A New Record of a Nonnative Bivalve Species in an Amazonian Environmental Protection Area: What Might Have Happened?
Abstract
:1. Introduction
2. Material and Methods
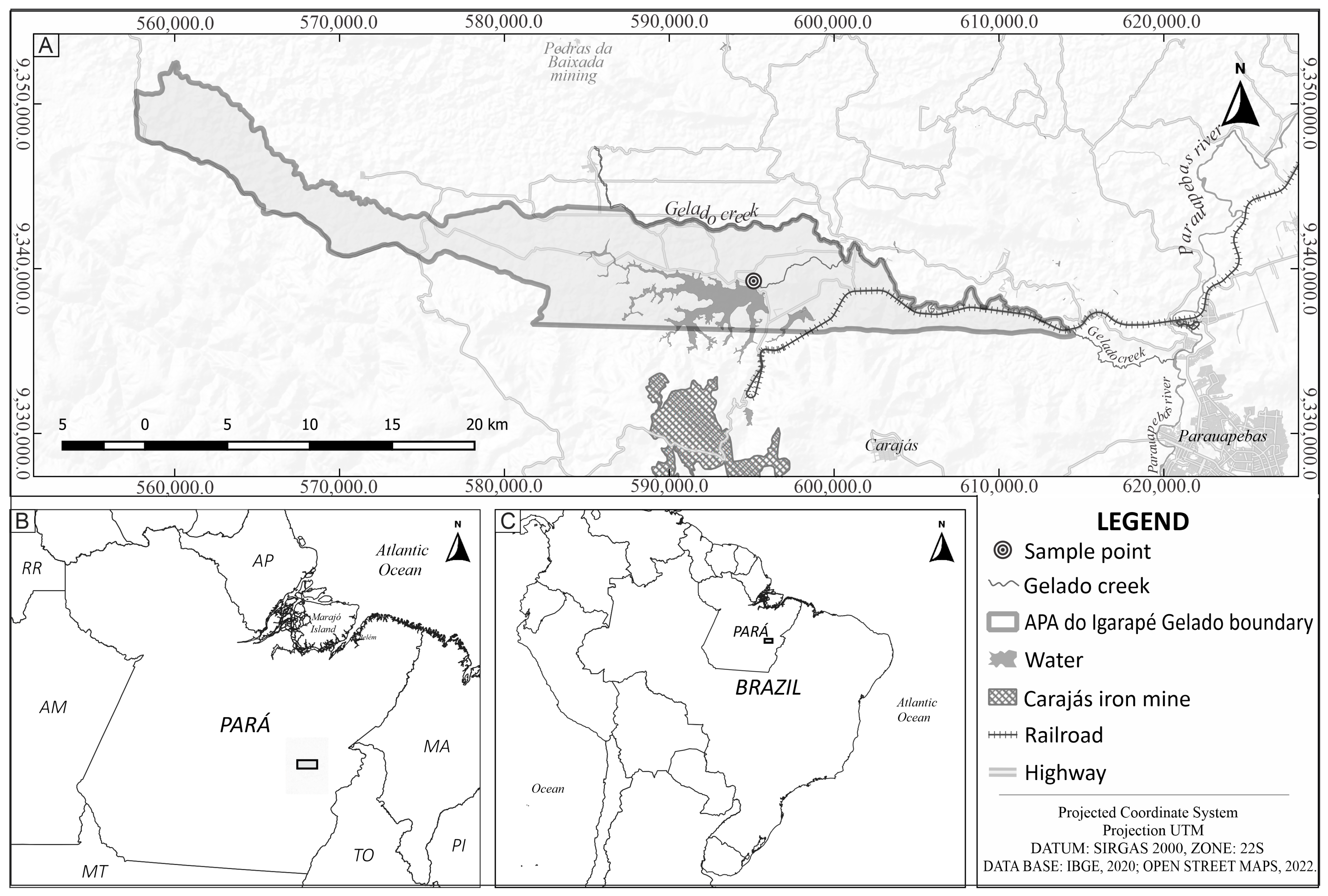
2.1. Study Area
2.2. Biological Sampling
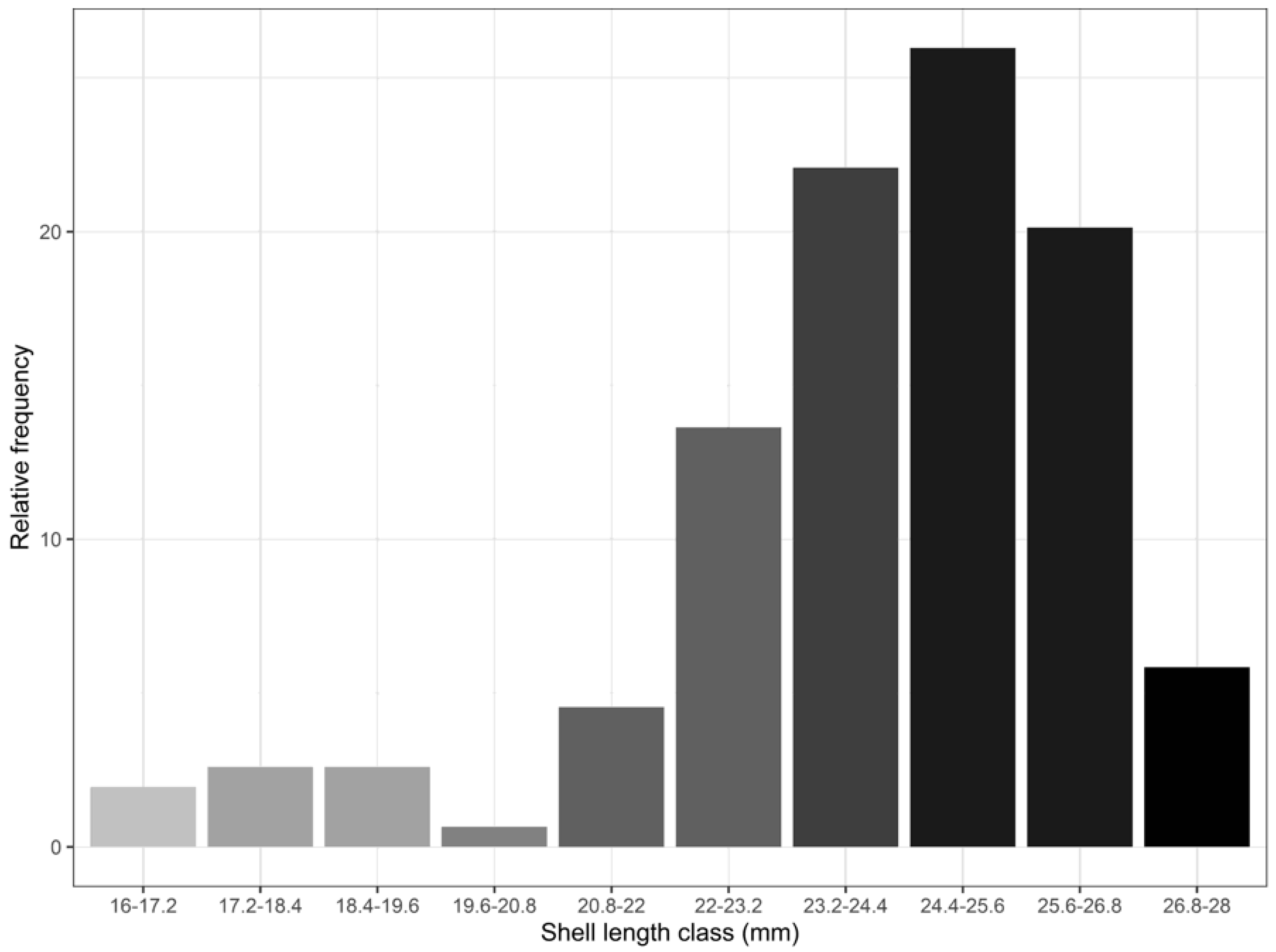
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veitenheimer-Mendes, I.L. Corbicula manilensis (Philippi, 1844) molusco asiático, na bacia do Jacuí e do Guaíba, Rio Grande do Sul, Brasil (Bivalvia, Corbiculidae). Iheningia 1981, 60, 63–74. [Google Scholar]
- Mansur, M.C.D.; Garces, L. Ocorrência e densidade de Corbicula fluminea (Mueller, 1774) e Neocorbicula limosa (Maton,1811) na Estação Ecológica do Taim e áreas adjacentes, Rio Grande do Sul, Brasil (Mollusca, Bivalvia, Corbiculidae). Iheringia Zool. 1988, 68, 99–115. [Google Scholar]
- Avelar, W.E.P. Moluscos bivalves. Biodiversidade de Estado de São Paulo, Brasil: Síntese do conhecimento ao final do século XX. In Invertebrados de Água Doce; Ismael, D., Valenti, W.C., Matsumura-Tundisi, T., Rocha, O., Eds.; Fapesp: São Paulo, Brazil, 1999; Volume 4, pp. 65–68. [Google Scholar]
- Suriani, A.L.; Roberta, S.F.; Rocha, O. Benthic malacofauna of the reservoirs of the Middle River Tietê (São Paulo, Brazil) and an ecological evaluation of the invading exotic species, Melanoides tuberculata (Müller) and Corbicula fluminea (Müller). Rev. Bras. Zool. 2007, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Vianna, M.P.; Avelar, W.E.P. Ocorrência da espécie invasora Corbicula fluminea (Bivalvia, Corbiculidae) no rio Sapucaí (São Paulo, Brasil). Biotemas 2010, 23, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Lima, J.C.S. Novos registros de Corbicula fluminea (MÜLLER, 1774) (Bivalvia, Corbiculidae) no Sudeste do Brasil. Rev.De Ciências Ambient. RCA 2017, 7, 11–12. [Google Scholar] [CrossRef] [Green Version]
- Callil, C.; Mansur, M.C. Corbiculidae in the Pantanal: History of invasion in southeast and central South America and biometrical data. Amazoniana 2002, XVII, 153–167. [Google Scholar]
- Poleze, M.; Callil, C.T. Bivalvia, Cyrenidae, Corbicula fluminea (Müller, 1774): New record, density, and population structure in the Teles Pires River, northern Mato Grosso, Brazil. Check List. 2015, 11, 1–5. [Google Scholar] [CrossRef]
- Santana, D.O.; Silva, M.J.M.; Bocchiglieri, A.; Pantaleão, S.M.; Faria, R.G.; Souza, B.B.; Rocha, S.M.; Lima, L.F.O. Mollusca, Bivalvia, Corbiculidae, Corbicula fluminea (Müller, 1774): First record for the Caatinga biome, northeastern Brazil. Check List. 2013, 9, 1072–1074. [Google Scholar] [CrossRef] [Green Version]
- Rosa, L.C.; Dantas, J.O. First record of the Asian clam Corbicula fluminea (Müller, 1774) (Bivalvia: Cyrenidae) at Poxim-Açu River, northeastern Brazil. Acta Limnol. Bras. 2020, 32, 1–4. [Google Scholar] [CrossRef]
- Beasley, C.R.; Tagliaro, C.H.; Figueiredo, W.B. The occurrence of the Asian clam Corbicula fluminea in the lower Amazon basin. Acta Amaz. 2003, 33, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Pimpão, D.M.; Martins, D.S. Occurrence of the Asian mollusc Corbicula fluminea (Müller, 1774) (Bivalvia, Corbiculidae) in the lower Rio Negro, Central Amazon Region, Brazil. Acta Amaz. 2008, 38, 589–592. [Google Scholar] [CrossRef]
- Instituto Chico Mendes de Conservação da Biodiversidade Guia Técnico de Prevenção de Invasão Biológica Associada a Atividades de Empreendimentos Licenciáveis em Unidades de Conservação Federais. Available online: https://wwfbr.awsassets.panda.org/downloads/pub_guiatecprev_icmbio_v7_29abr22_final_web_governofederal_compactado.pdf (accessed on 7 September 2022).
- Guimarães, T.C.S. Espécies Exóticas Invasoras da Fauna em Unidades de Conservação Federais no Brasil: Sistematização do Conhecimento e Implicações para o Manejo. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 2015. Available online: https://repositorio.unb.br/handle/10482/18866 (accessed on 12 March 2023).
- Leal, M.F.; Simone, L.R.L.; Lacerda, A.C.F.; Silva, E.L.; Pinheiro, T.G. Current distribution of the invasive molluk Corbicula fluminea (O.F. Müller, 1774) (Bivalvia, Cyrenidae in Brazil, including a new record from the state of Piauí. Check List. 2021, 1, 151–157. [Google Scholar] [CrossRef]
- Instituto Chico Mendes de Conservação da Biodiversidade Plano de Manejo da Área de Proteção Ambiental do Igarapé Gelado (Org.). 2017. Available online: https://www.gov.br/icmbio/pt-br/assuntos/biodiversidade/unidade-de-conservacao/unidades-de-biomas/amaz-nia/lista-de-ucs/apa-do-iguarape-gelado/arquivos/resumo_executivo-planodemanejo_apa_igarape_gelado.pdf (accessed on 7 September 2020).
- Patrick, C.H.; Waters, M.N.; Golladay, S.W. The distribution and ecological role of Corbicula fluminea (Muller, 1774) in a large and shallow reservoir. BioInvasions Rec. 2017, 6, 39–48. [Google Scholar] [CrossRef]
- Sousa, R.; Antunes, C.; Guilhermino, L. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: An overview. Ann. Limnol. 2008, 44, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Geist, J.; Benedict, A.; Dobler, A.H.; Hoess, R.; Hoos, P. Functional interactions of non-native aquatic fauna with European freshwater bivalves: Implications for management. Hydrobiologia 2023, 1, 1–24. [Google Scholar] [CrossRef]
- Ilarri, M.I.; Souza, A.T.; Antunes, C.; Guilhermino, L.; Sousa, R. Influence of the invasive Asian clam Corbicula fluminea (Bivalvia: Corbiculidae) on estuarine epibenthic assemblages. Estuar. Coast. Shelf Sci. 2014, 143, 12–19. [Google Scholar] [CrossRef]
- Ferreira-Rodriguez, N.; Sousa, R.; Pardo, I. Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia 2018, 810, 85–95. [Google Scholar] [CrossRef]
- Kao, Y.C.; Adlerstein, S.A.; Rutherford, E.S. Assessment of top-down and bottom-up controls on the collapse of ale-wives (Alosa pseudoharengus) in Lake Huron. Ecosystems 2016, 19, 805–831. [Google Scholar] [CrossRef]
- Chiarello, M.; Bucholz, J.R.; McCauley, M.; Vaughn, S.N.; Garrett, W.; Hopper, G.W.; González, I.S.; Atkinson, C.L.; Lozier, J.D.; Jackson, C. Environment and Co-occurring Native Mussel Species, but Not Host Genetics, Impact the Microbiome of a Freshwater Invasive Species (Corbicula fluminea). Front. Microbiol. 2022, 13, 4–13. [Google Scholar] [CrossRef]
- Pigneur, L.M.; Etoundi, E.; Aldridge, D.C.; Marescaux, J.; Yasuda, N.; Doninck, K.V. Genetic uniformity and long-distance clonal dispersal in the invasive androgenetic Corbicula clams. Mol. Ecol. 2014, 23, 5102–5116. [Google Scholar] [CrossRef]
- Takeda, A.M.; Fujita, D.S.; Fontes, H.M., Jr. Perspectives on exotic bivalves proliferation in the Upper Paraná River Floodplain. In Structure and Functioning of the Paraná River and Its Floodplain; Agostinho, A.A., Rodrigues, L., Gomes, L.C., Thomaz, S.M., Miranda, L.E., Eds.; EDUEM: Maringá, Brazil, 2004; pp. 97–100. [Google Scholar]
- Lima, M.W.; Pereira, W.V.S.; Souza, E.S.; Teixeira, R.A.; Palheta, D.C.; Faial, K.C.F.; Costa, H.F.; Fernandes, A.R. Bioaccumulation and human health risks of potentially toxic elements in fish species from the southeastern Carajás Mineral Province, Brazil. Environ. Res. 2022, 204, 112024. [Google Scholar] [CrossRef] [PubMed]
- Instituto Chico Mendes de Conservação da Biodiversidade Plano de Conservação Estratégico Para o Território 172 de Carajás (Org.). 2020. Available online: https://www.gov.br/icmbio/pt-br/centrais-de-conteudo/publicacoes/planos/plano_de_conservacao_estra173tegico_para_o_territorio_de_carajascompactado.pdf (accessed on 7 September 2020).
- Sioli, H. The Amazon and Its Main Affluents: Hydrography, Morphology of the River Courses, and River Types; Monographiae Biologicae; Springer: Dordrecht, Switzerland, 1974. [Google Scholar] [CrossRef]
- Pontes, F.H.G.; Bringel, F.O. For whom is the environmental protection? Territorial disputes between Vale and rural peasant communits: The case of the Rio Gelado Environmental Protection. Ambientes 2021, 3, 330–359. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Kõppen’s climate classification map for Brazil. Meteorol. Z 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Silva Júnior, R.O.D.; Souza, E.B.D.; Tavares, A.L.; Mota, J.A.; Ferreira, D.B.S.; Souza-Filho, P.W.M.; Rocha, E.J.P.D.A. Three decades of reference evapotranspiration estimates for a tropical watershed in the eastern Amazon. An. Acad. Bras. Cienc 2017, 89, 1985–2002. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.F.; Maia, M.B.; Maneschy, M.C.; Matlaba, V.; Mota, J.A. Redes sociais ao longo da estrada de ferro Carajás na Amazônia Oriental. Finisterra 2018, 53, 149–166. [Google Scholar] [CrossRef]
- Caitano, T.B.S.; Silva, E.R.P.; Alves, C.N. Characterization and safety analysis of iron mining dams located in the state of Pará, Brazil. Res. Soc. Dev. 2021, 10, e35810313384. [Google Scholar] [CrossRef]
- Isaac, A.; Fernandes, A.; Ganassin, M.J.M.; Hahn, N.S. Ocorrência de três espécies invasoras nas dietas de peixes em uma planície de inundação Neotropical. Braz. J. Biol. 2014, 74, S16–S22. [Google Scholar] [CrossRef] [Green Version]
- Sazima, I.; D’Angelo, G.B. The Asian invasive freshwater clam Corbicula fluminea as prey of two native waterbirds in South-eastern Brazil. Folia Malacol. 2013, 21, 293–295. [Google Scholar] [CrossRef] [Green Version]
- Haubrock, P.J.; Cuthbert, R.N.; Ricciardi, A.; Diagne, C.; Courchamp, F. Economic costs of invasive bivalves in freshwater ecosystems. Divers. Distrib. 2022, 28, 1010–1021. [Google Scholar] [CrossRef]
- Lercari, D.; Bergamino, L. Impacts of two invasive mollusks, Rapana venosa (Gastropoda) and Corbicula fluminea (Bivalvia), on the food web structure of the Río de la Plata estuary and nearshore oceanic ecosystem. Biol. Invasions 2011, 13, 2053–2061. [Google Scholar] [CrossRef]
- Mansur, M.C.D.; Santos, C.P.; Pereira, D.; Paz, I.C.P.; Zurita, M.L.L.; Rodriguez, M.T.R.; Nehrke, M.V.; Bergonci, P.E.A. Moluscos Límnicos Invasores No Brasil: Biologia, Prevenção e Controle; Redes Editora: Porto Alegre, Brazil, 2012; 412p. [Google Scholar]
- Foster, A.M.; Fuller, P.; Benson, A.; Constant, S.; Raikow, D.; Larson, J.; Fusaro, A. USGS NAS—Corbicula fluminea Species Profile—United States Geological Survey Nonindigenous. Species Database. 2013. Available online: https://nas.er.usgs.gov (accessed on 12 March 2023).
- Mayfield, A.E., III; Seybold, S.J.; Haag, W.R.; Johnson, M.T.; Kerns, B.K.; Kilgo, J.C.; Larkin, D.J.; Lucardi, R.D.; Moltzan, B.D.; Pearson, D.E.; et al. Impacts of invasive species in terrestrial and aquatic systems of united states. In Invasive Species in Forests and Rangelands of the United States; Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., Lopez, V.M., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 5–40. [Google Scholar] [CrossRef]
- Paschoal, L.R.P.; Andrade, D.P.; Darrigran, G. How the fluctuations of water levels affect populations of invasive bivalve Corbicula fluminea (Müller, 1774) in a Neotropical reservoir? Braz. J. Biol. 2015, 75, 135–143. [Google Scholar] [CrossRef]
- Pereira, J.L.; Pinho, S.; Ré, A.; Costa, P.A.; Costa, R.; Gonçalves, F.; Castro, B.B. Biological control of the invasive Asian clam, Corbicula fluminea: Can predators tame the beast? Hydrobiologia 2016, 779, 209–226. [Google Scholar] [CrossRef]
- Henricksen, S.; Bollens, S.M. Abundance and growth of the invasive Asian clam, Corbicula fluminea, in the lower Columbia River, USA. Aquat. Invasions 2022, 17, 36–56. [Google Scholar] [CrossRef]
- Bielański, W. Next step of invasion: The Asian clam Corbicula fluminea (O. F. Müller, 1774) (Bivalvia: Cyrenidae) colonises smaller sandy rivers in Poland. Folia Malacol. 2022, 30, 99–108. [Google Scholar] [CrossRef]
- Gabriel, R.G.; Ré, A.; Gonçalves, F.; Costa, R.; Pereira, J.L. Monitorização e controlo da amêijoa invasora Corbicula fluminea em indústrias hidro-dependentes. Captar 2013, 4, 92–112. [Google Scholar] [CrossRef]
- Silva, C.M.D. Melhoramento de Métodos de Controlo da Amêijoa Asiática Corbicula fluminea. Master’s Thesis, Universidade de Aveiro, Aveiro, Portugal, 2014; 77p. Available online: https://core.ac.uk/download/32243857.pdf (accessed on 12 March 2023).
- Britton, J.C.; Morton, B. Corbicula in North America: The evidence reviewed and evaluated. In First International Corbicula symposium Christian; Britton, J.C., Mattice, J.S., Murphy, C.E., Newland, L.W., Eds.; Texas Christian University Reserarch Foudation: Fort Worth, TX, USA, 1979; pp. 249–287. [Google Scholar]
- McMahon, R.F. The occurrence and spread of the introduced Asiatic freshwater clam, Corbicula fluminea (Müller), in north America. Nautilus 1982, 96, 1924–1982. [Google Scholar]
- Linares, M.S.; Amaral, P.H.M.; Callisto, M. Corbicula fluminea (Corbiculidae, Bivalvia) alters the taxonomic and functional structure of benthic assemblages in neotropical hydropower reservoirs. Ecol. Indic. 2022, 141, 109114. [Google Scholar] [CrossRef]
- Reshaid, Y.; Cao, L.; Brea, F.; Blanche, M.O.; Torres, S.; Darrigran, G. Variation in the distribution of Corbicula species (Mollusca: Bivalvia: Corbiculidae) after 25 years of its introduction in the Río de la Plata, Argentina. Zool 2017, 34, 1–6. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, C.A.C.R.; Palheta, D.d.C.; Trindade, D.G.; Rodrigues, T.M.; Bentes, B. A New Record of a Nonnative Bivalve Species in an Amazonian Environmental Protection Area: What Might Have Happened? Water 2023, 15, 1123. https://doi.org/10.3390/w15061123
de Oliveira CACR, Palheta DdC, Trindade DG, Rodrigues TM, Bentes B. A New Record of a Nonnative Bivalve Species in an Amazonian Environmental Protection Area: What Might Have Happened? Water. 2023; 15(6):1123. https://doi.org/10.3390/w15061123
Chicago/Turabian Stylede Oliveira, Claudia Antonia Campos Rodrigues, Dulcidéia da Conceição Palheta, Diego Gomes Trindade, Tatiane Medeiros Rodrigues, and Bianca Bentes. 2023. "A New Record of a Nonnative Bivalve Species in an Amazonian Environmental Protection Area: What Might Have Happened?" Water 15, no. 6: 1123. https://doi.org/10.3390/w15061123
APA Stylede Oliveira, C. A. C. R., Palheta, D. d. C., Trindade, D. G., Rodrigues, T. M., & Bentes, B. (2023). A New Record of a Nonnative Bivalve Species in an Amazonian Environmental Protection Area: What Might Have Happened? Water, 15(6), 1123. https://doi.org/10.3390/w15061123






