Facilitating Wastewater Purification through Progressive Thawing by Microwave: Responses of Microbial Communities
Abstract
:1. Introduction
2. Materials and Methods
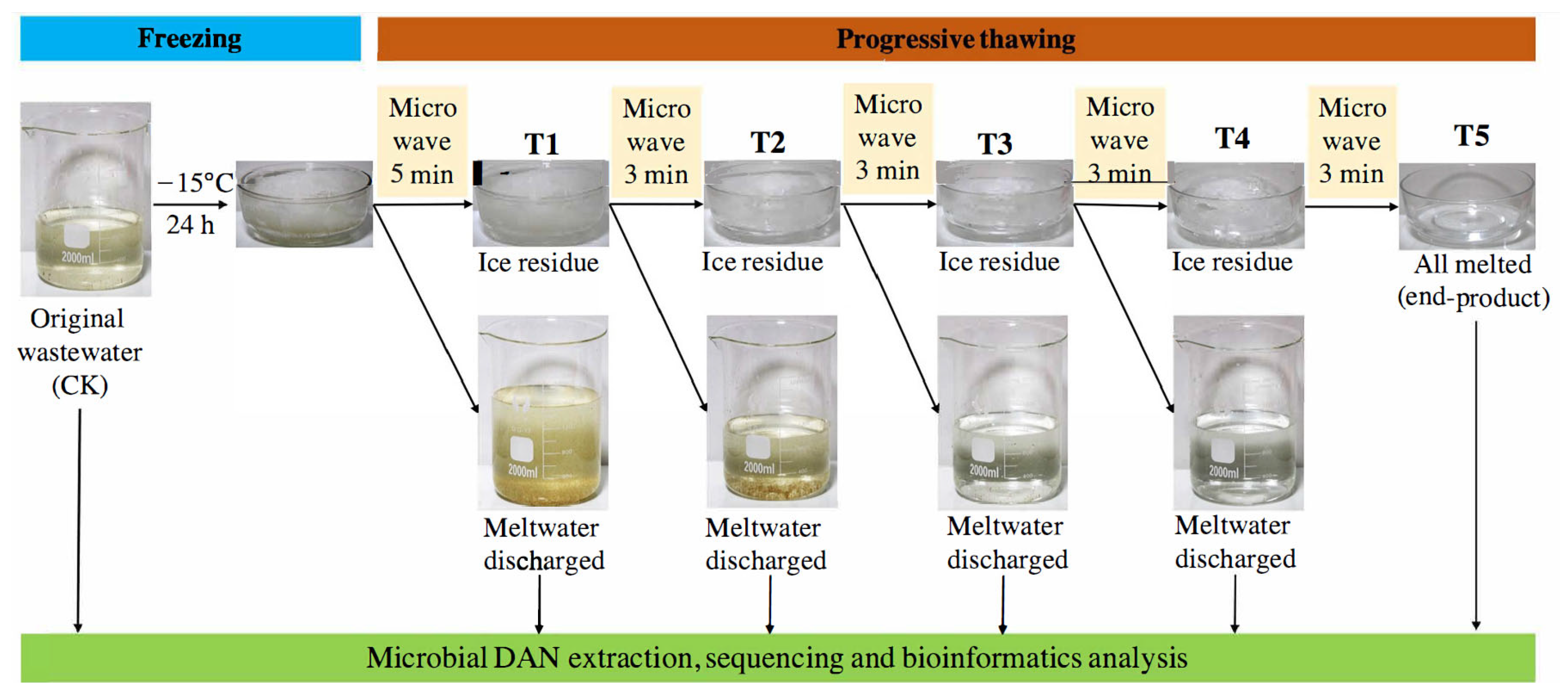
2.1. Wastewater Collection and Purification by Freeze–Thaw
2.2. Microbial DAN Extraction, Sequencing and Bioinformatics Analysis
2.3. Statistical Analysis and Plotting
3. Results
3.1. Purifications of Selected Materials over Progressive Thawing
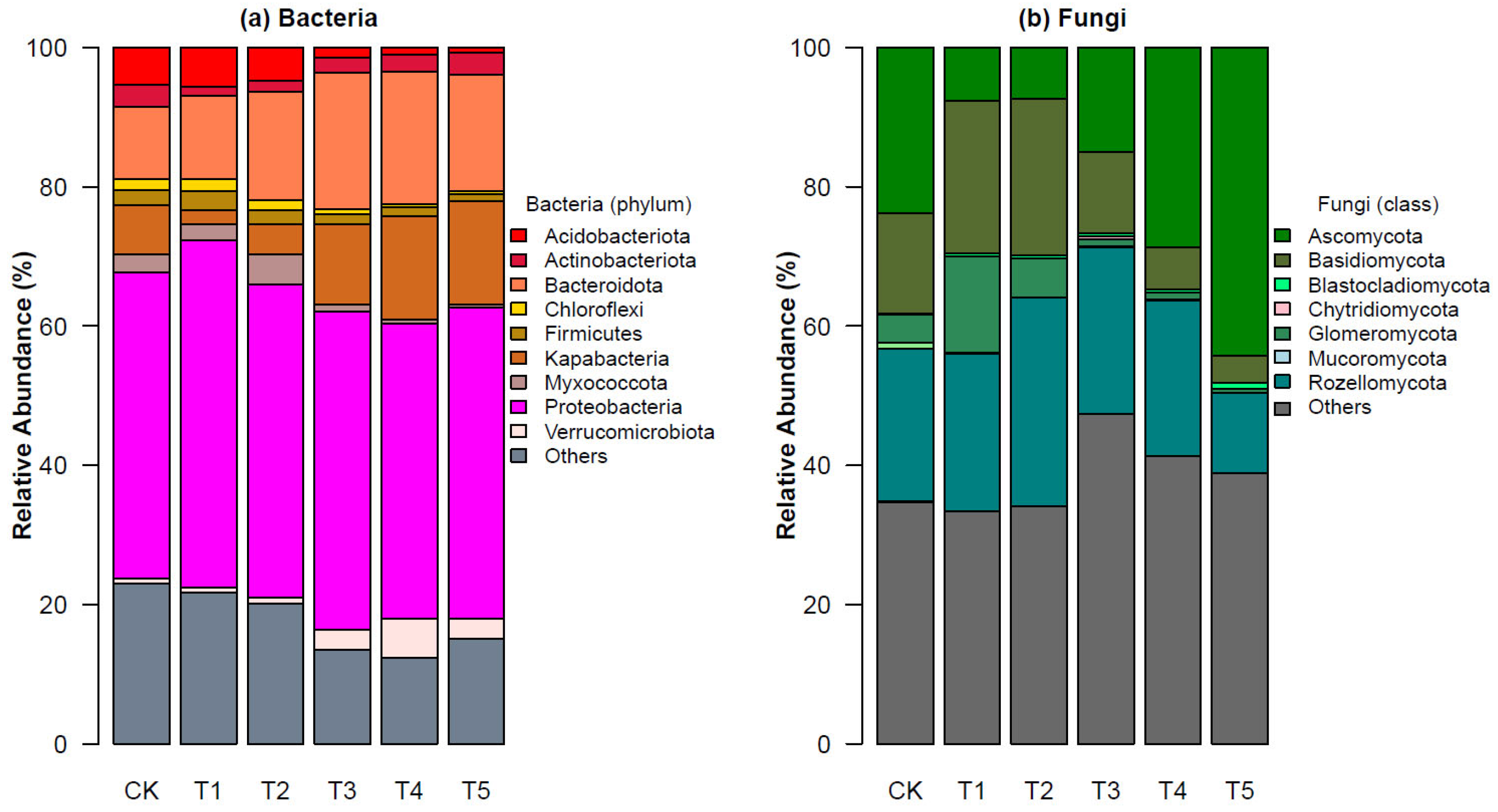
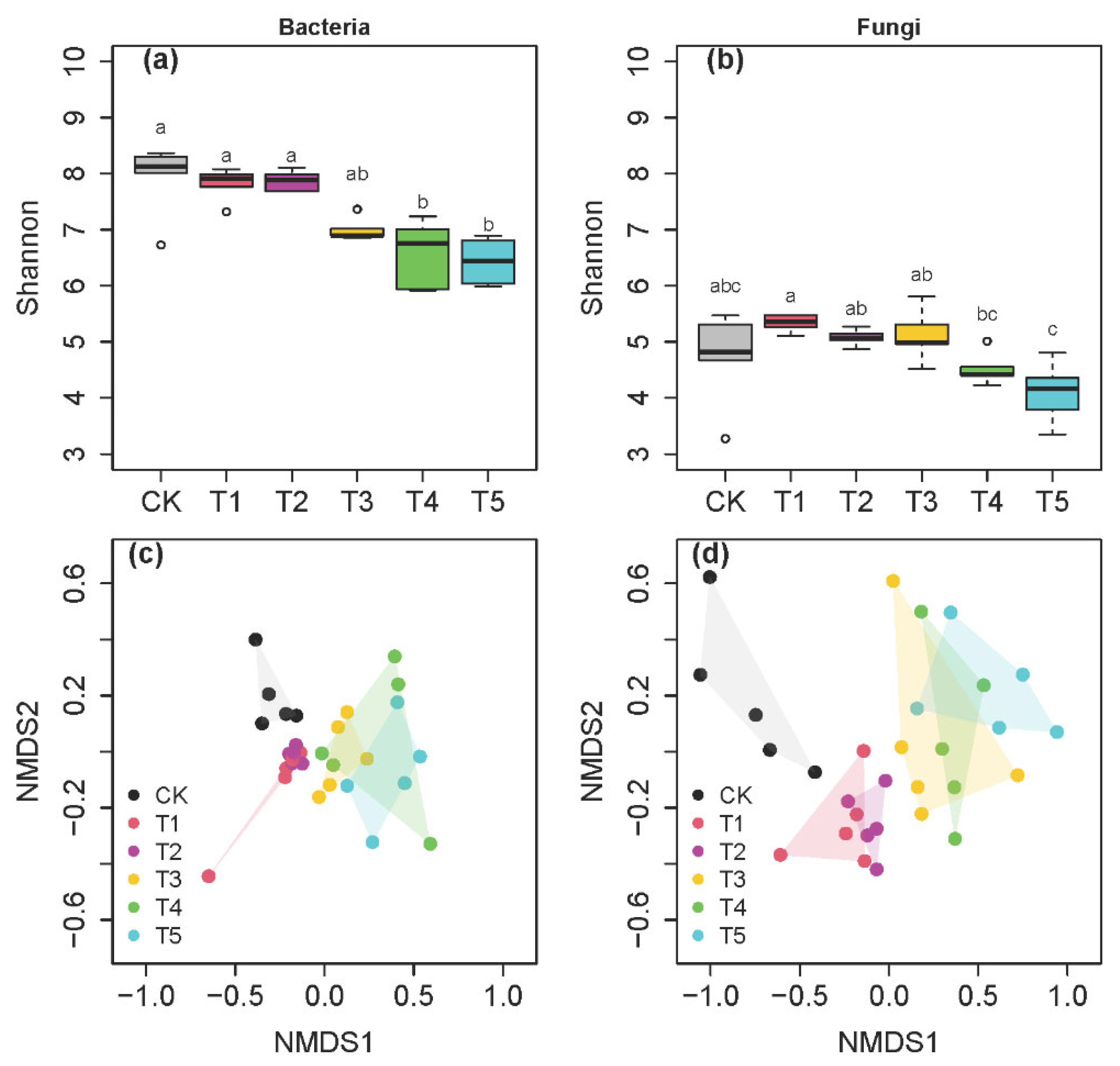
3.2. Bacterial and Fungal Compositions and Diversity across the Five Thawing Intervals
3.3. OTU-Based Co-Occurrence Networks
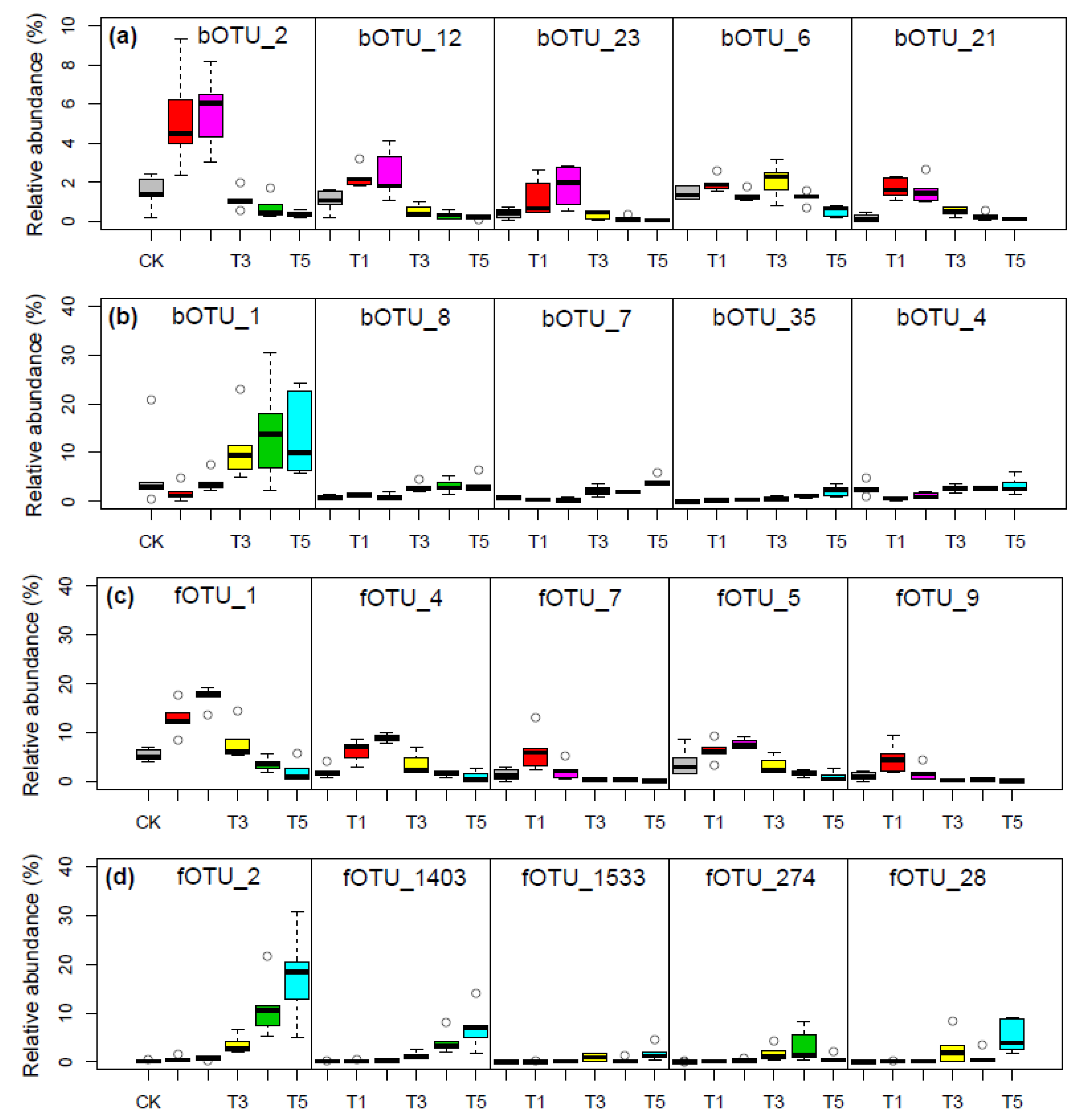
3.4. Removal and Enrichment of the Top-Abundant Microbes at the Five Thawing Intervals
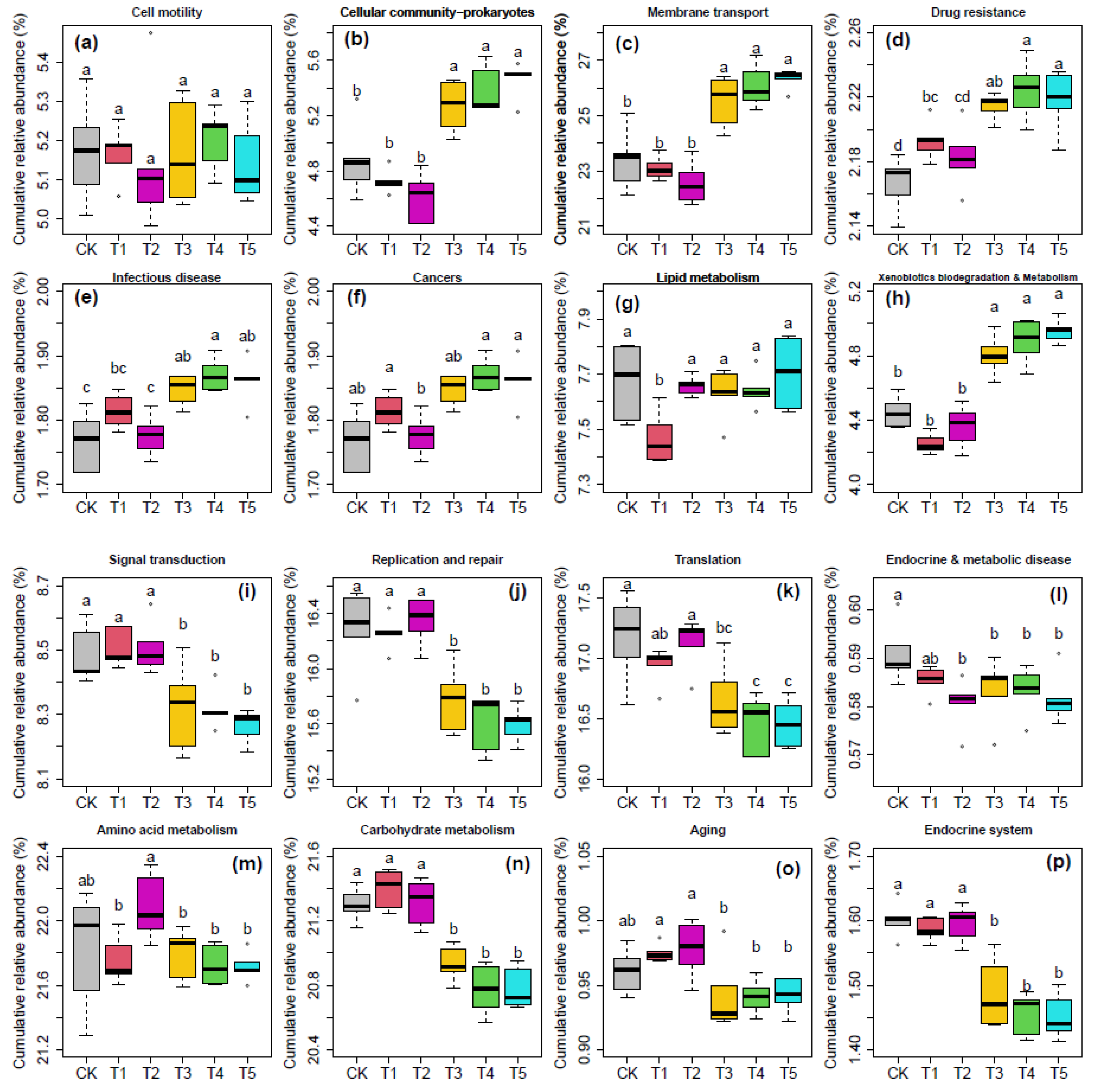
3.5. Cumulative Relative Abundances of Microbes with Different Functions
4. Discussion
4.1. Microwave Can Facilitate Wastewater Freeze–Thaw Purification Process
4.2. Unequal Responses of Different Microbial Subsets to Freezing and Progressive Thawing
4.3. Implications and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thebo, A.L.; Drechsel, P.; Lambin, E.F.; Nelson, K.L. A Global, Spatially-Explicit Assessment of Irrigated Croplands Influenced by Urban Wastewater Flows. Environ. Res. Lett. 2017, 12, 74008. [Google Scholar] [CrossRef]
- Zhu, Y.; Gillings, M.; Simonet, P.; Stekel, D.; Banwart, C.H. Microbial Mass Movements. Science 2017, 357, 1099–1100. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. A Review of Wastewater Irrigation: Environmental Implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar] [CrossRef]
- Chen, L.C.; Chian, C.Y.; Yen, P.S.; Chu, C.P.; Lee, D.J. High-Speed Sludge Freezing. Water Res. 2001, 35, 3502–3507. [Google Scholar] [CrossRef] [PubMed]
- Chu, C. Reduction of Microbial Density Level in Wastewater Activated Sludge via Freezing and Thawing. Water Res. 1999, 33, 3532–3535. [Google Scholar] [CrossRef]
- Gao, W.; Shao, Y. Freeze Concentration for Removal of Pharmaceutically Active Compounds in Water. Desalination 2009, 249, 398–402. [Google Scholar] [CrossRef]
- Melak, F.; Du Laing, G.; Ambelu, A.; Alemayehu, E. Application of Freeze Desalination for Chromium (VI) Removal from Water. Desalination 2016, 377, 23–27. [Google Scholar] [CrossRef]
- Mtombeni, T.; Maree, J.P.; Zvinowanda, C.M.; Asante, J.K.O.; Oosthuizen, F.S.; Louw, W.J. Evaluation of the Performance of a New Freeze Desalination Technology. Int. J. Environ. Sci. Technol. 2013, 10, 545–550. [Google Scholar] [CrossRef]
- Tao, T.; Peng, X.F.; Lee, D.J. Interaction between Wastewater-Sludge Floc and Moving Ice Front. Chem. Eng. Sci. 2006, 61, 5369–5376. [Google Scholar] [CrossRef]
- John, M.; Häkkinen, A.; Louhi-Kultanen, M. Purification Efficiency of Natural Freeze Crystallization for Urban Wastewaters. Cold Reg. Sci. Technol. 2020, 170, 102953. [Google Scholar] [CrossRef]
- Vrbka, L.; Jungwirth, P. Brine Rejection from Freezing Salt Solutions: A Molecular Dynamics Study. Phys. Rev. Lett. 2005, 95, 148501. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.-M. Advances in Seawater Desalination Technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Parker, L.V.; Yushak, M.L.; Martel, C.J.; Reynolds, C.M. Bacterial Survival in Snow Made from Wastewater; Cold Regions Research & Engineering Laboratory, US Army Corps of Engineers: Minneapolis, MN, USA, 2000; pp. 1–15. [Google Scholar]
- Gao, W.; Smith, D.W.; Li, Y. Natural Freezing as a Wastewater Treatment Method: E. Coli Inactivation Capacity. Water Res. 2006, 40, 2321–2326. [Google Scholar] [CrossRef]
- Gao, W.; Smith, D.W.; Li, Y. Effects of Freezing on the Survival of Escherichia Coli and Bacillus and Response to UV and Chlorine After Freezing. Water Environ. Res. 2007, 79, 507–513. [Google Scholar] [CrossRef]
- Parker, L.V.; Martel, C.J. Long-Term Survival of Enteric Microorganisms in Frozen Wastewater; Engineer Research and Development Center, US Army Corps of Engineers: Minneapolis, MN, USA, 2002; pp. 1–56. [Google Scholar]
- Gu, W.; Lin, Y.; Xu, Y.; Yuan, S.; Tao, J.; Li, L.; Liu, C. Sea Ice Desalination under the Force of Gravity in Low Temperature Environments. Desalination 2012, 295, 11–15. [Google Scholar] [CrossRef]
- Xie, L.; Ma, J.; Cheng, F.; Li, P.; Liu, J.; Chen, W.; Wang, S. Study on Sea Ice Desalination Technology. Desalination 2009, 245, 146–154. [Google Scholar] [CrossRef]
- Tang, W.; Shi, P.; Wang, J.; Zhang, H. Sea Ice Centrifugal Desalination Based on Microwave Heating. Desalination 2019, 449, 1–5. [Google Scholar] [CrossRef]
- Tang, W.; Tao, J.; Wang, J.; Liu, C.; Zhang, H. Sea Ice Desalination under Gravity Using Microwave Heating. Desalination 2018, 430, 159–164. [Google Scholar] [CrossRef]
- Liu, Y.; Kumblathan, T.; Uppal, G.K.; Zhou, A.; Moe, B.; Hrudey, S.E.; Li, X.-F. A Hidden Risk: Survival and Resuscitation of Escherichia Coli O157:H7 in the Viable but Nonculturable State after Boiling or Microwaving. Water Res. 2020, 183, 116102. [Google Scholar] [CrossRef]
- Zieliński, M.; Ciesielski, S.; Cydzik-Kwiatkowska, A.; Turek, J.; Dębowski, M. Influence of Microwave Radiation on Bacterial Community Structure in Biofilm. Process Biochem. 2007, 42, 1250–1253. [Google Scholar] [CrossRef]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.-C.; Schlaeppi, K. Cropping Practices Manipulate Abundance Patterns of Root and Soil Microbiome Members Paving the Way to Smart Farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, A.; Leyva-Diaz, J.C.; Gonzalez-Lopez, J.; Poyatos, J.M. Membrane Bioreactor and Hybrid Moving Bed Biofilm Reactor-Membrane Bioreactor for the Treatment of Variable Salinity Wastewater: Influence of Biomass Concentration and Hydraulic Retention Time. Chem. Eng. J. 2018, 336, 102–111. [Google Scholar] [CrossRef]
- Rodrigues, K.M.; Rodrigues, B.F. Chapter 27—Glomus. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 561–569. ISBN 978-0-12-823414-3. [Google Scholar]
- Powell, M.J.; Letcher, P.M.; James, T.Y. Ultrastructural Characterization of the Host–Parasite Interface between Allomyces Anomalus (Blastocladiomycota) and Rozella Allomycis (Cryptomycota). Fungal Biol. 2017, 121, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Dilek Sanin, F.; Aarne Vesilind, P.; James Martel, C. Pathogen Reduction Capabilities of Freeze/Thaw Sludge Conditioning. Water Res. 1994, 28, 2393–2398. [Google Scholar] [CrossRef]
- Rosenberg, E.; DeLong, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. (Eds.) The Prokaryotes: Alphaproteobacteria and Betaproteobacteria; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-30196-4. [Google Scholar]
- Bartram, J. (Ed.) Legionella and the Prevention of Legionellosis; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-156297-3. [Google Scholar]
- Shen, Q.; Wang, X.; Qu, F.; Xiao, Z.; Zhang, X.; Zhang, S. Responses of Soil Total Phosphorus to Freeze and Thaw Cycles in a Mollisol Watershed. Geoderma 2020, 376, 114571. [Google Scholar] [CrossRef]
- Yergeau, E.; Kowalchuk, G.A. Responses of Antarctic Soil Microbial Communities and Associated Functions to Temperature and Freeze–Thaw Cycle Frequency. Environ. Microbiol. 2008, 10, 2223–2235. [Google Scholar] [CrossRef]
- Zhou, Y.; Hiller, C.; Andersson, S.; Jakobsson, E.; Zhou, L.; Hawkes, J.A.; Kothawala, D.N.; Tranvik, L. Selective Exclusion of Aromatic Organic Carbon During Lake Ice Formation. Geophys. Res. Lett. 2023, 50, e2022GL101414. [Google Scholar] [CrossRef]
- Fujii, K.; Hayakawa, C. Fluxes of Dissolved Organic Matter and Nitrate and Their Contribution to Soil Acidification across Changing Permafrost Landscapes in Northwestern Canada. Geoderma 2023, 430, 116306. [Google Scholar] [CrossRef]


| pH | Turbidity (NTU) | Alkalinity (CaCO3 mg L−1) | Total Cation (mg L−1) | Total Anion (mg L−1) | Electrical Conductivity (μS cm−1) | Relative abundance of Escherichia coli (%) |
|---|---|---|---|---|---|---|
| 7.6 ± 0.1 | 12.6 ± 0.4 | 182.6 ± 1.2 | 185.9 ± 6.1 | 447.8 ± 5.7 | 804.7 ± 4.2 | 0.22 |
| T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|
| Interval (min) | 5 | 3 | 3 | 3 | 3 |
| Volume (mL) | 463.5 ± 53.9 a | 302.5 ± 38.7 c | 359.4 ± 21.7 b | 376.4 ± 22.3 b | 448.7 ± 82.1 a |
| Temperature (°C) | 6.7 ± 4.7 b | 19.3 ± 5.6 a | 20.6 ± 3.4 a | 20.4 ± 3.2 a | 20.2 ± 8.2 a |
| Turbidity (NTU) | 21.2 ± 2.3 | 17.1 ± 1.8 | 10 ± 1.3 | 8.67 ± 1.0 | 3.1 ± 0.2 |
| Electrical Conductivity (μS cm−1) | 1795.5 ± 182.6 a | 1163.4 ± 267.4 b | 676.8 ± 122.5 c | 294.6 ± 72.3 d | 66.1 ± 19.6 e |
| Removal rate of dissolved salt (%) | 53.1 ± 6.7 a | 22.1 ± 4.2 b | 15.6 ± 3.4 c | 7.1 ± 2.0 d | 1.99 ± 0.9 e |
| Removal rate of E. coli (%) | 40.4 ± 32.6 | 30.9 ± 31.3 | 18.5 ± 18.8 | 5.9 ± 5.8 | 4.4 ± 4.1 |
| Removal Rates (%) | ||||||
|---|---|---|---|---|---|---|
| Bacteria | T1 | T2 | T3 | T4 | T5 | |
| bOTU_2 | 47.60 ± 11.68 | 34.26 ± 8.23 | 7.83 ± 2.09 | 6.63 ± 7.14 | 3.68 ± 2.47 | |
| bOTU_12 | 49.63 ± 7.28 | 32.38 ± 8.12 | 8.01 ± 2.87 | 5.71 ± 5.00 | 4.27 ± 1.68 | |
| bOTU_23 | 41.03 ± 13.61 | 41.01 ± 10.07 | 8.15 ± 4.42 | 7.50 ± 11.82 | 2.31 ± 1.99 | |
| bOTU_6 | 33.68 ± 6.02 | 15.26 ± 4.31 | 26.48 ± 9.46 | 16.65 ± 1.42 | 7.94 ± 6.54 | |
| bOTU_21 | 50.38 ± 13.55 | 30.04 ± 9.38 | 11.09 ± 3.20 | 5.29 ± 3.65 | 3.20 ± 1.61 | |
| bOTU_1 | 5.90 ± 4.86 | 7.97 ± 2.94 | 23.58 ± 6.31 | 28.62 ± 10.07 | 33.92 ± 7.55 | |
| bOTU_8 | 12.44 ± 3.66 | 7.73 ± 3.67 | 22.91 ± 7.27 | 25.45 ± 7.87 | 31.47 ± 9.23 | |
| bOTU_7 | 4.70 ± 1.65 | 3.28 ± 2.06 | 20.60 ± 7.69 | 21.39 ± 3.92 | 50.03 ± 7.10 | |
| bOTU_35 | 6.13 ± 3.61 | 5.50 ± 2.61 | 13.82 ± 4.91 | 25.01 ± 9.29 | 49.54 ± 13.85 | |
| bOTU_4 | 7.24 ± 3.67 | 8.55 ± 3.39 | 23.09 ± 2.11 | 27.54 ± 9.25 | 33.58 ± 10.68 | |
| Fungi | fOTU_1 | 36.52 ± 8.04 | 32.24 ± 5.24 | 17.53 ± 8.67 | 8.12 ± 3.08 | 5.58 ± 5.51 |
| fOTU_4 | 36.14 ± 11.32 | 34.79 ± 5.84 | 16.13 ± 11.01 | 7.94 ± 2.39 | 5.00 ± 4.49 | |
| fOTU_7 | 76.30 ± 10.95 | 16.34 ± 8.86 | 3.05 ± 2.68 | 2.52 ± 2.13 | 1.79 ± 2.28 | |
| fOTU_5 | 38.48 ± 10.20 | 31.29 ± 5.90 | 16.12 ± 9.47 | 8.34 ± 2.61 | 5.77 ± 4.71 | |
| fOTU_9 | 76.15 ± 11.11 | 16.56 ± 9.14 | 2.57 ± 1.84 | 2.73 ± 2.40 | 1.99 ± 2.74 | |
| fOTU_2 | 2.31 ± 2.43 | 1.60 ± 0.78 | 9.46 ± 4.78 | 34.02 ± 22.47 | 52.61 ± 20.52 | |
| fOTU_1403 | 2.22 ± 2.69 | 1.55 ± 0.77 | 9.55 ± 5.95 | 33.58 ± 23.49 | 53.10 ± 21.50 | |
| fOTU_1533 | 2.34 ± 2.44 | 2.02 ± 1.61 | 27.01 ± 23.68 | 8.40 ± 6.93 | 60.22 ± 24.59 | |
| fOTU_274 | 4.27 ± 1.74 | 6.12 ± 5.31 | 31.42 ± 18.67 | 42.10 ± 30.82 | 16.09 ± 15.20 | |
| fOTU_28 | 1.78 ± 1.94 | 1.15 ± 0.76 | 22.10 ± 19.64 | 7.13 ± 7.67 | 67.84 ± 21.42 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Li, X.; Jiang, S.; Chen, J.; Yan, B. Facilitating Wastewater Purification through Progressive Thawing by Microwave: Responses of Microbial Communities. Water 2023, 15, 3664. https://doi.org/10.3390/w15203664
Hu Y, Li X, Jiang S, Chen J, Yan B. Facilitating Wastewater Purification through Progressive Thawing by Microwave: Responses of Microbial Communities. Water. 2023; 15(20):3664. https://doi.org/10.3390/w15203664
Chicago/Turabian StyleHu, Yaxian, Xianwen Li, Simin Jiang, Junying Chen, and Baowen Yan. 2023. "Facilitating Wastewater Purification through Progressive Thawing by Microwave: Responses of Microbial Communities" Water 15, no. 20: 3664. https://doi.org/10.3390/w15203664
APA StyleHu, Y., Li, X., Jiang, S., Chen, J., & Yan, B. (2023). Facilitating Wastewater Purification through Progressive Thawing by Microwave: Responses of Microbial Communities. Water, 15(20), 3664. https://doi.org/10.3390/w15203664







