1. Introduction
Currently, the behavior of such rare-earth elements (REEs) as Li, Sr, Rb, Th, W, and U in natural water is the subject of detailed studies [
1,
2]. The interest in REEs’ abundance in natural water is associated with their potential use as indicators of geochemical processes; this requires an understanding of the migration features and fractionation rules of certain rare-earth elements [
3,
4,
5]. The unambiguous interpretation of REEs’ behavior in natural water is complicated by various processes involving the formation of water complexes, colloid transport, ion exchange, and adsorption, leading to the fractionation of the elements in aqueous solutions in comparison with the source rock [
6,
7,
8,
9,
10].
Over the last years, the mineral stocks of large salt lakes have become objects of increased interest and intense study as sources of the commercial extraction of potassium salt, magnesium salt, soda, and sodium sulfate [
11,
12,
13,
14], as well as lithium compounds, bromine, potassium, boron, and rare elements. Salars in South America and salt lakes in China and Mongolia are the classic examples, since their reserves of valuable components (i.e., lithium, boron, bromine, etc.) amount to hundreds or thousands of tons [
15,
16].
The attractiveness of lake water’s use as a mineral stock depends upon the following factors: the availability of large renewable field reserves within large hydrogeological systems; environmental cleanliness in the context of extraction and production; the possibility of applying evaporative water concentration with the sequenced separation of valuable components; and the low cost of the products owing to the integrated use of hydro-mineral stocks [
17].
Recently, the hydro-mineral resources of both large and smaller salt lakes, which occur widely in Central Asia, have attracted increasing attention [
18,
19]. This interest results from the gradual depletion of large lake basins on one hand and, on the other, the large number of small salt lakes with an extended spectrum of elements for potential raw-material recovery (i.e., uranium, lithium, and rare elements) [
20,
21]. Zadereev et al. [
22] presented research reviewing the status, trends, and drivers of inland saline lakes in Europe and Central Asia, assessing the effects of direct drivers on ecosystem health and biodiversity and highlighting the need for improved water-management and conservation measures. In [
23], the authors present a study on the extraction of lithium from salt-lake brines in China using a membrane process. Li et al. [
24] investigated the origin and enrichment mechanisms of lithium in a lithium-rich salt lake in West Kunlun, Xinjiang, China, using hydrochemical and lithium-isotope analyses to uncover the genesis of brine-type lithium deposits and to contribute to a deeper understanding of lithium sources and their enrichment processes. Xu et al. [
25] investigated the effects of salinity on the composition of dissolved organic matter in salt lakes at the molecular level. Specifically, their study focused on two salt lakes, Qinghai Lake and Daihai Lake, which have similar geographical and climatic conditions but differ in their salinity levels. The results showed that at higher salinity levels, the dissolved organic matter had a larger average molecular weight, a higher oxidation degree, and lower aromaticity. Additionally, the proportion of vulnerable dissolved organic-matter compounds reduced, while the proportion of refractory compounds increased.
The purpose of [
26] was to comprehensively utilize magnesium and lithium resources in salt-lake brine for resource sustainability. The study reviewed the latest advances in magnesium/lithium separation and lithium recovery from salt-lake brines, including extraction, adsorption, membrane, and electrochemical methods, as well as reaction-coupled separation technology. The review also discusses the roles of various electrode materials in lithium recovery using electrochemical methods and the potential of reaction-coupled separation technology for highly efficient magnesium/lithium separation and the simultaneous preparation of high-value magnesium-based functional materials. Borzenko et al. [
27] developed a new concept to explain the diverse geochemical types of salt lakes, with a particular focus on those in the Transbaikal region. The study uses hydrogeochemical-field data and thermodynamic calculations to demonstrate that in addition to evaporation, interactions with rocks play a significant role in the formation of salt-lake composition. The study provides evidence that such processes are most common in soda lakes, which have high pH values, carbonate-ion concentrations, and high precipitation of Ca, Mg, and Fe carbonates. Additionally, the study highlights the role of sulphate reduction in the formation of sulphate-type lakes and how the presence of an oxidizing environment and sulphides in rocks can provide additional sources for sulphates. Overall, the study presents a new understanding of how landscape–climatic and geological–geochemical conditions shape the chemical compositions of salt lakes.
The studies described above provide further insights into the potential uses of mineralized waters, the factors that contribute to their mineral content, and their environmental impact. This is why small salt lakes in Kazakhstan are of considerable interest. A significant part of the state’s territory has a dry and almost arid climate [
28]. Brackish and salt lakes located in arheic basins are widespread within the land. The salt-clay rocks and brine from the Aral Sea, the brine from the solonchak and drying lakes of the Caspian Depression, and the territory of the Chu River basin and other regions of the Republic of Kazakhstan arouse considerable interest. The Zaisan depression in eastern Kazakhstan is one of these regions. The hydromineral resources of the objects are almost unexplored. Therefore, the purpose of this paper is to study the macro- and microcomponent composition of small salt lakes in eastern Kazakhstan [
29,
30,
31,
32].
The territory of Eastern Kazakhstan is part of the Late Paleozoic Altai collision system formed upon the collision of the Siberian and Kazakhstan continents [
33]. The geological structure was shaped in the Late Carboniferous–Early Permian; it contains sedimentary formations and intrusions of granitoid composition. The largest intrusion is the Kalba–Narym batholiths, stretching from North-West to South-East for more than 300 km (
Figure 1).
In
Figure 1, the inset shows the position of batholith on a terranes map of the Late Paleozoic Altai collision system. The granitoids are from the early Permian age; they cut through Middle–Late Palaeozoic sedimentary and metamorphic rocks [
18]. The formation of the Kalba batholith took place in the Early Permian (in the interval of 300–276 Ma), as follows: phase 1, biotite medium, coarse-grained porphyritic granites and, rarely, biotite granodiorites; phase 2, sub-phase 1, medium-grained, fine- and medium-grained biotite muscovitized granites; sub-phase 2, biotite and biotite-muscovite fine-grained granites; and phase 2, bastard granites, aplites, granitaplite, aplite-pegmatites, pegmatites, and quartz veins. The survey area in this paper is within the southern part of the Kalba–Narym batholith. A significant share of the territory is occupied by the Narym pluton, which combines several large granite-rock masses. The subject of our research is the territory of the Burabay rock mass located south of the Kurchum River (
Figure 2).
According to the geological-mapping data [
18], the Burabay rock mass has a concentric zonal structure. It is shape by rocks of the following three intrusive phases:
(1) biotite medium- and coarse-grained granites and granodiorites;
(2) biotite medium-grained granites; and
(3) biotite-muscovite fine-grained granites of granite-porphyry, aplite, and pegmatite veins.
Regarding the sedimentary rocks, the geological structure of the territory involves stratified Carboniferous stages. The rocks form an alternation of gray, medium- and fine-grained, quartz-feldspathic, polymictic, sometimes initially low-in-lime sandstones, and black carbonaceous–argillaceous siltstones.
The region relief is complex, spanning low-mountain and high-mountain terrain. The absolute elevation varies from 700–800 up to 1500 m. The planation surfaces within the relief are developed on granitoid intrusions. The true altitude falls in the range of 600–900 m; the relative altitude is 50–60 m (rarely achieving 100 m) [
34]. Morphologically, the relief type is a more or less oriented system of slightly elongated, highly deplanated hills; less commonly, it is represented by ridges with smooth (5–80) bare slopes and degradations, with bottoms that are either occupied by lakes or covered with a layer of gruss–sand material. As a rule, the relative hill elevations are 20–30 m. Due to weathering, various bizarre forms are present on the hills, as well as niches and depressions of various sizes [
35]. A satellite image of the research area is shown in
Figure 2a; the layout of the drainless lakes on the geological map is shown in
Figure 2b.
The area features a unique climate and relief, as well as a significant number of small lakes with various mineralization degrees, all of which make it an attractive location in which to study the processes of the formation of macro- and microcomponent compositions [
36]. The conditions for the formation of the chemical compositions of water bodies depend on many natural factors (i.e., climate patterns, relief features, water-exchange intensity, the nature of the geochemical environment, etc.).
The region within the Burabay rock mass is characterized by a good and uniformly developed hydrographic system with numerous lakes and marshes. During field studies in 2020, the hydrochemical state of the water bodies was assessed. In the course of the research, 15 lakes were identified in the survey area. They were taken as the basis to analyze both the content and changes in the key parameters and components determining the geochemical outlook of the survey area. The formation of the hydrochemical composition of the surface water took place in the survey area under the influence of natural and climatic conditions [
37].
A number of the features of the region’s hydrography should be mentioned. Watershed swampiness contributes to the accumulation of a wide range of organic substances in surface water, which are the products of the incomplete decomposition of plant litter. Therefore, the presence of intermediate decomposition products of plant biomass in the natural water determines a weak acid reaction of the environment, which bodes well for an increase in the mobility of a range of metals in the composition of organo-mineral complexes.
Our research purpose is to study the chemical composition of lake water and bottom deposits to identify the mineralization of rare metals, as well as other types of mineral. For this purpose, we carried out field studies to geometrize the lakes and obtained samples of the water and bottom deposits; we then performed a chemical analysis using high-resolution and supersensitive methods (inductively coupled plasma mass spectrometry). Finally, we tried to evaluate the possibility of using water and salt-brine development for the exploration of rare metals.
2. Materials and Methods
This paper is based on the results of hydrogeochemical sampling of small lakes in the area of Burabay rock mass (
Figure 2a) conducted in 2020–2022. The sampling was carried out during the driest summer period, which helped exclude influence of dilution processes through atmospheric precipitation. The studies included selection of 15 water samples (1–15) and 15 bottom-deposit samples (B1–B15). To identify the source of salt in lake water and deposits, the chemical compositions of the rocks of Burabay rock mass were analyzed in similar ways.
Figure 2b demonstrates position of the sampling points. Seven samples of coarse-grained and porphyritic granites were collected.
During the field studies, geographical reference of the sampling points was made by means of special software, FieldMove Clino.
To geometrize lakes and determine water-surface area, the field studies involved aerial photography [
38]. Unmanned aerial vehicle DJI Mavic was used for the purpose. The DJI Mavic quadcopter is a small but high-tech unmanned aerial vehicle with 24 high-capacity computing cores, state-of-the-art system of signal transmission for a distance of up to 7 km, five video transmitters, and 4K camera stabilized by a 3-axis mechanical gimbal. This made it possible to obtain geo-referenced high-quality aerial photographs. The georeferenced aerial photographs were taken using the Agisoft PhotoScan program.
The water was sampled according to the standard method [
39]. Clean-polymer, single-use 1.5-L bottles were applied. Walls of the containers were previously moistened with a solution of nitric acid (10%) and left for 2 h, thoroughly washed with clean water, neutralized with a solution of sodium bicarbonate (2%) and, finally, washed with distilled water. Water for chemical analysis was sampled at depths of 30–50 cm at a distance from the lakeside with the help of a sterile 200-milliliter sampler. To prepare the selected sample for storage, we conserved water and loaded it into a cooling box to transport the samples. Water samples were delivered to the laboratory within 24 h [
40,
41].
The set of bottom-deposit samples obtained with a MPK G01 N 1/02 sampler contained a water-uptake tank, a load, and a cable. Bottom deposits were sampled at depths of 10–15 cm and weighed up to 2.0 kg; they involved the destructed soil composition. Excess water was drained; the samples were packed into plastic buckets and transported to the laboratory. After drying at a 23–25 °C temperature, the samples were quartered by standard methods.
The granite samples were prepared in the sample-preparation sector of the regional university’s engineering laboratory, ‘IRGETAS’, by means of a fine crushing area. The procedure featured two stages: a ShD 6 jaw-breaker crushed samples to 1-mm fractions, after which they were bucked down to 0.07 mm using vibratory pulverator IV-4.
Tests with water and bottom-deposit samples were carried out in the laboratory of the Engineering Center for Advanced Development, ‘VERITAS’, at D. Serikbayev East Kazakhstan Technical University. The ICP-MS spectrometry methods were applied. The samples were dried in a draft chamber and the sediment was ground. A 200-mg sub-sample was taken and dissolved in concentrated hydrofluoric, nitric, and hydrochloric acids. The resulting solution was analyzed by means of a mass spectrometer for following elements: Ag, As, Ba, Be, Bi, Cd, Co, Cr, Cu, Ga, Ge, In, La, Li, Mn, Mo, Nb, Ni, P, Pb, Sb, Sc, Sn, Sr, Ta, Te, Ti, Tl, V, W, Zn, Zr. The minimum detectable concentration of elements was up to 10−9 g/dm3 in liquid objects and up to 1 ppb in solids.
4. Discussion
Over the past decades, Kalba–Narym batholith granites were studied by many researchers [
54,
55,
56,
57,
58,
59,
60,
61]. Their interest was mainly caused by the availability of large granite-pegmatite deposits with Li, Ta, Cs, Nb, and Be mineralization in the northern and central parts of the batholith [
62,
63]. In the southern part of the batholiths, Cherdoyak deposits associated with granitoids from the Narym Sn-W rock mass are present [
64,
65,
66,
67,
68].
The Kalba–Narym batholith is mainly composed of Kalba-complex granites. According to the data from previous geochemical studies [
60,
61], the granitoids in the Kalba complex contain 63–77% wt of SiO and 2–7% wt of K
2O; in addition, they belong to high-potassium calcium–alkali series. Furthermore, LREEs prevail over HREEs; in the majority of the analyzed samples, there was a negative Eu anomaly (Eu/Eu* from 0.7 to 0.3). The multielement diagrams showed negative Ba, Sr, Eu, and Ti anomalies, as well as positive Th and P anomalies with multidirectional U and Ta behavior. The set of petrogeochemical data allows the suggestion that Kalba-complex granitoids are similar to S-granites.
The geochemical characteristics of the Burabay rock-mass granites corresponded to those of the Kalba-complex granites. The behavior of the rare-earth and rare elements was also similar to that of Kalyubin granites. The four analyses of the granites in terms of the behavior of the rare-earth elements showed that these were fully consistent with those of Kalba granites. Two analyses of granites (#3 and #6 in
Table 2) showed reduced concentrations of LREEs. In analysis #3, the HREE concentrations were also reduced. This phenomenon can be explained by local manifestations of the post-magmatic alteration of granites.
Compared to Central Kalba, the granitoid mass within the Narym ore district was formed under Quarternary tectonic conditions, which resulted in the lesser development of intra-intrusive fractures, fault structures, and vein fields, a weaker manifestation of metasomatic processes, pegmatite veins with simpler compositions, and lower ore contents. Separate ore manifestations of block microcline and quartz–albite–muscovite pegmatites with no practical significance were established in the study area [
69,
70,
71,
72].
A geological survey within Burabay rock mass identified rare-metal specialization, including three complex halos. The largest of these, with an area of about 20 km
2 [
61], is located in the central part of the rock mass. The rocks are characterized by lithium content of 0.3–0.9 ppm. Two other complex halos, with 0.5 ppm of tin content, 4 ppm of copper content, less than 0.5 ppm of molybdenum content, and 0.1 ppm of silver content, of smaller sizes (up to 1 km
2), are located in the eastern part of the studied area.
According to the data from another geological survey [
1], two complex rare-metal anomalies were found in the territory of the Burabay rock mass: one northern (about 12 km
2) and one southern (about 5 km
2). The former is characterized by 0.0008–0.002% niobium content, 0.0002–0.0005% bismuth content, 0.0005–0.0008% beryllium content, 0.0015–0.002% lithium content, and 0.001–0.0015% tin content. The latter consists of three local halos, containing 0.0005% beryllium, 0.001–0.0012% tin, and 0.002–0.008% lithium; they are united by a 0.0003–0.0004% beryllium halo [
73].
Considering the findings of previous studies [
1], which include the rather significant saturation of the area with small (but low-in-contrast) halos, as well as its localization within a field with several points of tantalite–columbite, tin ore, and beryl (Pridorozhny section) mineralization, in addition to the proximity of Cherdoyak tin–tungsten deposits, the study of the chemical lake composition is of practical interest [
74].
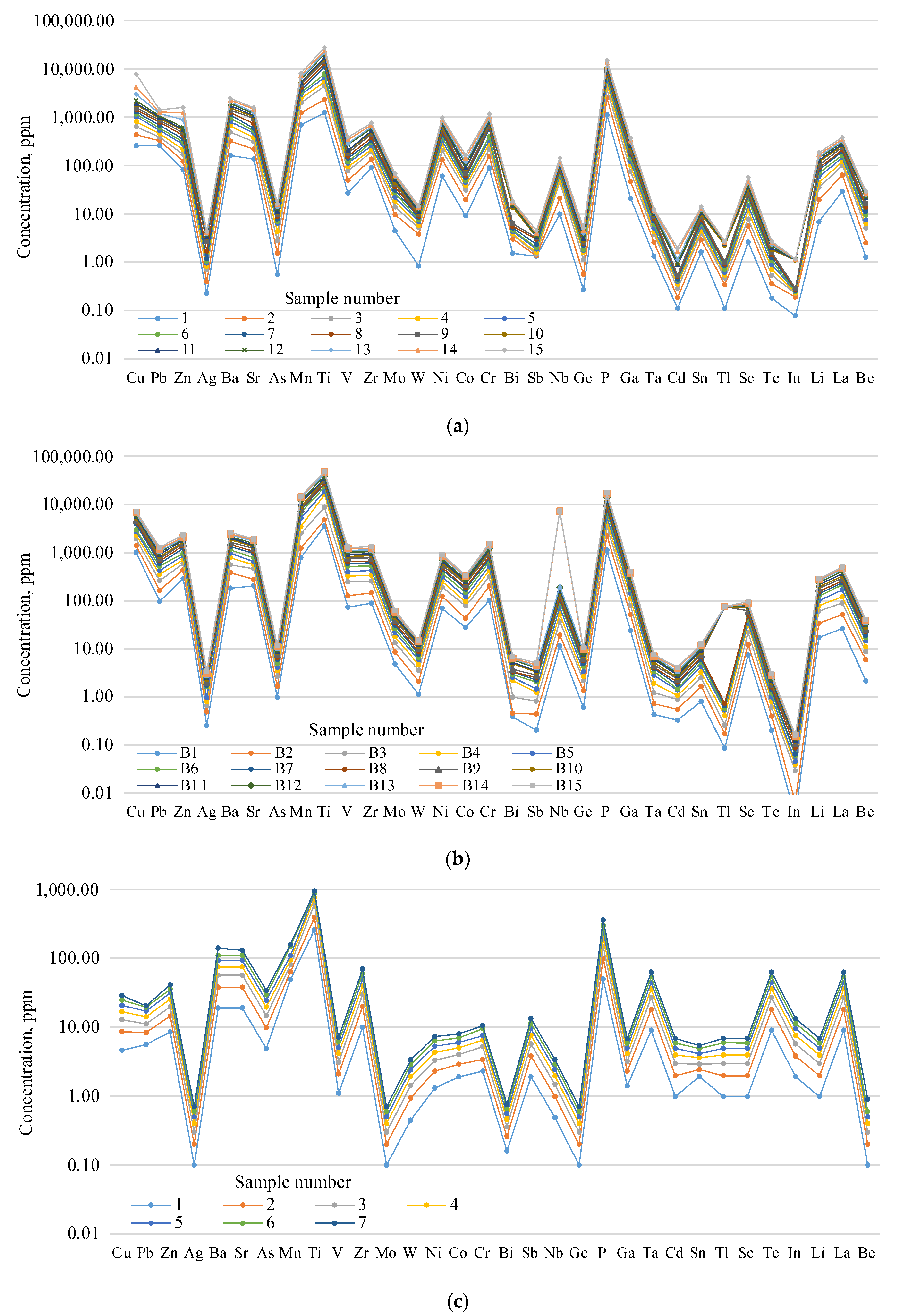
A comparison of the graphs concerning the distribution of the microcomponents in the lake water and bottom deposits within the bedrock of the Burabay rock mass (
Table 2,
Table 3 and
Table 4;
Figure 5,
Figure 6,
Figure 7 and
Figure 8) showed that the general tendency is preserved in their content by the main maxima and minima. Along with this similar general tendency in the content according to the graphs, there were certain differences in the nature of the microcomponents. For example, the following were higher in the bottom deposits (bottom deposits; water, ppm): V (up to 120.4; up to 64.17); Ti (up to 4597.3; 3800.6); Ge (up to 0.82; up to 0.6); Ta (up to 0.90; up to 1.3); Cd (0.551); and Co (35.68 23. 88). However, the following substances were significantly (i.e., up to the first tens) reduced (bottom deposit; water, ppm): In (0.02; 0.8) and Sb (0.54; 1.3).
It can be concluded from the analysis of the graphs in
Figure 5a–c that the contents of the microcomponents in the lake water and bottom deposits were tens and hundreds of times higher than those in the bedrock (except for Ti and P, which were almost equal). On the other hand, the aqueous solutions and bottom deposits have sharply reduced Ta, Cd, Sn, and In contents. This can be explained by the different solubility levels of the microcomponents in different types of water, as well as by the different sedimentation rates in the lakes.
The elevated REE concentrations in the lake waters may have resulted from the presence of erosion and the passage of the migration flow in the zone of influence of the geochemical anomalies established by [
72]. This scientific source proposes many possible reasons for the occurrence of anomalies in various rare-earth elements.
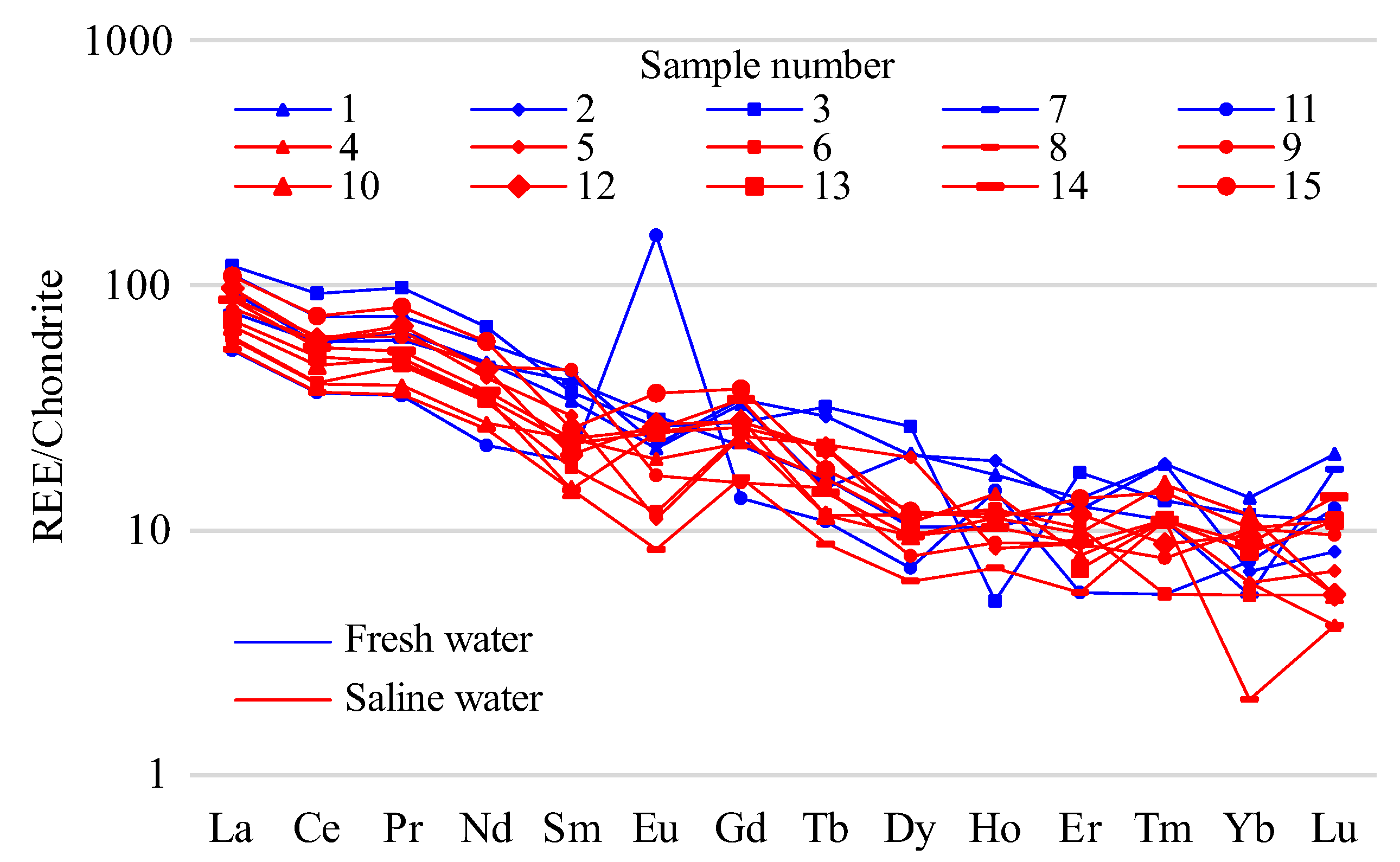
To study the behavior of the rare-earth elements, distribution graphs of rare-earth elements in the analyzed water were constructed, with chondrite-content specification in terms of the rock standards (
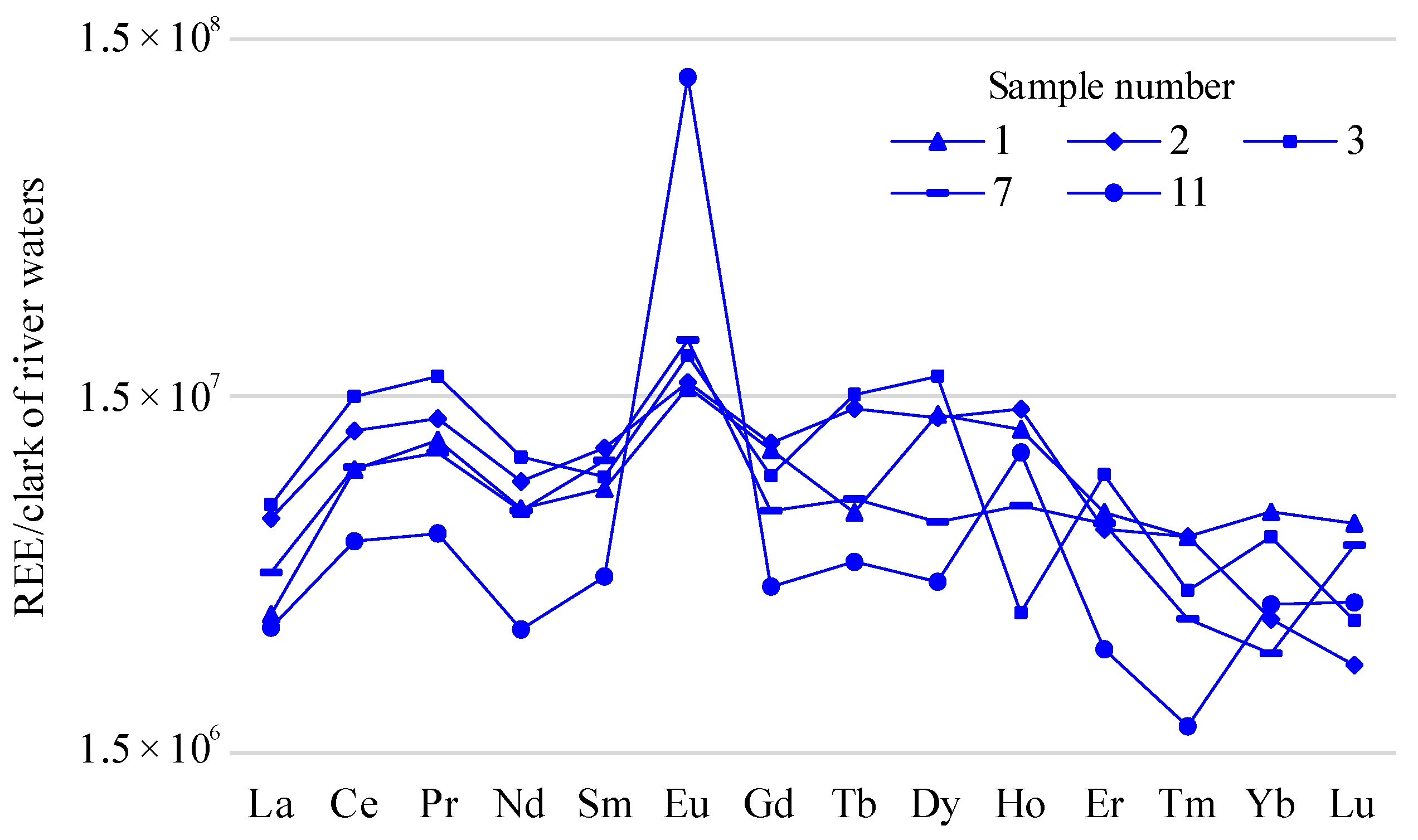
Figure 6). To take into consideration behavioral features of the elements in the solutions, the REE content in the fresh water was specified for lakes 1, 2, 3, 7, and 11 (river-water Clarke) (
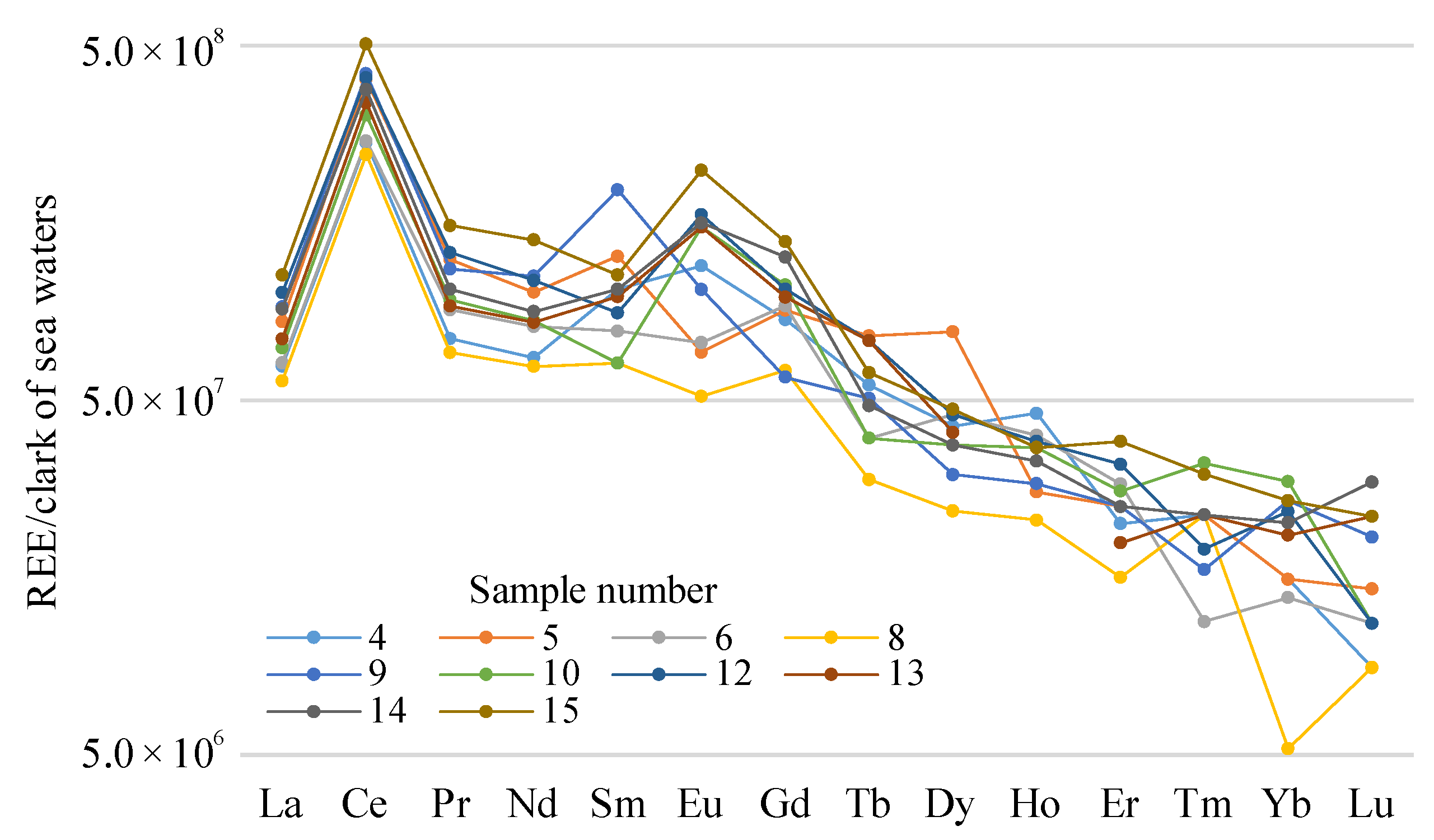
Figure 7). In the salt water (lakes 4–6, 8–10, and 12–15), the sea-water Clarke was used as the gauge (
Figure 8) [
53].
The graphs of the REE distribution standardized by the chondrite (
Figure 6), as well as by the sea-water Clarkes (
Figure 8), demonstrate clearly that the content of the light REEs prevailed over that of the heavy REEs. For the fresh water, the distribution of HREE and LREE was roughly the same. In the salt-lake water, prominent Ce maxima were seen; in the fresh water, prominent positive La, Ce, and Pr anomalies were seen. At the same time, the content value of the elements in the fresh water was dozens of times lower. In the graphs of the REE content in the salt water, slightly positive Sm anomalies were shown. There was a clear positive europium anomaly in both the fresh and the salt water; this was especially apparent in the fresh alkaline water from lake 11.
The study of the chemical compositions of the samples showed that the Li contents in the lake-bottom deposits were 1.7–2.7 10−3 wt%, and they were 0.6 to 1.9 10−3 wt% in the lake water. The obtained values are comparable to the Li content the in sea water (1.5–2.0 10−3), or slightly higher; nevertheless, they were lower than the Li Clarke in the sedimentary rocks (7.0–7.4 10−3). The proportions of Sr and Rb in the bottom deposits were −7.7–20.6 10−3 and 15.7–22.4 10−3 wt%, respectively, exceeding the Clarkes of the elements in the sedimentary rocks (7.5–15.3 10−3) and sea water (0.7–8.4 10−3). Similar shares were seen in the lake water for the Sr (10.7–13.6 10−3) and Rb (9.5–19.6 10−3 wt%).
The comparison of the REE contents in the lakes with the Clarke contents in the hydrosphere (
Figure 6) demonstrated that they were slightly higher than the Clarkes of the sea and fresh water, although the geochemical-environment standard for lake water (alkaline pH value, and high mineralization) is unfavorable for REE accumulation and migration in water [
46,
47,
49,
51,
75,
76,
77,
78].
It should be noted that interpretation of the REE behavior in natural water is complicated by such processes as the generation conditions and the hydrodynamic regime of water complexes, the prevailing form of element migration in water, ion exchange, and adsorption by host-rock complexes which, apparently, leads to the fractionation of these elements in aqueous solutions compared to source rocks. However, the data in this study are very important for identifying the direction of migration flows and determining the areas of microelement subtraction. The possibility of using the lake water within the Burabay rock mass as a source of hydromineral resources has been defined (
Table 7). The Sr, Rb, Th, and U contents are tens or even hundreds times higher (Th, U) than their respective Clarke contents in seawater and sedimentary rocks.
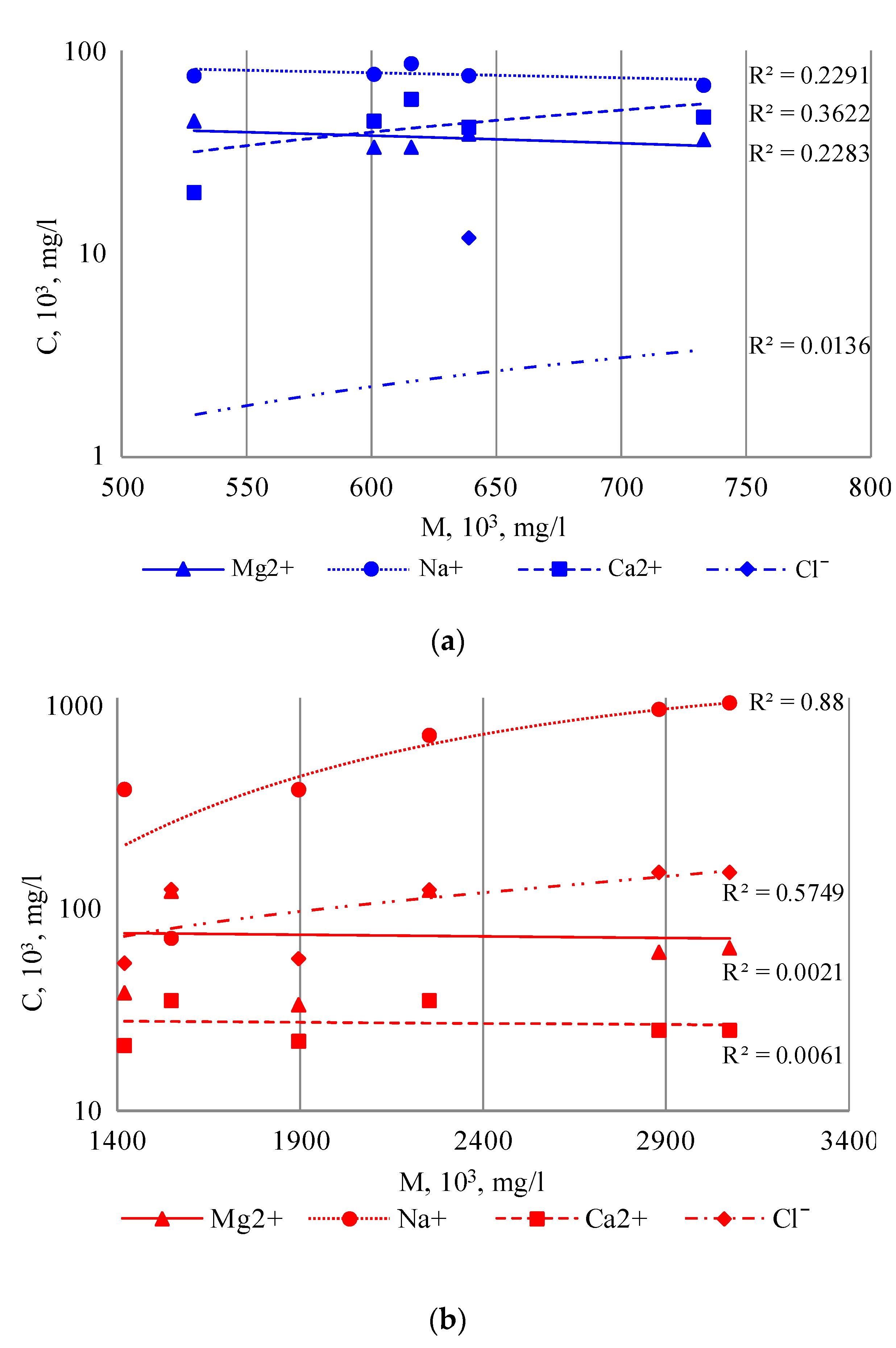
The results of the comparative analysis of the mineralization and macro-component composition of the lake water of the Burabay rock mass for fresh and salty water showed different correlations between the mineralization indicators and the levels of Na, Mg, Ca, and Cl accumulation. There was practically no correlation with fresh water (
Figure 9a). As expected, the Na content increased for the salty water, along with an increase in the mineralization indicator (
Figure 9b).
The Cl content showed a weak correlation with the mineralization; in turn, the Ca and Mg demonstrated no correlation. The fact that the correlation was high for the Na and medium for the Cl supports the idea that the Na originated from the surface-water runoff. The Na also potentially originated from the alkaline granites of the Burabay granitic rock mass, as their most soluble component.
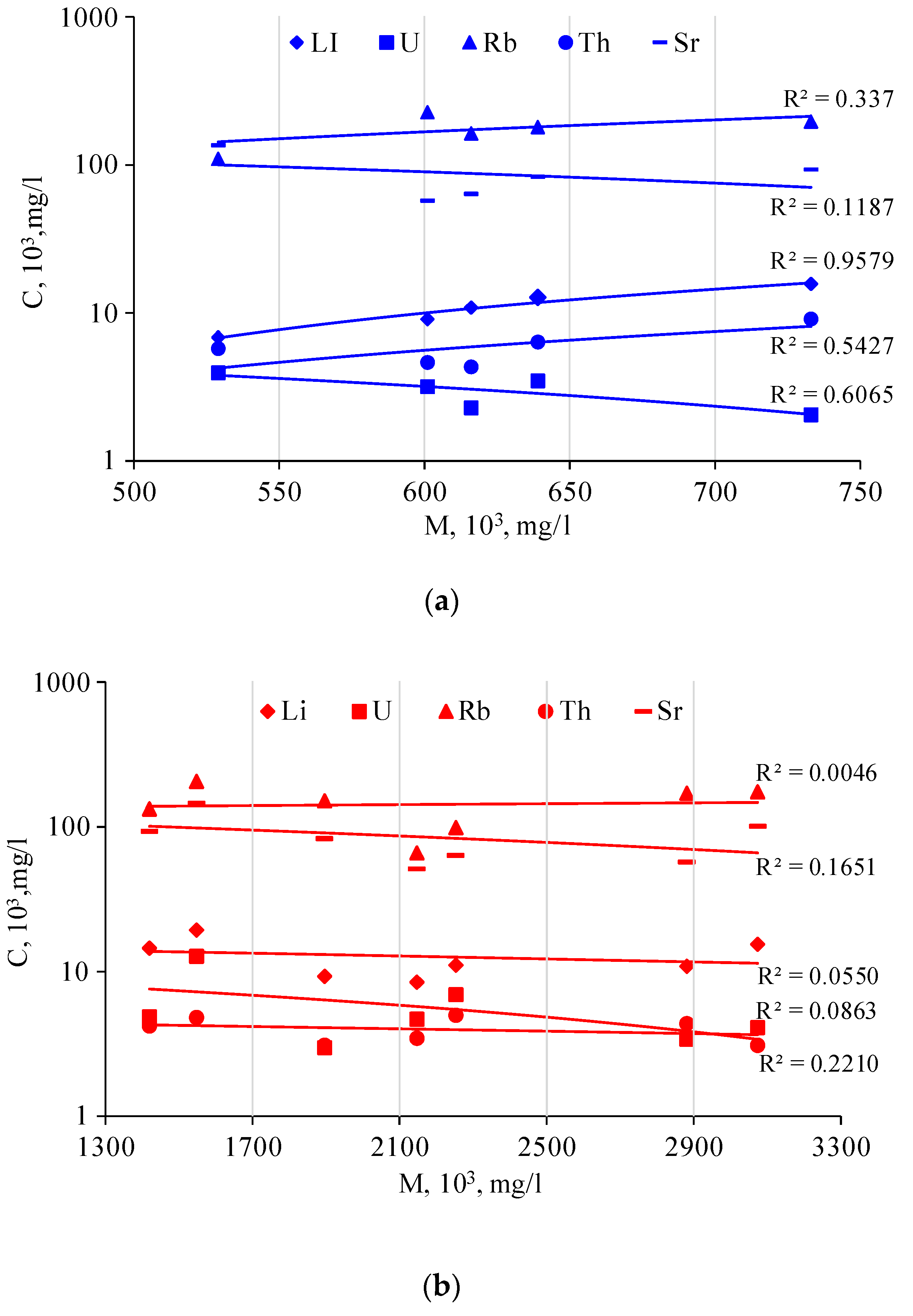
A positive correlation was also established between the water mineralization and the contents of some of the rare elements. In the fresh lakes of the Burabay rock mass, the Li shows a high correlation (R
2 = 0.957), the Th showed a moderate correlation (R
2 = 0.543), and the Rb showed a rather poor positive correlation (R
2 = 0.337). There was a negative content correlation for U (R
2 = 0.607), and there was no correlation for Sr (R
2 = 0.12) (
Figure 10a). These correlations were not expressed in the salty lakes (
Figure 10b). It is quite possible that the Li and Na were leached from the same mineral within the surrounding granitic rocks. The area is also of interest owing to the demonstrated correlation between the parent rocks of the enclosing formation and the lake-water composition.
The interpretation of the analytical data from within the area helped to establish the relationship between the water mineralization and the weathering products of the parent rock mass; moreover, the influence on the ultimate composition of the studied lake water was observed. This may have resulted from areal losses.
It was identified that the lake water accumulated elevated concentrations of commercial components (Sr, Rb, and U), which may become the basis for specific studies of the small lakes within the area. It is possible to apply the findings of this study for future geological prospecting activities and operations within the promising sites identified.
The main sources of rare-alkaline elements are rare-metal and rare-earth-mineral occurrences, which are associated with Permian granites in the eastern Kazakhstan region. During the orogenic (post-collisional) stage of the Hercynian cycle, the survey area experienced extremely high mobility, which is reflected in the occurrence of powerful granite magmatism. In this regard, the following promising survey areas are distinguished, In these areas, we plan to conduct further research on the chemical composition of the lake waters, salt brines, and bittern and bottom sediments to identify the mineralization of rare metals and other types of mineral:
(1) Delbegeteysky area (the lakes in the north-west of the Delbegeteysky massif);
(2) Shagan–Charyn area (between the Shagan and Charyn rivers);
(3) Kaskabulak area (near the Maksut field), where there is a fault zone in which an intrusion and a Suurly tungsten deposit are known to the south of the lakes.
Large-scale granite outcrops on the daylight surface of the Kalba complex (P1) were noted in the identified survey areas. These are genetically related to the rare-metal type of mineralization. Quartz-veined-greisen and quartz-veined tin, tin–tungsten, and tungsten formations are also widely evident. Given the intense geochemical migration ability of rare-alkaline elements in the thickness of loose deposits as a result of intense geodynamic processes in the eastern Kazakhstan region, we can assume the possibility of their migration to the upper horizons and their accumulation in salt lakes localized within the area of the development of Permian granite intrusions and associated deep tectonic faults.
The planned scientific research makes a certain scientific contribution to the problem of modeling rare-metal ore-forming systems and improving prediction methods in order to create a modern scientific base for the further development of the mineral and raw-materials sector in the world economy.