The Role of Oxygenated Functional Groups on Cadmium Removal using Pyrochar and Hydrochar Derived from Guadua angustifolia Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Synthesis and Characterization
2.2. Cd Adsorption Experiments
3. Results and Discussion
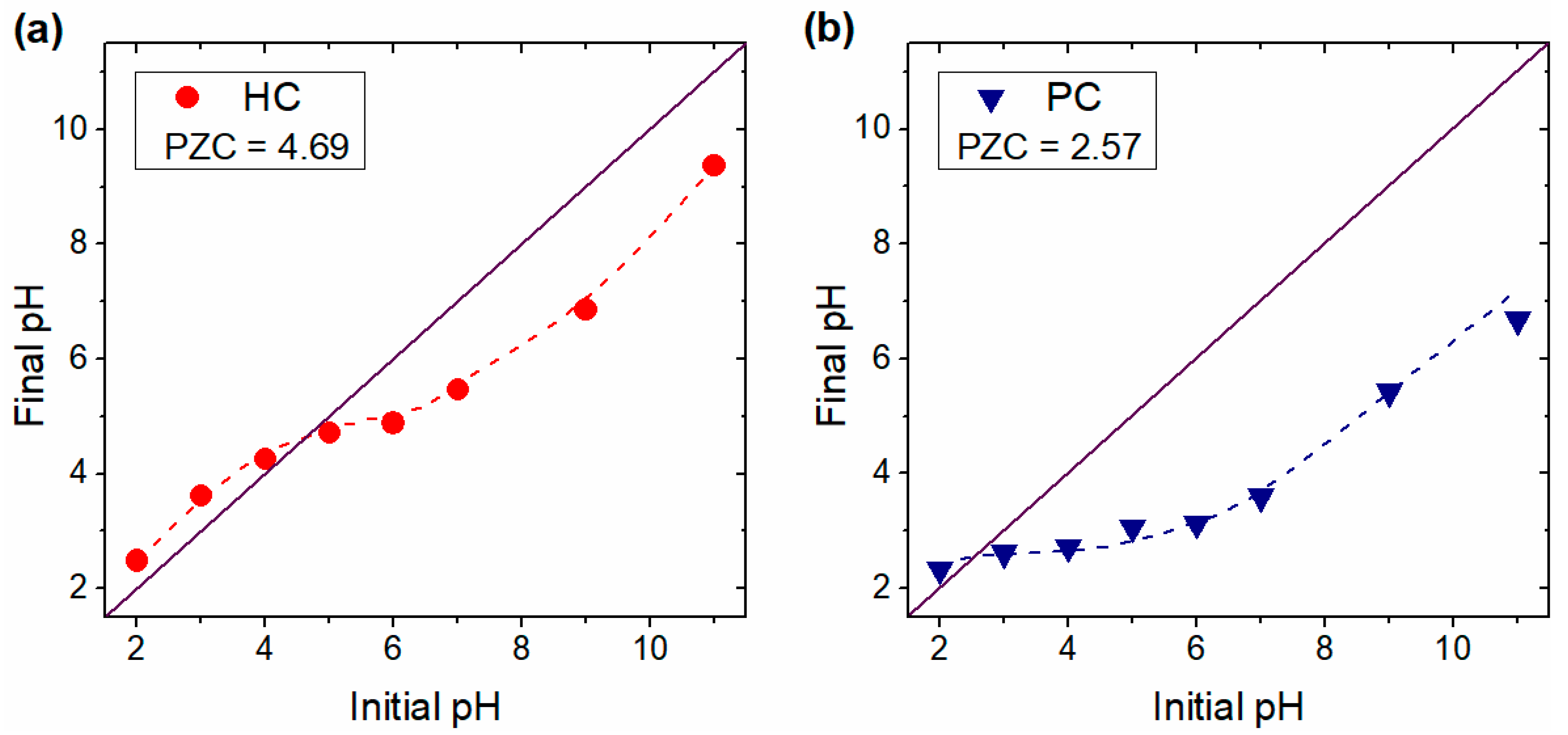
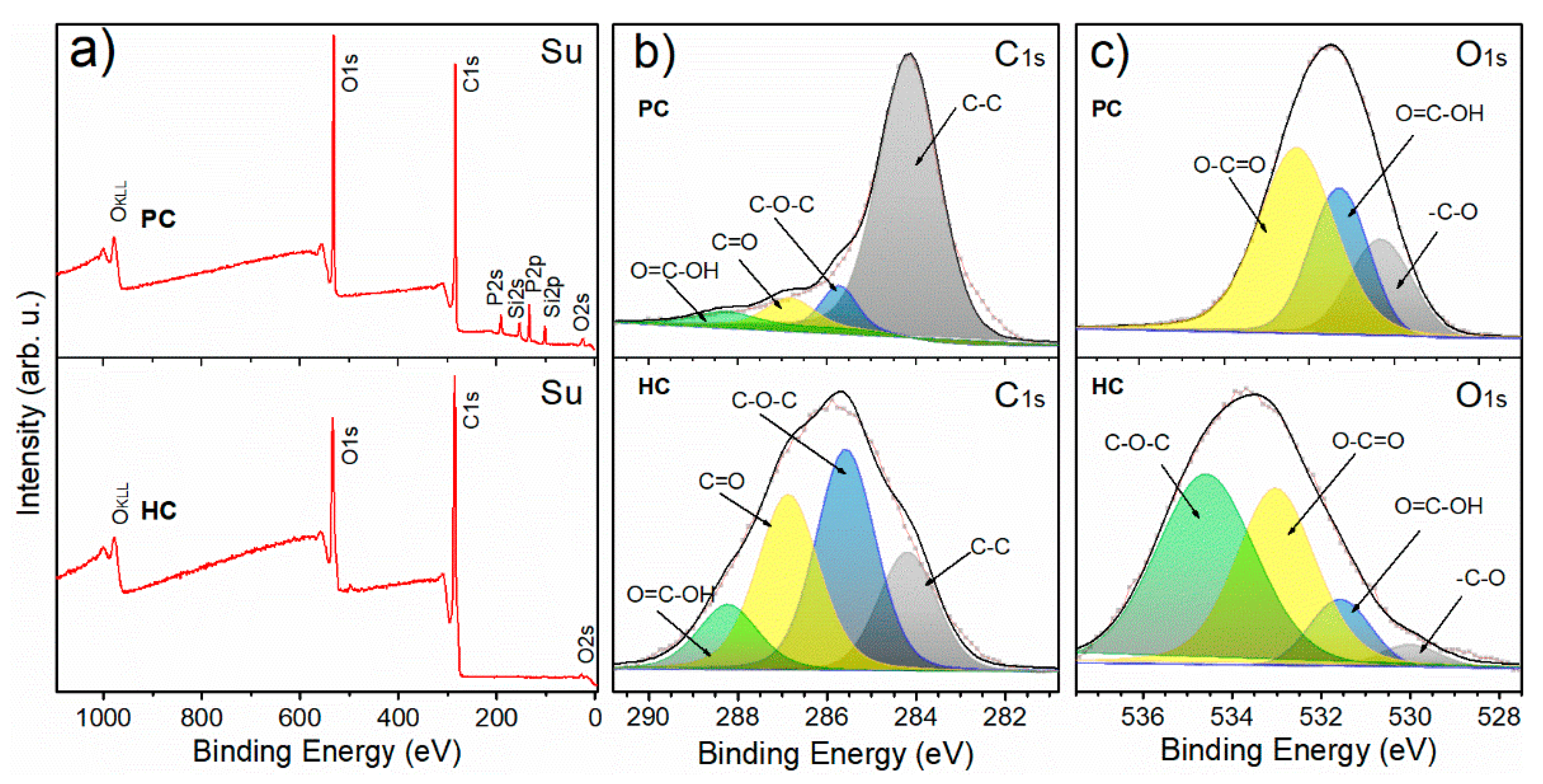
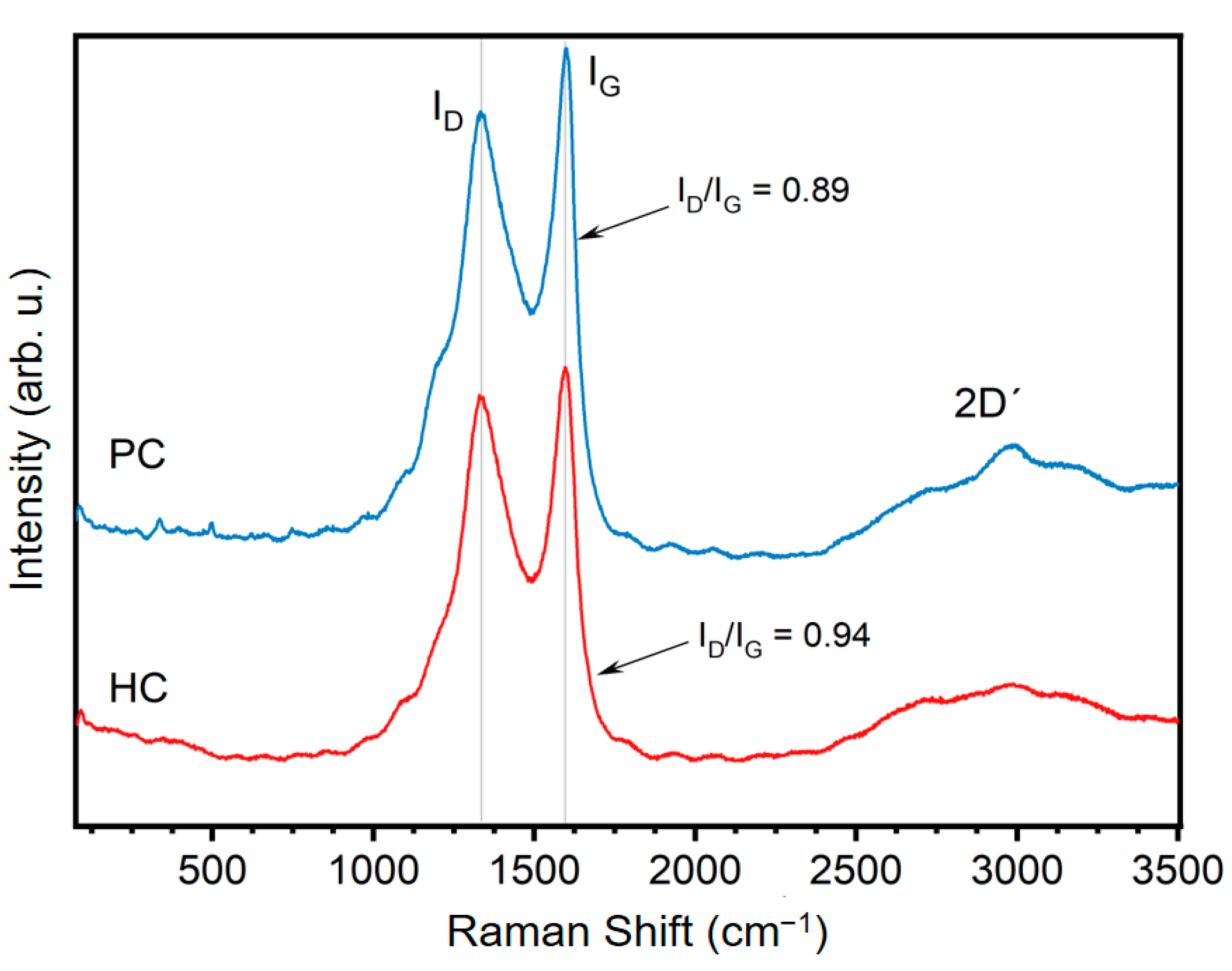
3.1. Physicochemical Properties of G. angustifolia Residues and Biochar Samples
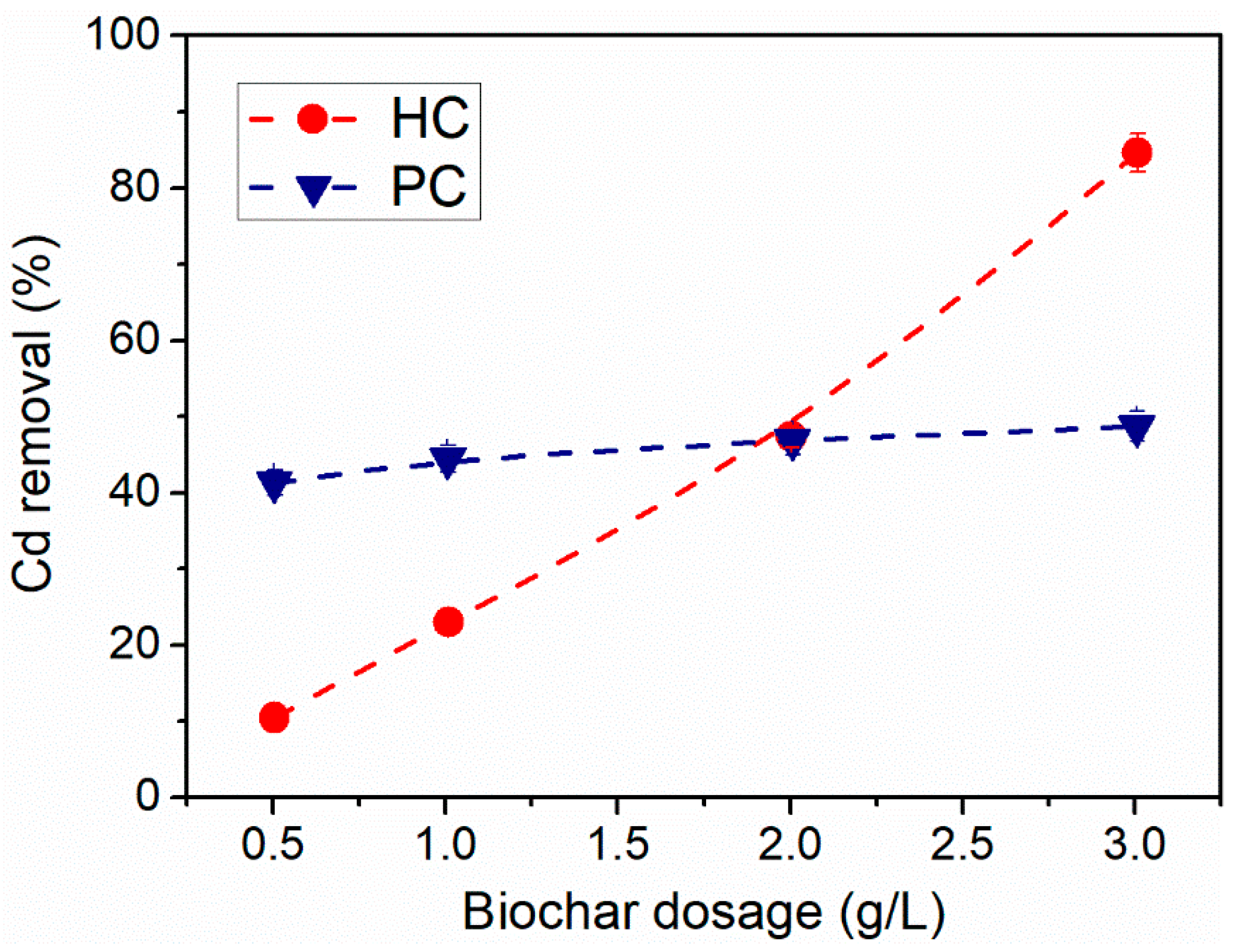
3.2. Effect of Biochar Dosage
3.3. Cd Adsorption Kinetics
3.4. Adsorption Isotherms
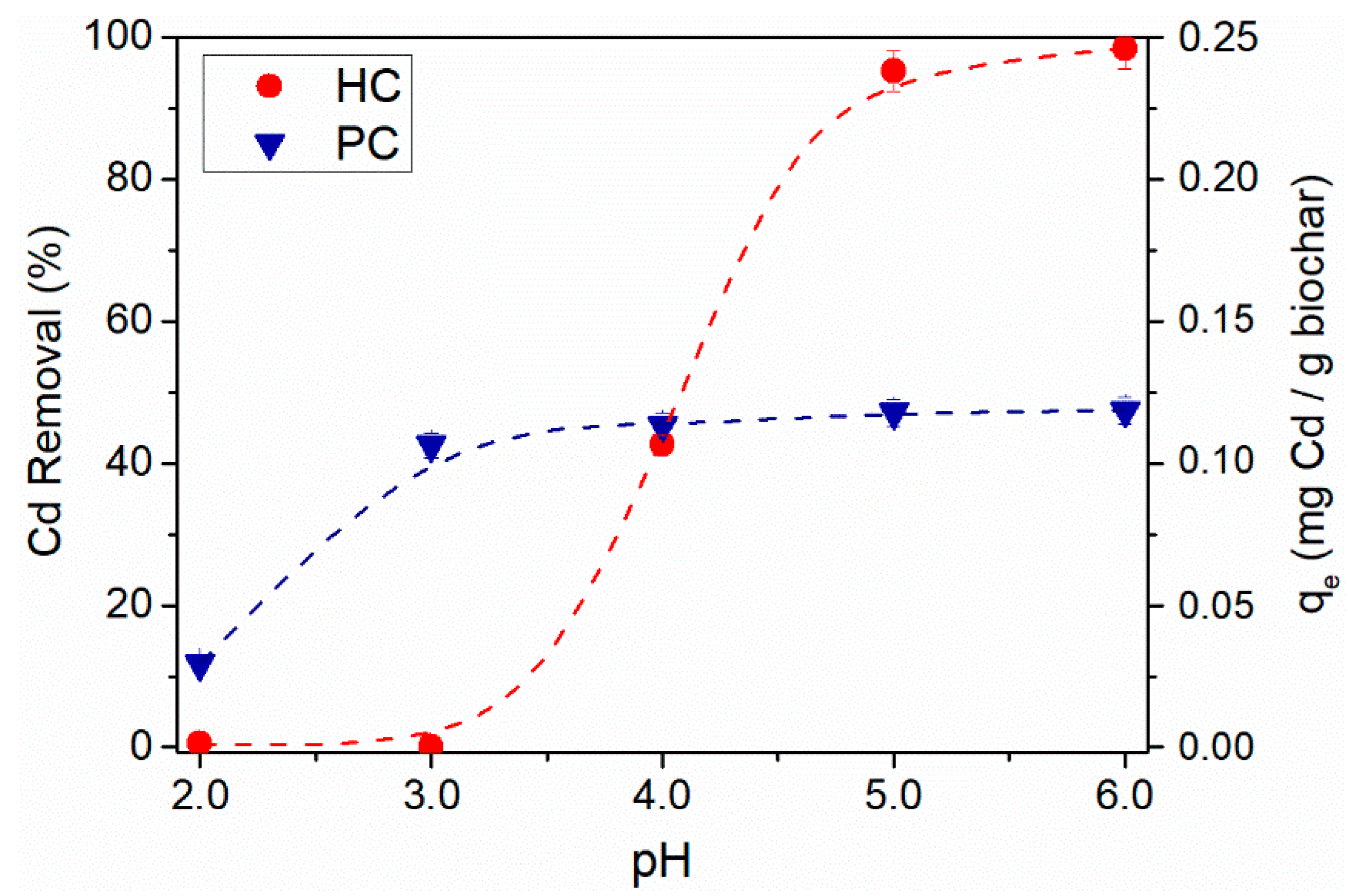
3.5. Effect of Solution Initial pH
3.6. Cost Analysis of HC and PC Preparation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capparelli, M.V.; Cabrera, M.; Rico, A.; Lucas-Solis, O.; Alvear, S.D.; Vasco, S.; Galarza, E.; Shiguango, L.; Pinos-Velez, V.; Pérez-González, A.; et al. An Integrative Approach to Assess the Environmental Impacts of Gold Mining Contamination in the Amazon. Toxics 2021, 9, 149. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Moulatlet, G.M.; de Abessa, D.M.S.; Lucas-Solis, O.; Rosero, B.; Galarza, E.; Tuba, D.; Carpintero, N.; Ochoa-Herrera, V.; Cipriani-Avila, I. An Integrative Approach to Identify the Impacts of Multiple Metal Contamination Sources on the Eastern Andean Foothills of the Ecuadorian Amazonia. Sci. Total Environ. 2020, 709, 136088. [Google Scholar] [CrossRef] [PubMed]
- Cumbal, L.H.; Bundschuh, J.; Aguirre, V.; Murgueitio, E.; Tipán, I.; Chavez, C. The Origin of Arsenic in Waters and Sediments from Papallacta Lake Area in Ecuador; Taylor & Francis: Abingdon-on-Thames, UK, 2009. [Google Scholar]
- Jiménez-Oyola, S.; García-Martínez, M.J.; Ortega, M.F.; Chavez, E.; Romero, P.; García-Garizabal, I.; Bolonio, D. Ecological and Probabilistic Human Health Risk Assessment of Heavy Metal(Loid)s in River Sediments Affected by Mining Activities in Ecuador. Environ. Geochem. Health 2021, 43, 4459–4474. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Removal of Cadmium from Wastewaters with Low-Cost Adsorbents. J. Environ. Chem. Eng. 2019, 7, 40. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; WHO Library: Geneva, Switzerland, 2017; ISBN 9789241549950. [Google Scholar]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Jellouli Ennigrou, D.; Gzara, L.; Ramzi Ben Romdhane, M.; Dhahbi, M. Cadmium Removal from Aqueous Solutions by Polyelectrolyte Enhanced Ultrafiltration. Desalination 2009, 246, 363–369. [Google Scholar] [CrossRef]
- Islamoglu, S.; Yilmaz, L.; Ozbelge, H.O. Development of a Precipitation Based Separation Scheme for Selective Removal and Recovery of Heavy Metals from Cadmium Rich Electroplating Industry Effluents. Sep. Sci. Technol. 2006, 41, 3367–3385. [Google Scholar] [CrossRef]
- Qdais, H.A.; Moussa, H. Removal of Heavy Metals from Wastewater by Membrane Processes: A Comparative Study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Zhuo, Q.; Xu, Z.; Guo, Q. Electrochemical Recovery of Metals from Cadmium Wastewater. Chem. Lett. 2014, 43, 1312–1314. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The Adsorption, Regeneration and Engineering Applications of Biochar for Removal Organic Pollutants: A Review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- He, P.; Zhang, H.; Duan, H.; Shao, L.; Lü, F. Continuity of Biochar-Associated Biofilm in Anaerobic Digestion. Chem. Eng. J. 2020, 390, 124605. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Gascó, G.; Palacios, T.; Paz-Ferreiro, J.; Méndez, A. Fe Oxides-Biochar Composites Produced by Hydrothermal Carbonization and Pyrolysis of Biomass Waste. J. Anal. Appl. Pyrolysis 2020, 151, 104893. [Google Scholar] [CrossRef]
- Ameen Hezam Saeed, A.; Yub Harun, N.; Mahmoud Nasef, M.; Al-Fakih, A.; Abdulhakim Saeed Ghaleb, A.; Kolawole Afolabi, H. Removal of Cadmium from Aqueous Solution by Optimized Rice Husk Biochar Using Response Surface Methodology. Ain Shams Eng. J. 2022, 13, 101516. [Google Scholar] [CrossRef]
- Cai, T.; Liu, X.; Zhang, J.; Tie, B.; Lei, M.; Wei, X.; Peng, O.; Du, H. Silicate-Modified Oiltea Camellia Shell-Derived Biochar: A Novel and Cost-Effective Sorbent for Cadmium Removal. J. Clean. Prod. 2021, 281, 125390. [Google Scholar] [CrossRef]
- Ge, Q.; Tian, Q.; Wang, S.; Zhang, J.; Hou, R. Highly Efficient Removal of Lead/Cadmium by Phosphoric Acid-Modified Hydrochar Prepared from Fresh Banana Peels: Adsorption Mechanisms and Environmental Application. Langmuir, 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, H.; He, Y.; Li, B.; Dong, Y.; Wang, L. Efficient Simultaneous Removal of Cadmium and Arsenic in Aqueous Solution by Titanium-Modified Ultrasonic Biochar. Bioresour. Technol. 2019, 284, 333–339. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and Utilization of Biochar: A Review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Salgado, M.A.H.; Tarelho, L.A.C.; Matos, A. Analysis of Combined Biochar and Torrefied Biomass Fuel Production as Alternative for Residual Biomass Valorization Generated in Small-Scale Palm Oil Mills. Waste Biomass Valoriz. 2020, 11, 343–356. [Google Scholar] [CrossRef]
- Glaser, B.; Parr, M.; Braun, C.; Kopolo, G. Biochar Is Carbon Negative. Nat. Geosci. 2009, 2, 2. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of Solid Fuel Biochar from Waste Biomass by Low Temperature Pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life Cycle Assessment of Biochar Systems: Estimating the Energetic, Economic, and Climate Change Potential. Environ. Sci. Technol. 2010, 44, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, N.; Yausup, S. A Mini Review of Biochar Synthesis, Characterization, and Related Standardization and Legislation. In Applications of Biochar for Environmental Safety; IntechOpen: London, UK, 2020. [Google Scholar]
- Liu, Z.; Wang, Z.; Chen, H.; Cai, T.; Liu, Z. Hydrochar and Pyrochar for Sorption of Pollutants in Wastewater and Exhaust Gas: A Critical Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 268, ISBN 8610627373. [Google Scholar]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal Carbonization of Lignocellulosic Biomass: Effect of Process Conditions on Hydrochar Properties. Appl. Energy 2015, 155, 576–584. [Google Scholar] [CrossRef]
- Donar, Y.O.; Çağlar, E.; Sınağ, A. Preparation and Characterization of Agricultural Waste Biomass Based Hydrochars. Fuel 2016, 183, 366–372. [Google Scholar] [CrossRef]
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in Water and Wastewater Treatment—A Sustainability Assessment. Chem. Eng. J. 2021, 420, 129946. [Google Scholar] [CrossRef]
- Hagemann, N.; Spokas, K.; Schmidt, H.P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an Eco-Friendly and Economical Adsorbent for the Removal of Colorants (Dyes) from Aqueous Environment: A Review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of Cadmium and Lead Ions by Phosphoric Acid-Modified Biochar Generated from Chicken Feather: Selective Adsorption and Influence of Dissolved Organic Matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Removal of Copper and Lead Using Banana Biochar in Batch Adsorption Systems: Isotherms and Kinetic Studies. Arab. J. Sci. Eng. 2018, 43, 5711–5722. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K.; Brink, H. Hydrothermal Synthesis of Magnetic-Biochar Nanocomposite Derived from Avocado Peel and Its Performance as an Adsorbent for the Removal of Methylene Blue from Wastewater. Mater. Today Sustain. 2022, 18, 100123. [Google Scholar] [CrossRef]
- Luo, M.; Lin, H.; Li, B.; Dong, Y.; He, Y.; Wang, L. A Novel Modification of Lignin on Corncob-Based Biochar to Enhance Removal of Cadmium from Water. Bioresour. Technol. 2018, 259, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.A.; Imran, M.; Amjad, M.; Abbas, G.; Tahir, M.; Murtaza, B.; Zakir, A.; Shahid, M.; Bulgariu, L.; Ahmad, I. Batch and Column Scale Removal of Cadmium from Water Using Raw and Acid Activated Wheat Straw Biochar. Water 2019, 11, 1438. [Google Scholar] [CrossRef] [Green Version]
- Arbelaez Breton, L.; Mahdi, Z.; Pratt, C.; El Hanandeh, A. Modification of Hardwood Derived Biochar to Improve Phosphorus Adsorption. Environments 2021, 8, 41. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U. Cadmium and Lead Remediation Using Magnetic Oak Wood and Oak Bark Fast Pyrolysis Biochars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Pattnaik, D.; Kumar, S.; Bhuyan, S.K.; Mishra, S.C. Effect of Carbonization Temperatures on Biochar Formation of Bamboo Leaves. IOP Conf. Ser. Mater. Sci. Eng. 2018, 338, 54. [Google Scholar] [CrossRef]
- Alchouron, J.; Navarathna, C.; Chludil, H.D.; Dewage, N.B.; Perez, F.; Hassan, E.B.; Pittman, C.U.; Vega, A.S.; Mlsna, T.E. Assessing South American Guadua Chacoensis Bamboo Biochar and Fe3O4 Nanoparticle Dispersed Analogues for Aqueous Arsenic(V) Remediation; Elsevier: Amsterdam, The Netherlands, 2020; Volume 706, ISBN 6623251618. [Google Scholar]
- Zhang, S.; Zhang, H.; Cai, J.; Zhang, X.; Zhang, J.; Shao, J. Evaluation and Prediction of Cadmium Removal from Aqueous Solution by Phosphate-Modified Activated Bamboo Biochar. Energy Fuels 2018, 32, 4469–4477. [Google Scholar] [CrossRef]
- Olivier, J.; Otto, T.; Roddaz, M.; Antoine, P.O.; Londoño, X.; Clark, L.G. First Macrofossil Evidence of a Pre-Holocene Thorny Bamboo Cf. Guadua (Poaceae: Bambusoideae: Bambuseae: Guaduinae) in South-Western Amazonia (Madre de Dios—Peru). Rev. Palaeobot. Palynol. 2009, 153, 1–7. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Livestock of Ecuador. International Bamboo and Rattan Network INBAR. In Ecuador: Estrategia Nacional del Bambú; Ministry of Agriculture and Livestock of Ecuador: Quito, Ecuador, 2018. [Google Scholar]
- Añasco, M.; Rojas, S. Estudio de La Cadena Desde La Producción Al Consumo del Bambú en Ecuador Con Énfasis en La Especie Guadua Angustifolia; Ministry of Agriculture and Livestock of Ecuador: Quito, Ecuador, 2015.
- Gatóo, A.; Sharma, B.; Bock, M.; Mulligan, H.; Ramage, M.H. Sustainable Structures: Bamboo Standards and Building Codes. Proc. Inst. Civ. Eng. Eng. Sustain. 2014, 167, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Dehkhoda, A.M.; Ellis, N. Biochar-Based Catalyst for Simultaneous Reactions of Esterification and Transesterification. Catal. Today 2013, 207, 86–92. [Google Scholar] [CrossRef]
- Li, M.; Zheng, Y.; Chen, Y.; Zhu, X. Biodiesel Production from Waste Cooking Oil Using a Heterogeneous Catalyst from Pyrolyzed Rice Husk. Bioresour. Technol. 2014, 154, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, Bioavailability and PH-Dependent Leaching of Cadmium, Zinc and Lead in a Contaminated Soil Amended with Biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 183. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- González, A.S.; Plaza, M.G.; Rubiera, F.; Pevida, C. Sustainable Biomass-Based Carbon Adsorbents for Post-Combustion CO2 Capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental Methods in Chemical Engineering: Specific Surface Area and Pore Size Distribution Measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Villasana, Y.; Moradi, N.; Navas-Cárdenas, C.; Patience, G.S. Experimental Methods in Chemical Engineering: PH. Can. J. Chem. Eng. 2022, 100, 24393. [Google Scholar] [CrossRef]
- Ardila, C.R.; Folgueras, M.B.; Fernández, F.J. Oxidative Pyrolysis of Guadua Angustifolia Kunth. Energy Rep. 2020, 6, 738–743. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E.W. Phosphoric Acid Pretreatment Enhances the Specific Surface Areas of Biochars by Generation of Micropores. Environ. Pollut. 2018, 240, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marsh, H.; Rodríguez-Reinoso, F. Activation Processes (Chemical). Act. Carbon 2006, 322–365. [Google Scholar] [CrossRef]
- Chen, L.; Huang, Q.; Wang, Y.; Xiao, H.; Liu, W.; Zhang, D.; Yang, T. Tailoring Performance of Co–Pt/MgO–Al2O3 Bimetallic Aerogel Catalyst for Methane Oxidative Carbon Dioxide Reforming: Effect of Pt/Co Ratio. Int. J. Hydrog. Energy 2019, 44, 19878–19889. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore Size Distribution Analysis of Microporous Carbons: A Density Functional Theory Approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Porosity in Carbons: Modeling. In Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006; pp. 87–142. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent Advances in Utilization of Biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.J.; Hao, X.; Peng, P.; Shi, J.Y.; Peng, F.; Sun, R.C. Hydrothermal Synthesis and Applications of Advanced Carbonaceous Materials from Biomass: A Review. Adv. Compos. Hybrid Mater. 2020, 3, 267–284. [Google Scholar] [CrossRef]
- Da Medeiros, D.C.C.S.; Nzediegwu, C.; Benally, C.; Messele, S.A.; Kwak, J.H.; Naeth, M.A.; Ok, Y.S.; Chang, S.X.; Gamal El-Din, M. Pristine and Engineered Biochar for the Removal of Contaminants Co-Existing in Several Types of Industrial Wastewaters: A Critical Review. Sci. Total Environ. 2022, 809, 151120. [Google Scholar] [CrossRef]
- Gupta, S.; Sireesha, S.; Sreedhar, I.; Patel, C.M.; Anitha, K.L. Latest Trends in Heavy Metal Removal from Wastewater by Biochar Based Sorbents. J. Water Process Eng. 2020, 38, 101561. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A Review of Biochar-Based Sorbents for Separation of Heavy Metals from Water. Int. J. Phytoremediat. 2020, 22, 111–126. [Google Scholar] [CrossRef]
- Brunelle, J.P. Preparation of Catalysts by Metallic Complex Adsorption on Mineral Oxides. Pure Appl. Chem. 1978, 50, 1211–1229. [Google Scholar] [CrossRef]
- Kosmulski, M. Compilation of PZC and IEP of Sparingly Soluble Metal Oxides and Hydroxides from Literature. Adv. Colloid Interface Sci. 2009, 152, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Pham, T.H.; Nguyen Thi, H.T.; Nguyen, T.N.; Nguyen, M.V.; Tran Dinh, T.; Nguyen, M.P.; Do, T.Q.; Phuong, T.; Hoang, T.T.; et al. Synthesis of Iron-Modified Biochar Derived from Rice Straw and Its Application to Arsenic Removal. J. Chem. 2019, 2019, 5295610. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, B.; Guo, Y.; Guo, Y.; Zheng, Z.; Pak, T.; Li, G. Effect of Hydrothermal Pretreatment on Pyrolyzed Sludge Biochars for Tetracycline Adsorption. J. Environ. Chem. Eng. 2021, 9, 106557. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface Chemistry Variations among a Series of Laboratory-Produced Biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, X.; Bi, E. Predicting the Effect of Dissolved Humic Acid on Sorption of Benzotriazole to Biochar. Biochar 2022, 4, 15. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Ding, Y.; Zeng, G.; Xu, Y.; Zeng, W.; Zheng, B. Removal of Metformin Hydrochloride by: Alternanthera Philoxeroides Biomass Derived Porous Carbon Materials Treated with Hydrogen Peroxide. RSC Adv. 2016, 6, 79275–79284. [Google Scholar] [CrossRef]
- Gai, C.; Guo, Y.; Peng, N.; Liu, T.; Liu, Z. N-Doped Biochar Derived from Co-Hydrothermal Carbonization of Rice Husk and: Chlorella Pyrenoidosa for Enhancing Copper Ion Adsorption. RSC Adv. 2016, 6, 53713–53722. [Google Scholar] [CrossRef]
- Saha, N.; Saba, A.; Reza, M.T. Effect of Hydrothermal Carbonization Temperature on PH, Dissociation Constants, and Acidic Functional Groups on Hydrochar from Cellulose and Wood. J. Anal. Appl. Pyrolysis 2019, 137, 138–145. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Chao, H.P. Effect of Pyrolysis Temperatures and Times on the Adsorption of Cadmium onto Orange Peel Derived Biochar. Waste Manag. Res. 2016, 34, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, H.; Thring, R.W.; Arocena, J.M. Chemical Pretreatment of Rice Straw Biochar: Effect on Biochar Properties and Hexavalent Chromium Adsorption. Int. J. Environ. Res. 2019, 13, 91–105. [Google Scholar] [CrossRef]
- Jedynak, K.; Charmas, B. Preparation and Characterization of Physicochemical Properties of Spruce Cone Biochars Activated by CO2. Materials 2021, 14, 3859. [Google Scholar] [CrossRef]
- Singh, P.; Sarswat, A.; Pittman, C.U.; Mlsna, T.; Mohan, D. Sustainable Low-Concentration Arsenite [As(III)] Removal in Single and Multicomponent Systems Using Hybrid Iron Oxide-Biochar Nanocomposite Adsorbents—A Mechanistic Study. ACS Omega 2020, 5, 2575–2593. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Seo, Y.D. Sorption of Halogenated Phenols and Pharmaceuticals to Biochar: Affecting Factors and Mechanisms. Environ. Sci. Pollut. Res. 2016, 23, 951–961. [Google Scholar] [CrossRef]
- Trakal, L.; Veselská, V.; Šafařík, I.; Vítková, M.; Číhalová, S.; Komárek, M. Lead and Cadmium Sorption Mechanisms on Magnetically Modified Biochars. Bioresour. Technol. 2016, 203, 318–324. [Google Scholar] [CrossRef]
- Burbano-Patiño, A.A.; Lassalle, V.L.; Horst, M.F. Magnetic Hydrochar Nanocomposite Obtained from Sunflower Husk: A Potential Material for Environmental Remediation. J. Mol. Struct. 2021, 1239, 130509. [Google Scholar] [CrossRef]
- Zhang, H.; Hay, A.G. Magnetic Biochar Derived from Biosolids via Hydrothermal Carbonization: Enzyme Immobilization, Immobilized-Enzyme Kinetics, Environmental Toxicity. J. Hazard. Mater. 2020, 384, 121272. [Google Scholar] [CrossRef]
- Kazak, O.; Tor, A. In Situ Preparation of Magnetic Hydrochar by Co-Hydrothermal Treatment of Waste Vinasse with Red Mud and Its Adsorption Property for Pb(II) in Aqueous Solution. J. Hazard. Mater. 2020, 393, 122391. [Google Scholar] [CrossRef]
- Lou, Y.; Joseph, S.; Li, L.; Graber, E.R.; Liu, X.; Pan, G. Water Extract from Straw Biochar Used for Plant Growth Promotion: An Initial Test. BioResources 2016, 11, 249–266. [Google Scholar] [CrossRef]
- Leithaeuser, A.; Gerber, M.; Span, R.; Schwede, S. Bioresource Technology Comparison of Pyrochar, Hydrochar and Lignite as Additive in Anaerobic Digestion and NH4+ Adsorbent. Bioresour. Technol. 2022, 361, 127674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Deng, X.; Gao, H. A Facile Pyrolysis Synthesis of Biochar/ZnO Passivator: Immobilization Behavior and Mechanisms for Cu (II) in Soil. Environ. Sci. Pollut. Res. 2020, 27, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, V.; Gomis-Berenguer, A.; Giudicianni, P.; Ania, C.O.; Ragucci, R.; Alfè, M. Assessing the Potential of Biochars Prepared by Steam-Assisted Slow Pyrolysis for CO2 Adsorption and Separation. Energy Fuels 2018, 32, 10218–10227. [Google Scholar] [CrossRef] [PubMed]
- Kwan, Y.C.G.; Ng, G.M.; Huan, C.H.A. Identification of Functional Groups and Determination of Carboxyl Formation Temperature in Graphene Oxide Using the XPS O 1s Spectrum. Thin Solid Film. 2015, 590, 40–48. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Z.; Jiang, Y.; Li, F.; Xue, B.; Dong, Z.; Ding, M.; Chen, R.; Yang, Q.; An, T.; et al. Micro-Structure, Surface Properties and Adsorption Capacity of Ball-Milled Cellulosic Biomass Derived Biochar Based Mineral Composites Synthesized via Carbon-Bed Pyrolysis. Appl. Clay Sci. 2020, 199, 105877. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Liu, L.; Ju, M.; Zheng, K. Adsorption Behavior of Selective Recognition Functionalized Biochar to Cd(II) in Wastewater. Materials 2018, 11, 299. [Google Scholar] [CrossRef] [Green Version]
- Huson, M.G.; Church, J.S.; Kafi, A.A.; Woodhead, A.L.; Khoo, J.; Kiran, M.S.R.N.; Bradby, J.E.; Fox, B.L. Heterogeneity of Carbon Fibre. Carbon N. Y. 2014, 68, 240–249. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.; Xue, B.; Huang, P.; Hao, Y.; Tang, J.; Maletić, S.P.; Rončević, S.D.; Sun, H. Preparation of Graphite-like Biochars Derived from Straw and Newspaper Based on Ball-Milling and TEMPO-Mediated Oxidation and Their Supersorption Performances to Imidacloprid and Sulfadiazine. Chem. Eng. J. 2021, 411, 128502. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, C.; Zhang, P.; Yue, Q.; Li, Y.; Gao, B.; Xu, X. Removal of Sulfamethoxazole from Water via Activation of Persulfate by Fe3C@NCNTs Including Mechanism of Radical and Nonradical Process. Chem. Eng. J. 2019, 375, 122004. [Google Scholar] [CrossRef]
- Gao, L.; Li, Z.; Yi, W.; Li, Y.; Zhang, P.; Zhang, A.; Wang, L. Impacts of Pyrolysis Temperature on Lead Adsorption by Cotton Stalk-Derived Biochar and Related Mechanisms. J. Environ. Chem. Eng. 2021, 9, 105602. [Google Scholar] [CrossRef]
- Yu, J.; Feng, H.; Tang, L.; Pang, Y.; Wang, J.; Zou, J.; Xie, Q.; Liu, Y.; Feng, C.; Wang, J. Insight into the Key Factors in Fast Adsorption of Organic Pollutants by Hierarchical Porous Biochar. J. Hazard. Mater. 2021, 403, 123610. [Google Scholar] [CrossRef] [PubMed]
- Deveci, H.; Kar, Y. Adsorption of Hexavalent Chromium from Aqueous Solutions by Bio-Chars Obtained during Biomass Pyrolysis. J. Ind. Eng. Chem. 2013, 19, 190–196. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Han, R.; Meng, R.; Wang, H.; Lu, W. Adsorption of Cadmium by Biochar Derived from Municipal Sewage Sludge: Impact Factors and Adsorption Mechanism. Chemosphere 2015, 134, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, H.; Yu, R.; Hu, G.; Chen, F. Removal of Aquatic Cadmium Ions Using Thiourea Modified Poplar Biochar. Water 2020, 12, 1117. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Suddique, M.; Shah, G.M.; Ahmad, I.; Murtaza, B.; Shah, N.S.; Mubeen, M.; Ahmad, S.; Zakir, A.; Schotting, R.J. Kinetic and Equilibrium Studies for Cadmium Biosorption from Contaminated Water Using Cassia Fistula Biomass. Int. J. Environ. Sci. Technol. 2019, 16, 3099–3108. [Google Scholar] [CrossRef]
- Ahmad, M.; Moon, D.H.; Vithanage, M.; Koutsospyros, A.; Lee, S.S.; Yang, J.E.; Lee, S.E.; Jeon, C.; Ok, Y.S. Production and Use of Biochar from Buffalo-Weed (Ambrosia trifida L.) for Trichloroethylene Removal from Water. J. Chem. Technol. Biotechnol. 2014, 89, 150–157. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.; Zhang, Q.; Liu, Q.; Li, Y.; Xiao, R. Adsorption of Cd(II) from Aqueous Solutions by Rape Straw Biochar Derived from Different Modification Processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Wang, S.; Kwak, J.-H.; Islam, M.S.; Naeth, M.A.; Gamal El-Din, M.; Chang, S.X. Biochar Surface Complexation and Ni(II), Cu(II), and Cd(II) Adsorption in Aqueous Solutions Depend on Feedstock Type. Sci. Total Environ. 2020, 712, 136538. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Kalsoom, T.; Yasmeen, S.; Kalsoom, A.; Raina, S.; Zhuang, Q.; Kong, J. Animal Manure-Derived Biochars Produced via Fast Pyrolysis for the Removal of Divalent Copper from Aqueous Media. J. Environ. Manage. 2018, 213, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich Equation Used for the Analysis of Adsorption Kinetics in Dye-Chitosan Systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of Linear and Nonlinear Equations of Pseudo-First Order and Pseudo-Second Order Kinetic Models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Romero-Cano, L.A.; García-Rosero, H.; Gonzalez-Gutierrez, L.V.; Baldenegro-Pérez, L.A.; Carrasco-Marín, F. Functionalized Adsorbents Prepared from Fruit Peels: Equilibrium, Kinetic and Thermodynamic Studies for Copper Adsorption in Aqueous Solution. J. Clean. Prod. 2017, 162, 195–204. [Google Scholar] [CrossRef]
- Saeed, A.A.H.; Harun, N.Y.; Sufian, S.; Bilad, M.R.; Zakaria, Z.Y.; Jagaba, A.H.; Abdulhakim, A.; Ghaleb, S.; Mohammed, H.G. Pristine and Magnetic Kenaf Fiber Biochar for Cd 2 + Adsorption from Aqueous Solution. Int. J. Environ. Res. Public Health 2021, 18, 7949. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Removal of Cd(II) from Aqueous Solutions Using Clarified Sludge. J. Colloid Interface Sci. 2008, 325, 48–56. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem 1906, 57, 1100–1107. [Google Scholar]
- Temkin, M.I. Adsorption Equilibrium and the Kinetics of Processes on Nonhomogeneous Surfaces and in the Interaction between Adsorbed Molecules. Zh. Fiz. Chim. 1941, 15, 296–332. [Google Scholar]
- Kumar, P.; Kumar, P. Removal of Cadmium (Cd-II) from Aqueous Solution Using Gas Industry-Based Adsorbent. SN Appl. Sci. 2019, 1, 872. [Google Scholar] [CrossRef] [Green Version]
- Abedpour, M.; Kamyab Moghadas, B.; Tamjidi, S. Equilibrium and Kinetic Study of Simultaneous Removal of Cd(II) and Ni(II) by Acrylamide-Based Polymer as Effective Adsorbent: Optimisation by Response Surface Methodology (RSM). Int. J. Environ. Anal. Chem. 2022, 102, 3524–3541. [Google Scholar] [CrossRef]
- Yousif, A.M.; El-Afandy, A.H.; Abdel Wahab, G.M.; Mubark, A.E.; Ibrahim, I.A. Selective Separation of Uranium(VI) from Aqueous Solutions Using Amine Functionalized Cellulose. J. Radioanal. Nucl. Chem. 2015, 303, 1821–1833. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of Arsenic, Cadmium, and Lead by Chars Produced from Fast Pyrolysis of Wood and Bark during Bio-Oil Production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Morales-Simfors, N.; Bundschuh, J.; Herath, I.; Inguaggiato, C.; Caselli, A.T.; Tapia, J.; Choquehuayta, F.E.A.; Armienta, M.A.; Ormachea, M.; Joseph, E.; et al. Arsenic in Latin America: A Critical Overview on the Geochemistry of Arsenic Originating from Geothermal Features and Volcanic Emissions for Solving Its Environmental Consequences. Sci. Total Environ. 2020, 716, 135564. [Google Scholar] [CrossRef] [PubMed]
- Inguaggiato, S.; Hidalgo, S.; Beate, B.; Bourquin, J. Geochemical and Isotopic Characterization of Volcanic and Geothermal Fluids Discharged from the Ecuadorian Volcanic Arc. Geofluids 2010, 10, 525–541. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A Review of Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Ni, F.; Liu, Q.; Yang, Y.; Wang, M.; Ao, T.; Chen, W. Synthesis of Magnesium Modified Biochar for Removing Copper, Lead and Cadmium in Single and Binary Systems from Aqueous Solutions: Adsorption Mechanism. Water 2021, 13, 599. [Google Scholar] [CrossRef]
- Park, C.M.; Han, J.; Chu, K.H.; Al-Hamadani, Y.A.J.; Her, N.; Heo, J.; Yoon, Y. Influence of Solution PH, Ionic Strength, and Humic Acid on Cadmium Adsorption onto Activated Biochar: Experiment and Modeling. J. Ind. Eng. Chem. 2017, 48, 186–193. [Google Scholar] [CrossRef]
- Banerjee, S.; Barman, S.; Halder, G. Sorptive Elucidation of Rice Husk Ash Derived Synthetic Zeolite towards Deionization of Coalmine Waste Water: A Comparative Study. Groundw. Sustain. Dev. 2017, 5, 137–151. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Baghel, P.; Anand, A.; Vijay, V.K.; Kaushal, P. A Comparative Study of Physical and Chemical Activation of Rice Straw Derived Biochar to Enhance Zn+2 Adsorption. Bioresour. Technol. Rep. 2021, 15, 100774. [Google Scholar] [CrossRef]
- Campbell, R.M.; Anderson, N.M.; Daugaard, D.E.; Naughton, H.T. Financial Viability of Biofuel and Biochar Production from Forest Biomass in the Face of Market Price Volatility and Uncertainty. Appl. Energy 2018, 230, 330–343. [Google Scholar] [CrossRef]
- Nematian, M.; Keske, C.; Ng’ombe, J.N. A Techno-Economic Analysis of Biochar Production and the Bioeconomy for Orchard Biomass. Waste Manag. 2021, 135, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Bilek, E.; Bergman, R.; Mani, S. Techno-Economic Analysis of Producing Solid Biofuels and Biochar from Forest Residues Using Portable Systems. Appl. Energy 2019, 235, 578–590. [Google Scholar] [CrossRef]
- Li, F.; Cao, X.; Zhao, L.; Wang, J.; Ding, Z. Effects of Mineral Additives on Biochar Formation: Carbon Retention, Stability, and Properties. Environ. Sci. Technol. 2014, 48, 11211–11217. [Google Scholar] [CrossRef]
- Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic Study of the Oxidation Resistance of Phosphorus-Containing Activated Carbons. Carbon N. Y. 2012, 50, 1523–1537. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, W.; Mašek, O.; Chen, X.; Gu, B.; Sharma, B.K.; Cao, X. Roles of Phosphoric Acid in Biochar Formation: Synchronously Improving Carbon Retention and Sorption Capacity. J. Environ. Qual. 2017, 46, 393–401. [Google Scholar] [CrossRef]
- Niu, H.; Jin, H.; Sun, Q.; Shi, Y.; Zhang, X.; Cai, Y. Activation of Biochars by Waste Phosphoric Acids: An Integrated Disposal Route of Waste Acids and Solid Waste. ACS Sustain. Chem. Eng. 2021, 9, 16403–16414. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Preparation and Characterization of High Surface Area Activated Carbon from Fox Nut (Euryale ferox) Shell by Chemical Activation with H3PO4. Results Phys. 2016, 6, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Yihunu, E.W.; Minale, M.; Abebe, S.; Limin, M. Preparation, Characterization and Cost Analysis of Activated Biochar and Hydrochar Derived from Agricultural Waste: A Comparative Study. SN Appl. Sci. 2019, 1, 873. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.; Show, S.; Banerjee, S.; Halder, G. Mechanistic Insight into Sorptive Elimination of Ibuprofen Employing Bi-Directional Activated Biochar from Sugarcane Bagasse: Performance Evaluation and Cost Estimation. J. Environ. Chem. Eng. 2018, 6, 5287–5300. [Google Scholar] [CrossRef]





| Parameter | Content (wt.%) |
|---|---|
| Moisture | 6.4 ± 0.6 |
| Volatile Matter | 83.9 ± 2.3 |
| Ash | 2.9 ± 0.4 |
| Fixed carbon | 13.2 ± 2.2 |
| Sample | Specific Surface Area (m2/g) | Pore Volume (77 K) (cm3/g) | Pore Diameter (nm) | PZC |
|---|---|---|---|---|
| HC | 28 | 0.072 | 3.5 | 4.7 |
| PC | 238 | 0.234 | 0.6 | 2.6 |
| XPS Spectrum | Functional Group | HC | PC | ||
|---|---|---|---|---|---|
| BE (eV) | Concentration (%) | BE (eV) | Concentration (%) | ||
| O1s | -C-O | 530.03 | 3.61 | 530.32 | 20.06 |
| O=C-OH | 531.58 | 9.75 | 531.39 | 28.09 | |
| O-C=O | 533.02 | 38.22 | 532.60 | 51.85 | |
| C-O-C | 534.59 | 48.42 | - | - | |
| C1s | C-C | 284.29 | 19.43 | 284.29 | 85.75 |
| C-O-C | 285.57 | 36.73 | 285.72 | 5.24 | |
| C=O | 286.87 | 31.78 | 286.83 | 5.56 | |
| O=C-OH | 288.22 | 12.06 | 288.15 | 3.44 | |
| Samples | Pseudo First Order | Pseudo Second Order | Elovich | Qe,exp (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe,first (mg/g) | k1 | RSE | Qe,second (mg/g) | k2 | RSE | Qe,elovich (mg/g) | α | β | RSE | ||
| HC | 0.108 | 0.080 | 0.0074 | 0.118 | 1.04 | 0.0083 | 0.124 | 0.17 | 68.13 | 0.0123 | 0.108 |
| PC | 0.110 | 0.080 | 0.0080 | 0.119 | 1.04 | 0.0042 | 0.119 | 0.12 | 61.15 | 0.0068 | 0.118 |
| Sample | Freundlich | Langmuir | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | KF (mg/g) | RSE | qm (mg/g) | QL (L/mg) | RSE | AT (L/mg) | BT (J/mg) | RSE | |
| HC | 7.95 | 0.15 | 0.0075 | 0.16 | 18.56 | 0.0070 | 22.64 | 0.015 | 0.0093 |
| PC | 0.97 | 0.37 | 0.0198 | 0.59 | 0.90 | 0.0165 | 10.00 | 0.116 | 0.0108 |
| Sample | a μg/m2 | b μg/m2 | c μg/m2 |
|---|---|---|---|
| HC | 5.14 | 5.71 | 5.32 |
| PC | 0.92 | 0.003 | 1.55 |
| Particulars | Sub Sections | Cost Break Up | Total Cost (USD) | |
|---|---|---|---|---|
| HC | PC | |||
| Raw material processing | Collection or purchase of raw material cost | Collected from local companies with free of cost | 0.00 | 0.00 |
| Drying | Sunlight drying | 0.00 | 0.00 | |
| Biochar preparation | HTC/Pyrolysis cost | hours × units × cost per max heat level HTC cost = 5 × 1 × 0.11 Pyrolysis cost = 1.5 × 1 × 0.29 | 0.55 | 0.44 |
| Washing cost | material was cleaned by distilled water: hours × units × per unit cost = 1 × 1 × 0.054 | 0.05 | 0.05 | |
| Drying cost | hours × unit × per unit cost = 12 × 1 × 0.054 | 0.65 | 0.65 | |
| Cost of H3PO4 | 1.71 | 1.71 | ||
| Net cost | 2.96 | 2.85 | ||
| 10% overall cost | 0.30 | 0.29 | ||
| Total cost | 3.26 | 3.14 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navas-Cárdenas, C.; Caetano, M.; Endara, D.; Jiménez, R.; Lozada, A.B.; Manangón, L.E.; Navarrete, A.; Reinoso, C.; Sommer-Márquez, A.E.; Villasana, Y. The Role of Oxygenated Functional Groups on Cadmium Removal using Pyrochar and Hydrochar Derived from Guadua angustifolia Residues. Water 2023, 15, 525. https://doi.org/10.3390/w15030525
Navas-Cárdenas C, Caetano M, Endara D, Jiménez R, Lozada AB, Manangón LE, Navarrete A, Reinoso C, Sommer-Márquez AE, Villasana Y. The Role of Oxygenated Functional Groups on Cadmium Removal using Pyrochar and Hydrochar Derived from Guadua angustifolia Residues. Water. 2023; 15(3):525. https://doi.org/10.3390/w15030525
Chicago/Turabian StyleNavas-Cárdenas, Carlos, Manuel Caetano, Diana Endara, Rocío Jiménez, Ana B. Lozada, Lucía E. Manangón, Angélica Navarrete, Carlos Reinoso, Alicia E. Sommer-Márquez, and Yanet Villasana. 2023. "The Role of Oxygenated Functional Groups on Cadmium Removal using Pyrochar and Hydrochar Derived from Guadua angustifolia Residues" Water 15, no. 3: 525. https://doi.org/10.3390/w15030525







