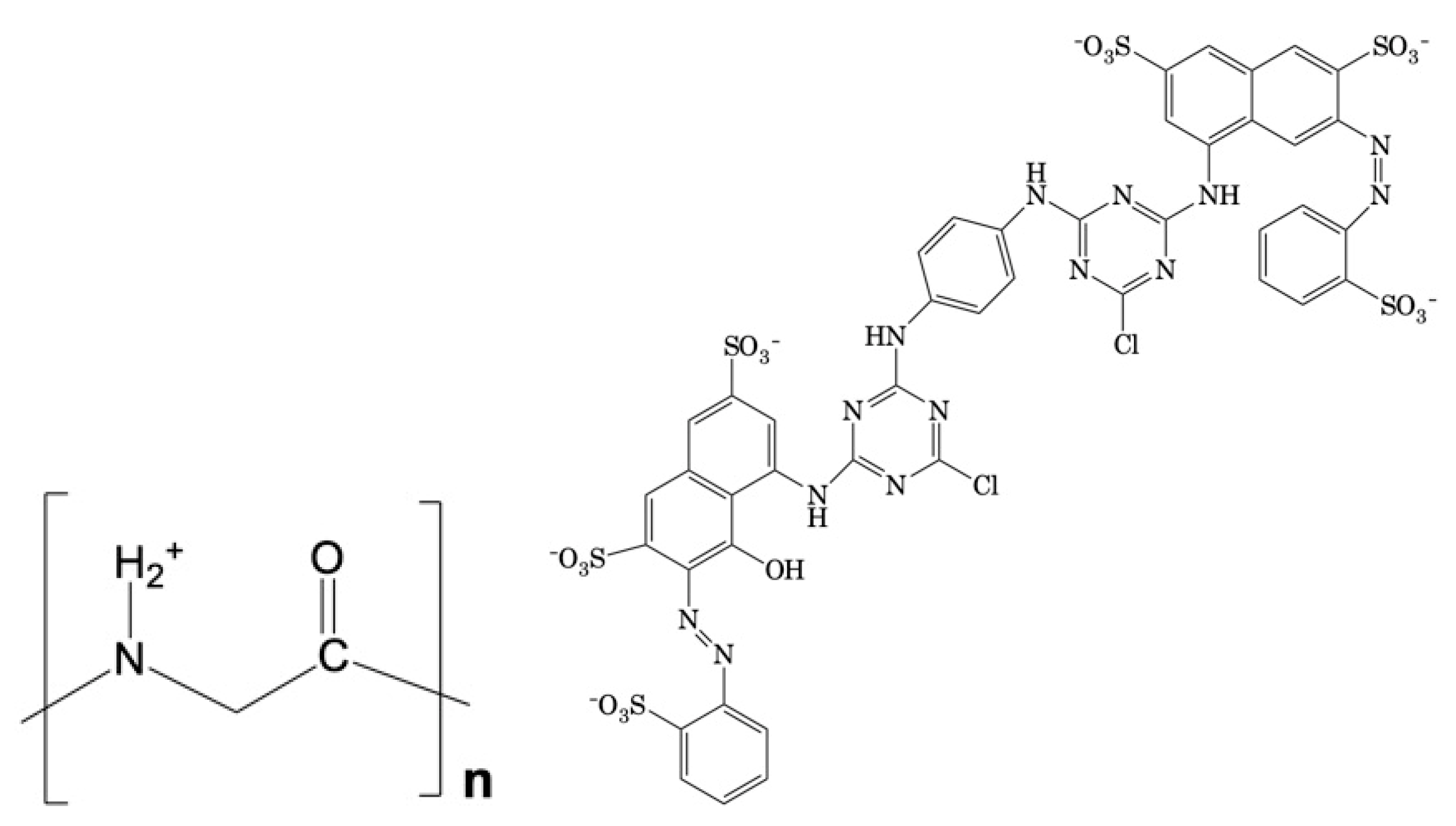
Adsorption of Reactive Red 120 Dye by Polyamide Nylon 6 Microplastics: Isotherm, Kinetic, and Thermodynamic Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
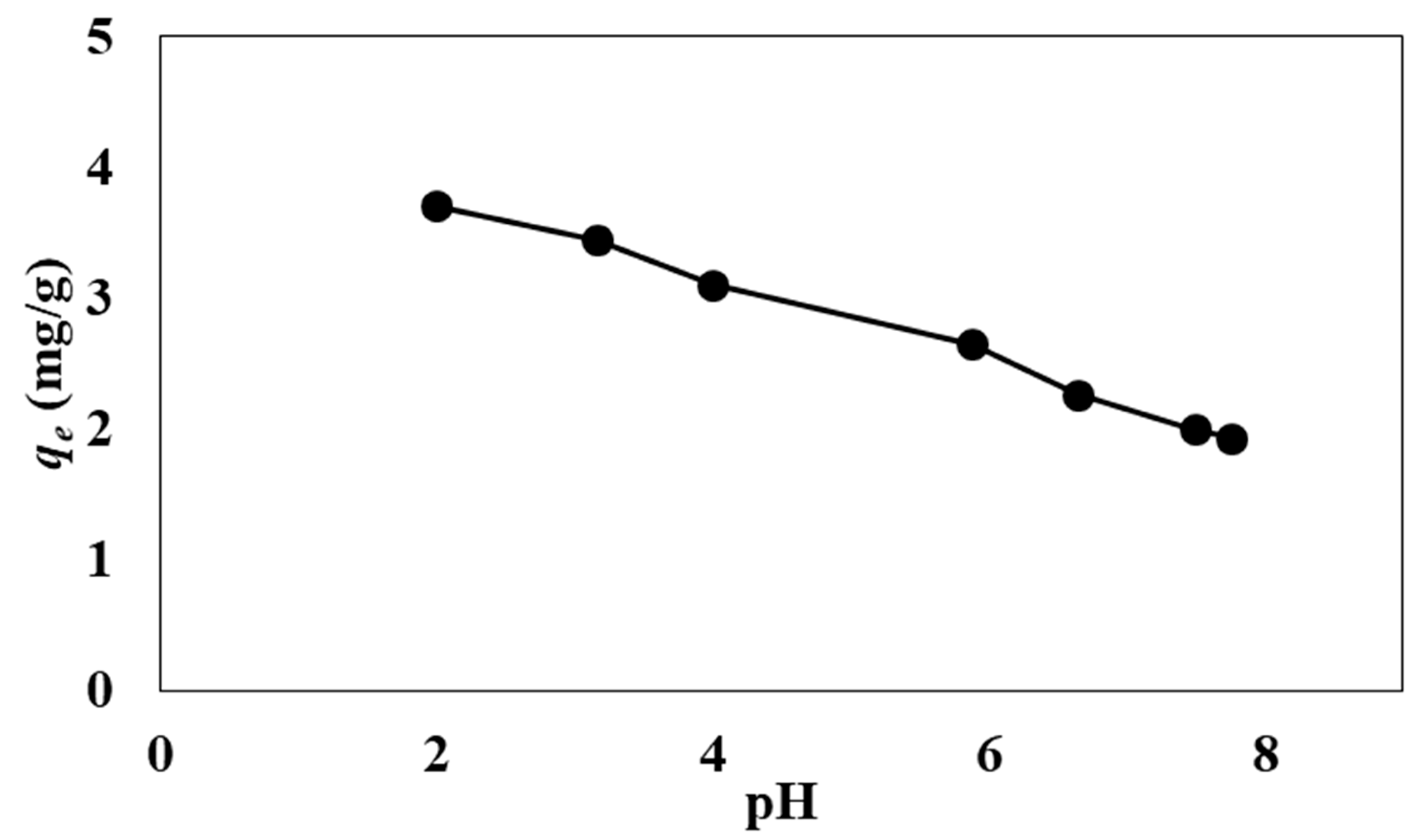
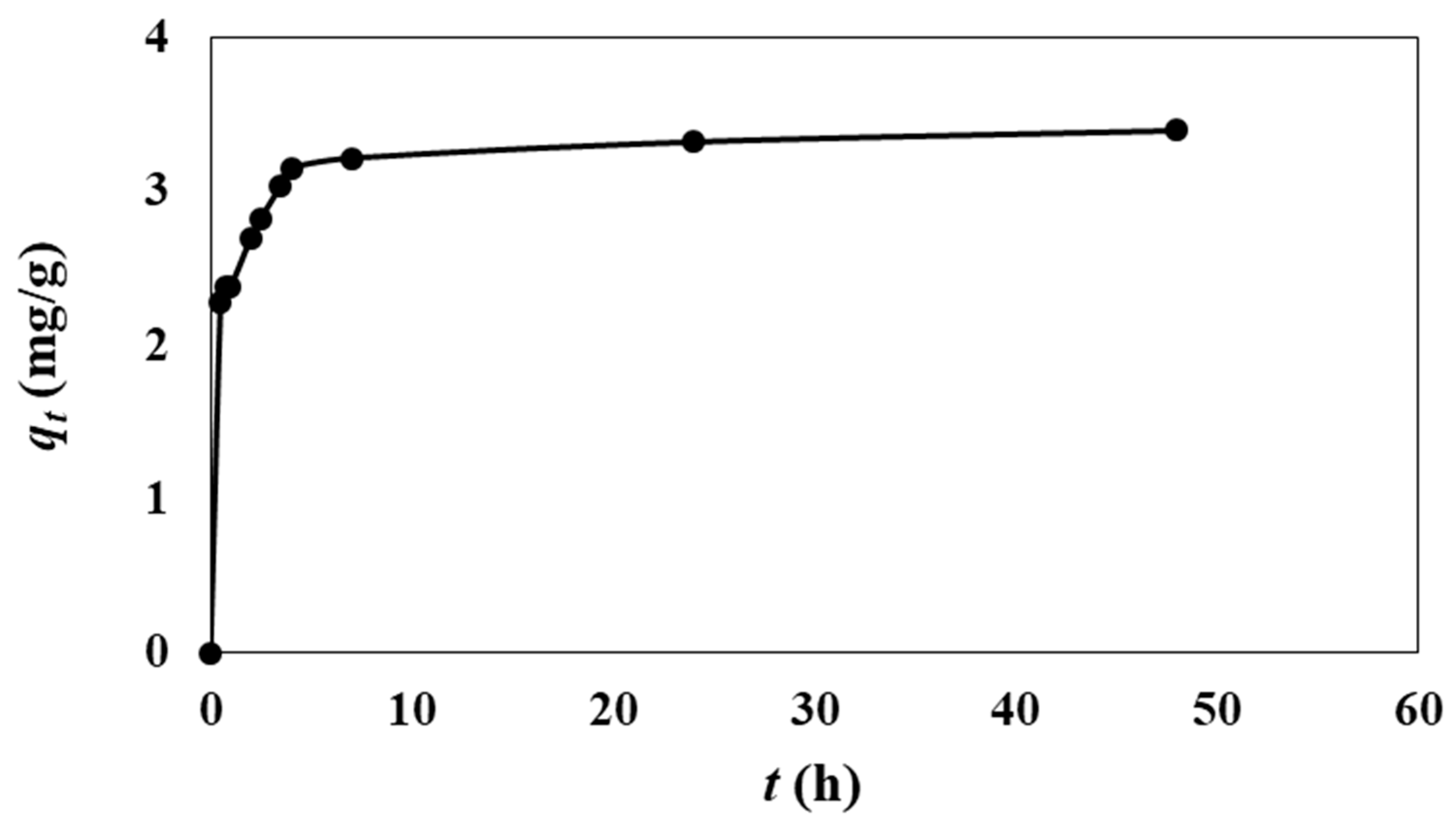
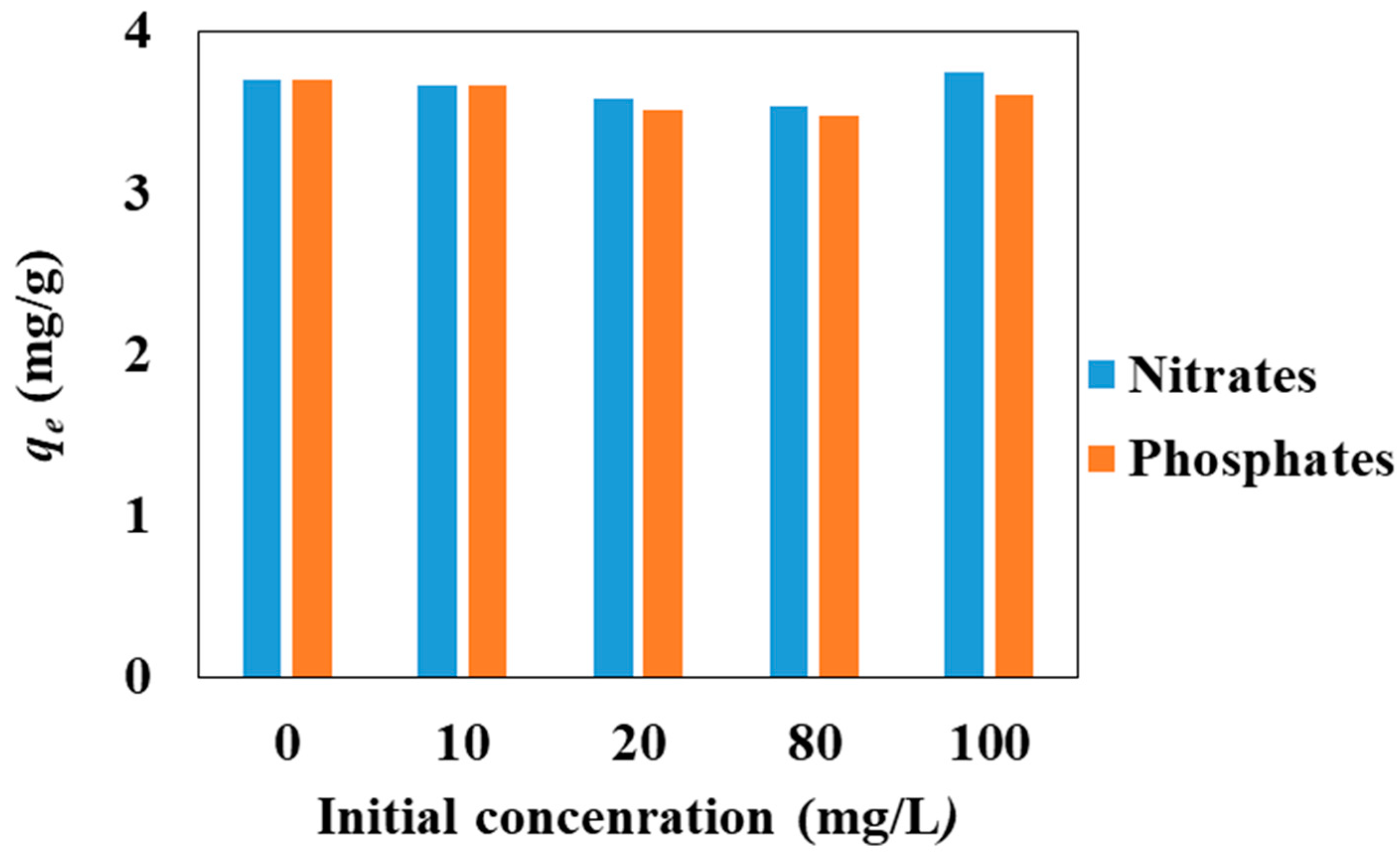
3.1. Effect of Adsorption Parameters (pH, Contact Time, Temperature, and Co-Existing Anions)
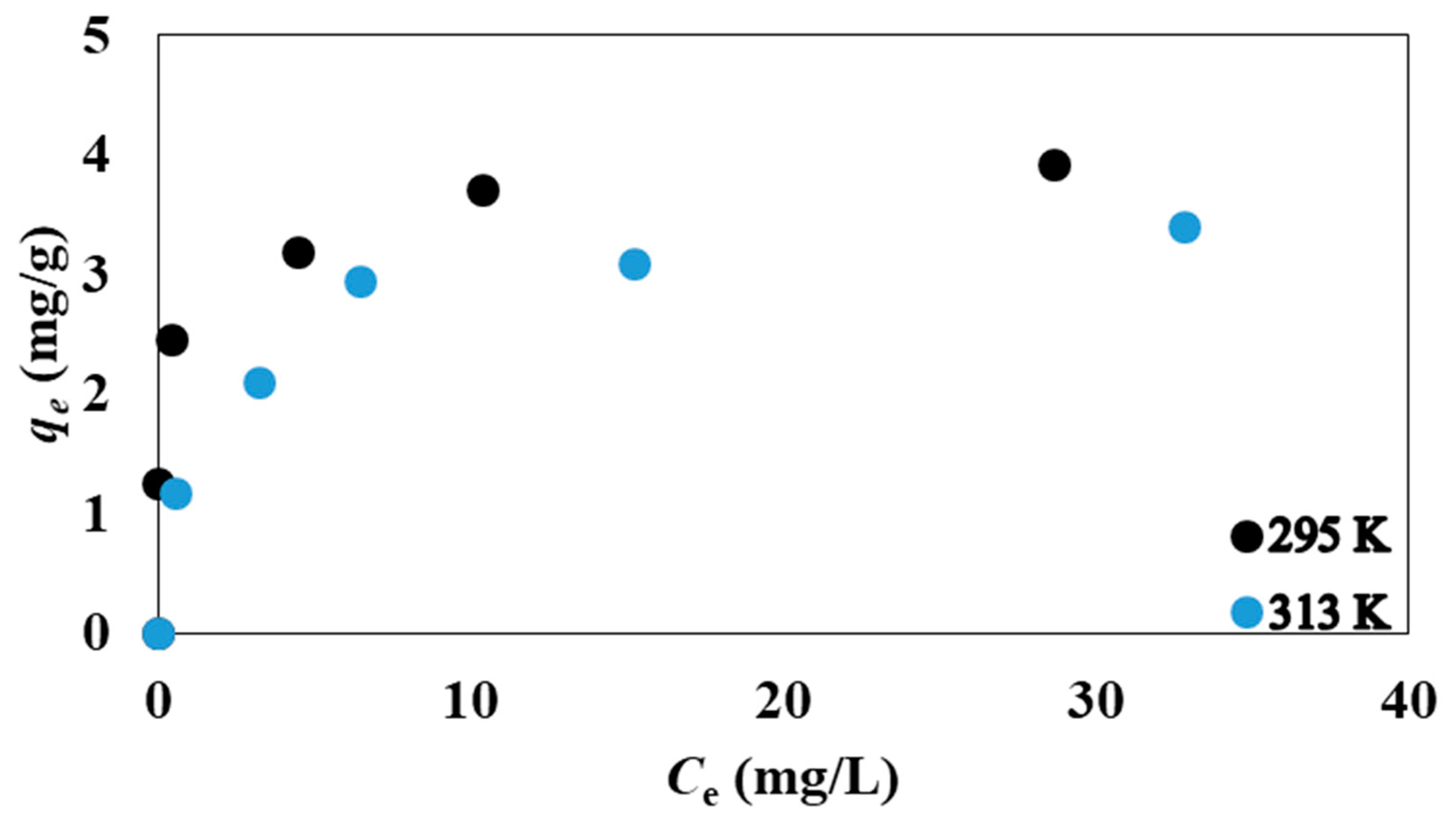
3.2. Isotherm, Kinetic and Thermodynamic Modeling
4. Conclusions and Future Works
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumari, A.; Rajput, V.D.; Mandzhieva, S.S.; Rajput, S.; Minkina, T.; Kaur, R.; Sushkova, S.; Kumari, P.; Ranjan, A.; Kalinitchenko, V.P. Microplastic Pollution: An Emerging Threat to Terrestrial Plants and Insights into Its Remediation Strategies. Plants 2022, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, L.; Arulmani, S.R.B.; Yan, J.; Wu, L.; Wu, T.; Zhang, H.; Xiao, T. Adsorption of different pollutants by using microplastic with different influencing factors and mechanisms in wastewater: A review. Nanomaterials 2022, 12, 2256. [Google Scholar] [CrossRef] [PubMed]
- Winnacker, M. Polyamides and their functionalization: Recent concepts for their applications as biomaterials. Biomater. Sci. 2017, 5, 1230–1235. [Google Scholar] [CrossRef]
- Didovets, Y.; Brela, M.Z. Theoretical Study on the Thermal Degradation Process of Nylon 6 and Polyhydroxybutyrate. Physchem 2022, 2, 334–346. [Google Scholar] [CrossRef]
- Völtz, L.R.; Geng, S.; Teleman, A.; Oksman, K. Influence of Dispersion and Orientation on Polyamide-6 Cellulose Nanocomposites Manufactured through Liquid-Assisted Extrusion. Nanomaterials 2022, 12, 818. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, M.; Yasid, N.A.; Othman, A.R.; Gunasekaran, B.; Halmi, M.I.E.; Shukor, M.Y.A. Biodecolourisation of Reactive Red 120 as a sole carbon source by a bacterial consortium—Toxicity assessment and statistical optimisation. Int. J. Environ. Res. Public Health 2021, 18, 2424. [Google Scholar] [CrossRef] [PubMed]
- Aliasghar Navaeia, M.; Alidadid, H.; Dankooba, M.; Bonyadid, Z.; Dehghand, A.; Hosseinie, A. Biosorption of Reactive Red 120 dye from aqueous solution using Saccharomyces cerevisiae: RSM analysis, isotherms and kinetic studies. Desalination Water Treat. 2019, 171, 418–427. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Kayan, B.; Kalderis, D. Microplastics as carriers of hydrophilic pollutants in an aqueous environment. J. Mol. Liq. 2021, 350, 118182. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Anastopoulos, I.; Barczak, M.; Antoniou, Ε.; Terpiłowski, K.; Mohammadi, E.; Shams, M.; Coy, E.; Bakandritsos, A.; Katsoyiannis, I.A.; et al. Enhanced uranium removal from acidic wastewater by phosphonate-functionalized ordered mesoporous silica: Surface chemistry matters the most. J. Hazard. Mater. 2021, 413, 125279. [Google Scholar] [CrossRef] [PubMed]
- Munagapati, V.S.; Wen, J.-C.; Pan, C.-L.; Gutha, Y.; Wen, J.-H. Enhanced adsorption performance of Reactive Red 120 azo dye from aqueous solution using quaternary amine modified orange peel powder. J. Mol. Liq. 2019, 285, 375–385. [Google Scholar] [CrossRef]
- Ioannidis, I.; Xenofontos, A.; Anastopoulos, I.; Pashalidis, I. Americium Sorption by Microplastics in Aqueous Solutions. Coatings 2022, 12, 1452. [Google Scholar] [CrossRef]
- Cardoso, N.F.; Lima, E.C.; Royer, B.; Bach, M.V.; Dotto, G.L.; Pinto, L.A.; Calvete, T. Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of Reactive Red 120 dye from aqueous effluents. J. Hazard. Mater. 2012, 241, 146–153. [Google Scholar] [CrossRef]
- Ay, Ç.; Sarpaşar, Z. Using zeolite and Fe3O4@ zeolite composites in removal of Reactive Red 120 from wastewater: Isotherm, kinetic, thermodynamic and adsorption behaviors. J. Dispers. Sci. Technol. 2022, 44, 370–381. [Google Scholar] [CrossRef]
- Jawad, A.H.; Shazwani, N.; Mubarak, A.; Sabar, S. Adsorption and mechanism study for reactive red 120 dye removal by cross-linked chitosan-epichlorohydrin biobeads. Compos. Part B Eng 2019, 75, 415–418. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mostafapour, F.K.; Hosseini, A.R.; Raksh Khorshid, A.; Mahvi, A.H. Decolorisation of reactive red 120 dye by using single-walled carbon nanotubes in aqueous solutions. J. Chem. 2013, 2013, 938374. [Google Scholar] [CrossRef] [Green Version]
- Çelekli, A.; Al-Nuaimi, A.I.; Bozkurt, H. Adsorption kinetic and isotherms of Reactive Red 120 on Moringa oleifera seed as an eco-friendly process. J. Mol. Struct. 2019, 1195, 168–178. [Google Scholar] [CrossRef]
- Tabak, A.; Baltas, N.; Afsin, B.; Emirik, M.; Caglar, B.; Eren, E. Adsorption of Reactive Red 120 from aqueous solutions by cetylpyridinium-bentonite. J. Chem. Technol. Biotechnol. 2010, 85, 1199–1207. [Google Scholar] [CrossRef]
- Al Rubai, H.F.; Hassan, A.K.; Sultan, M.S.; Abood, W.M. Kinetics of Adsorption of Reactive Red 120 Using Bentonite Modified by CTAB and Study the Effect of Salts. Nat. Environ. Pollut. Technol. 2021, 20, 281–289. [Google Scholar] [CrossRef]
| Starting Concentration (mg/L) | pH | Time of Contact (h) | T (K) | ||
|---|---|---|---|---|---|
| Initial concentration (mg/L) | 10–60 | 2 | 24 | 295 K | - |
| pH | 40 | 2–7.8 | 24 | 295 K | - |
| Contact time (min or h) | 35 | 2 | 0.5–48 | 295 K | - |
| Temperature (K) | 10–60 | 2 | 24 | 295 Κ and 313 Κ | - |
| Presence of or | 40 | 2 | 24 | 295 K | 10–100 |
| Expression | Equation | Constants |
|---|---|---|
| Langmuir (L) [10] | (mg/g): maximum monolayer adsorption capacity (L/mg): constant related to the energy of sorption and equilibrium constant | |
| Freundlich (F) [11] | (mg/g)(L/mg)1/n: Freundlich constant 1/n (dimensionless): adsorption intensity parameter | |
| Pseudo-first-order kinetic (PS1) [12] | qt (mg/g): the amount adsorbed at time t (min) k1 (min−1): PS1 rate constant | |
| Pseudo-second-order kinetic (PS2) [13] | qt (mg/g): the amount adsorbed at time t (min) k2 (g/mg min): PS2 rate constant | |
| Gibbs [14] | Free energy change |
| T (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | KF (mg/g)(L/mg)1/n | n | R2 | |
| 295 | 3.96 | 2.148 | 1 | 2.32 | 5.34 | 0.89 |
| 313 | 3.54 | 0.607 | 1 | 1.50 | 3.75 | 0.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afmataj, D.; Kordera, O.; Maragkaki, A.; Tzanakakis, V.A.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Adsorption of Reactive Red 120 Dye by Polyamide Nylon 6 Microplastics: Isotherm, Kinetic, and Thermodynamic Analysis. Water 2023, 15, 1137. https://doi.org/10.3390/w15061137
Afmataj D, Kordera O, Maragkaki A, Tzanakakis VA, Pashalidis I, Kalderis D, Anastopoulos I. Adsorption of Reactive Red 120 Dye by Polyamide Nylon 6 Microplastics: Isotherm, Kinetic, and Thermodynamic Analysis. Water. 2023; 15(6):1137. https://doi.org/10.3390/w15061137
Chicago/Turabian StyleAfmataj, Desara, Olympia Kordera, Angeliki Maragkaki, Vasileios A. Tzanakakis, Ioannis Pashalidis, Dimitrios Kalderis, and Ioannis Anastopoulos. 2023. "Adsorption of Reactive Red 120 Dye by Polyamide Nylon 6 Microplastics: Isotherm, Kinetic, and Thermodynamic Analysis" Water 15, no. 6: 1137. https://doi.org/10.3390/w15061137
APA StyleAfmataj, D., Kordera, O., Maragkaki, A., Tzanakakis, V. A., Pashalidis, I., Kalderis, D., & Anastopoulos, I. (2023). Adsorption of Reactive Red 120 Dye by Polyamide Nylon 6 Microplastics: Isotherm, Kinetic, and Thermodynamic Analysis. Water, 15(6), 1137. https://doi.org/10.3390/w15061137










