Removal of Arsenic from Wastewater Using Hydrochar Prepared from Red Macroalgae: Investigating Its Adsorption Efficiency and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Hydrochar (HC)
2.3. Activation of Hydrochar
2.4. Surface Morphology and Characterization of Hydrochar (HC) and Activated Hydrochar (AHC) Samples
2.5. Batch Adsorption
2.6. Calculations
2.6.1. Isotherm Models
2.6.2. Kinetic Models
- q t is the amount of arsenic adsorbed at time t;
- Kdiff is the intra-particle diffusion rate constant;
- C is the intercept.
3. Results
3.1. SEM Analysis of Hydrochar
3.2. Elemental Composition and Changes during Activation of Hydrochar
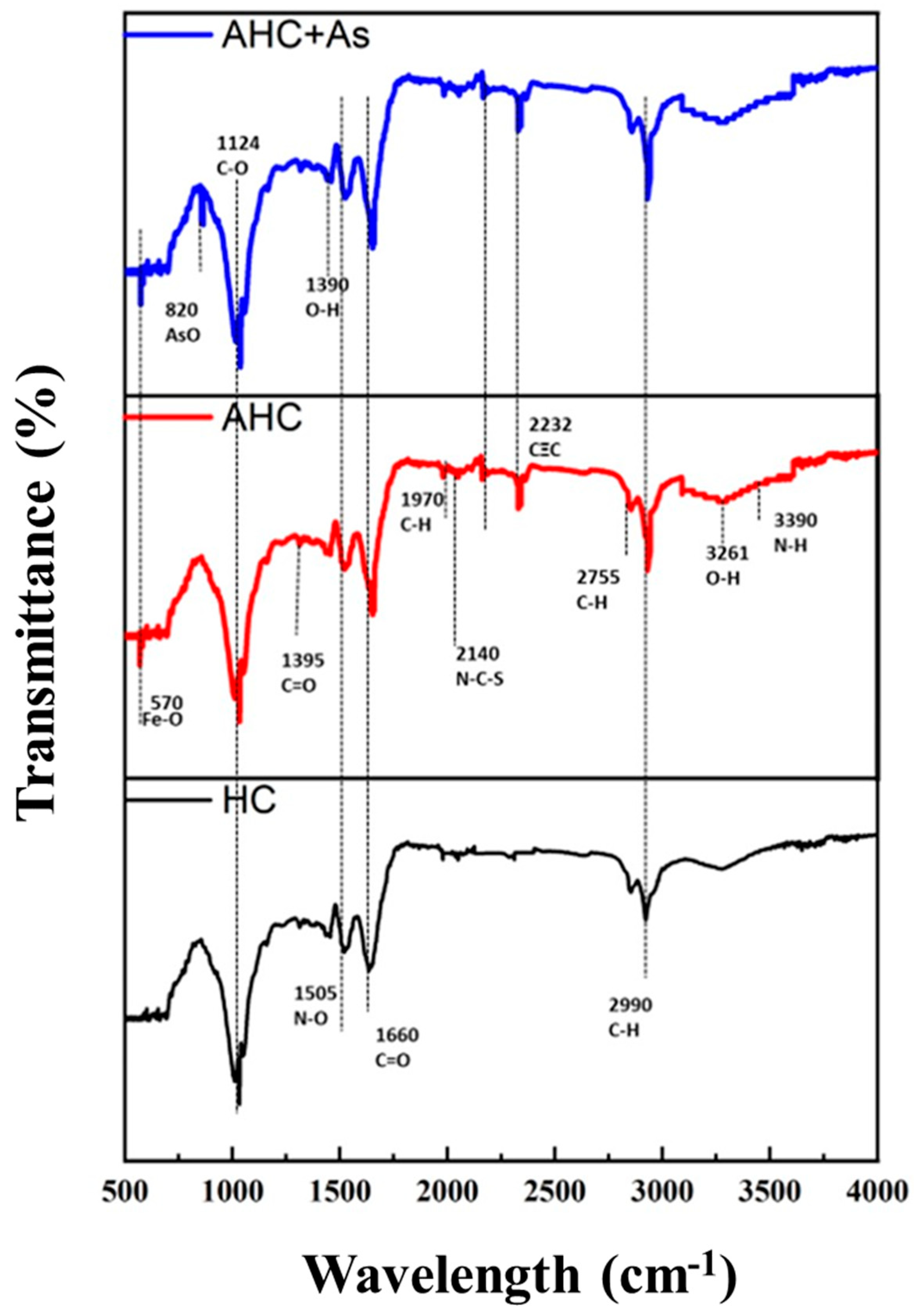
3.3. Identification and Impact of Surface Functional Groups of HC, AHC during As Adsorption, and AHC after As Adsorption
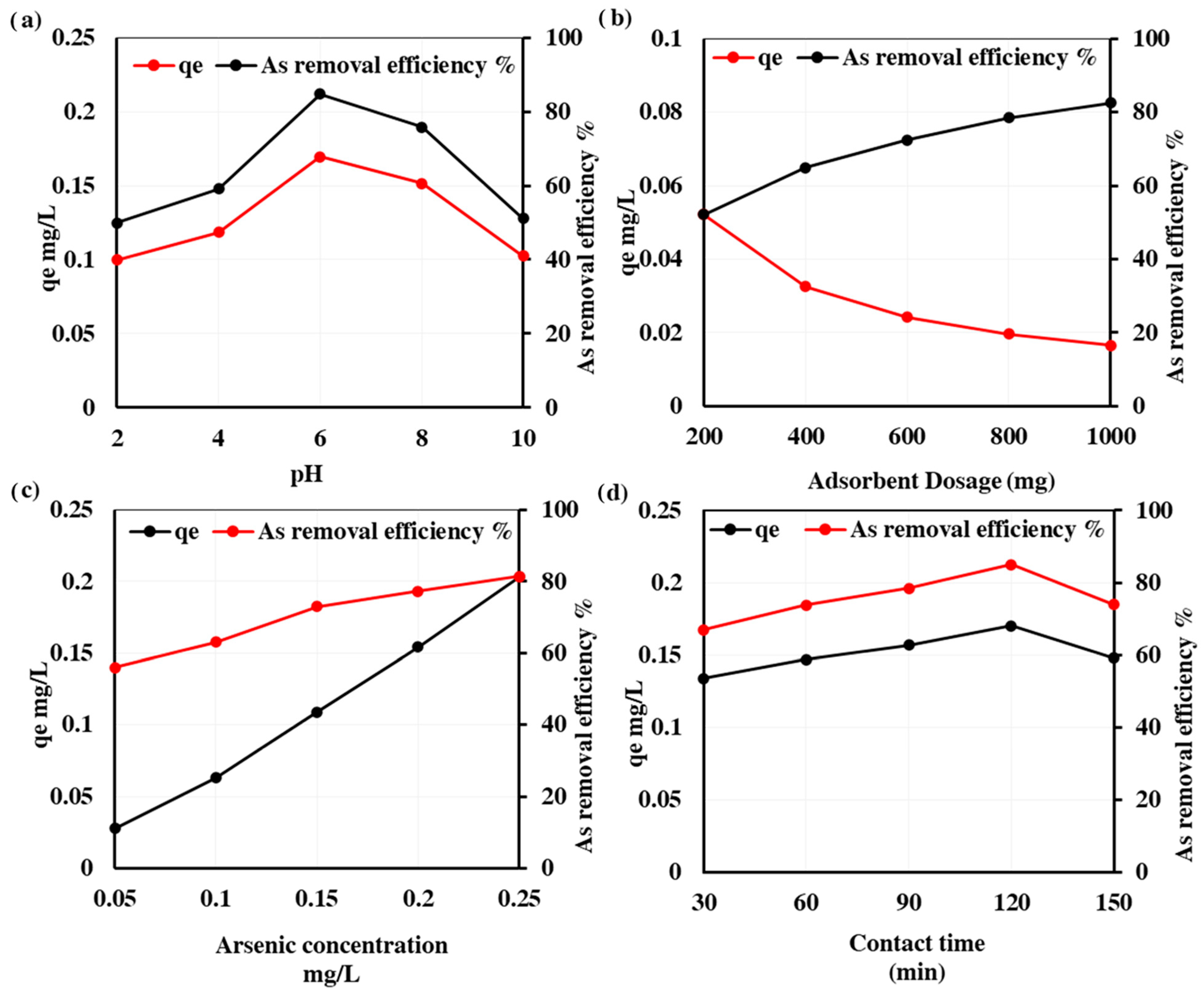
3.4. Factors Affecting As Removal
3.4.1. Effect of pH
3.4.2. Effect of Adsorbent Dosage
3.4.3. Effect of Initial As Concentration
3.4.4. Effect of Contact Time
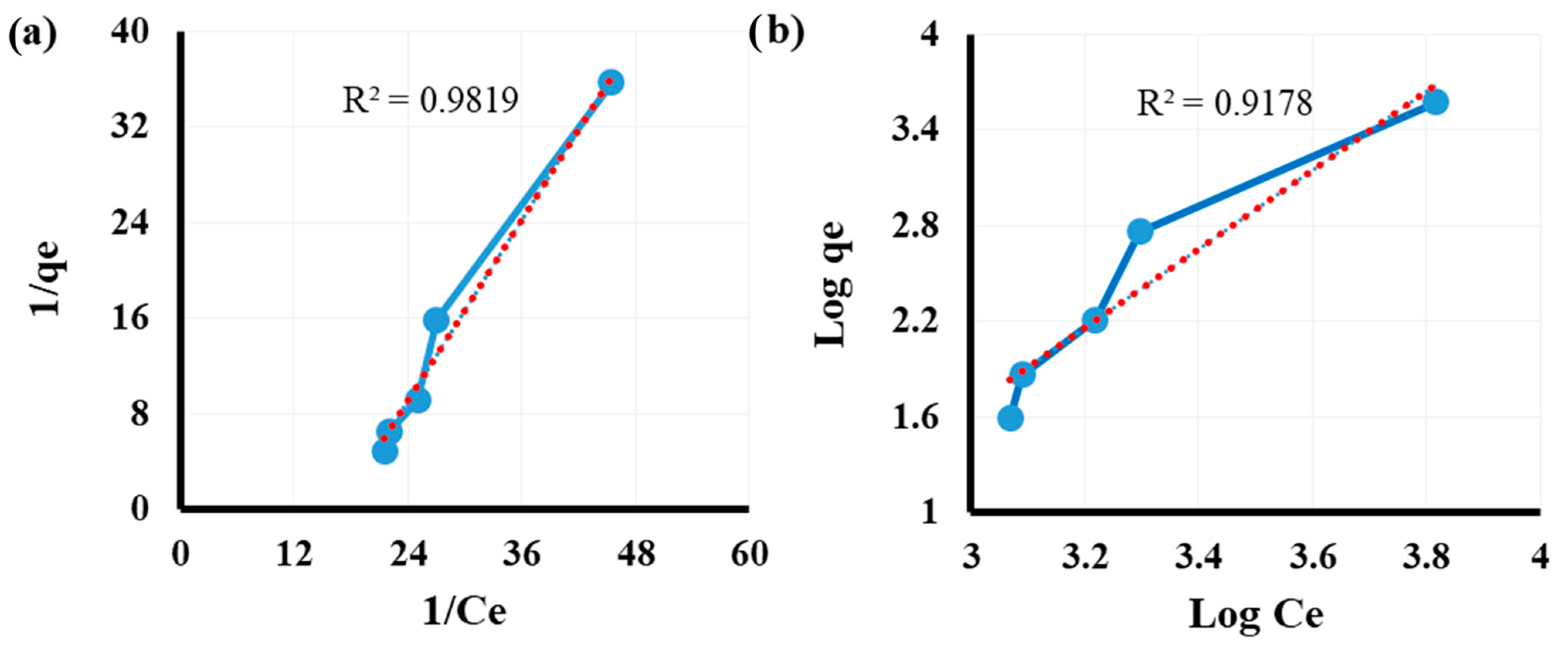
3.5. Equilibrium Investigation
| Material | As Species | Langmuir Isotherm Model Parameters | Freundlich Isotherm Model Parameters | References | ||||
|---|---|---|---|---|---|---|---|---|
| KL (L/mg) | Q max (mg/g) | R2 | K F (mg/g) | N | R2 | |||
| Activated hydrochar | As (III/V) | 0.0123 | 3.8314 | 0.981 | 0.031 | 0.403 | 0.917 | This study |
| Multi-amino-functionalized cellulose | As (III) | 0.21 | 5.71 | 0.970 | 2.11 | 3.94 | 0.926 | [77] |
| As (V) | 0.078 | 75.13 | 0.992 | 26.85 | 5.02 | 0.934 | ||
| Iron–zirconium binary oxide-coated sand (27, 35 and 45 °C) | As (V) | 0.0104 | 45.05 | 0.957 | 1.19 | 1.7 | 0.997 | [78] |
| As (V) | 0.0083 | 66.22 | 0.856 | 1.10 | 1.5 | 0.999 | ||
| As (V) | 0.0077 | 84.75 | 0.955 | 1.15 | 1.3 | 0.997 | ||
| Iron oxide nanoparticles | As (III) | 0.4 | 42 | 0.978 | 11.3 | 2.1 | 0.921 | [79] |
| Iron oxide nanoparticles | As (V) | 0.8 | 83 | 0.998 | 28.6 | 3.1 | 0.828 | |
| Magnetic graphene oxide nanocomposites | As (V) | 0.385 | 69.44 | 0.905 | 26.57 | 4.787 | 0.819 | [80] |
| Iron oxide nanoparticles | As (V) | 0.11 | 28.57 | _ | 2.6 | 0.68 | _ | [81] |
| Magnetic gelatin-modified biochar | As (V) | 0.81 | 43.15 | 0.89 | 19.27 | 0.24 | 0.70 | [82] |
| Phosphorus (P)-modified biochar (PLBC) (Taraxacum mongolicum Hand-Mazz) | As (III) | 0.08 | 30.76 | 0.843 | 6.76 | 0.33 | 0.818 | [83] |
| Ultisol | As (V) | 137.3 | 24.27 | 0.995 | 31.3 | 5.00 | 0.997 | [84] |
| Ultisol + biochar | As (V) | 66.4 | 21.51 | 0.996 | 26.1 | 4.67 | 0.995 | |
| Ultisol + biochar derived from aluminum-treated rice straw | As (V) | 77.0 | 25.97 | 0.994 | 38.8 | 3.62 | 0.992 | |
| Ultisol + aluminum-treated biochar form rice straw | As (V) | 185.5 | 26.95 | 0.996 | 42.9 | 4.08 | 0.988 | |
| Rice-husk biochar-stabilized iron and copper oxide nanoparticles | As (III/V) | 0.19 | 20.32 | 0.555 | 2.84 | 1.28 | 0.975 | [85] |
3.6. Kinetic Study
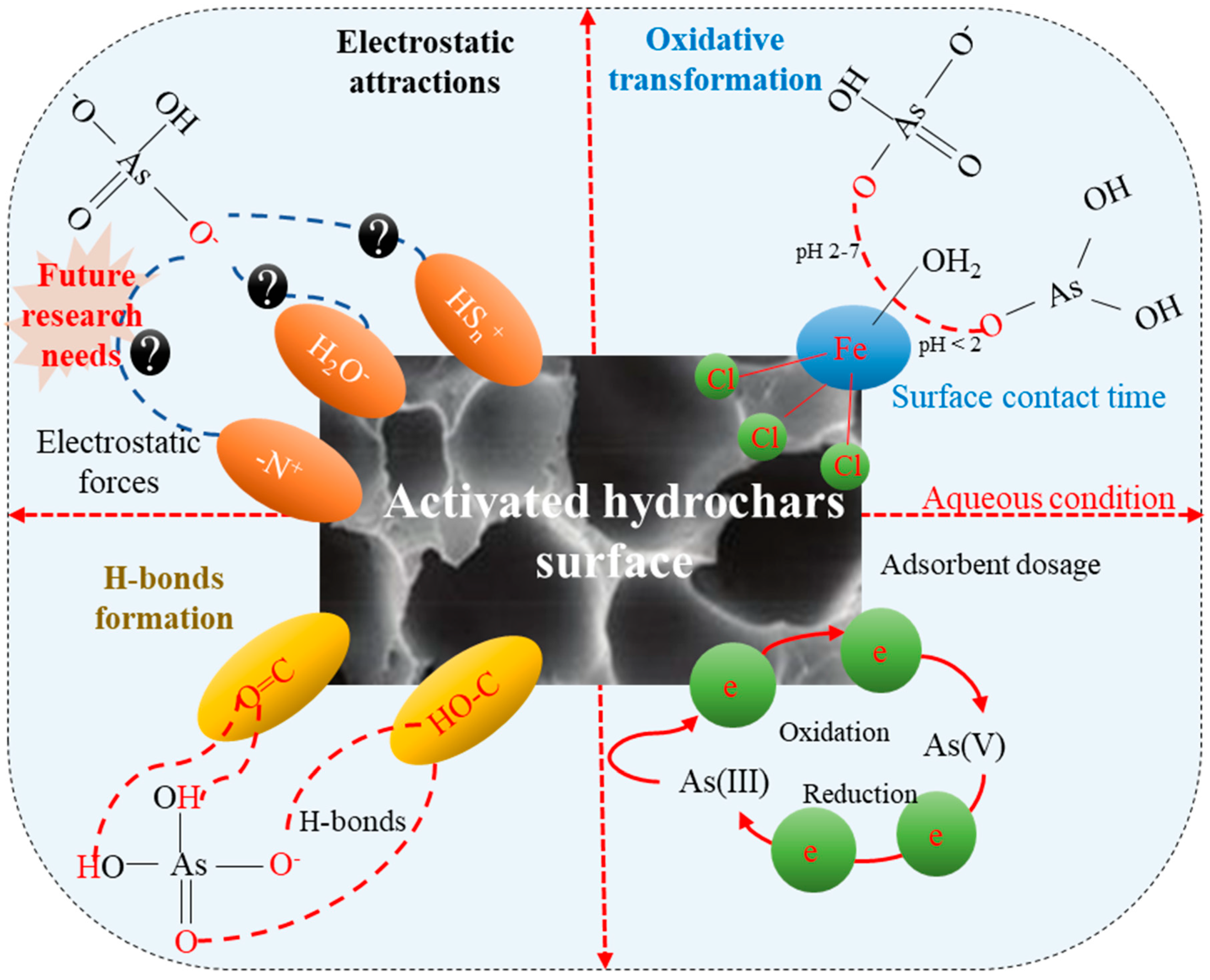
4. As Removal Mechanisms
5. Future Outlook and Perspectives Trends
- The removal of As from wastewater using activated hydrochar extracted from macro-algae has been shown to be a highly effective method. The increasing demand for sustainable and cost-effective methods for treating As-contaminated wastewater is driving the growth of this technology. Macro-algae are abundant and could be harvested in large quantities, making them an attractive option for the production of hydrochar. In addition, the production process is low-cost and energy-efficient, making it an attractive option for small-scale operations.
- Another research direction should be the development of advanced techniques for the activation of hydrochar, such as chemical, thermal, and microwave activation. These techniques could improve the adsorption capacity and efficiency of hydrochar, making it an even more practical solution for As removal from wastewater.
- It is expected that the use of activated hydrochar extracted from macro-algae will continue to grow as the demand for sustainable and effective methods for treating As-contaminated wastewater increases. In addition, the development of new and improved methods for the activation of hydrochar and the use of other sustainable materials for the production of hydrochar is expected to drive the growth of this technology in the future.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Hazmi, H.E.; Mohammadi, A.; Hejna, A.; Majtacz, J.; Esmaeili, A.; Habibzadeh, S.; Saeb, M.R.; Badawi, M.; Lima, E.C.; Mąkinia, J. Wastewater Reuse in Agriculture: Prospects and Challenges. Environ. Res. 2023, 236, 116711. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Hassan, G.K.; Maktabifard, M.; Grubba, D.; Majtacz, J.; Mąkinia, J. Integrating Conventional Nitrogen Removal with Anammox in Wastewater Treatment Systems: Microbial Metabolism, Sustainability and Challenges. Environ. Res. 2022, 215, 114432. [Google Scholar] [CrossRef]
- Joseph, T.M.; Al-Hazmi, H.E.; Śniatała, B.; Esmaeili, A.; Habibzadeh, S. Nanoparticles and Nanofiltration for Wastewater Treatment: From Polluted to Fresh Water. Environ. Res. 2023, 238, 117114. [Google Scholar] [CrossRef]
- Afify, A.A.; Hassan, G.K.; Al-Hazmi, H.E.; Kamal, R.M.; Mohamed, R.M.; Drewnowski, J.; Majtacz, J.; Mąkinia, J.; El-Gawad, H.A. Electrochemical Production of Sodium Hypochlorite from Salty Wastewater Using a Flow-by Porous Graphite Electrode. Energies 2023, 16, 4754. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Lu, X.; Grubba, D.; Majtacz, J.; Badawi, M.; Mąkinia, J. Sustainable Nitrogen Removal in Anammox-Mediated Systems: Microbial Metabolic Pathways, Operational Conditions and Mathematical Modelling. Sci. Total Environ. 2023, 868, 161633. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Shokrani, H.; Shokrani, A.; Jabbour, K.; Abida, O.; Mousavi Khadem, S.S.; Habibzadeh, S.; Sonawane, S.H.; Saeb, M.R.; Bonilla-Petriciolet, A.; et al. Recent Advances in Aqueous Virus Removal Technologies. Chemosphere 2022, 305, 135441. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Kot-Wasik, A.; Shokrani, A.; Majtacz, J.; Vatanpour, V.; Munir, M.T.; Habibzadeh, S.; Hejna, A.; Hasanpour, M.; Mohammadi, A.; et al. Diving Boldly into COVID-19 Contaminated Wastewater: Eyes at Nanotechnology-Assisted Solutions. Clin. Transl. Discov. 2023, 3, e195. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Maktabifard, M.; Grubba, D.; Majtacz, J.; Hassan, G.K.; Lu, X.; Piechota, G.; Mannina, G.; Bott, C.B.; Mąkinia, J. An Advanced Synergy of Partial Denitrification-Anammox for Optimizing Nitrogen Removal from Wastewater: A Review. Bioresour. Technol. 2023, 381, 129168. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Rajput, R.; Misra, K. Chapter 9—Status of Arsenic Remediation in India. In Advances in Water Purification Techniques; Ahuja, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–258. ISBN 978-0-12-814790-0. [Google Scholar]
- Algieri, C.; Pugliese, V.; Coppola, G.; Curcio, S.; Calabro, V.; Chakraborty, S. Arsenic Removal from Groundwater by Membrane Technology: Advantages, Disadvantages, and Effect on Human Health. Groundw. Sustain. Dev. 2022, 19, 100815. [Google Scholar] [CrossRef]
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of Uncontrolled Fertilization and Heavy Metal Toxicity Associated with Arsenic(As), Lead(Pb) and Cadmium (Cd), and Possible Remediation. Toxicology 2022, 477, 153274. [Google Scholar] [CrossRef]
- Vasireddy, D. Arsenic Adsorption onto Iron-Chitosan Composite from Drinking Water. Ph.D. Thesis, University of Missouri, Columbia, MO, USA, 2005. [Google Scholar]
- Hashmi, M.Z.; Ahmed, Z.; Rehman, S.U.; Reka, A.A. A Review on Arsenic Status in Environmental Compartments from Pakistan. Arab. J. Geosci 2023, 16, 130. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Batbayar, G.; Hofmann, J.; Siegfried, K.; Karthe, D.; Hahn-Tomer, S. Investigating Arsenic (As) Occurrence and Sources in Ground, Surface, Waste and Drinking Water in Northern Mongolia. Environ. Earth Sci. 2015, 73, 649–662. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A Meta-Analysis of the Distribution, Sources and Health Risks of Arsenic-Contaminated Groundwater in Pakistan. Environ. Pollut. 2018, 242, 307–319. [Google Scholar] [CrossRef]
- Pezeshki, H.; Hashemi, M.; Rajabi, S. Removal of Arsenic as a Potentially Toxic Element from Drinking Water by Filtration: A Mini Review of Nanofiltration and Reverse Osmosis Techniques. Heliyon 2023, 9, e14246. [Google Scholar] [CrossRef]
- Fu, D.; Kurniawan, T.A.; Li, H.; Wang, H.; Wang, Y.; Li, Q. Co-Oxidative Removal of Arsenite and Tetracycline Based on a Heterogeneous Fenton-like Reaction Using Iron Nanoparticles-Impregnated Biochar. Environ. Pollut. 2021, 290, 118062. [Google Scholar] [CrossRef]
- Ghorbanzadeh, N.; Jung, W.; Halajnia, A.; Lakzian, A.; Kabra, A.N.; Jeon, B.-H. Removal of Arsenate and Arsenite from Aqueous Solution by Adsorption on Clay Minerals. Geosyst. Eng. 2015, 18, 302–311. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and Adsorption Capacities of Low-Cost Sorbents for Wastewater Treatment: A Review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Khan Khanzada, A.; Al-Hazmi, H.E.; Śniatała, B.; Muringayil Joseph, T.; Majtacz, J.; Abdulrahman, S.A.M.; Albaseer, S.S.; Kurniawan, T.A.; Rahimi-Ahar, Z.; Habibzadeh, S.; et al. Hydrochar-Nanoparticle Integration for Arsenic Removal from Wastewater: Challenges, Possible Solutions, and Future Horizon. Environ. Res. 2023, 238, 117164. [Google Scholar] [CrossRef]
- Yoganandham, S.T.; Sathyamoorthy, G.; Renuka, R.R. Chapter 8—Emerging Extraction Techniques: Hydrothermal Processing. In Sustainable Seaweed Technologies; Advances in Green and Sustainable Chemistry; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 191–205. ISBN 978-0-12-817943-7. [Google Scholar]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal Carbonization of Renewable Waste Biomass for Solid Biofuel Production: A Discussion on Process Mechanism, the Influence of Process Parameters, Environmental Performance and Fuel Properties of Hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, D.; Jian, Q.; Wang, X.; Zheng, X.; Xue, G.; Liu, Y.; Li, X.; Hassan, G.K. Treatment of Industrial Ferric Sludge through a Facile Acid-Assisted Hydrothermal Reaction: Focusing on Dry Mass Reduction and Hydrochar Recyclability Performance. Sci. Total Environ. 2023, 869, 161879. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Fu, Y.; Sun, X.; Li, T.; Yang, C. Characterization of Dissolved Organic Matter in Biochar Derived from Various Macroalgae (Phaeophyta, Rhodophyta, and Chlorophyta): Effects of Pyrolysis Temperature and Extraction Solution pH. Sci. Total Environ. 2023, 869, 161786. [Google Scholar] [CrossRef]
- Yameen, M.Z.; AlMohamadi, H.; Naqvi, S.R.; Noor, T.; Chen, W.-H.; Amin, N.A.S. Advances in Production & Activation of Marine Macroalgae-Derived Biochar Catalyst for Sustainable Biodiesel Production. Fuel 2023, 337, 127215. [Google Scholar] [CrossRef]
- Xia, C.; Cai, L.; Zhang, H.; Zuo, L.; Shi, S.Q.; Lam, S.S. A Review on the Modeling and Validation of Biomass Pyrolysis with a Focus on Product Yield and Composition. Biofuel Res. J. 2021, 8, 1296–1315. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.-S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A Comprehensive Review of Engineered Biochar: Production, Characteristics, and Environmental Applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Peng, W.; Wong, C.C.; Liew, R.K.; Ho, Y.L.; Wan Mahari, W.A.; Azwar, E.; Yuan, T.Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Engineered Biochar via Microwave CO2 and Steam Pyrolysis to Treat Carcinogenic Congo Red Dye. J. Hazard. Mater. 2020, 395, 122636. [Google Scholar] [CrossRef]
- Wen, Z.; Xi, J.; Lu, J.; Zhang, Y.; Cheng, G.; Zhang, Y.; Chen, R. Porous Biochar-Supported MnFe2O4 Magnetic Nanocomposite as an Excellent Adsorbent for Simultaneous and Effective Removal of Organic/Inorganic Arsenic from Water. J. Hazard. Mater. 2021, 411, 124909. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lamb, D.; Rahman, M.M.; Bahar, M.M.; Sanderson, P.; Abbasi, S.; Bari, A.S.M.F.; Naidu, R. Removal of Arsenate from Contaminated Waters by Novel Zirconium and Zirconium-Iron Modified Biochar. J. Hazard. Mater. 2021, 409, 124488. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Chen, H.; Cai, T.; Liu, Z. Hydrochar and Pyrochar for Sorption of Pollutants in Wastewater and Exhaust Gas: A Critical Review. Environ. Pollut. 2021, 268, 115910. [Google Scholar] [CrossRef]
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical Activation of Biochar for Energy and Environmental Applications: A Comprehensive Review. Rev. Chem. Eng. 2019, 35, 777–815. [Google Scholar] [CrossRef]
- Capobianco, L.; Di Caprio, F.; Altimari, P.; Astolfi, M.L.; Pagnanelli, F. Production of an Iron-Coated Adsorbent for Arsenic Removal by Hydrothermal Carbonization of Olive Pomace: Effect of the Feedwater pH. J. Environ. Manag. 2020, 273, 111164. [Google Scholar] [CrossRef]
- Chen, H.; Xu, J.; Lin, H.; Zhao, X.; Shang, J.; Liu, Z. Arsenic Removal via a Novel Hydrochar from Livestock Waste Co-Activated with Thiourea and γ-Fe2O3 Nanoparticles. J. Hazard. Mater. 2021, 419, 126457. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Hydrothermal Processing of Algal Biomass for the Production of Biofuels and Chemicals. Biofuels 2012, 3, 603–623. [Google Scholar] [CrossRef]
- Patel, N.; Acharya, B.; Basu, P. Hydrothermal Carbonization (HTC) of Seaweed (Macroalgae) for Producing Hydrochar. Energies 2021, 14, 1805. [Google Scholar] [CrossRef]
- Rasam, S.; Talebkeikhah, F.; Talebkeikhah, M.; Salimi, A.; Moraveji, M.K. Physico-Chemical Properties Prediction of Hydrochar in Macroalgae Sargassum Horneri Hydrothermal Carbonisation. Int. J. Environ. Anal. Chem. 2021, 101, 2297–2318. [Google Scholar] [CrossRef]
- Spagnuolo, D.; Iannazzo, D.; Len, T.; Balu, A.M.; Morabito, M.; Genovese, G.; Espro, C.; Bressi, V. Hydrochar from Sargassum Muticum: A Sustainable Approach for High-Capacity Removal of Rhodamine B Dye. RSC Sustain. 2023, 1, 1404–1415. [Google Scholar] [CrossRef]
- Spagnuolo, D.; Bressi, V.; Chiofalo, M.T.; Morabito, M.; Espro, C.; Genovese, G.; Iannazzo, D.; Trifilò, P. Using the Aqueous Phase Produced from Hydrothermal Carbonization Process of Brown Seaweed to Improve the Growth of Phaseolus Vulgaris. Plants 2023, 12, 2745. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Xing, Y.; Liu, B.; Zhou, Y.; Lu, P. Role of Nitrate in the Production of Iron-Modified Hydrochar for Arsenic Removal. Res. Sq. 2023. preprint. [Google Scholar] [CrossRef]
- Ghanizadeh, G.; Ehrampoush, M.H.; Ghaneian, M.T. Application of iron impregnated activated carbon for removal of arsenic from water. J. Environ. Health Sci. Eng. 2010, 7, 145–156. [Google Scholar]
- Park, Y.R.; Hong, S.H.; Kim, J.H.; Park, J.Y. Arsenic Removal Using the Surface Modified Granular Activated Carbon Treated with Ferric Chloride. J. Korean Soc. Water Wastewater 2012, 26, 77–85. [Google Scholar] [CrossRef]
- Xu, Q.; Qian, Q.; Quek, A.; Ai, N.; Zeng, G.; Wang, J. Hydrothermal Carbonization of Macroalgae and the Effects of Experimental Parameters on the Properties of Hydrochars. ACS Sustain. Chem. Eng. 2013, 1, 1092–1101. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation Review of Lagergren Kinetic Rate Equation on Adsorption Reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Uçar, S.; Erdem, M.; Tay, T.; Karagöz, S. Preparation and Characterization of Activated Carbon Produced from Pomegranate Seeds by ZnCl2 Activation. Appl. Surf. Sci. 2009, 255, 8890–8896. [Google Scholar] [CrossRef]
- Petrović, J.T.; Stojanović, M.D.; Milojković, J.V.; Petrović, M.S.; Šoštarić, T.D.; Laušević, M.D.; Mihajlović, M.L. Alkali Modified Hydrochar of Grape Pomace as a Perspective Adsorbent of Pb2+ from Aqueous Solution. J. Environ. Manag. 2016, 182, 292–300. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, H.; Sheng, K.; Mustafa, A.M.; Yu, Y. Evaluation of Engineered Hydrochar from KMnO4 Treated Bamboo Residues: Physicochemical Properties, Hygroscopic Dynamics, and Morphology. Bioresour. Technol. 2018, 250, 806–811. [Google Scholar] [CrossRef]
- Wu, L.M.; Zhou, C.H.; Tong, D.S.; Yu, W.H.; Wang, H. Novel Hydrothermal Carbonization of Cellulose Catalyzed by Montmorillonite to Produce Kerogen-like Hydrochar. Cellulose 2014, 21, 2845–2857. [Google Scholar] [CrossRef]
- Ma, Q.; Cui, L.; Zhou, S.; Li, Y.; Shi, W.; Ai, S. Iron Nanoparticles in Situ Encapsulated in Lignin-Derived Hydrochar as an Effective Catalyst for Phenol Removal. Environ. Sci. Pollut. Res. 2018, 25, 20833–20840. [Google Scholar] [CrossRef]
- Lei, Y.; Su, H.; Tian, F. A Novel Nitrogen Enriched Hydrochar Adsorbents Derived from Salix Biomass for Cr (VI) Adsorption. Sci. Rep. 2018, 8, 4040. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, Y.; Gu, X.; Zhao, Y. Adsorption Behavior and Mechanism of Different Arsenic Species on Mesoporous MnFe2O4 Magnetic Nanoparticles. Chemosphere 2017, 181, 328–336. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Xionghui, J.; Ma, L. Efficient Arsenate Removal by Magnetite-Modified Water Hyacinth Biochar. Environ. Pollut. 2016, 216, 575–583. [Google Scholar] [CrossRef]
- Xu, M.; Qin, Y.; Huang, Q.; Beiyuan, J.; Li, H.; Chen, W.; Wang, X.; Wang, S.; Yang, F.; Yuan, W.; et al. Arsenic Adsorption by Different Fe-Enriched Biochars Conditioned with Sulfuric Acid. Environ. Sci. Pollut. Res. 2022, 30, 16398–16407. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Saqib, Z.A.; Nawaz, M.F.; Shaheen, S.M.; Wang, H.; Tsang, D.C.W.; Bundschuh, J.; et al. Exploring the Arsenic Removal Potential of Various Biosorbents from Water. Environ. Int. 2019, 123, 567–579. [Google Scholar] [CrossRef]
- Gräfe, M. Arsenic Adsorption on Iron Oxides in the Presence of Soluble Organic Carbon and the Influence of Arsenic on Radish and Lettuce Plant Development. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2002. [Google Scholar]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-Functionalized Graphene Oxide: A Novel Adsorbent an Efficient Adsorption of Arsenic from Aqueous Solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Jin, X.; Yu, C.; Li, Y.; Qi, Y.; Yang, L.; Zhao, G.; Hu, H. Preparation of Novel Nano-Adsorbent Based on Organic–Inorganic Hybrid and Their Adsorption for Heavy Metals and Organic Pollutants Presented in Water Environment. J. Hazard. Mater. 2011, 186, 1672–1680. [Google Scholar] [CrossRef]
- Vindevoghel, P.; Guyot, A. Suspended Emulsion Copolymerization of Acrylonitrile and Methyl Acrylate. Polym. React. Eng. 1995, 3, 23–42. [Google Scholar] [CrossRef]
- Kamala, C.T.; Chu, K.H.; Chary, N.S.; Pandey, P.K.; Ramesh, S.L.; Sastry, A.R.K.; Sekhar, K.C. Removal of Arsenic(III) from Aqueous Solutions Using Fresh and Immobilized Plant Biomass. Water Res. 2005, 39, 2815–2826. [Google Scholar] [CrossRef]
- Abtahi, M.; Mesdaghinia, A.; Saeedi, R.; Nazmara, S. Biosorption of As(III) and As(V) from Aqueous Solutions by Brown Macroalga Colpomenia Sinuosa Biomass: Kinetic and Equilibrium Studies. Desalination Water Treat. 2013, 51, 3224–3232. [Google Scholar] [CrossRef]
- Genç-Fuhrman, H.; Tjell, J.C.; McConchie, D. Adsorption of Arsenic from Water Using Activated Neutralized Red Mud. Environ. Sci. Technol. 2004, 38, 2428–2434. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Ghaffari, H.R.; Samadi, M.T. A comparative study on arsenic (III) removal from aqueous solution using nano and micro sized zero-valent iron. J. Environ. Health Sci. Eng. 2011, 8, 157–166. [Google Scholar]
- Gao, Q.; Wang, L.; Li, Z.; Xie, Y.; He, Q.; Wang, Y. Adsorptive Removal of Pyridine in Simulation Wastewater Using Coke Powder. Processes 2019, 7, 459. [Google Scholar] [CrossRef]
- Irshad, S.; Xie, Z.; Mehmood, S.; Nawaz, A.; Ditta, A.; Mahmood, Q. Insights into Conventional and Recent Technologies for Arsenic Bioremediation: A Systematic Review. Environ. Sci. Pollut. Res. 2021, 28, 18870–18892. [Google Scholar] [CrossRef]
- Mandal, S.; Sahu, M.K.; Patel, R.K. Adsorption Studies of Arsenic(III) Removal from Water by Zirconium Polyacrylamide Hybrid Material (ZrPACM-43). Water Resour. Ind. 2013, 4, 51–67. [Google Scholar] [CrossRef]
- Li, Q.; Xu, X.; Cui, H.; Pang, J.; Wei, Z.; Sun, Z.; Zhai, J. Comparison of Two Adsorbents for the Removal of Pentavalent Arsenic from Aqueous Solutions. J. Environ. Manag. 2012, 98, 98–106. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; Hefny, M.; El-Chaghaby, G.A.F. Removal of Lead from Aqueous Solution Using Low Cost Abundantly Available Adsorbents. Int. J. Environ. Sci. Technol. 2007, 4, 67–73. [Google Scholar] [CrossRef]
- Dudek, S.; Kołodyńska, D. Enhanced Arsenic(V) Removal on an Iron-Based Sorbent Modified by Lanthanum(III). Materials 2020, 13, 2553. [Google Scholar] [CrossRef]
- Attinti, R.; Sarkar, D.; Barrett, K.R.; Datta, R. Adsorption of Arsenic(V) from Aqueous Solutions by Goethite/Silica Nanocomposite. Int. J. Environ. Sci. Technol. 2015, 12, 3905–3914. [Google Scholar] [CrossRef]
- Patiha; Heraldy, E.; Hidayat, Y.; Firdaus, M. The Langmuir Isotherm Adsorption Equation: The Monolayer Approach. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012067. [Google Scholar] [CrossRef]
- Gunasundari, E.; Senthil Kumar, P. Adsorption Isotherm, Kinetics and Thermodynamic Analysis of Cu(II) Ions onto the Dried Algal Biomass (Spirulina Platensis). J. Ind. Eng. Chem. 2017, 56, 129–144. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Saad, E.A.; Soliman, M.A.; Abdelwahab, M.S. Synthesis and Surface Protection of Nano Zerovalent Iron (NZVI) with 3-Aminopropyltrimethoxysilane for Water Remediation of Cobalt and Zinc and Their Radioactive Isotopes. RSC Adv. 2016, 6, 66242–66251. [Google Scholar] [CrossRef]
- Altundoğan, H.S.; Altundoğan, S.; Tümen, F.; Bildik, M. Arsenic Adsorption from Aqueous Solutions by Activated Red Mud. Waste Manag. 2002, 22, 357–363. [Google Scholar] [CrossRef]
- Goswami, A.; Raul, P.K.; Purkait, M.K. Arsenic Adsorption Using Copper (II) Oxide Nanoparticles. Chem. Eng. Res. Des. 2012, 90, 1387–1396. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C.T. Mechanisms of Arsenic Adsorption on Amorphous Oxides Evaluated Using Macroscopic Measurements, Vibrational Spectroscopy, and Surface Complexation Modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Aghdasinia, H.; Mohammadi, R.; Ramavandi, B. Modification of Bio-Hydroxyapatite Generated from Waste Poultry Bone with MgO for Purifying Methyl Violet-Laden Liquids. Environ. Sci. Pollut. Res. 2020, 27, 44218–44229. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Synthesis and Characterization of Multi-Amino-Functionalized Cellulose for Arsenic Adsorption. Carbohydr. Polym. 2013, 92, 380–387. [Google Scholar] [CrossRef]
- Chaudhry, S.A.; Zaidi, Z.; Siddiqui, S.I. Isotherm, Kinetic and Thermodynamics of Arsenic Adsorption onto Iron-Zirconium Binary Oxide-Coated Sand (IZBOCS): Modelling and Process Optimization. J. Mol. Liq. 2017, 229, 230–240. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, W.; Hu, L.; Ding, W.; Wu, F.; Li, J. Etching Synthesis of Iron Oxide Nanoparticles for Adsorption of Arsenic from Water. RSC Adv. 2016, 6, 15900–15910. [Google Scholar] [CrossRef]
- Thy, L.T.M.; Thuong, N.H.; Tu, T.H.; My, N.H.T.; Tuong, H.H.P.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Fabrication and Adsorption Properties of Magnetic Graphene Oxide Nanocomposites for Removal of Arsenic (V) from Water. Adsorpt. Sci. Technol. 2020, 38, 240–253. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y.; Adil, S. Polymeric Nanocomposites of Iron–Oxide Nanoparticles (IONPs) Synthesized Using Terminalia Chebula Leaf Extract for Enhanced Adsorption of Arsenic(V) from Water. Colloids Interfaces 2019, 3, 17. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Liu, S.; Liu, H.; Zeng, G.; Tan, X.; Yang, C.; Ding, Y.; Yan, Z.; Cai, X. Sorption Performance and Mechanisms of Arsenic(V) Removal by Magnetic Gelatin-Modified Biochar. Chem. Eng. J. 2017, 314, 223–231. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Núñez-Delgado, A.; Ali, S.; Qaswar, M.; Shakoor, A.; Maitlo, A.A.; Chen, D.-Y. Adsorption of Arsenic (III) from Aqueous Solution by a Novel Phosphorus-Modified Biochar Obtained from Taraxacum Mongolicum Hand-Mazz: Adsorption Behavior and Mechanistic Analysis. J. Environ. Manag. 2021, 292, 112764. [Google Scholar] [CrossRef]
- He, X.; Jiang, J.; Hong, Z.; Pan, X.; Dong, Y.; Xu, R. Effect of Aluminum Modification of Rice Straw–Based Biochar on Arsenate Adsorption. J. Soils Sediments 2020, 20, 3073–3082. [Google Scholar] [CrossRef]
- Priyadarshni, N.; Nath, P.; Nagahanumaiah; Chanda, N. Sustainable Removal of Arsenate, Arsenite and Bacterial Contamination from Water Using Biochar Stabilized Iron and Copper Oxide Nanoparticles and Associated Mechanism of the Remediation Process. J. Water Process Eng. 2020, 37, 101495. [Google Scholar] [CrossRef]
- Wu, C.; Huang, L.; Xue, S.-G.; Huang, Y.-Y.; Hartley, W.; Cui, M.; Wong, M.-H. Arsenic Sorption by Red Mud-Modified Biochar Produced from Rice Straw. Environ. Sci. Pollut. Res. 2017, 24, 18168–18178. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Karunanayake, A.G.; Gunatilake, S.R.; Pittman, C.U.; Perez, F.; Mohan, D.; Mlsna, T. Removal of Arsenic(III) from Water Using Magnetite Precipitated onto Douglas Fir Biochar. J. Environ. Manag. 2019, 250, 109429. [Google Scholar] [CrossRef]
- Alkurdi, S.S.A.; Herath, I.; Bundschuh, J.; Al-Juboori, R.A.; Vithanage, M.; Mohan, D. Biochar versus Bone Char for a Sustainable Inorganic Arsenic Mitigation in Water: What Needs to Be Done in Future Research? Environ. Int. 2019, 127, 52–69. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y. Enhanced Arsenic Removal by Biochar Modified with Nickel (Ni) and Manganese (Mn) Oxyhydroxides. J. Ind. Eng. Chem. 2016, 37, 361–365. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption Mechanisms of Ibuprofen and Naproxen to UiO-66 and UiO-66-NH2: Batch Experiment and DFT Calculation. Chem. Eng. J. 2019, 360, 645–653. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, Y.; Qiu, W.; Sun, Z.; Liu, Z.; Song, Z. Adsorption Properties of Nano-MnO2–Biochar Composites for Copper in Aqueous Solution. Molecules 2017, 22, 173. [Google Scholar] [CrossRef]
- Chen, H.; Xu, J.; Lin, H.; Wang, Z.; Liu, Z. Multi-Cycle Aqueous Arsenic Removal by Novel Magnetic N/S-Doped Hydrochars Activated via One-Pot and Two-Stage Schemes. Chem. Eng. J. 2022, 429, 132071. [Google Scholar] [CrossRef]
- Huang, Y.; Gao, M.; Deng, Y.; Khan, Z.H.; Liu, X.; Song, Z.; Qiu, W. Efficient Oxidation and Adsorption of As(III) and As(V) in Water Using a Fenton-like Reagent, (Ferrihydrite)-Loaded Biochar. Sci. Total Environ. 2020, 715, 136957. [Google Scholar] [CrossRef]


| Materials | As Species | Pseudo-First-Order Model Parameters | Pseudo-Second-Order Model Parameters | Intra-Particle Diffusion Model Parameters | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K1 (min−1) | qe. Cal. (mg/g) | R2 | K2 (mg g−1 min−1) | qe. Cal. (mg/g) | R2 | K(diff) (mg g−1 min−1/2) | C (mg/g) | R2 | |||
| Activated hydrochar | As (III/V) | −0.0053 | 0.078 | 0.9567 | 2.3452 | 0.156 | 0.991 | 0.00322 | 0.17051 | 0.6189 | This study |
| Untreated red-mud biochar | As (V) | 1.195 | 451.4 | 0.983 | 0.00357 | 482.9 | 0.987 | 0.062 | 0.233 | 0.6664 | [86] |
| Red-mud-modified biochar | As (V) | 1.446 | 1656.5 | 0.900 | 0.00126 | 1758.6 | 0.957 | 0.212 | 0.9087 | 0.7298 | |
| Untreated red-mud biochar | As (III) | 0.805 | 296.0 | 0.948 | 0.00366 | 319.5 | 0.960 | 0.048.95 | 0.115 | 0.7662 | |
| Red-mud-modified biochar | As (III) | 0.686 | 377.9 | 0.911 | 0.00236 | 412.0 | 0.927 | 0.6657 | 0.132 | 0.8842 | |
| Iron oxide nanoparticles | As (V) | 0.50 | 0.66 | 0.92 | 4.3 | 4.0 | 0.99 | N.A. | N.A. | N.A. | [81] |
| Phosphorus (P)-modified biochar (PLBC)/Taraxacum mongolicum Hand-Mazz | As (III) | 0.23 ± 0.028 | 16.3 ± 0.4 | 0.972 | 0.020 ± 0.002 | 17.1 ± 0.4 | 0.997 | N.A. | N.A. | N.A. | [83] |
| Magnetic gelatin-modified biochar | As (V) | 0.03 | 26.64 | 0.87 | 0.00142 | 28.389 | 0.92 | N.A. | N.A. | N.A. | [82] |
| Magnetic Fe3O4/Douglas fir biochar composites | As (III) | N.A. | N.A. | N.A. | 0.337 | 1.30 | 0.9960 | N.A. | N.A. | N.A. | [87] |
| Magnetic Fe3O4/Douglas fir biochar composites | As (III) | N.A. | N.A. | N.A. | 0.319 | 3.75 | 0.9999 | N.A. | N.A. | N.A. | |
| Magnetic Fe3O4/Douglas fir biochar composites | As (III) | N.A. | N.A. | N.A. | 0.049 | 6.15 | 0.9894 | N.A. | N.A. | N.A. | |
| Rice-husk biochar-stabilized iron and copper oxide nanoparticles | As (III/V) | 0.17 | 1.75 | 0.839 | 0.09 | 6.84 | 0.999 | [85] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanzada, A.K.; Rizwan, M.; Al-Hazmi, H.E.; Majtacz, J.; Kurniawan, T.A.; Mąkinia, J. Removal of Arsenic from Wastewater Using Hydrochar Prepared from Red Macroalgae: Investigating Its Adsorption Efficiency and Mechanism. Water 2023, 15, 3866. https://doi.org/10.3390/w15213866
Khanzada AK, Rizwan M, Al-Hazmi HE, Majtacz J, Kurniawan TA, Mąkinia J. Removal of Arsenic from Wastewater Using Hydrochar Prepared from Red Macroalgae: Investigating Its Adsorption Efficiency and Mechanism. Water. 2023; 15(21):3866. https://doi.org/10.3390/w15213866
Chicago/Turabian StyleKhanzada, Aisha Khan, Muhammad Rizwan, Hussein E. Al-Hazmi, Joanna Majtacz, Tonni Agustiono Kurniawan, and Jacek Mąkinia. 2023. "Removal of Arsenic from Wastewater Using Hydrochar Prepared from Red Macroalgae: Investigating Its Adsorption Efficiency and Mechanism" Water 15, no. 21: 3866. https://doi.org/10.3390/w15213866








