Photosynthetic Physiological Response of Porphyra yezoensis to Light Change at Different CO2 Concentrations
Abstract
:1. Background
2. Methods
2.1. Sample Collection and Culture Conditions
2.2. Experimental Design
2.3. Determination of Carbonate System Parameters
2.4. Growth Measurements
2.5. Determination of Photosynthesis and Respiration Rates
2.6. Determination of Chlorophyll Fluorescence Parameters
2.7. Pigment Determination
2.8. Data Analysis
3. Results
3.1. Carbonate System Parameters
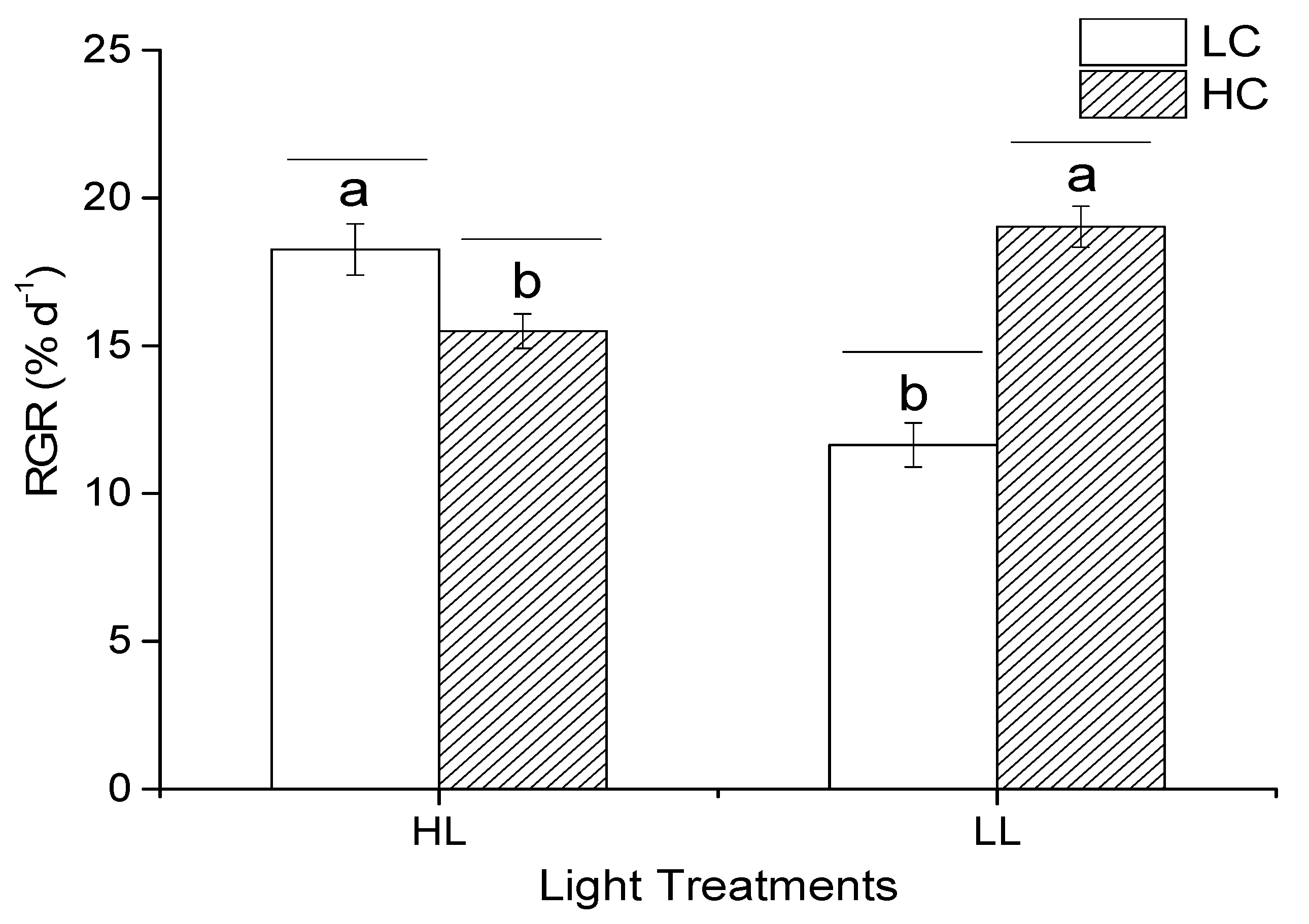
3.2. Relative Growth Rate
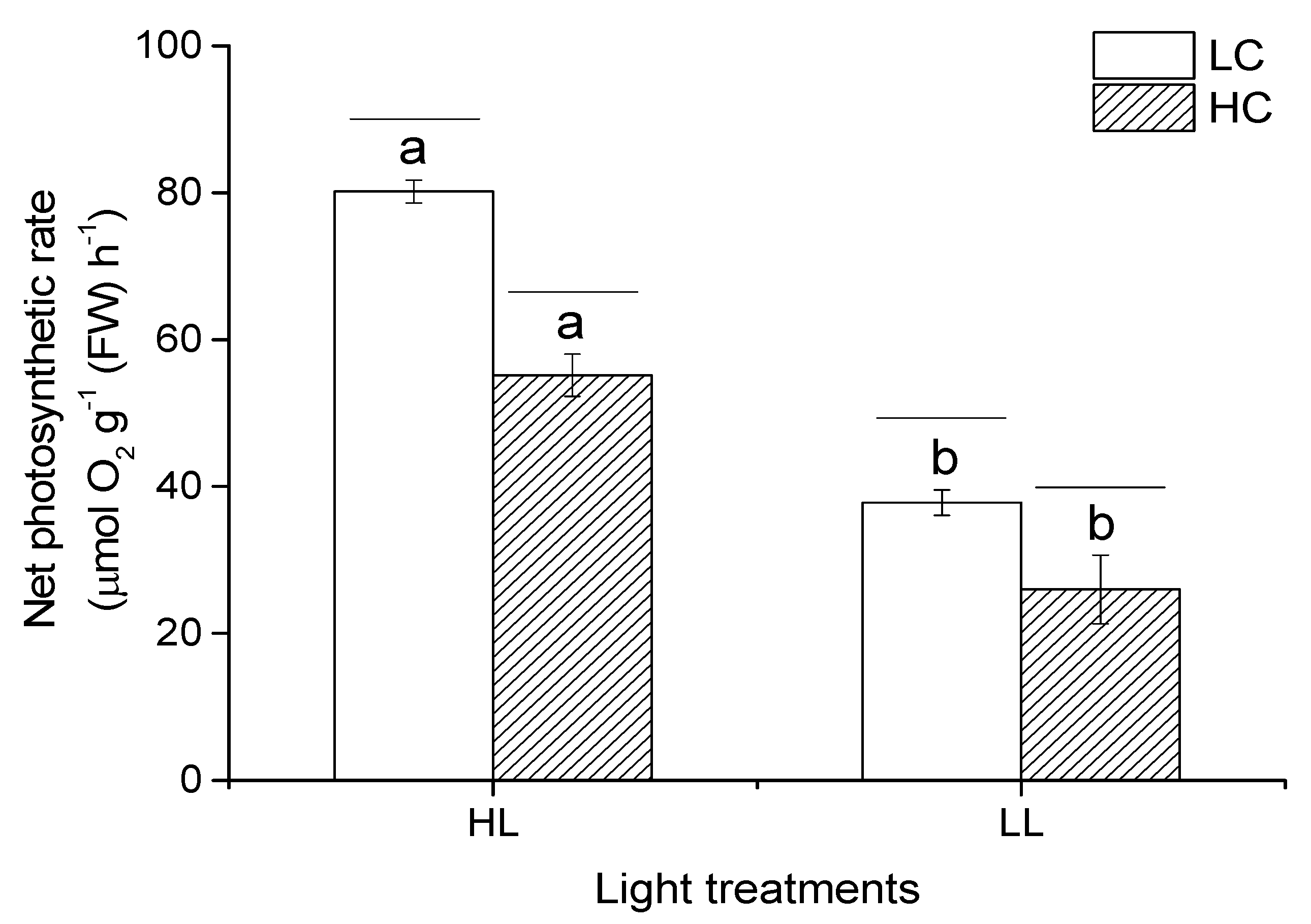
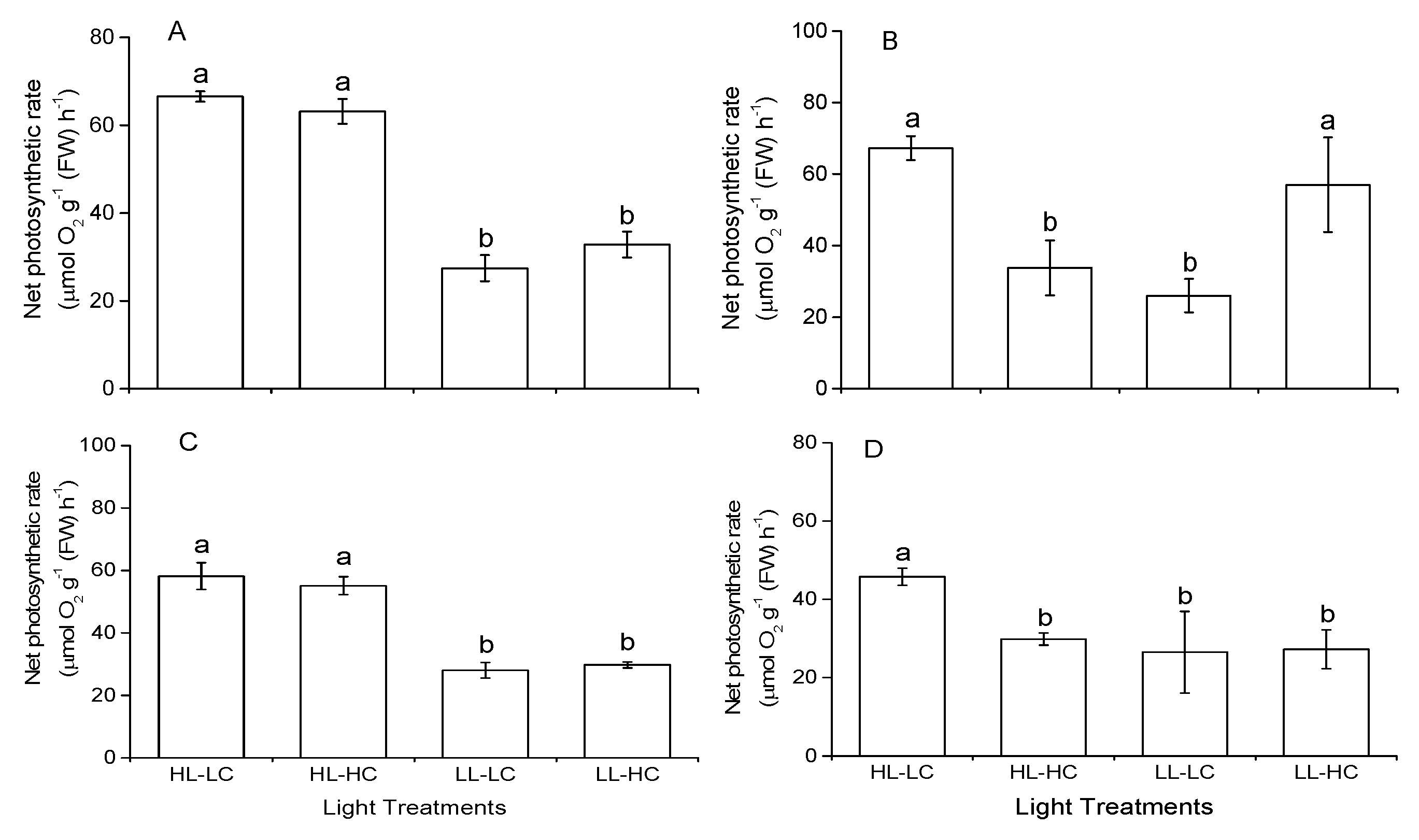
3.3. Photosynthesis Rate
3.4. Instantaneous Respiratory Rate
3.5. Chlorophyll Fluorescence Parameters
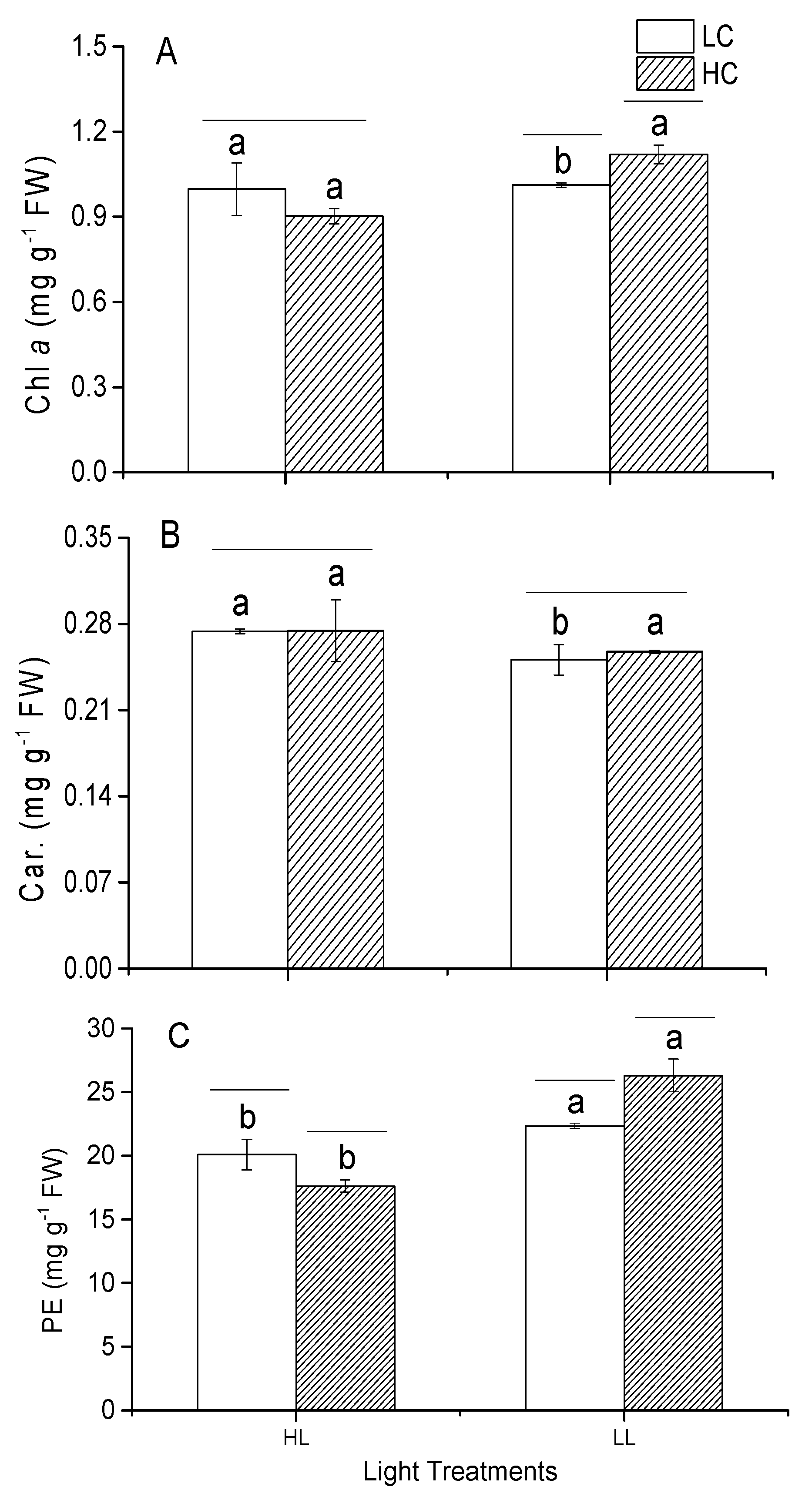
3.6. Photosynthetic Pigments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, K.S. Approaches and involved principles to control pH/pCO2 stability in algal cultures. J. Appl. Phycol. 2021, 33, 3497–3505. [Google Scholar] [CrossRef]
- Gao, G.; Xu, Z.G.; Shi, Q.; Wu, H.Y. Increased CO2 exacerbates the stress of ultraviolet radiation on photosystem ii function in the diatom thalassiosira weissflogii. Environ. Exp. Bot. 2018, 156, 96–105. [Google Scholar] [CrossRef]
- Zhou, M.J.; Liu, D.Y.; Anderson, D.M.; Valiela, I. Introduction to the special issue on green tides in the Yellow Sea. Estuar. Coast. Shelf Sci. 2015, 163, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Qu, L.M.; Xu, T.P.; Burgess, J.G.; Li, X.S.; Xu, J.T.; Norkko, J. Future CO2-induced ocean acidification enhances resilience of a green tide alga to low-salinity stress. ICES J. Mar. Sci. 2019, 76, 2437–2445. [Google Scholar] [CrossRef]
- Ma, J.; Wang, W.; Cao, J.Y.; Xu, T.P.; Chen, C.; Xu, J.T. Response of the green alga Ulva prolifera grown at different irradiance levels under ocean acidification at different life cycle stages. Bot. Mar. 2022, 65, 347–356. [Google Scholar] [CrossRef]
- Lin, H.; Jiang, P.; Zhang, J.; Wang, J.; Qin, S.; Sun, S. Genetic and marine cyclonic eddy analyses on the largest macroalgal bloom in the world. Environ. Sci. Technol. 2011, 45, 5996–6002. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresource Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, H.; Huang, J.J.; Wang, J.G.; Zhen, W.; Wang, J.W.; Ni, J.X.; Xu, J.T. Elevated-CO2 and nutrient limitation synergistically reduce the growth and photosynthetic performances of a commercial macroalga Gracilariopsis lemaneiformis. Aquaculture 2022, 550, 737878. [Google Scholar] [CrossRef]
- Gao, S.; Shen, S.; Wang, G.; Niu, J.; Lin, A.; Pan, G.H. PSI-driven cyclic electron flow allows intertidal macro-algae Ulva sp. (Chlorophyta) to survive in desiccated conditions. Plant Cell Physiol. 2011, 52, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Bergkemper, V.; Stadler, P.; Weisse, T. Moderate weather extremes alter phytoplankton diversity-a microcosm study. Freshw. Biol. 2018, 63, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.X.; Wang, H.T.; Zang, X.N.; Liu, Z.; Xu, D.; Jin, Y.M.; Zhang, F.; Wang, Z.D. Changes in the photosynthetic pigment contents and transcription levels of phycoerythrin-related genes in three Gracilariopsis lemaneiformis strains under different light intensities. J. Ocean Univ. China 2021, 20, 661–668. [Google Scholar] [CrossRef]
- Bao, M.L.; Wang, J.H.; Xu, T.P.; Wu, H.L.; Li, X.S.; Xu, J.T. Rising CO2 levels alter the responses of the red macroalga Pyropia yezoensis under light stress. Aquaculture 2019, 501, 325–330. [Google Scholar] [CrossRef]
- Li, J.J.; Pang, Y.L.; Qin, S.; Liu, Z.Y.; Zhong, Z.H.; Song, W.L.; Zhuang, L.C. Comparison of the photo-acclimation potential of floating and benthic thalli of Sargassum horneri (Phaeophyta) during autumn and winter. J.Oceanol. Limnol. 2021, 40, 195–205. [Google Scholar] [CrossRef]
- Gao, G.; Liu, Y.M.; Li, X.S.; Feng, Z.H.; Xu, J.T. An ocean acidification acclimatized green tide alga is robust to changes of seawater carbon chemistry but vulnerable to light stress. PLoS ONE 2016, 11, e0169040. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.H.; Sun, L.; Liu, Z.Y.; Song, Z.M.; Liu, M.Y.; Tong, S.Y.; Qin, S. Ocean acidification exacerbates the inhibition of fluctuating light on the productivity of Ulva prolifera. Mar. Pollut. Bull. 2022, 175, 113367. [Google Scholar] [CrossRef]
- Riebesell, U.; Gattuso, J.P. Lessons learned from ocean acidification research. Nat. Clim. Chang. 2014, 5, 12–14. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Li, Z.; Xu, J.; Gao, K. Effects of seawater acidification on the growth rates of the diatom Thalassiosira (Conticribra) weissflogii under different nutrient, light, and UV radiation regimes. J. Appl. Phycol. 2016, 29, 133–142. [Google Scholar] [CrossRef]
- Wu, H.L.; Feng, J.C.; Li, X.S.; Zhao, C.Y.; Liu, Y.H.; Yu, J.T.; Xu, J.T. Effects of increased CO2 and temperature on the physiological characteristics of the golden tide blooming macroalgae Sargassum horneri in the Yellow Sea, China. Mar. Pollut. Bull. 2019, 146, 639–644. [Google Scholar] [CrossRef]
- Xu, J.T.; Gao, K.S. Photosynthetic contribution of UV-A to carbon fixation by macroalgae. Phycologia 2016, 55, 318–322. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, C.M.; Zhou, W.; Deng, Y.Y.; Xu, G.P.; Tian, C.C.; Lu, Q.Q.; Lu, S.; Zhang, M.R.; Yang, L.E. Carotenoids participate in adaptation/resistance of daily desiccation in the intertidal red alga neopyropia yezoensis (bangiales, rhodophyta). Algal Res. 2022, 61, 102606. [Google Scholar] [CrossRef]
- Pierrot, D.; Lewis, E.; Wallace, D.W.R. ORNL/CDIAC-105; CO2SYS DOS Program Developed for CO2 System Calculations. Carbon Dioxide Information Analysis Center; Oak Ridge National Laboratory; U.S. Department of Energy: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Eilers, P.; Peeters, J.J.E.M. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- Jassby, A.D.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.; Thompson, W.; Kriedemann, P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. BBA-Bioenergetics 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Siegelman, H.W.; Kycia, J.H. Algal biliproteins. In Handbook of Phycological Methods. Physiological and Biochemical Methods; Hellebust, J.A., Craigie, J.S., Eds.; Cambridge University Press: Cambridge, MA, USA, 1978; pp. 71–79. [Google Scholar]
- Xie, X.J.; Lu, X.P.; Wang, L.P.; He, L.W.; Wang, G.C. High light intensity increases the concentrations of β-carotene and zeaxanthin in marine red macroalgae. Algal Res. 2020, 47, 101852. [Google Scholar] [CrossRef]
- Olischläger, M.; Wiencke, C. Ocean acidification alleviates low-temperature effects on growth and photosynthesis of the red alga Neosiphonia harveyi (Rhodophyta). J. Exp. Bot. 2013, 64, 5587–5597. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Xu, T.P.; Bao, M.L.; Zhou, H.M.; Zhang, T.Z.; Li, Z.Z.; Gao, G.; Li, X.S.; Xu, J.T. Response of the red algae Pyropia yezoensis grown at different light intensities to CO2-induced seawater acidification at different life cycle stages. Algal Res. 2020, 49. [Google Scholar] [CrossRef]
- Passow, U.; Laws, E.A. Ocean acidification as one of multiple stressors: Growth response of Thalassiosira weissflogii (diatom) under temperature and light stress. Mar. Ecol. Prog. Ser. 2015, 541, 75–90. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.J.; Kwon, E.H.; Choi, J.S.; Hong, S.Y.; Shin, H.W.; Yong, Y.K. Antifouling activity of seaweed extracts on the green alga Enteromorpha prolifera and the mussel Mytilus edulis. J. Appl. Phycol. 2001, 13, 117–125. [Google Scholar] [CrossRef]
- Harker, M.; Berkaloff, C.; Lemoine, Y.; Britton, G.; Young, A.; Duval, J.C.; Rmiki, N.-E.; Rousseau, B. Effects of high light and desiccation on the operation of the xanthophyll cycle in two marine brown algae. Eur. J. Phycol. 2010, 34, 35–42. [Google Scholar] [CrossRef]
- Warner, M.E.; Madden, M.L. The impact of shifts to elevated irradiance on the growth and photochemical activity of the harmful algae Chattonella subsalsa and Prorocentrum minimum from Delaware. Harmful Algae 2007, 6, 332–342. [Google Scholar] [CrossRef]
- Bao, N.; Gao, K.S. Interactive effects of elevated CO2 concentration and light on the picophytoplankton Synechococcus. Front. Mar. Sci. 2021, 8, 634189. [Google Scholar] [CrossRef]
- Gao, K.S.; Xu, J.T.; Gao, G.; Li, Y.; Hutchins, D.A.; Huang, B.; Wang, L.; Zheng, Y.; Jin, P.; Cai, X.; et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat. Clim. Chang. 2012, 2, 519–523. [Google Scholar] [CrossRef]

| pH | pCO2 (μatm) | DIC | HCO3− (μmol kg−1) | CO2 | TA | |
|---|---|---|---|---|---|---|
| HL–HC | 7.79 ± 0.01 a | 1060.25 ± 14 a | 2047.85 ± 24 a | 1934.05 ± 32 a | 32.4 ± 3 a | 2145.91 ± 13 a |
| HL–LC | 8.14 ± 0.01 b | 438.69 ± 18 b | 2010.73 ± 27 a | 1816.57 ± 39 b | 13.62 ± 1 b | 2244.2 ± 27 b |
| LL–HC | 7.82 ± 0.01 a | 1035.15 ± 26 a | 2143.59 ± 34 a | 2021.51 ± 42 a | 31.73 ± 2 a | 2253.54 ± 17 a |
| LL–LC | 8.13 ± 0.01 b | 444.291 ± 28 b | 2015.16 ± 11 b | 1839.67 ± 10 b | 13.72 ± 1 b | 2253.34 ± 30 a |
| rETRmax | α | Ik | |
|---|---|---|---|
| HL–HC | 24.65 ± 0.59 a | 0.06 ± 0.01 a | 251.14 ± 12.13 a |
| HL–LC | 24.93 ± 3.51 a | 0.09 ± 0.01 b | 304.08 ± 11.04 b |
| LL–HC | 25.40 ± 5.25 a | 0.09 ± 0.02 b | 311.65 ± 49.25 a |
| LL–LC | 20.32 ± 1.16 b | 0.05 ± 0.02 a | 228.71 ± 39.94 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhang, Y.; Feng, Z.; Wu, M.; Xu, T.; Qiao, S.; Wang, W.; Ma, J.; Xu, J. Photosynthetic Physiological Response of Porphyra yezoensis to Light Change at Different CO2 Concentrations. Water 2023, 15, 781. https://doi.org/10.3390/w15040781
Chen C, Zhang Y, Feng Z, Wu M, Xu T, Qiao S, Wang W, Ma J, Xu J. Photosynthetic Physiological Response of Porphyra yezoensis to Light Change at Different CO2 Concentrations. Water. 2023; 15(4):781. https://doi.org/10.3390/w15040781
Chicago/Turabian StyleChen, Cheng, Yanyan Zhang, Zhenjie Feng, Miaomiao Wu, Tianpeng Xu, Sen Qiao, Wen Wang, Jing Ma, and Juntian Xu. 2023. "Photosynthetic Physiological Response of Porphyra yezoensis to Light Change at Different CO2 Concentrations" Water 15, no. 4: 781. https://doi.org/10.3390/w15040781




