Mesoporous Zr-G-C3N4 Sorbent as an Exceptional Cu (II) Ion Adsorbent in Aquatic Solution: Equilibrium, Kinetics, and Mechanisms Study
Abstract
:1. Introduction
2. Experimental
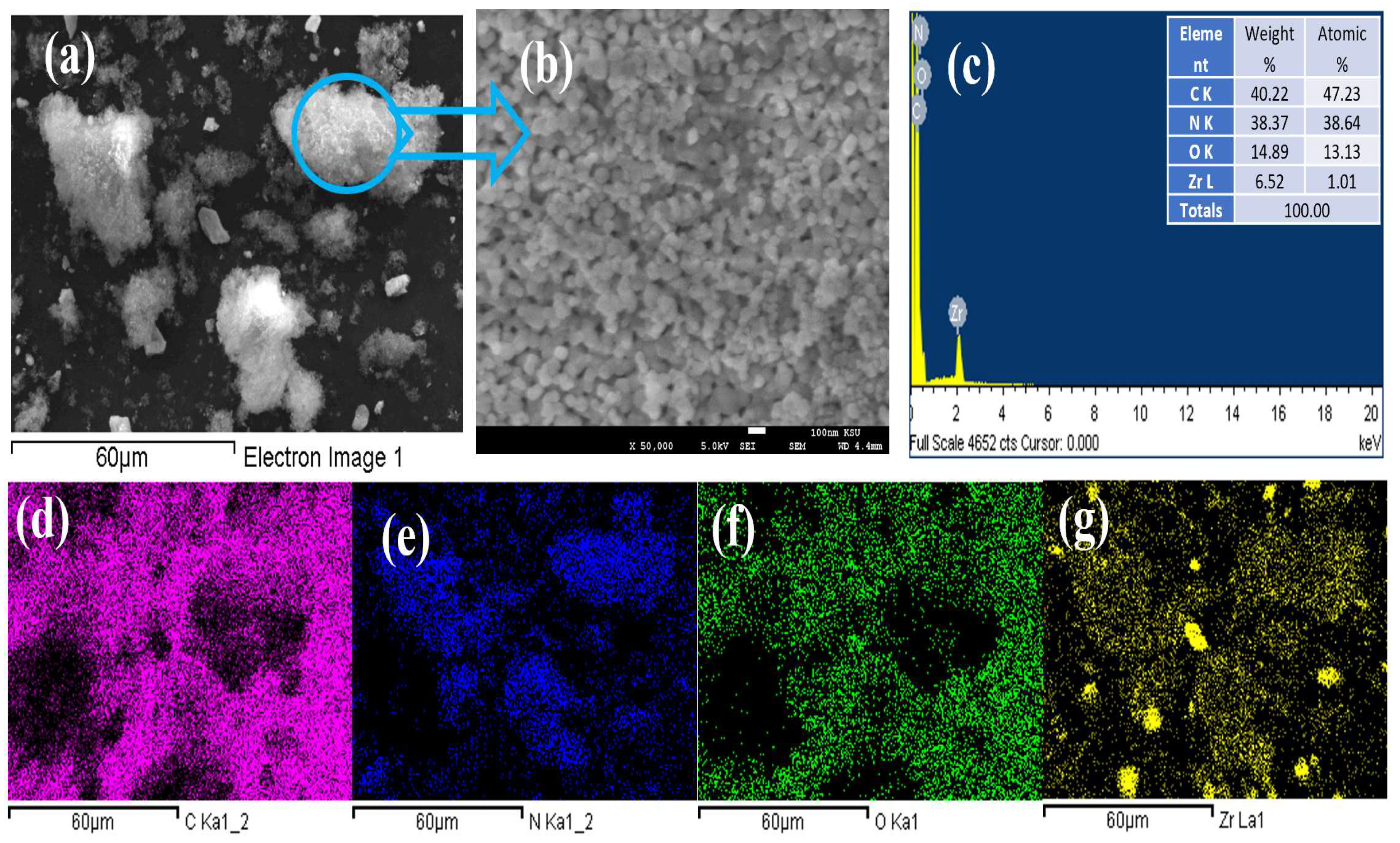
2.1. Nanomaterial Fabrication, Morphological, and Structural Characterization
2.2. Cu2+ Ion Removal Experiments
3. Results and Discussion
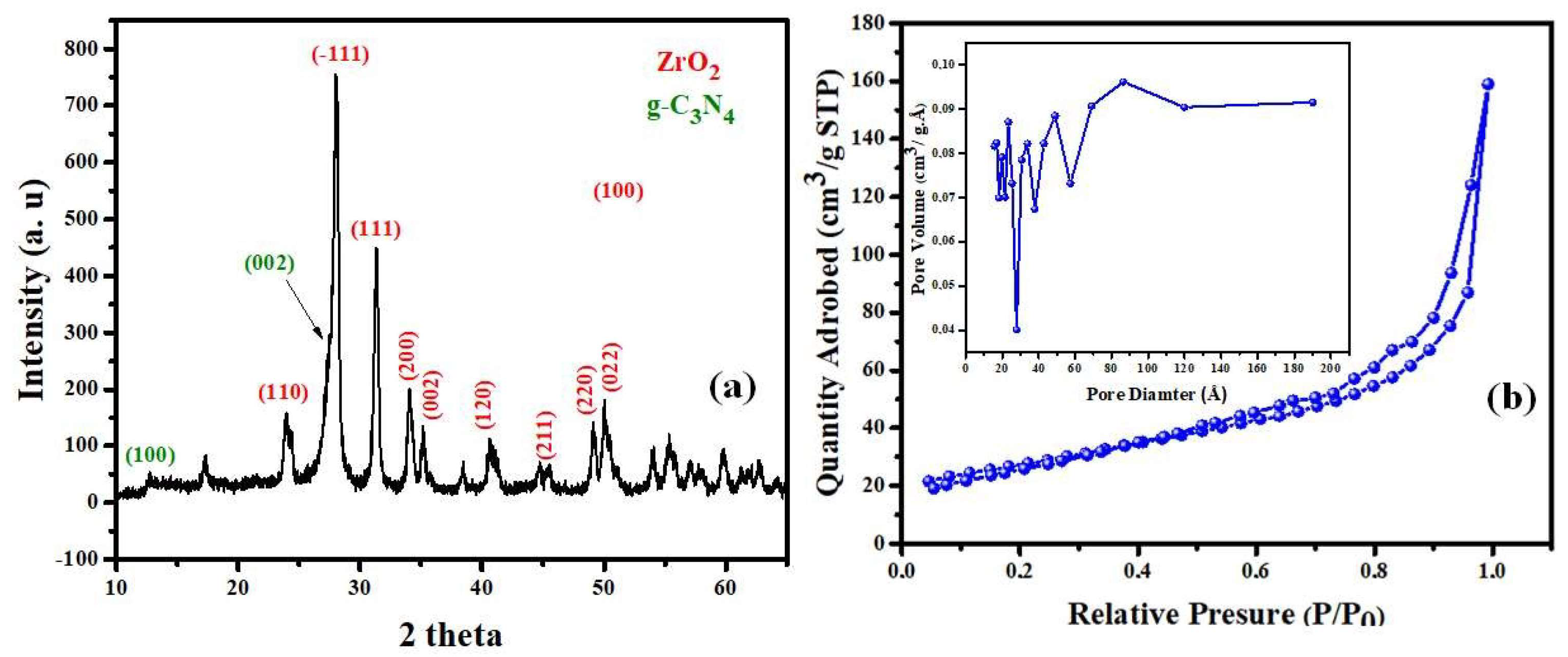
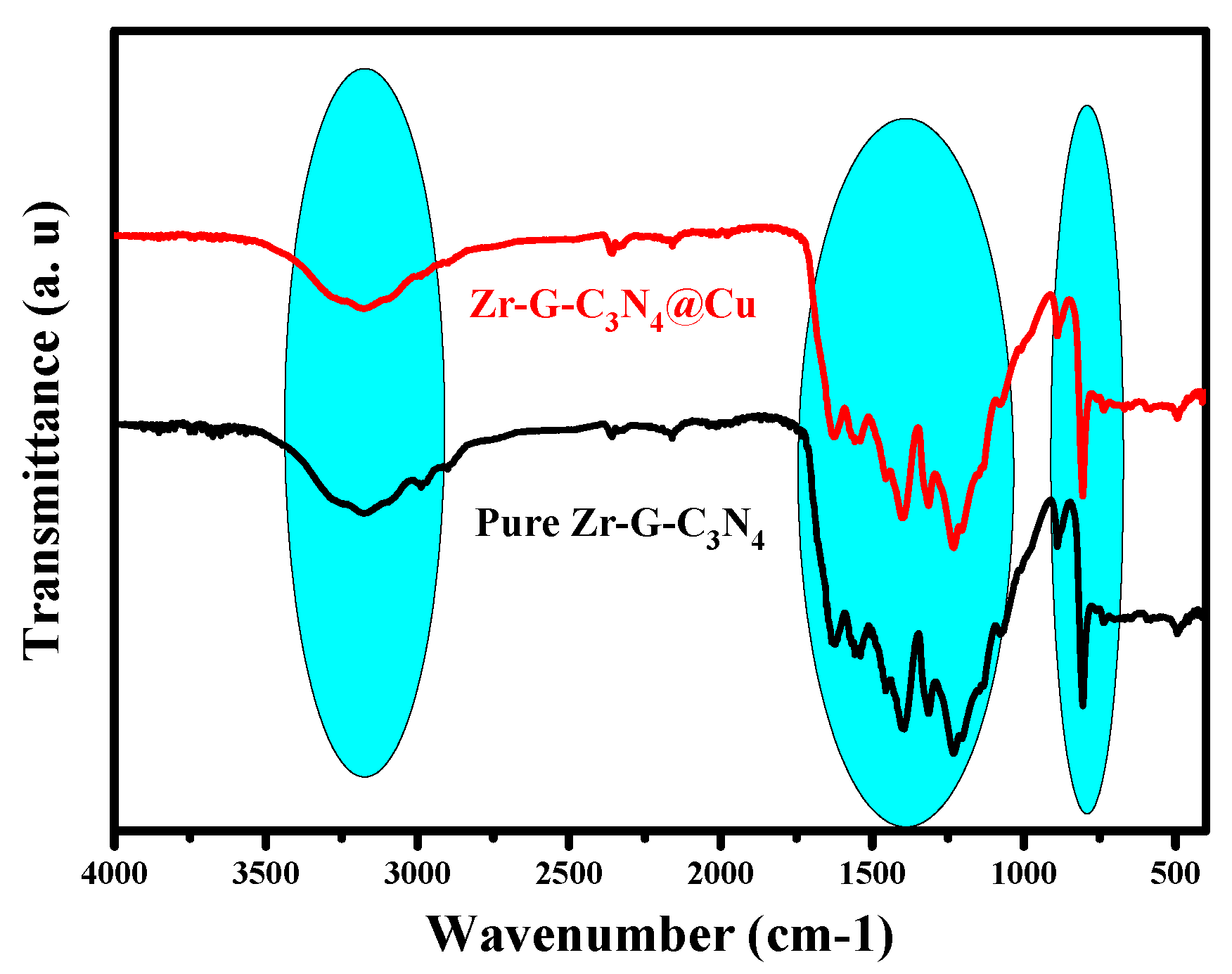
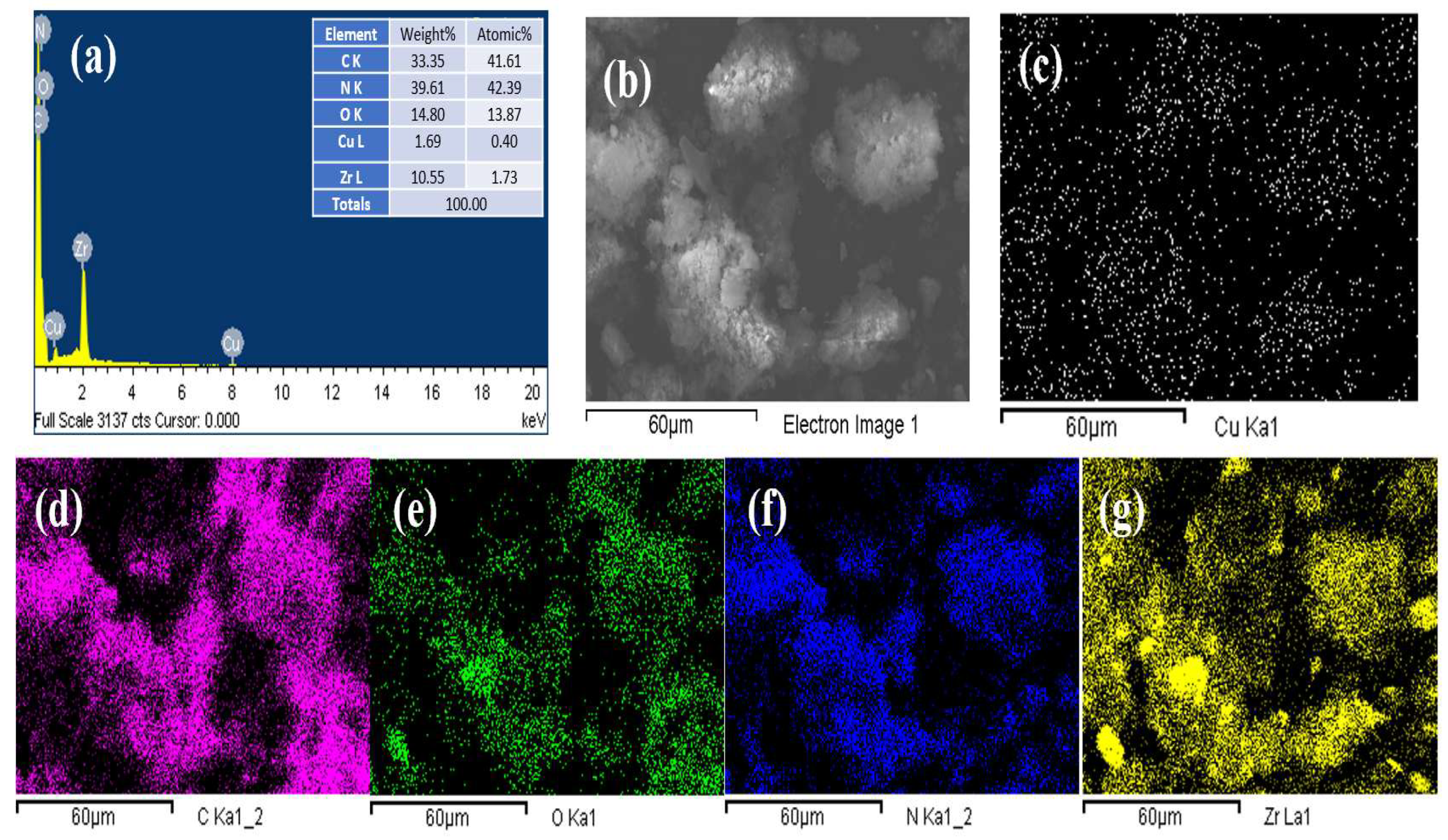
3.1. Composition Phase and Surface Characteristics of Mesoporous Zr-G-C3N4 Sorbent
3.2. Adsorption Measurements of Zr-G-C3N4 sorbent
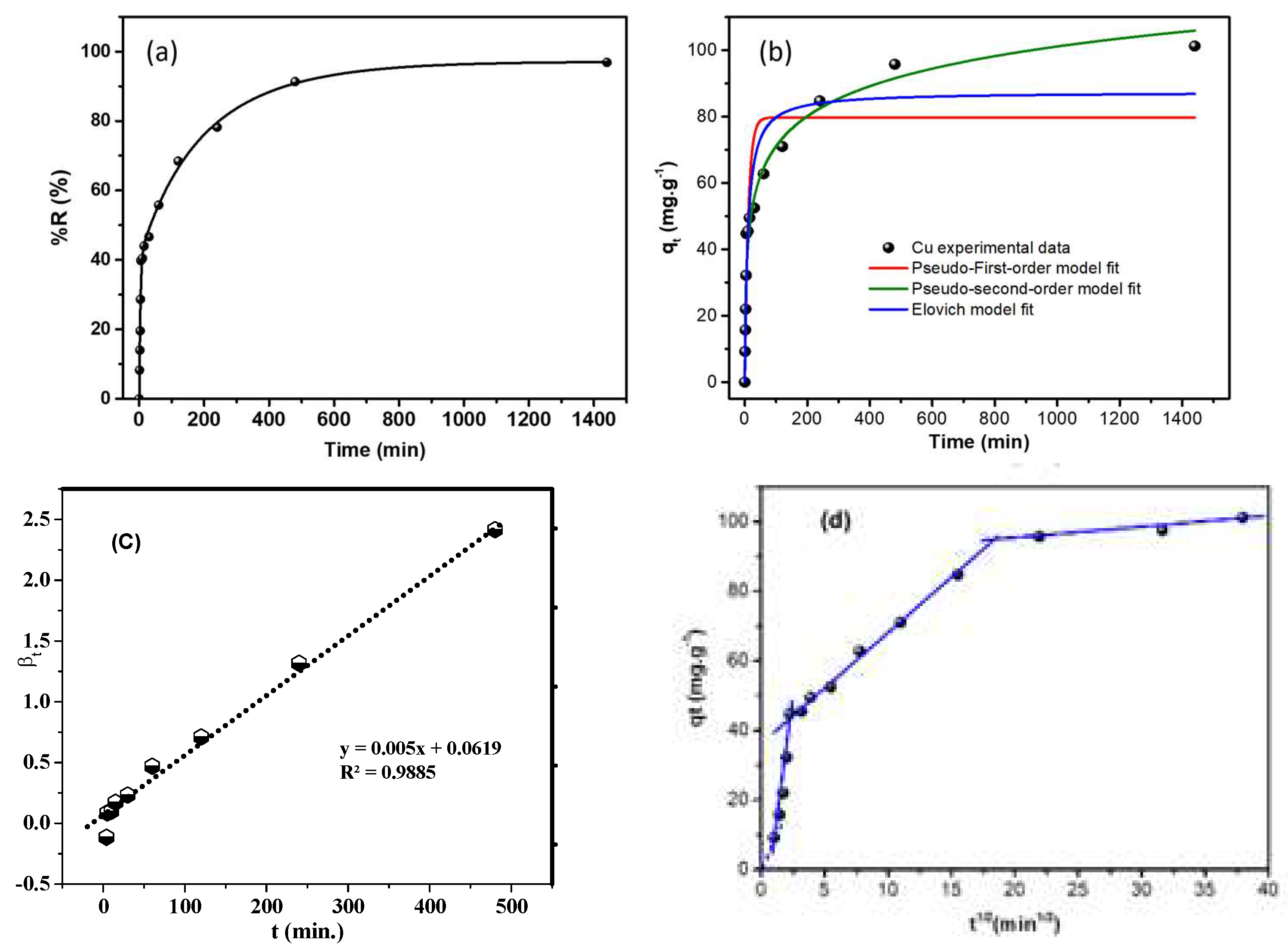
3.2.1. Impact of Adsorption Time
3.2.2. Kinetic Study
| Kinetics Model | Linear and Nonlinear Kinetic Equations | Equation No. | Refs. | Parameters | Values |
|---|---|---|---|---|---|
| Pseudo-first-order | (1 − ) | (4) | [37,38] | qm (mg·g−1) | 51.5 |
| k1 (min−1) | 1.4 × 10−3 | ||||
| R2 | 0.8207 | ||||
| Pseudo-second-order | (5) | [37] | qm (exp.) (mg·g−1) | 87 | |
| qm (cal.) (mg·g−1) | 90 | ||||
| k2 (g·mg−1·min−1) | 5.6 × 10−3 | ||||
| h0 (mg·(g−1·min−1)) | 4.65 | ||||
| t1/2 (min−1) | 21.95 | ||||
| R2 | 0.9984 | ||||
| Elovich | (6) | [39] | β (g·mg−1) | 0.0863 | |
| α (mg·g−1·min−1) | 57.37 | ||||
| R2 | 0.9687 | ||||
| Intraparticle diffusion | (7) | [39] | kdif1 (mg·g−1·min1/2) | 70.89 | |
| C1 (mg·g−1) | 41.76 | ||||
| R2 | 0.9737 | ||||
| kdif2 (mg·(g−1·min−1/2)) | 1.14 | ||||
| C2 (mg·g−1) | 81.88 | ||||
| R2 | 0.9527 | ||||
| kdif3 (mg·(g−1·min−1/2)) | 0.046 | ||||
| C3 (mg·g−1) | 115.32 | ||||
| R2 | 0.9612 | ||||
| Film diffusion | −0.4977 − ln(1 − F) | (8) | [40] | k (min−1) | 5.00 × 10−3 |
| Di (cm−2·g−1·min−1) | 9.166 × 10−9 | ||||
| R2 | 0.9885 |
3.2.3. Intraparticle Diffusion/Transport Model (IPDT)
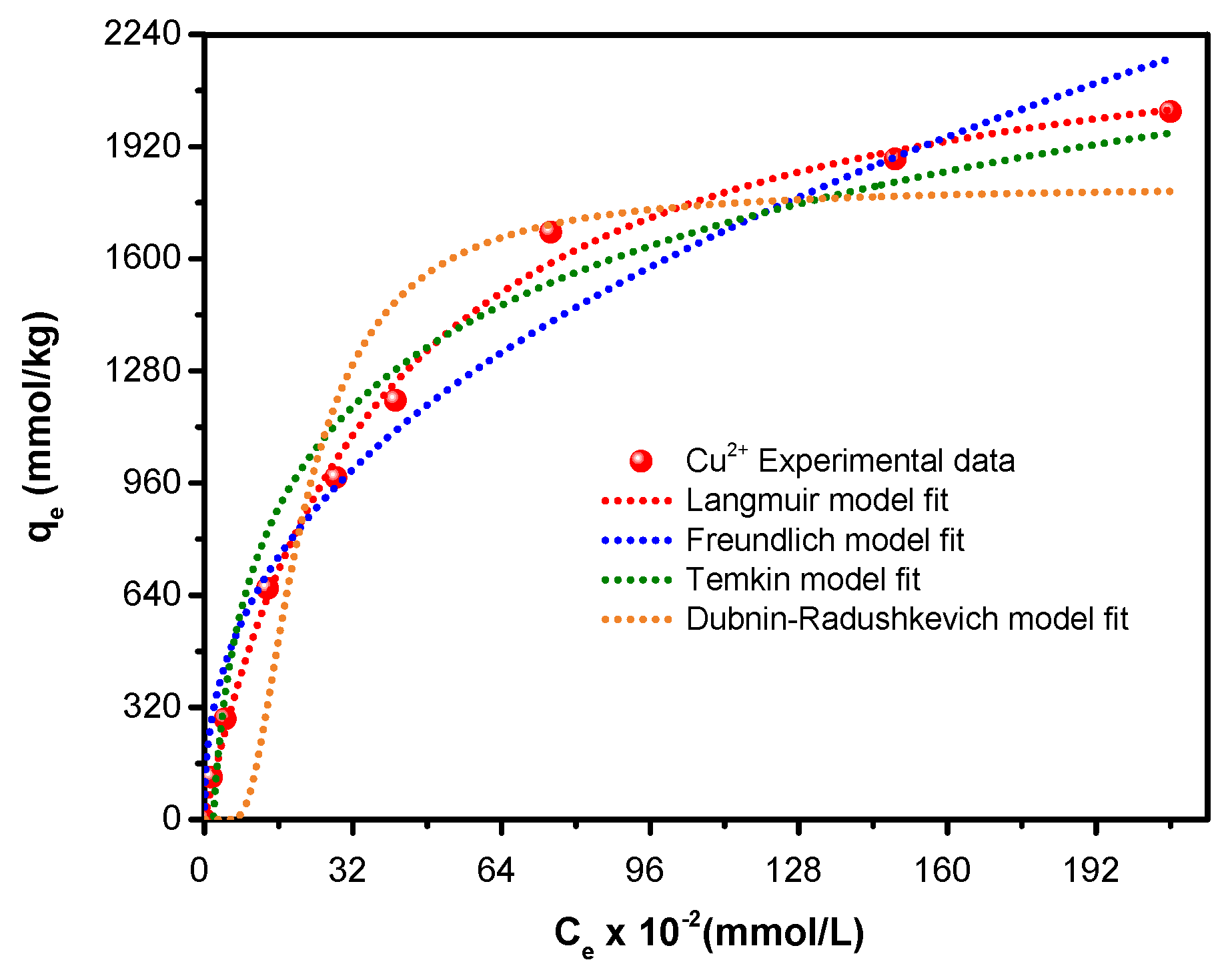
3.2.4. Uptake Isotherms of Copper Ions
| Equilibrium Model | Linear and Nonlinear Equilibrium Equations | Equation No. | Refs. | Parameters | Cu2+ |
|---|---|---|---|---|---|
| Langmuir | , | (9) | [26] | qm (mol·kg−1) | 2.262 |
| b (L·mol−1) | 5.5 × 10−6 | ||||
| RL | 0.9514 | ||||
| R2 | 0.9907 | ||||
| Freundlich | )1/n | (10) | [48] | n | 1.73 |
| kF | 9.87 | ||||
| R2 | 0.9634 | ||||
| Temkin | ) | (11) | [49] | βT (J·mol−1) | 563.2 |
| kT (L·mmol−1) | 5.85 | ||||
| R2 | 0.9612 | ||||
| Dubinin–Radushkevich | (12) | [50] | β (mol2·J−2) | 1.95 × 10−8 | |
| q (mol·kg−1) | 18.6 | ||||
| E (J·mol−1) | 5064 | ||||
| R2 | 0.9864 |
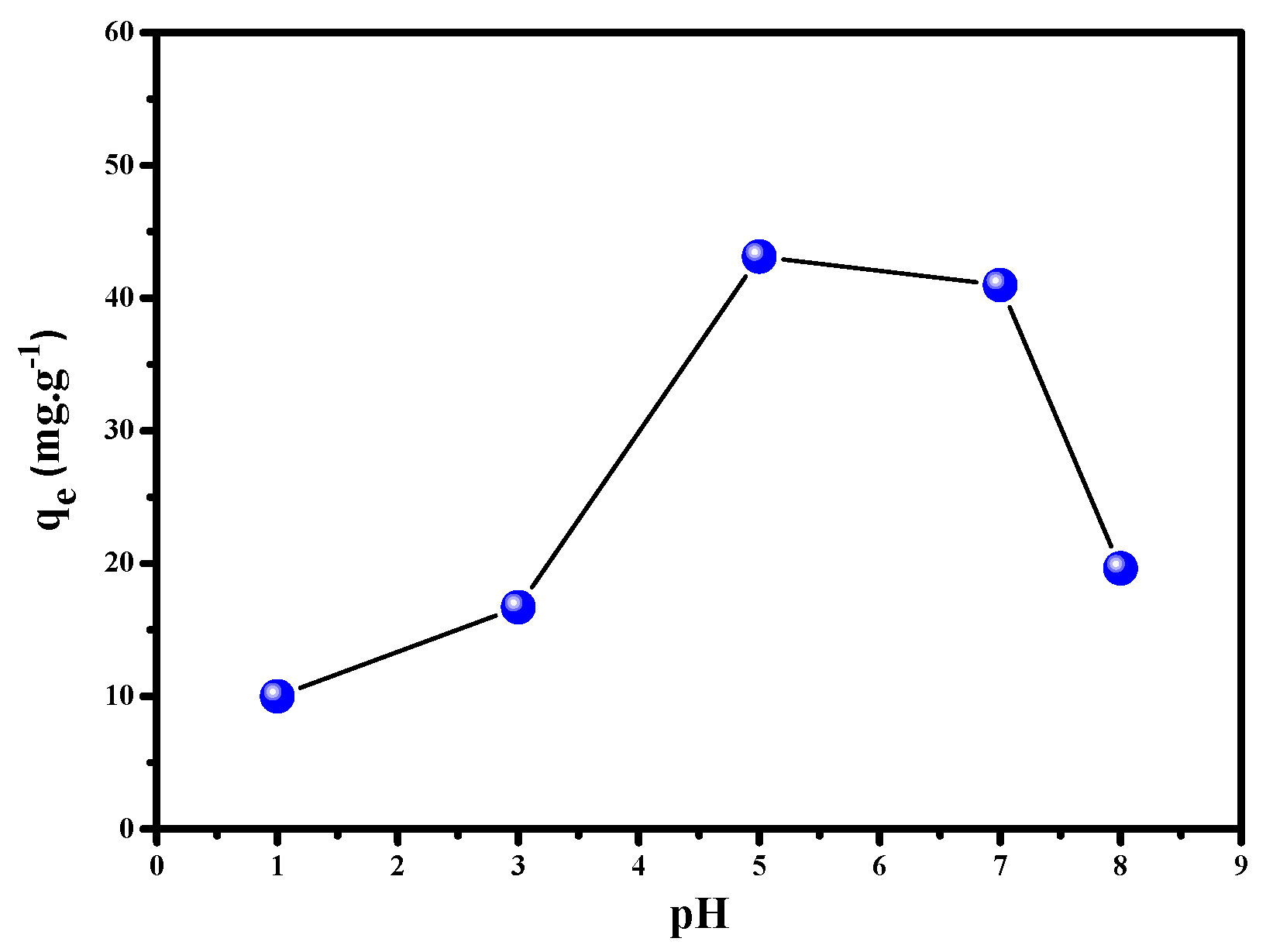
3.2.5. The Impact of pH on Cu2+ (II) Ion Uptake
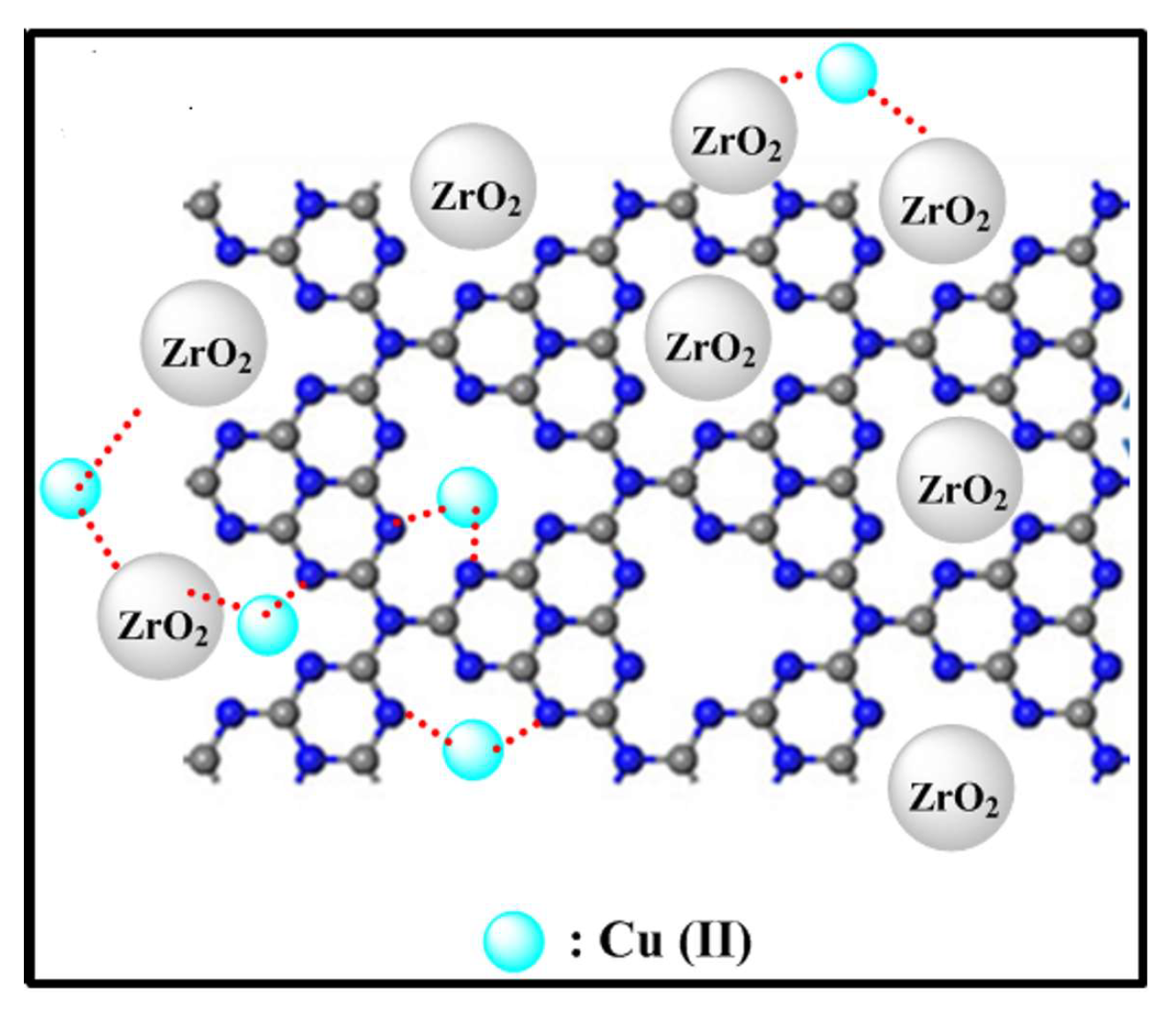
3.2.6. Uptake Mechanism of Copper Ions
3.2.7. Regeneration Tests for Zr-g-C3N4 Adsorbent
| Adsorbents | Cu2+ Uptake (mg/g) | t (min) | pH | References |
|---|---|---|---|---|
| GFLE | 193.40 | 105 | 1 and 40 °C | [22] |
| ɣ Fe2O3 nanoparticles | 26.00 | 240 | 6 | [77] |
| Graphene oxide | 75.00 | 1440 | <5.95 | [59] |
| MgO-CaO-Al2O3 -SiO2-CO2 system | 16.70 | 168 | - | [78] |
| MnO2 nanowires | 75.48 | 30 | 1.9 to 5.3 | [79] |
| CO3·Mg-Al LDH | 70.7 | - | 4 | [80] |
| COSAC | 17.67 | 60 | 5 | [45] |
| Zr-G-C3N4 | 144.1 | 48 | 5 | This work |
3.2.8. Comparative Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anbia, M.; Ghassemian, Z. Removal of Cd(II) and Cu(II) from aqueous solutions using mesoporous silicate containing zirconium and iron. Chem. Eng. Res. Des. 2011, 89, 2770–2775. [Google Scholar] [CrossRef]
- Anbia, M.; Haqshenas, M. Adsorption studies of Pb(II) and Cu(II) ions on mesoporous carbon nitride functionalized with melamine-based dendrimer amine. Int. J. Environ. Sci. Technol. 2015, 12, 2649–2664. [Google Scholar] [CrossRef]
- Teodoro, F.S.; Soares, L.C.; Filgueiras, J.G.; de Azevedo, E.R.; Patiño-Agudelo, J.; Adarme, O.F.H.; da Silva, L.H.M.; Gurgel, L.V.A. Batch and continuous adsorption of Cu(II) and Zn(II) ions from aqueous solution on bi-functionalized sugarcane-based biosorbent. Environ. Sci. Pollut. Res. 2021, 29, 26425–26448. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-H.; Zhu, X.-F.; Qian, W.; Yu, Y.-C.; Xu, R.-K. Effect of pectin on adsorption of Cu(II) by two variable-charge soils from southern China. Environ. Sci. Pollut. Res. 2015, 22, 19687–19694. [Google Scholar] [CrossRef]
- Meng, J.; Feng, X.; Dai, Z.; Liu, X.; Wu, J.; Xu, J. Adsorption characteristics of Cu(II) from aqueous solution onto biochar derived from swine manure. Environ. Sci. Pollut. Res. 2014, 21, 7035–7046. [Google Scholar] [CrossRef]
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of copper ion from solution by polyethylenimine modified wheat straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Adsorption of copper ions onto chitosan/poly(vinyl alcohol) beads functionalized with poly(ethylene glycol). Carbohydr. Polym. 2020, 234, 115890. [Google Scholar] [CrossRef]
- Khan, J.; Lin, S.; Nizeyimana, J.C.; Wu, Y.; Wang, Q.; Liu, X. Removal of copper ions from wastewater via adsorption on modified hematite (α-Fe2O3) iron oxide coated sand. J. Clean. Prod. 2021, 319, 128687. [Google Scholar] [CrossRef]
- Ye, X.; Shang, S.; Zhao, Y.; Cui, S.; Zhong, Y.; Huang, L. Ultra-efficient adsorption of copper ions in chitosan–montmorillonite composite aerogel at wastewater treatment. Cellulose 2021, 28, 7201–7212. [Google Scholar] [CrossRef]
- Su, C.; Berekute, A.K.; Yu, K.-P. Chitosan@ TiO2 composites for the adsorption of copper (II) and antibacterial applications. Sustain. Environ. Res. 2022, 32, 1–15. [Google Scholar] [CrossRef]
- Reddad, Z.; Gerente, C.; Andres, Y.; Le Cloirec, P. Adsorption of Several Metal Ions onto a Low-Cost Biosorbent: Kinetic and Equilibrium Studies. Environ. Sci. Technol. 2002, 36, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Removal of Heavy Metals from the Environment by Biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Wan, J.; Chen, L.; Li, Q.; Ye, Y.; Feng, X.; Zhou, A.; Long, X.; Xia, D.; Zhang, T.C. A novel hydrogel for highly efficient adsorption of Cu (II): Synthesis, characterization, and mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 26621–26630. [Google Scholar] [CrossRef] [PubMed]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Yang, L.; Li, P.; Li, S.-J.; Li, L.; Pang, X.-X.; Ye, F.; Fu, Y. A novel colorimetric and “turn-off” fluorescent probe based on catalyzed hydrolysis reaction for detection of Cu2+ in real water and in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117540. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Obregón, S. Exploring nanoengineering strategies for the preparation of graphitic carbon nitride nanostructures. FlatChem 2023, 38, 100473. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; An, X.; Hou, L.-A. A critical review of g-C3N4-based photocatalytic membrane for water purification. Chem. Eng. J. 2021, 412, 128663. [Google Scholar] [CrossRef]
- Shahbazi, A.; Younesi, H.; Badiei, A. Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb (II), Cu (II) and Cd (II) heavy metal ions in batch and fixed bed column. Chem. Eng. J. 2011, 168, 505–518. [Google Scholar] [CrossRef]
- Khezami, L.; Elamin, N.; Modwi, A.; Taha, K.K.; Amer, M.S.; Bououdina, M. Mesoporous Sn@ TiO2 nanostructures as excellent adsorbent for Ba ions in aqueous solution. Ceram. Int. 2022, 48, 5805–5813. [Google Scholar] [CrossRef]
- Mustafa, B.; Modwi, A.; Ismail, M.; Makawi, S.; Hussein, T.; Abaker, Z.; Khezami, L. Adsorption performance and Kinetics study of Pb(II) by RuO2–ZnO nanocomposite: Construction and Recyclability. Int. J. Environ. Sci. Technol. 2022, 19, 327–340. [Google Scholar] [CrossRef]
- Abdulkhair, B.; Salih, M.; Modwi, A.; Adam, F.; Elamin, N.; Seydou, M.; Rahali, S. Adsorption behavior of barium ions onto ZnO surfaces: Experiments associated with DFT calculations. J. Mol. Struct. 2021, 1223, 128991. [Google Scholar] [CrossRef]
- Ghiloufi, I.; El Ghoul, J.; Modwi, A.; El Mir, L. Ga-doped ZnO for adsorption of heavy metals from aqueous solution. Mater. Sci. Semicond. Process. 2016, 42, 102–106. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Taha, K.; Al-Duaij, O.; Houas, A. Fast and high efficiency adsorption of Pb(II) ions by Cu/ZnO composite. Mater. Lett. 2017, 195, 41–44. [Google Scholar] [CrossRef]
- Khezami, L.; Taha, K.K.; Modwi, A. Efficient Removal of Cobalt from Aqueous Solution by Zinc Oxide Nanoparticles: Kinetic and Thermodynamic Studies. Z. Nat. A 2017, 72, 409–418. [Google Scholar] [CrossRef]
- Khezami, L.; Modwi, A.; Ghiloufi, I.; Taha, K.K.; Bououdina, M.; ElJery, A.; El Mir, L. Effect of aluminum loading on structural and morphological characteristics of ZnO nanoparticles for heavy metal ion elimination. Environ. Sci. Pollut. Res. 2020, 27, 3086–3099. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Singh, S.; Gill, R. Applications of biopolymer coatings in biomedical engineering. J. Electrochem. Sci. Eng. 2022, 13, 63–81. [Google Scholar] [CrossRef]
- Nevarez-Rascon, A.; González-Lopez, S.; Acosta-Torres, L.S.; Nevarez-Rascon, M.M.; Borunda, E.O. Synthesis, biocompatibility and mechanical properties of ZrO2-Al2O3 ceramics composites. Dent. Mater. J. 2016, 35, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Sayed, E.T.; Abdelkareem, M.A.; Alawadhi, H.; Elsaid, K.; Wilberforce, T.; Olabi, A. Graphitic carbon nitride/carbon brush composite as a novel anode for yeast-based microbial fuel cells. Energy 2021, 221, 119849. [Google Scholar] [CrossRef]
- Svoboda, L.; Bednář, J.; Dvorský, R.; Panáček, A.; Hochvaldová, L.; Kvítek, L.; Malina, T.; Konvičková, Z.; Henych, J.; Němečková, Z.; et al. Crucial cytotoxic and antimicrobial activity changes driven by amount of doped silver in biocompatible carbon nitride nanosheets. Colloids Surf. B Biointerfaces 2021, 202, 111680. [Google Scholar] [CrossRef]
- Xavier, J.R. Electrochemical and Mechanical Investigation of Newly Synthesized NiO-ZrO 2 Nanoparticle–Grafted Polyure-thane Nanocomposite Coating on Mild Steel in Chloride Media. J. Mater. Eng. Perform. 2021, 30, 1554–1566. [Google Scholar] [CrossRef]
- Channu, V.R.; Kalluru, R.R.; Schlesinger, M.; Mehring, M.; Holze, R. Synthesis and characterization of ZrO2 nanoparticles for optical and electrochemical applications. Colloids Surf. A Physicochem. Eng. Asp. 2011, 386, 151–157. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, W.; Wang, X.; Li, P.; Gao, W.; Zou, H.; Wu, S.; Ding, K. Surface engineering for extremely enhanced charge separation and photocatalytic hydrogen evolution on g-C3N4. Adv. Mater. 2018, 30, 1705060. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.; Carvalho, J.; Lima, Z.; Sasaki, J. Size–strain study of NiO nanoparticles by X-ray powder diffraction line broadening. Mater. Lett. 2012, 72, 36–38. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Hu, W.; Cai, J.; Zhang, L.; Dong, L.; Zhao, L.; He, Y. Synthesis and photocatalytic activity of SiO2/g-C3N4 composite photocatalyst. Mater. Lett. 2014, 115, 53–56. [Google Scholar] [CrossRef]
- Modwi, A.; Abbo, M.A.; Hassan, E.A.; Houas, A. Effect of annealing on physicochemical and photocatalytic activity of Cu5% loading on ZnO synthesized by sol–gel method. J. Mater. Sci. Mater. Electron. 2016, 27, 12974–12984. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898, 24, 1–39. [Google Scholar]
- Abdelrahman, E.A.; El-Reash, Y.A.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 2021, 401, 123813. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Ye, T.; Naz, I. Sorption of cationic malachite green dye on phytogenic magnetic nanoparticles functionalized by 3-marcaptopropanic acid. RSC Adv. 2018, 8, 8878–8897. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cai, W.; Lin, Y.; Wang, G.; Liang, C. Mass production of micro/nanostructured porous ZnO plates and their strong structurally enhanced and selective adsorption performance for environmental remediation. J. Mater. Chem. 2010, 20, 8582. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.; Mohanty, C.; Meikap, B. Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Rahman, N.K. Equilibrium, kinetics and thermodynamic of Remazol Brilliant Orange 3R dye adsorption on coffee husk-based activated carbon. Chem. Eng. J. 2011, 170, 154–161. [Google Scholar] [CrossRef]
- Kumar, K.V.; Ramamurthi, V.; Sivanesan, S. Biosorption of malachite green, a cationic dye onto Pithophora sp., a fresh water algae. Dye. Pigment. 2006, 69, 102–107. [Google Scholar] [CrossRef]
- Podder, M.; Majumder, C. Biosorption of As (III) and As (V) on the surface of TW/MnFe2O4 composite from wastewater: Kinetics, mechanistic and thermodynamics. Appl. Water Sci. 2017, 7, 2689–2715. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.; Jobara, A.; Bekouche, H.; Allateef, M.A.; Ben Aissa, M.A.; Modwi, A. Impact of Cu Ions removal onto MgO nanostructures: Adsorption capacity and mechanism. J. Mater. Sci. Mater. Electron. 2022, 33, 12500–12512. [Google Scholar] [CrossRef]
- Brdar, M.; Šćiban, M.; Takači, A.; Došenović, T. Comparison of two and three parameters adsorption isotherm for Cr(VI) onto Kraft lignin. Chem. Eng. J. 2012, 183, 108–111. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Temkin, M.I. Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zh. Fiz. Chim. 1941, 15, 296–332. [Google Scholar]
- Dubinin, M. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR. 1947, 55, 327–329. [Google Scholar]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Nikiforova, T.E.; Kozlov, V.A.; Telegin, F.Y. Chemisorption of copper ions in aqueous acidic solutions by modified chitosan. Mater. Sci. Eng. B 2021, 263, 114778. [Google Scholar] [CrossRef]
- Bae, M.; Lee, H.; Yoo, K.; Kim, S. Copper(I) selective chemisorption on magnetite (Fe3O4) over gold(I) ions in chloride solution with cyanide. Hydrometallurgy 2021, 201, 105560. [Google Scholar] [CrossRef]
- Gao, X.; Wu, L.; Xu, Q.; Tian, W.; Li, Z.; Kobayashi, N. Adsorption kinetics and mechanisms of copper ions on activated carbons derived from pinewood sawdust by fast H3PO4 activation. Environ. Sci. Pollut. Res. 2018, 25, 7907–7915. [Google Scholar] [CrossRef] [PubMed]
- Buema, G.; Trifas, L.-M.; Harja, M. Removal of Toxic Copper Ion from Aqueous Media by Adsorption on Fly Ash-Derived Zeolites: Kinetic and Equilibrium Studies. Polymers 2021, 13, 3468. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Kumar, P.A.; Chakraborty, S.; Ray, M. Uptake and desorption of copper ion using functionalized polymer coated silica gel in aqueous environment. Sep. Purif. Technol. 2007, 57, 47–56. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Zhrani, G.; Kosa, S.A. Simultaneous removal of copper (II), lead (II), zinc (II) and cadmium (II) from aqueous solutions by multi-walled carbon nanotubes. Comptes Rendus Chim. 2012, 15, 398–408. [Google Scholar] [CrossRef]
- Toscano, G.; Caristi, C.; Cimino, G. Sorption of heavy metal from aqueous solution by volcanic ash. Comptes Rendus Chim. 2008, 11, 765–771. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Tan, X.; Wang, X. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper de-contamination. Dalton Trans. 2013, 42, 5266–5274. [Google Scholar] [CrossRef]
- Bohli, T.; Ouederni, A.; Fiol, N.; Villaescusa, I. Evaluation of an activated carbon from olive stones used as an adsorbent for heavy metal removal from aqueous phases. Comptes Rendus Chim. 2015, 18, 88–99. [Google Scholar] [CrossRef]
- Mao, N.; Jiang, J.-X. MgO/g-C3N4 nanocomposites as efficient water splitting photocatalysts under visible light irradiation. Appl. Surf. Sci. 2019, 476, 144–150. [Google Scholar] [CrossRef]
- Ge, L.; Han, C. Synthesis of MWNTs/g-C3N4 composite photocatalysts with efficient visible light photocatalytic hydrogen evolution activity. Appl. Catal. B Environ. 2012, 117-118, 268–274. [Google Scholar] [CrossRef]
- Ji, H.; Chang, F.; Hu, X.; Qin, W.; Shen, J. Photocatalytic degradation of 2,4,6-trichlorophenol over g-C3N4 under visible light irradiation. Chem. Eng. J. 2013, 218, 183–190. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Gopinath, C.S.; Yoshida, H.; Hattori, T.X.P.S. XPS, XANES and EXAFS investigations of CuO/ZnO/Al2O3/ZrO2 mixed oxide catalysts. Phys. Chem. Chem. Phys. 2002, 4, 1990–1999. [Google Scholar] [CrossRef]
- Ragupathy, P.; Park, D.H.; Campet, G.; Vasan, H.N.; Hwang, S.-J.; Choy, J.-H.; Munichandraiah, N. Remarkable Capacity Retention of Nanostructured Manganese Oxide upon Cycling as an Electrode Material for Supercapacitor. J. Phys. Chem. C 2009, 113, 6303–6309. [Google Scholar] [CrossRef]
- Toghan, A.; Modwi, A. Boosting unprecedented indigo carmine dye photodegradation via mesoporous MgO@ g-C3N4 nanocomposite. J. Photochem. Photobiol. A Chem. 2021, 419, 113467. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F.; Khan, M.A. Efficient and stable ZrO2/Fe modified hollow-C 3 N 4 for photodegradation of the herbicide MTSM. RSC Adv. 2017, 7, 3966–3974. [Google Scholar] [CrossRef] [Green Version]
- Ismael, M.; Wu, Y.; Wark, M. Photocatalytic activity of ZrO2 composites with graphitic carbon nitride for hydrogen production under visible light. New J. Chem. 2019, 43, 4455–4462. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Liu, J.; Wei, X.; Yang, T.; Chen, M.; Wang, J. Rare-Earth Doping Graphitic Carbon Nitride Endows Distinctive Multiple Emissions with Large Stokes Shifts. CCS Chem. 2022, 4, 1990–1999. [Google Scholar] [CrossRef]
- Guo, J.; Chen, T.; Zhou, X.; Xia, W.; Zheng, T.; Zhong, C.; Liu, Y. Synthesis, Cr(VI) removal performance and mechanism of nanoscale zero-valent iron modified potassium-doped graphitic carbon nitride. Water Sci. Technol. 2020, 81, 1840–1851. [Google Scholar] [CrossRef]
- Zou, X.; Silva, R.; Goswami, A.; Asefa, T. Cu-doped carbon nitride: Bio-inspired synthesis of H2-evolving electrocatalysts using graphitic carbon nitride (g-C3N4) as a host material. Appl. Surf. Sci. 2015, 357, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Guo, J.; Huang, X.; Wang, W.; Sun, P.; Li, Y.; Han, J. Functionalized biochar-supported magnetic MnFe2O4 nanocomposite for the removal of Pb (ii) and Cd (ii). RSC Adv. 2019, 9, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Chen, C.; Wen, T.; Zhao, Z.; Wang, X.; Xu, A. Superior adsorption capacity of g-C3N4 for heavy metal ions from aqueous solutions. J. Colloid Interface Sci. 2015, 456, 7–14. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Suzuki, S.; Shiwaku, H. Ultimate selenium (IV) monitoring and removal from water using a new class of organic ligand based composite adsorbent. J. Hazard. Mater. 2015, 291, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F.; El Achaby, M.; Lissaneddine, A.; Aziz, K.; Ouazzani, N.; Mamouni, R.; Mandi, L. Composites with alginate beads: A novel design of nano-adsorbents impregnation for large-scale continuous flow wastewater treatment pilots. Saudi J. Biol. Sci. 2020, 27, 2499–2508. [Google Scholar] [CrossRef]
- Şimşek, S.; Derin, Y.; Kaya, S.; Şenol, Z.M.; Katin, K.P.; Özer, A.; Tutar, A. High-performance material for the effective removal of uranyl ion from solution: Computationally sup-ported experimental studies. Langmuir 2022, 38, 10098–10113. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, S.; Turkan, Z.; Kilinc, E.; Bayat, R.; Soylak, M.; Sen, F. Preconcentrations of Cu (II) and Mn (II) by magnetic solid-phase extraction on Bacillus cereus loaded γ-Fe2O3 nanomaterials. Environ. Res. 2022, 209, 112766. [Google Scholar] [CrossRef]
- Morozova, A.G.; Lonzinger, T.M.; Skotnikov, V.A.; Mikhailov, G.G.; Kapelyushin, Y.; Khandaker, M.U.; Alqahtani, A.; Bradley, D.A.; Sayyed, M.I.; Tishkevich, D.I.; et al. Insights into sorption–mineralization mechanism for sustainable granular composite of MgO-CaO-Al2O3-SiO2-CO2 based on nanosized adsorption centers and its effect on aqueous Cu (II) removal. Nanomaterials 2021, 12, 116. [Google Scholar] [CrossRef]
- Claros, M.; Kuta, J.; El-Dahshan, O.; Michalička, J.; Jimenez, Y.P.; Vallejos, S. Hydrothermally synthesized MnO2 nanowires and their application in Lead (II) and Copper (II) batch ad-sorption. J. Mol. Liq. 2021, 325, 115203. [Google Scholar] [CrossRef]
- Baaloudj, O.; Nasrallah, N.; Kebir, M.; Guedioura, B.; Amrane, A.; Nguyen-Tri, P.; Nanda, S.; Assadi, A.A. Artificial neural network modeling of cefixime photodegradation by synthesized CoBi2O4 na-noparticles. Environ. Sci. Pollut. Res. 2021, 28, 15436–15452. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Jellali, S.; Akrout, H.; Assadi, A.A.; Bousselmi, L. Dynamic investigations on cationic dye desorption from chemically modified lignocellulosic material using a low-cost eluent: Dye recovery and anodic oxidation efficiencies of the desorbed solutions. J. Clean. Prod. 2018, 201, 28–38. [Google Scholar] [CrossRef]
- Yang, X.; Kameda, T.; Saito, Y.; Kumagai, S.; Yoshioka, T. Investigation of the mechanism of Cu(II) removal using Mg-Al layered double hydroxide intercalated with carbonate: Equilibrium and pH studies and solid-state analyses. Inorg. Chem. Commun. 2021, 132, 108839. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khezami, L.; Modwi, A.; Taha, K.K.; Bououdina, M.; Ben Hamadi, N.; Assadi, A.A. Mesoporous Zr-G-C3N4 Sorbent as an Exceptional Cu (II) Ion Adsorbent in Aquatic Solution: Equilibrium, Kinetics, and Mechanisms Study. Water 2023, 15, 1202. https://doi.org/10.3390/w15061202
Khezami L, Modwi A, Taha KK, Bououdina M, Ben Hamadi N, Assadi AA. Mesoporous Zr-G-C3N4 Sorbent as an Exceptional Cu (II) Ion Adsorbent in Aquatic Solution: Equilibrium, Kinetics, and Mechanisms Study. Water. 2023; 15(6):1202. https://doi.org/10.3390/w15061202
Chicago/Turabian StyleKhezami, Lotfi, Abueliz Modwi, Kamal K. Taha, Mohamed Bououdina, Naoufel Ben Hamadi, and Aymen Amine Assadi. 2023. "Mesoporous Zr-G-C3N4 Sorbent as an Exceptional Cu (II) Ion Adsorbent in Aquatic Solution: Equilibrium, Kinetics, and Mechanisms Study" Water 15, no. 6: 1202. https://doi.org/10.3390/w15061202








