Sorption Behavior and Prediction of Tetracycline on Sediments from the Yangtze Estuary and Its Coastal Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area

2.2. Sample Collection and Preparation
2.2.1. Sediment Samples
2.2.2. Seawater
2.3. Chemical Reagents
2.4. Measurement and Characterization of Sediment Properties
2.5. Sorption Experiments
2.5.1. Sorption Isotherm Experiments
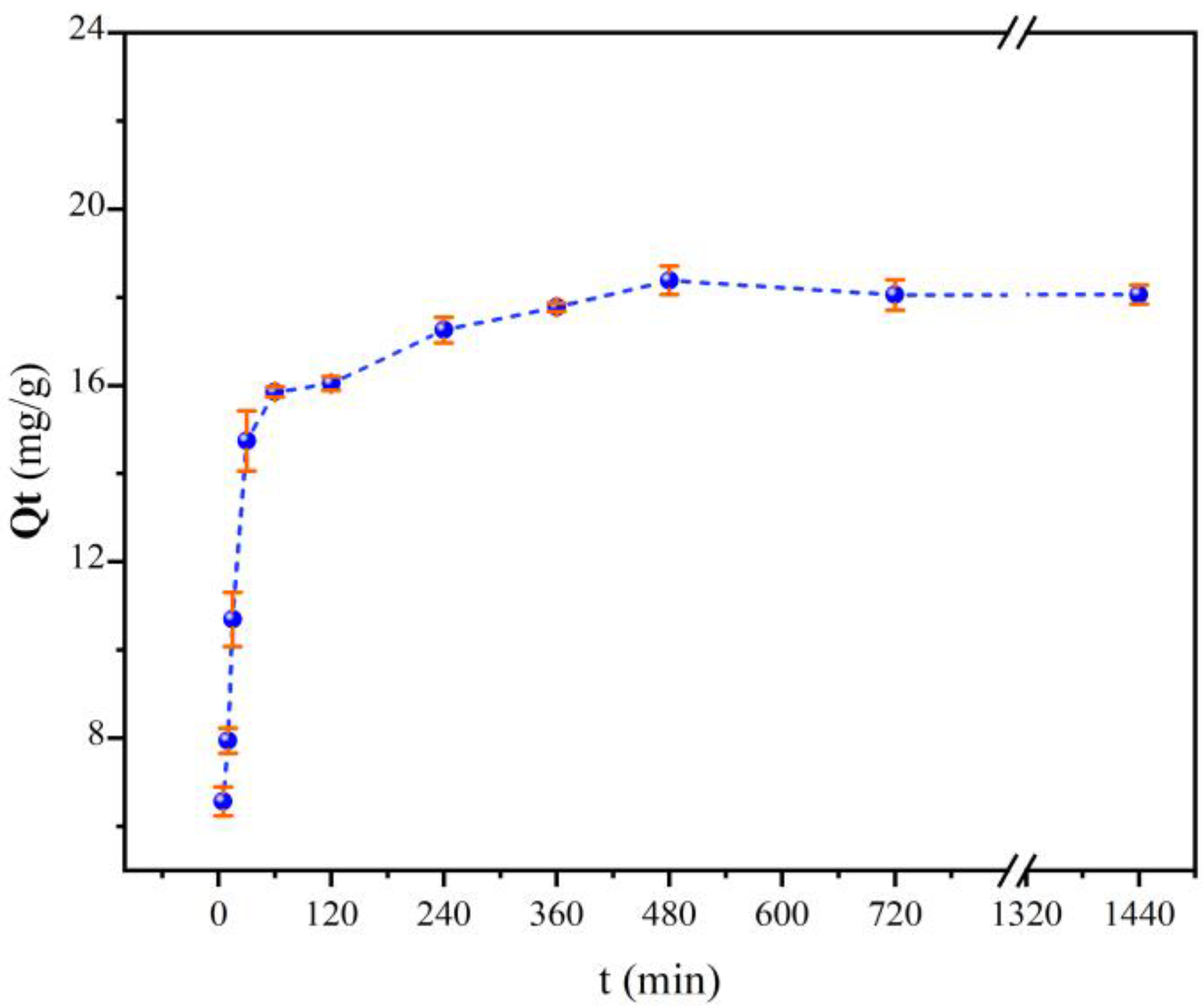
2.5.2. Kinetic Sorption Experiments
2.5.3. Effects of Salinity
2.5.4. Data Analysis Method
3. Results and Discussion
3.1. Physicochemical Properties of Sediments
3.2. Adsorption Isotherms
3.3. Pearson Correlation Analyses
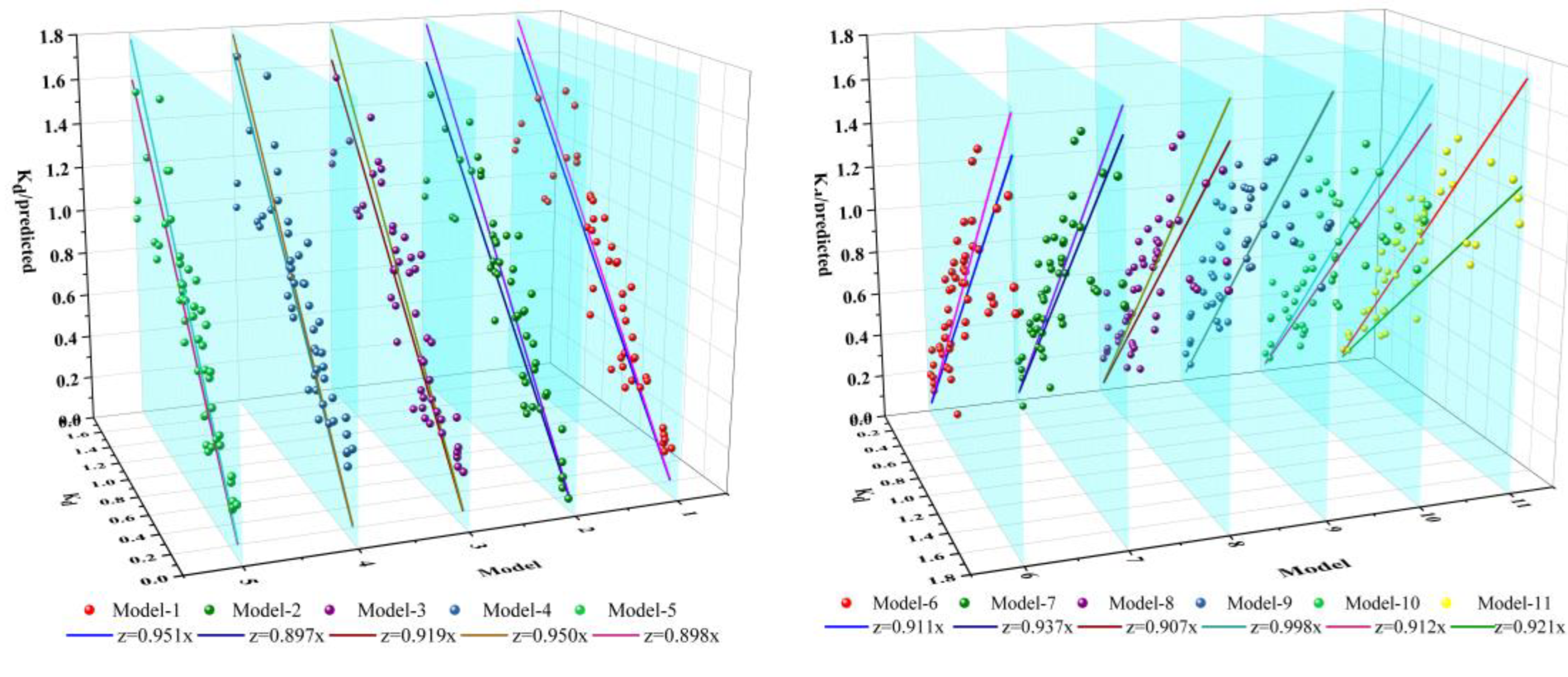
3.4. Model Fitting
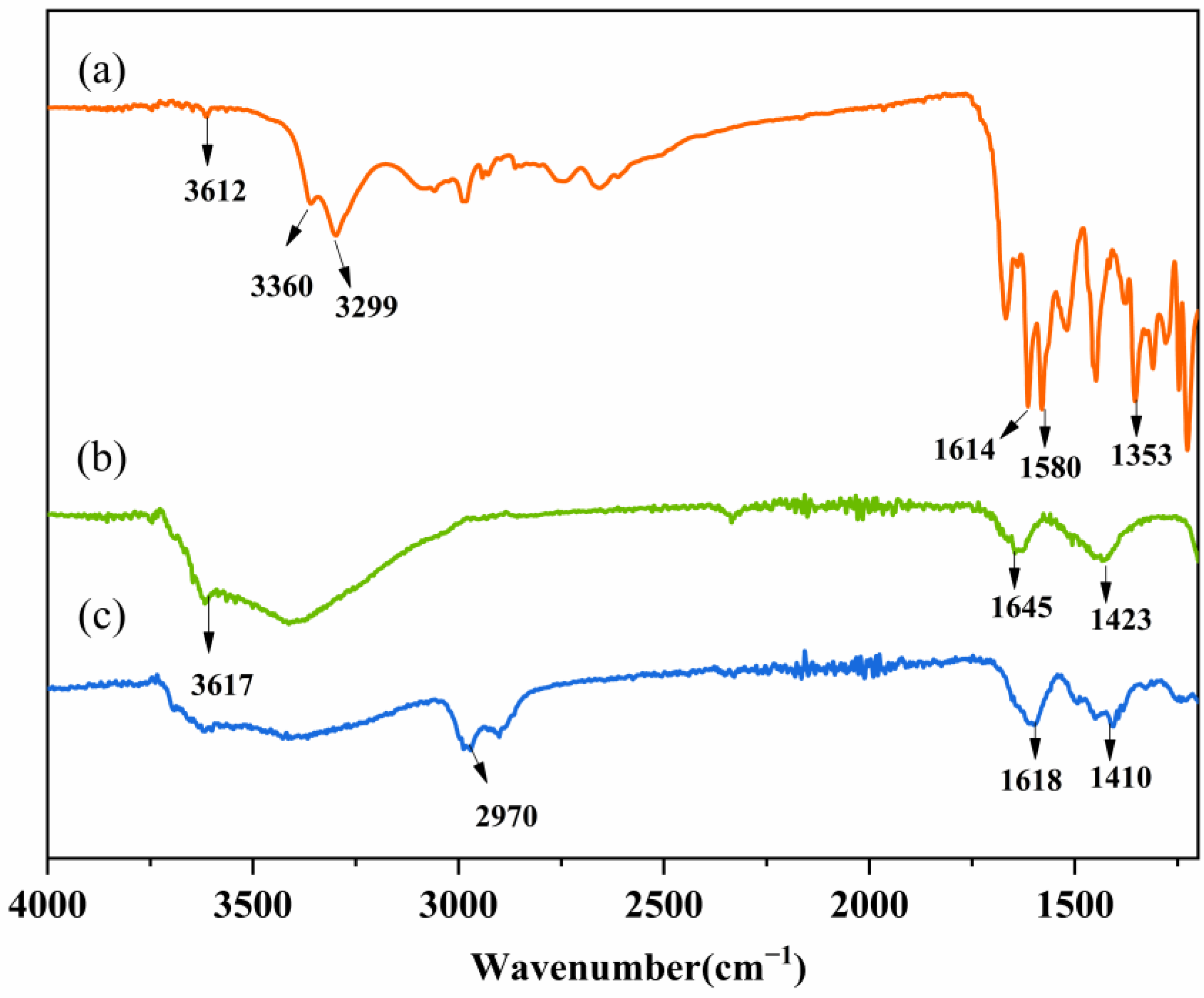
3.5. Fourier-Transform Infrared Analysis
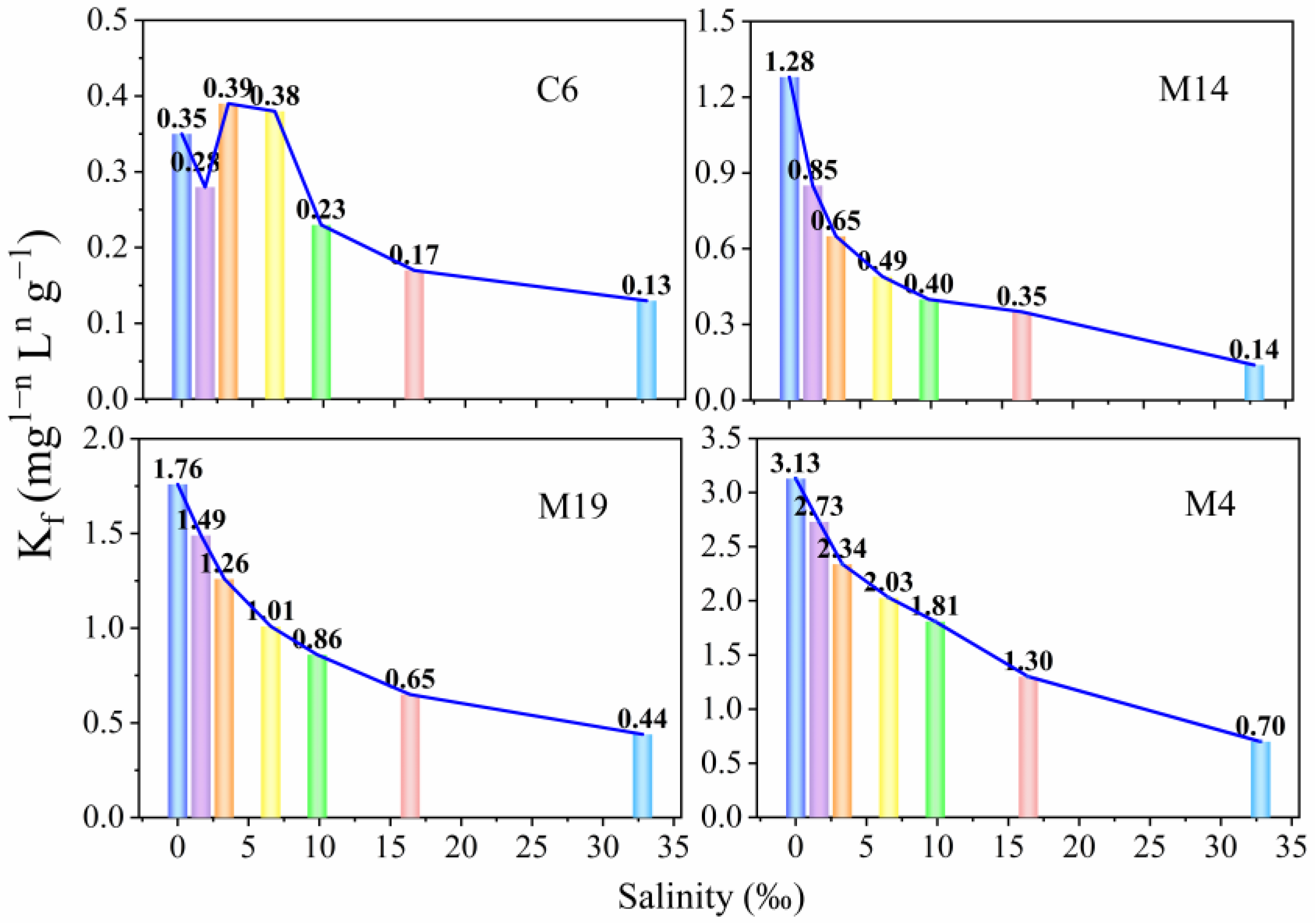
3.6. Effect of Salinity on TC Adsorption
4. Conclusions
- (a)
- The TC adsorption capacity was closely related to the physical and chemical properties of sediments, varying with sediment location. Sediments in the B and M areas displayed a relatively high adsorption capacity, and sediments in the O area showed moderate adsorption capacity. In addition, sediments in the C area displayed the lowest adsorption capacity of the four studied areas.
- (b)
- The physicochemical properties (CEC, OM, Clay, Cu, Al, Fe and K) of sediments were positively correlated with Kd and negatively correlated with SiO2, quartz and D50. Among them, CEC and clay displayed the highest correlation coefficient, indicating that they play key roles in the adsorption behaviors of TC by these sediments.
- (c)
- The ten Kd models obtained in the present research provided the tools for predicting the Kd values of contaminants similar in structure to TC by sediments of the Yangtze River estuary and its adjacent aquatic ecosystems.
- (d)
- The adsorption capacity of TC by estuary and marine sediments decreased as salinity increased. Because of the low salinity and high adsorption capacity of the sediments, significant amounts of TC are adsorbed by sediment B in the estuary. Moreover, in the M area near the coast, TC adsorbed by suspended sediment particles flows into the seawater. However, most TC is continuously desorbed and released to seawater upon salinity changes. Therefore, antibiotic risk in the B and M areas needs to be focused.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ying, G.; Pan, C.; Liu, Y.; Zhao, J. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and Transport of Tetracycline, Sulfonamide, Quinolone, and Macrolide Antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, Y.; Yang, C.; Liu, X.; Zhang, J.Q.; Li, E.H.; Zhang, Q.; Wang, X.L. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017, 609, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, M.; Wang, B.; Zhao, X.; Guo, R.; Bu, Y.; Zhang, S.; Chen, J. Distribution and potential risk assessment of antibiotic pollution in the main drinking water sources of Nanjing, China. Environ. Sci. Pollut. Res. 2020, 27, 21429–21441. [Google Scholar] [CrossRef]
- Wei, R.; He, T.; Zhang, S.; Zhu, L.; Shang, B.; Li, Z.; Wang, R. Occurrence of seventeen veterinary antibiotics and resistant bacterias in manure-fertilized vegetable farm soil in four provinces of China. Chemosphere 2019, 215, 234–240. [Google Scholar] [CrossRef]
- Shi, H.; Yang, Y.; Liu, M.; Yan, C.X.; Yue, H.Y.; Zhou, J.L. Occurrence and distribution of antibiotics in the surface sediments of the Yangtze Estuary and nearby coastal areas. Mar. Pollut. Bull. 2014, 83, 317–323. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Li, L.; Fu, C.; Tu, C.; Huang, Y.; Wu, L.; Tang, J.; Luo, Y.; Christie, P. Levels, distributions and sources of veterinary antibiotics in the sediments of the Bohai Sea in China and surrounding estuaries. Mar. Pollut. Bull. 2016, 109, 597–602. [Google Scholar] [CrossRef]
- Liu, K.; Yin, X.; Zhang, D.; Yan, D.; Cui, L.; Zhu, Z.; Wen, L. Distribution, sources, and ecological risk assessment of quinotone antibiotics in the surface sediments from Jiaozhou Bay wetland, China. Mar. Pollut. Bull. 2018, 129, 859–865. [Google Scholar] [CrossRef]
- Nava, A.R.; Daneshian, L.; Sarma, H. Antibiotic resistant genes in the environment-exploring surveillance methods and sustainable remediation strategies of antibiotics and ARGs. Environ. Res. 2022, 215, 114212. [Google Scholar] [CrossRef]
- Luo, Y.; Mao, D.Q.; Michal, R.; Zhou, Q.X.; Zhang, H.J.; Xu, L.; Alvarez, P.J.J. Trends in Antibiotic Resistance Genes Occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 44, 7220–7225. [Google Scholar] [CrossRef]
- Li, S.; Shi, W.; Liu, W.; Li, H.; Zhang, W.; Hu, J.; Ke, Y.; Sun, W.; Ni, J. A duodecennial national synthesis of antibiotics in China’s major rivers and seas (2005–2016). Sci. Total Environ. 2018, 615, 906–917. [Google Scholar] [CrossRef]
- Na, C.-J.; Yoo, M.-J.; Tsang, D.C.W.; Kim, H.W.; Kim, K.-H. High-performance materials for effective sorptive removal of formaldehyde in air. J. Hazard. Mater. 2019, 366, 452–465. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Ferreira-Coelho, G.; Núñez-Delgado, A.; Fernández-Calviño, D.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, E.M.J. Competitive adsorption of tetracycline, oxytetracycline and chlortetracycline on soils with different pH value and organic matter content. Environ. Res. 2019, 178, 1–12. [Google Scholar] [CrossRef]
- Ji, L.; Zhao, T.; Deng, L.; Ashraf, M.A. Sorption of tetracycline, oxytetracycline and tylosin to eight surface sediments of Taihu Lake. J. Environ. Biol. 2016, 37, 1087–1095. [Google Scholar]
- Sassman, S.A.; Lee, L.S. Sorption of Three Tetracyclines by Several Soils: Assessing the Role of pH and Cation Exchange. Environ. Sci. Technol. 2005, 39, 7452. [Google Scholar] [CrossRef]
- Li, Y.; Pan, T.; Miao, D.; Chen, Z.; Tao, Y. Sorption–Desorption of Typical Tetracyclines on Different Soils: Environment Hazards Analysis with Partition Coefficients and Hysteresis Index. Environ. Eng. Sci. 2015, 32, 865–871. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H. Adsorption-desorption of oxytetracycline on marine sediments: Kinetics and influencing factors. Chemosphere 2016, 164, 156–163. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Sun, K.; Zhao, Y.; Lin, C. Sorption of tetracycline to sediments and soils: Assessing the roles of pH, the presence of cadmium and properties of sediments and soils. Front. Environ. Sci. Eng. China 2010, 4, 421–429. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.; Cheng, D.; Liu, G.; Liang, B.; Cui, B.; Bai, J. Temporal–spatial variation and partitioning prediction of antibiotics in surface water and sediments from the intertidal zones of the Yellow River Delta, China. Sci. Total Environ. 2016, 569–570, 1350–1358. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Huang, Y.; Zhang, L.; Ye, F.; Wang, J.; Shang, J.; Liao, Q. Impact of sediment characteristics on adsorption behavior of typical antibiotics in Lake Taihu, China. Sci. Total Environ. 2020, 718, 137329. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, Y.; Zhou, J.; Liu, M.; Nie, M.; Shi, H.; Gu, L. Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environ. Pollut. 2013, 175, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zou, H.; Wang, J. Pollution status and potential sources of main heavy metals in the main stream of the Yangtze River. East China Geol. 2021, 42, 21–28. [Google Scholar]
- Cong, J.; Hu, G.; Jonell, T.N.; Yuan, Z.; Kong, X.; Zhang, Y.; Wang, Y. Centurial Evolution of an Offshore Mud Deposition Area in the Changjiang (Yangtze) Estuary and Its Links to Environmental and Anthropogenic Activities. J. Ocean. Univ. China 2020, 19, 790–800. [Google Scholar] [CrossRef]
- Hu, L.; Shi, X.; Yu, Z.; Lin, T.; Wang, H.; Ma, D.; Guo, Z.; Yang, Z. Distribution of sedimentary organic matter in estuarine–inner shelf regions of the East China Sea: Implications for hydrodynamic forces and anthropogenic impact. Mar. Chem. 2012, 142–144, 29–40. [Google Scholar] [CrossRef]
- Liu, J.P.; Xu, K.H.; Li, A.C.; Milliman, J.D.; Velozzi, D.M.; Xiao, S.B.; Yang, Z.S. Flux and fate of Yangtze River sediment delivered to the East China Sea. Geomorphology 2007, 85, 208–224. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Z.; Liu, J.; Xu, N.; Li, H. The Sr/Ba ratio response to salinity in clastic sediments of the Yangtze River Delta. Chem. Geol. 2021, 559, 119923. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H. Factors influencing adsorption and desorption of trimethoprim on marine sediments: Mechanisms and kinetics. Environ. Sci. Pollut. Res. 2017, 24, 21929–21937. [Google Scholar] [CrossRef]
- Sumner, D.M.; Belaine, G. Evaporation, Precipitation, and Associated Salinity Changes at a Humid, Subtropical Estuar. Estuaries 2005, 28, 844–855. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J.; Wu, C.; Zhang, J.; Tang, J.; Shang, J.; Liao, Q. Effect of particle size on adsorption of norfloxacin and tetracycline onto suspended particulate matter in lake. Environ. Pollut. 2019, 244, 549–559. [Google Scholar] [CrossRef]
- Xu, X.; Li, X. Sorption and desorption of antibiotic tetracycline on marine sediments. Chemosphere 2010, 78, 430–436. [Google Scholar] [CrossRef]
- Teixidó, M.; Granados, M.; Prat, M.D.; Beltrán, J.L. Sorption of tetracyclines onto natural soils: Data analysis and prediction. Environ. Sci. Pollut. Res. 2012, 19, 3087–3095. [Google Scholar] [CrossRef]
- Conde-Cida, M.; Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Experimental data and model prediction of tetracycline adsorption and desorption in agricultural soils. Environ. Res. 2019, 177, 108607. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Ahmad, M.; Usman, A.R.A.; Sallam, A.S.; Hussain, Q.; Binyameen, R.B.; Shehu, M.R.; Ok, Y.S. Evaluating the efficiency of different natural clay sediments for the removal of chlortetracycline from aqueous solutions. J. Hazard. Mater. 2020, 384, 121500. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Sun, P.; Li, D.; Huang, C.-H. Transformation of Tetracycline Antibiotics and Fe(II) and Fe(III) Species Induced by Their Complexation. Environ. Sci. Technol. 2015, 50, 145–153. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Sun, P.; Pei, J.; Li, D.; Huang, C.-H. Oxidation of tetracycline antibiotics induced by Fe(III) ions without light irradiation. Chemosphere 2015, 119, 1255–1261. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef]
- Bao, Y.; Zhou, Q.; Wang, Y. Adsorption characteristics of tetracycline by two soils: Assessing role of soil organic matter. Aust. J. Soil Res. 2009, 47, 286–295. [Google Scholar] [CrossRef]
- Kulshrestha, P.; Giese, R.F.; AGA, D.S. Investigating the Molecular Interactions of Oxytetracycline in Clay and Organic Matter: Insights on Factors Affecting Its Mobility in Soil. Environ. Sci. Technol. 2004, 38, 4097–4105. [Google Scholar] [CrossRef]
- Al-Khazrajy, O.S.A.; Boxall, A.B.A. Impacts of compound properties and sediment characteristics on the sorption behaviour of pharmaceuticals in aquatic systems. J. Hazard. Mater. 2016, 317, 198–209. [Google Scholar] [CrossRef]
- Wan, Y.; Bao, Y.; Zhou, Q. Simultaneous adsorption and desorption of cadmium and tetracycline on cinnamon soil. Chemosphere 2010, 80, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Shanmukh, S.; Dluhy, R.A. 2D IR analyses of rate processes in lipid–antibiotic monomolecular films. Vib. Spectrosc. 2004, 36, 167–177. [Google Scholar] [CrossRef]
- Turku, I.; Sainio, T.; Paatero, E. Thermodynamics of tetracycline adsorption on silica. Environ. Chem. Lett. 2007, 5, 225–228. [Google Scholar] [CrossRef]
| Area | CEC (cmol/kg) | D50 | OM | Mineralogical Composition (%) | Major Elements (%) | Trace Elements (µg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (μm) | (%) | Clay | Quartz | Others | SiO2 | Al2O3 | Fe2O3 | K2O | Cu | Zn | Pb | ||
| B Area | 11.98 | 36.35 | 0.98 | 47.86 | 29.71 | 17.43 | 57.28 | 13.25 | 5.42 | 2.76 | 30.00 | 91.99 | 27.19 |
| C Area | 4.75 | 130.71 | 0.42 | 16.14 | 56.00 | 24.71 | 65.89 | 9.24 | 4.12 | 2.11 | 12.61 | 58.31 | 20.20 |
| M Area | 14.16 | 45.61 | 0.93 | 41.00 | 30.20 | 23.35 | 53.82 | 13.09 | 5.45 | 2.87 | 30.22 | 90.97 | 28.29 |
| O Area | 7.36 | 154.81 | 0.61 | 21.80 | 46.20 | 29.07 | 59.98 | 10.14 | 3.96 | 2.43 | 10.76 | 59.83 | 24.09 |
| Sediments | Langmuir | Freundlich | Kd-mean (L/g) | ||||
|---|---|---|---|---|---|---|---|
| Kl (L/mg) | qm (mg/g) | R2 | Kf (mg1−nLng−1) | n | R2 | ||
| B1 | 0.07 ± 0.01 | 14.37 ± 1.63 | 0.98 | 1.42 ± 0.17 | 0.60 ± 0.05 | 0.96 | 0.57 |
| B2 | 0.17 ± 0.02 | 12.76 ± 0.85 | 0.98 | 2.65 ± 0.35 | 0.46 ± 0.07 | 0.90 | 0.92 |
| B3 | 0.03 ± 0.01 | 44.63 ± 6.66 | 1.00 | 1.70 ± 0.17 | 0.82 ± 0.04 | 0.99 | 1.21 |
| B4 | 0.07 ± 0.01 | 23.96 ± 2.27 | 0.99 | 1.91 ± 0.13 | 0.71 ± 0.03 | 0.99 | 1.11 |
| B5 | 0.49 ± 0.12 | 13.05 ± 1.11 | 0.89 | 5.30 ± 0.63 | 0.30 ± 0.060 | 0.82 | 1.70 |
| B6 | 0.10 ± 0.02 | 16.38 ± 1.50 | 0.98 | 1.99 ± 0.33 | 0.59 ± 0.06 | 0.96 | 0.88 |
| B7 | 0.07 ± 0.03 | 11.29 ± 1.88 | 0.96 | 1.06 ± 0.32 | 0.60 ± 0.10 | 0.92 | 0.44 |
| C1 | 0.06 ± 0.02 | 3.76 ± 0.91 | 0.90 | 0.29 ± 0.08 | 0.62 ± 0.10 | 0.85 | 0.13 |
| C2 | 0.03 ± 0.02 | 7.21 ± 3.14 | 0.93 | 0.29 ± 0.08 | 0.76 ± 0.11 | 0.91 | 0.15 |
| C3 | 0.04 ± 0.01 | 5.45 ± 1.26 | 0.95 | 0.32 ± 0.08 | 0.68 ± 0.10 | 0.89 | 0.14 |
| C4 | 0.31 ± 0.06 | 1.13 ± 0.07 | 0.90 | 0.45 ± 0.02 | 0.26 ± 0.02 | 0.97 | 0.07 |
| C5 | 0.10 ± 0.03 | 3.03 ± 0.33 | 0.92 | 0.53 ± 0.15 | 0.43 ± 0.09 | 0.86 | 0.13 |
| C6 | 0.09 ± 0.03 | 3.24 ± 0.39 | 0.94 | 0.48 ± 0.07 | 0.49 ± 0.05 | 0.96 | 0.14 |
| C7 | 0.15 ± 0.05 | 12.98 ± 1.52 | 0.95 | 2.46 ± 0.58 | 0.48 ± 0.09 | 0.91 | 0.89 |
| M1 | 0.03 ± 0.01 | 31.87 ± 6.26 | 0.98 | 1.24 ± 0.18 | 0.77 ± 0.06 | 0.98 | 0.77 |
| M2 | 0.07 ± 0.02 | 23.93 ± 4.03 | 0.97 | 2.07 ± 0.43 | 0.66 ± 0.09 | 0.94 | 1.07 |
| M3 | 0.06 ± 0.03 | 22.38 ± 6.15 | 0.92 | 1.80 ± 0.48 | 0.66 ± 0.11 | 0.90 | 0.88 |
| M4 | 0.06 ± 0.02 | 35.44 ± 8.69 | 0.97 | 2.41 ± 0.5 | 0.72 ± 0.09 | 0.95 | 1.51 |
| M5 | 0.10 ± 0.02 | 16.78 ± 1.84 | 0.98 | 2.03 ± 0.34 | 0.58 ± 0.07 | 0.96 | 0.91 |
| M6 | 0.21 ± 0.07 | 16.53 ± 3.23 | 0.94 | 3.20 ± 0.15 | 0.60 ± 0.04 | 0.98 | 1.75 |
| M7 | 0.11 ± 0.04 | 26.65 ± 4.31 | 0.96 | 3.69 ± 0.75 | 0.56 ± 0.08 | 0.94 | 1.77 |
| M8 | 0.08 ± 0.02 | 27.36 ± 4.20 | 0.97 | 2.58 ± 0.36 | 0.67 ± 0.07 | 0.94 | 1.37 |
| M9 | 0.03 ± 0.02 | 67.53 ± 56.51 | 0.95 | 1.88 ± 0.35 | 0.84 ± 0.09 | 0.96 | 1.41 |
| M10 | 0.03 ± 0.02 | 37.04 ± 21.6 | 0.93 | 1.32 ± 0.44 | 0.79 ± 0.13 | 0.93 | 0.85 |
| M11 | 0.05 ± 0.02 | 25.78 ± 5.43 | 0.97 | 1.56 ± 0.12 | 0.74 ± 0.04 | 0.98 | 0.93 |
| M12 | 0.05 ± 0.02 | 15.00 ± 4.39 | 0.91 | 1.05 ± 0.33 | 0.65 ± 0.12 | 0.89 | 0.49 |
| M13 | 0.01 ± 0.01 | 57.69 ± 31.31 | 0.98 | 0.84 ± 0.13 | 0.70 ± 0.09 | 0.98 | 0.69 |
| M14 | 0.01 ± 0.01 | 64.86 ± 44.8 | 0.98 | 0.86 ± 0.18 | 0.64 ± 0.04 | 0.98 | 0.63 |
| M15 | 0.05 ± 0.03 | 30.74 ± 11.87 | 0.91 | 1.97 ± 0.43 | 0.79 ± 0.05 | 0.94 | 1.18 |
| M16 | 0.11 ± 0.02 | 21.36 ± 1.81 | 0.99 | 2.50 ± 0.17 | 0.62 ± 0.11 | 0.98 | 1.33 |
| M17 | 0.03 ± 0.01 | 34.77 ± 6.91 | 0.99 | 1.39 ± 0.18 | 0.81 ± 0.03 | 0.99 | 0.89 |
| M18 | 0.09 ± 0.04 | 16.94 ± 4.61 | 0.88 | 1.79 ± 0.47 | 0.63 ± 0.06 | 0.86 | 0.71 |
| M19 | 0.04 ± 0.01 | 38.17 ± 5.59 | 0.99 | 1.55 ± 0.11 | 0.89 ± 0.06 | 0.99 | 1.08 |
| M20 | 0.07 ± 0.02 | 15.59 ± 1.99 | 0.98 | 1.44 ± 0.23 | 0.88 ± 0.07 | 0.97 | 0.65 |
| O1 | 0.08 ± 0.02 | 11.34 ± 1.37 | 0.98 | 1.21 ± 0.17 | 0.60 ± 0.06 | 0.96 | 0.53 |
| O2 | 0.15 ± 0.05 | 14.48 ± 1.99 | 0.90 | 3.06 ± 0.76 | 0.44 ± 0.10 | 0.82 | 0.94 |
| O3 | 0.03 ± 0.00 | 16.15 ± 1.28 | 1.00 | 0.75 ± 0.09 | 0.71 ± 0.04 | 0.99 | 0.37 |
| O4 | 0.03 ± 0.01 | 25.86 ± 3.90 | 0.99 | 0.83 ± 0.09 | 0.79 ± 0.04 | 0.99 | 0.53 |
| O5 | 0.03 ± 0.01 | 21.48 ± 3.78 | 0.98 | 1.02 ± 0.11 | 0.71 ± 0.04 | 0.99 | 0.54 |
| O6 | 0.03 ± 0.01 | 25.59 ± 5.15 | 0.99 | 0.94 ± 0.14 | 0.76 ± 0.05 | 0.99 | 0.56 |
| O7 | 0.10 ± 0.02 | 6.64 ± 0.57 | 0.97 | 1.09 ± 0.11 | 0.48 ± 0.05 | 0.94 | 0.31 |
| O8 | 0.11 ± 0.04 | 9.55 ± 1.50 | 0.86 | 1.75 ± 0.35 | 0.44 ± 0.08 | 0.82 | 0.51 |
| O9 | 0.02 ± 0.01 | 30.40 ± 15.01 | 0.97 | 0.69 ± 0.13 | 0.81 ± 0.07 | 0.98 | 0.45 |
| O10 | 0.10 ± 0.01 | 12.40 ± 0.92 | 0.98 | 1.78 ± 0.07 | 0.53 ± 0.02 | 0.99 | 0.65 |
| O11 | 0.09 ± 0.02 | 9.83 ± 0.69 | 0.98 | 1.31 ± 0.18 | 0.52 ± 0.05 | 0.98 | 0.46 |
| O12 | 0.03 ± 0.02 | 16.78 ± 5.45 | 0.95 | 0.80 ± 0.16 | 0.72 ± 0.07 | 0.97 | 0.43 |
| O13 | 0.04 ± 0.03 | 13.01 ± 6.33 | 0.84 | 0.66 ± 0.25 | 0.71 ± 0.15 | 0.82 | 0.32 |
| O14 | 0.08 ± 0.03 | 12.64 ± 1.96 | 0.95 | 1.54 ± 0.43 | 0.55 ± 0.1 | 0.90 | 0.56 |
| O15 | 0.07 ± 0.03 | 20.05 ± 3.97 | 0.97 | 1.95 ± 0.42 | 0.63 ± 0.09 | 0.95 | 0.86 |
| Parameters | CEC | Clay | OM | D50 | Quartz | SiO2 | Al2O3 | Fe2O3 | K2O | MgO | Cu | Pb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kd-mean | 0.816 ** | 0.795 ** | 0.692 ** | −0.612 ** | −0.739 ** | −0.724 ** | 0.750 ** | 0.717 ** | 0.713 ** | 0.612 ** | 0.751 ** | 0.596 ** |
| Kf | 0.558 ** | 0.666 ** | 0.528 ** | −0.380 ** | −0.577 ** | −0.528 ** | 0.540 ** | 0.506 ** | 0.487 ** | 0.401 ** | 0.540 ** | 0.445 ** |
| Qm | 0.345 * | 0.322 * | 0.219 | −0.255 | −0.310 * | −0.252 | 0.299 * | 0.302 * | 0.346 * | 0.339 * | 0.228 | 0.186 |
| Models | Fitting Equations | Properties | R2 |
|---|---|---|---|
| Model 1 | Kd = −0.080 + 0.011Clay + 0.048CEC | Clay, CEC | 0.726 |
| Model 2 | Kd = 2.039 + 0.015Clay − 0.031SiO2 | Clay, SiO2 | 0.716 |
| Model 3 | Kd = −0.052 + 0.014Clay + 0.016Cu | Clay, Cu | 0.723 |
| Model 4 | Kd = 0.307 + 0.018Clay − 0.001D50 | Clay, D50 | 0.657 |
| Model 5 | Kd = −0.127 + 0.016Clay + 0.456OM | Clay, OM | 0.648 |
| Model 6 | Kd = −0.593 + 0.014Clay + 0.076Al2O3 | Clay, Al2O3 | 0.698 |
| Model 7 | Kd = −0.693 + 0.016Clay + 0.362K2O | Clay, K2O | 0.688 |
| Model 8 | Kd = −0.480 + 0.015Clay + 0.154Fe2O3 | Clay, Fe2O3 | 0.691 |
| Model 9 | Kd = 1.449 + 0.098Al2O3 − 0.030SiO2 | Al2O3, SiO2 | 0.634 |
| Model 10 | Kd = 2.147 − 0.031SiO2 + 0.019Cu | SiO2, Cu | 0.628 |
| Model 11 | Kd = −0.499 + 0.030CEC + 0.012Clay + 0.021Pb | Clay, CEC, Pb | 0.764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zheng, W.; Zhang, F.; Li, W.; Shen, X.; Huang, H.; Shi, L.; Shi, R.; Zhang, S.; Lu, M. Sorption Behavior and Prediction of Tetracycline on Sediments from the Yangtze Estuary and Its Coastal Areas. Water 2023, 15, 671. https://doi.org/10.3390/w15040671
Chen H, Zheng W, Zhang F, Li W, Shen X, Huang H, Shi L, Shi R, Zhang S, Lu M. Sorption Behavior and Prediction of Tetracycline on Sediments from the Yangtze Estuary and Its Coastal Areas. Water. 2023; 15(4):671. https://doi.org/10.3390/w15040671
Chicago/Turabian StyleChen, Haiying, Wenfang Zheng, Fei Zhang, Wenxi Li, Xiaoming Shen, Haibo Huang, Lei Shi, Rui Shi, Shuai Zhang, and Ming Lu. 2023. "Sorption Behavior and Prediction of Tetracycline on Sediments from the Yangtze Estuary and Its Coastal Areas" Water 15, no. 4: 671. https://doi.org/10.3390/w15040671
APA StyleChen, H., Zheng, W., Zhang, F., Li, W., Shen, X., Huang, H., Shi, L., Shi, R., Zhang, S., & Lu, M. (2023). Sorption Behavior and Prediction of Tetracycline on Sediments from the Yangtze Estuary and Its Coastal Areas. Water, 15(4), 671. https://doi.org/10.3390/w15040671





