Application of Response Surface Methodology on Brewery Wastewater Treatment Using Chitosan as a Coagulant
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Brewery Wastewater Sample
2.3. Jar Test Method
2.4. Design of Experiment
3. Results and Discussion
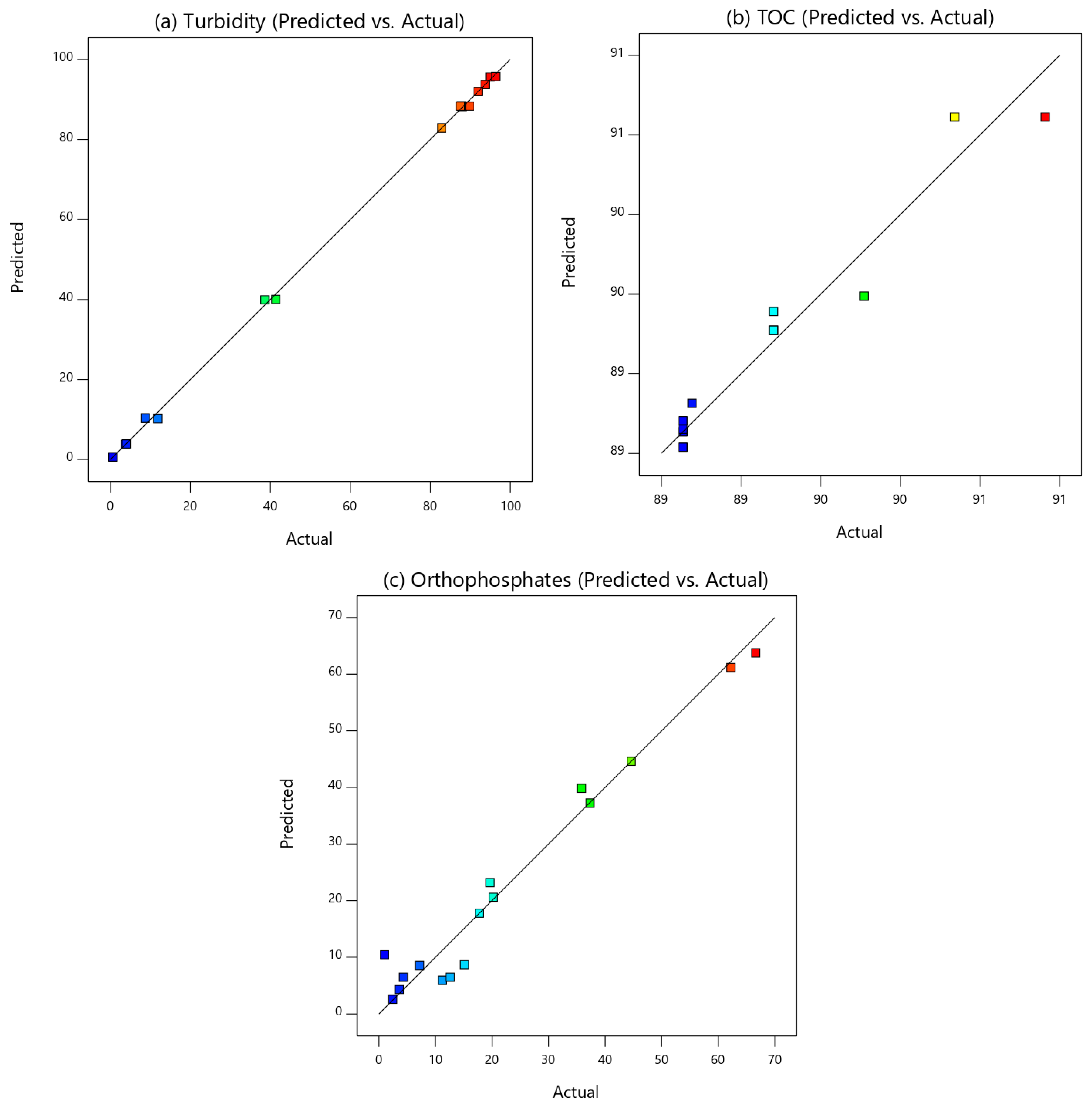
3.1. Central Composite Design (CCD)
3.2. Analysis of Variance (ANOVA)
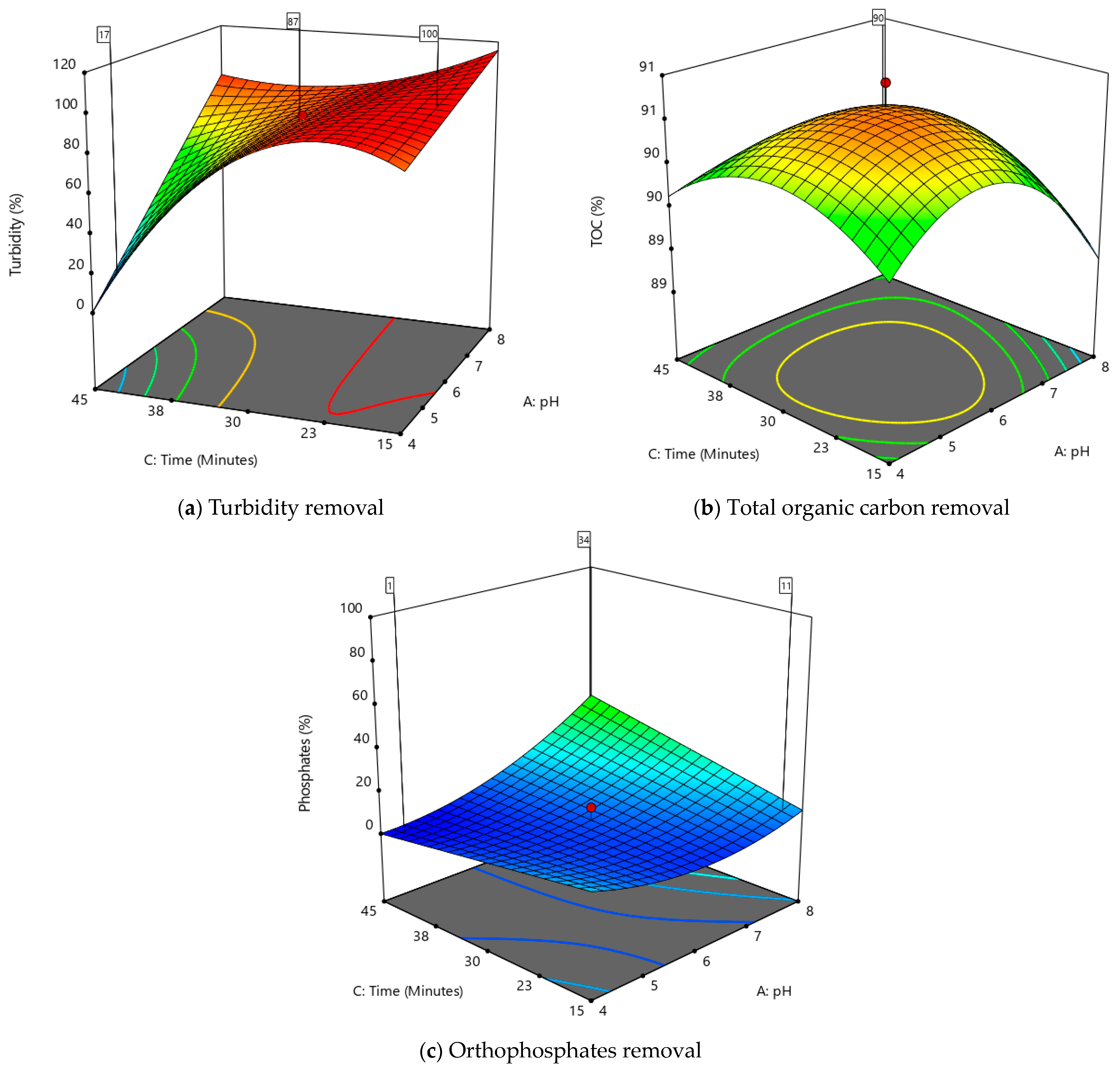
3.3. Response Surface Plots
3.4. Numerical Optimization
3.5. pH Optimisation
3.6. Comparison with Previous Studies
| Treatment Process | Removal Efficiency | HRT | Reference | |||
|---|---|---|---|---|---|---|
| Turbidity (%) | TOC, (%) | COD, (%) | ||||
| SBR | - | - | 54 | 69 | 18 h | [5] |
| - | - | 87 | 85 | 19.5 h | [32] | |
| - | - | 90 | - | 8 h | [33] | |
| CC | 91 | - | 59 | - | - | [34] |
| 75 | - | 50 | - | - | ||
| - | - | 66 | - | 40 min | [37] | |
| ECC | - | - | 26 | 74 | 30 min | [35] |
| - | - | 68 | - | 6 h | [38] | |
| - | - | 72 | - | 2 h | [39] | |
| Chitosan coagulation | 90 | - | 84 | - | 60 min | [36] |
| 91 | 89 | - | 65 | 43 min | Current study | |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simate, G.S.; Cluett, J.; Iyuke, S.E.; Musapatika, E.T.; Ndlovu, S.; Walubita, L.F.; Alvarez, A.E. The treatment of brewery wastewater for reuse: State of the art. Desalination 2011, 273, 235–247. [Google Scholar] [CrossRef]
- Enitan, A.M.; Adeyemo, J.; Kumari, S.K.; Swalaha, F.M.; Bux, F. Characterization of brewery wastewater composition. Int. J. Environ. Ecol. Eng. 2015, 9, 1073–1076. [Google Scholar] [CrossRef]
- Khumalo, S.M.; Bakare, B.F.; Rathilal, S.; Tetteh, E.K. Characterization of South African Brewery Wastewater: Oxidation-Reduction Potential Variation. Water 2022, 14, 1604. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef]
- Khumalo, S.M.; Bakare, B.F.; Tetteh, E.K.; Rathilal, S. Sequencing Batch Reactor Performance Evaluation on Orthophosphates and COD Removal from Brewery Wastewater. Fermentation 2022, 8, 296. [Google Scholar] [CrossRef]
- Bakare, B.; Shabangu, K.; Chetty, M. Brewery wastewater treatment using laboratory scale aerobic sequencing batch reactor. S. Afr. J. Chem. Eng. 2017, 24, 128–134. [Google Scholar] [CrossRef]
- Wang, S.-G.; Liu, X.-W.; Gong, W.-X.; Gao, B.-Y.; Zhang, D.-H.; Yu, H.-Q. Aerobic granulation with brewery wastewater in a sequencing batch reactor. Bioresour. Technol. 2007, 98, 2142–2147. [Google Scholar] [CrossRef]
- Moe, N.S.; Aung, E.M. A laboratory scale up-flow anaerobic sludge blanket (UASB) reactor for distillery wastewater treatment. Int. J. Sci. Eng. Technol. Res. 2014, 3, 4050–4055. [Google Scholar]
- Basandorj, D. Anaerobic Treatment of Brewery Wastewater Using UASB (Up Flow Anaerobic Sludge Blanket) Reactors Seeded with Activated Sludge. In Proceedings of the 2007 International Forum on Strategic Technology, Ulaanbaatar, Mongolia, 3–6 October 2007; pp. 630–631. [Google Scholar]
- Joshi, A. Performance Evaluation of Up-flow Anaerobic Sludge Blanket Reactor and Aerobic Digestor of Raj Brewery, Bhairahawa, Nepal. Master’s Thesis, Pulchowk Campus, Patan, Nepal, 2019. [Google Scholar]
- Meshksar, M.; Roostaee, T.; Rahimpour, M.R. Membrane technology for brewery wastewater treatment. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 289–303. [Google Scholar]
- Deschamps, L.; Merlet, D.; Lemaire, J.; Imatoukene, N.; Filali, R.; Clément, T.; Lopez, M.; Theoleyre, M.-A. Excellent performance of anaerobic membrane bioreactor in treatment of distillery wastewater at pilot scale. J. Water Process. Eng. 2021, 41, 102061. [Google Scholar] [CrossRef]
- Werkneh, A.A.; Beyene, H.D.; Osunkunle, A.A. Recent advances in brewery wastewater treatment; approaches for water reuse and energy recovery: A review. Environ. Sustain. 2019, 2, 199–209. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Maran, J.P. Application of chitosan as an adsorbent to treat rice mill wastewater—Mechanism, modelling and optimization. Carbohydr. Polym. 2013, 97, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Shabangu, K.P.; Bakare, B.F. Study of an SBR Treating Brewery Wastewater: Case of COD-HRT and BOD Removal. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 22–24 October 2019; pp. 22–24. [Google Scholar]
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Hassan, M.A.; Li, T.P.; Noor, Z.Z. Coagulation and flocculation treatment of wastewater in textile industry using chitosan. J. Chem. Nat. Resour. Eng. 2009, 4, 43–53. [Google Scholar]
- Onsøyen, E.; Skaugrud, O. Metal recovery using chitosan. J. Chem. Technol. Biotechnol. 1990, 49, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Nechita, P. Applications of chitosan in wastewater treatment. Biol. Act. Appl. Mar. Polysacch. 2017, 1, 209–228. [Google Scholar]
- Abd El-Monaem, E.M.; Eltaweil, A.S.; Elshishini, H.M.; Hosny, M.; Abou Alsoaud, M.M.; Attia, N.F.; El-Subruiti, G.M.; Omer, A.M. Sustainable adsorptive removal of antibiotic residues by chitosan composites: An insight into current developments and future recommendations. Arab. J. Chem. 2022, 15, 103743. [Google Scholar] [CrossRef]
- De Farias, B.S.; Grundmann, D.D.R.; Rizzi, F.Z.; Martins, N.S.S.; Junior, T.R.S.A.C.; de Almeida Pinto, L.A. Production of low molecular weight chitosan by acid and oxidative pathways: Effect on physicochemical properties. Food Res. Int. 2019, 123, 88–94. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Surgutskaia, N.S.; Di Martino, A.; Zednik, J.; Ozaltin, K.; Lovecka, L.; Bergerova, E.D.; Kimmer, D.; Svoboda, J.; Sedlarik, V. Efficient Cu2+, Pb2+ and Ni2+ ion removal from wastewater using electrospun DTPA-modified chitosan/polyethylene oxide nanofibers. Sep. Purif. Technol. 2020, 247, 116914. [Google Scholar] [CrossRef]
- Dong, L.; Shan, C.; Liu, Y.; Sun, H.; Yao, B.; Gong, G.; Jin, X.; Wang, S. Characterization and mechanistic study of heavy metal adsorption by facile synthesized magnetic xanthate-modified chitosan/polyacrylic acid hydrogels. Int. J. Environ. Res. Public Health 2022, 19, 11123. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; El-Tawil, A.M.; Abd El-Monaem, E.M.; El-Subruiti, G.M. Zero valent iron nanoparticle-loaded nanobentonite intercalated carboxymethyl chitosan for efficient removal of both anionic and cationic dyes. ACS Omega 2021, 6, 6348–6360. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Bridgewater, L.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Nair, A.T.; Makwana, A.R.; Ahammed, M.M. The use of response surface methodology for modelling and analysis of water and wastewater treatment processes: A review. Water Sci. Technol. 2014, 69, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Cheng, W.P.; Chi, F.H.; Yu, R.F.; Lee, Y.C. Using chitosan as a coagulant in recovery of organic matters from the mash and lauter wastewater of brewery. J. Polym. Environ. 2005, 13, 383–388. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y. Coagulation of colloidal particles in water by chitosan. J. Chem. Technol. Biotechnol. 1996, 66, 227–232. [Google Scholar] [CrossRef]
- Domard, A.; Rinaudo, M.; Terrassin, C. Adsorption of chitosan and a quaternized derivative on kaolin. J. Appl. Polym. Sci. 1989, 38, 1799–1806. [Google Scholar] [CrossRef]
- Khan, N.A.; Morabet, R.E.; Khan, R.A.; Alsubih, M.; Gaurav, G.K.; Klemeš, J.J.; Thakur, A.K. Modelling and parameter optimisation for performance evaluation of sequencing batch reactor for treating hospital wastewater. Biomass. Convers. Biorefin. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Peng, D.; Teng, Z.; Ju, X. Treatment of brewery wastewater using anaerobic sequencing batch reactor (ASBR). Bioresour. Technol. 2008, 99, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Shabangu, K.P.; Bakare, B.F.; Bwapwa, J.K. The treatment effect of chemical coagulation process in South African brewery wastewater: Comparison of polyamine and aluminum-chlorohydrate coagulants. Water 2022, 14, 2495. [Google Scholar] [CrossRef]
- Swain, K.; Abbassi, B.; Kinsley, C. Combined electrocoagulation and chemical coagulation in treating brewery wastewater. Water 2020, 12, 726. [Google Scholar] [CrossRef]
- Ferral-Pérez, H.; Torres Bustillos, L.; Méndez, H.; Rodríguez-Santillan, J.; Chairez, I. Sequential treatment of tequila industry vinasses by biopolymer-based coagulation/flocculation and catalytic ozonation. Ozone Sci. Eng. 2016, 38, 279–290. [Google Scholar] [CrossRef]
- Wagh, M.P.; Nemade, P. Treatment of distillery spent wash by using chemical coagulation (CC) and electro-coagulation (EC). Am. J. Environ. Prot. 2015, 3, 159–163. [Google Scholar]
- Farshi, R.; Priya, S.; Saidutta, M. Reduction of color and COD of anaerobically treated distillary wastewater by electrochemical method. In Proceedings of the National Conference on Women in Science & Engineering (SCWSE), Dharwad, India, 5 July 2013. [Google Scholar]
- Krishna, B.; Murthy, U.N.; Manoj Kumar, B.; Lokesh, K. Electrochemical pretreatment of distillery wastewater using aluminum electrode. J. Appl. Electrochem. 2010, 40, 663–673. [Google Scholar] [CrossRef]

| Physicochemical Property | Value |
|---|---|
| pH | 7.15 |
| Dissolved oxygen | 8.33 mg/L |
| Turbidity | 160 NTU |
| Total organic carbon | 176 mg/L |
| Orthophosphates | 139 mg/L |
| Total dissolved solids | 1042 mg/L |
| Range and Level of Factors | |||
|---|---|---|---|
| Factor | Code | Low −1 | High +1 |
| pH | 4 | 8 | |
| Contact time (min) | 15 | 45 | |
| Chitosan dosage (g/L) | 2 | 4 | |
| Std | Run | Factors | Actual Values | RSM Predicted Values | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 1 | Response 2 | Response 3 | ||
| pH, (X1) | Coagulant Dosage, g/L (X2) | Time, Minutes (X3) | Turbidity Removal, % (Y1) | TOC Removal, % (Y2) | Removal, % (Y3) | Turbidity Removal, % (Y1) | TOC Removal, % (Y2) | Removal, % (Y3) | ||
| 14 | 1 | 6 | 3 | 55 | 0.625 | 88.64 | 1.00 | 0.6250 | 88.71 | 10.43 |
| 9 | 2 | 3 | 3 | 30 | 82.88 | 89.20 | 17.77 | 82.86 | 89.27 | 17.77 |
| 11 | 3 | 6 | 1 | 30 | 88.00 | 88.69 | 3.60 | 88.20 | 88.82 | 4.34 |
| 16 | 4 | 6 | 3 | 30 | 89.88 | 90.34 | 4.32 | 88.30 | 90.61 | 6.51 |
| 7 | 5 | 4 | 4 | 45 | 4.00 | 88.64 | 7.19 | 3.94 | 88.54 | 8.55 |
| 6 | 6 | 8 | 2 | 45 | 94.99 | 89.77 | 62.23 | 95.62 | 89.49 | 61.18 |
| 5 | 7 | 4 | 2 | 45 | 3.75 | 88.64 | 11.22 | 3.81 | 88.64 | 5.97 |
| 10 | 8 | 9 | 3 | 30 | 93.75 | 88.64 | 44.60 | 93.74 | 88.71 | 44.60 |
| 4 | 9 | 8 | 4 | 15 | 41.38 | 88.64 | 35.83 | 40.06 | 88.54 | 39.82 |
| 1 | 10 | 4 | 2 | 15 | 11.88 | 88.64 | 20.22 | 10.25 | 88.64 | 20.59 |
| 15 | 11 | 6 | 3 | 5 | 87.56 | 90.91 | 12.59 | 88.30 | 90.61 | 6.51 |
| 8 | 12 | 8 | 4 | 45 | 96.39 | 89.20 | 66.62 | 95.75 | 89.39 | 63.76 |
| 13 | 13 | 6 | 3 | 5 | 92.00 | 89.20 | 2.45 | 92.00 | 89.27 | 2.59 |
| 2 | 14 | 8 | 2 | 15 | 38.63 | 88.64 | 37.34 | 39.94 | 88.64 | 37.24 |
| 12 | 15 | 6 | 5 | 30 | 87.75 | 88.64 | 15.11 | 88.41 | 88.65 | 8.68 |
| 3 | 16 | 4 | 4 | 15 | 8.75 | 88.64 | 19.64 | 10.38 | 88.54 | 23.17 |
| Response | Turbidity | TOC | |
|---|---|---|---|
| p-values | <0.0001 | 0.0023 | <0.0001 |
| F-values | 1604.51 | 13.80 | 29.90 |
| Mean of squares | 2988.77 | 0.7822 | 896.25 |
| Sum of squares errors | 23,910.18 | 0.7822 | 896.25 |
| Standard deviation | 1.36 | 0.2381 | 5.48 |
| Mean | 57.64 | 89.07 | 22.61 |
| Coefficient of variance (C.V., %) | 2.37 | 0.2673 | 24.22 |
| Coefficient of determination (R2) | 0.9995 | 0.9539 | 0.9632 |
| Adjusted R2 | 0.9988 | 0.8848 | 0.9310 |
| Adequate precision | 92.9304 | 11.0195 | 15.800 |
| Response | Experimental Values (%) | RSM Predicted Values (%) | Standard Deviation |
|---|---|---|---|
| Turbidity | 91 | 90.168 | 0.588 |
| TOC | 89 | 89.559 | 0.395 |
| Orthophosphates | 65 | 59.904 | 3.603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumalo, S.M.; Bakare, B.F.; Tetteh, E.K.; Rathilal, S. Application of Response Surface Methodology on Brewery Wastewater Treatment Using Chitosan as a Coagulant. Water 2023, 15, 1176. https://doi.org/10.3390/w15061176
Khumalo SM, Bakare BF, Tetteh EK, Rathilal S. Application of Response Surface Methodology on Brewery Wastewater Treatment Using Chitosan as a Coagulant. Water. 2023; 15(6):1176. https://doi.org/10.3390/w15061176
Chicago/Turabian StyleKhumalo, Siphesihle Mangena, Babatunde Femi Bakare, Emmanuel Kweinor Tetteh, and Sudesh Rathilal. 2023. "Application of Response Surface Methodology on Brewery Wastewater Treatment Using Chitosan as a Coagulant" Water 15, no. 6: 1176. https://doi.org/10.3390/w15061176
APA StyleKhumalo, S. M., Bakare, B. F., Tetteh, E. K., & Rathilal, S. (2023). Application of Response Surface Methodology on Brewery Wastewater Treatment Using Chitosan as a Coagulant. Water, 15(6), 1176. https://doi.org/10.3390/w15061176










