Effects of Shelter on the Hatching, Immune Performance, and Profitability of the Ovigerous Red Swamp Crayfish Procambarus clarkii under High Stocking Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Design
2.2. Sample Collection
2.3. Nonspecific Immune Parameter Determination
2.4. Profitability Analysis
2.5. Statistical Analysis
3. Results
3.1. Water Quality
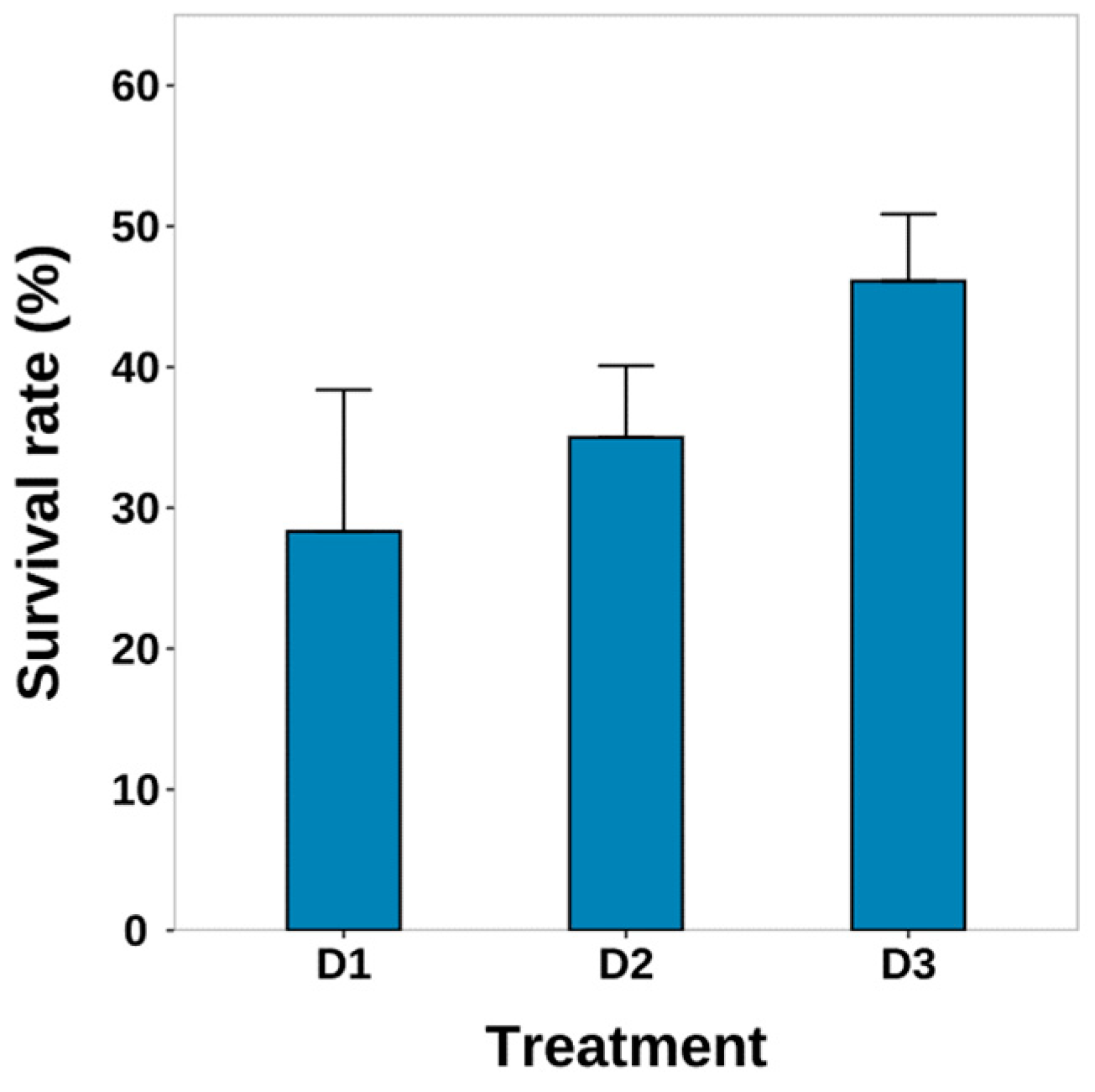
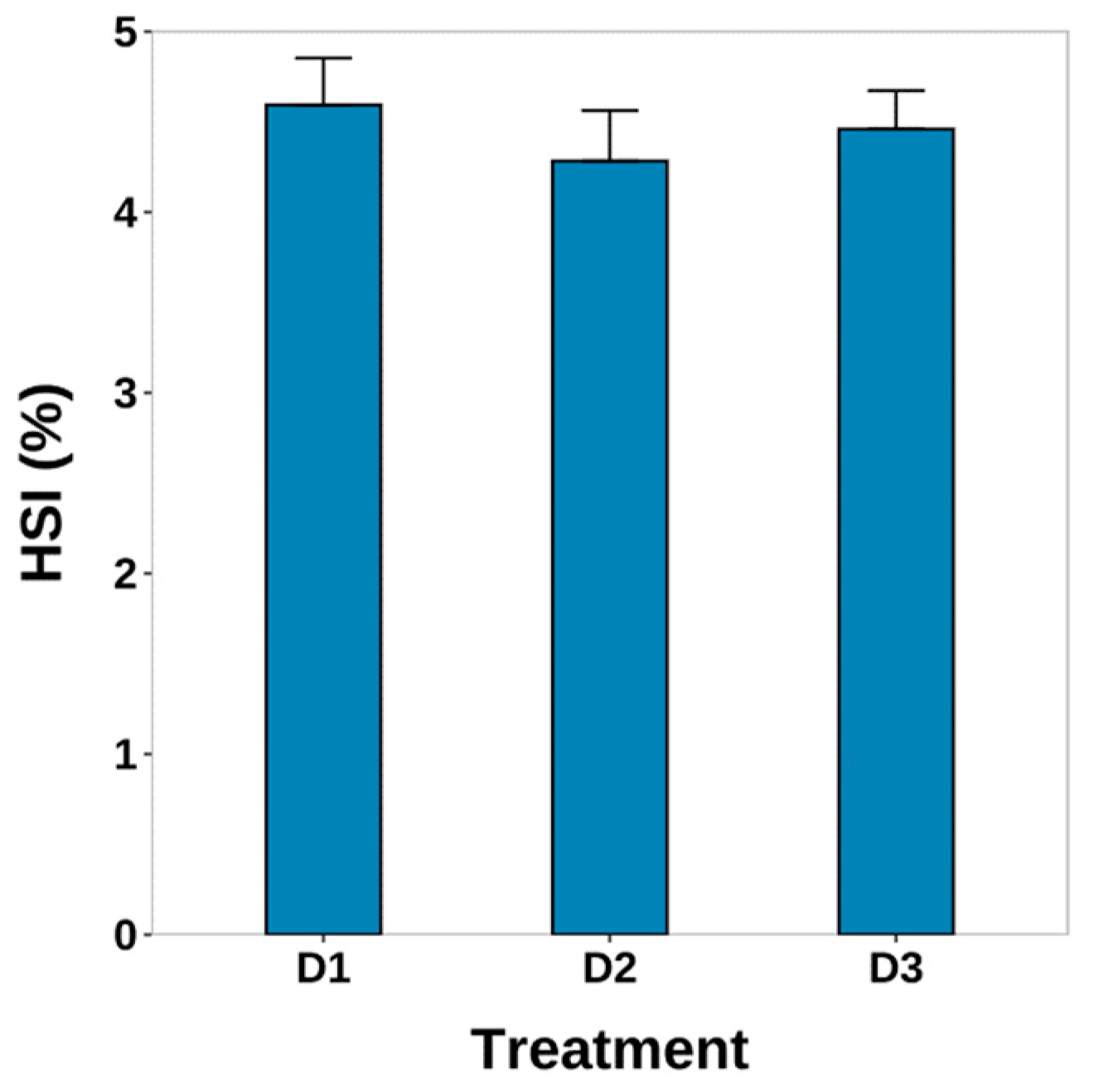
3.2. Survival and HSI
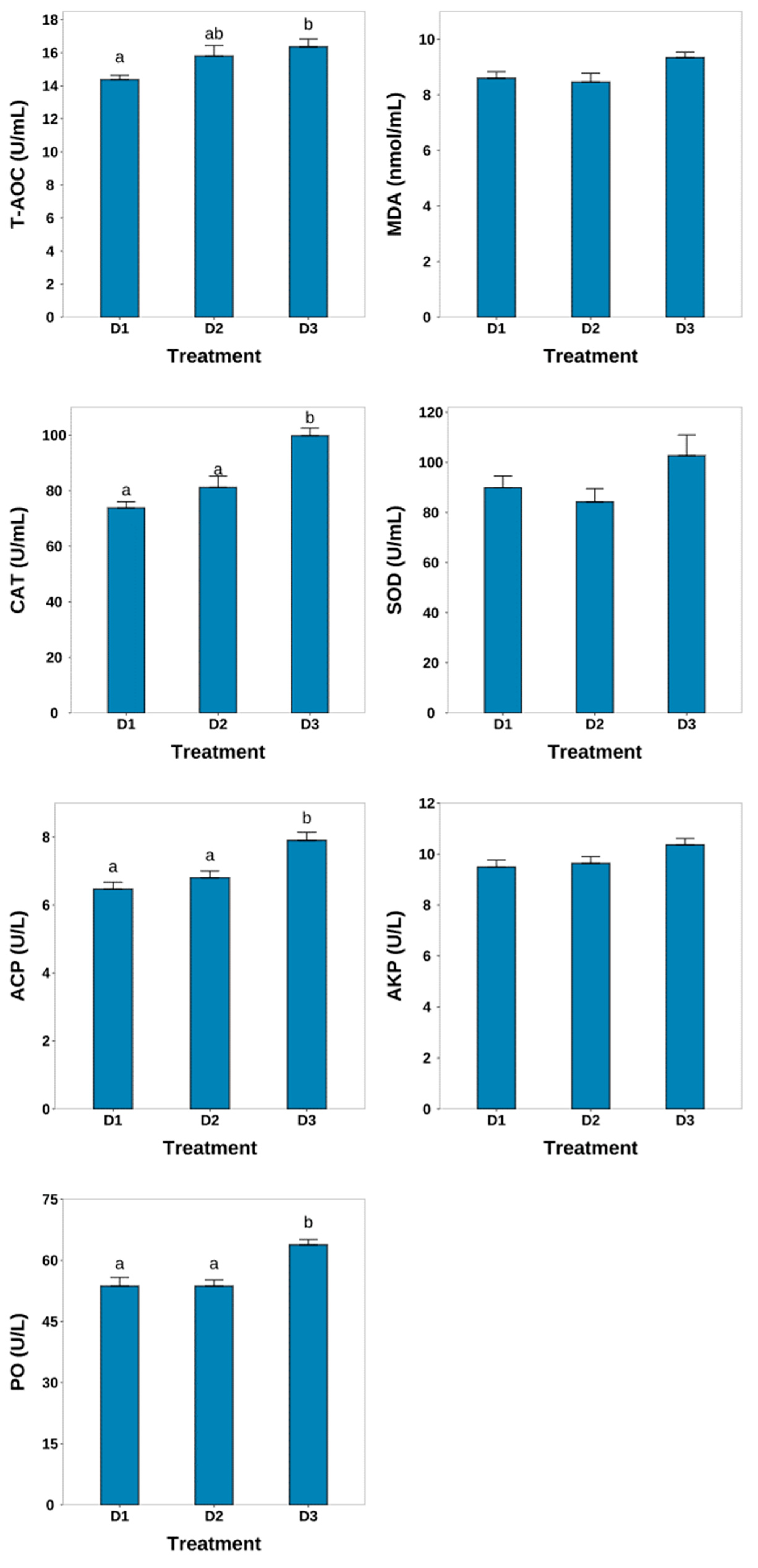
3.3. Nonspecific Immune Condition
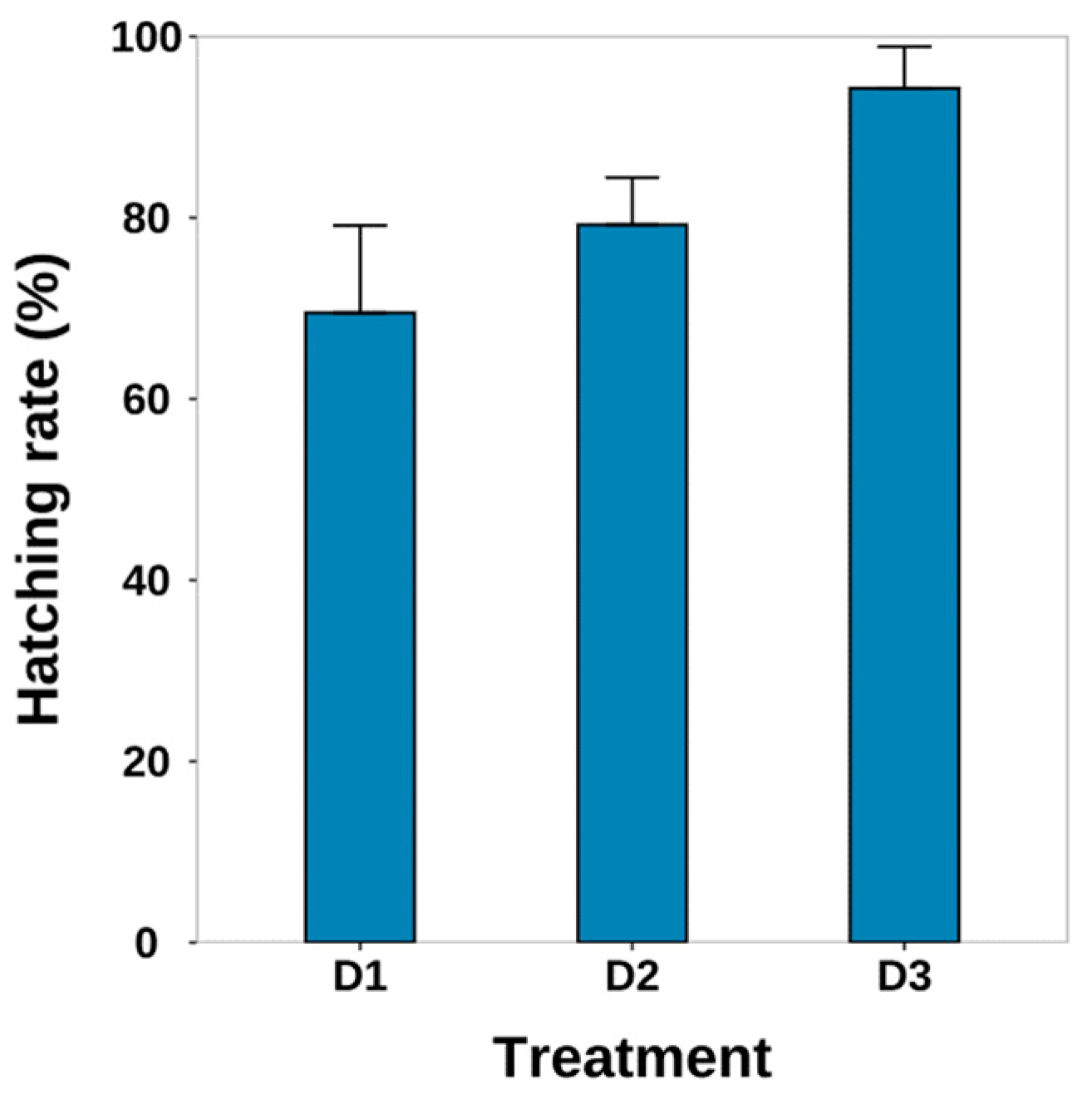
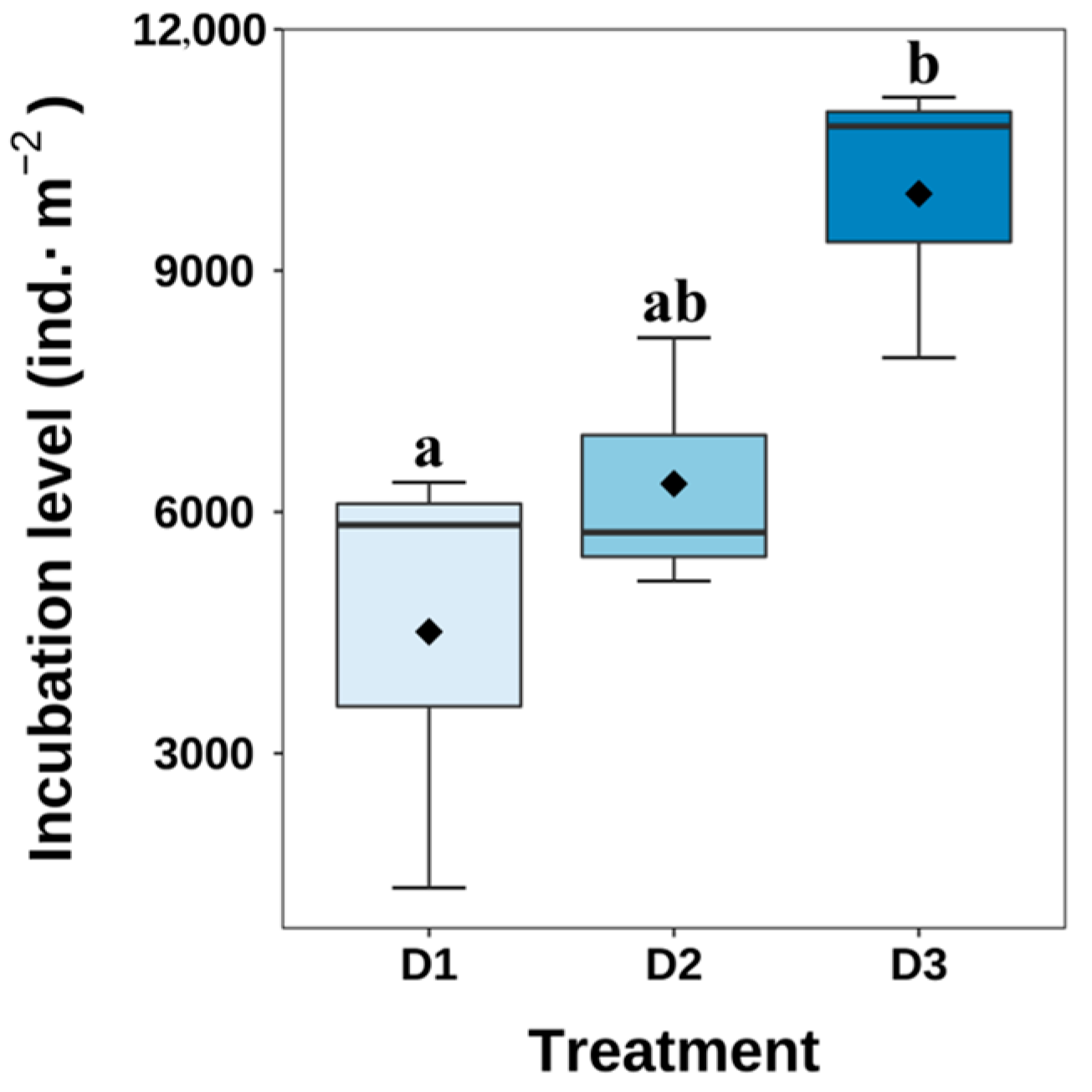
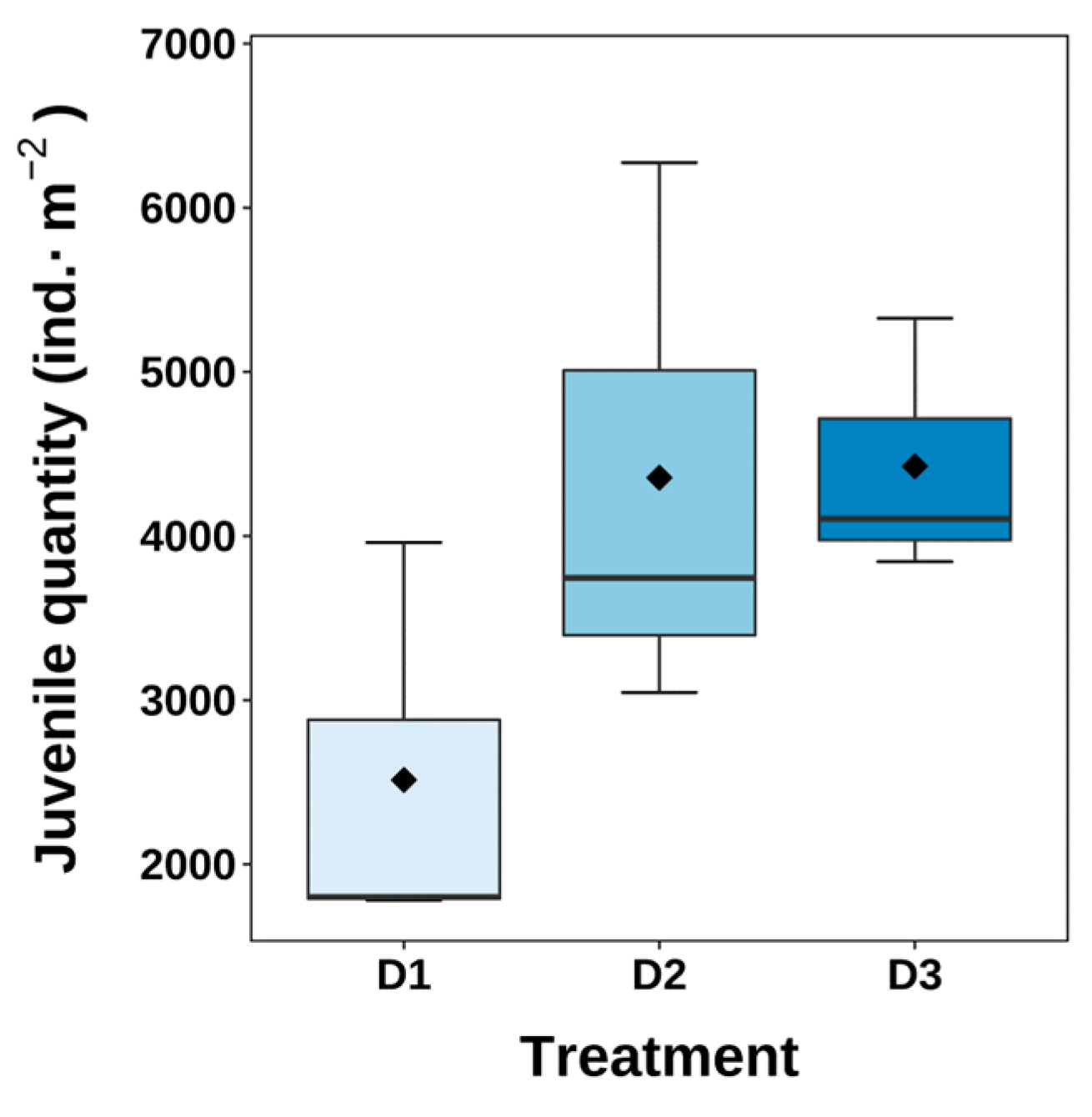
3.4. Hatching Condition
3.5. Profitability Analysis
4. Discussion
4.1. Survival
4.2. Nonspecific Immune Condition
4.3. Hatching Conditions
4.4. Profitability Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Sustainability in Action; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Hobbs, H.H. Biota of freshwater ecosystems, identification manual no. 9. In Crayfishes (Astacidae) of North and Middle America; Environmental Protection Agency: Washington, DC, USA, 1972. [Google Scholar]
- Center, C.F.T.E. China Fisheries Technology Extension Center. Chinese crayfish industry development report (2020). China Fish. 2020, 536, 8–17. (In Chinese) [Google Scholar]
- Dai, Y.; Gong, X.J.; Li, B.; Wang, Y.F.; Huang, W.G. Reproductive Period of Procambarus clarkii in Wuhan Area. Chin. J. Zool. 2008, 43, 21–27. (In Chinese) [Google Scholar] [CrossRef]
- Xu, Z.H.; Zhou, X.; Zhao, C.Y. Preliminary study on artificial breeding technology of Procambarus clarkii. Fish. Sci. Technol. Inf. 2010, 37, 43–45. (In Chinese) [Google Scholar]
- Song, G.T.; Ding, F.Q.; Wu, S.; Chen, J.; Wang, X.; Hou, G.J. Studies of crucial artificial techniques of red swamp crayfish Procambarus clarkii. In: Fisheries Science and Technology. Fish. Sci. Technol. Inf. 2015, 42, 108–112. (In Chinese) [Google Scholar] [CrossRef]
- Barki, A.; Karplus, I. Crowding female red claw crayfish, Cherax quadricarinatus, under small-tanks hatchery conditions: What is the limit? Aquaculture 2000, 181, 235–240. [Google Scholar] [CrossRef]
- Xu, J.Y.; Yue, C.F.; Ying, D.; Wang, Y.F. Effects of water temperature, photoperiod and diet on survival rate and ovarian development of the crayfish, Procambarus clarkill. J. Cent. China Norm. Univ. (Nat. Sci.) 2008, 42, 97–101. (In Chinese) [Google Scholar] [CrossRef]
- Song, G.T.; Ding, F.Q.; Chen, J.; Wu, S.; Wang, X. Effects of broodstock sizes, shelter, Illumination and stocking density on breeding in Red Swamp Crayfish Procambarus clarkii. Fish. Sci. 2012, 31, 549–553. (In Chinese) [Google Scholar] [CrossRef]
- Jin, S.Y.; Jacquin, L.; Huang, F.; Xiong, M.T.; Li, R.J.; Lek, S.; Li, W.; Liu, J.S.; Zhang, T.L. Optimizing reproductive performance and embryonic development of red swamp crayfish Procambarus clarkii by manipulating water temperature. Aquaculture 2019, 510, 32–42. [Google Scholar] [CrossRef]
- Tong, L.J.; Moss, G.A.; Pickering, T.D.; Paewai, M.P. Temperature effects on embryo and early larval development of the spiny lobster Jasus edwardsii, and description of a method to predict larval hatch times. Mar. Freshw. Res. 2000, 51, 243–248. [Google Scholar] [CrossRef]
- Rotllant, G.; Simeo, C.G.; Macia, G.; Estevez, A. High environmental salinity reduces the reproductive potential of the spider crab Maja brachydactyla (Decapoda, Majidae). Mar. Ecol.-Evol. Perspect. 2015, 36, 496–505. [Google Scholar] [CrossRef]
- Djunaidah, I.S.; Wille, M.; Kontara, E.K.; Sorgeloos, P. Reproductive performance and offspring quality in mud crab (Scylla paramamosain) broodstock fed different diets. Aquac. Int. 2003, 11, 3–15. [Google Scholar] [CrossRef]
- Laurenz, J.; Georg, A.; Brendelberger, H.; Lehmann, K. Effects of nitrate on early life stages of Astacus astacus (Linnaeus, 1758) and Procambarus virginalis (Lyko, 2017). Int. Aquat. Res 2020, 12, 53–62. [Google Scholar]
- Seginer, I. Are restricted periods of over-stocking of recirculating aquaculture systems advisable? A simulation study. Aquac. Eng. 2009, 41, 194–206. [Google Scholar] [CrossRef]
- Yuan, J.; Liao, C.A.S.; Zhang, T.L.; Guo, C.A.B.; Liu, J.S. Advances in Ecology Research on Integrated Rice Field Aquaculture in China. Water 2022, 14, 2333. [Google Scholar] [CrossRef]
- Xiao, M.H.; Xiao, Y.P.; Wu, Z.Q.; Hu, X.P. Effects of stocking density on growth, digestive enzyme activities and biochemical indices of juvenile Procambarus clarkii. J. Fish. China 2012, 36, 1088–1093. (In Chinese) [Google Scholar] [CrossRef]
- Chen, Y. Effect of stocking density on growth and survival rate of Procambarus clarkill. J. Anhui Agric. Univ. 2016, 43, 37–41. (In Chinese) [Google Scholar]
- Naranjo-Paramo, J.; Hernandez-Llamas, A.; Vargas-Mendieta, M.; Villarreal-Colmenares, H. Stochastic dynamic model analysis of the effect of stocking density on the monosex production of male redclaw crayfish Cherax quadricarinatus reared in commercial gravel-lined ponds. Aquaculture 2021, 535, 736351. [Google Scholar] [CrossRef]
- McClain, W.R. Effects of population density and feeding rate on growth and feed consumption of red swamp crawfish Procambarus clarkii. J. World Aquac. Soc. 1995, 26, 14–23. [Google Scholar] [CrossRef]
- Kouba, A.; Buřič, M.; Policar, T.; Kozák, P. Evaluation of body appendage injuries to juvenile signal crayfish (Pacifastacus leniusculus) relationships and consequences. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 4. [Google Scholar] [CrossRef] [Green Version]
- He, M.D.; Liu, F.; Wang, F. Quantitative analysis of density dependent resource utilization, cannibalism, and competition of the red swamp crayfish (Procambarus clarkii) in rice-crayfish cocultures without supplementary food. Aquaculture 2021, 543, 736966. [Google Scholar] [CrossRef]
- Martin, A.L.; Moore, P.A. Field observations of agonism in the crayfish, Orconectes rusticus: Shelter use in a natural environment. Ethology 2007, 113, 1192–1201. [Google Scholar] [CrossRef]
- Yu, J.X.; Xiong, M.T.; Ye, S.W.; Li, W.; Xiong, F.; Liu, J.S.; Zhang, T.L. Effects of stocking density and artificial macrophyte shelter on survival, growth and molting of juvenile red swamp crayfish (Procambarus clarkii) under experimental conditions. Aquaculture 2020, 521, 735001. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhang, X.L.; Li, S.Q.; Wang, G.Z.; Lin, Q.W. Shelter preference of megalopae and first juvenile of mud crab, Scylla paramamosain (Estampador, 1949). J. Xiamen Univ. (Nat. Sci.) 2009, 48, 594–599. (In Chinese) [Google Scholar]
- Moksnes, P.O.; Pihl, L.; van Montfrans, J. Predation on postlarvae and juveniles of the shore crab Carcinus maenas: Importance of shelter, size and cannibalism. Mar. Ecol. Prog. Ser. 1998, 166, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Halwart, M.; Gupta, M.V. Culture of Fish in Rice Fields; FAO: Rome, Italy; WorldFish Center: Lusaka, Zambia, 2004. [Google Scholar]
- Miao, W.M. Recent developments in rice-fish culture in China: A holistic approach for livelihood improvement in rural areas. In Success Stories in Asian Aquaculture; De Silva, S.S., Davy, F.B., Eds.; Springer Science: Dordrecht, The Netherlands; New York, NY, USA, 2010; pp. 15–40. [Google Scholar] [CrossRef]
- Long, H.P. A preliminary study on the artificial induction breeding technology of Procambarus clarkii. Fish. Guide Be Rich 2010, 298, 27–29. (In Chinese) [Google Scholar]
- Figler, M.H.; Cheverton, H.M.; Blank, G.S. Shelter competition in juvenile red swamp crayfish (Procambarus clarkii): The influences of sex differences, relative size, and prior residence. Aquaculture 1999, 178, 63–75. [Google Scholar] [CrossRef]
- Flint, N.; Pearson, R.G.; Crossland, M.R. Use of aquatic plants to create fluctuating hypoxia in an experimental environment. Mar. Freshw. Res. 2012, 63, 351–360. [Google Scholar] [CrossRef]
- Tang, X.S. Red swamp crayfish (Procambarus clarkii). Bull. Biol. 2001, 09, 19–20. (In Chinese) [Google Scholar]
- Sun, R.J.; Gong, S.Y.; Ma, Y.X.; He, X.G.; Zhang, X.P. A study on the suitable eco-environment for spawning of Procambarus clarkii. Freshw. Fish. 2008, 38, 16–19. (In Chinese) [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992; Volume 6. [Google Scholar]
- Jia, E.; Li, Z.Q.; Xue, Y.F.; Jiang, G.Z.; Li, X.F.; Liu, W.B.; Zhang, D.D. Effects of dietary fructooligosaccharide on the growth, antioxidants, immunity and disease resistance of Chinese mitten crab. Aquaculture 2017, 481, 154–161. [Google Scholar] [CrossRef]
- Luo, S.; Li, X.Q.; Onchari, M.M.; Li, W.; Bu, Y.Y.; Lek, S.; Zhang, T.L.; Wang, Z.Y.; Jin, S.Y. High feeding level alters physiological status but does not improve feed conversion efficiency and growth performance of juvenile red swamp crayfish Procambarus clarkii (Girard, 1852). Aquaculture 2021, 537, 736507. [Google Scholar] [CrossRef]
- Xu, D.F.; Wu, J.X.; Sun, L.J.; Qin, X.M.; Fan, X.P.; Zheng, X.X. Combined stress of acute cold exposure and waterless duration at low temperature induces mortality of shrimp Litopenaeus vannamei through injuring antioxidative and immunological response in hepatopancreas tissue. J. Therm. Biol. 2021, 100, 103080. [Google Scholar] [CrossRef] [PubMed]
- Shawon, N.; Prodhan, M.M.; Khan, M.; Mitra, S. Financial profitability of small scale shrimp farming in a coastal area of Bangladesh. J. Bangladesh Agric. Univ. 2018, 16, 104. [Google Scholar] [CrossRef] [Green Version]
- Andrea, S.; Marco, R.; Terry, A.; Francesca, C.; Jessica, C.; Debora, G.; Michaela, S.; Stefano, T. Sensitivity Analysis: From Theory to Practice. In Global Sensitivity Analysis. The Primer; Wiley Online Library: New York, NY, USA, 2007; pp. 237–275. [Google Scholar]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Package Version 1.8.4-1.; The Comprehensive R Archive Network: London, UK, 2022; Available online: https://CRAN.R-project.org/package=emmeans (accessed on 7 November 2022).
- Hothorn, T.; Hornik, K.; Van De Wiel, M.A.; Zeileis, A. A lego system for conditional inference. Am. Stat. 2008, 60, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Rcompanion: Functions to Support Extension Education Program Evaluation; R Package Version 2.4.6.; The Comprehensive R Archive Network: London, UK, 2016; Available online: https://CRAN.R-project.org/package=rcompanion (accessed on 7 November 2022).
- Nemenyi, P. Distribution-Free Multiple Comparisons; Princeton University: Princeton, NJ, USA, 1963. [Google Scholar]
- PMCMRplus: Calculate Pairwise Multiple Comparisons of Mean Rank Sums; R Package Version 1.9.6.; The Comprehensive R Archive Network: London, UK, 2022; Available online: https://CRAN.R-project.org/package=PMCMRplus (accessed on 7 November 2022).
- Luo, S.; Wang, Z.Y.; Li, X.Q.; Onchari, M.M.; Song, C.W.; Yuan, X.Y.; Li, W.; John, C.K.; Zhang, T.L.; Lek, S.; et al. Feed deprivation over 16 days followed by refeeding until 75 days fails to elicit full compensation of Procambarus clarkii. Aquaculture 2022, 547, 737490. [Google Scholar] [CrossRef]
- Harrison, K.E. The role of nutrition in maturation, reproduction and embryonic development of decapod crustacean: A review. J. Shellfish Res. 1990, 9, 1–28. [Google Scholar]
- Tian, J.; Chen, Y.P.; Qi, Z.A.; Huang, C. Effects of space and rearing mode on growth performance of juvenile Red Swamp Crayfish Procambarus clarkii. Fish. Sci. 2018, 37, 825–829. (In Chinese) [Google Scholar] [CrossRef]
- Corkum, L.D.; Cronin, D.J. Habitat complexity reduces aggression and enhances consumption in crayfish. J. Ethol. 2004, 22, 23–27. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.B.; Wu, W.H.; Yin, J.S.; Mou, Z.B.; Hao, F.H. Effects of stocking density on growth performance and metabolism of juvenile Lenok (Brachymystax lenok). Aquaculture 2019, 504, 107–113. [Google Scholar] [CrossRef]
- Li, Y.Q.; Li, J.; Wang, Q.Y. The effects of dissolved oxygen concentration and stocking density on growth and non-specific immunity factors in Chinese shrimp, Fenneropenaeus chinensis. Aquaculture 2006, 256, 608–616. [Google Scholar] [CrossRef]
- Yang, Y.H.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef]
- Cammarata, M.; Parrinello, N. The ascidian prophenoloxidase activating system. Invertebr. Surviv. J. 2009, 6, S67–S76. [Google Scholar]
- Chen, Y.Y.; Huang, X.H.; Wang, J.Z.; Li, C.L. Effect of pure microcystin-LR on activity and transcript level of immune-related enzymes in the white shrimp (Litopenaeus vannamei). Ecotoxicology 2017, 26, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Dörr, A.J.M.; Pacini, N.; Abete, M.C.; Prearo, M.; Elia, A.C. Effects of a selenium-enriched diet on antioxidant response in adult crayfish (Procambarus clarkii). Chemosphere 2008, 73, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.L.; Wan, J.J.; Meng, X.L.; Zhou, Z.H.; Sun, L.S.; Tang, J.Q. Effects of acute and chronic high pH stress on non-specific immunity and antioxidant capacity in Procambarus clarkii. Freshw. Fish. 2021, 51, 101–107. (In Chinese) [Google Scholar] [CrossRef]
- Ruan, G.L.; Li, S.X.; He, N.J.; Fang, L.; Wang, Q. Short-term adaptability to non-hyperthermal stress: Antioxidant, immune and gut microbial responses in the red swamp crayfish, Procambarus clarkii. Aquaculture 2022, 560, 738497. [Google Scholar] [CrossRef]
- Le Moullac, G.; Haffner, P. Environmental factors affecting immune responses in Crustacea. Aquaculture 2000, 191, 121–131. [Google Scholar] [CrossRef]
- Scherping, F.D.; Watson, M.J. A standardized protocol for measuring phenoloxidase in captive and wild Murray crayfish Euastacus armatus. Fish Shellfish Immunol. 2021, 111, 140–144. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.Y.; Yu, Y.H.; Sun, N.; Li, Y.D.; Wei, H.; Yang, Z.Q.; Li, X.D.; Li, L. Effect of stocking density on growth performance, digestive enzyme activities, and nonspecific immune parameters of Palaemonetes sinensis. Fish Shellfish Immunol. 2018, 73, 37–41. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Zhang, C.; Duan, Z.Q. Pollutants’ Release, Redistribution and Remediation of Black Smelly River Sediment Based on Re-Suspension and Deep Aeration of Sediment. Int. J. Environ. Res. Public Health 2017, 14, 374. [Google Scholar] [CrossRef]
- Fossat, P.; Bacque-Cazenave, J.; De Deurwaerdere, P.; Delbecque, J.P.; Cattaert, D. Anxiety-like behavior in crayfish is controlled by serotonin. Science 2014, 344, 1293–1297. [Google Scholar] [CrossRef]
- Bacque-Cazenave, J.; Cattaert, D.; Delbecque, J.P.; Fossat, P. Social harassment induces anxiety-like behaviour in crayfish. Sci. Rep. 2017, 7, 39935. [Google Scholar] [CrossRef] [Green Version]
- de Abreu, M.S.; Maximino, C.; Banha, F.; Anastacio, P.M.; Demin, K.A.; Kalueff, A.V.; Soares, M.C. Emotional behavior in aquatic organisms? Lessons from crayfish and zebrafish. J. Neurosci. Res. 2020, 98, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, M.D. Composition, accumulation and utilization of yolk lipids in teleost fish. Rev. Fish Biol. Fish. 1996, 6, 259–286. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Soderhall, I.; Soderhall, K.; Jiravanichpaisal, P. Expression of immune-related genes in one phase of embryonic development of freshwater crayfish, Pacifastacus leniusculus. Fish Shellfish Immunol. 2010, 28, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Sopinka, N.M.; Wilson, S.M.; Hinch, S.G.; Patterson, D.A.; Cooke, S.J.; Willmore, W.G. Examining the relationships between egg cortisol and oxidative stress in developing wild sockeye salmon (Oncorhynchus nerka). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 200, 87–93. [Google Scholar] [CrossRef]
- Li, T.G. The effects of salinity on embryonic development of crayfish Procambarus clarkii. Fish. Sci. 2009, 28, 789–791. (In Chinese) [Google Scholar]
- Rosemore, B.J.; Welsh, C.A. The effects of rearing density, salt concentration, and incubation temperature on Japanese medaka (Oryzias latipes) embryo development. Zebrafish 2012, 9, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Scholtz, G.; Kawai, T. Aspects of embryonic and postembryonic development of the Japanese freshwater crayfish Cambaroides japonicus (Crustacea, Decapoda) including a hypothesis on the evolution of maternal care in the Astacida. Acta Zool. 2002, 83, 203–212. [Google Scholar] [CrossRef]
- Nightingale, J.; Stebbing, P.; Taylor, N.; McCabe, G.; Jones, G. Determining an effective density regime for rearing juvenile Austropotamobius pallipes in a small-scale closed system hatchery. Aquac. Res. 2018, 49, 3055–3062. [Google Scholar] [CrossRef]
- Ramalho, R.O.; Correia, A.M.; Anastácio, P.M. Effects of density on growth and survival of juvenile Red Swamp Crayfish, Procambarus clarkii (Girard), reared under laboratory conditions. Aquac. Res. 2008, 39, 577–586. [Google Scholar] [CrossRef]

| Water Quality Parameters | At the Beginning of the Experiment | During the Experiment with Three Treatments | ||
|---|---|---|---|---|
| D1 | D2 | D3 | ||
| WT (°C) | 20.45 ± 0.24 | 19.99 ± 0.22 | 20.10 ± 0.29 | |
| DO (mg L−1) | 8.14 ± 0.14 | 8.43 ± 0.13 | 8.13 ± 0.12 | |
| pH | 8.14 ± 0.02 | 8.07 ± 0.02 | 8.13 ± 0.03 | |
| TN (mg L−1) | 2.18 ± 0.29 a | 6.33 ± 1.09 b | 5.19 ± 0.09 b | 6.75 ± 0.22 b |
| TP (mg L−1) | 0.34 ± 0.02 | 0.46 ± 0.11 | 0.65 ± 0.03 | 0.46 ± 0.02 |
| NH4+-N (mg L−1) | 0.43 ± 0.01 a | 1.58 ± 0.37 b | 1.24 ± 0.10 ab | 1.39 ± 0.09 b |
| NO2−-N (mg L−1) | −0.00 ± 0.00 | 0.53 ± 0.15 | 0.19 ± 0.07 | 0.71 ± 0.21 |
| NO3−-N (mg L−1) | 0.09 ± 0.00 a | 1.36 ± 0.56 ab | 1.99 ± 0.02 ab | 2.39 ± 0.17 b |
| CODMn (mg L−1) | 8.29 ± 2.38 a | 27.10 ± 0.63 c | 22.88 ± 1.95 bc | 18.80 ± 0.35 b |
| TUR (NTU) | 4.71 ± 0.45 | 10.08 ± 2.66 | 8.14 ± 0.84 | 10.03 ± 3.24 |
| Treatment | P. clarkii | Quantity | RMB. Unit−1 | Cost (RMB) | Gross Profit (RMB. m−2) | BCR (Ratio. m−2) | |
|---|---|---|---|---|---|---|---|
| D1 | Income | Juvenile | 1816 ind. | 0.15. ind.−1 | 272.42 | 275.24 ± 108.48 | 5.13 ± 1.48 |
| Expenditure | Adult | 0.83 kg | 70. kg−1 | 58.13 | |||
| Feed | 5.14 kg | 3. kg−1 | 15.42 | ||||
| D2 | Income | Juvenile | 3146 ind. | 0.15. ind.−1 | 471.97 | 548.99 ± 147.10 | 8.67 ± 1.95 |
| Expenditure | Adult | 0.83 kg | 70. kg−1 | 58.13 | |||
| Feed | 5.73 kg | 3. kg−1 | 17.19 | ||||
| D3 | Income | Juvenile | 3197 ind. | 0.15. ind.−1 | 479.60 | 559.46 ± 68.53 | 8.80 ± 0.91 |
| Expenditure | Adult | 0.83 kg | 70. kg−1 | 58.13 | |||
| Feed | 5.76 kg | 3. kg−1 | 17.27 |
| Scenario | BCR (Ratio. m−2) | ||
|---|---|---|---|
| D1 | D2 | D3 | |
| Business as usual | 5.13 ± 1.48 | 8.67 ± 1.95 | 8.80 ± 0.91 |
| If juvenile price reduced by 5% | 4.87 ± 1.40 | 8.24 ± 1.86 | 8.36 ± 0.86 |
| If juvenile price reduced by 10% | 4.61 ± 1.33 | 7.81 ± 1.76 | 7.92 ± 0.82 |
| If juvenile price reduced by 15% | 4.36 ± 1.25 | 7.37 ± 1.66 | 7.48 ± 0.77 |
| If juvenile price reduced by 20% | 4.10 ± 1.18 | 6.94 ± 1.56 | 7.04 ± 0.73 |
| If juvenile price increased by 5% | 5.38 ± 1.55 | 9.11 ± 2.05 | 9.24 ± 0.95 |
| If juvenile price increased by 10% | 5.64 ± 1.62 | 9.54 ± 2.15 | 9.68 ± 1.00 |
| If juvenile price increased by 15% | 5.90 ± 1.70 | 9.97 ± 2.25 | 10.12 ± 1.05 |
| If juvenile price increased by 20% | 6.15 ± 1.77 | 10.41 ± 2.34 | 10.57 ± 1.09 |
| If adult price reduced by 5% | 5.34 ± 1.54 | 9.02 ± 2.03 | 9.16 ± 0.95 |
| If adult price reduced by 10% | 5.57 ± 1.60 | 9.40 ± 2.12 | 9.54 ± 0.98 |
| If adult price reduced by 15% | 5.82 ± 1.67 | 9.81 ± 2.21 | 9.96 ± 1.03 |
| If adult price reduced by 20% | 6.09 ± 1.75 | 10.26 ± 2.31 | 10.41 ± 1.07 |
| If adult price increased by 5% | 4.93 ± 1.42 | 8.35 ± 1.88 | 8.48 ± 0.88 |
| If adult price increased by 10% | 4.75 ± 1.37 | 8.05 ± 1.81 | 8.17 ± 0.84 |
| If adult price increased by 15% | 4.58 ± 1.32 | 7.77 ± 1.75 | 7.89 ± 0.81 |
| If adult price increased by 20% | 4.43 ± 1.27 | 7.51 ± 1.69 | 7.63 ± 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, L.; Guo, C.; Xiong, M.; Gong, K.; Liu, J.; Zhang, T.; Li, W. Effects of Shelter on the Hatching, Immune Performance, and Profitability of the Ovigerous Red Swamp Crayfish Procambarus clarkii under High Stocking Density. Water 2023, 15, 907. https://doi.org/10.3390/w15050907
Qin L, Guo C, Xiong M, Gong K, Liu J, Zhang T, Li W. Effects of Shelter on the Hatching, Immune Performance, and Profitability of the Ovigerous Red Swamp Crayfish Procambarus clarkii under High Stocking Density. Water. 2023; 15(5):907. https://doi.org/10.3390/w15050907
Chicago/Turabian StyleQin, Lirong, Chao Guo, Mantang Xiong, Kun Gong, Jiashou Liu, Tanglin Zhang, and Wei Li. 2023. "Effects of Shelter on the Hatching, Immune Performance, and Profitability of the Ovigerous Red Swamp Crayfish Procambarus clarkii under High Stocking Density" Water 15, no. 5: 907. https://doi.org/10.3390/w15050907





