Multi-Analytical Approach to Evaluate Elements and Chemical Alterations in Pteris vittata Plants Exposed to Arsenic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth and Sample Preparation
2.2. The μ-XRF Device
2.3. The HSI Device
2.4. The FTIR Device
2.5. Principal Component Analysis (PCA) and Partial Least Square-Discriminant Analysis (PLS-DA)
2.6. Statistical and Spectral Analysis
3. Results and Discussion
3.1. Results by µ-XRF Analyses
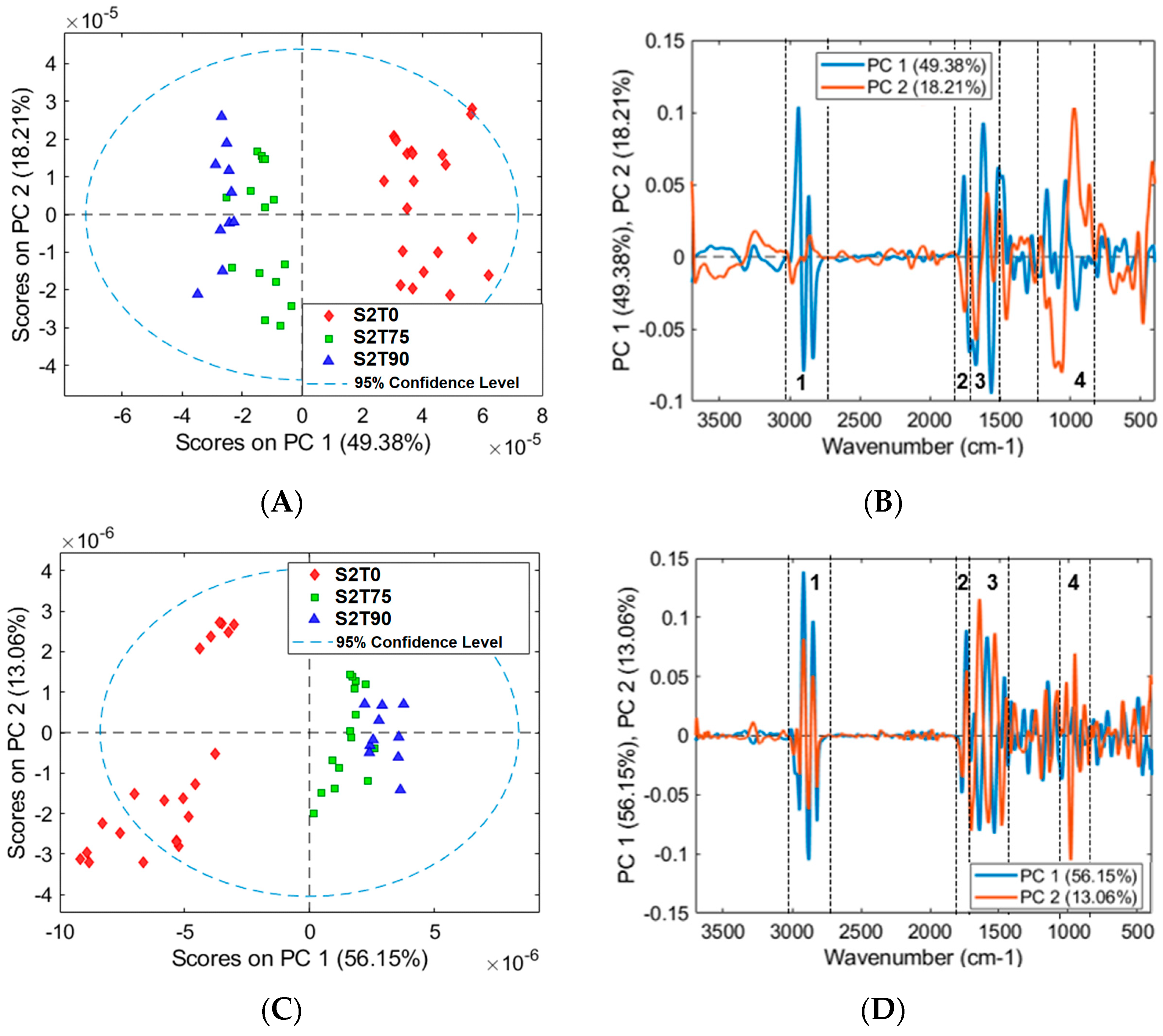
3.2. Mean Spectra and PCA Models of Samples for FTIR Analysis
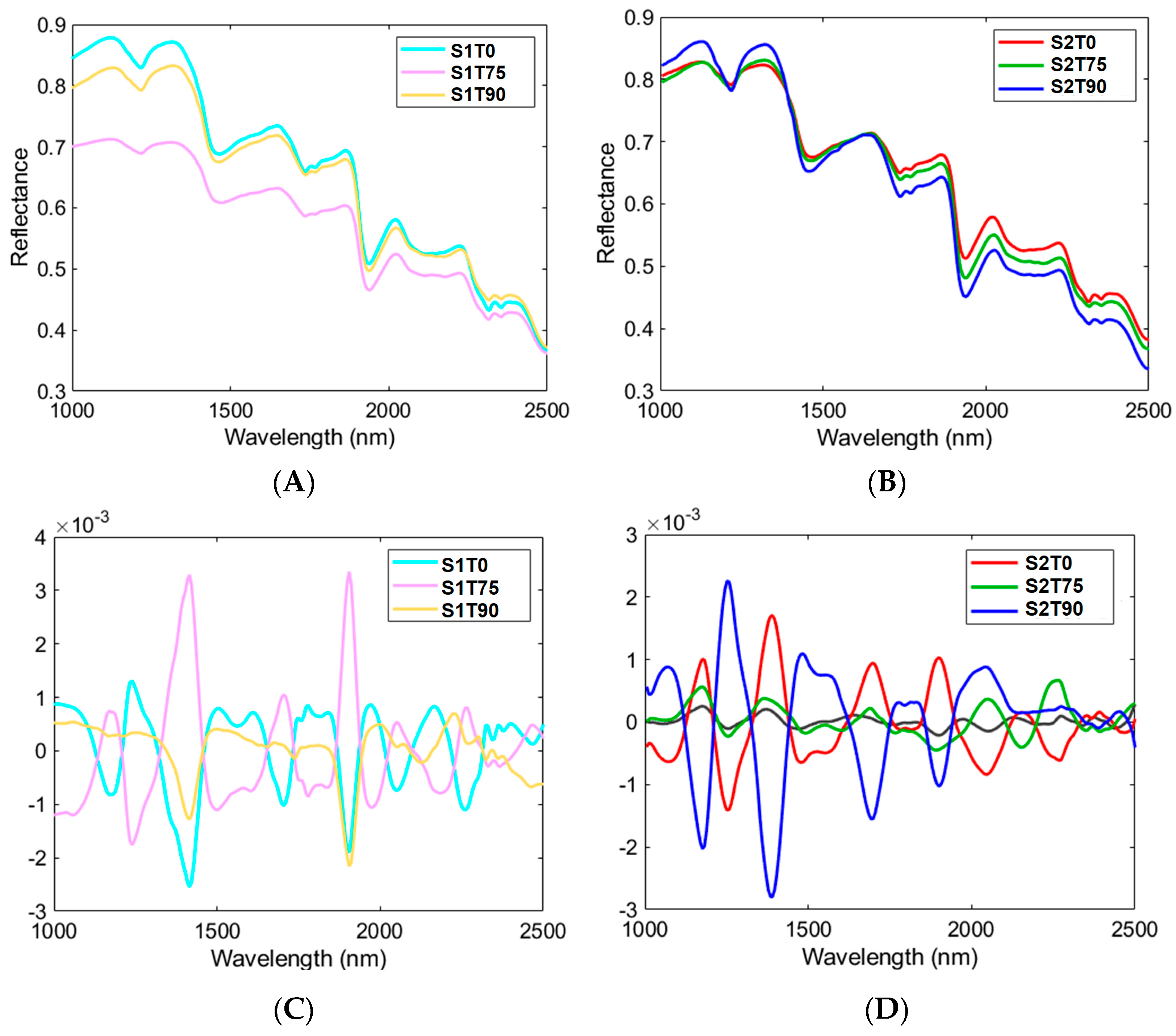
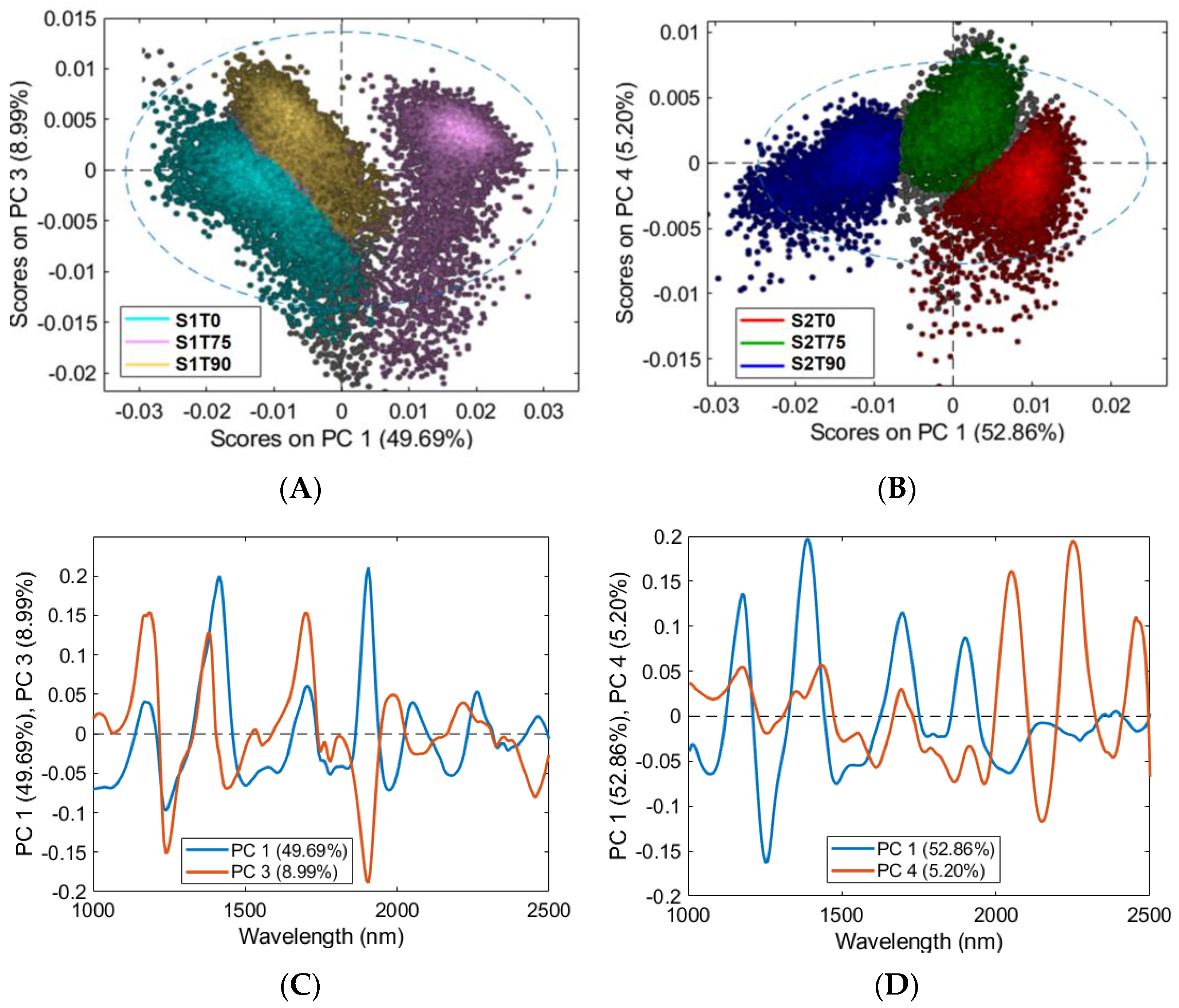
3.3. HSI Mean Spectra and PCA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cluis, C. Junk-greedy greens: Phytoremediation as a new option for soil decontamination. BioTeach J. 2004, 2, 61–67. [Google Scholar]
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal-contaminated land. A review. Environ. Chem. Lett. 2010, 8, 1–17. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef]
- Chen, T.; Wei, C.; Huang, Z.; Huang, Q.; Lu, Q.; Fan, Z. Arsenic hyperaccumulator Pteris vittata L. and its arsenic accumulation. Chin. Sci. Bull. 2002, 47, 902–905. [Google Scholar] [CrossRef]
- Antenozio, M.L.; Capobianco, G.; Costantino, P.; Vamerali, T.; Bonifazi, G.; Serranti, S.; Brunetti, P.; Cardarelli, M. Arsenic accumulation in Pteris vittata: Time course, distribution, and arsenic-related gene expression in fronds and whole plantlets. Environ. Pollut. 2022, 309, 119773. [Google Scholar] [CrossRef]
- Pickering, I.J.; Gumaelius, L.; Harris, H.H.; Prince, R.C.; Hirsch, G.; Banks, J.A.; Salt, D.E.; George, G.N. Localizing the biochemical transformations of arsenate in a hyperaccumulating fern. Environ. Sci. Technol. 2006, 40, 5010–5014. [Google Scholar] [CrossRef]
- van der Ent, A.; de Jonge, M.D.; Spiers, K.M.; Brueckner, D.; Montargès-Pelletier, E.; Echevarria, G.; Wan, X.M.; Lei, M.; Mak, R.; Lovett, J.H.; et al. Confocal Volumetric μXRF and Fluorescence Computed μ-Tomography Reveals Arsenic ThreeDimensional Distribution within Intact Pteris vittata Fronds. Environ. Sci. Technol. 2020, 54, 745–757. [Google Scholar] [CrossRef]
- Lahlali, R.; Karunakaran, C.; Wang, L.; Willick, I.; Schmidt, M.; Liu, X.; Borondics, F.; Forseille, L.; Fobert, P.R.; Tanino, K.; et al. Synchrotron based phase contrast X-ray imaging combined with FTIR spectroscopy reveals structural and biomolecular differences in spikelets play a significant role in resistance to Fusarium in wheat. BMC Plant Biol. 2015, 15, 24. [Google Scholar] [CrossRef] [Green Version]
- Comino, F.; Ayora-Cañada, M.J.; Aranda, V.; Díaz, A.; Domínguez-Vidal, A. Near-infrared spectroscopy and X-ray fluorescence data fusion for olive leaf analysis and crop nutritional status determination. Talanta 2018, 188, 676–684. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Natasha Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Ma, L.Q.; Srivastava, M.; Rathinasabapathi, B. Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci. 2006, 170, 274–282. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gill, R.A.; Ali, B.; Wang, J.; Islam, F.; Ali, S.; Zhou, W. Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology 2016, 25, 350–366. [Google Scholar] [CrossRef]
- Yan, X.L.; Lin, L.Y.; Liao, X.Y.; Zhang, W.B. Arsenic accumulation and resistance mechanism in Panax notoginseng, a traditional rare medicinal herb. Chemosphere 2012, 87, 31–36. [Google Scholar] [CrossRef]
- Leung, H.M.; Wu, F.Y.; Cheung, K.C.; Ye, Z.H.; Wong, M.H. Synergistic effects of arbuscular mycorrhizal fungi and phosphate rock on heavy metal uptake and accumulation by an arsenic hyperaccumulator. J. Hazard. Mater. 2010, 181, 497–507. [Google Scholar] [CrossRef]
- Chen, T.; Yan, X.; Liao, X.; Xiao, X.; Huang, Z.; Xie, H.; Zhai, L. Subcellular distribution and compartmentalization of arsenic in Pteris vittata L. Chin. Sci. Bull. 2005, 50, 2843–2849. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology & Development, 6th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2014. [Google Scholar]
- Huang, W.X.; Chen, X.W.; Wu, L.; Yu, Z.S.; Gao, M.Y.; Zhao, H.M.; Mo, C.H.; Li, Y.W.; Cai, Q.Y.; Wong, M.H.; et al. Root cell wall chemistry remodelling enhanced arsenic fixation of a cabbage cultivar. J. Hazard. Mater. 2021, 420, 126165. [Google Scholar] [CrossRef]
- Antenozio, M.L.; Giannelli, G.; Marabottini, R.; Brunetti, P.; Allevato, E.; Marzi, D.; Capobianco, G.; Bonifazi, G.; Serranti, S.; Visioli, G.; et al. Phytoextraction efficiency of Pteris vittata grown on a naturally As-rich soil and characterization of As-resistant rhizosphere bacteria. Sci. Rep. 2021, 11, 6794. [Google Scholar] [CrossRef]
- Marzi, D.; Antenozio, M.L.; Vernazzaro, S.; Sette, C.; Veschetti, E.; Lucentini, L.; Daniele, G.; Brunetti, P.; Cardarelli, M. Advanced Drinking Groundwater As Phytofiltration by the Hyperaccumulating Fern Pteris vittata. Water 2021, 13, 2187. [Google Scholar] [CrossRef]
- Capobianco, G.; Bonifazi, G.; Serranti, S.; Marabottini, R.; Antenozio, M.L.; Cardarelli, M.; Brunetti, P.; Stazi, S.R. A Green Approach Based on Micro-X-ray Fluorescence for Arsenic, Micro- and Macronutrients Detection in Pteris vittata. Water 2022, 14, 2202. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Ballabio, D.; Consonni, V. Classification tools in chemistry. Part 1: Linear models. PLS-DA. Anal. Methods 2013, 5, 3790–3798. [Google Scholar] [CrossRef]
- Rinnan, Å.; Berg, F.; Engelsen, S.B. Review of the most common preprocessing techniques for near-infrared spectra. TrAC-Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Grahn, H.; Geladi, P.; Burger, J.H. (Eds.) Techniques and Applications of Hyperspectral Image Analysis; John Wiley & Sons: West Sussex, UK, 2007; pp. 1–15. [Google Scholar]
- Amigo, J.M.; Martí, I.; Gowen, A. Hyperspectral imaging and chemometrics: A perfect combination for the analysis of food structure, composition and quality. In Data Handling in Science and Technology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 28, pp. 343–370. [Google Scholar]
- Vidal, M.; Amigo, J.M. Pre-processing of hyperspectral images. Essential steps before image analysis. Chemom. Intell. Lab. Syst. 2012, 117, 138–148. [Google Scholar] [CrossRef]
- Ribeiro da Luz, B. Attenuated total reflectance spectroscopy of plant leaves: A tool for ecological and botanical studies. New Phytol. 2006, 172, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Uttam, K.N. Nondestructive and Rapid Probing of Biochemical Response of Arsenic Stress on the Leaves of Wheat Seedlings Using Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy. Anal. Lett. 2019, 52, 268–287. [Google Scholar] [CrossRef]
- Giri, A.K.; Patel, R.K. Phytoaccumulation Potential and Toxicity of Arsenic Ions by Eichhornia Crassipes in Hydroponic System. J. Bioremediat. Biodegrad. 2012, 3, 137. [Google Scholar] [CrossRef] [Green Version]
- Topala, C.M.; Paunescu, A.; Soare, L.C. ATR—FTIR spectral analysis of ferns using as fingerprint for identification of fern species. Rev. Chim. 2019, 70, 875–880. [Google Scholar] [CrossRef]
- Garside, P.; Wyeth, P. Identification of Cellulosic Fibres by FTIR Spectroscopy—Thread and Single Fibre Analysis by Attenuated Total Reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Topală, C.M.; Rusea, I. Analysis of leaves using ftir spectroscopy and principal component analysis discrimination of different plant samples. Curr. Trends Nat. Sci. 2018, 7, 286–291. [Google Scholar]
- Baruah, S.; Borgohain, J.; Sarma, K.P. Phytoremediation of arsenic by Trapa natans in a hydroponic system. Water Env. Res. 2014, 86, 422–432. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef] [Green Version]
- Sturchio, E.; Napolitano, P.; Beni, C.; Mecozzi, M. Evaluation of arsenic effects in Vicia faba by FTIR and FTNIR spectroscopy. Glob. Nest J. 2012, 14, 86–92. [Google Scholar] [CrossRef]
- Thumanu, K.; Sompong, M.; Phansak, P.; Nontapot, K.; Buensanteai, N. Use of infrared microspectroscopy to determine leaf biochemical composition of cassava in response to Bacillus subtilis CaSUT007. J. Plant Interact. 2015, 10, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Kizewski, F.; Liu, Y.-T.; Morris, A.; Hesterberg, D. Spectroscopic Approaches for Phosphorus Speciation in Soils and Other Environmental Systems. J. Environ. Qual. 2011, 40, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.J. Ion exchange properties of roots and ionic interactions within the root apoplasm: Their role in ion accumulation by plants. Bot. Rev. 1980, 46, 75–99. [Google Scholar] [CrossRef]
- Allen, D.L.; Jarrell, W.M. Proton and copper adsorption to maize and soybean root cell walls. Plant Physiol. 1989, 89, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Nishizono, H.; Ichikawa, H.; Suziki, S.; Ishii, F. The role of the root cell wall in the heavy metal tolerance of Athyrium yokoscense. Plant Soil 1987, 101, 15–20. [Google Scholar] [CrossRef]
- Boominathan, R.; Doran, P.M. Organic acid complexation, heavy metal distribution and the effect of ATPase inhibition in hairy roots of hyperaccumulator plant species. J Biotechnol. 2003, 101, 131–146. [Google Scholar] [CrossRef]
- Osborne, B.G. Near-infrared spectroscopy in food analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists, Inc.: Paul, MN, USA, 1987. [Google Scholar]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Banwell, C.N. Fundamentals of Molecular Spectroscopy; McGrow Hill: Columbus, OH, USA, 1983. [Google Scholar]
- Barton, F.E.; Himmelsbach, D.S.; Duckworth, J.H.; Smith, M.J. Two-dimensional vibration spectroscopy: Correlation of mid-and near-infrared regions. Appl. Spectrosc. 1992, 46, 420–429. [Google Scholar] [CrossRef]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]



| S1T0 | ||||||||||
| Spectrum | Mg | Si | P | S | K | Ca | Mn | Fe | Zn | As |
| Mean value | 0.87 | 6.27 | 1.28 | 3.43 | 52.83 | 31.35 | 1.43 | 2.04 | 0.50 | 0 |
| Std. Abw. | 0.53 | 5.47 | 0.43 | 0.94 | 9.24 | 6.71 | 1.30 | 1.37 | 0.26 | 0 |
| Conf. interval | 0.06 | 0.58 | 0.04 | 0.10 | 0.97 | 0.71 | 0.14 | 0.14 | 0.03 | 0 |
| S2T0 | ||||||||||
| Spectrum | Mg | Si | P | S | K | Ca | Mn | Fe | Zn | As |
| Mean value | 0.99 | 6.12 | 1.49 | 3.26 | 56.59 | 27.48 | 1.83 | 1.83 | 0.42 | 0 |
| Std. Abw. | 0.47 | 5.17 | 0.56 | 0.78 | 7.74 | 5.96 | 0.84 | 1.11 | 0.16 | 0 |
| Conf. interval | 0.05 | 0.56 | 0.06 | 0.08 | 0.84 | 0.65 | 0.09 | 0.12 | 0.02 | 0 |
| S1T75 | ||||||||||
| Spectrum | Mg | Si | P | S | K | Ca | Mn | Fe | Zn | As |
| Mean value | 0.29 | 12.03 | 1.62 | 1.65 | 54.41 | 13.92 | 0.50 | 13.23 | 0.21 | 2.13 |
| Std. Abw. | 0.26 | 5.46 | 0.47 | 0.23 | 7.40 | 3.65 | 0.18 | 7.75 | 0.05 | 0.37 |
| Conf. interval | 0.04 | 0.92 | 0.08 | 0.04 | 1.25 | 0.62 | 0.03 | 1.31 | 0.01 | 0.06 |
| S2T75 | ||||||||||
| Spectrum | Mg | Si | P | S | K | Ca | Mn | Fe | Zn | As |
| Mean value | 1.12 | 9.60 | 1.11 | 1.66 | 45.75 | 11.51 | 0.20 | 0.40 | 0.09 | 28.56 |
| Std. Abw. | 0.33 | 3.37 | 0.24 | 0.61 | 5.80 | 6.91 | 0.07 | 0.72 | 0.05 | 5.39 |
| Conf. interval | 0.06 | 0.57 | 0.04 | 0.10 | 0.98 | 1.17 | 0.01 | 0.12 | 0.01 | 0.91 |
| S1T90 | ||||||||||
| Spectrum | Mg | Si | P | S | K | Ca | Mn | Fe | Zn | As |
| Mean value | 0.44 | 12.54 | 1.37 | 1.73 | 49.35 | 17.67 | 0.57 | 14.14 | 0.25 | 1.94 |
| Std. Abw. | 0.26 | 5.16 | 0.40 | 0.46 | 6.11 | 3.68 | 0.18 | 6.03 | 0.09 | 0.56 |
| Conf. interval | 0.04 | 0.87 | 0.07 | 0.08 | 1.03 | 0.62 | 0.03 | 1.02 | 0.01 | 0.09 |
| S2T90 | ||||||||||
| Spectrum | Mg | Si | P | S | K | Ca | Mn | Fe | Zn | As |
| Mean value | 1.26 | 12.26 | 0.90 | 1.86 | 37.96 | 13.33 | 0.27 | 2.43 | 0.11 | 29.62 |
| Std. Abw. | 0.37 | 3.68 | 0.23 | 0.34 | 5.12 | 3.09 | 0.10 | 2.85 | 0.03 | 6.32 |
| Conf. interval | 0.06 | 0.62 | 0.04 | 0.06 | 0.87 | 0.52 | 0.02 | 0.48 | 0.00 | 1.07 |
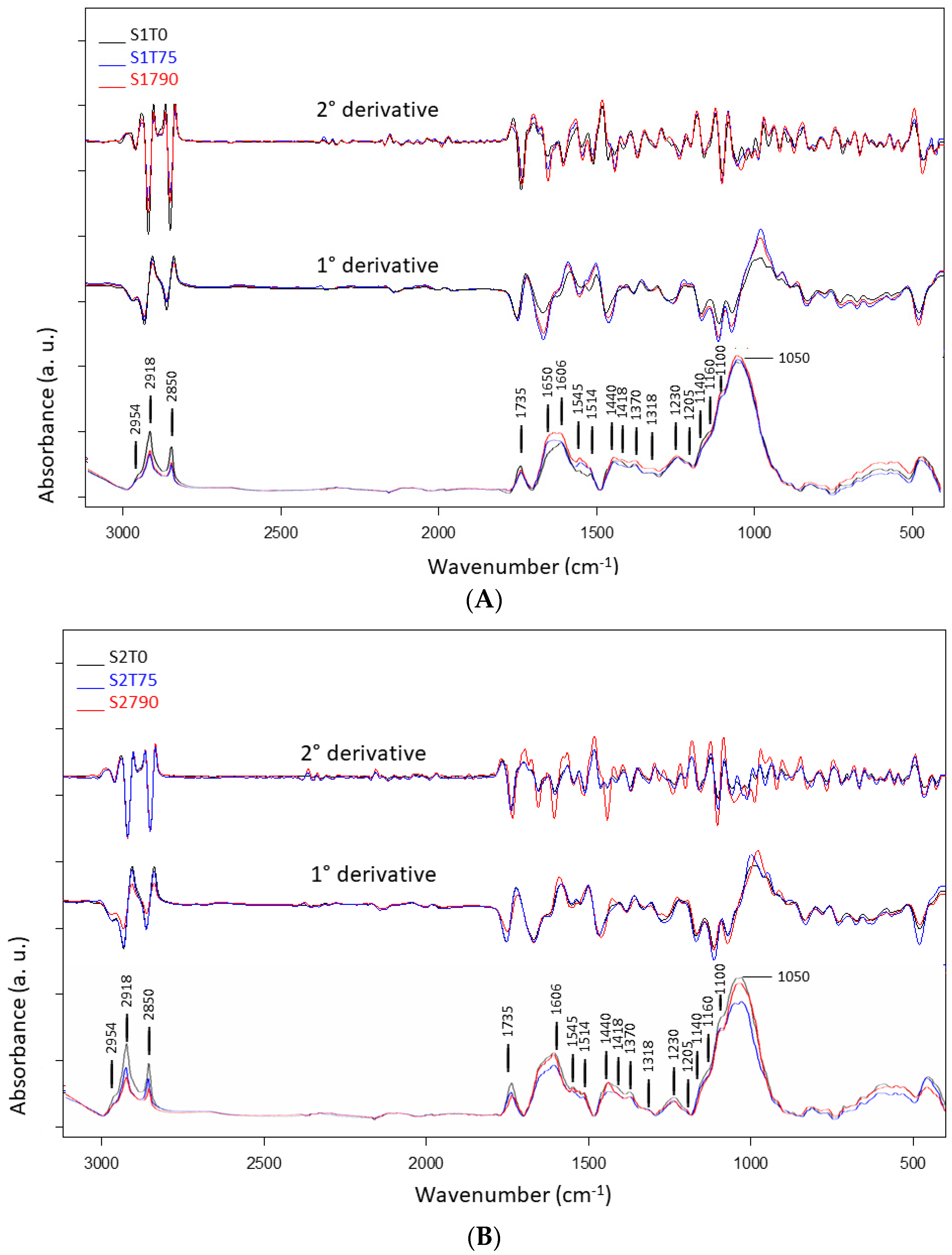
| Band Position (cm−1) | FTIR Band Assignments | References |
|---|---|---|
| 3300 | OH stretching (water), N-H stretching | [27,30] |
| 2954 | C-H asymmetric stretching | [28,31] |
| 2918 | C-H asymmetric stretching | [27,28] |
| 2850 | C-H symmetric stretching | [27,28,31] |
| 1735 | COOH stretching, carbonyl (C=O) stretching | [27,28] |
| 1650 (sh) | N-H stretching and C=O stretching of amide I | [32,33] |
| 1606 | Carbonyl (C=O) stretching, C-C aromatic stretching, NH2 group bending | [28,31,34] |
| 1545 | C-C aromatic ring stretching phenolic compounds, N-H bending and C-N stretching of protein | [28,34] |
| 1514 | C=C-C aromatic ring stretching | [28,30] |
| 1440 | C-C aromatic stretching (conjugated with C=O), asymmetric C-H bending from lipids, protein, lignin | [34,35,36] |
| 1418 | CH asymmetric bending, O-H bending: cell wall polysaccarides, alcohols and carboxylic acids, COO− symmetric stretching vibration of non-esterified uronic acid | [28,31,35,37] |
| 1370 | C-H deformation and CH2 bending | [28,31] |
| 1318 | CH symmetric bending of cellulose | [28] |
| 1230 | C-O stretching from hemicelluloses and lignin, amide III (C=N and N-H stretching): mainly proteins | [28,31,35,36] |
| 1140 (sh) | O-C-O asymmetric stretching, cellulose (β-1,4 glucan) | [31,35] |
| 1160 (sh) | C-C(C=O)-O stretching or C-O-C asymmetric stretching (hemicellulose) C-O bonds in the ester linkages of cutin | [27,28,34] |
| 1100 (sh) | Carbonyl (C=O) stretching (fatty acid)/C-O and C-C (pectin)/C-O-C symmetric stretching | [27,28,34] |
| 1050–1020 | C-O, C=C and C-C-O stretching of cellulose and hemicellulose | [27,28,37] |
| Classes | No As | Low As | High As |
|---|---|---|---|
| Sensitivity (Cal) | 0.994 | 0.903 | 0.886 |
| Specificity (Cal) | 0.993 | 0.931 | 0.869 |
| Sensitivity (CV) | 0.992 | 0.856 | 0.820 |
| Specificity (CV) | 0.992 | 0.890 | 0.788 |
| Sensitivity (Pred) | 0.887 | 0.995 | 0.919 |
| Specificity (Pred) | 0.989 | 1.000 | 0.876 |
| Class, Err (Cal) | 0.006 | 0.083 | 0.123 |
| Class, Err (CV) | 0.008 | 0.127 | 0.196 |
| Class, Err (Pred) | 0.062 | 0.003 | 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capobianco, G.; Antenozio, M.L.; Bonifazi, G.; Brunetti, P.; Cardarelli, M.; Cestelli Guidi, M.; Pronti, L.; Serranti, S. Multi-Analytical Approach to Evaluate Elements and Chemical Alterations in Pteris vittata Plants Exposed to Arsenic. Water 2023, 15, 1333. https://doi.org/10.3390/w15071333
Capobianco G, Antenozio ML, Bonifazi G, Brunetti P, Cardarelli M, Cestelli Guidi M, Pronti L, Serranti S. Multi-Analytical Approach to Evaluate Elements and Chemical Alterations in Pteris vittata Plants Exposed to Arsenic. Water. 2023; 15(7):1333. https://doi.org/10.3390/w15071333
Chicago/Turabian StyleCapobianco, Giuseppe, Maria Luisa Antenozio, Giuseppe Bonifazi, Patrizia Brunetti, Maura Cardarelli, Mariangela Cestelli Guidi, Lucilla Pronti, and Silvia Serranti. 2023. "Multi-Analytical Approach to Evaluate Elements and Chemical Alterations in Pteris vittata Plants Exposed to Arsenic" Water 15, no. 7: 1333. https://doi.org/10.3390/w15071333





