Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment
Abstract
1. Introduction
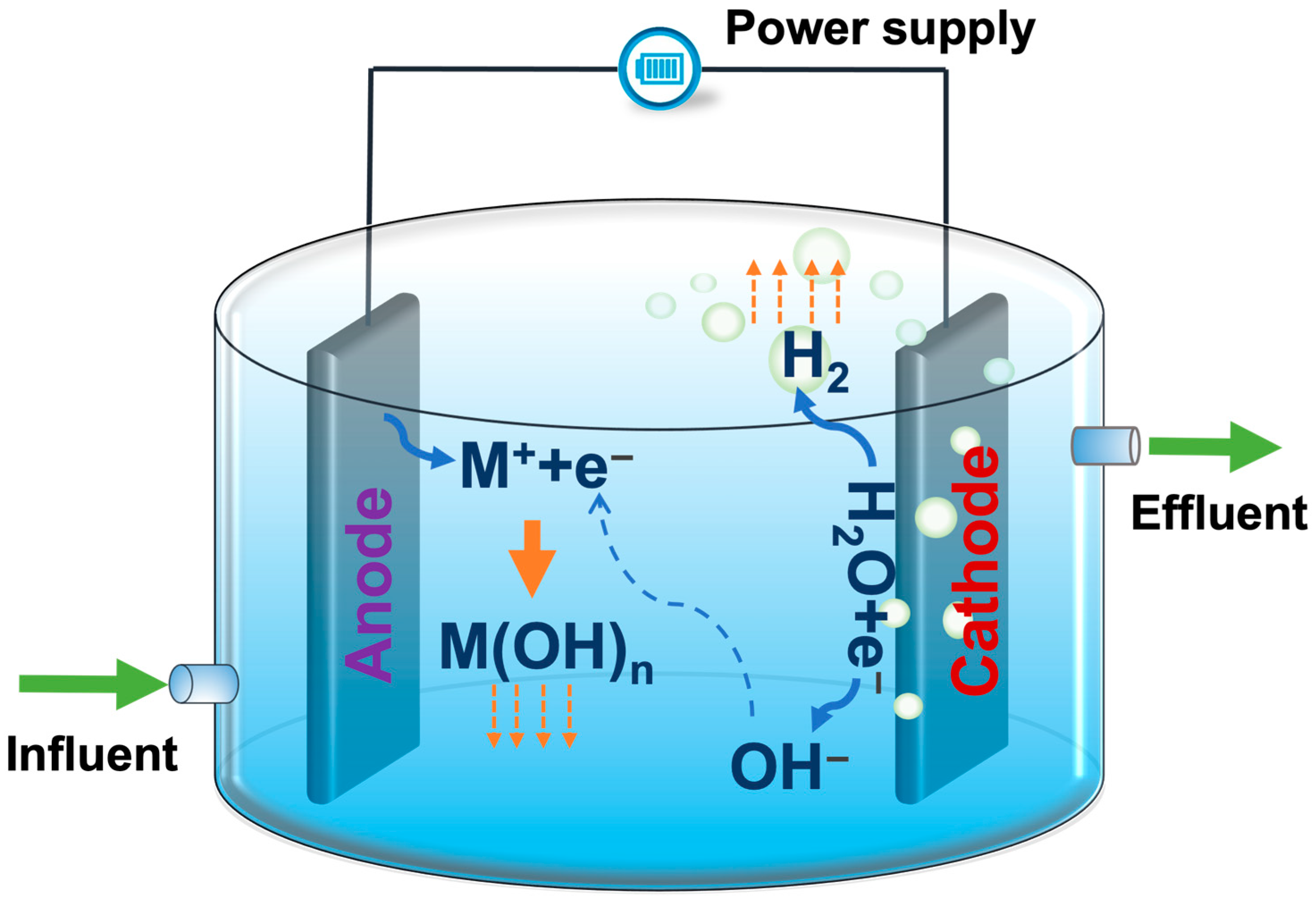
- (1)
- When an electric current is supplied from an external power source, the active coagulant cations (usually aluminum or iron) are released into the solution by the electrolytic oxidation of a sacrificial anode Equations (1) and (4);
- (2)
- Simultaneously, hydroxyl ions are produced due to cathode hydrolysis Equation (6);
- (3)
- The metal cations react with hydroxyls to form monomeric and polymeric species Equations (2), (3) and (5);
- (4)
- Neutralization of the surface charge of contaminants, suspended particulate matter, and emulsions is achieved due to its reaction with metal hydroxyls;
- (5)
- Agglomeration of neutralized particles and their coagulation occurs in the aqueous phase as flocs;
- (6)
- Precipitation of heavier flocs takes place by sedimentation via sweep coagulation;
- (7)
- Hydrogen bubbles are produced at the cathode by water electrolysis, resulting in the floatation of flocs at the surface of the solution via sweep coagulation Equation (6).
2. Nature of Electrocoagulation and Performance-Influencing Factors
2.1. Pros and Cons of Electrocoagulation
2.2. Removal Mechanisms
2.3. Influencing Factors
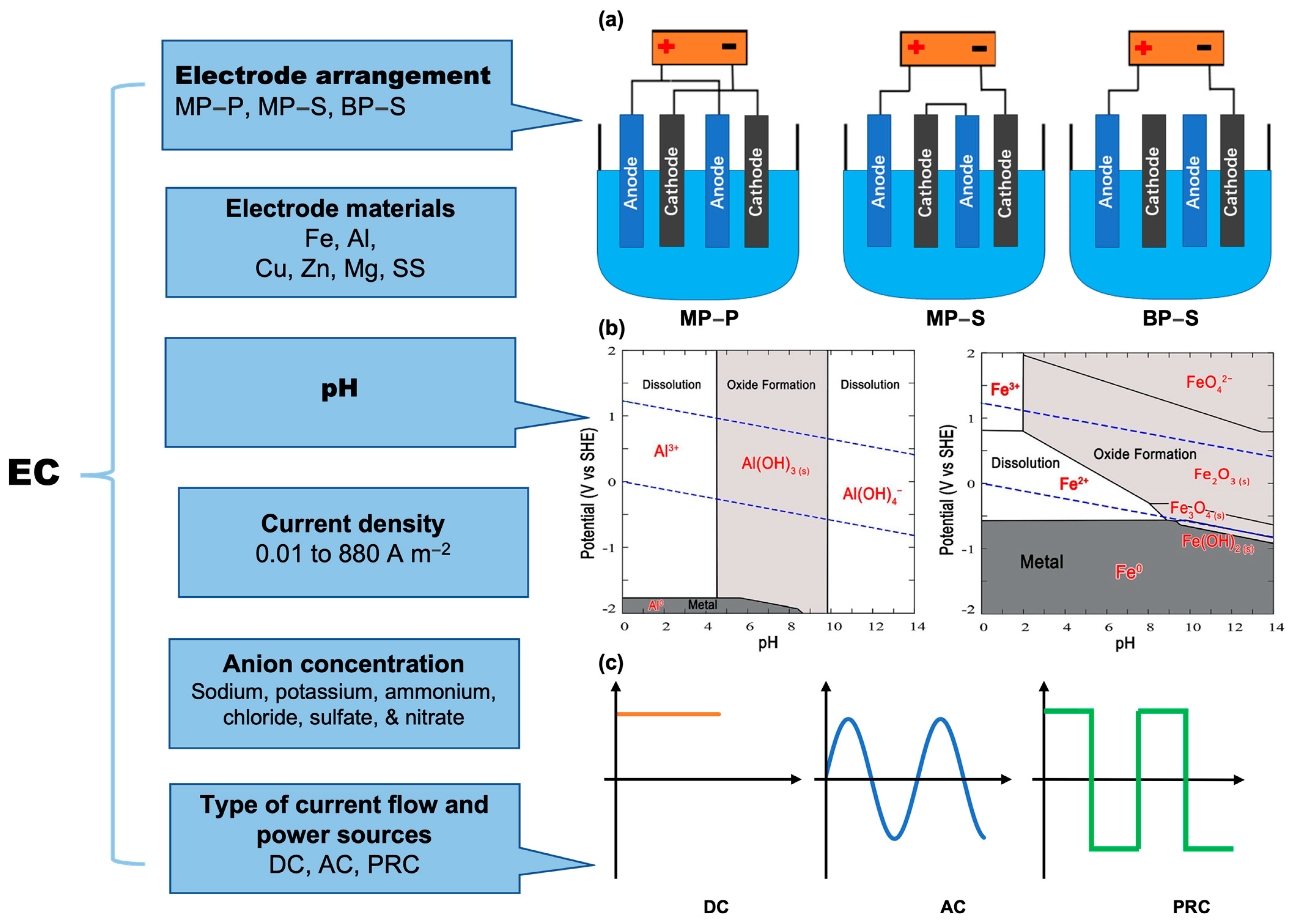
2.3.1. Electrode Materials and Its Arrangement
2.3.2. pH and Anion Concentration
2.3.3. Type of Power Sources and Current Density
| EC Configuration | Impactor Factors | Pollutants or Wastewater | Optimum Operation Conditions | Removal Performance (%) and Power Consumption | Operation Cost | Reference |
|---|---|---|---|---|---|---|
| Batch mode; Reaction volume: 2 L; Electrode: Al (5 cm × 16.5 cm × 0.2 cm) and SS (6 cm × 16.5 cm × 0.2 cm), 5 cm apart; Stir rate: 300-rpm | CD, initial concentration, electrolysis duration, and application mode | pharmaceuticals from municipal wastewater | 48%, 44%, and 36% of DCF, CBZ, and AMX removal when the CD of 1.8 mA/cm2 | 0.1, 0.2, 0.81, and 1.57 €/m3 when CD of 0.3, 0.5, 1.15, and 1.8 mA/m2 | [33] | |
| Electrode: Fe-SS, Al-SS (70 mm × 50 mm, distance: 5 mm); Reaction Volume: 500 mL; Stir rate: 200 rpm; Voltage: 40 V; Current: 5 A. | CD, Reaction time (RT) | uranium from mine water | RT: 101.6 min, CD: 59.9 mA/cm2 | Iron system: uranium concentration: 5 μg/L, cumulative uncertainty: 25 μg/L; Power consumption: 461.7 kWh/g-U; Aluminum systerm: 96 μg/L-U; | Iron system: in the United States: 60.0 USD/g-U, South Korea: 55.4 USD/g-U and Finland: 78.5 USD/g-U; Aluminum system: 9 747 USD/g-U) | [34] |
| Batch mode; Reaction volume: 500 mL, Electrode: AL-SS and Fe-SS (70 mm × 50 mm, 5 mm apart); Stir rate: 200 rpm. | Electrode type, CD and RT | uranium from mine water | CD: 70 mA/cm2, RT: 120 min | 99.7% and 97.7% of uranium removal in Fe-SS and AL-SS system | [35] | |
| Batch mode; Reaction volume: 0.35 L; Electrode: titanium, Al, Fe (4.4 cm × 4.7 cm, effective area of 62 cm2, MP-P); Stir rate: 300 rpm | Initial pH, CD, initial phosphorus concentration, and reaction | phosphorus from domestic wastewater | pHi: 4, CD: 20 A/m2, RT: 80 min | 99.99% of removal; Power consumption: 3.422 kWh/m3 | [36] | |
| Batch mode (180 min); Reaction volume: 1000 mL; Electrode: Zn-SS (effective initial area of 33.5 cm2, connect in parallel, distance: 5–20 mm) | pH, CD, distance between electrodes, nature of electrolyte, and kind of cathode | filtered real olive mill | Initial pH: 3.2, CD: 250 A/m2, distance between electrodes: 1.0 cm and NaCl: 1.5g/L | Removal of total phenolic (TPh) and COD: 84.2% and 40.3% with NaCl, 72.3% and 20.9% without NaCl addition; Power consumption: 40 kW h/m3 (simulated WW) and 34 kW h/m3 (real WW) | [37] | |
| Reaction volume: 3 L; Electrode: Four AL (0.15 m × 0.1 m × 0.002 m, distance 0.5 cm, BP/MP connection, effective surface area 4 × 10−3 m2). | Initial concentrations of fluoride, electrode connections | fluoride from drinking water | RT: 30 min, CD: 625 A/m2, using BP connection | Fluoride (1 mg/L) | 0.38 US$/m3 (MP) and 0.62 US$/m3 (BP) for the initial fluoride concentration of 10 mg/L1 | [39] |
| Reaction volume: 1L; Electrode: Al or Fe (60 mm × 60 mm × 3 mm, effective area: 96 cm2, distance: 0.8 cm). | MP-P, MP-S, BP-P | textile wastewater | MP-P mode | Turbidity removal of 88.6% and 84.1%, Color removal 90.9% and 80.0%, COD removal of 69.3% and 64.1% of Al and Fe-based EC | Al-based EC: 6.439 €/m3, Fe based EC: 4.732 €/m3 | [41] |
| EC followed by advanced oxidation by photoelectro-Fenton (PEF) process with in-situ H2O2 electrogeneration and UVA light irradiation; EC electrode: Fe or Al anode (3.0 cm × 1.5 cm × 0.25 cm), SS cathode, distance: 1.0 cm. | Anode material, supporting electrolyte, pH, and current | organic pollutants | 0.05 M NaCl, pH: 6.3, CD: 200 mA, RT: 15 min | [43] | ||
| Batch mode; Reaction volume: 2 L; Anode: 10 mesh horizontal Al, Cathode: H2 evolving Al plate (14 cm × 20 cm), distance: 0.5 cm | Initial NO3− concentration, initial pH, applied CD, and NaCl concentration | nitrates | Complete removal after 100 min, 80% removal in 60 min; Power consumption: 3.9 to 96.17 kWh/kg nitrates | [44] | ||
| Anode: Al-Mg (4.0 cm × 6.0 cm), cathode: SS (surface area of 35.1 cm2, Constant CD of 11.0 mA/cm2,300 cm3 of solution | Initial concentration of phosphate | phosphates | Maximum 100% removal in AL-Mg system, 97 ± 2% removal in Al-Al; Power consumption: 3.15 to 0.15 kWh/m3 | [45] | ||
| Batch mode; Reaction volume: 1 L; Electrode: seven Al electrodes (active area: 72 cm2 of each, distance 1 cm, BP connection); Stir rate: 400 rpm | SO42−, Cl−, NO3− | fluoride | Defluoridation = 100% w/o other ions. With other ions, bulk reaction happens, residual fluoride controlled by Al (III) amount. | [46] | ||
| Reactor size: 3 cm × 12 cm. Reaction volume: 40 mL; Electrode: Al plates (thickness of 2 mm, distance of 3 mm, effective surface area of 14 cm2 of each) | DC/AC, current densities, voltages, and operation modes, polarity changing frequency, AC current | Oil from synthetic bilge water | DC powered system: 98.8 ± 0.2%, 0.378 kWh/m3 to 0.977 kWh/m3; AC powered system: 99.2 ± 0.1%, 0.787 kWh/m3 to 0.936 kWh/m3 | [48] | ||
| Batch mode; Reaction volume: 0.9 L; Electrodes: AZ31 alloy and Al plates (effective area of 76.5 cm2 and 46.6 cm2, distance of 0.5 cm), Current intensity of 0.34 A | polarity reversal frequency | Indigo carmine and chloride ions | 69.1% of chloride ions removal and 90.4% of non-purgeable dissolved organic carbon removal with 0 and 2 min polarity changes. | [49] | ||
| Reaction volume: 400 mL; Electrodes: Al-Zn (60 mm × 40 mm × 2 mm, distance 2 cm); PREC: 9 V; reversing period of 10 s; Stir rate: 600 r/min, electrolyte concentration of NaCl: 1 g/L | Voltage, pH, stirring speed | PFAs from synthetic/natural groundwater samples | Voltage: 12.0 V, pH: 7.0, and stirring speed: 400 rpm | Removal of PFBS, PFHxS, and PFOS: 87.4%, 95.6%, and 100% in the synthetic aqueous solutions, and 59.0%, 88.2%, and 100% in the natural groundwater | [50] | |
| Reaction volume: 1.0 L; Electrode: Magnesium alloy (size of 2 dm2 and distance of 0.5 cm) | pH, initial ion concentration, CD, co-existing ions | Copper | CD: 0.025 A/dm2, pH: 7.0 | Removal of 97.8 and 97.2% with an energy consumption of 0.634 and 0.996 kWh/m3 for AC and DC | [51] | |
| Reaction volume: 1 L; Electrodes: Two L-shaped Al (surface area of 100 cm2 at 1 cm distance, and 20 pores with a diameter of 4 mm); Mixing speed of 50 rpm (≈10.47 rad/s). | pHi, process time, conductivity, initial HA concentration, pulse time, current type, electrode shape, electrode surfaces | HA | 0.57 kWh/g and 90% with DC and a simple electrode; 0.43 Wh/gand 91% with DC and a perforated electrode; 0.17 Wh/g and 85% with APC and a simple electrode; 0.18 Wh/g and 87% with APC and a perforated electrode | [31] | ||
| Reaction volume: 1 l; Electrode: 30 Fe and SS rods (50 mm × 5 mm, distance of 2 cm, MP connection) | Electrode type, current type, CD, RT. | Lead and zinc from battery-making industry wastewater | EC with AC and Fe: 96.7%, 95.2%, and 0.69 kWh/m3 of lead removal, zinc removal and power consumption; EC with AC and SS: 93.8%,93.3% and 0.98 kWh/m2; EC with DC and Fe: 97.2%, 95.5% and 1.97 kW h/m2; EC with DC and SS: 93.2%, 92.5% and 2.53 kWh/m3 respectively | [52] | ||
| Reaction volume: 1.0 L; Electrode: six Al plates (distance of 1 cm, effective area of 0.0210 m2, parallel connected) Power source: solar photovoltaic module | CD and detention time, | municipal wastewater | CD: 48 A/m2, hydraulic detention time: 16 min | 90% for COD, 94.56% for turbidity, and 49.78% for TDS; Power consumption: 2.27 kWh/m3 (20 min of RT and 40 A/m2 of CD) | [53] | |
| Reaction volume: 50 mL; Electrodes: Al-AL with a conversion circuit; Power sources: a wind energy harvesting triboelectric nanogenerator | RT | algae wastewater, dye wastewater | SPEC removes 90% of algae and 97% of organic dye with self-powered treatment for 72 h. | [54] | ||
| Reaction volume: 200 mL; Electrodes: two Al plates (effective electrode area of 25.3 cm2 and distance of 0.5 cm) Power sources: 3 MFC cells and DC | Power sources, electrode area, and distance | bilge water (EC) and municipal wastewater (MFC) | MFC stack-powered ECC removed 93% of oily organics | [56] | ||
| Batch mode (60 min of each run); Electrode: AL-SS (surface area: 60 cm2 of each, distance: 3 cm); Power sources: 6 MFCs with two 1.2 V rechargeable batteries | Synthetic and real municipal wastewater, | For synthetic wastewater treatment: 95.4%, 88.4%, and 93.8% of COD, TDS, and TSS removal; For real municipal treatment: 83.7%, 57.5%, and 85.8% of each. | 3600 $/m3 per year | [57] | ||
| Continuous mode; Reaction volume: 1 L; Stir rate: 250 rpm; Electrode: Al–Al (distance 2 cm, 6.3 cm × 7.9 cm) | HRT, drug concentration | NOM, acetaminophen | HRT at 40 min with 0.5 mg/L AP exhibited the best removal efficiency for NOM (55.9%) and AP (53.4%) removal. | US$ 0.03/m3, 0.05/m3, 0.08/m3, 0.10/m3 in 10 min, 20 min, 30 min, and 40 min HRT | [60] | |
| Reaction volume: 150 mL; Electrode: two rods (1.2 cm × 5 cm, 2.5 cm apart). | current intensity, TDS | Boron (B) from river water, oilfield produced water | 50% B removal from river water (C0 = 10 mg/L, current = 0.2 A) in 2 h; 80% B removal from produced water (C0 = 50 mg/L, current = 1.0 A) in 2 h. | [62] | ||
| EC–ultrafiltration; EC: two Fe anode (dimensions of 15 cm × 12 cm × 0.3 cm) and two graphite cathodes (15 cm × 12 cm × 1 cm), distance of electrode: 1.5 cm. | RT, CD, Initial pH | Sulfonated Humic Acid | RT: 7 min, CD: 10 mA/cm2, pH: 5. | Higher CD and operation time, lower pH, and improved SHA removal. Max removal: 89.12%. | [63] | |
| Batchwise mode: recirculation of the liquid medium at 1.0 L/min; Electrode: Fe plates (parallel connected, 10 cm × 17 cm × 0.2 cm, effective area: 170 cm2, 2.0 cm apart); CD of 318–481 A/m2 | CD, RT, and initial pH, cl− and Fe | shale gas wastewater | For turbidity removal: 318 mA/cm2, 20 min and 4.4 (pHi); for TOC removal: 481 mA/cm2, 20 min and 2.4, and for Ca2+ removal: 400 A/m2, 20 min, 3.9 (pHi) | 98.3%, 78.5%, and 56.5% for turbidity, TOC, and Ca2+ removal under the optimum conditions | 0.80 US$/m3 | [64] |
2.4. Process Economy of EC
3. Process Developments in the Past and Up to Now
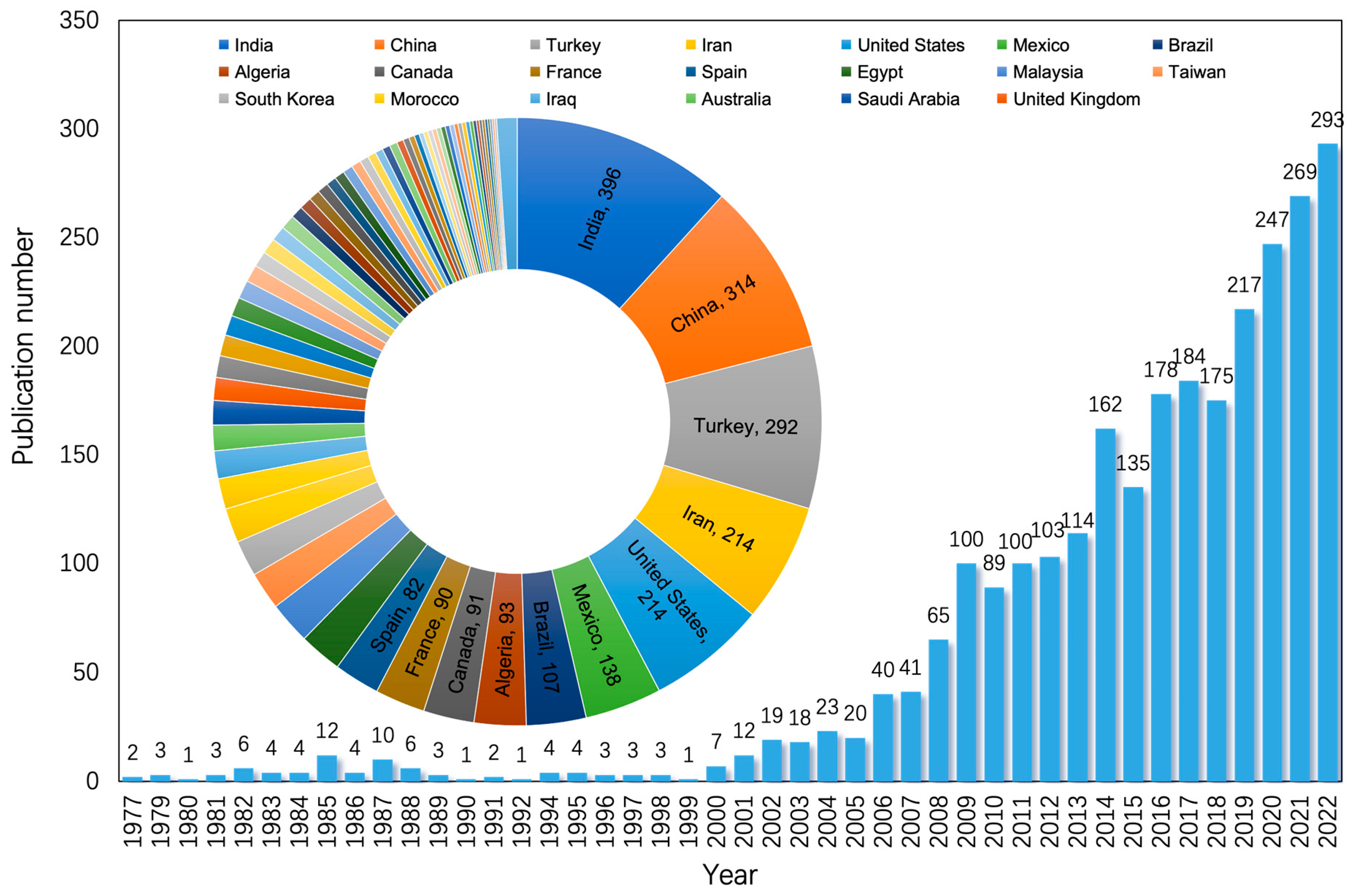
3.1. Earlier Work and Updated Studies
3.2. Application and Development Trends
4. New Developments
4.1. Emerging Pollutants Removal by EC
4.1.1. Removal of Microplastics
- (1)
- Sorption, electrical neutralization, and flotation, whereby the positive charge on the surface of the flocculants allows for the adsorption of negatively charged ionic MPs. The production of H2 and O2 bubbles during the EC process could also help bring the flocs to the surface, where they can be removed through skimming [23].
- (2)
- Oxidation reaction, where the reactive chlorine species (RCS, such as ClO− and Cl2) and reactive oxygen species (ROS, such as O2, O2•−, H2O2, and OH−) and Fe(IV) generated on the anode accompanied by the metal-ion precipitation, can oxidize the MPs into small molecules of non-toxic substrates [23].
4.1.2. Removal of PFASs
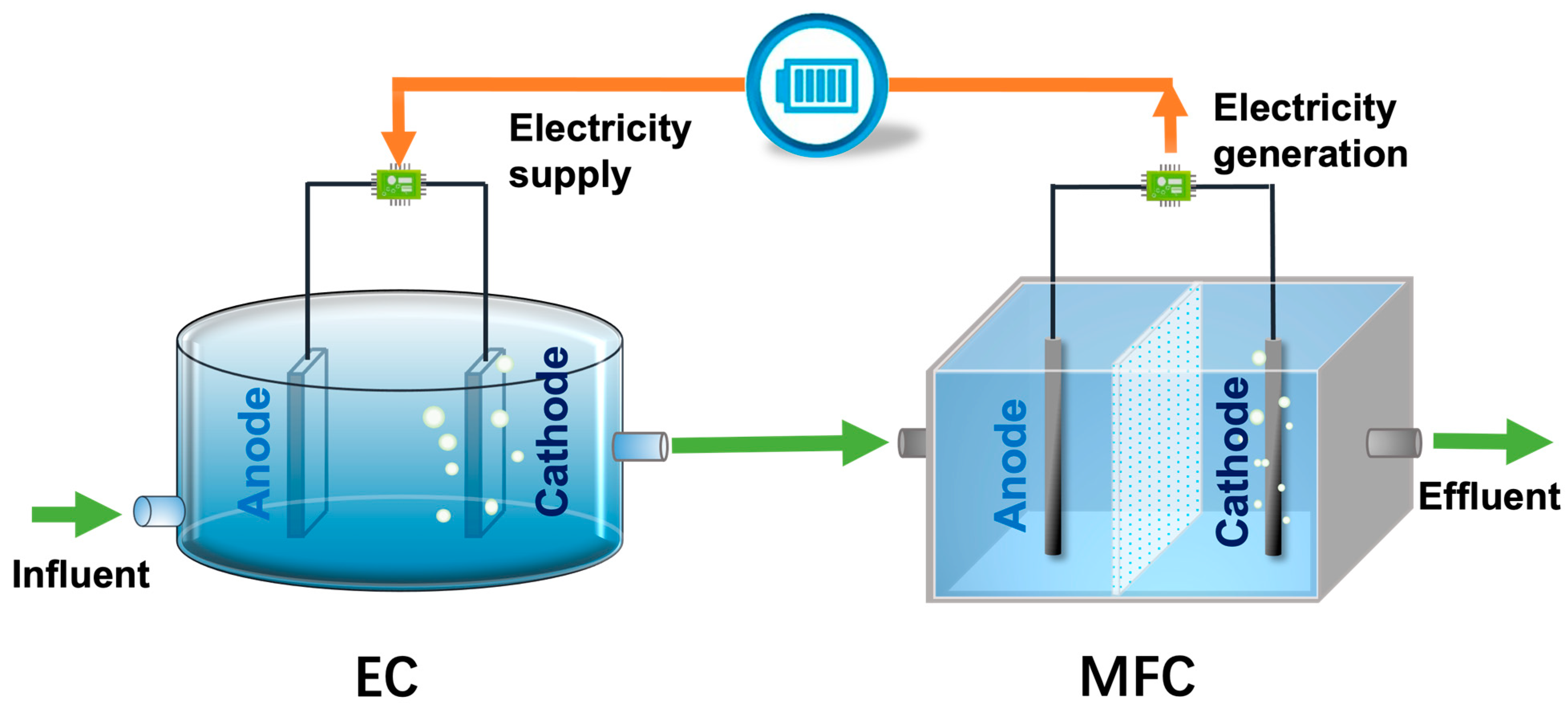
4.2. EC Powered by Bio-Current
4.2.1. Power Supply via Microbial Fuel Cells (MFCs)
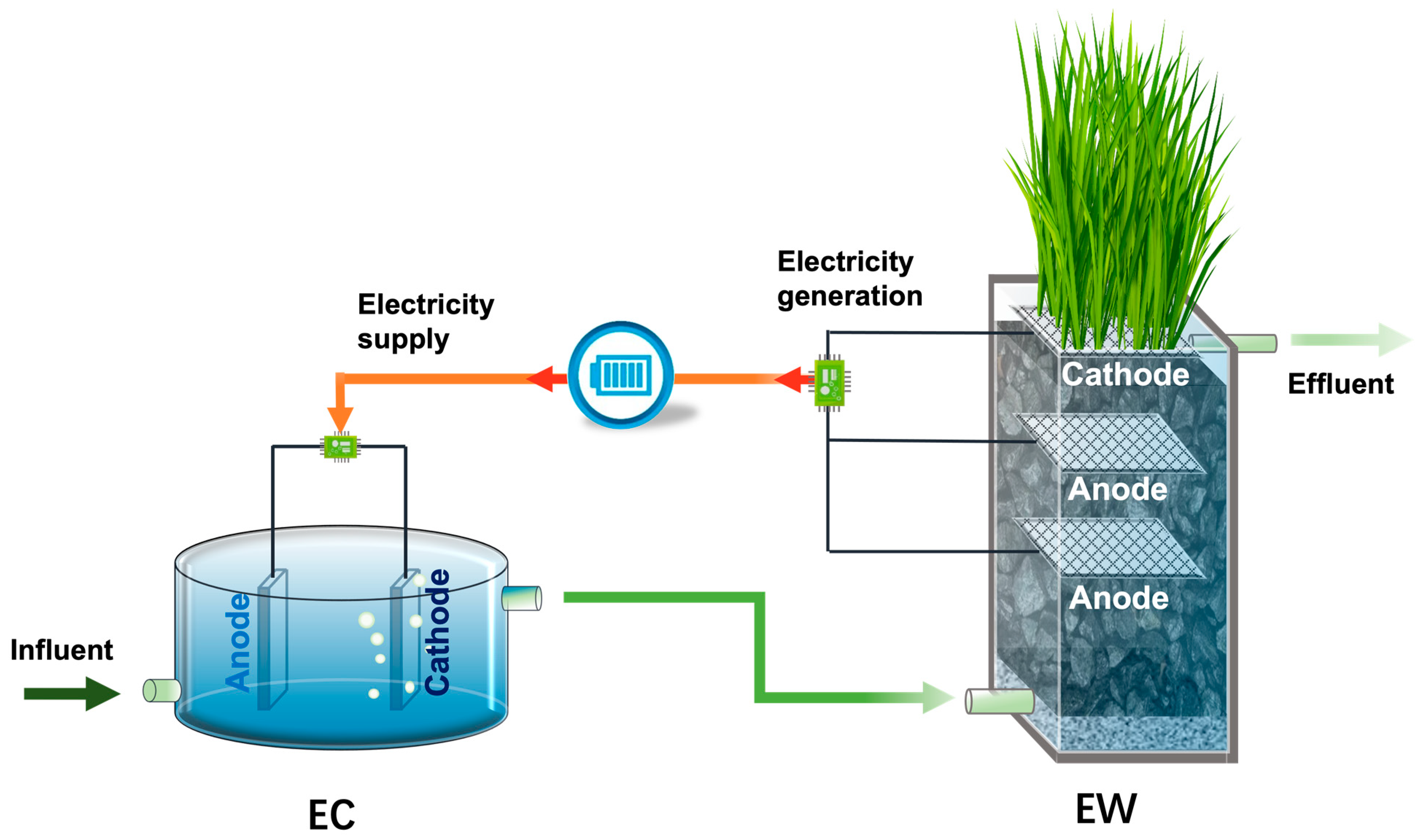
4.2.2. Power Supply via EW
4.3. Unilizaiton of Power Management Systems in EC
5. Research Gap and Further Research Direction of EC
6. Conclusions
- Optimizing operating conditions of EC to achieve both low power consumption and high removal efficiency;
- Exploring the techno-economic feasibility of coupling EC and EW/MFC systems;
- Identifying the energy pathways and energy application/management of hybrid systems;
- Focusing on the effective removal of emerging pollutants using a hybrid EC system.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations. The Sustainable Development Goals Report 2022; United Nations: New York, NY, USA, 2022. [Google Scholar]
- WHO. World Health Statistics 2022 (Monitoring Health of the SDGs); WHO: Geneva, Switzerland, 2022; ISBN 9789240051140. [Google Scholar]
- Ingelsson, M.; Yasri, N.; Roberts, E.P.L. Electrode Passivation, Faradaic Efficiency, and Performance Enhancement Strategies in Electrocoagulation—A Review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef] [PubMed]
- Almukdad, A.; Hafiz, M.; Yasir, A.T.; Alfahel, R.; Hawari, A.H. Unlocking the Application Potential of Electrocoagulation Process through Hybrid Processes. J. Water Process Eng. 2021, 40, 101956. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A Comprehensive Review of Electrocoagulation for Water Treatment: Potentials and Challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Vik, E.A.; Carlson, D.A.; Eikum, A.S.; Gjessing, E.T. Electrocoagulation of Potable Water. Water Res. 1984, 18, 1355–1360. [Google Scholar] [CrossRef]
- Dura, A. Electrocoagulation for Water Treatment: The Removal of Pollutants Using Aluminium Alloys, Stainless Steels and Iron Anodes; National University of Ireland: Maynooth, Ireland, 2013; pp. 1–306. [Google Scholar]
- Song, P.; Yang, Z.; Zeng, G.; Yang, X.; Xu, H.; Wang, L.; Xu, R.; Xiong, W.; Ahmad, K. Electrocoagulation Treatment of Arsenic in Wastewaters: A Comprehensive Review. Chem. Eng. J. 2017, 317, 707–725. [Google Scholar] [CrossRef]
- Abdulhadi, B.; Kot, P.; Hashim, K.; Shaw, A.; Muradov, M.; Al-Khaddar, R. Continuous-Flow Electrocoagulation (EC) Process for Iron Removal from Water: Experimental, Statistical and Economic Study. Sci. Total Environ. 2021, 760, 143417. [Google Scholar] [CrossRef] [PubMed]
- Sari-Erkan, H. Wastewater Treatment from the Biodiesel Production Using Waste Cooking Oil by Electrocoagulation: A Multivariate Approach. Water Sci. Technol. 2019, 79, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Criado, S.P.; Gonçalves, M.J.; Ballod Tavares, L.B.; Bertoli, S.L. Optimization of Electrocoagulation Process for Disperse and Reactive Dyes Using the Response Surface Method with Reuse Application. J. Clean. Prod. 2020, 275, 122690. [Google Scholar] [CrossRef]
- Ni’am, M.F.; Othman, F. Experimental Design of Electrocoagulation and Magnetic Technology for Enhancing Suspended Solids Removal from Synthetic Wastewater. Int. J. Sci. Eng. 2014, 7, 178–192. [Google Scholar] [CrossRef]
- Rahman, N.A.; Jol, C.J.; Linus, A.A.; Ismail, V. Emerging Application of Electrocoagulation for Tropical Peat Water Treatment: A Review. Chem. Eng. Process.-Process Intensif. 2021, 165, 108449. [Google Scholar] [CrossRef]
- McBeath, S.T.; Nouri-Khorasani, A.; Mohseni, M.; Wilkinson, D.P. In-Situ Determination of Current Density Distribution and Fluid Modeling of an Electrocoagulation Process and Its Effects on Natural Organic Matter Removal for Drinking Water Treatment. Water Res. 2020, 171, 115404. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Ji, M.; Zhao, Y.; Pedersen, T.H.; Wang, H. Optimization of Electrocoagulation Process Parameters for Enhancing Phosphate Removal in a Biofilm-Electrocoagulation System. Water Sci. Technol. 2021, 83, 2560–2574. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Verma, A.K.; Dash, A.K. Electrocoagulation for Removal of Phosphate from Aqueous Solution: Statistical Modeling and Techno-Economic Study. J. Clean. Prod. 2020, 246, 118988. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Ahmad, A.; Das, S.; Ghangrekar, M.M. Application of Innovative Electrochemical and Microbial Electrochemical Technologies for the Efficacious Removal of Emerging Contaminants from Wastewater: A Review. J. Environ. Chem. Eng. 2022, 10, 108230. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Niaragh, E.K.; Usman, M.; Khan, S.U.; Sandoval, M.A.; Al-Qodah, Z.; Khalid, Z.B.; Gilhotra, V.; Emamjomeh, M.M. A Critical Review of State-of-the-Art Electrocoagulation Technique Applied to COD-Rich Industrial Wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 43143–43172. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and Advanced Electrocoagulation Processes: A General Review about the Fundamentals, Emerging Applications and Its Association with Other Technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Ghaffarian Khorram, A.; Fallah, N. Comparison of Electrocoagulation and Photocatalytic Process for Treatment of Industrial Dyeing Wastewater: Energy Consumption Analysis. Environ. Prog. Sustain. Energy 2020, 39, 13288. [Google Scholar] [CrossRef]
- Akyol, A.; Can, O.T.; Demirbas, E.; Kobya, M. A Comparative Study of Electrocoagulation and Electro-Fenton for Treatment of Wastewater from Liquid Organic Fertilizer Plant. Sep. Purif. Technol. 2013, 112, 11–19. [Google Scholar] [CrossRef]
- Gao, T.; Lin, J.; Zhang, K.; Padervand, M.; Zhang, Y.; Zhang, W.; Shi, M.; Wang, C. Porous Defective Bi/Bi3NbO7 Nanosheets for Efficient Photocatalytic NO Removal under Visible Light. Processes 2023, 11, 115. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Li, H.; Offiong, N.-A.O.; Bi, Y.; Zhou, R.; Ren, H. A Systematic Review of Electrocoagulation Technology Applied for Microplastics Removal in Aquatic Environment. Chem. Eng. J. 2023, 456, 141078. [Google Scholar] [CrossRef]
- Padervand, M.; Rhimi, B.; Wang, C. One-Pot Synthesis of Novel Ternary Fe3N/Fe2O3/C3N4 Photocatalyst for Efficient Removal of Rhodamine B and CO2 Reduction. J. Alloys Compd. 2021, 852, 156955. [Google Scholar] [CrossRef]
- Belongia, B.M.; Haworth, P.D.; Baygents, J.C.; Raghavan, S. Treatment of Alumina and Silica Chemical Mechanical Polishing Waste by Electrodecantation and Electrocoagulation. J. Electrochem. Soc. 1999, 146, 4124. [Google Scholar] [CrossRef]
- Rahman, N.A.; Muhammad Firdaus Kumar, N.K.; Gilan, U.J.; Jihed, E.E.; Phillip, A.; Linus, A.A.; Nen@Shahinan, D.; Ismail, V. Kinetic Study & Statistical Modelling of Sarawak Peat Water Electrocoagulation System Using Copper and Aluminium Electrodes. J. Appl. Sci. Process Eng. 2020, 7, 439–456. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, R.N. River Water Treatment Using Electrocoagulation for Removal of Acetaminophen and Natural Organic Matter. Chemosphere 2021, 273, 128571. [Google Scholar] [CrossRef] [PubMed]
- Snehi, S.; Singh, H.; Priya, T.; Mishra, B.K. Understanding the Natural Organic Matter Removal Mechanism from Mine and Surface Water through the Electrocoagulation Method. J. Water Supply Res. Technol.-AQUA 2019, 68, 523–534. [Google Scholar] [CrossRef]
- Ulu, F.; Gengec, E.; Kobya, M. Removal of Natural Organic Matter from Lake Terkos by EC Process: Studying on Removal Mechanism by Floc Size and Zeta Potential Measurement and Characterization by HPSEC Method. J. Water Process Eng. 2019, 31, 100831. [Google Scholar] [CrossRef]
- El-Ghenymy, A.; Alsheyab, M.; Khodary, A.; Sirés, I.; Abdel-Wahab, A. Corrosion Behavior of Pure Titanium Anodes in Saline Medium and Their Performance for Humic Acid Removal by Electrocoagulation. Chemosphere 2020, 246, 125674. [Google Scholar] [CrossRef]
- Hasani, G.; Maleki, A.; Daraei, H.; Ghanbari, R.; Safari, M.; McKay, G.; Yetilmezsoy, K.; Ilhan, F.; Marzban, N. A Comparative Optimization and Performance Analysis of Four Different Electrocoagulation-Flotation Processes for Humic Acid Removal from Aqueous Solutions. Process Saf. Environ. Prot. 2019, 121, 103–117. [Google Scholar] [CrossRef]
- Chellam, S.; Sari, M.A. Aluminum Electrocoagulation as Pretreatment during Microfiltration of Surface Water Containing NOM: A Review of Fouling, NOM, DBP, and Virus Control. J. Hazard. Mater. 2016, 304, 490–501. [Google Scholar] [CrossRef]
- Ensano, B.M.B.; Borea, L.; Naddeo, V.; Belgiorno, V.; de Luna, M.D.G.; Balakrishnan, M.; Ballesteros, F.C. Applicability of the Electrocoagulation Process in Treating Real Municipal Wastewater Containing Pharmaceutical Active Compounds. J. Hazard. Mater. 2019, 361, 367–373. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Futalan, C.C.M.; Yee, J.-J. Fuzzy Optimization for the Removal of Uranium from Mine Water Using Batch Electrocoagulation: A Case Study. Nucl. Eng. Technol. 2020, 52, 1471–1480. [Google Scholar] [CrossRef]
- Nariyan, E.; Sillanpää, M.; Wolkersdorfer, C. Uranium Removal from Pyhäsalmi/Finland Mine Water by Batch Electrocoagulation and Optimization with the Response Surface Methodology. Sep. Purif. Technol. 2018, 193, 386–397. [Google Scholar] [CrossRef]
- Omwene, P.I.; Kobya, M.; Can, O.T. Phosphorus Removal from Domestic Wastewater in Electrocoagulation Reactor Using Aluminium and Iron Plate Hybrid Anodes. Ecol. Eng. 2018, 123, 65–73. [Google Scholar] [CrossRef]
- Fajardo, A.S.; Rodrigues, R.F.; Martins, R.C.; Castro, L.M.; Quinta-Ferreira, R.M. Phenolic Wastewaters Treatment by Electrocoagulation Process Using Zn Anode. Chem. Eng. J. 2015, 275, 331–341. [Google Scholar] [CrossRef]
- S.I. No. 122/2014-European Union (Drinking Water) Regulations 2014. Available online: https://www.irishstatutebook.ie/eli/2014/si/122/made/en/print (accessed on 11 January 2023).
- Ghosh, D.; Medhi, C.R.; Purkait, M.K. Treatment of Fluoride Containing Drinking Water by Electrocoagulation Using Monopolar and Bipolar Electrode Connections. Chemosphere 2008, 73, 1393–1400. [Google Scholar] [CrossRef]
- Das, P.P.; Sharma, M.; Purkait, M.K. Recent Progress on Electrocoagulation Process for Wastewater Treatment: A Review. Sep. Purif. Technol. 2022, 292, 121058. [Google Scholar] [CrossRef]
- Demirci, Y.; Pekel, L.C.; Alpbaz, M. Investigation of Different Electrode Connections in Electrocoagulation of Textile Wastewater Treatment. Int. J. Electrochem. Sci. 2015, 10, 2685–2693. [Google Scholar]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of Wastewater by Electrocoagulation: A Review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Thiam, A.; Zhou, M.; Brillas, E.; Sirés, I. Two-Step Mineralization of Tartrazine Solutions: Study of Parameters and by-Products during the Coupling of Electrocoagulation with Electrochemical Advanced Oxidation Processes. Appl. Catal. B Environ. 2014, 150–151, 116–125. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; El-Ashtoukhy, E.-S.Z.; Zoromba, M.S.; Bassyouni, M.; Sedahmed, G.H. Removal of Nitrates from Water by Electrocoagulation Using a Cell with Horizontally Oriented Al Serpentine Tube Anode. J. Ind. Eng. Chem. 2020, 82, 105–112. [Google Scholar] [CrossRef]
- Dura, A.; Breslin, C.B. The Removal of Phosphates Using Electrocoagulation with Al-Mg Anodes. J. Electroanal. Chem. 2019, 846, 113161. [Google Scholar] [CrossRef]
- Hu, C.Y.; Lo, S.L.; Kuan, W.H. Effects of Co-Existing Anions on Fluoride Removal in Electrocoagulation (EC) Process Using Aluminum Electrodes. Water Res. 2003, 37, 4513–4523. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, P.; Li, J. Electrocoagulation Technology for Water Purification: An Update Review on Reactor Design and Some Newly Concerned Pollutants Removal. J. Environ. Manag. 2021, 296, 113259. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Ge, Z.; Albano, C.; Lobo, F.L.; Ren, Z.J. Oily Bilge Water Treatment Using DC/AC Powered Electrocoagulation. Environ. Sci. Water Res. Technol. 2019, 5, 1654–1660. [Google Scholar] [CrossRef]
- Donneys-Victoria, D.; Marriaga-Cabrales, N.; Machuca-Martínez, F.; Benavides-Guerrero, J.; Cloutier, S.G. Indigo Carmine and Chloride Ions Removal by Electrocoagulation. Simultaneous Production of Brucite and Layered Double Hydroxides. J. Water Process Eng. 2020, 33, 101106. [Google Scholar] [CrossRef]
- Bao, J.; Yu, W.-J.; Liu, Y.; Wang, X.; Liu, Z.-Q.; Duan, Y.-F. Removal of Perfluoroalkanesulfonic Acids (PFSAs) from Synthetic and Natural Groundwater by Electrocoagulation. Chemosphere 2020, 248, 125951. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, R.; Ganesan, P.; Lakshmi, J.; Vasudevan, S. Removal of Copper from Water by Electrocoagulation Process—Effect of Alternating Current (AC) and Direct Current (DC). Environ. Sci. Pollut. Res. 2013, 20, 399–412. [Google Scholar] [CrossRef]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J. Removal of Lead and Zinc from Battery Industry Wastewater Using Electrocoagulation Process: Influence of Direct and Alternating Current by Using Iron and Stainless Steel Rod Electrodes. Sep. Purif. Technol. 2014, 135, 165–175. [Google Scholar] [CrossRef]
- Nawarkar, C.J.; Salkar, V.D. Solar Powered Electrocoagulation System for Municipal Wastewater Treatment. Fuel 2019, 237, 222–226. [Google Scholar] [CrossRef]
- Jeon, S.-B.; Kim, S.; Park, S.-J.; Seol, M.-L.; Kim, D.; Chang, Y.K.; Choi, Y.-K. Self-Powered Electro-Coagulation System Driven by a Wind Energy Harvesting Triboelectric Nanogenerator for Decentralized Water Treatment. Nano Energy 2016, 28, 288–295. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable Energies Driven Electrochemical Wastewater/Soil Decontamination Technologies: A Critical Review of Fundamental Concepts and Applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Mei, X.; Wang, H.; Hou, D.; Lobo, F.L.; Xing, D.; Ren, Z.J. Shipboard Bilge Water Treatment by Electrocoagulation Powered by Microbial Fuel Cells. Front. Environ. Sci. Eng. 2019, 13, 53. [Google Scholar] [CrossRef]
- Safwat, S.M. Coupling Microbial Fuel Cells with Electrocoagulation Cells to Form an Integrated System for Wastewater Treatment. Pol. J. Environ. Stud. 2018, 28, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Kim, I.; Park, J.; Kim, D. A Waterwheel Hybrid Generator with Disk Triboelectric Nanogenerator and Electromagnetic Generator as a Power Source for an Electrocoagulation System. Nano Energy 2022, 95, 107048. [Google Scholar] [CrossRef]
- Tegladza, I.D.; Xu, Q.; Xu, K.; Lv, G.; Lu, J. Electrocoagulation Processes: A General Review about Role of Electro-Generated Flocs in Pollutant Removal. Process Saf. Environ. Prot. 2021, 146, 169–189. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, R.N. River Water Treatment by Continuous Electrocoagulation: Insights into Removal of Acetaminophen, and Natural Organic Matter. Water Supply 2022, 22, 4055–4066. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Jiang, W.M.; Wu, M.R.; Li, Z.H. Comprehensive Review of Floc Growth and Structure Using Electrocoagulation: Characterization, Measurement, and Influencing Factors. Chem. Eng. J. 2021, 417, 129310. [Google Scholar] [CrossRef]
- Chen, M.; Dollar, O.; Shafer-Peltier, K.; Randtke, S.; Waseem, S.; Peltier, E. Boron Removal by Electrocoagulation: Removal Mechanism, Adsorption Models and Factors Influencing Removal. Water Res. 2020, 170, 115362. [Google Scholar] [CrossRef]
- Han, N.; Huang, G.; An, C.; Zhao, S.; Yao, Y.; Fu, H.; Li, W. Removal of Sulfonated Humic Acid through a Hybrid Electrocoagulation-Ultrafiltration Process. Ind. Eng. Chem. Res. 2015, 54, 5793–5801. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, B.; Li, X.; Zhang, X.; Wang, Y. Electrocoagulation Treatment of Shale Gas Drilling Wastewater: Performance and Statistical Optimization. Sci. Total Environ. 2021, 794, 148436. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sirés, I.; Scialdone, O. Single and Coupled Electrochemical Processes and Reactors for the Abatement of Organic Water Pollutants: A Critical Review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Abraham, T.; Luthra, A. Socio-Economic & Technical Assessment of Photovoltaic Powered Membrane Desalination Processes for India. Desalination 2011, 268, 238–248. [Google Scholar] [CrossRef]
- Matteson, M.J.; Dobson, R.L.; Glenn, R.W.; Kukunoor, N.S.; Waits, W.H.; Clayfield, E.J. Electrocoagulation and Separation of Aqueous Suspensions of Ultrafine Particles. Colloids Surfaces A Physicochem. Eng. Asp. 1995, 104, 101–109. [Google Scholar] [CrossRef]
- Karhu, M.; Kuokkanen, V.; Kuokkanen, T.; Rämö, J. Bench Scale Electrocoagulation Studies of Bio Oil-in-Water and Synthetic Oil-in-Water Emulsions. Sep. Purif. Technol. 2012, 96, 296–305. [Google Scholar] [CrossRef]
- Kobya, M.; Can, O.T.; Bayramoglu, M. Treatment of Textile Wastewaters by Electrocoagulation Using Iron and Aluminum Electrodes. J. Hazard. Mater. 2003, 100, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Karimifard, S.; Alavi Moghaddam, M.R. Application of Response Surface Methodology in Physicochemical Removal of Dyes from Wastewater: A Critical Review. Sci. Total Environ. 2018, 640–641, 772–797. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent Advances in the Treatment of Dye-Containing Wastewater from Textile Industries: Overview and Perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of Chromium from Wastewater by Membrane Filtration, Chemical Precipitation, Ion Exchange, Adsorption Electrocoagulation, Electrochemical Reduction, Electrodialysis, Electrodeionization, Photocatalysis and Nanotechnology: A Review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A Review on Industrial Wastewater Treatment via Electrocoagulation Processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of Electrocoagulation in Wastewater Treatment: A Developmental Review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Kobya, M.; Soltani, R.D.C.; Omwene, P.I.; Khataee, A. A Review on Decontamination of Arsenic-Contained Water by Electrocoagulation: Reactor Configurations and Operating Cost along with Removal Mechanisms. Environ. Technol. Innov. 2020, 17, 100519. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A Comprehensive Review on Contaminants Removal from Pharmaceutical Wastewater by Electrocoagulation Process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef] [PubMed]
- Perren, W.; Wojtasik, A.; Cai, Q. Removal of Microbeads from Wastewater Using Electrocoagulation. ACS Omega 2018, 3, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
- Hashim, K.S.; Al Khaddar, R.; Jasim, N.; Shaw, A.; Phipps, D.; Kot, P.; Pedrola, M.O.; Alattabi, A.W.; Abdulredha, M.; Alawsh, R. Electrocoagulation as a Green Technology for Phosphate Removal from River Water. Sep. Purif. Technol. 2019, 210, 135–144. [Google Scholar] [CrossRef]
- Khaled, B.; Wided, B.; Béchir, H.; Elimame, E.; Mouna, L.; Zied, T. Investigation of Electrocoagulation Reactor Design Parameters Effect on the Removal of Cadmium from Synthetic and Phosphate Industrial Wastewater. Arab. J. Chem. 2019, 12, 1848–1859. [Google Scholar] [CrossRef]
- Changmai, M.; Pasawan, M.; Purkait, M.K. Treatment of Oily Wastewater from Drilling Site Using Electrocoagulation Followed by Microfiltration. Sep. Purif. Technol. 2019, 210, 463–472. [Google Scholar] [CrossRef]
- Khorram, A.G.; Fallah, N. Treatment of Textile Dyeing Factory Wastewater by Electrocoagulation with Low Sludge Settling Time: Optimization of Operating Parameters by RSM. J. Environ. Chem. Eng. 2018, 6, 635–642. [Google Scholar] [CrossRef]
- Bener, S.; Bulca, Ö.; Palas, B.; Tekin, G.; Atalay, S.; Ersöz, G. Electrocoagulation Process for the Treatment of Real Textile Wastewater: Effect of Operative Conditions on the Organic Carbon Removal and Kinetic Study. Process Saf. Environ. Prot. 2019, 129, 47–54. [Google Scholar] [CrossRef]
- Núñez, J.; Yeber, M.; Cisternas, N.; Thibaut, R.; Medina, P.; Carrasco, C. Application of Electrocoagulation for the Efficient Pollutants Removal to Reuse the Treated Wastewater in the Dyeing Process of the Textile Industry. J. Hazard. Mater. 2019, 371, 705–711. [Google Scholar] [CrossRef]
- Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Coupling of Electrocoagulation and Ozone Treatment for Textile Wastewater Reuse. Chem. Eng. J. 2019, 358, 992–1001. [Google Scholar] [CrossRef]
- Chanikya, P.; Nidheesh, P.V.; Syam Babu, D.; Gopinath, A.; Suresh Kumar, M. Treatment of Dyeing Wastewater by Combined Sulfate Radical Based Electrochemical Advanced Oxidation and Electrocoagulation Processes. Sep. Purif. Technol. 2021, 254, 117570. [Google Scholar] [CrossRef]
- Zazou, H.; Afanga, H.; Akhouairi, S.; Ouchtak, H.; Addi, A.A.; Akbour, R.A.; Assabbane, A.; Douch, J.; Elmchaouri, A.; Duplay, J.; et al. Treatment of Textile Industry Wastewater by Electrocoagulation Coupled with Electrochemical Advanced Oxidation Process. J. Water Process Eng. 2019, 28, 214–221. [Google Scholar] [CrossRef]
- Luo, M.; Wang, Z.; Fang, S.; Song, B.; Cao, P.; Liu, H.; Yang, Y. Removal and Toxic Forecast of Microplastics Treated by Electrocoagulation: Influence of Dissolved Organic Matter. Chemosphere 2022, 308, 136309. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.-U.-R.; Zhang, L. A Review on Microplastics Separation Techniques from Environmental Media. J. Clean. Prod. 2022, 337, 130458. [Google Scholar] [CrossRef]
- Mohsen, P.; Eric, L.; Didier, R.; Wang, C. Removal of Microplastics from the Environment. A Review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Liu, S.; Song, H.; Wei, S.; Yang, F.; Li, X. Bio-Cathode Materials Evaluation and Configuration Optimization for Power Output of Vertical Subsurface Flow Constructed Wetland—Microbial Fuel Cell Systems. Bioresour. Technol. 2014, 166, 575–583. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhu, Y.; Zeng, G.; Zhang, Y.; Yang, Y.; Wen, X.; Chen, M.; Yi, H. Removal of Microplastics via Drinking Water Treatment: Current Knowledge and Future Directions. Chemosphere 2020, 251, 126612. [Google Scholar] [CrossRef]
- Elkhatib, D.; Oyanedel-Craver, V.; Carissimi, E. Electrocoagulation Applied for the Removal of Microplastics from Wastewater Treatment Facilities. Sep. Purif. Technol. 2021, 276, 118877. [Google Scholar] [CrossRef]
- Akarsu, C.; Kumbur, H.; Kideys, A.E. Removal of Microplastics from Wastewater through Electrocoagulation-Electroflotation and Membrane Filtration Processes. Water Sci. Technol. 2021, 84, 1648–1662. [Google Scholar] [CrossRef]
- Akarsu, C.; Deniz, F. Electrocoagulation/Electroflotation Process for Removal of Organics and Microplastics in Laundry Wastewater. CLEAN—Soil Air Water 2021, 49, 2000146. [Google Scholar] [CrossRef]
- Kim, K.T.; Park, S. Enhancing Microplastics Removal from Wastewater Using Electro-Coagulation and Granule-Activated Carbon with Thermal Regeneration. Processes 2021, 9, 617. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Almatrafi, E.; Hu, T.; Zhou, C.; Song, B.; Zeng, Z.; Zeng, G. Efficient Removal of Microplastics from Wastewater by an Electrocoagulation Process. Chem. Eng. J. 2022, 428, 131161. [Google Scholar] [CrossRef]
- Lenka, S.P.; Kah, M.; Padhye, L.P. A Review of the Occurrence, Transformation, and Removal of Poly- and Perfluoroalkyl Substances (PFAS) in Wastewater Treatment Plants. Water Res. 2021, 199, 117187. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Andersen, H.R.; Thomaidis, N.S.; Stasinakis, A.S. Sorption of Perfluorinated Compounds onto Different Types of Sewage Sludge and Assessment of Its Importance during Wastewater Treatment. Chemosphere 2014, 111, 405–411. [Google Scholar] [CrossRef]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for Grouping Per- and Polyfluoroalkyl Substances (PFAS) to Protect Human and Environmental Health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef]
- Boone, J.S.; Vigo, C.; Boone, T.; Byrne, C.; Ferrario, J.; Benson, R.; Donohue, J.; Simmons, J.E.; Kolpin, D.W.; Furlong, E.T.; et al. Per- and Polyfluoroalkyl Substances in Source and Treated Drinking Waters of the United States. Sci. Total Environ. 2019, 653, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.-M.; Guo, Y.; Zeng, L.; Liang-Ying, L.; Lu, X.; Wang, F.; Zeng, E.Y. Global Distribution of Perfluorochemicals (PFCs) in Potential Human Exposure Source—A Review. Environ. Int. 2017, 108, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Lalwani, D.; Kwok, K.Y.; Yamazaki, E.; Taniyasu, S.; Kumar, N.J.I.; Lam, P.K.S.; Yamashita, N. Assessing Exposure to Legacy and Emerging Per-and Polyfluoroalkyl Substances via Hair–The First Nationwide Survey in India. Chemosphere 2019, 229, 366–373. [Google Scholar] [CrossRef]
- Rand, A.A.; Mabury, S.A. Is There a Human Health Risk Associated with Indirect Exposure to Perfluoroalkyl Carboxylates (PFCAs)? Toxicology 2017, 375, 28–36. [Google Scholar] [CrossRef]
- Poothong, S.; Papadopoulou, E.; Padilla-Sánchez, J.A.; Thomsen, C.; Haug, L.S. Multiple Pathways of Human Exposure to Poly-and Perfluoroalkyl Substances (PFASs): From External Exposure to Human Blood. Environ. Int. 2020, 134, 105244. [Google Scholar] [CrossRef]
- Ryan, D.R.; Mayer, B.K.; Baldus, C.K.; McBeath, S.T.; Wang, Y.; McNamara, P.J. Electrochemical Technologies for Per- and Polyfluoroalkyl Substances Mitigation in Drinking Water and Water Treatment Residuals. AWWA Water Sci. 2021, 3, e1249. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Niu, J.; Yue, Z.; Huang, Q. Efficient Sorption and Removal of Perfluoroalkyl Acids (PFAAs) from Aqueous Solution by Metal Hydroxides Generated in Situ by Electrocoagulation. Environ. Sci. Technol. 2015, 49, 10562–10569. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Kim, T.; Kim, T.-K.; Joo, S.-W.; Zoh, K.-D. Degradation Mechanism of Perfluorooctanoic Acid (PFOA) during Electrocoagulation Using Fe Electrode. Sep. Purif. Technol. 2020, 247, 116911. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, H.; Jin, F.; Niu, J.; Zhao, J.; Bi, Y.; Li, Y. Electrocoagulation Mechanism of Perfluorooctanoate (PFOA) on a Zinc Anode: Influence of Cathodes and Anions. Sci. Total Environ. 2016, 557–558, 542–550. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.M.; Zhao, Y.; Wang, J.; Lu, M.X.; Peng, F.H.; Bao, J. Removal of Perfluorooctanoic Acid in Simulated and Natural Waters with Different Electrode Materials by Electrocoagulation. Chemosphere 2018, 201, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.; Park, M.; Kim, K.Y. Energy-Efficient Removal of PFOA and PFOS in Water Using Electrocoagulation with an Air-Cathode. Chemosphere 2021, 281, 130956. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Yang, J.; Xu, H.; Yan, W. Research Progress of Microbial Fuel Cell in Wastewater Treatment. Huagong Jinzhan/Chem. Ind. Eng. Prog. 2022, 41, 951–963. [Google Scholar] [CrossRef]
- Srivastava, P.; Abbassi, R.; Yadav, A.K.; Garaniya, V.; Asadnia, M. A Review on the Contribution of Electron Flow in Electroactive Wetlands: Electricity Generation and Enhanced Wastewater Treatment. Chemosphere 2020, 254, 126926. [Google Scholar] [CrossRef]
- Xu, L. Integrating Microbial Fuel Cell into Traditional Constructed Wetland: From Fundamentals towards Practical Applications. Ph.D. Thesis, University College Dublin, Dublin, Ireland, 2018. [Google Scholar]
- Degrenne, N. Power Management for Microbial Fuel Cells. Ph.D. Thesis, Ecole Centrale de Lyon, Écully, France, 2012. [Google Scholar]
- An, J.; Lee, Y.S.; Kim, T.; Chang, I.S. Significance of Maximum Current for Voltage Boosting of Microbial Fuel Cells in Series. J. Power Sources 2016, 323, 23–28. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Mungray, A.K.; Arkatkar, A.; Kumar, S.S. Recent Advancement in Scaling-up Applications of Microbial Fuel Cells: From Reality to Practicability. Sustain. Energy Technol. Assess. 2021, 45, 101226. [Google Scholar] [CrossRef]
- Santoro, C.; Winfield, J.; Theodosiou, P.; Ieropoulos, I. Supercapacitive Paper Based Microbial Fuel Cell: High Current/Power Production within a Low Cost Design. Bioresour. Technol. Rep. 2019, 7, 100297. [Google Scholar] [CrossRef] [PubMed]
- Mehravanfar, H.; Mahdavi, M.A.; Gheshlaghi, R. Economic Optimization of Stacked Microbial Fuel Cells to Maximize Power Generation and Treatment of Wastewater with Minimal Operating Costs. Int. J. Hydrogen Energy 2019, 44, 20355–20367. [Google Scholar] [CrossRef]
- Lovley, D.R. Bug Juice: Harvesting Electricity with Microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, W.; Graham, N.; Zhao, Y.; Qu, J. Application of Integrated Bioelectrochemical-Wetland Systems for Future Sustainable Wastewater Treatment. Environ. Sci. Technol. 2019, 53, 1741–1743. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.; Zhao, X.; Hu, Y.; Hao, X.; Xu, L.; Liu, R. A Review of a Recently Emerged Technology: Constructed Wetland—Microbial Fuel Cells. Water Res. 2015, 85, 38–45. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y.; Zhao, X.; Wang, W. Nutrient and Organics Removal from Swine Slurry with Simultaneous Electricity Generation in an Alum Sludge-Based Constructed Wetland Incorporating Microbial Fuel Cell Technology. Chem. Eng. J. 2015, 266, 74–81. [Google Scholar] [CrossRef]
- Yadav, A.K.; Dash, P.; Mohanty, A.; Abbassi, R.; Mishra, B.K. Performance Assessment of Innovative Constructed Wetland-Microbial Fuel Cell for Electricity Production and Dye Removal. Ecol. Eng. 2012, 47, 126–131. [Google Scholar] [CrossRef]
- Zhao, Y.; Collum, S.; Phelan, M.; Goodbody, T.; Doherty, L.; Hu, Y. Preliminary Investigation of Constructed Wetland Incorporating Microbial Fuel Cell: Batch and Continuous Flow Trials. Chem. Eng. J. 2013, 229, 364–370. [Google Scholar] [CrossRef]
- Aguirre-Sierra, A.; Bacchetti-De Gregoris, T.; Berná, A.; Salas, J.J.; Aragón, C.; Esteve-Núñez, A. Microbial Electrochemical Systems Outperform Fixed-Bed Biofilters in Cleaning up Urban Wastewater. Environ. Sci. Water Res. Technol. 2016, 2, 984–993. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Tang, C.; Mao, Y.; Shen, C. Significance of Water Level in Affecting Cathode Potential in Electro-Wetland. Bioresour. Technol. 2019, 285, 121345. [Google Scholar] [CrossRef]
- Doherty, L.; Zhao, Y. Operating a Two-Stage Microbial Fuel Cell-Constructed Wetland for Fuller Wastewater Treatment and More Efficient Electricity Generation. Water Sci. Technol. 2015, 72, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Qi, J.; Zhang, F.; Miwornunyuie, N.; Amaniampong, P.S.; Koomson, D.A.; Chen, L.; Yan, Y.; Dong, Y.; Setordjie, V.E.; et al. The Role of Wetland Plants on Wastewater Treatment and Electricity Generation in Constructed Wetland Coupled with Microbial Fuel Cell. Appl. Sci. 2021, 11, 7454. [Google Scholar] [CrossRef]
- Xu, L.; Wang, B.; Liu, X.; Yu, W.; Zhao, Y. Maximizing the Energy Harvest from a Microbial Fuel Cell Embedded in a Constructed Wetland. Appl. Energy 2018, 214, 83–91. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Y.; Kang, C.; Yang, Y.; Morgan, D.; Xu, L. Towards Concurrent Pollutants Removal and High Energy Harvesting in a Pilot-Scale CW-MFC: Insight into the Cathode Conditions and Electrodes Connection. Chem. Eng. J. 2019, 373, 150–160. [Google Scholar] [CrossRef]
- Gupta, S.; Nayak, A.; Roy, C.; Yadav, A.K. An Algal Assisted Constructed Wetland-Microbial Fuel Cell Integrated with Sand Filter for Efficient Wastewater Treatment and Electricity Production. Chemosphere 2021, 263, 128132. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Xu, L.; Wang, W.; Doherty, L.; Tang, C.; Ren, B.; Zhao, J. Constructed Wetland Integrated Microbial Fuel Cell System: Looking Back, Moving Forward. Water Sci. Technol. 2017, 76, 471–477. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.L.; Li, H.; Song, H.L.; Wang, R.C.; Dai, Z.Q. Degradation of Sulfamethoxazole in Bioelectrochemical System with Power Supplied by Constructed Wetland-Coupled Microbial Fuel Cells. Bioresour. Technol. 2017, 244, 345–352. [Google Scholar] [CrossRef]
- Zhang, S.; Song, H.L.; Yang, X.L.; Huang, S.; Dai, Z.Q.; Li, H.; Zhang, Y.Y. Dynamics of Antibiotic Resistance Genes in Microbial Fuel Cell-Coupled Constructed Wetlands Treating Antibiotic-Polluted Water. Chemosphere 2017, 178, 548–555. [Google Scholar] [CrossRef]
- Srivastava, P.; Belford, A.; Abbassi, R.; Asadnia, M.; Garaniya, V.; Yadav, A.K. Low-Power Energy Harvester from Constructed Wetland-Microbial Fuel Cells for Initiating a Self-Sustainable Treatment Process. Sustain. Energy Technol. Assess. 2021, 46, 101282. [Google Scholar] [CrossRef]
- Mao, Y.; Cotterill, S.; Morgan, D.; Regan, S.; Zhao, Y. Electrocoagulation of Peatland Runoff: Statistical Optimization and Economic Analysis. J. Water Process Eng. 2022, 49, 103113. [Google Scholar] [CrossRef]
- Dewan, A.; Ay, S.U.; Karim, M.N.; Beyenal, H. Alternative Power Sources for Remote Sensors: A Review. J. Power Sources 2014, 245, 129–143. [Google Scholar] [CrossRef]
- Wang, H.; Park, J.-D.; Ren, Z.J. Practical Energy Harvesting for Microbial Fuel Cells: A Review. Environ. Sci. Technol. 2015, 49, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Srivastava, P.; Patil, S.A.; Yadav, A.K. A Comprehensive Review on Emerging Constructed Wetland Coupled Microbial Fuel Cell Technology: Potential Applications and Challenges. Bioresour. Technol. 2021, 320, 124376. [Google Scholar] [CrossRef] [PubMed]
- Gajda, I.; Obata, O.; Jose Salar-Garcia, M.; Greenman, J.; Ieropoulos, I.A. Long-Term Bio-Power of Ceramic Microbial Fuel Cells in Individual and Stacked Configurations. Bioelectrochemistry 2020, 133, 107459. [Google Scholar] [CrossRef] [PubMed]
- Tamta, P.; Rani, N.; Yadav, A.K. Enhanced Wastewater Treatment and Electricity Generation Using Stacked Constructed Wetland–Microbial Fuel Cells. Environ. Chem. Lett. 2020, 18, 871–879. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Wang, T.; Liu, R.; Gao, F. Energy Capture and Nutrients Removal Enhancement through a Stacked Constructed Wetland Incorporated with Microbial Fuel Cell. Water Sci. Technol. 2017, 76, 28–34. [Google Scholar] [CrossRef]

| Pros | Cons |
|---|---|
| Produces larger and more stable flocs for easy filtration, compared with CC. | Cathode passivation can occur due to the hydroxides of calcium, magnesium, and other substances, inhibiting the flow of current and the release of hydrogen. |
| pH control without chemicals. | Sacrificial anodes must be replaced periodically due to corrosion. |
| Lower operating costs compared to CC, PC, and EF processes due to lower equipment and maintenance costs and no addition of chemical costs. | Post-treatment may be necessary to remove high concentrations of iron and aluminum ions from effluent. |
| No secondary pollution or added chemicals means less sludge production. | Electrochemical methods may be costly in areas with limited access to electricity. |
| Produces gas bubbles to aid pollutant removal. | Sludge buildup on electrodes can inhibit the electrolytic process during continuous operation. |
| Simple equipment with automation potential. | Electrochemical methods may not be as effective as biological processes for removing biodegradable pollutants and soluble organic substances. |
| Can remove even the smallest colloidal particles through faster collision and facilitated coagulation. | |
| Can operate under a wide range of conditions compared with PC and EF, including high salt and pH levels. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Zhao, Y.; Cotterill, S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water 2023, 15, 1455. https://doi.org/10.3390/w15081455
Mao Y, Zhao Y, Cotterill S. Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water. 2023; 15(8):1455. https://doi.org/10.3390/w15081455
Chicago/Turabian StyleMao, Yi, Yaqian Zhao, and Sarah Cotterill. 2023. "Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment" Water 15, no. 8: 1455. https://doi.org/10.3390/w15081455
APA StyleMao, Y., Zhao, Y., & Cotterill, S. (2023). Examining Current and Future Applications of Electrocoagulation in Wastewater Treatment. Water, 15(8), 1455. https://doi.org/10.3390/w15081455








