The Socio-Environmental and Human Health Problems Related to the Use of Pesticides and the Use of Advanced Oxidative Processes for Their Degradation: Brazil
Abstract
1. Introduction
2. Development
2.1. The Socio-Environmental and Human Health Issues Related to the Use of Agrochemicals in Brazil
2.2. Main Classes of Agrochemicals and Their Effects on the Environment
2.3. The Advanced Oxidative Processes (AOPs) in the Treatment of Pesticides
2.4. AOPs Using Hydrogen Peroxide
2.5. Ozone Process
2.6. Carbonate Anion Radical
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carraro, G. AGROTÓXICO E MEIO AMBIENTE: Uma Proposta de Ensino de Ciências e Química. Available online: http://www.quimica.seed.pr.gov.br/arquivos/File/AIQ_2011/agrotoxicos_ufrgs.pdf (accessed on 5 April 2023).
- Liang, J.; Tang, S.; Nieto, J.J.; Cheke, R.A. Analytical Methods for Detecting Pesticide Switches with Evolution of Pesticide Resistance. Math. Biosci. 2013, 245, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Birich, B.; El Hajjaji, S.; Ghandi, M.; Ait Daoud, N.; Badrane, N.; Soulaymani, R.B. A Simple Method of Detection of 15 Organochlorine Pesticides in Human Plasma Using Gas Chromatography. Chem. Data Collect. 2020, 30, 100562. [Google Scholar] [CrossRef]
- Patil, P.B.; Raut-Jadhav, S.; Pandit, A.B. Effect of Intensifying Additives on the Degradation of Thiamethoxam Using Ultrasound Cavitation. Ultrason. Sonochem. 2021, 70, 105310. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, L.B.; Carlos, T.D.; Nogueira das Neves, A.P.; Durães, W.A.; de Almeida Sarmento, R.; Pereira, D.H.; Cavallini, G.S. Theoretical-Experimental Study of the Advanced Oxidative Process Using Peracetic Acid and Solar Radiation: Removal Efficiency and Thermodynamic Elucidation of Radical Formation Processes. J. Photochem. Photobiol. A Chem. 2022, 423, 113615. [Google Scholar] [CrossRef]
- Carlos, T.D.; Bezerra, L.B.; Vieira, M.M.; Sarmento, R.A.; Pereira, D.H.; Cavallini, G.S. Fenton-Type Process Using Peracetic Acid: Efficiency, Reaction Elucidations and Ecotoxicity. J. Hazard. Mater. 2021, 403, 123949. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.M.; Pereira Dornelas, A.S.; Carlos, T.D.; Pallini, A.; Gravato, C.; Pereira, D.H.; Sarmento, R.A.; Cavallini, G.S. When Treatment Increases the Contaminant’s Ecotoxicity: A Study of the Fenton Process in the Degradation of Methylene Blue. Chemosphere 2021, 283, 131117. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; da Silveira, G.R.; Amazonas, J.C.; Gurgel, A.D.M.; de Almeida, V.E.S.; Sarpa, M. Situação Regulatória Internacional de Agrotóxicos Com Uso Autorizado No Brasil: Potencial de Danos Sobre a Saúde e Impactos Ambientais. Cad. Saude Publica 2021, 37, 1820. [Google Scholar] [CrossRef]
- Rigotto, R.M.; e Vasconcelos, D.P.; Rocha, M.M. Pesticide Use in Brazil and Problems for Public Health. Cad. Saude Publica 2014, 30, 1360–1362. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2020; FAO: Rome, Italy, 2020; ISBN 978-92-5-133441-6. [Google Scholar]
- Carneiro, L.; Faria, L.; Miiller, N.; Cavalcante, A.; Murata, A.; Vitule, J.R.S. Brazilian Pesticides Law Could Poison the World. Science 2022, 376, 362. [Google Scholar] [CrossRef]
- De Miranda, E.E. Areas cultivadas no brasil e no mundo. Agroanalysis 2018, 38, 25–27. [Google Scholar]
- Carneiro, F.F.; da Augusto, L.G.S.; Rigotto, R.M.; Friedrich, K.; Búrigo, A.C. Dossiê Abrasco: Um Alerta Sobre os Impactos dos Agrotóxicos na Saúde; Expressão Popular: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- EMBRAPA NASA. Confirms Embrapa’s Data on Planted Area in Brazil. Available online: https://www.embrapa.br/en/busca-de-noticias/-/noticia/30972114/nasa-confirms-embrapas-data-on-planted-area-in-brazil (accessed on 6 April 2023).
- Aulakh, C.S.; Sharma, S.; Thakur, M.; Kaur, P. A Review of the Influences of Organic Farming on Soil Quality, Crop Productivity and Produce Quality. J. Plant Nutr. 2022, 45, 1884–1905. [Google Scholar] [CrossRef]
- Pignati, W.A.; e Lima, F.A.N.D.S.; de Lara, S.S.; Correa, M.L.M.; Barbosa, J.R.; Leão, L.H.D.C.; Pignatti, M.G. Distribuição Espacial Do Uso de Agrotóxicos No Brasil: Uma Ferramenta Para a Vigilância Em Saúde. Cienc. Saude Colet. 2017, 22, 3281–3293. [Google Scholar] [CrossRef]
- MAPA DA ÁGUA—O Que Sai Da Sua Torneira? Cidades Com Água Imprópria Ao Menos Uma Vez Entre 2018 e 2020. Available online: https://mapadaagua.reporterbrasil.org.br/metodologia (accessed on 6 April 2023).
- Stoppelli, I.M.d.B.S.; Magalhães, C.P. Saúde e Segurança Alimentar: A Questão Dos Agrotóxicos. Cienc. Saude Colet. 2005, 10, 91–100. [Google Scholar] [CrossRef]
- Brasil Lei Federal Dos Agrotóxicos—LEI No 7.802 de 11 DE Julho de 1989. Available online: https://www.planalto.gov.br/ccivil_03/leis/l7802.htm (accessed on 6 April 2023).
- Brasil Decreto, n.° 4.074 de 04 de Janeiro de 2002. Regulamenta a Lei N° 7.802/89 (Lei Federal Dos Agrotóxicos). Available online: http://www.planalto.gov.br/ccivil_03/decreto/2002/d4074.htm (accessed on 6 April 2023).
- ANVISA (Agência Nacional de Vigilância Sanitária). Resíduos de Agrotóxicos Em Alimentos. Rev. Saúde Pública 2006, 40, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Brasil Ministério Da Saúde; Secretaria de Atenção à Saúde; Departamento de Ações Programáticas e Estratégicas; Área Temática de Saúde Do Trabalhador. Diretrizes Para Atenção Integral a Saúde Do Trabalhador de Complexidade Diferenciada; Protocolo de Atenção à Saú; UNESCO: Brasília, Brazil, 2010.
- ANVISA (Agência Nacional de Vigilância Sanitária). Programa de Análise de Resíduo de Agrotóxico Em Alimentos (PARA); Dados Da Coleta e Análise de Alimentos de 2010; ANVISA: Brasília, Brazil, 2011.
- ANVISA (Agência Nacional de Vigilância Sanitária). Resolução RDC n. 10 de 22 de Fevereiro de 2008. Que Estabelece a Reavaliação Toxicológica de 14 Agrotóxicos. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2008/res0010_22_02_2008.html (accessed on 6 April 2023).
- Ministério da Saúde. Saúde Pública Recomendações Para Tratamento de Intoxicação Por Agrotóxicos—Português (Brasil). Available online: https://www.gov.br (accessed on 6 April 2023).
- Klaassen, C.D.; Watkins, J.B., III. Fundamentos de Toxicologia de Casarett e Doull, 2nd ed.; AMGH: Porto Alegre, Brazil, 2012. [Google Scholar]
- Alves Filho, J.P. Uso de Agrotóxicos No Brasil: Controle Social e Interesses Corporativos; Annablume; Fapesp: São Paulo, Brazil, 2002. [Google Scholar]
- ABRASCO. Associação Brasileira de Saúde Coletiva—DOSSIÊ ABRASCO: Um Alerta Sobre Os Impactos Dos Agrotóxicos Na Saúde; ABRASCO: Rio de Janeiro, Brazil, 2012.
- Faria, Á.B.D.C. A Review of Some Insecticide Groups Used in Forest Pest Integrated Management. Ambiência Rev. Set. Ciênc. Agrárias Ambient. 2009, 5, 345–358. [Google Scholar]
- Mariconi, F.A.M. Inseticidas e Seu Emprego No Combate as Pragas: Com Uma Introdução Sobre o Estudo dos Insetos, 3rd ed.; Nobel: São Paulo, Brazil, 1977. [Google Scholar]
- Barra, C.M.; Santelli, R.E.; Abrão, J.J.; De La Guardia, M. Especiação de arsênio—UMA revisão. Quim. Nova 2000, 23, 58–70. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Pesticides Pollution: Classifications, Human Health Impact, Extraction and Treatment Techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde—Portaria GM/MS No 888, de 4 de Maio de 2021. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_07_05_2021.html (accessed on 6 April 2023).
- Ferracini, V.L.; Queiroz, S.C.N.; Rosa, M.A.; De Souza, D.R.C.; de Queiroz, J.F.; Paraiba, L.C. Análise de agrotóxicos organoclorados em camarão e pescado por cromatografia a gás com detector de micro captura de eletrons (GC-ΜECD). Pestic. Rev. Ecotoxicol. Meio Ambient. 2014, 24, 13–20. [Google Scholar] [CrossRef]
- Costabeber, I.; Jodral, M.; Angulo, R. Detectação de pesticidas organoclorados e bifenilos policlorados em amostras biológicas. Pestic. Rev. Ecotoxicol. Meio Ambient. 2000, 10, 1–12. [Google Scholar] [CrossRef]
- Jiang, Y.-F.; Wang, X.-T.; Jia, Y.; Wang, F.; Wu, M.-H.; Sheng, G.-Y.; Fu, J.-M. Occurrence, Distribution and Possible Sources of Organochlorine Pesticides in Agricultural Soil of Shanghai, China. J. Hazard. Mater. 2009, 170, 989–997. [Google Scholar] [CrossRef]
- INCA Instituto Nacional do Câncer. C2021. Causas e Prevenção: Agrotóxicos. Available online: https://www.inca.gov.br/exposicao-no-trabalho-e-no-ambiente/agrotoxicos (accessed on 6 April 2023).
- BASF. Insecticide Mode of Action—Technical Training Manual; BASF: Ludwigshafen, Germany, 2013. [Google Scholar]
- Bayer, S.A. CURBIX 200 SC—Bula Agrofit. Available online: https://s3.sa-east-1.amazonaws.com/bd-sp.canaldapeca.com.br/Terraverde/Bulas/27-08-2020/208852.pdf (accessed on 6 April 2023).
- Serafim, L.F.; Wang, L.; Rathee, P.; Yang, J.; Frenk Knaul, H.S.; Prabhakar, R. Remediation of Environmentally Hazardous Organophosphates by Artificial Metalloenzymes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100529. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, M.; Llompart, M.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Cela, R.; Dagnac, T. Simultaneous Determination of Traces of Pyrethroids, Organochlorines and Other Main Plant Protection Agents in Agricultural Soils by Headspace Solid-Phase Microextraction—Gas Chromatography. J. Chromatogr. A 2008, 1188, 154–163. [Google Scholar] [CrossRef] [PubMed]
- ANVISA. Agência Nacional de Vigilância Sanitária Nota Técnica. In Reavaliação Toxicológica Do Ingrediente Ativo Fosforado; ANVISA: Brasília, Brazil, 2012. [Google Scholar]
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, History, and Classification. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–42. [Google Scholar]
- Wang, Y.; An, P.; Li, S.; Zhou, L. The Oxidation Mechanism and Kinetics of 2′-Deoxyguanosine by Carbonate Radical Anion. Chem. Phys. Lett. 2020, 739, 136982. [Google Scholar] [CrossRef]
- Gallo, M.A.; Lawryk, N.J. Organic Phosphorus Pesticides. In Handbook of Pesticide Toxicology; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Scorza Júnior, R.P.; de Rigitano, R.L.O. Comportamento Ambiental Do Inseticida Thiamethoxam Em Um Latossolo Vermelho Distroférrico de Dourados, MS; Embrapa Agropecuária Oeste: Dourados, Brazil, 2009.
- Feo, M.L.; Eljarrat, E.; Barceló, D.; Barceló, D. Determination of Pyrethroid Insecticides in Environmental Samples. TrAC Trends Anal. Chem. 2010, 29, 692–705. [Google Scholar] [CrossRef]
- Li, Z.; Jennings, A. Worldwide Regulations of Standard Values of Pesticides for Human Health Risk Control: A Review. Int. J. Environ. Res. Public Health 2017, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, Y.; Gong, D.; Yang, L.; Li, L.; Zhou, Z.; Xiong, S.; Tang, R.; Zheng, J. Visible Light Excited Graphitic Carbon Nitride for Efficient Degradation of Thiamethoxam: Removal Efficiency, Factors Effect and Reaction Mechanism Study. J. Environ. Chem. Eng. 2021, 9, 105739. [Google Scholar] [CrossRef]
- Craddock, H.A.; Huang, D.; Turner, P.C.; Quirós-Alcalá, L.; Payne-Sturges, D.C. Trends in Neonicotinoid Pesticide Residues in Food and Water in the United States, 1999–2015. Environ. Health 2019, 18, 7. [Google Scholar] [CrossRef]
- Mörtl, M.; Vehovszky, Á.; Klátyik, S.; Takács, E.; Győri, J.; Székács, A. Neonicotinoids: Spreading, Translocation and Aquatic Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 2006. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.; Acayaba, R.; Montagner, C. A química na avaliação do impacto à saúde humana diante da exposição aos pesticidas. Quim. Nova 2021, 44, 584–598. [Google Scholar] [CrossRef]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Silva Martinez, S. Advanced Oxidation Processes for the Removal of Organophosphorus Pesticides in Aqueous Matrices: A Systematic Review and Meta-Analysis. Process Saf. Environ. Prot. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Dong, H.; Xu, L.; Mao, Y.; Wang, Y.; Duan, S.; Lian, J.; Li, J.; Yu, J.; Qiang, Z. Effective Abatement of 29 Pesticides in Full-Scale Advanced Treatment Processes of Drinking Water: From Concentration to Human Exposure Risk. J. Hazard. Mater. 2021, 403, 123986. [Google Scholar] [CrossRef] [PubMed]
- Skanes, B.; Ho, J.; Warriner, K.; Prosser, R.S. Degradation of Boscalid, Pyraclostrobin, Fenbuconazole, and Glyphosate Residues by an Advanced Oxidative Process Utilizing Ultraviolet Light and Hydrogen Peroxide. J. Photochem. Photobiol. A Chem. 2021, 418, 113382. [Google Scholar] [CrossRef]
- Badellino, C.; Rodrigues, C.A.; Bertazzoli, R. Oxidation of Pesticides by in Situ Electrogenerated Hydrogen Peroxide: Study for the Degradation of 2,4-Dichlorophenoxyacetic Acid. J. Hazard. Mater. 2006, 137, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Vasseghian, Y.; Almomani, F.; Le, V.T.; Moradi, M.; Dragoi, E.-N. Decontamination of Toxic Malathion Pesticide in Aqueous Solutions by Fenton-Based Processes: Degradation Pathway, Toxicity Assessment and Health Risk Assessment. J. Hazard. Mater. 2022, 423, 127016. [Google Scholar] [CrossRef]
- Zekkaoui, C.; Berrama, T.; Dumoulin, D.; Billon, G.; Kadmi, Y. Optimal Degradation of Organophosphorus Pesticide at Low Levels in Water Using Fenton and Photo-Fenton Processes and Identification of by-Products by GC-MS/MS. Chemosphere 2021, 279, 130544. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Oturan, N.; Romero, A.; Santos, A.; Oturan, M.A. Optimization of Electro-Fenton Process for Effective Degradation of Organochlorine Pesticide Lindane. Catal. Today 2018, 313, 196–202. [Google Scholar] [CrossRef]
- Wu, J.; Luan, T.; Lan, C.; Hung Lo, T.W.; Chan, G.Y.S. Removal of Residual Pesticides on Vegetable Using Ozonated Water. Food Control 2007, 18, 466–472. [Google Scholar] [CrossRef]
- Wu, J.G.; Luan, T.G.; Lan, C.Y.; Lo, W.H.; Chan, G.Y.S. Efficacy Evaluation of Low-Concentration of Ozonated Water in Removal of Residual Diazinon, Parathion, Methyl-Parathion and Cypermethrin on Vegetable. J. Food Eng. 2007, 79, 803–809. [Google Scholar] [CrossRef]
- Wu, C.; Linden, K.G. Phototransformation of Selected Organophosphorus Pesticides: Roles of Hydroxyl and Carbonate Radicals. Water Res. 2010, 44, 3585–3594. [Google Scholar] [CrossRef]
- Frangos, P.; Shen, W.; Wang, H.; Li, X.; Yu, G.; Deng, S.; Huang, J.; Wang, B.; Wang, Y. Improvement of the Degradation of Pesticide Deethylatrazine by Combining UV Photolysis with Electrochemical Generation of Hydrogen Peroxide. Chem. Eng. J. 2016, 291, 215–224. [Google Scholar] [CrossRef]
- Ahmed Ibrahim, K.E.; Şolpan, D. Removal of Carbofuran in Aqueous Solution by Using UV-Irradiation/Hydrogen Peroxide. J. Environ. Chem. Eng. 2019, 7, 102820. [Google Scholar] [CrossRef]
- Antony, J.; Niveditha, S.V.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Stabilized Landfill Leachate Treatment by Zero Valent Aluminium-Acid System Combined with Hydrogen Peroxide and Persulfate Based Advanced Oxidation Process. Waste Manag. 2020, 106, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, Y.G. Sonochemistry: Environmental Science and Engineering Applications. Ind. Eng. Chem. Res. 2001, 40, 4681–4715. [Google Scholar] [CrossRef]
- Kida, M.; Ziembowicz, S.; Koszelnik, P. Removal of Organochlorine Pesticides (OCPs) from Aqueous Solutions Using Hydrogen Peroxide, Ultrasonic Waves, and a Hybrid Process. Sep. Purif. Technol. 2018, 192, 457–464. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A Review on Fenton and Improvements to the Fenton Process for Wastewater Treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Aanchal; Basu, S. Synthesis of Fe2O3/TiO2 Monoliths for the Enhanced Degradation of Industrial Dye and Pesticide via Photo-Fenton Catalysis. J. Photochem. Photobiol. A Chem. 2019, 376, 32–42. [Google Scholar] [CrossRef]
- Zhang, M.M.; Rempel, D.L.; Gross, M.L. A Fast Photochemical Oxidation of Proteins (FPOP) Platform for Free-Radical Reactions: The Carbonate Radical Anion with Peptides and Proteins. Free Radic. Biol. Med. 2019, 131, 126–132. [Google Scholar] [CrossRef]
- Gonçalves, B.R.; Guimarães, R.O.; Batista, L.L.; Ueira-Vieira, C.; Starling, M.C.V.M.; Trovó, A.G. Reducing Toxicity and Antimicrobial Activity of a Pesticide Mixture via Photo-Fenton in Different Aqueous Matrices Using Iron Complexes. Sci. Total Environ. 2020, 740, 140152. [Google Scholar] [CrossRef]
- Mishra, N.; Reddy, R.; Kuila, A.; Rani, A.; Nawaz, A.; Pichiah, S. A Review on Advanced Oxidation Processes for Effective Water Treatment. Curr. World Environ. 2017, 12, 469–489. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater—A Review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Kaavya, R.; Jayanath, Y.; Veenuttranon, K.; Lueprasitsakul, P.; Divya, V.; Kothakota, A.; Ramesh, S.V. Ozone as a Novel Emerging Technology for the Dissipation of Pesticide Residues in Foods—A Review. Trends Food Sci. Technol. 2020, 97, 38–54. [Google Scholar] [CrossRef]
- Wang, H.; Zhan, J.; Gao, L.; Yu, G.; Komarneni, S.; Wang, Y. Kinetics and Mechanism of Thiamethoxam Abatement by Ozonation and Ozone-Based Advanced Oxidation Processes. J. Hazard. Mater. 2020, 390, 122180. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Mendret, J.; Roustan, M.; Brosillon, S. Ozonation Using Hollow Fiber Contactor Technology and Its Perspectives for Micropollutants Removal in Water: A Review. Sci. Total Environ. 2020, 729, 138664. [Google Scholar] [CrossRef]
- Lafi, W.K.; Al-Qodah, Z. Combined Advanced Oxidation and Biological Treatment Processes for the Removal of Pesticides from Aqueous Solutions. J. Hazard. Mater. 2006, 137, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, J.; Xia, L.; Wang, J.; Chen, S.; Zhang, Y.; Bai, J.; Li, L.; Zhou, T.; Rahim, M.; et al. The Synergic Generation of CO3− and O2− Radicals in a Novel Photocatalytic Fuel Cell for Efficient Oxidation of Carbonate-Containing Wastewater and Simultaneous Electricity Production. Appl. Catal. B Environ. 2020, 277, 119227. [Google Scholar] [CrossRef]
- Yadav, A.; Mishra, P.C. Carbonate Radical Anion as an Efficient Reactive Oxygen Species: Its Reaction with Guanyl Radical and Formation of 8-Oxoguanine. Chem. Phys. 2012, 405, 76–88. [Google Scholar] [CrossRef]
- Gao, J.; Duan, X.; O’Shea, K.; Dionysiou, D.D. Degradation and Transformation of Bisphenol A in UV/Sodium Percarbonate: Dual Role of Carbonate Radical Anion. Water Res. 2020, 171, 115394. [Google Scholar] [CrossRef]
- Buxton, G.V.; Elliot, A.J. Rate Constant for Reaction of Hydroxyl Radicals with Bicarbonate Ions. Int. J. Radiat. Appl. Instrum. Part C. Radiat. Phys. Chem. 1986, 27, 241–243. [Google Scholar] [CrossRef]
- Arnold, W.A. One Electron Oxidation Potential as a Predictor of Rate Constants of N-Containing Compounds with Carbonate Radical and Triplet Excited State Organic Matter. Environ. Sci. Process. Impacts 2014, 16, 832–838. [Google Scholar] [CrossRef]
- Chen, F.; Xia, L.; Zhang, Y.; Bai, J.; Wang, J.; Li, J.; Rahim, M.; Xu, Q.; Zhu, X.; Zhou, B. Efficient Degradation of Refractory Organics for Carbonate-Containing Wastewater via Generation Carbonate Radical Based on a Photoelectrocatalytic TNA-MCF System. Appl. Catal. B Environ. 2019, 259, 118071. [Google Scholar] [CrossRef]
- Xia, L.; Chen, F.; Li, J.; Chen, S.; Bai, J.; Zhou, T.; Li, L.; Xu, Q.; Zhou, B. Efficient Organic Pollutants Conversion and Electricity Generation for Carbonate-Containing Wastewater Based on Carbonate Radical Reactions Initiated by BiVO4-Au/PVC System. J. Hazard. Mater. 2020, 389, 122140. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.S.; Khan, J.A.; Al-Muhtaseb, A.H.; Sayed, M.; Khan, H.M. Gamma Radiolytic Decomposition of Endosulfan in Aerated Solution: The Role of Carbonate Radical. Environ. Sci. Pollut. Res. 2016, 23, 12362–12371. [Google Scholar] [CrossRef]
- Conrado, A.B.; Pecci, L.; Fontana, M. Effects of Hypotaurine on Carbonate Radical Anion and Nitrogen Dioxide Radical Generated by Peroxidase Activity of Cu,Zn-Superoxide Dismutase. Free Radic. Biol. Med. 2013, 65, S23–S24. [Google Scholar] [CrossRef]
- Sablas, M.M.; de Luna, M.D.G.; Garcia-Segura, S.; Chen, C.-W.; Chen, C.-F.; Dong, C.-D. Percarbonate Mediated Advanced Oxidation Completely Degrades Recalcitrant Pesticide Imidacloprid: Role of Reactive Oxygen Species and Transformation Products. Sep. Purif. Technol. 2020, 250, 117269. [Google Scholar] [CrossRef]
- Banti, C.N.; Giannoulis, A.D.; Kourkoumelis, N.; Owczarzak, A.M.; Poyraz, M.; Kubicki, M.; Charalabopoulos, K.; Hadjikakou, S.K. Mixed Ligand–Silver(I) Complexes with Anti-Inflammatory Agents Which Can Bind to Lipoxygenase and Calf-Thymus DNA, Modulating Their Function and Inducing Apoptosis. Metallomics 2012, 4, 545–560. [Google Scholar] [CrossRef]
- Fagan, W.P.; Villamena, F.A.; Zweier, J.L.; Weavers, L.K. In Situ EPR Spin Trapping and Competition Kinetics Demonstrate Temperature-Dependent Mechanisms of Synergistic Radical Production by Ultrasonically Activated Persulfate. Environ. Sci. Technol. 2022, 56, 3729–3738. [Google Scholar] [CrossRef]


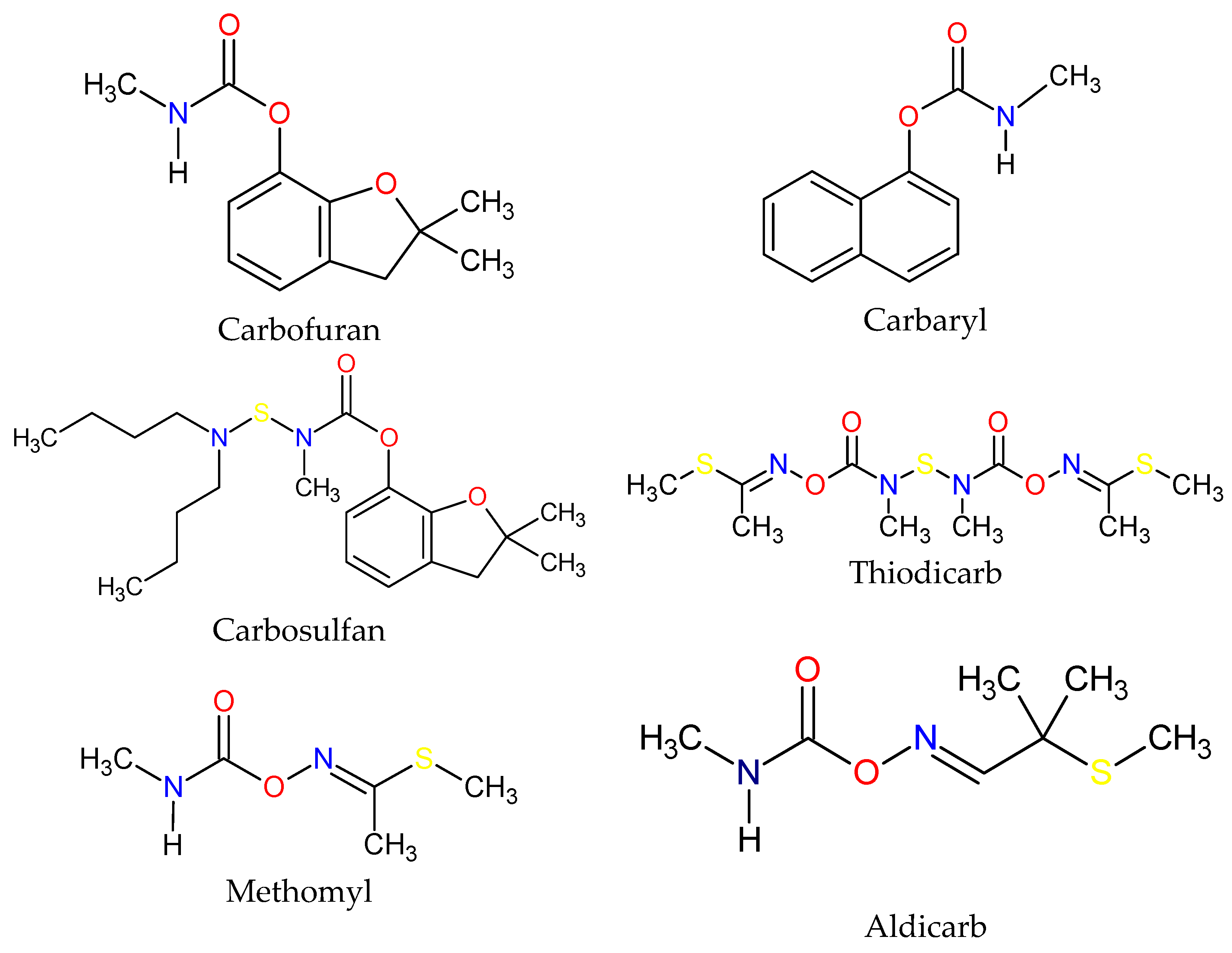

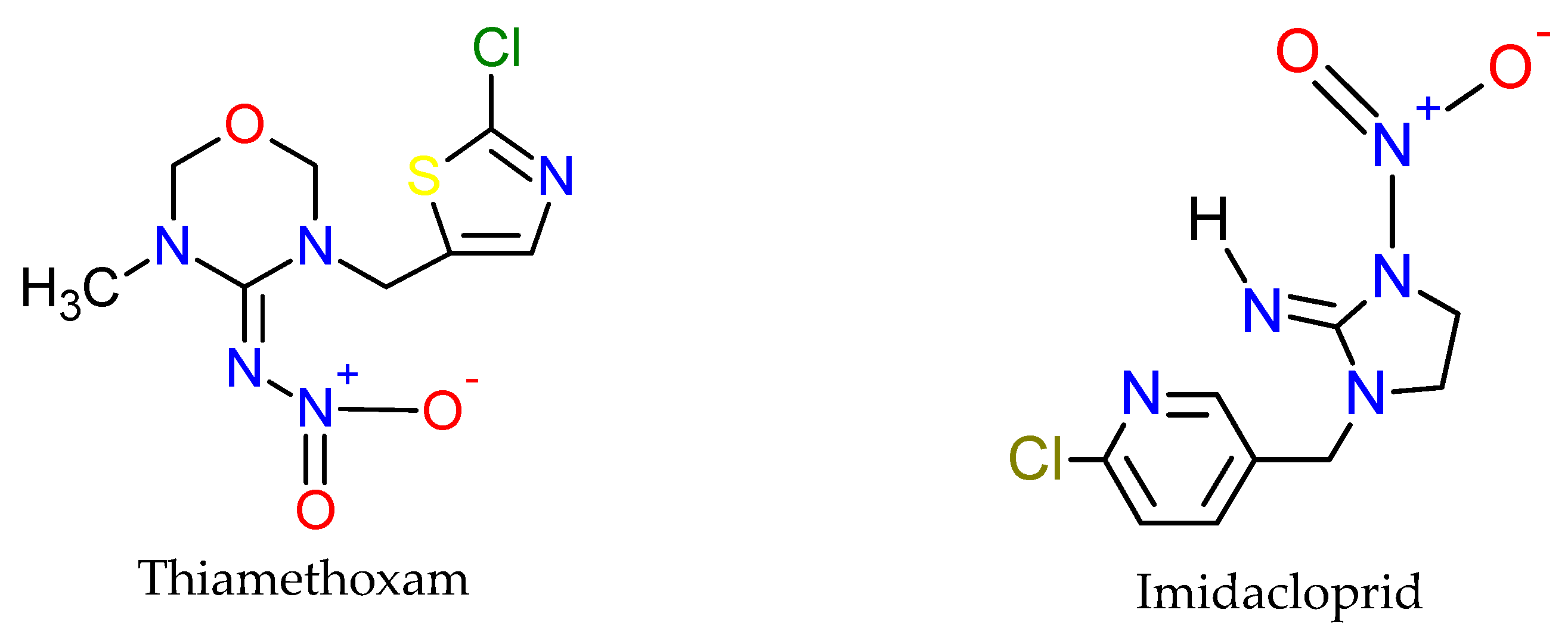
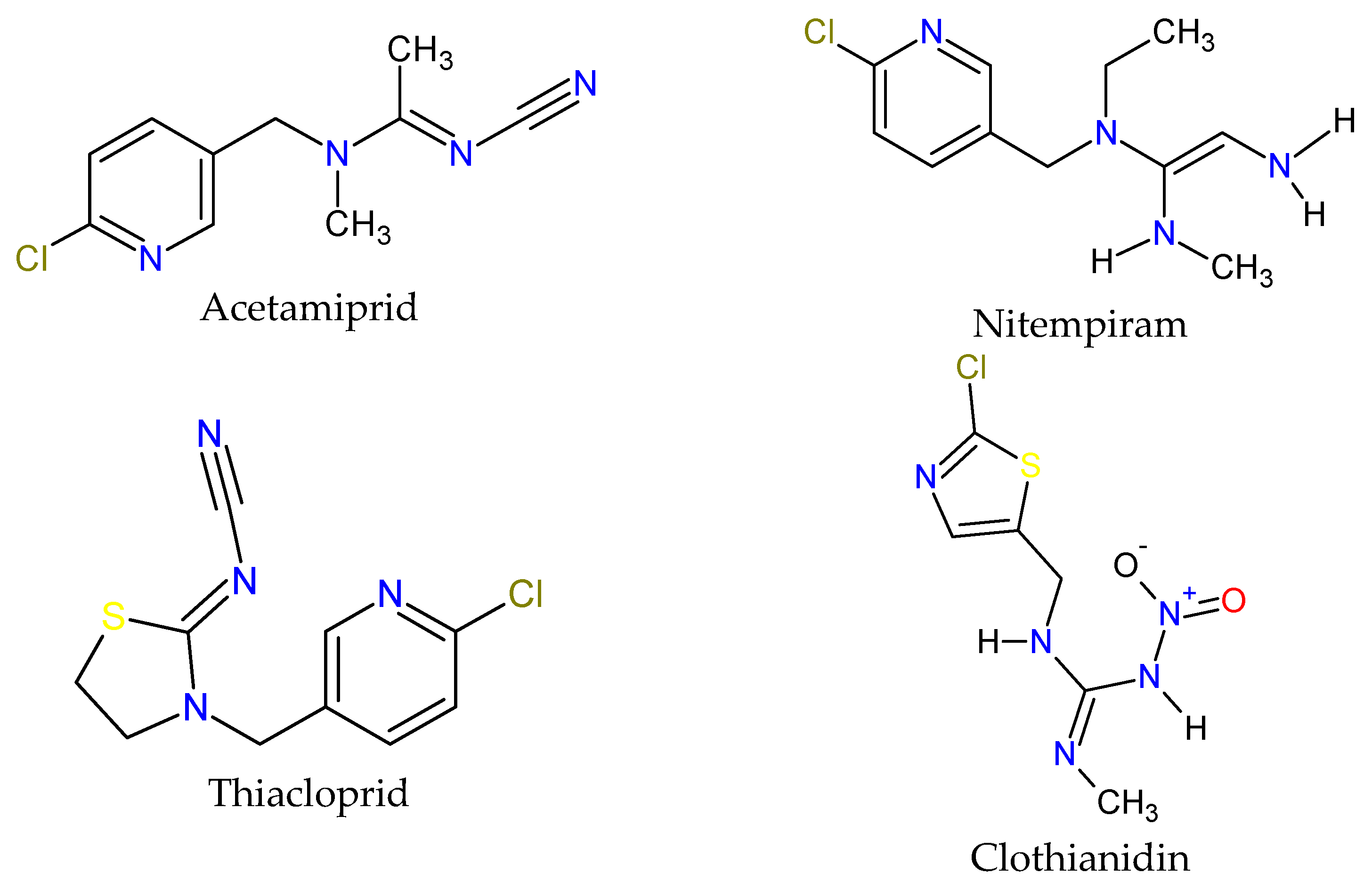
| Active Ingredient | Use | Chemical Group | * µg/L |
|---|---|---|---|
| Glyphosate | Herbicide | OP | 500 |
| Atrazine | Herbicide | Triazine | 2 |
| Parachat | Herbicide | Bipyridilium | 13 |
| DDT | Insecticidal | OCP | 1 |
| 2,4-D | Herbicide | OCP | 30 |
| Malation | Insecticidal | OP | 60 |
| Fipronil | Insecticidal | OCP | 1.2 |
| S-metolachlor | Herbicide | Chloroacetanilide | 10 |
| Lindane | Insecticidal | OCP | 2 |
| Thiamethoxam | Insecticidal | Neonicotinoid | 36 |
| Aldicarb | Insecticidal | Carbamate | 10 |
| AOPs | Pesticide | Efficiency | References |
|---|---|---|---|
| Fenton/Photo Fenton | OPS | >90% | [53] |
| 2,4-D | TOC Remove 69% | [56] | |
| Fenton | Malathion | 55% | [57] |
| Photo Fenton | 70% | ||
| Sono Feton | 98% | ||
| Photo Fenton | Diazion | 100% | [58] |
| Fenton | 85% | ||
| Eletro Fenton | Lindane | 92% | [59] |
| Ozone | Parathion, diazinon, cypermethrin | 75% | [60,61] |
| Carbonate radical | Paration | 4–10% | [62] |
| Chlorpyrifos | 15–45% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.K.S.; Silva, L.F.; Barbosa, G.A.F.; Miranda, T.G.; Sousa, R.R.; Sarmento, R.A.; Souza, N.L.G.D.; Pereira, D.H.; Cavallini, G.S. The Socio-Environmental and Human Health Problems Related to the Use of Pesticides and the Use of Advanced Oxidative Processes for Their Degradation: Brazil. Water 2023, 15, 1608. https://doi.org/10.3390/w15081608
Pereira AKS, Silva LF, Barbosa GAF, Miranda TG, Sousa RR, Sarmento RA, Souza NLGD, Pereira DH, Cavallini GS. The Socio-Environmental and Human Health Problems Related to the Use of Pesticides and the Use of Advanced Oxidative Processes for Their Degradation: Brazil. Water. 2023; 15(8):1608. https://doi.org/10.3390/w15081608
Chicago/Turabian StylePereira, Anna Karla Santos, Lívia Fernandes Silva, Gustavo Antonio Figueredo Barbosa, Thaynara Guimarães Miranda, Rayane Reis Sousa, Renato Almeida Sarmento, Nelson Luís Gonçalves Dias Souza, Douglas Henrique Pereira, and Grasiele Soares Cavallini. 2023. "The Socio-Environmental and Human Health Problems Related to the Use of Pesticides and the Use of Advanced Oxidative Processes for Their Degradation: Brazil" Water 15, no. 8: 1608. https://doi.org/10.3390/w15081608
APA StylePereira, A. K. S., Silva, L. F., Barbosa, G. A. F., Miranda, T. G., Sousa, R. R., Sarmento, R. A., Souza, N. L. G. D., Pereira, D. H., & Cavallini, G. S. (2023). The Socio-Environmental and Human Health Problems Related to the Use of Pesticides and the Use of Advanced Oxidative Processes for Their Degradation: Brazil. Water, 15(8), 1608. https://doi.org/10.3390/w15081608







