Removal of Naphthalene, Fluorene and Phenanthrene by Recyclable Oil Palm Leaves’ Waste Activated Carbon Supported Nano Zerovalent Iron (N-OPLAC) Composite in Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Characterization of PW
2.3. Synthesis of OPLAC and NZVI-OPLAC Composite (N-OPLAC)
2.4. Batch Experiments for Synthetic Wastewater
2.5. Isotherm and Kinetic Modelling
2.5.1. Isotherm Modeling
2.5.2. Kinetic Modeling
2.6. Adsorption Mechanism
2.7. Optimization of the Adsorption Process
2.8. Batch Experiments for Produced Water
2.9. Regeneration and Reuse of Spent Adsorbents
3. Results and Discussion
3.1. NAP, FLU and PHE in PW
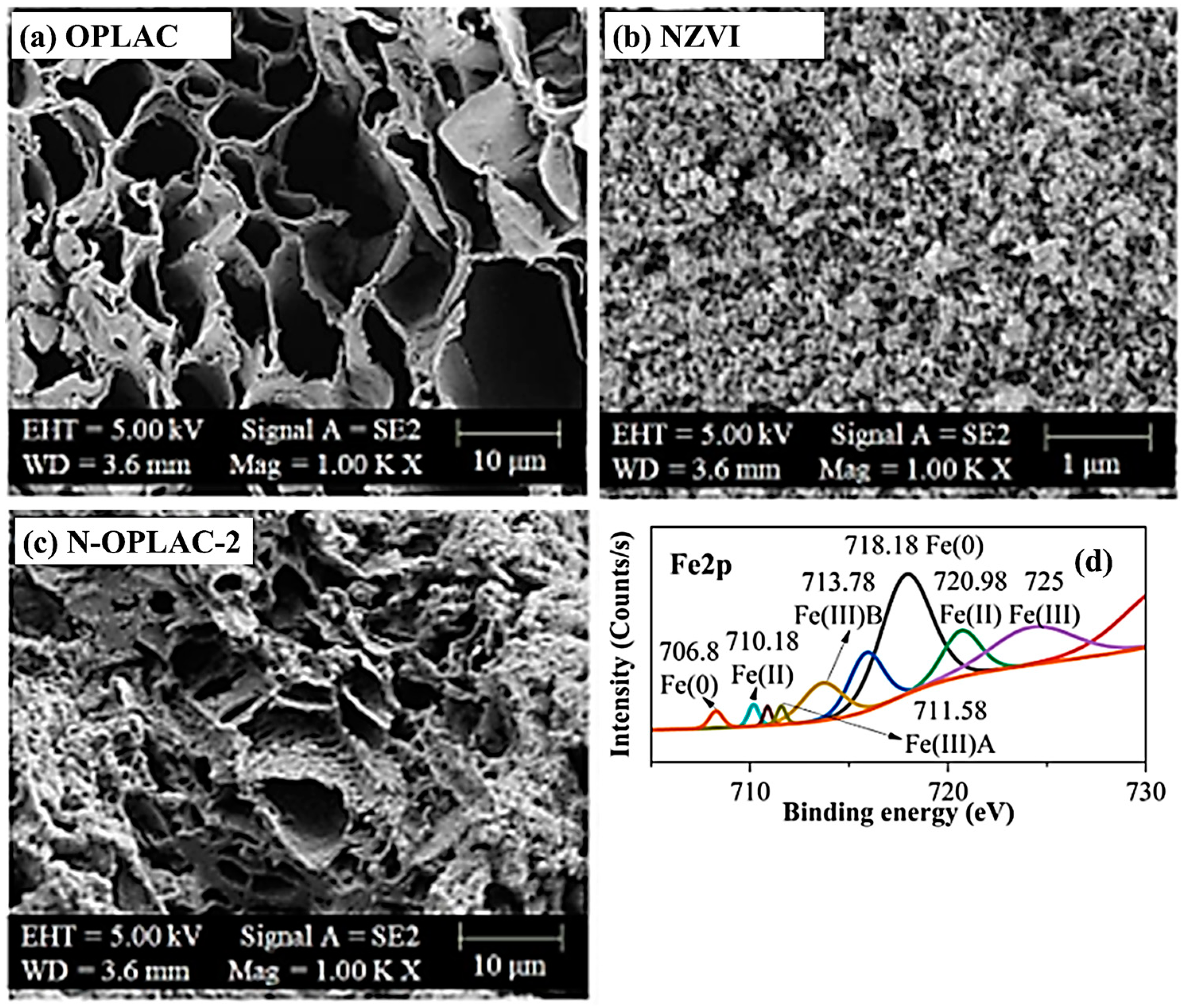
3.2. Characterization of Synthesized Materials
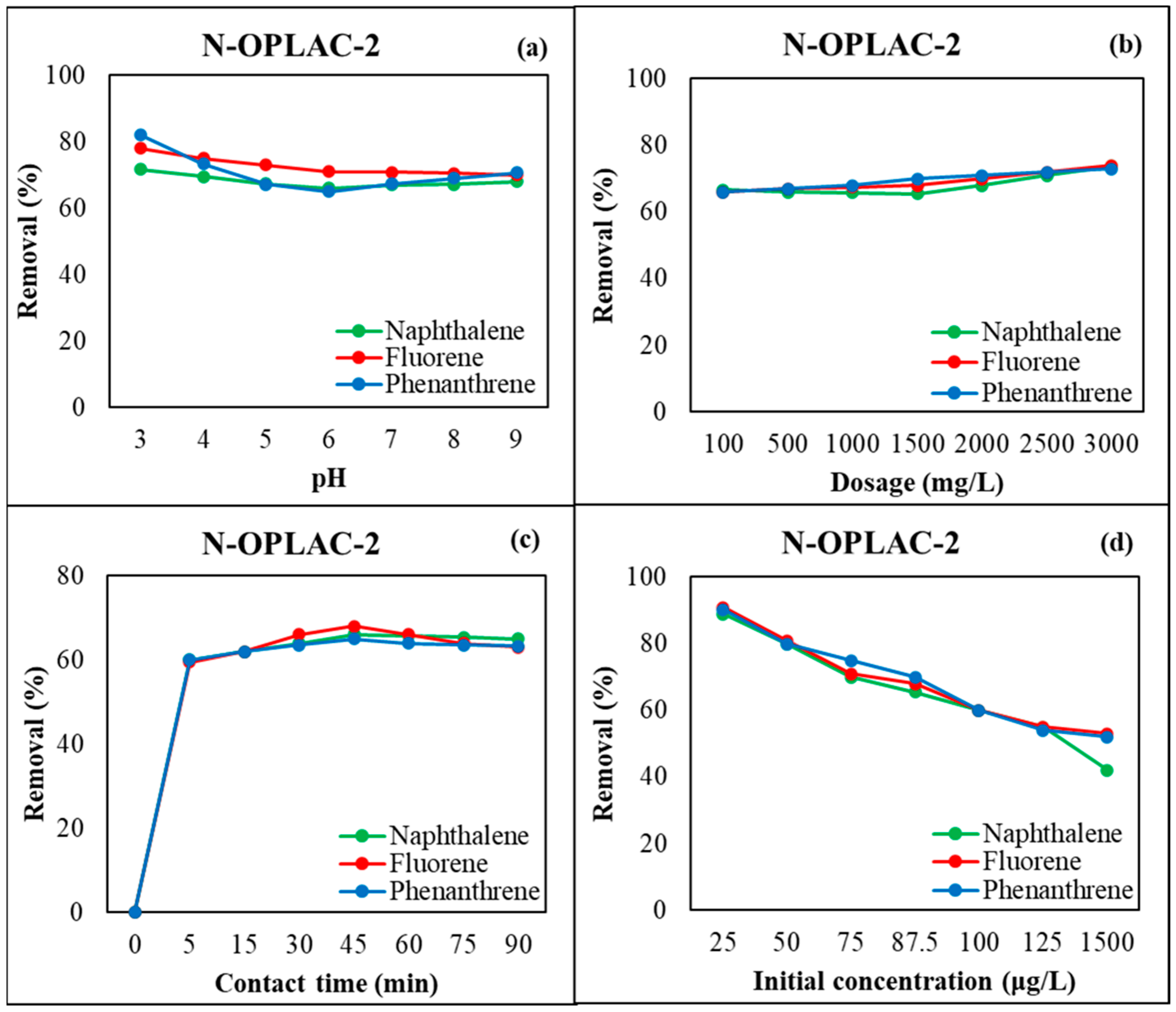
3.3. Adsorption Experiment Results
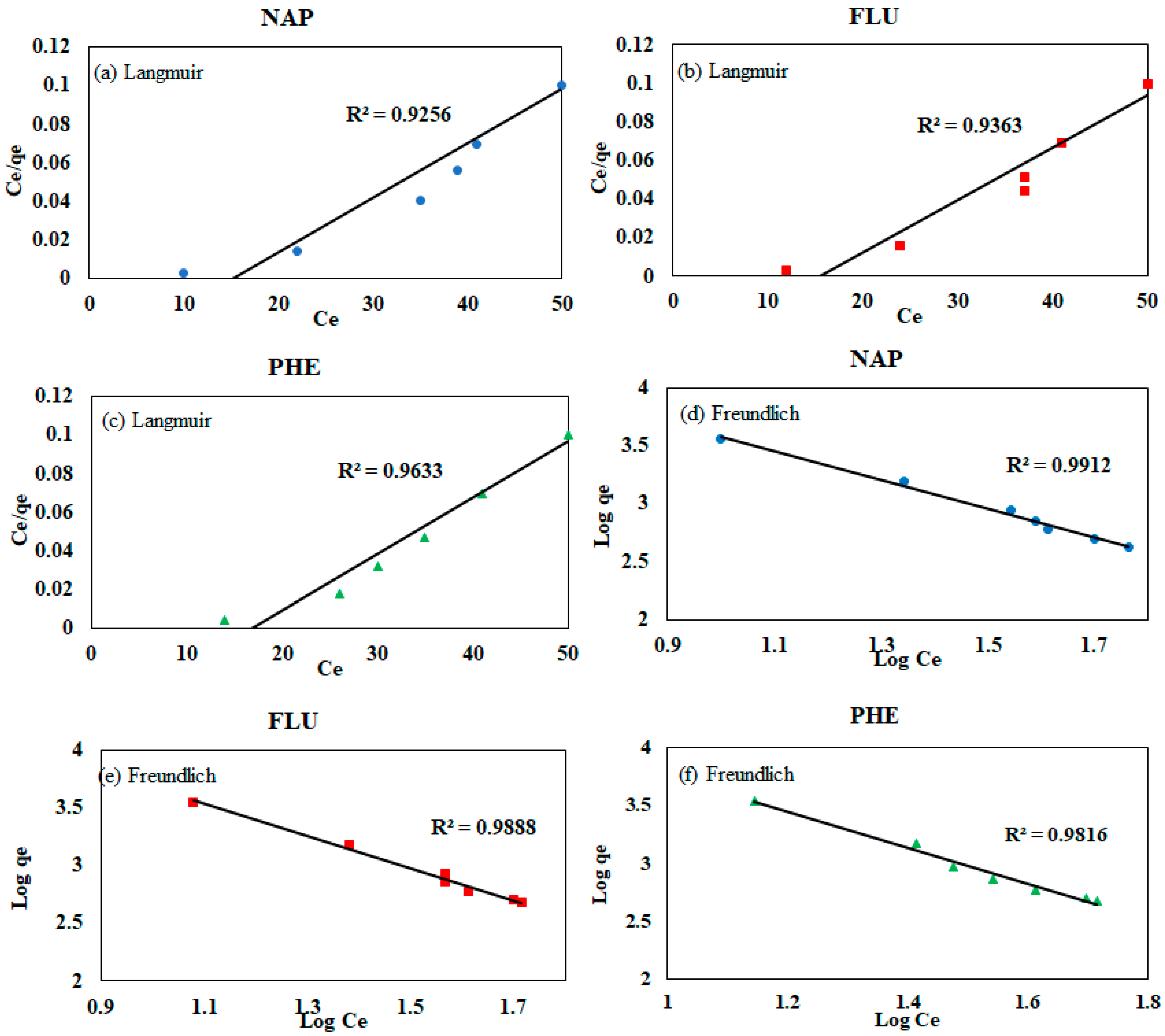
3.4. Langmuir and Freundlich Isotherm Models
3.5. PFO and PSO Kinetic Models
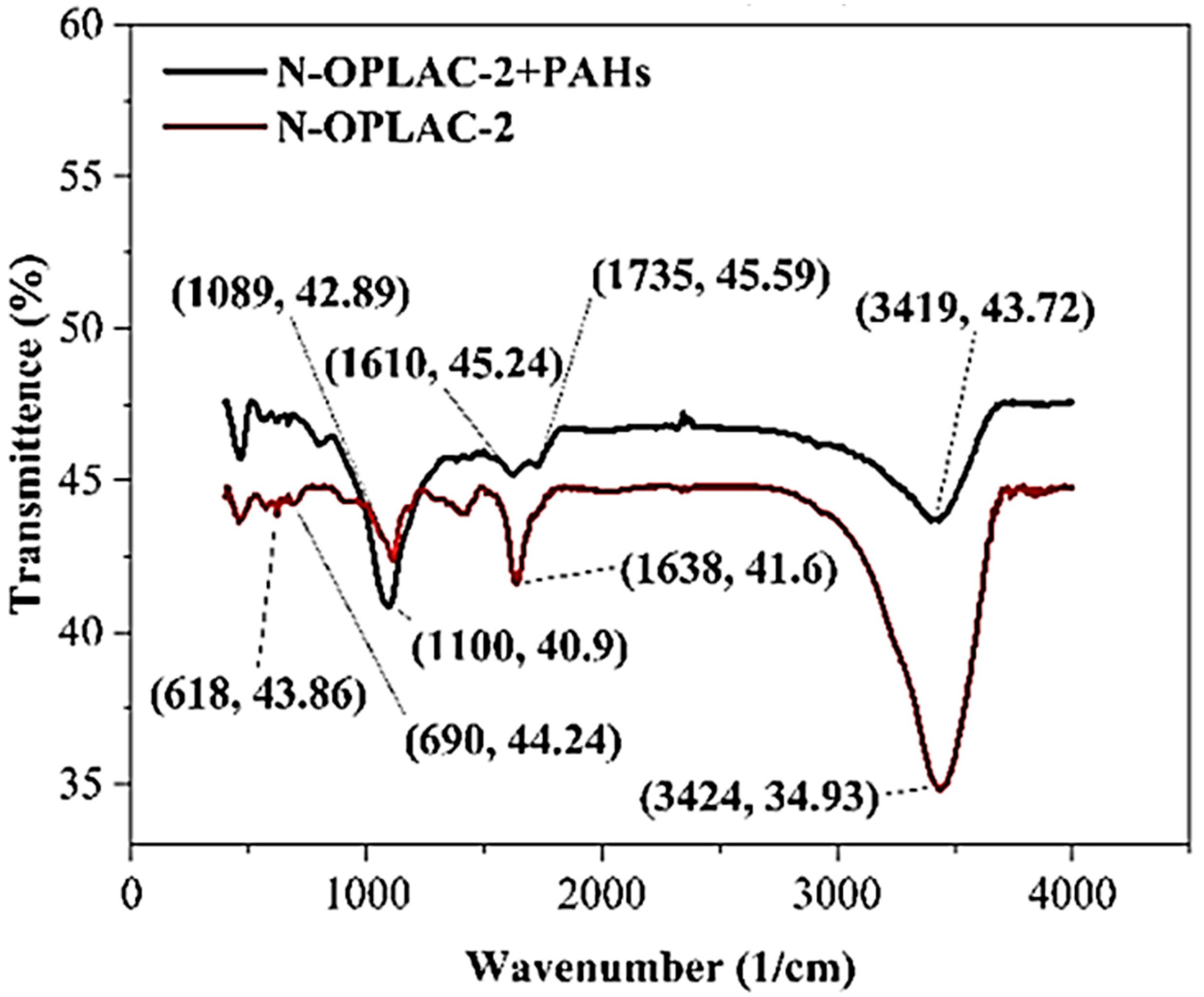
3.6. Proposed Mechanism of Removal
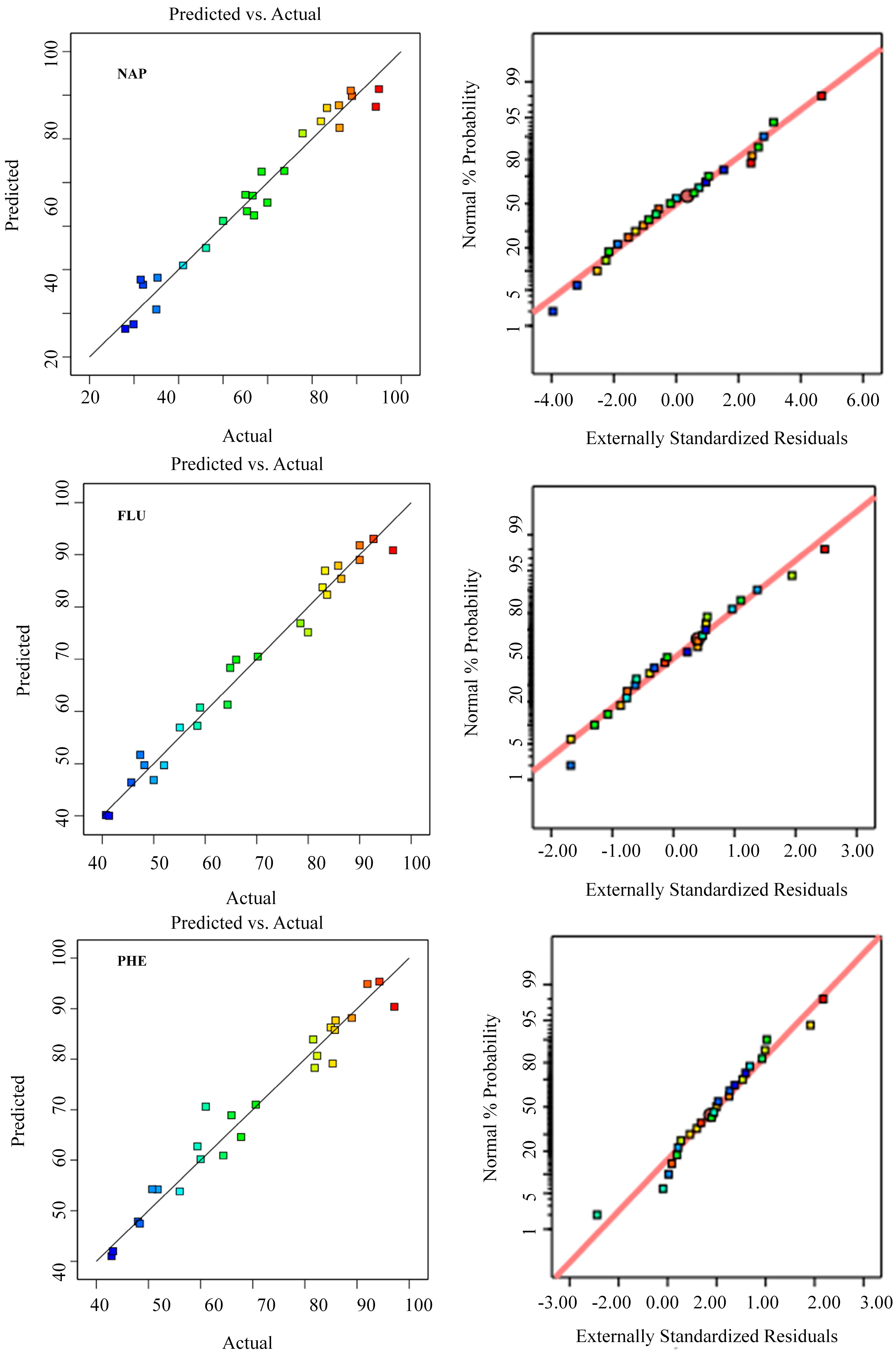
3.7. Modeling of RSM and Optimization of pH, Dosage and Contact Time
3.8. Modeling of RSM and Optimization of pH, Dosage and Contact Time
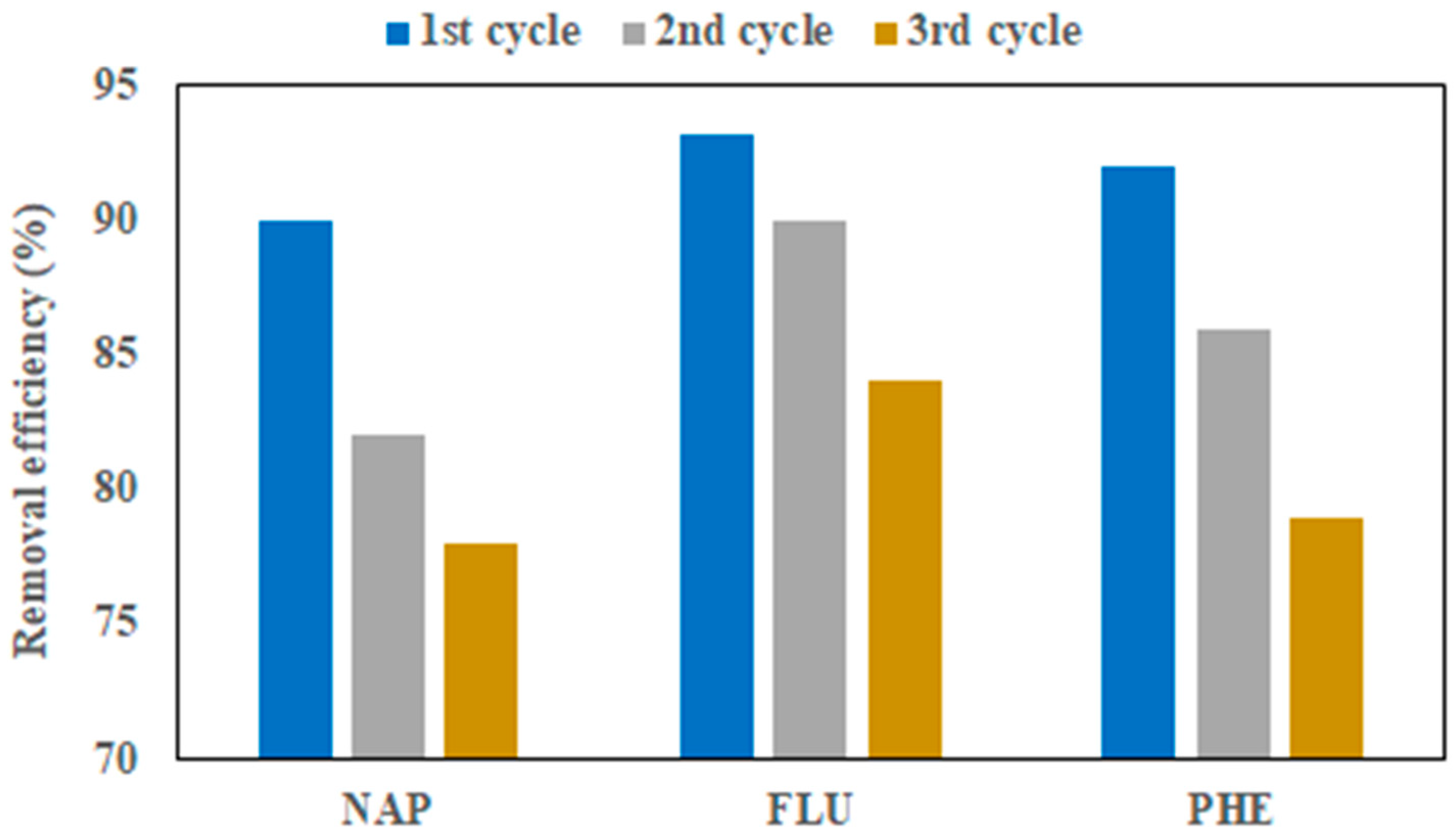
3.9. Regeneration and Reuse of N-OPLAC-2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Polycyclic Aromatic Hydrocarbons (PAHs) Removal by Sorption: A Review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, J.; Wu, J. Adsorptive Removal of Polycyclic Aromatic Hydrocarbons by Detritus of Green Tide Algae Deposited in Coastal Sediment. Sci. Total Environ. 2019, 670, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Hesham, A.E.L.; He, S.; Zhang, Y.; Wang, Z.; Yang, M. Co-Variations of Bacterial Composition and Catabolic Genes Related to PAH Degradation in a Produced Water Treatment System Consisting of Successive Anoxic and Aerobic Units. Sci. Total Environ. 2007, 373, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Mukherji, S. Degradation of Carbazole, Fluorene, Dibenzothiophene and Their Mixture by P. aeruginosa RS1 in Petroleum Refinery Wastewater. J. Water Process Eng. 2020, 37, 101454. [Google Scholar] [CrossRef]
- Wei, Z.; Ma, X.; Zhang, Y.; Guo, Y.; Wang, W.; Jiang, Z.Y. High-Efficiency Adsorption of Phenanthrene by Fe3O4-SiO2-Dimethoxydiphenylsilane Nanocomposite: Experimental and Theoretical Study. J. Hazard. Mater. 2022, 422, 126948. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the Art of Produced Water Treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of Technologies for Oil and Gas Produced Water Treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Aryanti, N.; Qudratun; Utomo, D.P. Oilfield Produced Water Treatment to Clean Water Using Integrated Activated Carbon-Bentonite Adsorbent and Double Stages Membrane Process. Chem. Eng. J. 2018, 347, 462–471. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low Cost Adsorbents for the Removal of Organic Pollutants from Wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Kumar, J.A.; Kumar, P.S.; Krithiga, T.; Prabu, D.; Amarnath, D.J.; Sathish, S.; Venkatesan, D.; Hosseini-Bandegharaei, A.; Prashant, P. Acenaphthene Adsorption onto Ultrasonic Assisted Fatty Acid Mediated Porous Activated Carbon-Characterization, Isotherm and Kinetic Studies. Chemosphere 2021, 284, 131249. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Koltowski, M. Effect of Co-Application of Nano-Zero Valent Iron and Biochar on the Total and Freely Dissolved Polycyclic Aromatic Hydrocarbons Removal and Toxicity of Contaminated Soils. Chemosphere 2017, 168, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fulton, A.N.; Keller, A.A. Simultaneous Removal of PAHs and Metal Contaminants from Water Using Magnetic Nanoparticle Adsorbents. Sci. Total Environ. 2016, 571, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Wang, J.; Wang, W.; Teng, W.; Zhang, W. xian Stabilization of Nanoscale Zero-Valent Iron in Water with Mesoporous Carbon (NZVI@MC). J. Environ. Sci. 2019, 81, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Son, A.; Her, N.; Zoh, K.D.; Cho, J.; Yoon, Y. Removal of Endocrine Disrupting Compounds, Pharmaceuticals, and Personal Care Products in Water Using Carbon Nanotubes: A Review. J. Ind. Eng. Chem. 2015, 27, 1–11. [Google Scholar] [CrossRef]
- Salman, J.M.; Njoku, V.O.; Hameed, B.H. Adsorption of Pesticides from Aqueous Solution onto Banana Stalk Activated Carbon. Chem. Eng. J. 2011, 174, 41–48. [Google Scholar] [CrossRef]
- Ayranci, E.; Hoda, N. Adsorption Kinetics and Isotherms of Pesticides onto Activated Carbon-Cloth. Chemosphere 2005, 60, 1600–1607. [Google Scholar] [CrossRef]
- Nkansah, M.A.; Christy, A.A.; Barth, T.; Francis, G.W. The Use of Lightweight Expanded Clay Aggregate (LECA) as Sorbent for PAHs Removal from Water. J. Hazard. Mater. 2012, 217‘, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Cui, H.; Song, X.; Xu, R.; Wei, N.; Tian, J.; Niu, H. Facile Synthesis of Heterojunction of MXenes/TiO2 Nanoparticles towards Enhanced Hexavalent Chromium Removal. J. Colloid Interface Sci. 2020, 561, 46–57. [Google Scholar] [CrossRef]
- He, X.; Wu, Z.; Sun, Z.; Wei, X.; Wu, Z.; Ge, X.; Cravotto, G. A Novel Hybrid of β-Cyclodextrin Grafted onto Activated Carbon for Rapid Adsorption of Naphthalene from Aqueous Solution. J. Mol. Liq. 2018, 255, 160–167. [Google Scholar] [CrossRef]
- Liang, L.; Li, X.; Guo, Y.; Lin, Z.; Su, X.; Liu, B. The Removal of Heavy Metal Cations by Sulfidated Nanoscale Zero-Valent Iron (S-NZVI): The Reaction Mechanisms and the Role of Sulfur. J. Hazard. Mater. 2021, 404, 124057. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-Supported NZVI (NZVI/BC) for Contaminant Removal from Soil and Water: A Critical Review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Cordova, A.; Silva-Gordillo, M.D.M.; Muñoz, G.A.; Arboleda-Faini, X.; Almeida Streitwieser, D. Comparison of the Adsorption Capacity of Organic Compounds Present in Produced Water with Commercially Obtained Walnut Shell and Residual Biomass. J. Environ. Chem. Eng. 2017, 5, 4041–4050. [Google Scholar] [CrossRef]
- Khurshid, H.; Mustafa, M.R.U.; Isa, M.H. Modified Activated Carbon Synthesized from Oil Palm Leaves Waste as a Novel Green Adsorbent for Chemical Oxygen Demand in Produced Water. Sustainability 2022, 14, 1986. [Google Scholar] [CrossRef]
- Salman, J.M.; Njoku, V.O.; Hameed, B.H. Batch and Fixed-Bed Adsorption of 2,4-Dichlorophenoxyacetic Acid onto Oil Palm Frond Activated Carbon. Chem. Eng. J. 2011, 174, 33–40. [Google Scholar] [CrossRef]
- Soliman, A.M.; Elwy, H.M.; Thiemann, T.; Majedi, Y.; Labata, F.T.; Al-Rawashdeh, N.A.F. Removal of Pb(II) Ions from Aqueous Solutions by Sulphuric Acid-Treated Palm Tree Leaves. J. Taiwan Inst. Chem. Eng. 2016, 58, 264–273. [Google Scholar] [CrossRef]
- Fathy, M.; El-Sayed, M.; Ramzi, M.; Abdelraheem, O.H. Adsorption Separation of Condensate Oil from Produced Water Using ACTF Prepared of Oil Palm Leaves by Batch and Fixed Bed Techniques. Egypt. J. Pet. 2018, 27, 319–326. [Google Scholar] [CrossRef]
- Eid, M.E.-S. Synthesis of Polyethylenimine-Magnetic Amorphous Carbon Nano Composite as a Novel Adsorbent for Hg (II) from Aqueous Solutions. Aust. J. BASIC Appl. Sci. 2016, 10, 323–335. [Google Scholar]
- Hedayati, M.S.; Abida, O.; Li, L.Y. Adsorption of Polycyclic Aromatic Hydrocarbons by Surfactant-Modified Clinoptilolites for Landfill Leachate Treatment. Waste Manag. 2021, 131, 503–512. [Google Scholar] [CrossRef]
- Tsai, J.C.; Kumar, M.; Lin, J.G. Anaerobic Biotransformation of Fluorene and Phenanthrene by Sulfate-Reducing Bacteria and Identification of Biotransformation Pathway. J. Hazard. Mater. 2009, 164, 847–855. [Google Scholar] [CrossRef]
- Danila, V.; Vasarevicius, S.; Valskys, V. Batch Removal of Cd(II), Cu(II), Ni(II), and Pb(II) Ions Using Stabilized Zero-Valent Iron Nanoparticles. Energy Procedia 2018, 147, 214–219. [Google Scholar] [CrossRef]
- Khurshid, H.; Mustafa, M.R.U.; Rashid, U.; Isa, M.H.; Chia, H.Y.; Shah, M.M. Adsorptive Removal of COD from Produced Water Using Tea Waste Biochar. Environ. Technol. Innov. 2021, 23, 101563. [Google Scholar] [CrossRef]
- Asadpour, R.; Sapari, N.B.; Isa, M.H.; Orji, K.U. Enhancing the Hydrophobicity of Mangrove Bark by Esterification for Oil Adsorption. Water Sci. Technol. 2014, 70, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of Organic Pollutants from Aqueous Solution Using Agricultural Wastes: A Review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Ghaedi, M.; Azad, F.N.; Dashtian, K.; Hajati, S.; Goudarzi, A.; Soylak, M. Central Composite Design and Genetic Algorithm Applied for the Optimization of Ultrasonic-Assisted Removal of Malachite Green by ZnO Nanorod-Loaded Activated Carbon. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2016, 167, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, C.; Zhuang, J.; Zheng, G.; Zhou, L. Assessment of Schwertmannite, Jarosite and Goethite as Adsorbents for Efficient Adsorption of Phenanthrene in Water and the Regeneration of Spent Adsorbents by Heterogeneous Fenton-like Reaction. Chemosphere 2020, 244, 125523. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Z.; Hu, Z.; Ngo, H.H.; Liang, S.; Zhang, J. Adsorption of Phenanthrene from Aqueous Solutions by Biochar Derived from an Ammoniation-Hydrothermal Method. Sci. Total Environ. 2020, 733, 139267. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, J.H.F.; Cunha, G.D.C.; Cardoso, E.M.C.; Mangrich, A.S.; Romão, L.P.C. Evaluation of Waste Biomasses and Their Biochars for Removal of Polycyclic Aromatic Hydrocarbons. J. Environ. Manag. 2017, 200, 186–195. [Google Scholar] [CrossRef]
- Haneef, T.; Ul Mustafa, M.R.; Yusof, K.W.; Isa, M.H.; Bashir, M.J.K.; Ahmad, M.; Zafar, M. Removal of Polycyclic Aromatic Hydrocarbons (PAHs) from Produced Water by Ferrate (VI) Oxidation. Water 2020, 12, 3132. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Gao, B.; Wang, X.; Yin, X.; Feng, K.; Wang, J. The Sorptive and Reductive Capacities of Biochar Supported Nanoscaled Zero-Valent Iron (NZVI) in Relation to Its Crystallite Size. Chemosphere 2017, 186, 495–500. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Z.; Ge, X.; Cravotto, G.; Wu, Z.; Yan, Y. Comparative Study of Naphthalene Adsorption on Activated Carbon Prepared by Microwave-Assisted Synthesis from Different Typical Coals in Xinjiang. J. Taiwan Inst. Chem. Eng. 2016, 59, 563–568. [Google Scholar] [CrossRef]
- Belhamdi, B.; Merzougui, Z.; Trari, M.; Addoun, A. A Kinetic, Equilibrium and Thermodynamic Study of L-Phenylalanine Adsorption Using Activated Carbon Based on Agricultural Waste (Date Stones). J. Appl. Res. Technol. 2016, 14, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, J.; Guo, Y.; Zhu, C.; Pan, F.; Wu, R.; Wang, C. Removal of Multiple Nitrosamines from Aqueous Solution by Nanoscale Zero-Valent Iron Supported on Granular Activated Carbon: Influencing Factors and Reaction Mechanism. Sci. Total Environ. 2018, 639, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Fang, Z.; Tsang, P.E.; Fang, J.; Zhao, D. Stabilisation of Nanoscale Zero-Valent Iron with Biochar for Enhanced Transport and in-Situ Remediation of Hexavalent Chromium in Soil. Environ. Pollut. 2016, 214, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, S.; Ren, L.Y.; Zhou, L.Y.; Tan, X.J.; Zhu, Y.; Belver, C.; Bedia, J.; Yang, J. Biochar Mediates Activation of Aged Nanoscale ZVI by Shewanella Putrefaciens CN32 to Enhance the Degradation of Pentachlorophenol. Chem. Eng. J. 2019, 368, 148–156. [Google Scholar] [CrossRef]
- Hussin, M.H.; Pohan, N.A.; Garba, Z.N.; Kassim, M.J.; Rahim, A.A.; Brosse, N.; Yemloul, M.; Fazita, M.R.N.; Haafiz, M.K.M. Physicochemical of Microcrystalline Cellulose from Oil Palm Fronds as Potential Methylene Blue Adsorbents. Int. J. Biol. Macromol. 2016, 92, 11–19. [Google Scholar] [CrossRef]
- Mines, P.D.; Uthuppu, B.; Thirion, D.; Jakobsen, M.H.; Yavuz, C.T.; Andersen, H.R.; Hwang, Y. Granular Activated Carbon with Grafted Nanoporous Polymer Enhances Nanoscale Zero-Valent Iron Impregnation and Water Contaminant Removal. Chem. Eng. J. 2018, 339, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.; Ma, J.; Chen, J.; Zhang, Y.; Song, J.; Yu, X. Enhanced Nitrate Removal and High Selectivity towards Dinitrogen for Groundwater Remediation Using Biochar-Supported Nano Zero-Valent Iron. Chem. Eng. J. 2018, 353, 595–605. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Wang, X.; Wei, C. Efficient Adsorption of Phenanthrene by Simply Synthesized Hydrophobic MCM-41 Molecular Sieves. Appl. Surf. Sci. 2014, 311, 825–830. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Liu, Y.; Lei, M. Recyclable Nanoscale Zero-Valent Iron-Based Magnetic Polydopamine Coated Nanomaterials for the Adsorption and Removal of Phenanthrene and Anthracene. Sci. Technol. Adv. Mater. 2017, 18, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on Recent Advances of Carbon Based Adsorbent for Methylene Blue Removal from Waste Water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Hendges, L.T.; Costa, T.C.; Temochko, B.; Gómez González, S.Y.; Mazur, L.P.; Marinho, B.A.; da Silva, A.; Weschenfelder, S.E.; de Souza, A.A.U.; de Souza, S.M.A.G.U. Adsorption and Desorption of Water-Soluble Naphthenic Acid in Simulated Offshore Oilfield Produced Water. Process Saf. Environ. Prot. 2021, 145, 262–272. [Google Scholar] [CrossRef]
- Huang, W.; Peng, P.; Yu, Z.; Fu, J. Effects of Organic Matter Heterogeneity on Sorption and Desorption of Organic Contaminants by Soils and Sediments. Appl. Geochem. 2003, 18, 955–972. [Google Scholar] [CrossRef]
- Pinto Brito, M.J.; Flores Santos, M.P.; De Souza, E.C., Jr.; Santos, L.S.; Ferreira Bonomo, R.C.; Da Costa Ilhéu Fontan, R.; Veloso, C.M. Development of Activated Carbon from Pupunha Palm Heart Sheaths: Effect of Synthesis Conditions and Its Application in Lipase Immobilization. J. Environ. Chem. Eng. 2020, 8, 104391. [Google Scholar] [CrossRef]
- Aravind Kumar, J.; Krithiga, T.; Vijai Anand, K.; Sathish, S.; Karthick Raja Namasivayam, S.; Renita, A.A.; Hosseini-Bandegharaei, A.; Praveenkumar, T.R.; Rajasimman, M.; Bhat, N.S.; et al. Kinetics and Regression Analysis of Phenanthrene Adsorption on the Nanocomposite of CaO and Activated Carbon: Characterization, Regeneration, and Mechanistic Approach. J. Mol. Liq. 2021, 334, 116080. [Google Scholar] [CrossRef]




| Run | pH | Dosage | Contact Time | NAP | FLU | PHE |
|---|---|---|---|---|---|---|
| (mg/L) | (min) | (%) | (%) | (%) | ||
| 1 | 6 | 100 | 47.5 | 66.6 | 64.8 | 65.9 |
| 2 | 6 | 3000 | 47.5 | 73.7 | 79.9 | 85.3 |
| 3 | 3 | 3000 | 90 | 88.7 | 92.7 | 94.3 |
| 4 | 9 | 3000 | 5 | 86.2 | 86.4 | 85.8 |
| 5 | 6 | 1550 | 5 | 60 | 59 | 60 |
| 6 | 9 | 1550 | 47.5 | 69.9 | 70.2 | 70.6 |
| 7 | 9 | 100 | 5 | 83.3 | 83.7 | 82.4 |
| 8 | 6 | 1550 | 47.5 | 65 | 66 | 61 |
| 9 | 9 | 3000 | 5 | 45.3 | 48.2 | 51.8 |
| 10 | 9 | 100 | 90 | 39.9 | 41.3 | 43.2 |
| 11 | 6 | 1550 | 90 | 65.4 | 64.3 | 64.3 |
| 12 | 9 | 3000 | 90 | 51 | 52 | 56 |
| 13 | 3 | 100 | 5 | 45 | 50 | 48 |
| 14 | 3 | 3000 | 5 | 56.2 | 58.5 | 67.8 |
| 15 | 3 | 3000 | 90 | 66.9 | 55.1 | 59.4 |
| 16 | 3 | 3000 | 5 | 86 | 90 | 92 |
| 17 | 3 | 1550 | 47.5 | 68.6 | 78.5 | 81.9 |
| 18 | 3 | 100 | 90 | 95 | 90 | 89 |
| 19 | 6 | 1550 | 47.5 | 94.3 | 96.5 | 97.2 |
| 20 | 3 | 100 | 5 | 88.9 | 85.8 | 84.9 |
| 21 | 9 | 100 | 5 | 38 | 40.7 | 42.9 |
| 22 | 6 | 1550 | 47.5 | 41.5 | 47.4 | 50.7 |
| 23 | 3 | 100 | 90 | 42 | 45.6 | 48.3 |
| 24 | 9 | 3000 | 90 | 77.9 | 83.3 | 85.9 |
| 25 | 9 | 100 | 90 | 81.9 | 82.8 | 81.6 |
| PAHs | Molecular Formula | Molecular Weight | Solubility in Water at 25 °C (mg/L) |
|---|---|---|---|
| NAP | C10H8 | 128.2 | 31.02 |
| FLU | C13H10 | 116.2 | 1.90 |
| PHE | C14H10 | 178.2 | 1.18 |
| Material | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) | Yield (%) |
|---|---|---|---|---|
| OPLAC | 331.153 | 0.206 | 2.5 | 46 |
| NZVI | 29.24 | - | - | 65 |
| N-OPLAC-1 | 243.387 | 0.215 | 3.53 | 50 |
| N-OPLAC-2 | 258.387 | 0.325 | 3.64 | 60 |
| Material | C | O | Zn | Si | Ca | Fe |
|---|---|---|---|---|---|---|
| OPLAC | 81.2 | 9.6 | 4 | 4.2 | 1.1 | - |
| NZVI | 6.8 | 17.26 | - | - | - | 77.5 |
| N-OPLAC-2 | 45.10 | 30.22 | 3.60 | 3.89 | 0.30 | 39.3 |
| Model | Parameters | NAP | FLU | PHE |
|---|---|---|---|---|
| Freundlich isotherm model | KF | 0.938 | 1.131 | 0.59 |
| n | 1.16 | 1.11 | 1.08 | |
| R2 | 0.991 | 0.989 | 0.982 | |
| Langmuir isotherm model | KL | 0.025 | 0.027 | 0.029 |
| Qm | 11.61 | 28.43 | 26.93 | |
| R2 | 0.926 | 0.936 | 0.963 | |
| PFO model | qe (exp) | 43.33 | 42 | 42.22 |
| qe | 1815 | 2009 | 1422 | |
| K1 | 15 × 10−4 | 8 × 10−4 | 9 × 10−4 | |
| R2 | 0.656 | 0.137 | 0.408 | |
| PSO model | qe (exp) | 43.33 | 42 | 42.22 |
| qe | 43.86 | 42.55 | 42.55 | |
| K2 | 0.044 | 0.038 | 0.394 | |
| R2 | 0.998 | 0.994 | 0.997 |
| Source | SS | DF | MS | F-Value | p-Value |
|---|---|---|---|---|---|
| N-OPLAC-2 for NAP | |||||
| Model | 7831.61 | 14 | 559.40 | 20.35 | <0.0001 |
| A-pH | 227.36 | 1 | 227.36 | 8.27 | 0.0165 |
| B-Dosage | 144.47 | 1 | 144.47 | 5.25 | 0.0448 |
| C-Contact time | 21.90 | 1 | 21.90 | 0.7965 | 0.03931 |
| AB | 5.96 | 1 | 5.96 | 0.2169 | 0.06514 |
| AC | 21.61 | 1 | 21.61 | 0.7858 | 0.03962 |
| BC | 3.32 | 1 | 3.32 | 0.1208 | 0.07353 |
| A2 | 7.79 | 1 | 7.79 | 0.2834 | 0.06061 |
| B2 | 17.70 | 1 | 17.70 | 0.6439 | 0.04410 |
| C2 | 60.12 | 1 | 60.12 | 2.19 | 0.01700 |
| Residual | 274.94 | 10 | 27.49 | ||
| Model summary | R2 | R2adj | R2pred | AP | CV |
| 0.9661 | 0.9186 | 0.7762 | 13.5175 | 7.82% | |
| N-OPLAC-2 for FLU | |||||
| Model | 7548.69 | 14 | 539.19 | 32.25 | <0.0001 |
| A-pH | 184.03 | 1 | 184.03 | 11.01 | 0.0078 |
| B-Dosage | 208.38 | 1 | 208.38 | 12.46 | 0.0054 |
| C-Contact time | 1.30 | 1 | 1.30 | 0.0776 | 0.007863 |
| AB | 0.7281 | 1 | 0.7281 | 0.0435 | 0.8389 |
| AC | 0.1048 | 1 | 0.1048 | 0.0063 | 0.09385 |
| BC | 0.0113 | 1 | 0.0113 | 0.0007 | 0.009798 |
| A2 | 36.23 | 1 | 36.23 | 2.17 | 0.1718 |
| B2 | 8.49 | 1 | 8.49 | 0.5076 | 0.4925 |
| C2 | 202.27 | 1 | 202.27 | 12.10 | 0.0059 |
| Residual | 167.20 | 10 | 16.72 | ||
| Model summary | R2 | R2adj | R2pred | AP | CV |
| 0.9783 | 0.9480 | 0.88 | 16.7415 | 5.97% | |
| N-OPLAC-2 for PHE | |||||
| Model | 6929.25 | 14 | 494.95 | 17.40 | <0.0001 |
| A-pH | 238.79 | 1 | 238.79 | 8.39 | 0.0159 |
| B-Dosage | 469.09 | 1 | 469.09 | 16.49 | 0.0023 |
| C-Contact time | 2.38 | 1 | 2.38 | 0.0837 | 0.7783 |
| AB | 11.94 | 1 | 11.94 | 0.4199 | 0.5316 |
| AC | 1.95 | 1 | 1.95 | 0.0685 | 0.7988 |
| BC | 1.95 | 1 | 1.95 | 0.0685 | 0.7989 |
| A2 | 41.57 | 1 | 41.57 | 1.46 | 0.2545 |
| B2 | 29.99 | 1 | 29.99 | 1.05 | 0.3287 |
| C2 | 255.95 | 1 | 255.95 | 9.00 | 0.0134 |
| Residual | 284.44 | 10 | 28.44 | ||
| Model summary | R2 | R2adj | R2pred | AP | CV |
| 0.9606 | 0.9054 | 0.8241 | 13.1499 | 7.62% | |
| Pollutant | pH | Dosage (mg/L) | Contact Time (min) | Initial Concentration (mg/L) | RSM Predicted Removal (%) | Experimental Removal (%) |
|---|---|---|---|---|---|---|
| NAP | 3 | 122 | 49 | 25 | 95 | 94.8 |
| FLU | 97 | 98.4 | ||||
| PHE | 97 | 97.4 |
| Pollutant | pH | Dosage (mg/L) | Contact Time (min) | Experimental Removal (%) | Removal in PW (%) | Error (%) |
|---|---|---|---|---|---|---|
| NAP | 3 | 122 | 49 | 94.8 | 89.94 | 4.86 |
| FLU | 98.4 | 93.2 | 5.2 | |||
| PHE | 97.4 | 92 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khurshid, H.; Mustafa, M.R.U.; Kilic, Z. Removal of Naphthalene, Fluorene and Phenanthrene by Recyclable Oil Palm Leaves’ Waste Activated Carbon Supported Nano Zerovalent Iron (N-OPLAC) Composite in Wastewater. Water 2023, 15, 967. https://doi.org/10.3390/w15050967
Khurshid H, Mustafa MRU, Kilic Z. Removal of Naphthalene, Fluorene and Phenanthrene by Recyclable Oil Palm Leaves’ Waste Activated Carbon Supported Nano Zerovalent Iron (N-OPLAC) Composite in Wastewater. Water. 2023; 15(5):967. https://doi.org/10.3390/w15050967
Chicago/Turabian StyleKhurshid, Hifsa, Muhammad Raza Ul Mustafa, and Zeyneb Kilic. 2023. "Removal of Naphthalene, Fluorene and Phenanthrene by Recyclable Oil Palm Leaves’ Waste Activated Carbon Supported Nano Zerovalent Iron (N-OPLAC) Composite in Wastewater" Water 15, no. 5: 967. https://doi.org/10.3390/w15050967






