945. [Google Scholar] [CrossRef]
- Edmunds, W.M.; Cook, J.M.; Darling, W.G.; Kinniburgh, D.G.; Miles, D.L.; Bath, A.H.; Morgan-Jones, M.; Andrews, J.N. Baseline geochemical conditions in the chalk aquifer, berkshire, U.K.: A basis for groundwater quality management. Appl. Geochem. 1987, 2, 251–274. [Google Scholar] [CrossRef]
- Zheng, M.; Tang, J.; Liu, J.; Zhang, F. Chinese saline lakes. Hydrobiologia 1993, 267, 23–36. [Google Scholar] [CrossRef]
- Bowler, J.M.; Huang, Q.; Kezao, C.; Head, M.J.; Baoyin, Y. Radiocarbon dating of playa-lake hydrologic changes: Examples from northwestern China and central Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1986, 54, 241–260. [Google Scholar] [CrossRef]
- Yapiyev, V.; Sagintayev, Z.; Inglezakis, V.J.; Samarkhanov, K.; Verhoef, A. Essentials of Endorheic Basins and Lakes: A Review in the Context of Current and Future Water Resource Management and Mitigation Activities in Central Asia. Water 2017, 9, 798. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, B.; Wan, L.; Cao, W.; Wu, S.; Feng, W. Occurrence of soluble salts and moisture in the unsaturated zone and groundwater hydrochemistry along the middle and lower reaches of the Heihe River in northwest China. Environ. Geol. 2006, 50, 1085–1093. [Google Scholar] [CrossRef]
- Xiao, J.; Jin, Z.D.; Wang, J.; Zhang, F. Hydrochemical characteristics, controlling factors and solute sources of groundwater within the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. Quat. Int. 2015, 380–381, 237–246. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, R.; Wang, Y.; Hu, Y.; Sun, L. Hydrogeological and hydrogeochemical control of groundwater salinity in an arid inland basin: Dunhuang basin, northwestern China. Hydrol. Process. 2016, 30, 1884–1902. [Google Scholar] [CrossRef]
- Tian, Y.; Wen, Z.; Cheng, M.; Xu, M. Evaluating the water quality characteristics and tracing the pollutant sources in the yellow river basin, China. Sci. Total Environ. 2022, 846, 157389. [Google Scholar] [CrossRef] [PubMed]
- GB/T 14848-2017; Standard for Groundwater Quality. Standardization Administration of the People’s Republic of China: Beijing, China, 2017.


| Index | Max. | Min. | Mean | CV (%) |
|---|---|---|---|---|
| Ca2+ (mg/L) | 557.80 | 28.10 | 78.15 | 126.66 |
| Mg2+ (mg/L) | 1605.00 | 9.30 | 74.99 | 313.07 |
| Na+ (mg/L) | 6105.00 | 10.20 | 275.13 | 328.47 |
| K+ (mg/L) | 165.40 | 0.60 | 11.27 | 221.51 |
| HCO3− (mg/L) | 373.40 | 92.70 | 190.17 | 40.09 |
| Cl− (mg/L) | 8260.00 | 14.20 | 365.57 | 333.56 |
| SO42− (mg/L) | 8039.00 | 37.50 | 422.46 | 287.68 |
| NO3− (mg/L) | 68.75 | 0.50 | 6.71 | 212.73 |
| TDS (mg/L) | 24,840.00 | 193.00 | 1341.67 | 274.25 |
| pH | 8.96 | 7.39 | 8.23 | 4.28 |
| Index | Max. | Min. | Mean | CV (%) |
|---|---|---|---|---|
| Ca2+ (mg/L) | 180.90 | 5.60 | 54.96 | 62.33 |
| Mg2+ (mg/L) | 77.80 | 6.20 | 27.66 | 70.91 |
| Na+ (mg/L) | 669.40 | 18.10 | 142.31 | 118.91 |
| K+ (mg/L) | 40.70 | 1.30 | 6.03 | 128.52 |
| HCO3− (mg/L) | 244.10 | 53.70 | 138.42 | 28.57 |
| Cl− (mg/L) | 820.40 | 24.50 | 194.02 | 98.06 |
| SO42− (mg/L) | 858.70 | 23.40 | 177.21 | 129.09 |
| NO3− (mg/L) | 36.17 | 0.50 | 8.90 | 92.08 |
| TDS (mg/L) | 2691.00 | 197.00 | 687.97 | 90.94 |
| pH | 8.67 | 7.73 | 8.14 | 2.63 |
| Factor | Extraction Sums of Squared Loadings | Rotated Component Matrix | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum | % of Variance | Cumulative (%) | Ca2+ | Mg2+ | Na+ | K+ | HCO3− | SO42− | Cl− | NO3− | TDS | pH | |
| 1 | 6.96 | 69.96 | 69.96 | 0.35 | 0.97 | 0.95 | 0.94 | 0.88 | 0.96 | 0.94 | 0.08 | 0.95 | −0.13 |
| 2 | 2.30 | 23.01 | 90.98 | 0.71 | 0.11 | 0.28 | 0.15 | −0.21 | 0.22 | 0.27 | 0.94 | 0.26 | 0.01 |
| I | II | III | IV | V | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| TH | 8 | 22.86 | 19 | 54.29 | 2 | 5.71 | 4 | 11.43 | 2 | 5.71 |
| TDS | 12 | 34.29 | 9 | 25.71 | 5 | 14.29 | 6 | 17.14 | 3 | 8.57 |
| Sulfate | 4 | 11.43 | 16 | 45.71 | 6 | 17.14 | 2 | 5.71 | 7 | 20.00 |
| Chloride | 14 | 40.00 | 10 | 28.57 | 2 | 5.71 | 3 | 8.57 | 6 | 17.14 |
| Fluoride | 34 | 97.14 | 0 | 0.00 | 0 | 0.00 | 1 | 2.86 | 0 | 0.00 |
| Nitrate | 22 | 62.86 | 10 | 28.57 | 3 | 8.57 | 0 | 0.00 | 0 | 0.00 |
| Nitrite | 31 | 88.57 | 3 | 8.57 | 1 | 2.86 | 0 | 0.00 | 0 | 0.00 |
| Na | 23 | 65.71 | 3 | 8.57 | 0 | 0.00 | 4 | 11.43 | 5 | 14.29 |
| Fe | 29 | 82.86 | 2 | 5.71 | 2 | 5.71 | 2 | 5.71 | 0 | 0.00 |
| Cu | 35 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Zn | 32 | 91.43 | 3 | 8.57 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Mn | 34 | 97.14 | 0 | 0.00 | 0 | 0.00 | 1 | 2.86 | 0 | 0.00 |
| Pb | 35 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Ni | 35 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Cd | 35 | 100.00 | 0 | 0.00 | 0 | 37.14 | 0 | 0.00 | 0 | 0.00 |
| As | 34 | 97.14 | 0 | 0.00 | 1 | 2.86 | 0 | 0.00 | 0 | 0.00 |
| Se | 35 | 100.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| Hg | 34 | 97.14 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 2.86 |
| Cr Ⅵ | 26 | 74.29 | 3 | 8.57 | 6 | 17.14 | 0 | 0.00 | 0 | 0.00 |
| pH | 33 | 94.29 | 0 | 0.00 | 0 | 0.00 | 2 | 5.71 | 0 | 0.00 |
| Result | 1 | 2.86 | 9 | 25.71 | 12 | 34.29 | 5 | 14.29 | 8 | 22.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Hu, L.; Ma, H.; Zhang, W. Hydrochemical Characteristics of Groundwater and Their Significance in Arid Inland Hydrology. Water 2023, 15, 1641. https://doi.org/10.3390/w15091641
Yang Z, Hu L, Ma H, Zhang W. Hydrochemical Characteristics of Groundwater and Their Significance in Arid Inland Hydrology. Water. 2023; 15(9):1641. https://doi.org/10.3390/w15091641
Chicago/Turabian StyleYang, Zhengqiu, Litang Hu, Haiyan Ma, and Wang Zhang. 2023. "Hydrochemical Characteristics of Groundwater and Their Significance in Arid Inland Hydrology" Water 15, no. 9: 1641. https://doi.org/10.3390/w15091641
APA StyleYang, Z., Hu, L., Ma, H., & Zhang, W. (2023). Hydrochemical Characteristics of Groundwater and Their Significance in Arid Inland Hydrology. Water, 15(9), 1641. https://doi.org/10.3390/w15091641







