Groundwater Circulation Mechanism of the Upstream Area of Beiniuchuan River Using Isotope–Hydrochemical Tracer
Abstract
:1. Introduction
2. Overview of the Study Area
3. Sampling and Testing
4. Isotopes and Hydrochemical Characteristics
4.1. Hydrochemical Characteristics
4.2. Hydrogen and Oxygen Stable Isotopic Characteristics
4.3. Radiogenic Isotope Characteristics
5. Discussion
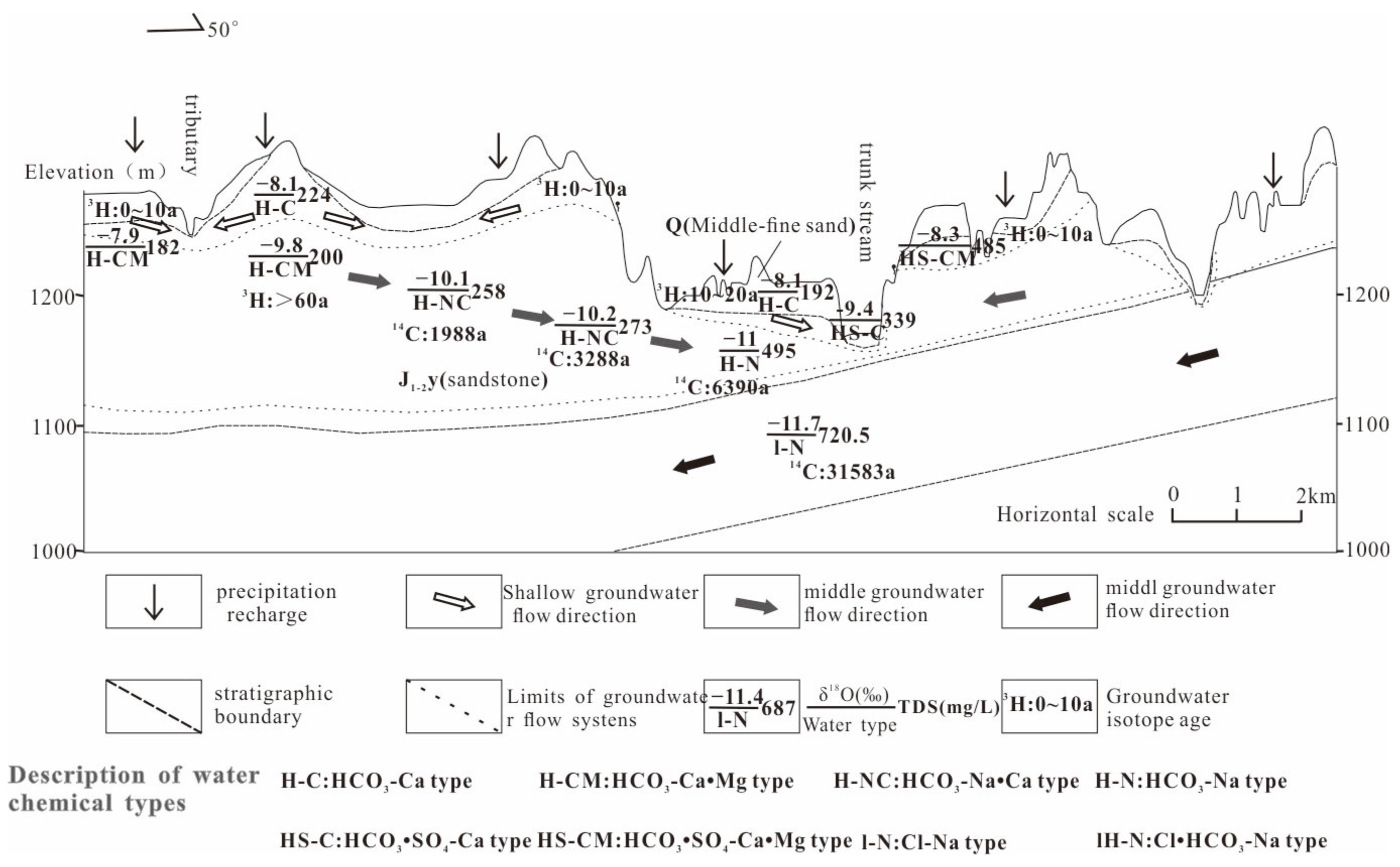
5.1. Characteristics of Groundwater Circulation
5.2. Conceptual Model of Groundwater Circulation
- (1)
- Shallow Quaternary Pore–Yan’an Formation Weathered Fracture Flow System: The shallow layer has a well-developed pore–fracture system, good conditions for infiltration recharge from precipitation, and fast groundwater circulation and renewal. The age of groundwater generally ranges from 0 to 20 years, with a circulation depth of 10 to 50 m. This flow system is scattered and mainly based on secondary valleys as discharge reference planes. The TDS concentration of groundwater is generally less than 300 mg/L, and the hydrochemical type is HCO3-Ca.
- (2)
- Intermediate Yan’an Formation Fracture–Pore Flow System: The development of pore–fracture is relatively poor and heterogeneous. Groundwater recharge takes a longer process, and the circulation renewal is slower. The age of groundwater increases gradually from the top of hills to river valleys, with a maximum age of approximately 6000 years and a circulation depth of 50 to 150 m. This flow system is continuously distributed, mainly relying on the Beiniuchuan River and its main tributaries as discharge reference planes. The TDS concentration of groundwater ranges from 200 to 500 mg/L, and the hydrochemical type is mainly HCO3.
- (3)
- Deep Yan’chang Formation Fracture–Pore Flow System: The development conditions for pore–fracture are extremely poor. Groundwater recharge takes a long time, and the renewal is very slow. The age of groundwater can reach tens of thousands of years. The main recharge of groundwater occurs in the eastern outcrop area of the Yan’chang Formation in the study area, flowing downstream along the stratigraphic dip. The circulation depth of groundwater is generally deeper than 100 to 150 m, and in the low-lying areas of valleys, it can be deeper than 50 m. The hydrochemical type of groundwater is Cl-Na.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, G.; Ma, F.S.; Guo, J.; Li, K.P.; Lu, R. Assessment of Water Sources and Mixing of Groundwater in a Coastal Mine: The Sanshandao Gold Mine, China. Mine Water Environ. 2018, 37, 351–365. [Google Scholar]
- Li, P.Y.; Qian, h.; Wu, J.H.; Zhang, Y.Q.; Zhang, H.B. Major Ion Chemistry of Shallow Groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ. 2013, 32, 195–206. [Google Scholar] [CrossRef]
- Yue, H.; Chen, C. Study of Hydrogeological Characteristics and roundwater Circulation Pattern in Shendong Mining Area. Coal Technol. 2017, 36, 113–115. [Google Scholar]
- Fu, C.C.; Li, X.Q.; Ma, J.F.; Liu, L.X.; Gao, M.; Bai, Z.X. A hydrochemistry and multi-isotopic study of groundwater origin and hydrochemical evolution in the middle reaches of the Kuye River basin. Appl. Geochem. 2018, 98, 82–93. [Google Scholar] [CrossRef]
- Fan, L.M.; Ma, X.D.; Ji, R.J. Progress in engineering practice of water-preserved coalmining in western eco-environment frangible area. J. China Coal Soc. 2015, 40, 1711–1717. [Google Scholar]
- Gu, D.Z. Theory framework and technological system of coal mine underground reservoir. China Coal Soc. 2015, 40, 239–246. [Google Scholar]
- Zhang, M.S.; Dong, Y.; Du, R.J.; Gu, X.F. The strategy and influence of coal mining on the groundwater resources at the energy and chemical base in the north of Shaanxi. Earth Sci. Front. 2010, 17, 235–246. [Google Scholar]
- Zhang, D.S.; Fan, G.W.; Ma, L.Q.; Wang, X.F. Aquifer protection during long wall mining of shallow coal seams: A case study in the Shendong Coalfield of China. Int. J. Coal Geol. 2011, 86, 190–196. [Google Scholar] [CrossRef]
- Wang, Z.R. Study on coal mine hydro-geological classification standards in Shendong Mining Area. Saf. Incoal Mines 2017, 47, 86–89. [Google Scholar]
- Ma, J.F.; Li, X.Q. Study on models of aquifer failure caused by coal mining in Shendong Mining Area. Coal Sci. Technol. 2019, 47, 207–213. [Google Scholar]
- Zhao, D.J.; Liu, Y.M.; Sun, Y.H.; Zhao, Y.; Bai, F.T. Experiments and simulations of underground artificial freezing with the use ofnatural cold resources in cold regions. Build. Environ. 2015, 87, 224–233. [Google Scholar] [CrossRef]
- Qiao, X.J.; Li, G.M.; Li, M.; Zhou, J.L.; Du, J.; Sun, Z.H. Influence of coal mining on regional karst groundwater system: A case study in west mountain area of Taiyuan city, nouthern China. Environ. Earth Sci. 2011, 64, 1525–1535. [Google Scholar] [CrossRef]
- Barbieri, M.; Barberio, M.D.; Banzato, F.; Billi, A.; Boschetti, T.; Franchini, S.; Gori, F.; Petitta, M. Climate change and its effect ongroundwater quality. Environ. Geochem. Health 2021, 45, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Herczeg, A.L.; Leaney, F.W. Review: Environmental-Tracers in Arid-Zone Hydrology. Hydrogel. J. 2011, 19, 17–29. [Google Scholar] [CrossRef]
- Liu, P.; Hoth, N.; Drebenstedt, C.; Sun, Y.J.; Xu, Z.M. Hydro-geochemical paths of ulti-layer groundwater system in coalmining regions—Using multivariate statistics and geochemical modeling approaches. Sci. Total Environ. 2017, 601–602, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Z.; Peng, Y.X.; Zhao, W.D.; Ma, L.; He, X.R.; Lu, Y.H. Hydrochemical processes and evolution of karst groundwater in thenortheastern Huaibei Plain, China. Hydrogeol. J. 2018, 26, 1721–1729. [Google Scholar] [CrossRef]
- Huang, Q.B.; Qin, X.Q.; Yang, Q.Y.; Liu, P.Y.; Zhang, J.S. Identification of dissolved sulfate sources and the role of sulfuric acid incarbonate weathering usingδ13CDICandδ34S in karst area, northern China. Environ. Earth Sci. 2016, 75, 51. [Google Scholar]
- Qu, S.; Wang, G.C.; Shi, Z.M.; Xu, Q.Y.; Guo, Y.Y.; Ma, L.; Sheng, Y.Z. Using stable isotopes (δD, δ18O, δ34S and δ87Sr/δ86Sr) to identify sources of water in abandoned mines in the Fengfeng coal mining district, northern China. Hydrogeol. J. 2018, 26, 1443–1453. [Google Scholar] [CrossRef]
- Chen, J.S.; Liu, X.Y.; Wang, C.Y.; Rao, W.B.; Tan, H.B.; Dong, H.Z.; Sun, X.X.; Wang, Y.S.; Su, Z.G. Isotopic constraints on the origin of groundwater in the Ordos Basin of northern China. Environ. Earth Sci. 2012, 66, 505–517. [Google Scholar] [CrossRef]
- Jia, Y.F.; Guo, H.M.; Xi, B.D.; Jiang, Y.H.; Zhang, Z.; Yuan, R.X.; Yi, W.X.; Xue, X.L. Sources ofgroundwater salinity and potential impact on arsenic mobility in the western Hetao Basin, Inner Mongolia. Sci. Total Environ. 2017, 601–602, 691–702. [Google Scholar] [CrossRef]
- Yin, L.H.; Hou, G.C.; Su, X.S. Isotopes (δD and δ18O) in precipitation, groundwater and surface water in the Ordos Plateau, China: Implications with respect to groundwater recharge and circulation. Hydrogeol. J. 2011, 19, 429–443. [Google Scholar] [CrossRef]
- Huang, T.M.; Ma, B.Q.; Pang, Z.H.; Li, Z.; Li, Z.B.; Yin, L. How does precipitation recharge groundwater in loess aquifers: Evidence from multiple environmental tracers. J. Hydrol. 2020, 583, 124532. [Google Scholar] [CrossRef]
- Dong, W.H.; Kang, B.; Du, S.H.; Shi, X.F. Estimation of shallow groundwater ages and circulation rates in the Henan Plain, China: CFC and deuterium excess methods. Geosci. J. 2013, 17, 479–488. [Google Scholar] [CrossRef]
- Sherif, M.I.; Sultan, M.; Sturchio, N.C. Chlorine isotopes astracers of solute origin and age of groundwaters from the EasternDesert of Egypt. Earth Planet. Sci. Lett. 2019, 510, 37–44. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Zhang, X.L.; He, D. A Study of 36Cl age for the deep geothermal water in the Guanzhong Basin. Hydrogeol. Eng. Geol. 2016, 43, 157–163. [Google Scholar]
- Wang, J.Y.; Chen, J.S.; Lu, B.H.; Tong, H.B.; Tan, Z.C.; Sun, Y.Y.; Lin, T.; Wang, Y.S. Review and prospect ofisotopehydrology. J. Hohai Univ. 2015, 43, 406–413. [Google Scholar]
- Shi, X.F.; Dong, W.H.; Li, M.Z.; Yan, Z. Evaluation of groundwater renewability in the Henan Plains, China. Geochem. J. 2012, 46, 107–115. [Google Scholar] [CrossRef]
- Huang, T.M.; Ma, B.Q. The origin of major ions of groundwater in a loess aquifer. Water 2019, 11, 2464. [Google Scholar] [CrossRef]
- Zhang, C.C.; Li, X.Q.; Ma, J.F.; Wang, Z.X.; Hou, X.W. Stable isotope and hydrochemical evolution of shallow groundwater in mining area of the Changzhi Basin, northern China. Environ. Earth Sci. 2022, 81, 294. [Google Scholar] [CrossRef]
- Su, X.S.; Xu, W.; Yang, F.T.; Zhu, P.C. Using new mass balance methods to estimate gross surface water and groundwater exchange with naturally occurring tracer222Rn in data poor regions: A case study in northwest China. Hydrol. Process. 2015, 29, 979–990. [Google Scholar] [CrossRef]
- Li, J.; Pang, Z.H.; Yang, G.M.; Tian, J.; Tong, A.L.; Zhang, X.Y.; Hu, S.M. Million-year-old groundwater revealed by krypton-81 dating in Guanzhong Basin, China. Sci. Bull. 2017, 62, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Song, X.F.; Yang, L.H.; Han, D.M.; Zhang, Y.H.; Ma, Y.; Bu, H.M. The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci. Total Environ. 2015, 538, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.H.; Hou, G.C.; Dou, Y.; Tao, Z.P.; Li, Y. Hydrogeochemical and isotopic study of groundwater in the habor lake basin of the Ordos plateau, NW China. Environ. EarthSci. 2011, 64, 1575–1584. [Google Scholar] [CrossRef]
- Chen, X.H.; Guo, Q.L.; Chen, Z.H. The runoff variation characteristic and influencing factors in Beiniuchuan River. J. Arid. Land Resour. Environ. 2015, 29, 186–191. [Google Scholar]
- Bian, Z.F.; Lei, S.G.; In, H.; Chang, L.Q.; Zhang, R.C.; Zhou, C.J.; He, X. Integrated method of RS and GPR for monitoring the changes in the soil moisture and groundwater environment due to underground coal mining. Environ. Geol. 2009, 57, 131–142. [Google Scholar] [CrossRef]
- Miao, X.X.; Wang, C.S.; Bai, H.B. Hydrogeologic characteristics of mine water hazards in Shendong mining area. Min. Saf. Eng. 2010, 27, 285–291. [Google Scholar]
- Ma, L.Q.; Zhang, D.S.; Jia, J.L.; Zhao, Y.S.; Cao, X.Q.; Bo, Z.P.; Zhao, Y.F. The recycling mode and technique of water in desertification area of Shendong Coal Mine. Energy Procedia 2012, 14, 20–25. [Google Scholar]
- Tao, X.; Zhang, G.; Qiao, J. A genetic discussion on coal sulfur and ash in Jurassic north Shaanxi and Huanglong coalfields. Coal Geol. China 2012, 24, 11–14. [Google Scholar]
- Lee, C.L.; Chin, K.L.; H’ng, P.S.; Rashid, U.; Maminski, M.; Khoo, P.S. Effect of pretreatment conditions on the chemical-structural characteristics of coconut and palm kernel shell: A potentially valuable precursor for eco-efficient activated carbon production. Environ. Technol. Innov. 2021, 21, 101309. [Google Scholar] [CrossRef]
- Li, T.K.; Liu, B.S.; Bi, X.H.; Wu, J.H.; Zhang, Y.F.; Feng, Y.C. Size and chemical characteristics of particles emitted from typical rural biomass cookstoves in North China. Atmos. Res. 2021, 249, 105295. [Google Scholar] [CrossRef]
- Liu, J.; Guo, H.L.; Liu, F.L.; Wei, W.; Zhang, L.; Zhang, X.Y. The variations of stable isotopes (δD and δ18O) inthe precipitation in Baotou area. J. Arid. Land. Resour. Environ. 2013, 27, 157–162. [Google Scholar]
- Guo, Q.L.; Xiong, X.Z.; Jiang, J.R. Analysis of isotopic and hydrochemical charicteristics of different water in Kuye River Basin. J. Soil. Water Conserv. 2016, 30, 237–242. [Google Scholar]
- Liu, F.; Wang, S.; Wang, L.S.; Shi, L.M.; Song, X.F.; Yeh, T.C.; Zhen, P.N. Coupling hydrochemistry and stable isotopes to identify the major factors affecting groundwater geochemical evolution in the Heilongdong Spring Basin, North China. J. Geochem. Explor. 2019, 205, 106352. [Google Scholar] [CrossRef]
- Ruan, Y.F.; Zhao, L.J.; Xiao, H.L.; Zhou, M.X.; Cheng, G.D. The groundwater in the Heihe River basin: Isotope age and renewability. J. Glaciol. Geocryol. 2015, 37, 767–782. [Google Scholar]
- Wan, Y.Y.; Su, X.S.; Dong, W.H. Evaluation of groundwater renewal ability in the Ordos Cretaceous groundwater basin. J. Jilin Univ. (Earth Sci. Ed.) 2010, 40, 623–630. [Google Scholar]
- Tang, C.L.; Jin, H.; Liang, Y.P. Using Isotopic and Hydrochemical Indicators to Identify Sources of Sulfate in Karst Groundwater of the Niangziguan Spring Field, China. Water 2021, 13, 390. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Lyu, Z.C.; Wang, G.C.; Ma, L.; Xu, Q.Y.; Huang, X.J.; Gao, S.Z. Hydrogeochemical simulation of groundwater in Eastern Fengfeng mining area. Coal Geol. Explor. 2016, 44, 101–105. [Google Scholar]
- Chen, L.; Zhang, F.W.; Yao, H.C.; Han, Z.T.; Qian, L.; Chen, L.; Jiang, C.C. Using of key stratum theory to study the structural development of roof aquifer. ACTC Geol. (Engl. Ed.) 2015, 89, 2090–2093. [Google Scholar]
- Zhang, F.W.; Chen, L.; Yao, H.C. Progress of hydrogeological research in mining areas and its mid-and long-term trend. ACTC Geologica Sinnca (Chin. Ed.) 2016, 90, 2464–24753. [Google Scholar]
- Huang, T.M.; Pang, Z.H.; Li, J.; Xiang, Y.; Zhao, Z.J. Mapping ground water renewability using age data in the Baiyang Alluvial Fan, NWChina. Hydrogeol. J. 2017, 25, 743–755. [Google Scholar] [CrossRef]
- Hou, G.C. Groundwater system and Water Circulation Patter in Ordos Cretaceous Groundwater Basin. Ph.D. Thesis, Jilin University, Changchun, China, 2008. [Google Scholar]
- Liu, F.T. Research on Groundwater Circulation and Hydrochemical Transport in the North of Ordos Cretaceous Basin Based on Isotope Technology. Ph.D. Thesis, Jilin University, Changchun, Chian, 2008. [Google Scholar]

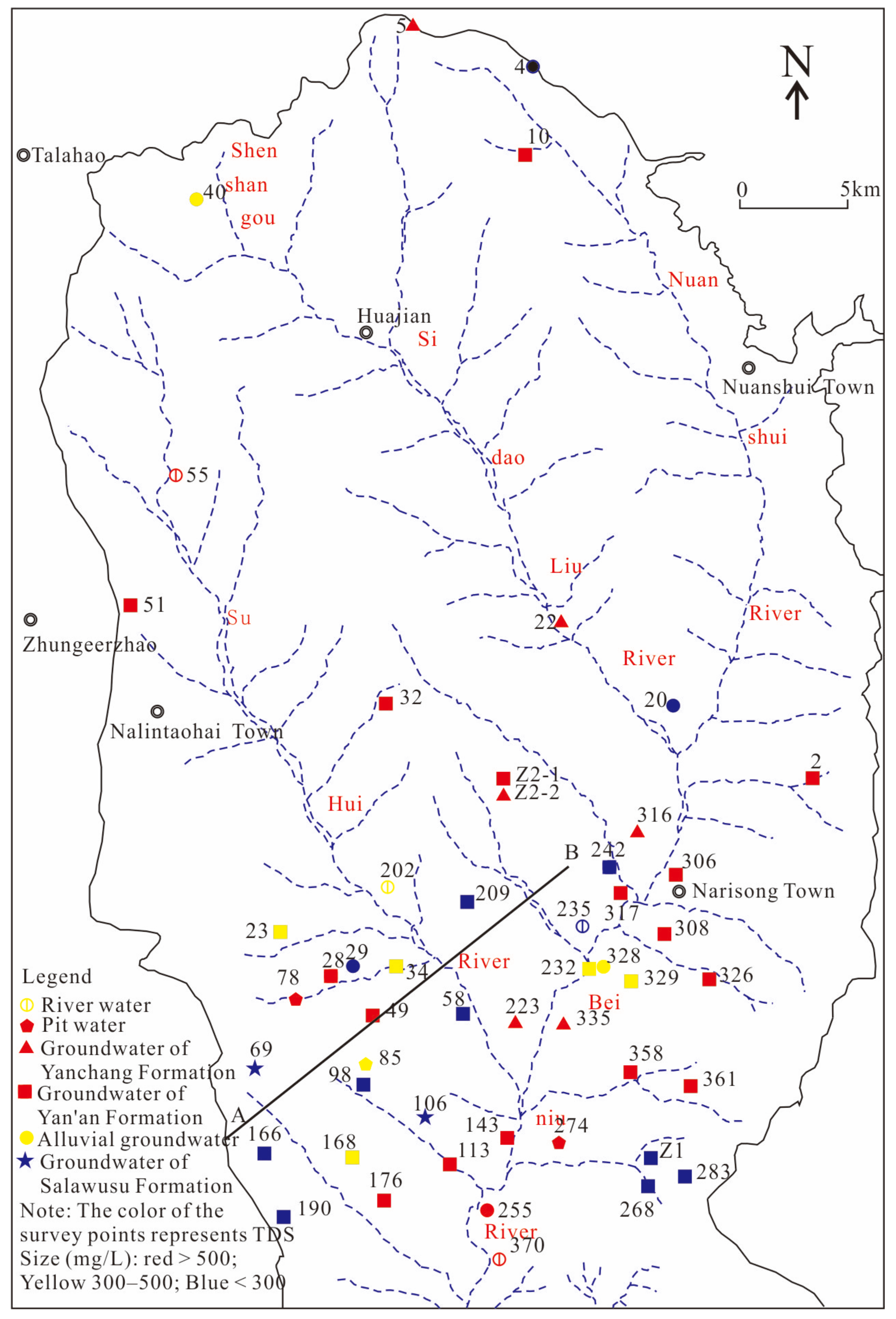
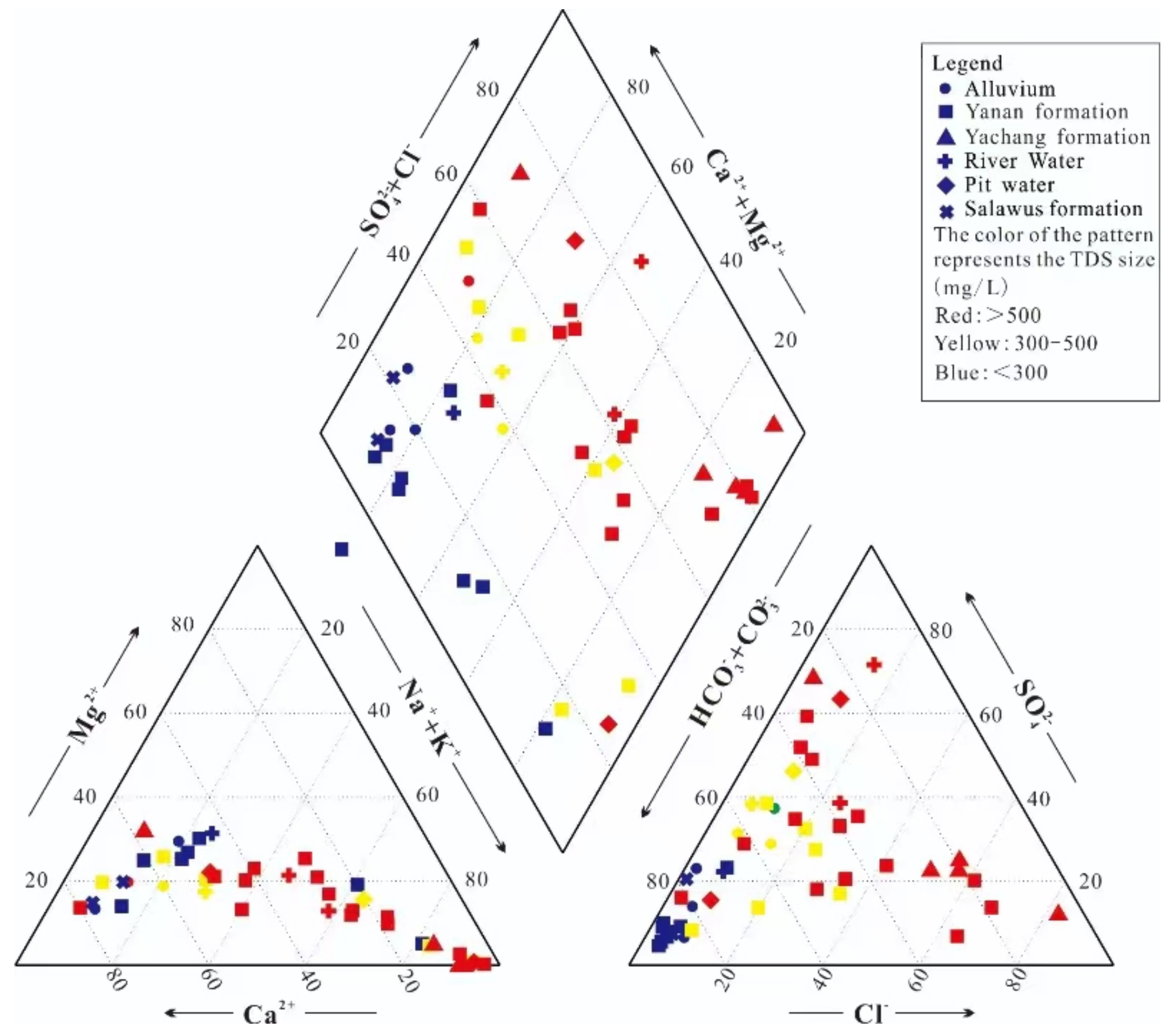
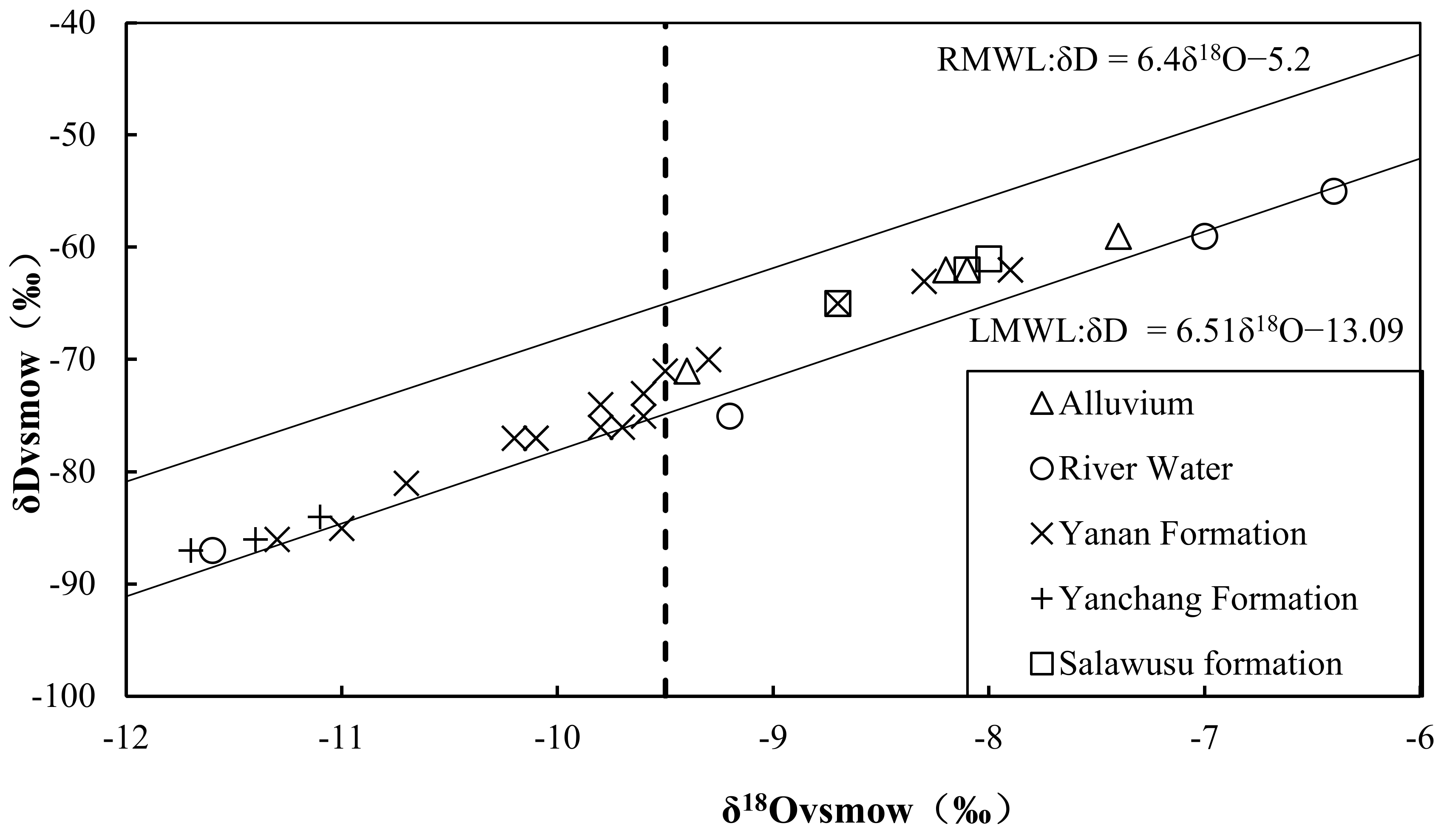
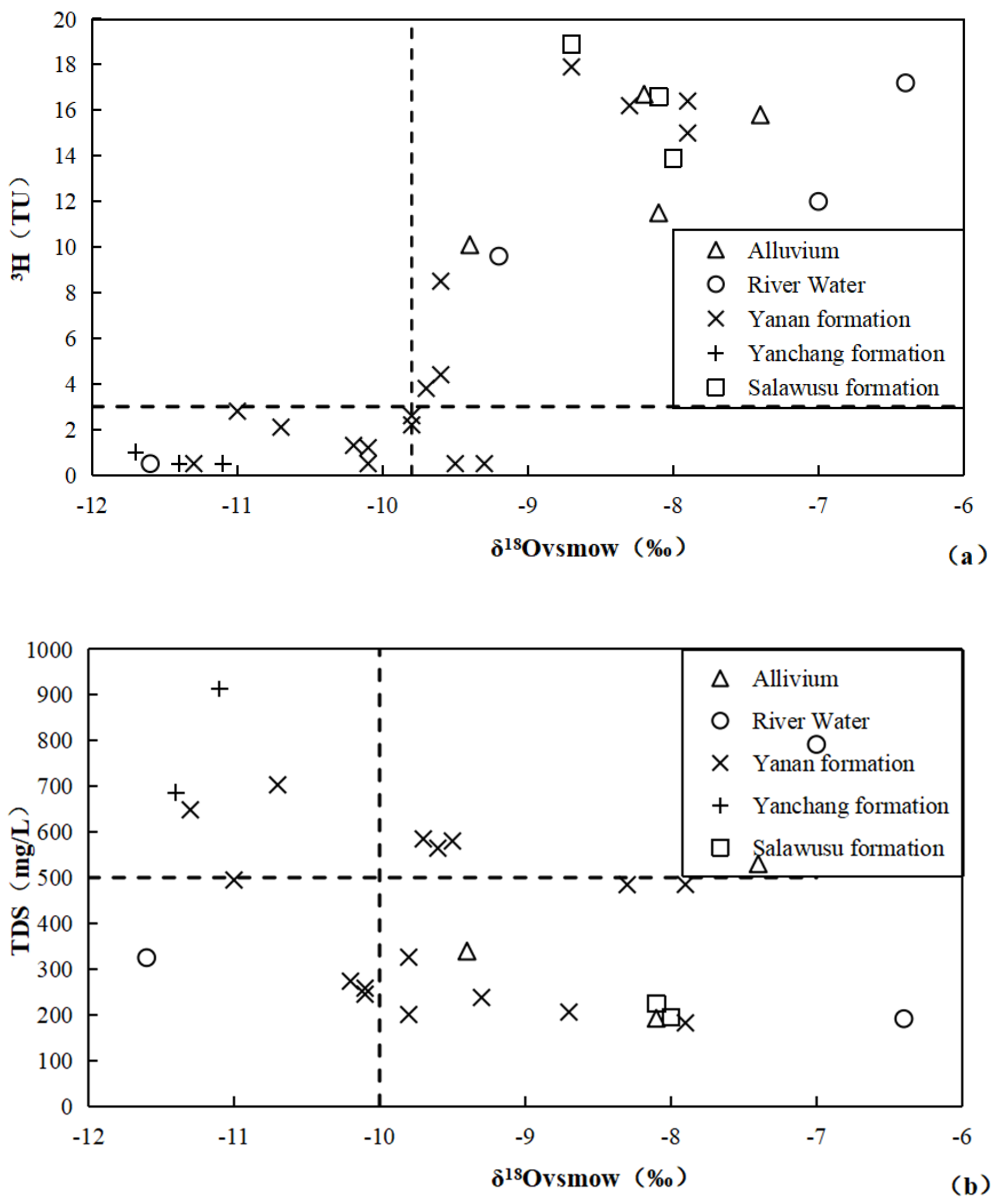
| Number | Sample | Well Depth | Water Depth | TDS | Hydrochemical Type | δ2H | δ18O | 3H | 14C |
|---|---|---|---|---|---|---|---|---|---|
| Layer | (m) | /m | (mg/L) | (‰) | (‰) | (TU) | (pmC) | ||
| 275 | River water | −75 | −9.2 | 9.6 | |||||
| 235 | River water | 191.1 | HCO3-Ca·Mg | −55 | −6.4 | 17.2 | |||
| 370 | River water | 791.6 | HCO3·SO4-Na·Ca | −59 | −7 | 12 | |||
| 202 | River water | 324.7 | HCO3·SO4-Ca·Na | −87 | −11.6 | <1 | |||
| 20 | Q4al+pl | 4 | 3.5 | 250.7 | HCO3-Ca·Mg | ||||
| 40 | Q4al+pl | 4.2 | 2.61 | 410.4 | HCO3·SO4-Ca·Na | ||||
| 29 | Q4al+pl | 16 | 2.19 | 192 | HCO3-Ca | −62 | −8.1 | 11.5 | |
| 232 | Q4al+pl | 8 | 3.95 | 339.1 | HCO3·SO4-Ca | −71 | −9.4 | 10.1 | |
| 255 | Q4al+pl | 12 | 7.27 | 530.2 | HCO3·NO3-Ca | −59 | −7.4 | 15.8 | |
| 106 | Q3s | 224 | HCO3-Ca | −62 | −8.1 | 16.6 | |||
| 328 | J1–2y | 120 | 6.9 | 485 | HCO3·SO4-Ca·Na | −62 | −7.9 | 15 | 54.14 |
| 176 | J1–2y | 12 | 5.77 | 580.1 | SO4·HCO3-Ca·Na | −71 | −9.5 | <1 | |
| 209 | J1–2y | 4.5 | 3 | 245.7 | HCO3-Ca·Mg | ||||
| 329 | J1–2y | 6 | 2.6 | 484.5 | HCO3·SO4-Ca·Mg | −63 | −8.3 | 16.2 | |
| 49 | J1–2y | 30 | 23.47 | 755.2 | HCO3·SO4-Ca | ||||
| 308 | J1–2y | 140 | 8 | 507.7 | HCO3·SO4-Na | ||||
| 32 | J1–2y | 100 | 5.3 | 624.9 | HCO3·SO4-Ca·Na | ||||
| 51 | J1–2y | 100 | 3.89 | 842.9 | SO4·HCO3-Ca·Na | ||||
| 28 | J1–2y | 130 | 3.9 | 564.4 | SO4·HCO3-Na | −75 | −9.6 | 4.4 | |
| 34 | J1–2y | 83 | 60.5 | 325.7 | HCO3-Na | −76 | −9.8 | 2.2 | 28.19 |
| 58 | J1–2y | 150 | 105 | 244.7 | HCO3-Na | −77 | −10.1 | <1 | 32.43 |
| 168 | J1–2y | 100 | 60.5 | 494.7 | HCO3-Na | −85 | −11 | 2.8 | 16.85 |
| 190 | J1–2y | 140 | 59.84 | 181.9 | HCO3-Ca·Mg | −62 | −7.9 | 16.4 | |
| 242 | J1–2y | 150 | 80 | 273.2 | HCO3-Na·Ca | −77 | −10.2 | 1.3 | 24.52 |
| 268 | J1–2y | 130 | 37.25 | 237.5 | HCO3-Ca·Mg | −70 | −9.3 | <1 | |
| 283 | J1–2y | 160 | 80 | 257.9 | HCO3-Na·Ca·Mg | −77 | −10.1 | 1.2 | 28.7 |
| 113 | J1–2y | 120 | 72.29 | 703.1 | Cl-HCO3·Na | −81 | −10.7 | 2.1 | 14.35 |
| 143 | J1–2y | 120 | 68.3 | 556.1 | SO4·HCO3·Cl-Ca·Na | ||||
| 358 | J1–2y | 150 | 17.44 | 584.7 | HCO3·Cl-Na | −76 | −9.7 | 3.8 | |
| Z1 | J1–2y | 200 | 35 | −65 | −8.7 | 18.9 | 39.92 | ||
| 361 | J1–2y | 150 | 65 | 557.8 | HCO3·Cl-Na | ||||
| Z2-1 | J1–2y | 80 | 18 | −73 | −9.6 | 8.5 | |||
| Z2-2 | T3y | 200 | 0 | 720.5 | Cl-Na | −87 | −11.7 | 1 | 0.8 |
| 306 | J1–2y + T3y | 200 | 32.4 | 648.4 | Cl-Na | −86 | −11.3 | <1 | 15.44 |
| 317 | J1–2y + T3y | 150 | 8.4 | 620.7 | Cl-Na | ||||
| 223 | T3y | 180 | 1.5 | 706.4 | Cl-Na | ||||
| 316 | T3y | 180 | 0 | 686.5 | Cl-Na | −86 | −11.4 | <1 |
| 3H content/TU | 1~3 | 3~8 | 8~13 | 12~20 |
| Groundwater age/a | >60 | 50~60 | 10~20 | 0~10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhu, P.; Liu, P.; Zhang, W.; Geng, X.; Ma, L. Groundwater Circulation Mechanism of the Upstream Area of Beiniuchuan River Using Isotope–Hydrochemical Tracer. Water 2023, 15, 4000. https://doi.org/10.3390/w15224000
Chen L, Zhu P, Liu P, Zhang W, Geng X, Ma L. Groundwater Circulation Mechanism of the Upstream Area of Beiniuchuan River Using Isotope–Hydrochemical Tracer. Water. 2023; 15(22):4000. https://doi.org/10.3390/w15224000
Chicago/Turabian StyleChen, Li, Pucheng Zhu, Pei Liu, Wei Zhang, Xinxin Geng, and Linna Ma. 2023. "Groundwater Circulation Mechanism of the Upstream Area of Beiniuchuan River Using Isotope–Hydrochemical Tracer" Water 15, no. 22: 4000. https://doi.org/10.3390/w15224000





