Occurrence and Removal of Pharmaceutical Contaminants in Urine: A Review
Abstract
:1. Introduction
2. Methods
3. Classification, Sources, and Metabolic Pathways of Pharmaceutical Contaminants
3.1. Classification of Pharmaceutical Contaminants in the Environment
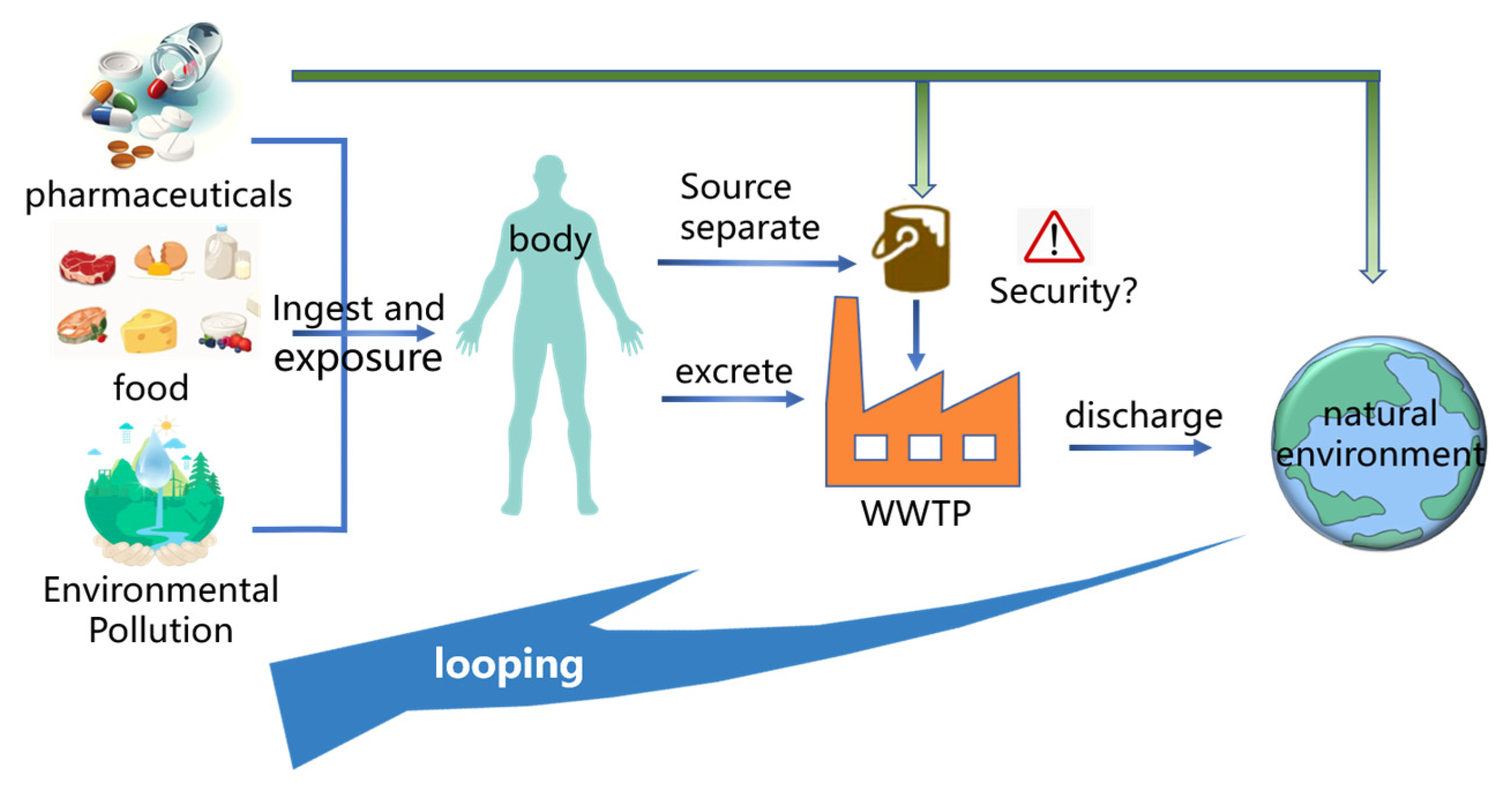
3.2. Sources of Pharmaceutical Contaminants in the Environment
3.3. Metabolic Pathway of Pharmaceuticals in Organisms
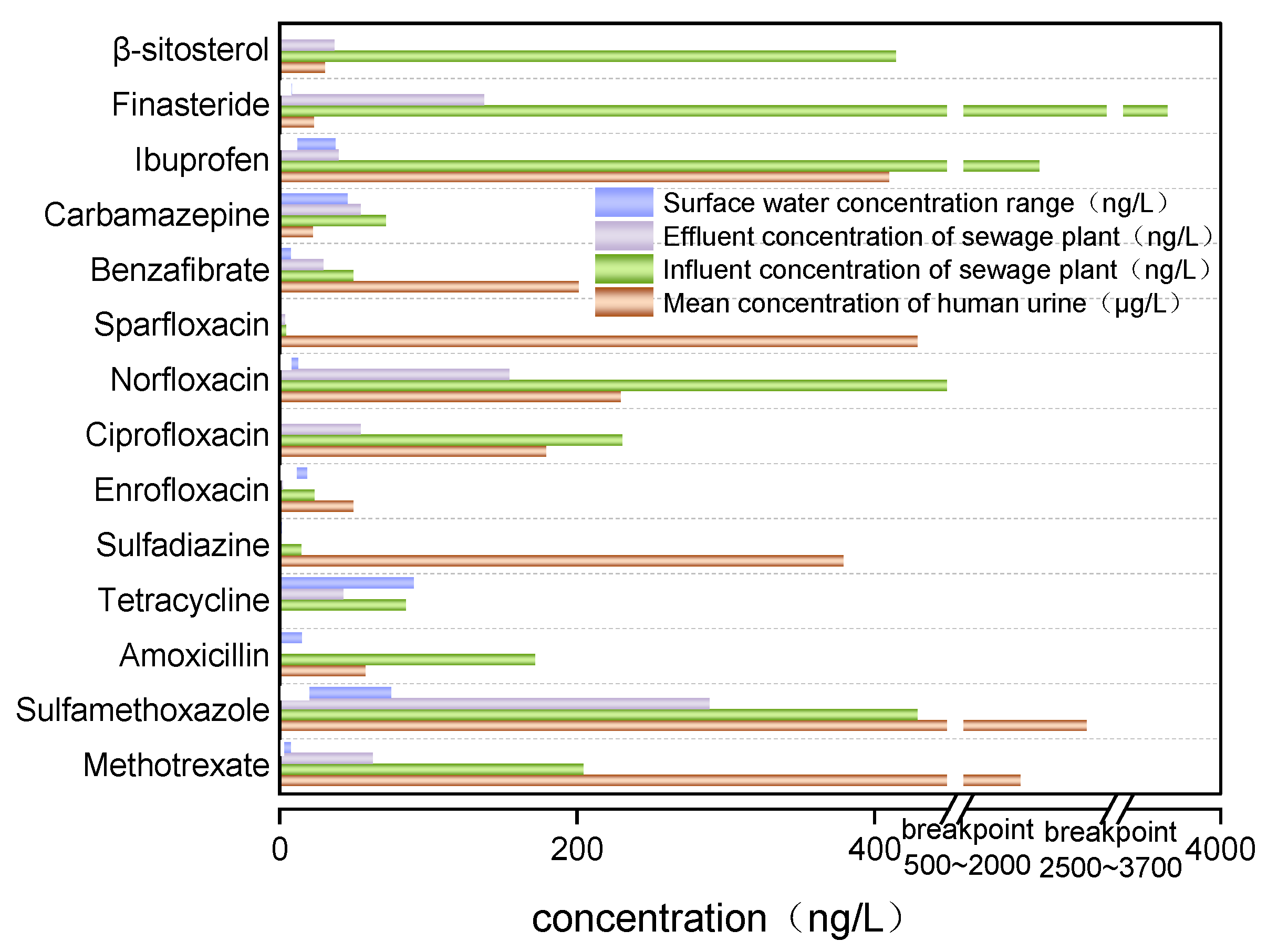
4. Pharmaceutical Contaminants in Human Urine and Other Different Environmental Media
| Pharmaceutical | Human Urine (μg/L) | Reference | Influent in WWTP (ng/L) | Effluent in WWTP (ng/L) | Reference | Surface Water (ng/L) | Reference |
|---|---|---|---|---|---|---|---|
| Methotrexate | 2199 (0.7–12800) a | [43] | 205 | 63 | [47] | 6–8 | [48] |
| Sulfamethoxazole | 2430 (ND–7740) a | [43] | 430 | 290 | [49] | 19.25–75.48 | [50] |
| Amoxicillin | 58.1 (ND–310) a | [43] | 172.6 | ND | [51] | 0–15.1 | [52] |
| Tetracycline | 1.4 (ND–2.8) a | [43] | 85.4 | 43.1 | [53] | ND–90.7 | [54] |
| Sulfadiazine | 380 b | [55] | 15 | ND | [56] | ND–1.898 | [57] |
| Enrofloxacin | 50 b | [55] | 23.93 | 2.47 | [58] | 10.5–18.7 | [59] |
| Ciprofloxacin | 180 b | [55] | 231 | 55 | [56] | 0.12–0.63 | [60] |
| Norfloxacin | 230 b | [55] | 468 | 155 | [56] | 7.0–12.9 | [59] |
| Sparfloxacin | 430 b | [55] | 4.7 | 4.1 | [61] | - | - |
| Benzafibrate | 202 b | [62] | 50 | 30 | [63] | 8 | [63] |
| Carbamazepine | 22.7 b | [62] | 72 | 55 | [48] | 46 | [64] |
| Ibuprofen | 411 b | [62] | 2265 | 40 | [48] | 11–38 | [65] |
| Finasteride | 23.3 b | [62] | 3840 | 138 | [66] | 7.7–8.6 | [67] |
| β-sitosterol | 30.8 b | [62] | 415.56 | 37.22 | [68] | ND | [69] |
5. Research Progress in Urine Treatment
5.1. Source Separation of Urine
5.2. Research Progress in Urine Treatment Technology
5.2.1. Physical Treatment
5.2.2. Biological Treatment
5.2.3. Chemical Treatment
5.2.4. Electrochemical Advanced Oxidation
Research Progress of Electrode Materials
Prospects for the Development of Electrochemical Technology
6. Conclusions
- (1)
- The vast majority of pharmaceuticals are excreted in the urine, and these pharmaceuticals enter the wastewater treatment plant with the domestic wastewater, so the wastewater treatment plant is the main source of pharmaceutical contaminants in the environment;
- (2)
- The results of the research show that pharmaceutical concentrations in urine are typically 2–3 orders of magnitude higher than those in municipal wastewater treatment plants and that pharmaceutical concentrations in wastewater treatment plants and surface water are generally at ng/L levels, posing potential risks to humans and the ecological environment;
- (3)
- Compared to physical and biological methods, the advanced electrochemical oxidation method is more effective and promising in treating pharmaceutical contaminants in urine. This technology is now maturing, but the cost is still too high, and in the future, it needs to be considered for coupling with other technologies to further reduce costs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, G.Q.; Zhang, C.X.; Zhang, D.D.; Xu, Y.; Yang, B.; Wei, X.C.; Zheng, X.Q. Prospects and problems in the agricultural utilization of source-separated urine as a substitute for chemical fertilizers. J. Agric. Resour. Environ. 2022, 39, 256–265. (In Chinese) [Google Scholar]
- Wilsenach, J.A.; Van Loosdrecht, M.C.M. Effects of separate urine collection on advanced nutrient removal processes. Environ. Sci. Technol. 2004, 38, 1208–1215. [Google Scholar] [CrossRef]
- Wang, J.Q.; Sun, F.Y.; Wang, Y.B.; Xiao, J.; Xue, J.R.; Zhang, Y.H.; Zhao, W.X.; Pan, S.C. Designation, Application and Evaluation of effectiveness of non-hazardous disposal of excreta. Chin. J. Public Health Eng. 2002, 01, 11–15. (In Chinese) [Google Scholar]
- Zhang, J.; Gao, S.B.; Zhang, J.; Joachim, B.; Nie, Z.Y. Concept and Application Demonstration for Ecological Sanitation. China Water Wastewater. 2008, 24, 10–14. (In Chinese) [Google Scholar]
- Tun, L.L.; Jeong, D.; Jeong, S.; Cho, K.; Lee, S.; Bae, H. Dewatering of source-separated human urine for nitrogen recovery by membrane distillation. J. Membr. Sci. 2016, 512, 13–20. [Google Scholar] [CrossRef]
- Guan, T.; Kuang, Y.; Li, X.D.; Fang, J.; Fang, W.K.; Wu, D.Y. The recovery of phosphorus from source-separated urine by repeatedly usable magnetic Fe3O4@ ZrO2 nanoparticles under acidic conditions. Environ. Int. 2020, 134, 105322. [Google Scholar] [CrossRef] [PubMed]
- Heinonen-Tanski, H.; Sjöblom, A.; Fabritius, H.; Päivi, K. Pure human urine is a good fertiliser for cucumbers. Bioresour. Technol. 2007, 98, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Adolfsson-Erici, M.; Parkkonen, J.; Pettersson, M.; Berg, A.H.; Olsson, P.E.; Förlin, L. Ethinyloestradiol—An undesired fish contraceptive? Aquat. Toxicol. 1999, 45, 91–97. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef]
- Escher, B.I.; Pronk, W.; Suter, M.J.F.; Maurer, M. Monitoring the removal efficiency of pharmaceuticals and hormones in different treatment processes of source-separated urine with bioassays. Environ. Sci. Technol. 2006, 40, 5095–5101. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef]
- Maurer, M.; Pronk, W.; Larsen, T.A. Treatment processes for source-separated urine. Water Res. 2006, 40, 3151–3166. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Cheng, S.; Zhang, J.; Saroj, D.P.; Mang, H.P.; Han, Y.Z.; Zhang, L.L.; Davaa, B.; Zheng, L.; Li, Z.F. Precipitation in urine source separation systems: Challenges for large-scale practical applications. Resour. Conserv. Recycl. 2021, 169, 105479. [Google Scholar] [CrossRef]
- Zheng, X.; Ye, H.; Cheng, T.; Zhang, Y.; Zhao, M.; Kong, H. Progress on the treatment of source separated urine. Technol. Water Treat. 2012, 38, 16–20. (In Chinese) [Google Scholar]
- Imwene, K.O.; Ngumba, E.; Kairigo, P.K. Emerging technologies for enhanced removal of residual antibiotics from source-separated urine and wastewaters: A review. J. Environ. Manag. 2022, 322, 116065. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Barceló, D.; Petrovic, M. Pharmaceuticals and personal care products (PPCPs) in the environment. Anal. Bioanal. Chem. 2007, 387, 1141–1142. [Google Scholar] [CrossRef]
- Wang, D.; Sui, Q.; Zhao, W.T.; Lǚ, S.G.; Qiu, Z.F.; Yu, G. Pharmaceutical and personal care products in the surface water of China: A review. Chin. Sci. Bull. 2014, 59, 743–751. [Google Scholar]
- Zhang, X.X.; Zhang, T.; Fang, H.H.P. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.M.; Scrimshaw, M.D.; Lester, J.N. The effects of natural and synthetic steroid estrogens in relation to their environmental occurrence. Crit. Rev. Toxicol. 2002, 32, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.P.; Otero, M.; Esteves, V. Processes for the elimination of estrogenic steroid hormones from water: A review. Environ. Pollut. 2012, 165, 38–58. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxiocl. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Zhou, X.F.; Dai, C.M.; Zhang, Y.L.; Surampalli, R.Y.; Zhang, T.C. A preliminary study on the occurrence and behavior of carbamazepine (CBZ) in aquatic environment of Yangtze River Delta, China. Environ. Monit. Assess. 2011, 173, 45–53. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.X.; Jia, Y.; Xiao, G.T.; Zhang, J.W.; Shan, W.J.; Zhou, S.X. Removal of Carbamazepine from water using persulfate catalyzed by N-doped sludge carbon. J. Qinghai Univ. 2022, 40, 9–17. (In Chinese) [Google Scholar]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change. Environ. Health Perspect. 1999, 107 (Suppl. S6), 907–938. [Google Scholar] [CrossRef] [PubMed]
- Lienert, J.; Bürki, T.; Escher, B.I. Reducing micropollutants with source control: Substance flow analysis of 212 pharmaceuticals in faeces and urine. Water Sci. Technol. 2007, 56, 87–96. [Google Scholar] [CrossRef]
- Sun, Y.Q. Disposition of Tritium Labeled Sulfamethoxazole in Swine, Broilers and Rats. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2020. (In Chinese). [Google Scholar]
- Zhao, H.H. The Disposition of Tritium Labeled Zaltoprofen in Pigs and Rats. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2020. (In Chinese). [Google Scholar]
- Wang, L.Y. Disposition and Residue Depletion of Aditoprim in Pig, Chicken, Carp and Rats. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2016. (In Chinese). [Google Scholar]
- Wen, L.H. The Disposition of Diaveridine in Swine, Broilers and Rats. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2013. (In Chinese). [Google Scholar]
- Tan, H.L. The Disposition of Olaquindox in Swine, Broilers, Craps and Rats. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2013. (In Chinese). [Google Scholar]
- Dudley, S.; Sun, C.; Jiang, J.; Gan, J. Metabolism of sulfamethoxazole in Arabidopsis thaliana cells and cucumber seedlings. Environ. Pollut. 2018, 242, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.C.; Wong, W.W.K. Chromatographic analysis of dithiocarbamate residues and their metabolites in foods employed in dietary exposure studies—A review. Food Addit. Contam. A 2022, 39, 1731–1743. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Yang, F.; Tang, Y. Janus Particles with pH Switchable Properties for High-Efficiency Adsorption of PPCPs in Water. Solid State Sci. 2021, 119, 106702. [Google Scholar] [CrossRef]
- Lishman, L.; Smyth, S.A.; Sarafin, K.; Kleywegt, S.; Toito, J.; Peart, T.; Lee, B.; Servos, M.; Beland, M.; Seto, P. Occurrence and reductions of pharmaceuticals and personal care products and estrogens by municipal wastewater treatment plants in Ontario, Canada. Sci. Total Environ. 2006, 367, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ. Int. 2007, 33, 596–601. [Google Scholar] [CrossRef]
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral drugs in aquatic environment and wastewater treatment plants: A review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020, 699, 134322. [Google Scholar] [CrossRef]
- Gulkowska, A.; Leung, H.W.; So, M.K.; Taniyasu, S.; Yamashita, N.; Yeung, L.W.Y.; Richardson, B.J.; Lei, A.P.; Giesy, J.P.; Lam, P.K.S. Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water Res. 2008, 42, 395–403. [Google Scholar] [CrossRef]
- Bayer, A.; Asner, R.; Schüssler, W.; Kopf, W.; Weiß, K.; Sengl, M.; Letzel, M. Behavior of sartans (antihypertensive drugs) in wastewater treatment plants, their occurrence and risk for the aquatic environment. Environ. Sci. Pollut. Res. 2014, 21, 10830–10839. [Google Scholar] [CrossRef] [PubMed]
- Ngumba, E.; Gachanja, A.; Nyirenda, J.; Maldonado, J.; Tuhkanen, T. Occurrence of antibiotics and antiretroviral drugs in source-separated urine, groundwater, surface water and wastewater in the peri-urban area of Chunga in Lusaka, Zambia. Water SA 2020, 46, 278–284. [Google Scholar]
- Wang, H.; Wang, N.; Qian, J.; Hu, L.Y.; Huang, P.X.; Su, M.F.; Yu, X.; Fu, C.W.; Jiang, F.; Zhao, Q.; et al. Urinary Antibiotics of Pregnant Women in Eastern China and Cumulative Health Risk Assessment. Environ. Sci. Technol. 2017, 51, 3518–3525. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, L.; Chen, Q.; Yu, K.; Zhao, S.S.; Zhang, L.; Zhang, J.; Zhang, W.X.; Huang, L.S. Maternal antibiotic concentrations in pregnant women in Shanghai and their determinants: A biomonitoring-based prospective study. Environ. Int. 2020, 138, 105638. [Google Scholar] [CrossRef]
- Ji, K.; Kho, Y.; Park, C.; Paek, D.; Ryu, P.; Paek, D.; Kim, M.; Kim, P.; Choi, K. Influence of water and food consumption on inadvertent antibiotics intake among general population. Environ. Res. 2010, 110, 641–649. [Google Scholar] [CrossRef]
- Behera, S.K.; Kim, H.W.; Oh, J.E.; Park, H.S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Meyer, M.T.; Liu, X.; Zhao, Q.; Chen, H.; Chen, J.A.; Qiu, Z.Q.; Yang, L.; Cao, J.; Shu, W.Q. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ. Pollut. 2010, 158, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Zhang, Z.; Li, P.; Zang, Y.; Liu, X. Antibiotics in aquatic environments of China: A review and meta-analysis. Ecotoxicol. Environ. Saf. 2020, 199, 110668. [Google Scholar] [CrossRef] [PubMed]
- Mutiyar, P.K.; Mittal, A.K. Occurrences and fate of an antibiotic amoxicillin in extended aeration-based sewage treatment plant in Delhi, India: A case study of emerging pollutant. Desalin. Water Treat. 2013, 51, 6158–6164. [Google Scholar] [CrossRef]
- Rico, A.; Arenas-Sánchez, A.; Alonso-Alonso, C.; López-Heras, I.; Nozal, L.; Rivas-Tabares, D.; Vighi, M. Identification of contaminants of concern in the upper Tagus river basin (central Spain). Part 1, Screening, quantitative analysis and comparison of sampling methods. Sci. Total Environ. 2019, 666, 1058–1070. [Google Scholar] [CrossRef]
- Li, K.Z.; Gao, P.; Wang, K.; Liu, Z.H.; Xue, G. Selective pressure of antibiotics and heavy metals on erythromycin resistance genes in wastewater. China Environ. Sci. 2015, 35, 889–896. (In Chinese) [Google Scholar]
- Zhang, Q.Q. Distribution Characteristics and Source Analysis of Antibiotic Pollution in Wenyu River Basin, Beijing. Master’s Thesis, Chongqing University, Chongqing, China, 2012. (In Chinese). [Google Scholar]
- Zhong, S.; Wu, X.; Zhang, D.; Du, S.J.; Shen, J.C.; Xiao, L.H.; Zhu, Y.; Xu, Y.Y.; Lin, Y.L.; Yin, L.Y.; et al. Antibiotics in urine from general adults in Shenzhen, China: Demographic-related difference in exposure levels. Sci. Total Environ. 2022, 843, 157070. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Okuda, T.; Yamashita, N.; Tanaka, H. Occurrence and elimination of antibiotics at four sewage treatment plants in Japan and their effects on bacterial ammonia oxidation. Water Sci. Technol. 2009, 59, 779–786. [Google Scholar] [CrossRef]
- Ma, X.Y.; Zheng, H.; Wang, Q.Q.; Ye, W.J.; Ding, Z.; Tang, W. Current Status of Antibiotic Contamination and its Ecological Risk Evaluation in Different Water Bodies of Jiangsu Province. J. Environ. Hyg. 2020, 10, 131–137. (In Chinese) [Google Scholar]
- Wu, M.H.; Que, C.J.; Xu, G.; Sun, Y.F.; Ma, J.; Xu, H.; Sun, R.; Tang, L. Occurrence, fate and interrelation of selected antibiotics in sewage treatment plants and their receiving surface water. Ecotoxicol. Environ. Saf. 2016, 132, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.L.; Zhuo, X.J.; Guo, Y. Occurrence and risk assessment of four typical fluoroquinolone antibiotics in raw and treated sewage and in receiving waters in Hangzhou, China. J. Agric. Food Chem. 2011, 59, 7303–7309. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xiao, X.B.; Tang, W.H.; Ge, C.J.; Yang, Y. Concentration Characteristics of Antibiotics in Urban Aquatic Environment of Haikou. Environ. Sci. Technol. 2013, 36, 60–65. (In Chinese) [Google Scholar]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J.Y. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Tettenborn, F.; Behrendt, J.; Otterpohl, R. Exemplary Treatment Processes for Yellow Water-Nutrients and Pharmaceutical Residues; International Water Association (IWA): London, UK, 2006. [Google Scholar]
- Garcia-Ac, A.; Segura, P.A.; Gagnon, C.; Sauvé, S. Determination of bezafibrate, methotrexate, cyclophosphamide, orlistat and enalapril in waste and surface waters using on-line solid-phase extraction liquid chromatography coupled to polarity-switching electrospray tandem mass spectrometry. J. Environ. Monit. 2009, 11, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fu, J.; Peng, H.; Hou, L.; Liu, M.; Zhou, J.L. Occurrence and phase distribution of selected pharmaceuticals in the Yangtze Estuary and its coastal zone. J. Hazard. Mater. 2011, 190, 588–596. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef]
- Fu, J.T. Occurrence and Removal Rules of Pharmaceutical Contaminants in the Third Wastewater Treatment Plant of Xi’an City. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, 2012. (In Chinese). [Google Scholar]
- Gumbi, B.P.; Moodley, B.; Birungi, G.; Ndungu, P.G. Detection and quantification of acidic drug residues in South African surface water using gas chromatography-mass spectrometry. Chemosphere 2017, 168, 1042–1050. [Google Scholar] [CrossRef]
- Furtula, V.; Liu, J.; Chambers, P.; Osachoff, H.; Kennedy, C.; Harkness, J. Sewage Treatment Plants Efficiencies in Removal of Sterols and Sterol Ratios as Indicators of Fecal Contamination Sources. Water Air Soil Pollut. 2012, 223, 1017–1031. [Google Scholar] [CrossRef]
- Focazio, M.J.; Kolpin, D.W.; Barnes, K.K.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Barber, L.B.; Thurman, M.E. A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States—II Untreated drinking water sources. Sci. Total Environ. 2008, 402, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.J. Status, problems and countermeasures of antibiotic use in livestock agriculture. China Anim. Health 2009, 9, 55–57. [Google Scholar]
- Kyriakides, D.; Lazaris, A.C.; Arsenoglou, K.; Emmanouil, M.; Kyriakides, O.; Kavantzas, N.; Panderi, I. Dietary Exposure Assessment of Veterinary Antibiotics in Pork Meat on Children and Adolescents in Cyprus. Foods 2020, 9, 1479. [Google Scholar] [CrossRef]
- The Ministry of Agriculture plans to ban four kinds of antibiotics, and hundreds of enterprises’ veterinary drug approvals will be canceled. Sichuan Anim. Vet. Sci. 2015, 42, 8. (In Chinese)
- Sim, W.J.; Lee, J.W.; Oh, J.E. Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea. Environ. Pollut. 2010, 158, 1938–1947. [Google Scholar] [CrossRef]
- Larsen, T.A.; Gruendl, H.; Binz, C. The potential contribution of urine source separation to the SDG agenda—A review of the progress so far and future development options. Environ. Sci. Water Res. Technol. 2021, 7, 1161–1176. [Google Scholar] [CrossRef]
- Ohlinger, K.N.; Young, T.M.; Schroeder, E.D. Predicting struvite formation in digestion. Water Res. 1998, 32, 3607–3614. [Google Scholar] [CrossRef]
- Chipako, T.L.; Randall, D.G. Urine treatment technologies and the importance of pH. J. Environ. Chem. Eng. 2020, 8, 103622. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Abdel-Shafy, H.I.; Mansour, M.S.M. Removal of pharmaceutical compounds from urine via chemical coagulation by green synthesized ZnO-nanoparticles followed by microfiltration for safe reuse. Arab. J. Chem. 2019, 12, 4074–4083. [Google Scholar] [CrossRef] [Green Version]
- Antonini, S.; Paris, S.; Eichert, T.; Clemens, J. Nitrogen and Phosphorus Recovery from Human Urine by Struvite Precipitation and Air Stripping in Vietnam. Clean Soil Air Water 2011, 39, 1099–1104. [Google Scholar] [CrossRef]
- Paredes-Laverde, M.; Silva-Agredo, J.; Torres-Palma, R.A. Removal of norfloxacin in deionized, municipal water and urine using rice (Oryza sativa) and coffee (Coffea arabica) husk wastes as natural adsorbents. J. Environ. Manag. 2018, 213, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.Z.; Li, Y.X.; Meng, T.; Zhang, R.C.; Song, M.; Ren, J. Removal of sulfonamide antibiotics and human metabolite by biochar and biochar/H2O2 in synthetic urine. Water Res. 2018, 147, 91–100. [Google Scholar] [CrossRef]
- Pronk, W.; Palmquist, H.; Biebow, M.; Boller, M. Nanofiltration for the separation of pharmaceuticals from nutrients in source-separated urine. Water Res. 2006, 40, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Landry, K.A.; Boyer, T.H. Diclofenac removal in urine using strong-base anion exchange polymer resins. Water Res. 2013, 47, 6432–6444. [Google Scholar] [CrossRef]
- Udert, K.M.; Fux, C.; Münster, M.; Larsen, T.A.; Siegrist, H.; Gujer, W. Nitrification and autotrophic denitrification of source-separated urine. Water Sci. Technol. 2003, 48, 119–130. [Google Scholar] [CrossRef]
- Köpping, I.; McArdell, C.S.; Borowska, E.; Böhler, M.A.; Udert, K.M. Removal of pharmaceuticals from nitrified urine by adsorption on granular activated carbon. Water Res. X 2020, 9, 100057. [Google Scholar] [CrossRef]
- Shang, K.; Wang, X.; Li, J.; Wang, H.; Lu, N.; Jiang, N.; Wu, Y. Synergetic degradation of Acid Orange 7 (AO7) dye by DBD plasma and persulfate. Chem. Eng. J. 2017, 311, 378–384. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.J.; Hong, Y.X.; Chelme-Ayala, P.; Meng, L.J.; Wu, Z.R.; El-Din, M.G. The treatment of electroplating wastewater using an integrated approach of interior microelectrolysis and Fenton combined with recycle ferrite. Chemosphere 2022, 286, 131543. [Google Scholar] [CrossRef]
- Cotillas, S.; Lacasa, E.; Sáez, C.; Cañizares, P.; Rodrigo, M.A. Electrolytic and electro-irradiated technologies for the removal of chloramphenicol in synthetic urine with diamond anodes. Water Res. 2018, 128, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Guateque-Londoño, J.F.; Serna-Galvis, E.A.; Ávila-Torres, Y.; Torres-Palma, R.A. Degradation of Losartan in Fresh Urine by Sonochemical and Photochemical Advanced Oxidation Processes. Water 2020, 12, 3398. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, D.M.; Serna-Galvis, E.A.; Ferraro, F.; Torres-Palma, R.A. Degradation of the emerging concern pollutant ampicillin in aqueous media by sonochemical advanced oxidation processes—Parameters effect, removal of antimicrobial activity and pollutant treatment in hydrolyzed urine. J. Environ. Manag. 2020, 261, 110224. [Google Scholar] [CrossRef] [PubMed]
- Sebuso, D.P.; Kuvarega, A.T.; Lefatshe, K.; King’ondu, C.K.; Numan, N.; Maaza, M.; Muiva, C.M. Corn husk multilayered graphene/ZnO nanocomposite materials with enhanced photocatalytic activity for organic dyes and doxycycline degradation. Mater. Res. Bull. 2022, 151, 111800. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 2019, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Jasper, J.T.; Yang, Y.; Hoffmann, M.R. Toxic Byproduct Formation during Electrochemical Treatment of Latrine Wastewater. Environ. Sci. Technol. 2017, 51, 7111–7119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.F.; Zhang, J.; Zhou, K.H.; Feng, Y.J. Control and removal of disinfection by-products (DBPs) during electrochemical oxidation of urine. Chin. J. Environ. Eng. 2021, 15, 2973–2984. [Google Scholar]
- Loaiza-Ambuludi, S.; Panizza, M.; Oturan, N.; Özcan, A.; Oturan, M.A. Electro-Fenton degradation of anti-inflammatory drug ibuprofen in hydroorganic medium. J. Electroanal. Chem. 2013, 702, 31–36. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous photocatalytic degradation of ibuprofen in ultrapure water, municipal and pharmaceutical industry wastewaters using a TiO2/UV-LED system. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Quero-Pastor, M.J.; Garrido-Perez, M.C.; Acevedo, A.; Quiroga, J.M. Ozonation of ibuprofen: A degradation and toxicity study. Sci. Total Environ. 2014, 466, 957–964. [Google Scholar] [CrossRef]
- Illés, E.; Takács, E.; Dombi, A.; Gajda-Schrantz, K.; Rácz, G.; Gonter, K.; Wojnárovits, L. Hydroxyl radical induced degradation of ibuprofen. Sci. Total. Environ. 2013, 447, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, J.J.; Zeng, G.M.; Liu, Y.N.; Deng, Y.C.; Zhou, Y.Y.; Tang, J.; Wang, J.J.; Guo, J. Enhanced photocatalytic degradation of norfloxacin in aqueous Bi2WO6 dispersions containing nonionic surfactant under visible light irradiation. J. Hazard. Mater. 2016, 306, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Palma-Goyes, R.E.; Guzmán-Duque, F.L.; Peñuela, G.; González, I.; Nava, J.L.; Torres-Palma, R.A. Electrochemical degradation of crystal violet with BDD electrodes: Effect of electrochemical parameters and identification of organic by-products. Chemosphere 2010, 81, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Surenjan, A.; Pradeep, T.; Philip, L. Application and performance evaluation of a cost-effective vis-LED based fluidized bed reactor for the treatment of emerging contaminants. Chemosphere 2019, 228, 629–639. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gao, L.X.; Yang, W.L. Study on the COD(Cr) removal efficiency from printing dyeing wastewater by electrochemical process. Ind. Water Treat. 2012, 32, 47–50. [Google Scholar]
- Sun, M.; Qin, M.H.; Wang, C.; Weng, G.M.; Huo, M.X.; Taylor, A.D.; Qu, J.H.; Elimelech, M. Electrochemical-Osmotic Process for Simultaneous Recovery of Electric Energy, Water, and Metals from Wastewater. Environ. Sci. Technol. 2020, 54, 8430–8442. [Google Scholar] [CrossRef]
- Chaplin, B.P. Advantages, disadvantages, and future challenges of the use of electrochemical technologies for water and wastewater treatment. In Electrochemical Water and Wastewater Treatment; Butterworth-Heinemann: Oxford, UK, 2018; pp. 451–494. [Google Scholar]
- Larsen, T.A.; Riechmann, M.E.; Udert, K.M. State of the art of urine treatment technologies: A critical review. Water Res. 2021, 13, 100114. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.F.; Deng, Y.Y.; Zhang, Q.; Jiang, G.M.; Liu, H. Recent progress on the recovery of valuable resources from source-separated urine on-site using electrochemical technologies: A review. Chem. Eng. J. 2022, 442, 136200. [Google Scholar] [CrossRef]
- Zhang, Z.F. Optimization and Application of Water Treatment Technologyby Boron-Doped Diamond Anode. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2018. (In Chinese). [Google Scholar]
- Özcan, A.; Özcan, A.A.; Demirci, Y. Evaluation of mineralization kinetics and pathway of norfloxacin removal from water by electro-Fenton treatment. Chem. Eng. J. 2016, 304, 518–526. [Google Scholar] [CrossRef]
- Liang, S.T.; Lin, H.; Habteselassie, M.; Huang, Q.Q. Electrochemical inactivation of bacteria with a titanium sub-oxide reactive membrane. Water Res. 2018, 145, 172–180. [Google Scholar] [CrossRef]
- Gonzaga, I.M.D.; Dória, A.R.; Moratalla, A.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Cañizares, P.; Rodrigo, M.A.; Saez, C. Electrochemical systems equipped with 2D and 3D microwave-made anodes for the highly efficient degradation of antibiotics in urine. Electrochim. Acta 2021, 392, 139012. [Google Scholar] [CrossRef]
- Jojoa-Sierra, S.D.; Silva-Agredo, J.; Herrera-Calderon, E.; Torres-Palma, R.A. Elimination of the antibiotic norfloxacin in municipal wastewater, urine and seawater by electrochemical oxidation on IrO2 anodes. Sci. Total Environ. 2017, 575, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Ji, Q.H.; Hu, C.Z.; Qu, J.H. Recent advances in electro-oxidation technology for water treatment. J. Civ. Environ. Eng. 2022, 44, 104–118. [Google Scholar]
- Salazar-Banda, G.R.; Santos, G.O.S.; Gonzaga, I.M.D.; Dória, A.R.; Eguiluz, K.I.B. Developments in electrode materials for wastewater treatment. Curr. Opin. Electrochem. 2021, 26, 100663. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Yin, L.; Niu, J.F.; Hou, L.A. Insights of ibuprofen electro-oxidation on metal-oxide-coated Ti anodes: Kinetics, energy consumption and reaction mechanisms. Chemosphere 2016, 163, 584–591. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Wang, Y.P.; Tang, S.Y.; Wang, Y.; Yu, H.Y.; Niu, J.F. Insights into the electrochemical degradation of triclosan from human urine: Kinetics, mechanism and toxicity. Chemosphere 2021, 264, 128598. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.; Xu, J.; Li, Y.; Pan, Y.H. Electrochemical mineralization of sulfamethoxazole by Ti/SnO2-Sb/Ce-PbO2 anode: Kinetics, reaction pathways, and energy cost evolution. Electrochim. Acta 2013, 97, 167–174. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, Q. Electrochemical degradation of antibiotic levofloxacin by PbO2 electrode: Kinetics, energy demands and reaction pathways. Chemosphere 2018, 205, 215–222. [Google Scholar] [CrossRef]
- Parra, K.N.; Gul, S.; Aquino, J.M.; Miwa, D.W.; Motheo, A.J. Electrochemical degradation of tetracycline in artificial urine medium. J. Solid State Electrochem. 2016, 20, 1001–1009. [Google Scholar] [CrossRef]
- Perea, A.; Palma-Goyes, R.E.; Vazquez-Arenas, J.; Romero-Ibarra, I.; Ostos, C.; Torres-Palma, R.A. Efficient cephalexin degradation using active chlorine produced on ruthenium and iridium oxide anodes: Role of bath composition, analysis of degradation pathways and degradation extent. Sci. Total Environ. 2019, 648, 377–387. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Fortunato, G.V.; Kronka, M.S.; Vernasqui, L.G.; Ferreira, N.G.; Lanza, M.R.V. Electrochemical oxidation of ciprofloxacin in different aqueous matrices using synthesized boron-doped micro and nano-diamond anodes. Environ. Res. 2022, 204, 112027. [Google Scholar] [CrossRef]
- Sordello, F.; Fabbri, D.; Rapa, L.; Minero, C.; Minella, M.; Vione, D. Electrochemical abatement of cefazolin: Towards a viable treatment for antibiotic-containing urine. J. Clean. Prod. 2021, 289, 125722. [Google Scholar] [CrossRef]
- Gonzaga, I.M.D.; Moratalla, A.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Cañizares, P.; Rodrigo, M.A.; Saez, C. Novel Ti/RuO2IrO2 anode to reduce the dangerousness of antibiotic polluted urines by Fenton-based processes. Chemosphere 2021, 270, 129344. [Google Scholar] [CrossRef]
- Singla, J.; Verma, A.; Sangal, V.K. Applications of doped mixed metal oxide anode for the electro-oxidation treatment and mineralization of urine metabolite, uric acid. J. Water Process Eng. 2019, 32, 100944. [Google Scholar] [CrossRef]
- Gonzaga, I.M.D.; Moratalla, A.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Cañizares, P.; Rodrigo, M.A.; Saez, C. Influence of the doping level of boron-doped diamond anodes on the removal of penicillin G from urine matrixes. Sci. Total Environ. 2020, 736, 139536. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, W.J.; Lu, Z.D.; Ao, X.W.; Li, S.M. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef]
- Gattani, A.; Singh, S.V.; Agrawal, A.; Khan, M.H.; Singh, P. Recent progress in electrochemical biosensors as point of care diagnostics in livestock health. Anal. Biochem. 2019, 579, 25–34. [Google Scholar] [CrossRef] [PubMed]

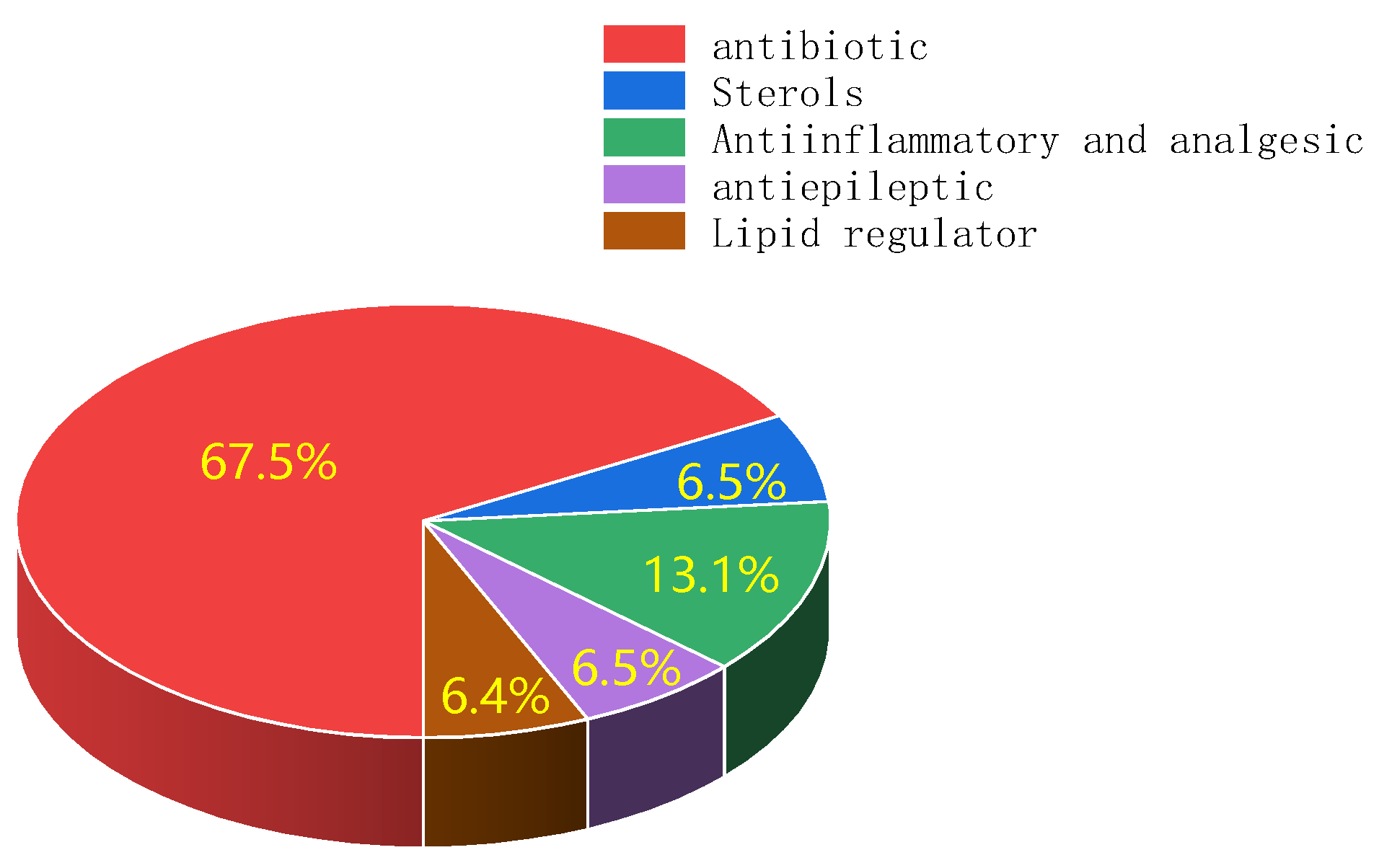

| Subgroups | Representative Compounds | |
|---|---|---|
| Pharmaceuticals | Antibiotics | Clarithromycin |
| Sulfamethoxazole | ||
| Sulfadimethoxine | ||
| Norfloxacin | ||
| Ciprofloxacin | ||
| Hormones | estrone (E1) | |
| estradiol (E2) | ||
| ethinylestradiol (EE2) | ||
| estradiol (E3) | ||
| antiepileptics | Carbamazepine | |
| Primidone | ||
| Analgesics and anti-inflammatory drugs | Ibuprofen | |
| Diclofenac | ||
| Acetaminophen | ||
| Blood lipid regulators | Gemfibrozil | |
| Clofibrate | ||
| β-blockers | Propanolol | |
| Metoprolol | ||
| Stimulants | Caffeine Cocaine |
| Drug Name | Recovery Rate in Urine (%) | Recovery Rate in Feces (%) | Total Recovery Rate (%) | Reference | |
|---|---|---|---|---|---|
| Drug recovery after a single intramuscular drug injection in pigs | Sulfamethoxazole | 80.59 ± 5.72 | 14.72 ± 1.31 | 95.31 ± 4.41 | [30] |
| Zaltoprofen | 74.80 ± 2.52 | 21.13 ± 1.90 | 95.82 ± 0.51 | [31] | |
| Adiprin | 78.28 ± 1.86 | 17.29 ± 2.54 | 95.57 ± 1.16 | [32] | |
| Diaveridine | 81.7 ± 3.61 | 11.00 ± 0.97 | 92.70 ± 4.23 | [33] | |
| Olaquindox | 93.08 ± 2.87 | 1.98 ± 0.61 | 95.07 ± 2.93 | [34] | |
| Drug recovery in male rats after a single intramuscular drug injection | Sulfamethoxazole | 75.32 ± 4.54 | 23.24 ± 1.79 | 98.56 ± 2.82 | [30] |
| Zaltoprofen | 17.23 ± 1.70 | 79.73 ± 5.65 | 96.97 ± 7.28 | [31] | |
| Adiprin | 81.12 ± 13.03 | 15.7 ± 9.27 | 96.82 ± 3.81 | [32] | |
| Diaveridine | 81.50 ± 8.81 | 11.30 ± 2.01 | 92.80 ± 6.81 | [33] | |
| Olaquindox | 88.48 ± 0.56 | 6.82 ± 1.57 | 94.89 ± 2.09 | [34] | |
| Drug recovery in female rats after a single intramuscular drug injection | Sulfamethoxazole | 77.9 ± 5.93 | 19.58 ± 2.09 | 97.48 ± 5.56 | [30] |
| Zaltoprofen | 26.61 ± 0.73 | 68.16 ± 5.06 | 94.77 ± 5.76 | [31] | |
| Adiprin | 73.53 ± 1.40 | 19.18 ± 8.73 | 92.7 ± 10.01 | [32] | |
| Diaveridine | 80.98 ± 9.92 | 13.00 ± 3.88 | 93.98 ± 7.14 | [33] | |
| Olaquindox | 85.45 ± 2.08 | 6.87 ± 1.86 | 91.79 ± 1.03 | [34] |
| Pharmaceuticals | Processing Technology | Treatment Effect | Urine Type | Reference |
|---|---|---|---|---|
| Ibuprofen, ephedrine and propranolol | ZnO nanoparticles for chemical coagulation | Removal rates all over 99% | Real urine | [78] |
| Norfloxacin | RH adsorption CH adsorption | Removal rates were 30.6% and 83.54%, respectively | Synthetic urine | [80] |
| Sulfonamides | Biochar/H2O2 | Removal rates all over 80% | Hydrolysis of urine | [81] |
| Propranolol, ethinyl estradiol, ibuprofen, diclofenac, and carbamazepine | Nanofiltration Membrane | Fresh urine: drug retention > 92% Synthetic urine: drug retention > 73% | Fresh urine/synthetic urine | [82] |
| Diclofenac | Ion exchange resin | Removal rate over 90% | Synthetic urine | [83] |
| 11 pharmaceuticals including carbamazepine and metoprolol | Nitrification + Adsorption | Removal rate of 90% | Synthetic urine | [85] |
| Chloramphenicol | Photodissolution | Chloramphenicol fully mineralized | Synthetic urine | [88] |
| Clozaril | Acoustic Chemistry/ UVC/H2O2 | Removal rate of 90% | Synthetic urine | [89] |
| Ampicillin | Acoustic Chemistry | Removal rate of 92% | Synthetic urine | [90] |
| Penicillin G, Meropenem and Chloramphenicol | Electrolysis/ Light-Electrolysis | Removal rate of > 70/82–100% | Synthetic urine | [106] |
| Norfloxacin | Electrolysis | Removal rate up to 100% | Synthetic urine | [107] |
| Ibuprofen | Electrolysis | Fully mineralized | Synthetic urine | [110] |
| Anode Type | Processing Objects | Operating Conditions | Main Results | Energy Consumption Analysis | Reference |
|---|---|---|---|---|---|
| Ti/SnO2eSb/PbO2 | Simulated urine wastewater containing 5 mg/L triclosan | Electrode spacing: 10 mm Current density: 10 mA/cm2 | Triclosan removal rate: 90% | Ton of water power consumption: 4.5~47.8 kWh | [116] |
| Ti/Ru0.3Ti0.7O2 | Simulated urine wastewater containing 200 mg/L tetracycline | Electrode spacing: 6 mm Current density: 10–40 mA/cm2 Electrolysis time: 3 h | Tetracycline removal rate: 50% | Electricity consumption per ton of water: 2.85–4.1 kWh | [119] |
| Ti/RuO2-IrO2 | Simulated urine wastewater containing cephalexin | Current density: 6 mA/cm2 Electrolysis time: 2 h | Degradation rate of ciprofloxacin: 80% | Electricity consumption per ton of water: 0.088 kWh | [120] |
| Nanocrystalline Diamond (NCD) | simulated urine wastewater containing 15 mg/L ciprofloxacin | Current density: 40 mA/cm2 Electrolysis time: 60 min Temperature: 25 °C | Degradation rate of ciprofloxacin: 90.4% | Electricity consumption per ton of water: 22.9 kWh | [121] |
| Ag/AgCl/KCl | Simulated urine wastewater containing 10 μM-1 mM cefazolin | Current density: 0.5–150 mA/cm2 Electrolysis time: 0–500 min | Current density: 150 mA/cm2, electrolysis: 20 min, cefazolin residue < 0.5‰ | Maximum power consumption of 3.7 kWh per ton of water | [122] |
| MMO-RuO2-IrO2 | Simulated urine wastewater containing 50 mg/L penicillin G | Current density: 30 mA/cm2 Electrolysis time: 2 h | Penicillin G removal rate ≥ 99% | SEC: 0.5 kWh (% inhibition)-1 | [123] |
| Sb-Sn-Ta-Ir/Ti | Simulated urine wastewater containing 50 mg/L uric acid | Electrode spacing: about 20 mm Current density: 7.46 mA/cm2 Electrolysis time: 42.79 min | COD removal rate: 92% TOC removal rate: 89% | Electricity consumption per ton of water: 2.479 kWh | [124] |
| 100–8000 ppm BDD anode | Simulated urine wastewater containing 50 mg/L penicillin G | Current density: 30 mA/cm2 Electrolysis time: 8 h Charge through: 6.4 Ah dm−3 | 200 ppm-BDD has the best effect, a 100% removal rate of penicillin G, and a 90% reduction of toxicity | SEC: 0.15 kWh (% inhibition)-1 | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, B.; Liu, F.; Yu, G. Occurrence and Removal of Pharmaceutical Contaminants in Urine: A Review. Water 2023, 15, 1517. https://doi.org/10.3390/w15081517
Li X, Wang B, Liu F, Yu G. Occurrence and Removal of Pharmaceutical Contaminants in Urine: A Review. Water. 2023; 15(8):1517. https://doi.org/10.3390/w15081517
Chicago/Turabian StyleLi, Xiaolin, Bin Wang, Feng Liu, and Gang Yu. 2023. "Occurrence and Removal of Pharmaceutical Contaminants in Urine: A Review" Water 15, no. 8: 1517. https://doi.org/10.3390/w15081517
APA StyleLi, X., Wang, B., Liu, F., & Yu, G. (2023). Occurrence and Removal of Pharmaceutical Contaminants in Urine: A Review. Water, 15(8), 1517. https://doi.org/10.3390/w15081517






