Abstract
Copepods are the dominant crustacean group in groundwater, where they perform valuable ecosystem services related to carbon recycling. The life-history traits of stygobitic (groundwater-obligate dweller) copepods, however, have only been casually studied in the past. In addition, next to nothing is known about the responses of stygobitic copepods to climate change. In this study, we investigated the life-history traits and respiratory metabolism of a species of harpacticoid copepods, Moraria sp., endemic to the Corchia Cave in the Apuan Alps (Italy). We collected the specimens of Moraria sp. from the dripping waters of the cave and observed their development, survival, and reproduction rates in the laboratory for one year. We also evaluated the acclimation ability of adult females of Moraria sp. by measuring their oxygen consumption in a temperature range from 8 °C (average annual temperature of the dripping water in the Stalactites Gallery of the Corchia Cave) to 12.5 °C (maximum temperature of the dripping water of the cave expected according to climate change scenarios in 2100). Our results indicate that Moraria sp. Is a stenothermal species showing remarkable stygobitic traits (long life span, low metabolic rates). We noted that the metabolism of this species is significantly affected by small (+1.5 °C) thermal changes. Our results showed no metabolic compensation occurring in this species over two weeks of exposure to temperatures higher than 8 °C. The outcomes of this study suggest that Moraria sp. May not be able to tolerate thermal changes brought on by climate change.
1. Introduction
Life-history traits of stygobitic (aquatic and strictly subterranean) species are still mostly indeterminate due to several obstacles, the first of which is the challenging access to their habitats [1,2]. Subterranean habitats are limited in energy and thermally buffered [3]. Stygobitic animals have, therefore, lower energy consumption and metabolic rates than their epigean phylogenetic relatives [4,5]. In addition, stygobitic species have long life spans and development cycles, which make laboratory culturing challenging [6,7]. Accordingly, we lack even the most fundamental knowledge of the physiology, ecological requirements, and behavior of most stygobitic species. Understanding how stygobitic species react when facing new thermal conditions is critical in the present global climate change scenario [1].
Animal metabolic rate scales with the 3/4-power of body mass and increases exponentially with temperature [8]. Rises in environmental temperatures during ontogenetic development may have a profound effect on metabolism and generate variations in metabolic scaling with body mass through a decrease in the allometric slope [9]. Furthermore, the effect of temperature on metabolism is mass-dependent at high latitudes, where the metabolism of small/juvenile individuals increases at greater rates than that of large/adult ones, while, at low latitudes, the variation in metabolism with temperature may not be observed in large/adult individuals [10]. Most animal species can gradually adjust their metabolic rates to thermal changes. Most studies refer to this adjustment as “acclimatization”, or “acclimation” if it occurs in the laboratory [11]. Acclimation acts to keep biological processes stable at all temperatures [11]. The expression “acclimation ability” refers to the rates at which some physiological functions (e.g., respiration) vary after exposure to shifting environmental conditions (e.g., temperature increase). According to the type of animal involved, acclimation takes anywhere from a few hours to many days to complete in a laboratory setting [11,12]. Species that live in thermally stable environments (such as caves) have slower acclimation rates than species that reside in thermally changing habitats [13,14]. Species that inhabit continental subterranean ecosystems, such as ice caves where temperatures range from 0 to 5.5 °C [15], accumulate cryoprotective molecules, such as glycerol and free amino acids, to endure extreme thermal conditions; however, they cannot acclimate to warmer conditions [16,17]. Pallarées et al. [18] claimed that a “loss of heat acclimation capacity” could be an adaptative trait of stygobitic and troglobitic (terrestrial and subterranean) fauna. However, there are still few experimental studies that have demonstrated this inability [5].
Groundwater food webs are dominated by crustaceans [3,19], mostly copepods [20,21]. Stygobitic copepods contribute to the biogeochemical cycles in groundwater through feeding, the formation of fecal pellets, and respiration processes [3]. Changes in environmental temperature have a significant impact on copepod physiology [22]. Since several stygobitic copepod species are stenotherm and show restricted distributional ranges [15,23,24], we expect that temperature rises due to climate change could be detrimental for most of them. However, compared to the massive number of studies on freshwater and marine copepods, the life-history traits and physiological characteristics of stygobitic copepods have been poorly investigated in recent years [25,26,27,28].
The aims of our study were to i) perform an extensive, long-term culturing of a stygobitic harpacticoid species, Moraria sp., to assess its life-history traits; and (ii) explore the acclimation ability of adult females of Moraria sp. by examining how their oxygen consumption rates vary with environmental temperature changes. Moraria sp. is endemic to a limited sector of a wide karst cave in Italy, where the environmental temperature is about 8 °C and varies by less than 0.2 °C annually [29]. We hypothesized that this species would have lower metabolic rates than epigean (surface water) copepod species, which would be evidenced by a long lifespan and low reproduction rates. We further predicted that Moraria sp. would have a limited ability to adapt to climate change.
2. Materials and Methods
2.1. Study Area
Mount Corchia (1677.7 m above sea level—a.s.l.; Figure 1) is an isolated limestone massif within the Apuan Alps Regional Park (south-western part of the Apuan Alps, NW Tuscany, Italy; Figure 1), which is a member of the UNESCO global network of geoparks (Tuscany, Italy). From a geological point of view, Mount Corchia represents a multiphase, over-turned syncline belonging to the largest fold structure of the Apuan Alps. The syncline core consists here only of Early Mesozoic, carbonate formations represented by dolostones (“Grezzoni”), marbles, and cherty metalimestone and represents the northernmost part of a relatively isolated hydrogeological structure, surrounded by the impermeable rocks of the Palaeozoic basement [30]. Carbonate formations are intensively karstified and characterized by a high infiltration rate, despite the steep surface morphological gradient. The main spring fed by this karst system is located in the Stazzema valley and is represented by several outlets close to each other located around 175 m above sea level, just upstream of the village of Ponte Stazzemese (Figure 1). The overall average discharge is estimated to be around 180–200 L/s [30,31]. In the area, there are other minor springs, which are part of the same hydrogeological structure.
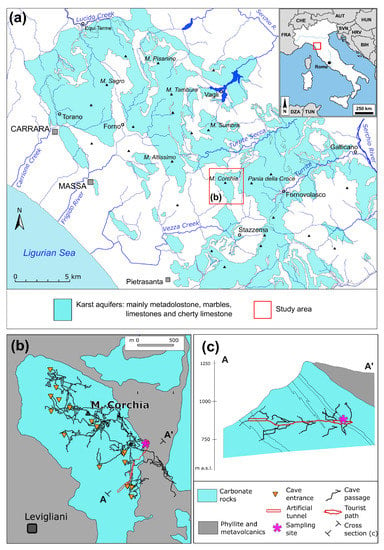
Figure 1.
(a) Location of Monte Corchia and schematic map of Apuan Alps’ karst area; (b) plan view of Corchia Cave karst system; (c) projected cross-section through the tourist path of Corchia Cave. Note that the sampling site is about 400 m below the surface and overlayed by low-permeable rocks.
The karst complex of Mount Corchia, i.e., the part of the system known thanks to speleological explorations, is affected by a dense network of percolation and concentrated runoff routes, which gathers in a single collector which can be followed by the north-western part of the karst complex, up to the bottom of the cave, at an altitude of 450 m a.s.l. (Figure 1b). The Corchia Cave (44°01′31.98″ N 10°17′59.64″ E; Figure 1), the subterranean system (more than 70 km in length and 1185 m in vertical range) of Mount Corchia (the formation of which dates back to the Late Pliocene, about 2.6 million years ago), is one of the most extensive and deepest caves in Europe [30]. About half of the underground three-dimensional network (from 1550 to 450 m a.s.l.) consists of relict phreatic and epiphreatic tubes intersected by a system of predominantly vertical cavities, mainly deep pits and narrow meandering passages [32].
The vertical cavities represent the current rapid drainage system for infiltrating water into the saturated zone [30,32]. The Corchia Cave (partially exploited for tourism since 2001) has been monitored by universities and national research bodies under the coordination of the Regional Agency for the Environment Protection of Tuscany (ARPAT) [33] since 1998. The Stalactites Gallery (where we collected the individuals of Moraria sp. used in this study) is at 840 m and about 1.2 km far from the artificial entrance (Figure 1c). This sector of the cave is located in the north-eastern portion of the karst complex, at a depth of about 400 m from the surface and partially covered by the overturned flank of the syncline, formed by the phyllites of the Paleozoic basement (Figure 1b,c). This condition determines a supply of dripping water characterized by a very slow seepage and is not influenced by seasonal variability [34]. For this reason, this sector of the Corchia Cave is characterized by very stable environmental conditions both from a thermal and hydrological point of view (Figure 2). The average air temperature in the gallery is 7.8 ± 0.3 °C (min-max: 7.3–8.4 °C [34]), whereas the mean drip-water temperature is about 7.8 ± 0.2 °C (min-max: 7.3–8.6 °C [29]). Humidity fluctuates between 98 and 100% [34]. The physical-chemical parameters of the dripping water of the Stalactites Gallery are in the following ranges [30]: electrical conductivity (317–323 μS/cm), pH (8.1–8.3), ammonium and nitrite ions (<0.1 mg/L), nitrate (0.25–1.9 mg/L), fluoride (0.20–0.42 mg/L), chloride (4.5–6.4 mg/L), sulfate (33.0–37.6 mg/L), hydrogen carbonate (165–171 mg/L), sodium (3.5–4.8 mg/L), potassium (0.2–0.5 mg/L), calcium (30.5–33.7 mg/L), and magnesium (21.9–25.5 mg/L). The concentrations of heavy metals are in line with the natural background, and the drip waters are not polluted by fecal bacteria [34]. Drip counting ranges from 60 to 130 drips/h, with a few exceptions (from 23–24 September 2009) when a rate of 450 drips/h was recorded [26]. In the Stalactites Gallery, a slow flow-discharging system regulates the dripping, which is not directly correlated with the rainfalls [29]. An impermeable basement of only-superficially-fractured phyllites, metavolcanics, and clay minerals, produced by silicates’ alteration [30], partially overlaps the Stalactites Gallery (Figure 1 and Figure 2) [29]. Such structural conditions delay the vertical infiltration of recharging meteoric waters and suggest a long residence time of the waters dripping in this sector of the Corchia Cave [29].

Figure 2.
(a) Panoramic view of Monte Corchia from the South; the red arrow indicates the probable infiltration area feeding the Stalactites Gallery drips; (b) the Stalactites Gallery where the ARPAT monitoring station is placed. The red circle shows the main sampling site of Moraria sp.; (c) plastic container (mouth diameter 5 cm, volume 500 mL) used to collect the dripping water in the Corchia Cave.
2.2. Biological Monitoring and Rearing
We began monitoring the biological assemblages of the epikarst of Mount Corchia in 2016. We collected and filtered the dripping water in the Corchia Cave following the methods in Pipan [35] and Pipan and Culver [36,37]. Between 2016 and 2021, we collected the dripping waters from at least 10 ceiling drips in the Corchia Cave’s touristic sector. In 2017, we selected, for regular monitoring, two drips in the Stalactites Gallery (Figure 2b) where individuals of Moraria sp. had been found in relevant numbers in a reasonable time (max 52 specimens in max 30 days). We collected the dripping water in two plastic containers (mouth diameter: 5 cm; volume: 500 mL; Figure 2c), where a 2 × 3 cm portion was cut out and covered with a net with a mesh size of 60 µm. At regular intervals (30/60 days), we poured the contents of the containers (drip water and animals) into 50 mL plastic falcons and transported them to the laboratory within 4 h.
Following the collection in April 2017, we started rearing 26 individuals of Moraria sp. (10 females, 8 males, 6 CIII-CIV copepodites, and 2 NV-NVI nauplii) to study the life-history traits of this species in a single cohort in the laboratory for a year. For a whole year, we kept the animals in a 250 mL glass container filled with 100 mL of the dripping water of the Stalactites Gallery, which ensured a minimum living space of 1 cc per individual, as recommended in Di Lorenzo et al. [38]. We provided no artificial food because Moraria sp. feeds on the microbes naturally contained in the dripping water. The water was changed once every four weeks by replacing half the volume with 50 mL of new drip water. We kept the animals in the dark in a thermostatic cabinet (Mod. ST 3, Pol-Eko-Aparatura, Wodzisław Śląski, Poland; precision: ± 0.2 °C) at a temperature of 8.0 °C, which was close to the average annual temperature of the dripping water in the Stalactites Gallery [29]. We covered the glass container with a glass lid, but we did not seal it. We measured the dissolved oxygen on the occasion of water changing by using the device described in paragraph 2.3 and noted that it remained equal to about 6 mg/L throughout the year. We carried out life-history trait observations (development stage, survival, mating, etc.) every week for a year. At the end of a year of rearing, we drafted the protocol for the respirometry trials (described in Section 2.3) based on the life-history traits we had observed during rearing.
2.3. Protocol of Respirometry Trials
We run four respirometry trials at four temperatures (8.0, 9.5, 11.0, and 12.5 °C), where 8.0 °C is about the annual average temperature of the dripping water at the Stalactites Gallery and 12.5 °C is consistent with the worst climate-change scenario depicted for groundwaters worldwide within the next century (3–5 °C projected thermal increase [39,40,41]). The specimens used in the trials were collected in November and December 2018 and in February and March 2019, by leaving the containers in place for a minimum of 30 days and a maximum of 56 days. The trials were run about six weeks after collection: on December 11th, 2018 (trial at 8.0 °C), 15 January 2019 (trial at 9.5 °C), 15 March 2019 (trial at 11.0 °C), and 12 April 2019 (trial at 12.5 °C). The specimens were transported in a cooling box to the laboratory within 3 h of each collection (Figure 3). In the laboratory, we let the specimens acclimate to the laboratory conditions at the temperature of the collection site (8.0 °C), keeping them in 100 mL of drip water in a 250 mL glass container in permanent darkness for four weeks (Figure 3). During this period, we did not provide sediments (because we never found deposits in the collection containers) and never changed the water. At the end of the four-week acclimation at the laboratory conditions and the temperature of the collection site, we picked up the adult females with a glass pipette and loaded them into a glass vial (250 mL) with 50 mL of drip water and 50 mL of MILLIPORE MILLI-Q® ultrafiltered water (Elix®, Merck KGaA, Darmstadt, Germany), remineralized with the following reagent grade chemicals: 0.06 g of MgSO4, 0.096 g of NaHCO3, 0.004 g of KCl, and 0.06 g of CaSO42H2O [42,43]. For the measurements at 8 °C, we did not change the temperature and kept the animals in the darkness for seven days (Figure 3). For the trials run at temperatures >8.0 °C, we slowly increased the temperature by 0.041 °C/h. The desired temperatures of 9.5, 11.0, and 12.5 °C were reached in 1–3 days following Di Lorenzo and Reboleira [5]. Once the new temperature was reached, we let the specimens acclimate in the darkness for seven days. Afterward, we moved the adult females to a new 250 mL container previously filled with 100 mL of the remineralized MILLI-Q water (Figure 3). We let them acclimate for seven more days to the new medium before measuring the oxygen consumption rates at the appropriate temperature (Figure 3). Acclimation in MILLI-Q remineralized water was necessary to ensure gut emptying and avoid the overshoot of oxygen consumption by fecal bacteria during measurements [4,5,38]. We used adult females only to minimize the effect of mass, gender, and developmental stage on oxygen consumption.
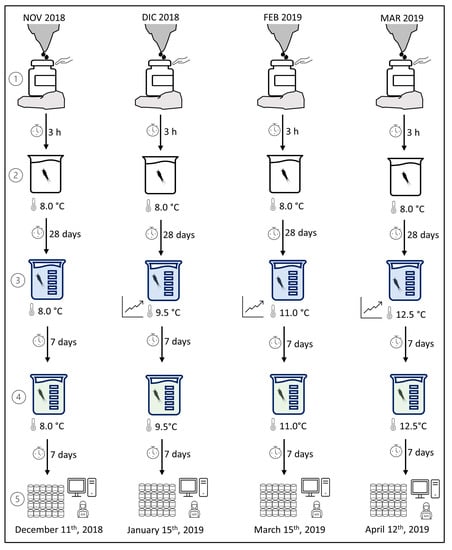
Figure 3.
Phases of the experiment performed with adult females of Moraria sp. The specimens were: (1) collected from the dripping waters of the Corchia Cave (Apuan Alps; Italy) and transported to the laboratory within 3 h of collection; (2) acclimated at the laboratory conditions at the temperature of the collection site for 28 days; (3) acclimated to the new medium (50 mL of drip water and 50 mL of remineralized MILLI-Q water) and, eventually, temperature for 7 days; (4) acclimated to the new medium (100 mL of remineralized MILLI-Q water) for 7 more days and (5) loaded in the 80 µL glass wells for measurements of the oxygen consumption rates.
We measured the individual oxygen consumption rates (OCRs) of female copepods in 80 µL glass wells, each provided with an oxygen sensor spot (4 mm in diameter) glued to the bottom. The wells were drilled in a microplate (Loligo Systems in Viborg, Denmark), which we placed on a Sensor Dish Reader (SDR) consisting of 24 fluorescence-based channels (PreSens Precision Sensing GmbH, Regensburg, Germany). Before animal loading, we kept the microplate and the SDR inside a thermostatic cabinet at the desired testing temperature (8.0, 9.5, 11.0, 12.5 °C; accuracy: ± 0.1 °C) for at least 12 h [5]. We filled the wells with MILLI-Q remineralized water and then individually loaded the female copepods by using a soft brush. Three wells were left without animals and used as blank controls. After animal loading, we sealed the wells and let the temperature re-equilibrate for at least 2 h [42]. Oxygen concentration (in mg/L) was recorded in each well every 5 min for 18 h. We calculated the oxygen consumption rates by determining the slope of the oxygen concentration decrease over time in each well. Finally, we corrected the obtained values for the mean oxygen consumption of the control wells. At the beginning of the trials, the dissolved oxygen concentration was 6 ± 0.5 mg/L. It never dropped below 80% at the end of the trials.
We could not conduct a balanced experiment due to the different number of female individuals collected in the four sampling campaigns. Overall, we used 10 adult females in the trial at 8.0 °C, 21 at 9.5 °C, 8 at 11.0 °C, and 15 at 12. 5 °C. All individuals were alive at the end of the trials. At the end of the measurements, we narcotized the animals with CO2 and photographed them with a camera integrated into a Leica M80 stereomicroscope. We measured the length (from the tip of the cephalic shield to the end of the caudal rami) and the width (at the larger somite-bearing legs) of every individual by using ImageJ software [44]. Afterward, we converted the body dimensions (in mm) to dry weight by assuming a dry/wet weight ratio equal to 0.25 [45,46,47] and a wet weight equal to biovolume/1.1 [45]. We calculated the biovolume (BV) as in Equation (1) [45]:
where a is the length (mm), b is the width (mm), and CF is a correction factor equal to 490 [46]. We expressed OCRs in ng O2/mg DW × h where DW is the dry weight.
BV = a × b2 (1) × CF,
2.4. Statistical Analyses
To identify significant variations in mass-corrected OCRs (ng O2/mg DW × h) at the four testing temperatures, we used a one-way permutational analysis of variance (PERMANOVA [48]), based on the Euclidean distances of the raw data, followed by a permutational paired t-test [48]. We looked at the model using Type I partitioning of the sum of squares. To obtain the best power and the most accurate Type I error with unbalanced data, we used the permutation of residuals under a reduced model. Potential heterogeneity of the variances is not strictly necessary to achieve prior to using PERMANOVA [48]. However, we included an analysis of the homogeneity of dispersions by performing Levene’s test as part of the general null hypothesis of “no differences” among groups tested by PERMANOVA [48]. We set the significance level (α) equal to 0.05 since permutational tests do not require alpha correction for multiple groups. We performed all analyses with E-PRIMER and PERMANOVA + software v.7 [49].
3. Results
3.1. Life-History Traits
Moraria sp. shows stygobitic morphological traits such as lack of eyes, depigmentation, small (adult female length in the range of 0.53–0.58 mm) and slender (adult female width at the largest somite bearing legs in the range of 0.13–0.15 mm) body size, and long antennules (Figure 4). As with many cave species [50], populations of Moraria sp. typically show low densities. In April 2018, one year after the start of the laboratory rearing, 25 out of the 26 initial cohort specimens were still alive, indicating that Moraria sp. does not show cannibalistic behavior in captivity. The dead individual was a male. All nauplii and copepodids had reached the adult stage within the year. At the end of the observation period, the cohort consisted of 18 females and 7 males. The development of the two NV-NVI nauplii to the adult stage took 6–7 weeks at 8 °C. We found the exuviae in the cohort, indicating that the molting individuals did not eat them. During the rearing period, we observed two couples with the male attached to the female (Video S1). However, we never found ovigerous females nor new nauplii in the cohort.

Figure 4.
Adult female of Moraria sp. (right) and its fecal pellets (left). Moraria sp. shows stygobitic morphological traits such as lack of eyes, depigmentation, small body size, and long antennules and does not ingest the fecal pellets, which remain visible in the vial.
We did not collect ovigerous females in our surveys; therefore this ontogenetic stage remains still unknown for this species. Unlike other stygobitic crustaceans [51], individuals of Moraria sp. did not eat their fecal pellets, which were numerous and always clearly visible in the cohort vial (Figure 4). Overall, in the absence of any sediments, the individuals of Moraria sp. swim adherent to the bottom of the vial. When the water was changed every 30 days, we noticed that some individuals swam vertically by wriggling or writhing movements typical of some harpacticoid species [52], i.e., twisting their bodies to produce propulsive thrusts without using supports. In our previous observations, we had put some individuals in a Petri dish with a 4 mm sediment layer. When provided with some sediments, individuals of Moraria sp. quickly dig in the sediments layer, disappearing from the observer’s view in a few seconds (Video S2), thus showing an evident burrowing behavior. We assume that the specimens of Moraria sp. in our cohort fed on the microbial biofilm of the dripping water. As already observed for other stygobitic copepods [53], individuals of Moraria sp. tended to stay still for at least 50% of the observation time. After one year, we could no longer observe the individuals on a weekly schedule but continued to change the water about once a month until the first Italian lockdown due to the Covid-19 pandemic, which occurred on 9 March 2020, when we could no longer enter the laboratories. Three out of the 26 initial individuals were still alive on that date, about three years from the start of cohort rearing. We did not find any ovigerous females or new nauplii over this period.
3.2. Oxygen Consumption Rates
The dry weight of the adult females of Moraria sp. used in the respirometry trials (Table S1) ranged from 0.0011 to 0.0016 mg (mean ± SD = 0.0014 ± 0.0001 mg). Mass-corrected OCRs (Table S1) ranged from 104.37 ng O2/mg DW × h to 4285.61 ng O2/mg DW × h (Table 1). The average OCRs at the test temperatures are reported in Table 1.

Table 1.
Mean (μ), standard deviation (sd), minimum (Min), and maximum (Max) values of oxygen consumption rates (ng O2/mg DW × h) and number (N) of the female individuals of Moraria sp. used in this study at four assay temperatures (T; °C).
We assessed a significant heterogeneity among group variance, which was due to the higher data dispersion at 11.0 and 12.5 °C than at 8.0 and 9.5 °C. However, OCRs significantly varied with temperature (PERMANOVA; Pseudo-F3,53 = 3.63, p-value = 0.016, perms = 999; Table S1). The post hoc comparisons indicated significant differences (p-values < 0.05) in OCRs, except for 9.5 vs. 12.5 °C (t = 2.2533; p-value = 0.033) and 11.0 vs. 12.5 °C (t = 0.8791; p-value = 0.061). Average OCRs at 9.5 °C were 1.5-fold the OCRs at 8.0 °C (t = 2.9108; p-value = 0.007), at 11.5 °C were 2.2-fold (t = 2.3638; p-value = 0.022) and at 12.0 °C were 2.1-fold the OCRs at 8.0 °C (t = 2.2986; p-value = 0.028; Figure 5).
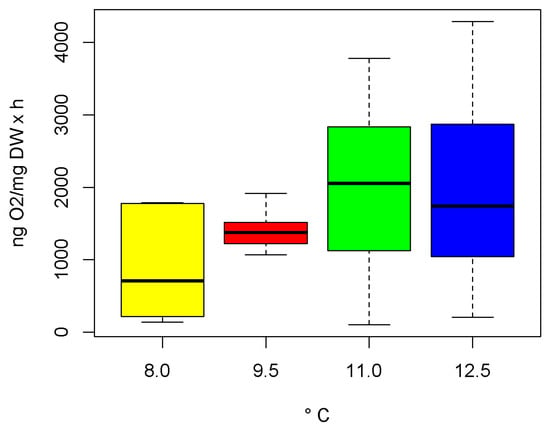
Figure 5.
Boxplot of oxygen consumption rates (OCR in ng O2/mg DW × h) of adult females of Moraria sp. at 8.0 °C (average annual temperature of the collection site), 9.5, 11.0, and 12.5 °C. Boxplots are delimited by the first and third quartiles and divided internally by the median. Whiskers indicate variability outside the upper and lower quartiles.
4. Discussion
4.1. Life-History Traits
Our attempts to rear specimens of Moraria sp. in the laboratory resulted in long-term and almost 100% survival of the initial cohort. The development from the naupliar stages NV-NVI to the adult stage seems more extended than that of epigean (surface water) copepod species. For instance, the post-embryonic development of the epigean harpacticoid species Canthocamptus (Canthocamptus) staphylinus staphylinus (Jurine, 1820) requires 4–6 weeks at 12 °C [54]. We found no naupliar stage NI in our samples or cohort, and therefore, we could not estimate the whole post-embryonic development of Moraria sp.; nevertheless, the development of Moraria sp. from the naupliar stages NV-NVI to the adult stage took 6–7 weeks. We assume that the development from the naupliar stage NI to NV/NVI could require as many. Rouch [55] and Glatzel [56] observed that some stygobitic harpacticoid species have a post-embryonic development of 13–16 weeks, which is, overall, significantly longer than the development of epigean copepods (from 3 to 9 weeks [55,56]).
The life span of the adults of Moraria sp. is longer than two years in the laboratory conditions we set up in this study. Accordingly, Di Lorenzo et al. [57] observed that the adult individuals of the stygobitic harpacticoid species Nitocrella achaiae Pesce, 1981 can survive for almost two years in the laboratory at 15 °C (average annual temperature of the collection site). Conversely, the life span of epigean freshwater copepods seems to vary from one month to a maximum of one year (though it may be longer at lower temperatures [58]. For instance, the life spans of the epigean cyclopoid species Megacyclops viridis viridis (Jurine, 1820) and Eucyclops serrulatus serrulatus (Fischer, 1851) are of 6 months, while the epigean harpacticoid species Bryocamptus (Rheocamptus) zschokkei zschokkei (Schmeil, 1893) can live up to 11 months [23]. Some cyclopoid species of cold habitats may extend their life spans up to 1–2 years through a diapause [58]. Cyclops scutifer scutifer Sars, 1863 can live more than 2–3 years by delaying its post-embryonic development during the long Norwegian winter when water temperatures are mostly below 2 °C [59]. However, notwithstanding some species in extreme environments, we can safely assume that most epigean copepod species live much shorter lives than stygobitic copepod species.
We did not observe cannibalism in our cohort, which, on the contrary, induces increased naupliar death rates in the populations of epigean copepod species [58]. For instance, van den Bosch and Santer [60] observed that the adult females of the epigean cyclopoid species Cyclops abyssorum abyssorum Sars G.O., 1863 cannibalize their nauplii, which represent up to 45% of the female dry weight. However, we did not collect naupliar stages earlier than NV/NVI (likely because the 60 µ-mesh net did not retain them), so we cannot exclude that adults of Moraria sp. cannibalize naupliar stages earlier than NV-NVI.
On two occasions only, we found two couples of Moraria sp. where the male was grasping the setae of both caudal rami of the female. We observed the couples for 15 min, but we did not observe any copulation (i.e., the male rolling its abdomen and pressing it to the female’s ventral side for spermatophore transfer to the copulatory pore of the female) as it happens for other stygobitic harpacticoid species, such as Proserpinocaris phyllura (Kiefer, 1938) [61]. The females of Moraria sp. in our cohort did not produce egg sacs, nor did we find any ovigerous females in four years of drip water sampling. Similarly, no females bearing egg sacs have ever been found for the stygobitic harpacticoid species Pseudectinosoma janineae Galassi, Dole-Olivier & De Laurentiis, 1999, Nitocrellopsis rouchi Galassi, De Laurentiis & Dole-Olivier, 1999 and Nitocrella pescei Galassi & De Laurentiis, 1997 [20]. Therefore, the most probable hypothesis is that the females of these species release their eggs one at a time freely in the water and do not retain them in any sac, as it also happens for the species of some calanoid genera such as Limnocalanus and Senecella [62]. Therefore, we might suppose that Moraria sp. has the same behavior as these species. However, we should also assume that if the females of our cohort released the eggs one at a time freely in the container, they also cannibalized the nauplii as soon as they hatched because we never found any of them. In our study, the cohort individuals did not show any coprophagous behavior. Rütz et al. [7] also observed the absence of coprophagy in the laboratory cohorts of Niphargus aquilex Schiödte, 1855. The traits observed in this study (absence of coprophagy or cannibalistic behavior, the fact that the individuals did not eat their exuviae, the long and successful survival in the laboratory, and the regular ontogenetic development of the juvenile stages) indicated that the nutritional conditions created in the laboratory were adequate. Therefore, we conclude that our respirometry trials were not affected by a bias due to nutritional stress.
4.2. Oxygen Consumption Rates
This is the first study on oxygen consumption rates of a stygobitic harpacticoid species. We measured the OCRs of Moriaria sp. by microsensor respirometry, which allowed individual, continual, and non-invasive measurements of the oxygen consumption of individuals with a considerably small body mass (average dry weight = 1.4 μg). Ikeda [63] and Ikeda et al. [64] ran a metanalysis of the OCRs of several marine copepods worldwide from the Arctic to the Antarctic and boreal, subtropical, and tropical regions. They concluded that body weight and environmental temperature explained >90% of the OCR variance, where body weight explained the most. In particular, Ikeda et al. [64] examined the OCRs of 35 marine copepod species with body weights in the range of 39 μg to 3.9 mg, observing an allometric relation between OCRs and body weight with an increase factor of approximately 0.8. The OCR values of Moraria sp. do not fit this model. The average OCR of Moraria sp. at 8.0 °C is 2.2 ng O2/ind × h only. This value is much lower than the OCRs of the 35 species analyzed by Ikeda et al. [64]. However, the female individuals of Moraria sp. had an average dry weight that fell outside the weight range (39 μg to 3.9 mg) examined by Ikeda [63] and Ikeda et al. [64]. More importantly, the low OCRs of Moraria sp. could be related to low metabolism, which seems to be one of the most distinctive traits of stygobitic species [3]. Low metabolic rates could be an adaptive trait to the conditions of low energy and oxygen availability in groundwater, which are typical of many subterranean environments, caves, in particular [4,5]. Comparative studies have shown that stygobitic crustaceans (which live in environments where the trophic resource is allochthonous, scarce, and discontinuous [3]) show significantly lower OCRs than their surface water relatives living in energy-rich environments [4,5,65,66].
Groundwater environments also show substantial thermal inertia with consequent low variation in the annual average temperature [3]. We might assume that species residing in such environments should exhibit physiological adaptations to thermal stability, being significantly affected by temperature variations [3]. They might, for instance, increase their OCRs with temperature, but not be able to compensate by resetting them to their initial values after an acclimation period, as epigean species usually do. For example, the intertidal harpacticoid species Tigriopus californicus (Baker, 1912), which inhabits an environment where water temperature changes by 5 °C in 30 min, does not significantly vary its OCRs with changing temperatures; this indicates a rapid metabolic compensation [67]. On the contrary, we observed that the OCRs of Moraria sp. varied significantly with increasing temperatures, and the values remained altered even after 15 days of acclimation. The standard deviations of the OCRs, especially those measured at 11.0 and 12.5 °C, reflected a high within-subject variability. Some individual variability is expected in experiment set-ups such as that used in this study, where the variation is mainly represented by genetic differences between individuals of the same species [68]. In addition, high data variability in individual OCRs has already been assessed in previous studies [66,69] and especially when animals are under stress [65,70]. Overall, OCR variation with temperature indicated that Moraria sp. cannot rapidly acclimate to changing thermal conditions. Pallarées et al. [18] assumed that a “loss of heat acclimation capacity” could likely be an adaptive trait of stygobitic and troglobitic fauna. Accordingly, Beasley-Hall et al. [71] observed that a subterranean species of dytiscid, Paroster macrosturtensis (Watts & Humphreys), is poorly equipped to respond to thermal stress because it mounts a weaker (in terms of expressed genes) response to heat shock than its epigean relative species confined to the same aquifer. Lack of acclimation capacity was also found in some polar fishes inhabiting highly thermal stable environments [72]. Elevated OCRs at temperatures higher than that of the collection site entail elevated metabolic costs and energy (food) requirements [73,74]. However, despite the increased energy requirement at temperatures >8 °C and one week of fasting (acclimation in remineralized Milli-Q water), all females of Moraria sp. remained alive until the end of the trials and beyond. This indicates that the adult females of Moraria sp. could not compensate their OCRs for increasing temperatures, in 2 weeks at least. Nevertheless, they showed remarkable resistance, remaining alive for the duration of the experiments and beyond. The stygobitic amphipod species Niphargus rhenorhodanensis Schellenberg, 1937 exhibits 100% survival at a thermal change of 4 °C compared to the temperature of its collection site [16,75]. Conversely, Proasellus lusitanicus (Frade, 1938), a stygobitic isopod endemic to the Estremanho Massif in Portugal, cannot compensate its OCRs at temperatures >18 °C (average annual temperature of the collection site), at least in 2 days, and its survival decreases at temperatures 1 °C higher than 18 °C [5]. The same phenomenon is observed for the stygobitic amphipod species Niphargus virei Chevreux, 1896 [16,75]. It is unknown why some stygobitic species seem more resistant to thermal change than others. In crustaceans, thermal acclimation may induce several physiological changes in hemolymph [76] and enzyme properties [16], gene expression [17], or hemoglobin affinity [77], which confer protection against potential injuries induced by temperature changes [78]. We speculate that such physiological protection might be more effective in some stygobitic species (such as Moraria sp. and N. rhenorhodanensis) than in others. However, further physiological studies are needed to shed light on this subject.
5. Conclusions
In this study, we demonstrated that Moraria sp., a narrow endemic species restricted to the Corchia Cave, is a stenothermal species showing remarkable stygobitic traits such as a long life span. We noted that the metabolism of this species is significantly affected by small (+1.5 °C) thermal changes. We also demonstrated that no metabolic compensation occurs in this species over 2 weeks of exposure to temperatures higher than 8 °C. The results suggest that Moraria sp. may not be able to tolerate thermal changes brought on by climate change. However, future studies are required to understand the underlying mechanisms of compensation in stygobitic animals. Our findings suggest that the timing of metabolic measures after a temperature shift is important to understand animal acclimation and compensation mechanisms, especially in those species that reside in habitats with low levels of natural thermal variability. Further metabolic studies concerning stygobitic species could be extremely valuable for understanding mechanisms of temperature acclimation and the impact of climate change in caves.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w15071356/s1, Table S1: oxygen consumption rates in ng O2/mg DW × h of female individuals of Moraria sp. at four acclimation temperatures. T: temperature in °C; L: body length in mm; W: body width in mm; BV: biovolume in nL; FW: fresh weight in μg; DW: dry weight in μg. ID indicates the individuals of Moraria sp. used in this study; Table S2: PERMANOVA results based on Euclidean distances of oxygen consumption rates ng O2/mg DW × h of female individuals of Moraria sp. at four acclimation temperatures (T: 8.0, 9.5, 11.0, 12.5 °C). Video S1: Moraria sp. mating individuals with the male attached to the female; Video S2: three individuals of Moraria sp. quickly digging in a fine-sediments layer (burrowing).
Author Contributions
Conceptualization, T.D.L. and L.P.; methodology, T.D.L.; software, T.D.L.; validation, D.M.P.G. and S.I.; formal analysis, T.D.L.; investigation, L.P.; resources, T.D.L., L.P. and S.I.; data curation, A.T.D.C.; writing—original draft preparation, T.D.L. and L.P.; writing—review and editing, M.M.P., A.T.D.C., S.I. and D.M.P.G.; project administration, T.D.L. and S.I.; funding acquisition, T.D.L., D.M.P.G. and S.I. All authors have read and agreed to the published version of the manuscript.
Funding
T.L.D. acknowledges support from National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4-Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of the Italian Ministry of University and Research funded by the European Union–NextGenerationEU (Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP B83C22002930006, Project title “National Biodiversity Future Center-NBFC”). T.D.L., A.T.D.C., and D.M.P.G. acknowledge support from the Biodiversa+ 2021–2022 program (project DarCo; BIODIV21_0006). S.I. and M.M.P. were supported by a grant from the Romanian Ministry of Education and Research, CNCS-UEFISCDI (project number: PN-III-P4-PCE-2020-2843; title: Evo-Devo-Cave), within PNCDI III.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
We are grateful to Coop. Sviluppo e Futuro Levigliani, Soc. Coop. A.r.l., Via Della Chiesa, 8 55040 STAZZEMA (LU), for allowing free access to the cave.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mammola, S.; Amorim, I.R.; Bichuette, M.E.; Borges, P.A.; Cheeptham, N.; Cooper, S.J.B.; Culver, D.C.; Deharveng, L.; Eme, D.; Ferreira, R.L.; et al. Fundamental research questions in subterranean biology. Biol. Rev. 2020, 95, 1855–1872. [Google Scholar] [CrossRef] [PubMed]
- Mammola, S.; Lunghi, E.; Bilandžija, H.; Cardoso, P.; Grimm, V.; Schmidt, S.I.; Hesselberg, T.; Martínez, A. Collecting eco-evolutionary data in the dark: Impediments to subterranean research and how to overcome them. Ecol. Evol. 2021, 11, 5911–5926. [Google Scholar] [CrossRef] [PubMed]
- Hose, G.C.; Chariton, A.A.; Daam, M.A.; Di Lorenzo, T.; Galassi, D.M.P.; Halse, S.A.; Reboleira, A.S.P.; Robertson, A.L.; Schmidt, S.I.; Korbel, K.L. Invertebrate traits, diversity and the vulnerability of groundwater ecosystems. Funct. Ecol. 2022, 36, 2200–2214. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Di Marzio, W.D.; Spigoli, D.; Baratti, M.; Messana, G.; Cannicci, S.; Galassi, D.M.P. Metabolic rates of a hypogean and an epigean species of copepod in an alluvial aquifer. Freshw. Biol. 2015, 60, 426–435. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Reboleira, A.S.P. Thermal acclimation and metabolic scaling of a groundwater asellid in the climate change scenario. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Carpenter, J.H. Forty-year natural history study of Bahalana geracei Carpenter, 1981, an anchialine cave-dwelling isopod (Crustacea, Isopoda, Cirolanidae) from San Salvador Island, Bahamas: Reproduction, growth, longevity, and population structure. Subterran. Biol. 2021, 37, 105–156. [Google Scholar] [CrossRef]
- Rütz, N.K.; Marxsen, J.; Wolters, V. Long-term cultivation of the groundwater amphipod Niphargus aquilex (Crustacea). Hydrobiologia 2023, 850, 269–281. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef]
- Fossen, E.I.F.; Pélabon, C.; Einum, S. Genetic and environmental effects on the scaling of metabolic rate with body size. J. Exp. Biol. 2019, 222, jeb193243. [Google Scholar] [CrossRef]
- Shokri, M.; Cozzoli, F.; Vignes, F.; Bertoli, M.; Pizzul, E.; Basset, A. Metabolic rate and climate change across latitudes: Evidence of mass-dependent responses in aquatic amphipods. J. Exp. Biol. 2022, 225, jeb244842. [Google Scholar] [CrossRef]
- Willmer, P.; Stone, G.; Johnston, I. Environmental Physiology of Animals, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–768. [Google Scholar]
- Huey, R.B.; Kingsolver, J.G. Evolution of thermal sensitivity of ectotherm performance. Trends Ecol. Evol. 1989, 4, 131–135. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F.; Lefour, C.; Lalouette, L.; Renault, D.; Malard, F.; Simon, L.; Douady, C.J. Thermal tolerance breadths among groundwater crustaceans living in a thermally constant environment. J. Exp. Biol. 2013, 216, 1683–1694. [Google Scholar] [CrossRef]
- Peck, L.S.; Morley, S.A.; Richard, J.; Clark, M.S. Acclimation and thermal tolerance in Antarctic marine ectotherms. J. Exp. Biol. 2014, 217, 16–22. [Google Scholar] [CrossRef]
- Iepure, S. Ice cave fauna. In Ice Caves, 1st ed.; Perşoiu, A., Lauritzen, A.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–171. [Google Scholar] [CrossRef]
- Issartel, J.; Hervant, F.; Voituron, Y.; Renault, D.; Vernon, P. Behavioural, ventilatory and respiratory responses of epigean and hypogean crustaceans to different temperatures. Comp. Biochem. Physiol. Mol. Amp Integr. Physiol. 2005, 141, 1–7. [Google Scholar] [CrossRef]
- Colson-Proch, C.; Morales, A.; Hervant, F.; Konecny, L.; Moulin, C.; Douady, C.J. First cellular approach of the effects of global warming on groundwater organisms: A study of the HSP70 gene expression. Cell Stress Chaperones 2010, 15, 259–270. [Google Scholar] [CrossRef]
- Pallarés, S.; Colado, R.; Botella-Cruz, M.; Montes, A.; Balart-García, P.; Bilton, D.T.; Millán, A.; Ribera, I.; Sánchez-Fernández, D. Loss of heat acclimation capacity could leave subterranean specialists highly sensitive to climate change. Anim. Conserv. 2021, 24, 482–490. [Google Scholar] [CrossRef]
- Deharveng, L.; Stoch, F.; Gibert, J.; Bedos, A.; Galassi, D.M.P.; Zagmajster, M.; Brancelj, A.; Camacho, A.; Fiers, F.; Martin, P.; et al. Groundwater biodiversity in Europe. Freshw. Biol. 2009, 54, 709–726. [Google Scholar] [CrossRef]
- Galassi, D.M.P. Groundwater copepods: Diversity patterns over ecological and evolutionary scales. Hydrobiologia 2001, 453, 227–253. [Google Scholar] [CrossRef]
- Galassi, D.M.P.; Huys, R.; Reid, J.W. Diversity, ecology and evolution of groundwater copepods. Freshw. Biol. 2009, 54, 691–708. [Google Scholar] [CrossRef]
- Ikeda, T. Metabolism in mesopelagic and bathypelagic copepods: Reply to Childress et al. (2008). Mar. Ecol. Prog. Ser. 2008, 373, 193–198. [Google Scholar] [CrossRef]
- Dole-Olivier, M.J.; Galassi, D.M.P.; Marmonier, P.; Creuzé des Châtelliers, M. The biology and ecology of lotic microcrustaceans. Freshw. Biol. 2000, 44, 63–91. [Google Scholar] [CrossRef]
- Fattorini, S.; Borges, P.A.V.; Fiasca, B.; Galassi, D.M.P. Trapped in the web of water: Groundwater-fed springs are island-like ecosystems for the meiofauna. Ecol. Evol. 2006, 6, 8389–8401. [Google Scholar] [CrossRef] [PubMed]
- Pleşa, C. Recherches sur la périodicité de reproduction chez les cavernicoles. Spelunca Mémoires 1967, 5, 295–299. [Google Scholar]
- Pleşa, C. Cercetări asupra periodicităţii reproductive la unele crustacee cavernicole troglobionte. In Résumé de Thése de Doctorat en Biologie; Inst. Biologie “Tr. Săvulescu”: București, Romania, 1969; p. 30. [Google Scholar]
- Lescher-Moutoue, F. Sur la biologie et l’écologie des copépodes cyclopides hypogés (Crustacés). Ann. Spéliol. 1973, 28, 429–502. [Google Scholar]
- Notenboom, J.; Boessenkool, J.J. Toxicity of Selected Pesticides to the Groundwater Copepod Parastenocaris germanica (Crustacea); RIVM Rapport 710302005; RIVM: Bilthoven, The Nederlands, 1994. [Google Scholar]
- Baneschi, I.; Piccini, L.; Regattieri, E.; Isola, I.; Guidi, M.; Lotti, L.; Mantelli, F.; Menichetti, M.; Drysdale, R.N.; Zanchetta, G. Hypogean microclimatology and hydrology of the 800–900 m asl in the Monte Corchia Cave (Tuscany, Italy): Preliminary considerations and implications for paleoclimatological studies. Acta Carsologica 2011, 40, 175–187. [Google Scholar] [CrossRef]
- Piccini, L.; Zanchetta, G.; Drysdale, R.N.; Hellstrom, J.; Isola, I.; Fallick, A.E.; Leone, G.; Doveri, M.; Mussi, M.; Mantelli, F.; et al. The environmental features of the Monte Corchia cave system (Apuan Alps, central Italy) and their effects on speleothem growth. Int. J. Speleol. 2008, 37, 153–172. [Google Scholar] [CrossRef]
- Doveri, M.; Piccini, L.; Menichini, M. Hydrodynamic and geochemical features of metamorphic carbonate aquifers and implications for water management: The Apuan Alps (NW Tuscany, Italy) case study. In The Handbook of Environmental Chemistry; Younos, T., Schreiber, M., Kosič Ficco, K., Eds.; Springer International Publishing: New York, NY, USA, 2018; Volume 68, pp. 209–249. [Google Scholar] [CrossRef]
- Piccini, L. Speleogenesis in highly geodynamic contexts: The quaternary evolution of Monte Corchia multi-level karst system (Alpi Apuane, Italy). Geomorphology 2011, 134, 49–61. [Google Scholar] [CrossRef]
- Piccini, L.; Mantelli, F.; Montigiani, A.; Cecconi, E.; Lotti, L. Idrogeologia del sistema carsico del Monte Corchia: Sintesi dei dati e delle attuali conoscenze. Acta Apuana 2015, 2012, 23–32. [Google Scholar]
- Mantelli, F.; Piccini, L.; Scala, C.; Menichetti, S.; Lotti, L.; Montigiani, A.; De Sio, F.; Occhini, F. Antro Del Corchia-1997–2017 20 Anni Di Monitoraggio e Ricerche; ARPAT: Florence, Italy, 2021; ISBN 978-88-96693-27-8. Available online: https://www.arpat.toscana.it/documentazione/catalogo-pubblicazioni-arpat/antro-del-corchia-1997-2017 (accessed on 21 February 2023).
- Pipan, T. Epikarst—A promising habitat: Copepod fauna, its diversity and ecology: A case study from Slovenia (Europe). In Carsologica, 1st ed.; Gabrovšek, F., Ed.; Založba ZRC: Ljubljana, Slovenia, 2005; Volume 5. [Google Scholar] [CrossRef]
- Pipan, T.; Culver, D.C. Estimating biodiversity in the epikarstic zone of a West Virginia cave. J. Cave Karst. Stud. 2005, 67, 103–1091. [Google Scholar]
- Pipan, T.; Culver, D.C. Regional species richness in an obligate subterranean dwelling fauna–epikarst copepods. J. Biogeogr. 2007, 34, 854–861. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Di Marzio, W.D.; Fiasca, B.; Galassi, D.M.P.; Korbel, K.; Iepure, S.; Hose, G.C. Recommendations for ecotoxicity testing with stygobiotic species in the framework of groundwater environmental risk assessment. Sci. Total Environ. 2019, 681, 292–304. [Google Scholar] [CrossRef]
- Taylor, C.A.; Stefan, H.G. Shallow groundwater temperature response to climate change and urbanization. J. Hydrol. 2009, 375, 601–612. [Google Scholar] [CrossRef]
- Bovolo, C.I.; Parkin, G.; Sophocleous, M. Groundwater Resources, Climate and Vulnerability. Environ. Res. Lett. 2009, 4, 035001. [Google Scholar] [CrossRef]
- Menberg, K.; Blum, P.; Kurylyk, B.L.; Bayer, P. Observed groundwater temperature response to recent climate change. Hydrol. Earth Syst. Sci. 2014, 18, 4453–4466. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Galassi, D.M.P. Effect of temperature rising on the stygobitic crustacean species Diacyclops belgicus: Does global warming affect groundwater populations? Water 2017, 9, 951. [Google Scholar] [CrossRef]
- Cifoni, M.; Galassi, D.M.P.; Faraloni, C.; Di Lorenzo, T. Test procedures for measuring the (sub) chronic effects of chemicals on the freshwater cyclopoid Eucyclops serrulatus. Chemosphere 2017, 173, 89–98. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Feller, R.J.; Warwick, R.M. Energetics. In Introduction to the Study of Meiofauna, 1st ed.; Higgins, R.P., Thiel, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 181–196. [Google Scholar]
- Reiss, J.; Schmid-Araya, J.M. Existing in plenty: Abundance, biomass and diversity of ciliates and meiofauna in small streams. Freshw. Biol. 2008, 53, 652–668. [Google Scholar] [CrossRef]
- Reiss, J.; Schmid-Araya, J.M. Life history allometries and production of small fauna. Ecology 2010, 91, 497–507. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2015. [Google Scholar]
- Galassi, D.M.P.; Fiasca, B.; Di Lorenzo, T.; Montanari, A.; Porfirio, S.; Fattorini, S. Groundwater biodiversity in a chemoautotrophic cave ecosystem: How geochemistry regulates microcrustacean community structure. Aquat. Ecol. 2017, 51, 75–90. [Google Scholar] [CrossRef]
- Dickson, G.W. The Importance of Cave Mud Sediments in Food Preference, Growth and Mortality of the Troglobitic Amphipod Crustacean Crangonyx antennatus Packard (Crangonyctidae). Crustaceana 1979, 36, 129–140. [Google Scholar] [CrossRef]
- Giere, O. Meiobenthology: The Microscopic Motile Fauna of Aquatic Sediments, 1st ed.; Springer Science: Berlin/Heidelberg, Germany, 2009; pp. 1–527. [Google Scholar]
- Di Lorenzo, T.; Di Cicco, M.; Di Censo, D.; Galante, A.; Boscaro, F.; Messana, G.; Galassi, D.M.P. Environmental risk assessment of propranolol in the groundwater bodies of Europe. Environ. Pollut. 2019, 255, 113189. [Google Scholar] [CrossRef] [PubMed]
- Sarvala, J. A parthenogenetic life cycle in a population of Canthocamptus staphylinus (Copepoda, Harpacticoida). Hydrobiologia 1979, 62, 113–129. [Google Scholar] [CrossRef]
- Rouch, R. Sur l’ecologie des eaux souterraines dans la karst. Stygologia 1986, 2, 352–398. [Google Scholar]
- Glatzel, T. On the biology of Parastenocaris phyllura Kiefer (Copepoda, Harpacticoida). Stygologia 1990, 5, 131–136. [Google Scholar]
- Di Lorenzo, T.; Cifoni, M.; Baratti, M.; Pieraccini, G.; Di Marzio, W.D.; Galassi, D.M.P. Four scenarios of environmental risk of diclofenac in European groundwater ecosystems. Environ. Pollut. 2021, 287, 117315. [Google Scholar] [CrossRef]
- Suárez-Morales, E. Class Maxillopoda. In Thorp and Covich’s Freshwater Invertebrates, 1st ed.; Thorp, H., Covich, A.P., Eds.; Academic Press: New York, NY, USA, 2015; pp. 709–755. [Google Scholar]
- Elgmork, K.; Eie, J.A. Two-and three-year life cycles in the planktonic copepod Cyclops scutifer in two high mountain lakes. Ecography 1989, 12, 60–69. [Google Scholar] [CrossRef]
- Van den Bosch, F.; Santer, B. Cannibalism in Cyclops abyssorum. Oikos 1993, 67, 19–28. [Google Scholar] [CrossRef]
- Glatzel, T.; Schminke, H.K. Mating behaviour of the groundwater copepod Parastenocaris phyllura Kiefer, 1938 (Copepoda: Harpacticoida). Bijdr. Dierkd. 1996, 66, 103–108. [Google Scholar] [CrossRef]
- Reid, J.W.; Williamson, C.E. Copepoda. In Ecology and Classification of North American Freshwater Invertebrates, 2nd ed.; Thorp, H., Covich, A.P., Eds.; Academic Press: New York, NY, USA, 2010; pp. 829–899. [Google Scholar]
- Ikeda, T. Metabolic rates of epipelagic marine zooplankton as a function of body mass and temperature. Mar. Biol. 1985, 85, 1–11. [Google Scholar] [CrossRef]
- Ikeda, T.; Kanno, Y.; Ozaki, K.; Shinada, A. Metabolic rates of epipelagic marine copepods as a function of body mass and temperature. Mar. Biol. 2001, 139, 587–596. [Google Scholar] [CrossRef]
- Hervant, F.; Mathieu, J.; Barré, H.; Simon, K.; Pinon, C. Comparative study on the behavioral, ventilatory, and respiratory responses of hypogean and epigean crustaceans to long-term starvation and subsequent feeding. Comp. Biochem. Physiol. Mol. Amp Integr. Physiol. 1997, 118, 1277–1283. [Google Scholar] [CrossRef]
- Wilhelm, F.M.; Taylor, S.J.; Adams, G.L. Comparison of routine metabolic rates of the stygobite, Gammarus acherondytes (Amphipoda: Gammaridae) and the stygophile, Gammarus troglophilus. Freshw. Biol. 2006, 51, 1162–1174. [Google Scholar] [CrossRef]
- Scheffler, M.L.; Barreto, F.S.; Mueller, C.A. Rapid metabolic compensation in response to temperature change in the intertidal copepod, Tigriopus californicus. Comp. Biochem. Physiol. Mol. Amp Integr. Physiol. 2019, 230, 131–137. [Google Scholar] [CrossRef]
- Olkova, A. Intraspecific sensitivity to toxicants—A methodological problem of bioassay. J. Ecol. Eng. 2021, 22, 113–122. [Google Scholar] [CrossRef]
- Culver, D.C.; Poulson, T.L. Oxygen consumption and activity in closely related amphipod populations from cave and surface habitats. Am. Midl. Nat. 1971, 85, 74–84. [Google Scholar] [CrossRef]
- Hervant, F.; Mathieu, J.; Messana, G. Oxygen consumption and ventilation in declining oxygen tension and posthypoxic recovery in epigean and hypogean crustaceans. J. Crustac. Biol. 1998, 18, 717–727. [Google Scholar] [CrossRef]
- Beasley-Hall, P.G.; Bertozzi, T.; Bradford, T.M.; Foster, C.S.P.; Jones, K.; Tierney, S.M.; Humphreys, W.F.; Austin, A.D.; Cooper, S.J.B. Differential transcriptomic responses to heat stress in surface and subterranean diving beetles. Sci. Rep. 2022, 12, 16194. [Google Scholar] [CrossRef]
- Wilson, R.; Franklin, C.; Davison, W.; Kraft, P. Stenotherms at sub-zero temperatures: Thermal dependence of swimming performance in Antarctic fish. J. Comp. Physiol. 2001, 171, 263–269. [Google Scholar] [CrossRef]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Peck, L.S.; Conway, L.Z. The myth of metabolic cold adaptation: Oxygen consumption in stenothermal Antarctic bivalves. Geol. Soc. Spec. Publ. 2000, 177, 441–450. [Google Scholar] [CrossRef]
- Issartel, J.; Voituron, Y.; Hervant, F. Impact of temperature on the survival, the activity and the metabolism of the cave-dwelling Niphargus virei, the ubiquitous stygobiotic N. rhenorhodanensis and the surface-dwelling Gammarus fossarum (Crustacea, Amphipoda). Subterr. Bio. 2007, 5, 9–14. [Google Scholar]
- Tanaka, K.; Udagawa, T. Cold adaptation of the terrestrial isopod, Porcellio scaber, to subnivean environments. J. Comp. Physiol. 1993, 163, 439–444. [Google Scholar]
- Paul, R.J.; Zeis, B.; Lamkemeyer, T.; Seidl, M.; Pirow, R. Control of oxygen transport in the microcrustacean Daphnia: Regulation of haemoglobin expression as central mechanism of adaptation to different oxygen and temperature conditions. Acta Physiol. Scand. 2004, 182, 259–275. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution, 1st ed.; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).