Micro- and Nano-Plastics Contaminants in the Environment: Sources, Fate, Toxicity, Detection, Remediation, and Sustainable Perspectives
Abstract
:1. Introduction
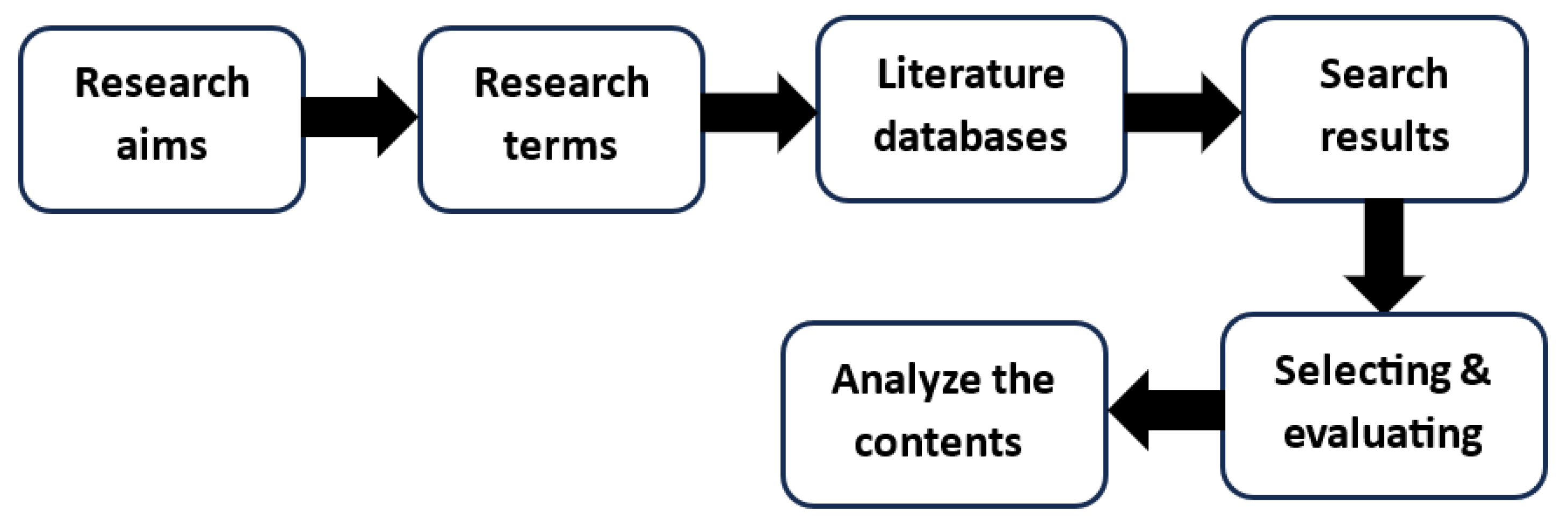
2. Method
3. The Occurrence and Sources of Microplastics and Nanoplastics in the Environment
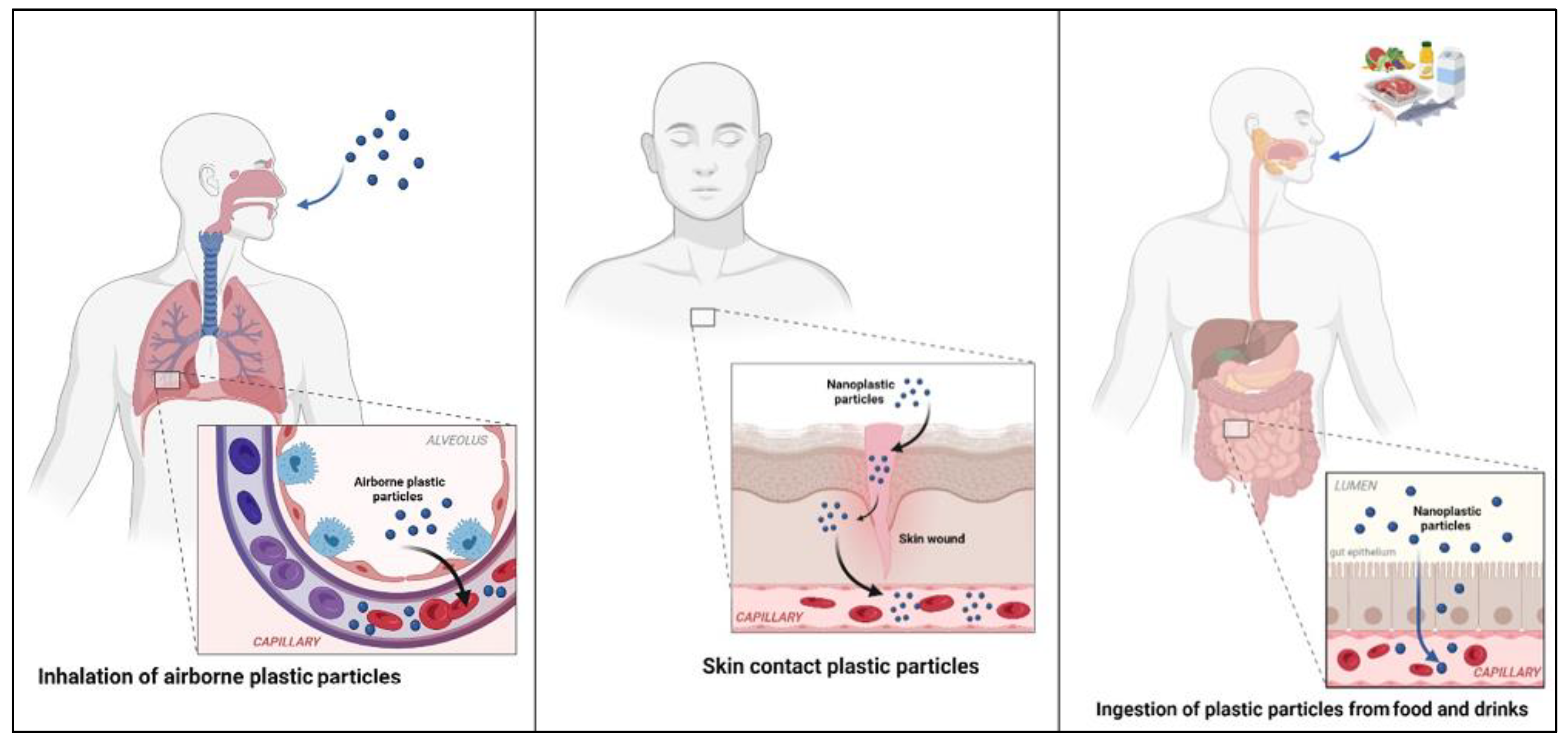
4. Uptake and Bioaccumulation of Microplastics and Nanoplastics in the Human Body
4.1. Gastric Exposure
4.2. Pulmonary Exposure
4.3. Dermal Exposure
5. Toxic Effects of MPs and NPs on Human Health
6. Methods of Microplastics Analysis
6.1. Visual Inspection Methods
6.2. Thermal Analytical Methods
6.3. Spectral Analytical Method
6.4. Other Analytical Methods
6.5. The Evaluation of Analysis Methods
7. Sampling of MPs and NPs in the Aquatic Environment
8. Remediation Strategies and Methods
8.1. Physical Remediation Methods
8.2. Chemical Remediation Methods
8.3. Bioremediation
8.4. Nanoremediation
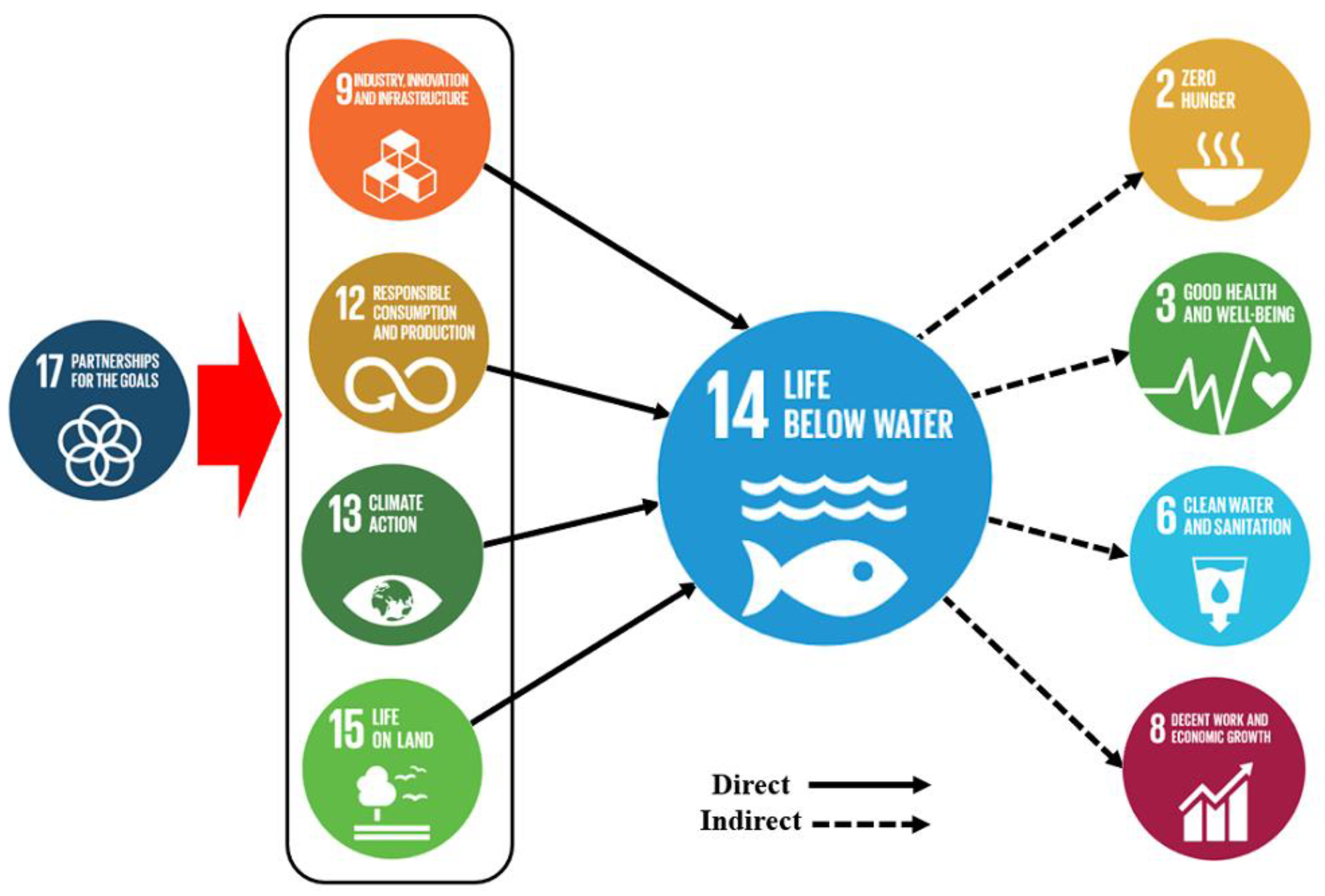
9. Plastic Pollution in the Context of SDGs
10. Future Prospectives and Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Garside, M. Global Plastic Production Statistics. Retrieved from Statista. Available online: https://www.statista.com/statistics/282732/global (accessed on 1 August 2020).
- Plastics Europe. Plastics—The Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 27 September 2022).
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Wagner, S.; Reemtsma, T. Things we know and don’t know about nanoplastic in the environment. Nat. Nanotechnol. 2019, 14, 300–301. [Google Scholar] [CrossRef]
- Shrivastava, A. Polymerization. In Introduction to Plastics Engineering; William Andrew Publishing: New York, NY, USA, 2018; pp. 17–48. [Google Scholar]
- Liu, T.; Guo, X.; Liu, W.; Hao, C.; Wang, L.; Hiscox, W.C.; Liu, C.; Jin, C.; Xin, J.; Zhang, J. Selective cleavage of ester linkages of anhydride-cured epoxy using a benign method and reuse of the decomposed polymer in new epoxy preparation. Green Chem. 2017, 19, 4364–4372. [Google Scholar] [CrossRef]
- Ambrogi, V.; Carfagna, C.; Cerruti, P.; Marturano, V. Additives in Polymers. In Modification of Polymer Properties; William Andrew Publishing: New York, NY, USA, 2017; pp. 87–108. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- da Costa, J.P. Micro-and nanoplastics in the environment: Research and policymaking. Curr. Opin. Environ. Sci. Health 2018, 1, 12–16. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- European Food Safety Authority; EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nano plastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar] [CrossRef]
- Tang, Y.; Hady, T.J.; Yoon, J. Receptor–based detection of microplastics and nanoplastics: Current and future. Biosens. Bioelectron. 2023, 234, 115361. [Google Scholar] [CrossRef]
- Mariano, S.; Tacconi, S.; Fidaleo, M.; Rossi, M.; Dini, L. Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques. Front. Toxicol. 2021, 3, 636640. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify plastic waste as hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Rashed, A.H. Bahrain’s Environmental Legal Tools for Giving Effect to Sustainable Development Goals: An Assessment. Environ. Policy Law 2022, 52, 39–54. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are there nanoplastics in your personal care products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Ter Halle, A.; Jeanneau, L.; Martignac, M.; Jardé, E.; Pedrono, B.; Brach, L.; Gigault, J. Nanoplastic in the North Atlantic Subtropical Gyre. Environ. Sci. Technol. 2017, 51, 13689–13697. [Google Scholar] [CrossRef]
- Gigault, J.; Pedrono, B.; Maxit, B.; Ter Halle, A. Marine plastic litter: The unanalyzed nano-fraction. Environ. Sci. Nano 2016, 3, 346–350. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Zitko, V.; Hanlon, M.J. Another source of pollution by plastics: Skin cleaners with plastic scrubbers. Mar. Pollut. Bull. 1991, 22, 41–42. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Plastic Production, Waste, and Legislation. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 39–56. [Google Scholar]
- Mendoza, L.M.; Vargas, D.L.; Balcer, M. Microplastics occurrence and fate in the environment. Curr. Opin. Green Sustain. Chem. 2021, 32, 100523. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, E.; Singh, S.; Pandey, A.; Bhargava, P.C. Micro-and nano-plastics (MNPs) as emerging pollutant in ground water: Environmental impact, potential risks, limitations and way forward towards sustainable management. Chem. Eng. J. 2023, 459, 141568. [Google Scholar] [CrossRef]
- Golwala, H.; Zhang, X.; Iskander, S.M.; Smith, A.L. Solid waste: An overlooked source of microplastics to the environment. Sci. Total Environ. 2021, 769, 144581. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. WIREs Water 2018, 5, e1268. [Google Scholar] [CrossRef]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Kochleus, C.; Stock, F.; Reifferscheid, G. Tyre and road wear particles-a calculation of generation, transport and release to water and soil with special regard to German roads. Sci. Total Environ. 2021, 752, 141939. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Beriot, N.; Corradini, F.; Silva, V.; Yang, X.; Baartman, J.; Rezaei, M.; van Schaik, L.; Riksen, M.; Geissen, V. Review of microplastic sources, transport pathways and correlations with other soil stressors: A journey from agricultural sites into the environment. Chem. Biol. Technol. Agric. 2022, 9, 20. [Google Scholar] [CrossRef]
- Michel, E.; Néel, M.C.; Capowiez, Y.; Sammartino, S.; Lafolie, F.; Renault, P.; Pelosi, C. Making Waves: Modeling bioturbation in soils–are we burrowing in the right direction? Water Res. 2022, 216, 118342. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics and nanoplastics: Emerging contaminants in food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Zylstra, E.R. Accumulation of wind-dispersed trash in desert environments. J. Arid. Environ. 2013, 89, 13–15. [Google Scholar] [CrossRef]
- Barboza, L.G.; Cózar, A.; Gimenez, B.C.; Barros, T.L.; Kershaw, P.J.; Guilhermino, L. Macroplastics Pollution in the Marine Environment. In World Seas: An Environmental Evaluation; Academic Press: Cambridge, MA, USA, 2019; pp. 305–328. [Google Scholar]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Wirzberger, V.; Krumpen, T.; Lorenz, C.; Primpke, S.; Tekman, M.B.; Gerdts, G. High quantities of microplastic in Arctic deep-sea sediments from the HAUSGARTEN observatory. Environ. Sci. Technol. 2017, 51, 11000–11010. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Alzona, J.B.; Cohen, B.L.; Rudolph, H.; Jow, H.N.; Frohliger, J.O. Indoor-outdoor relationships for airborne particulate matter of outdoor origin. Atmos. Environ. (1967) 1979, 13, 55–60. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Löder, M.G.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef]
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Vieira, M.N. Microplastic pollution in commercial salt for human consumption: A review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168. [Google Scholar] [CrossRef]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef]
- Mason, S.A.; Welch, V.G.; Neratko, J. Synthetic polymer contamination in bottled water. Front. Chem. 2018, 6, 407. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Leslie, H.A.; Quinn, B. Microplastics in drinking water: A review and assessment. Curr. Opin. Environ. Sci. Health 2019, 7, 69–75. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Wufuer, R.; Duo, J.; Pan, X. Distinct soil microplastic distributions under various farmland-use types around Urumqi, China. Sci. Total Environ. 2023, 857, 159573. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Jesuraja, K.; Venkatramanan, S.; Roy, P.D.; Kumari, V.J. Hazardous microplastic characteristics and its role as a vector of heavy metal in groundwater and surface water of coastal south India. J. Hazard. Mater. 2021, 402, 123786. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.R.; Lu, H.C.; Sharley, D.J.; Pettigrove, V. Associations between microplastic pollution and land use in urban wetland sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Zhang, K.; Yang, R.; Li, R.; Li, Y. Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the Qinzhou Bay, China. Mar. Pollut. Bull. 2018, 136, 401–406. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, S.; Zhao, L.; Duan, X.; Xie, S.; Zhang, H.; Liu, Y.; Peng, Y.; Liu, C.; Wang, L. Microplastics in Yellow River Delta wetland: Occurrence, characteristics, human influences, and marker. Environ. Pollut. 2020, 258, 113232. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Uddin, S.; Lyons, B. Evidence of microplastics (MP) in gut content of major consumed marine fish species in the State of Kuwait (of the Arabian/Persian Gulf). Mar. Pollut. Bull. 2020, 154, 111052. [Google Scholar] [CrossRef]
- Saeed, T.; Al-Jandal, N.; Al-Mutairi, A.; Taqi, H. Microplastics in Kuwait marine environment: Results of first survey. Mar. Pollut. Bull. 2020, 152, 110880. [Google Scholar] [CrossRef]
- Habib, R.Z.; Thiemann, T. Microplastic in Commercial Fish in the Mediterranean Sea, the Red Sea and the Arabian/Persian Gulf. Part 3. The Arabian/Persian Gulf. J. Water Resour. Prot. 2022, 14, 474–500. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Behbehani, M. An assessment of microplastic inputs into the aquatic environment from wastewater streams. Mar. Pollut. Bull. 2020, 160, 111538. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano-and microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef] [PubMed]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermato-endocrinology 2009, 1, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Camacho, M.; Herrera, A.; Gómez, M.; Acosta-Dacal, A.; Martínez, I.; Henríquez-Hernández, L.A.; Luzardo, O.P. Organic pollutants in marine plastic debris from Canary Islands beaches. Sci. Total Environ. 2019, 662, 22–31. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Viršek, M.K.; Lovšin, M.N.; Koren, Š.; Kržan, A.; Peterlin, M. Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar. Pollut. Bull. 2017, 125, 301–309. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Ge, H.; Yan, Y.; Wu, D.; Huang, Y.; Tian, F. Potential role of LINC00996 in colorectal cancer: A study based on data mining and bioinformatics. OncoTargets Ther. 2018, 11, 4845–4855. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Cell Junctions. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer Nature: Cham, Switzerland, 2015. [Google Scholar]
- Tomazic-Jezic, V.J.; Merritt, K.; Umbreit, T.H. Significance of the type and the size of biomaterial particles on phagocytosis and tissue distribution. J. Biomed. Mater. Res. 2001, 55, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.E.; Smyth, S.H.; McCullough, M.T.; Morris, J.F.; Moyes, S.M. Morphological aspects of interactions between microparticles and mammalian cells: Intestinal uptake and onward movement. Prog. Histochem. Cytochem. 2012, 46, 185–252. [Google Scholar] [CrossRef] [PubMed]
- Walczak, A.P.; Kramer, E.; Hendriksen, P.J.; Tromp, P.; Helsper, J.P.; van der Zande, M.; Rietjens, I.M.; Bouwmeester, H. Translocation of differently sized and charged polystyrene nanoparticles in in vitro intestinal cell models of increasing complexity. Nanotoxicology 2015, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.; Halbert, G.W.; Langridge, J.; Florence, A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. J. Pharm. Pharmacol. 1990, 42, 821–826. [Google Scholar] [CrossRef]
- des Rieux, A.; Fievez, V.; Théate, I.; Mast, J.; Préat, V.; Schneider, Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid Formation of Plasma Protein Corona Critically Affects Nanoparticle Pathophysiology. In Nano-Enabled Medical Applications; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2020; pp. 251–278. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G.E. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef]
- Stapleton, P.A. Toxicological considerations of nano-sized plastics. AIMS Environ. Sci. 2019, 6, 367–378. [Google Scholar] [CrossRef]
- Vethaak, A.D.; Leslie, H.A. Plastic Debris Is a Human Health Issue. Environ. Sci. Technol. 2016, 50, 6825–6826. [Google Scholar] [CrossRef] [PubMed]
- Ohlwein, S.; Kappeler, R.; Kutlar Joss, M.; Künzli, N.; Hoffmann, B. Health effects of ultrafine particles: A systematic literature review update of epidemiological evidence. Int. J. Public Health 2019, 64, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.W.; Hubbs, A.F.; Mercer, R.R.; Wu, N.; Wolfarth, M.G.; Sriram, K.; Leonard, S.; Battelli, L.; Schwegler-Berry, D.; Friend, S.; et al. Mouse pulmonary dose-and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 2010, 269, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Almroth, B.C.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef]
- Varela, J.A.; Bexiga, M.G.; Åberg, C.; Simpson, J.C.; Dawson, K.A. Quantifying size-dependent interactions between fluorescently labeled polystyrene nanoparticles and mammalian cells. J. Nanobiotechnol. 2012, 10, 39. [Google Scholar] [CrossRef]
- Deville, S.; Penjweini, R.; Smisdom, N.; Notelaers, K.; Nelissen, I.; Hooyberghs, J.; Ameloot, M. Intracellular dynamics and fate of polystyrene nanoparticles in A549 Lung epithelial cells monitored by image (cross-) correlation spectroscopy and single particle tracking. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 2411–2419. [Google Scholar] [CrossRef]
- Yacobi, N.R.; DeMaio, L.; Xie, J.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.J.; Crandall, E.D. Polystyrene nanoparticle trafficking across alveolar epithelium. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 139–145. [Google Scholar] [CrossRef]
- Salvati, A.; Åberg, C.; dos Santos, T.; Varela, J.; Pinto, P.; Lynch, I.; Dawson, K.A. Experimental and theoretical comparison of intracellular import of polymeric nanoparticles and small molecules: Toward models of uptake kinetics. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 818–826. [Google Scholar] [CrossRef]
- Som, C.; Wick, P.; Krug, H.; Nowack, B. Environmental and health effects of nanomaterials in nanotextiles and façade coatings. Environ. Int. 2011, 37, 1131–1142. [Google Scholar] [CrossRef]
- Bouwstra, J.; Pilgram, G.; Gooris, G.; Koerten, H.; Ponec, M. New aspects of the skin barrier organization. Ski. Pharmacol. Physiol. 2001, 14 (Suppl. 1), 52–62. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control Release 2004, 99, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.S.; Contreras-Rojas, L.R.; Delgado-Charro, M.B.; Guy, R.H. Objective assessment of nanoparticle disposition in mammalian skin after topical exposure. J. Control Release 2012, 162, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J. Investig. Dermatol. 2006, 126, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef]
- Mortensen, L.J.; Oberdörster, G.; Pentland, A.P.; DeLouise, L.A. In vivo skin penetration of quantum dot nanoparticles in the murine model: The effect of UVR. Nano Lett. 2008, 8, 2779–2787. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Jatana, S.; Callahan, L.M.; Pentland, A.P.; DeLouise, L.A. Impact of cosmetic lotions on nanoparticle penetration through ex vivo C57BL/6 hairless mouse and human skin: A comparison study. Cosmetics 2016, 3, 6. [Google Scholar] [CrossRef]
- Kuo, T.R.; Wu, C.L.; Hsu, C.T.; Lo, W.; Chiang, S.J.; Lin, S.J.; Dong, C.Y.; Chen, C.C. Chemical enhancer induced changes in the mechanisms of transdermal delivery of zinc oxide nanoparticles. Biomaterials 2009, 30, 3002–3008. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, S.; Yin, L.; Pu, Y.; Liang, G. Recent consequences of micro-nanaoplastics (MNPLs) in subcellular/molecular environmental pollution toxicity on human and animals. Ecotoxicol. Environ. Saf. 2023, 249, 114385. [Google Scholar] [CrossRef]
- Khan, A.; Jia, Z. Recent insights into uptake, toxicity, and molecular targets of microplastics and nanoplastics relevant to human health impacts. iScience 2023, 26, 106061. [Google Scholar] [CrossRef]
- Lee, H.S.; Amarakoon, D.; Wei, C.I.; Choi, K.Y.; Smolensky, D.; Lee, S.H. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem. Toxicol. 2021, 154, 112356. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Li, H.; Ren, H.; Zhang, X.; Huang, F.; Zhang, D.; Huang, Q.; Zhang, X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard. Mater. 2022, 421, 126770. [Google Scholar] [CrossRef] [PubMed]
- Visalli, G.; Facciolà, A.; Pruiti Ciarello, M.; De Marco, G.; Maisano, M.; Di Pietro, A. Acute and sub-chronic effects of microplastics (3 and 10 µm) on the human intestinal cells HT-29. Int. J. Environ. Res. Public Health 2021, 18, 5833. [Google Scholar] [CrossRef] [PubMed]
- Domenech, J.; de Britto, M.; Velázquez, A.; Pastor, S.; Hernández, A.; Marcos, R.; Cortés, C. Long-term effects of polystyrene nanoplastics in human intestinal Caco-2 cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef]
- Stock, V.; Laurisch, C.; Franke, J.; Dönmez, M.H.; Voss, L.; Böhmert, L.; Braeuning, A.; Sieg, H. Uptake and cellular effects of PE, PP, PET and PVC microplastic particles. Toxicol. In Vitro 2021, 70, 105021. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cao, X.; Bitounis, D.; Singh, D.; Llopis, P.M.; Buckley, B.; Demokritou, P. Toxicity, uptake, and nuclear translocation of ingested micro-nanoplastics in an in vitro model of the small intestinal epithelium. Food Chem. Toxicol. 2021, 158, 112609. [Google Scholar] [CrossRef]
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y.; et al. Disturbed Gut-Liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ. Int. 2022, 164, 107273. [Google Scholar] [CrossRef]
- Menéndez-Pedriza, A.; Jaumot, J.; Bedia, C. Lipidomic analysis of single and combined effects of polyethylene microplastics and polychlorinated biphenyls on human hepatoma cells. J. Hazard. Mater. 2022, 421, 126777. [Google Scholar] [CrossRef]
- Zheng, T.; Yuan, D.; Liu, C. Molecular toxicity of nanoplastics involving in oxidative stress and desoxyribonucleic acid damage. J. Mol. Recognit. 2019, 32, e2804. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Goodman, K.E.; Hare, J.T.; Khamis, Z.I.; Hua, T.; Sang, Q.X. Exposure of human lung cells to polystyrene microplastics significantly retards cell proliferation and triggers morphological changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, S.; Ge, Y.; Wan, X.; Zhu, Y.; Li, J.; Yin, L.; Pu, Y.; Liang, G. Polystyrene nanoplastics induce lung injury via activating oxidative stress: Molecular insights from bioinformatics analysis. Nanomaterials 2022, 12, 3507. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef]
- Annangi, B.; Villacorta, A.; Vela, L.; Tavakolpournegari, A.; Marcos, R.; Hernández, A. Effects of true-to-life PET nanoplastics using primary human nasal epithelial cells. Environ. Toxicol. Pharmacol. 2023, 100, 104140. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Hwang, J.; Bang, J.; Han, S.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. In vitro toxicity from a physical perspective of polyethylene microplastics based on statistical curvature change analysis. Sci. Total Environ. 2021, 752, 142242. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Schwiebs, A.; Solhaug, H.; Stenvik, J.; Nilsen, A.M.; Wagner, M.; Relja, B.; Radeke, H.H. Nanoplastics affect the inflammatory cytokine release by primary human monocytes and dendritic cells. Environ. Int. 2022, 163, 107173. [Google Scholar] [CrossRef]
- Sarma, D.K.; Dubey, R.; Samarth, R.M.; Shubham, S.; Chowdhury, P.; Kumawat, M.; Verma, V.; Tiwari, R.R.; Kumar, M. The biological effects of polystyrene nanoplastics on human peripheral blood lymphocytes. Nanomaterials 2022, 12, 1632. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.; Liu, Y.; Mei, A.; Wang, X.; Shi, Q. Cellular absorption of polystyrene nanoplastics with different surface functionalization and the toxicity to RAW264. 7 macrophage cells. Ecotoxicol. Environ. Saf. 2023, 252, 114574. [Google Scholar] [CrossRef]
- Caputi, S.; Diomede, F.; Lanuti, P.; Marconi, G.D.; Di Carlo, P.; Sinjari, B.; Trubiani, O. Microplastics affect the inflammation pathway in human gingival fibroblasts: A study in the Adriatic Sea. Int. J. Environ. Res. Public Health 2022, 19, 7782. [Google Scholar] [CrossRef]
- Choi, D.; Bang, J.; Kim, T.; Oh, Y.; Hwang, Y.; Hong, J. In vitro chemical and physical toxicities of polystyrene microfragments in human-derived cells. J. Hazard. Mater. 2020, 400, 123308. [Google Scholar] [CrossRef]
- Fahrenfeld, N.L.; Arbuckle-Keil, G.; Beni, N.N.; Bartelt-Hunt, S.L. Source tracking microplastics in the freshwater environment. TrAC Trends Anal. Chem. 2019, 112, 248–254. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Filella, M. Questions of size and numbers in environmental research on microplastics: Methodological and conceptual aspects. Environ. Chem. 2015, 12, 527–538. [Google Scholar] [CrossRef]
- Hanvey, J.S.; Lewis, P.J.; Lavers, J.L.; Crosbie, N.D.; Pozo, K.; Clarke, B.O. A review of analytical techniques for quantifying microplastics in sediments. Anal. Methods 2017, 9, 1369–1383. [Google Scholar] [CrossRef]
- Xu, J.L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949–1956. [Google Scholar] [CrossRef]
- Tianniam, S.; Bamba, T.; Fukusaki, E. Pyrolysis GC-MS-based metabolite fingerprinting for quality evaluation of commercial Angelica acutiloba roots. J. Biosci. Bioeng. 2010, 109, 89–93. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–5784. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Celina, M.; Braun, U. Automated thermal extraction-desorption gas chromatography mass spectrometry: A multifunctional tool for comprehensive characterization of polymers and their degradation products. J. Chromatogr. A 2019, 1592, 133–142. [Google Scholar] [CrossRef]
- Shishkin, Y.L. The effect of sample mass and heating rate on DSC results when studying the fractional composition and oxidative stability of lube base oils. Thermochim. Acta 2006, 444, 26–34. [Google Scholar] [CrossRef]
- Rodríguez Chialanza, M.; Sierra, I.; Pérez Parada, A.; Fornaro, L. Identification and quantitation of semi-crystalline microplastics using image analysis and differential scanning calorimetry. Environ. Sci. Pollut. Res. 2018, 25, 16767–16775. [Google Scholar] [CrossRef] [PubMed]
- Huppertsberg, S.; Knepper, T.P. Instrumental analysis of microplastics—Benefits and challenges. Anal. Bioanal. Chem. 2018, 410, 6343–6352. [Google Scholar] [CrossRef] [PubMed]
- Dierkes, G.; Lauschke, T.; Becher, S.; Schumacher, H.; Földi, C.; Ternes, T. Quantification of microplastics in environmental samples via pressurized liquid extraction and pyrolysis-gas chromatography. Anal. Bioanal. Chem. 2019, 411, 6959–6968. [Google Scholar] [CrossRef]
- Steinmetz, Z.; Kintzi, A.; Muñoz, K.; Schaumann, G.E. A simple method for the selective quantification of polyethylene, polypropylene, and polystyrene plastic debris in soil by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2020, 147, 104803. [Google Scholar] [CrossRef]
- Peters, C.A.; Hendrickson, E.; Minor, E.C.; Schreiner, K.; Halbur, J.; Bratton, S.P. Pyr-GC/MS analysis of microplastics extracted from the stomach content of benthivore fish from the Texas Gulf Coast. Mar. Pollut. Bull. 2018, 137, 91–95. [Google Scholar] [CrossRef]
- Funck, M.; Yildirim, A.; Nickel, C.; Schram, J.; Schmidt, T.C.; Tuerk, J. Identification of microplastics in wastewater after cascade filtration using Pyrolysis-GC–MS. MethodsX 2020, 7, 100778. [Google Scholar] [CrossRef]
- El Hayany, B.; El Fels, L.; Quénéa, K.; Dignac, M.F.; Rumpel, C.; Gupta, V.K.; Hafidi, M. Microplastics from lagooning sludge to composts as revealed by fluorescent staining-image analysis, Raman spectroscopy and pyrolysis-GC/MS. J. Environ. Manag. 2020, 275, 111249. [Google Scholar] [CrossRef]
- Hendrickson, E.; Minor, E.C.; Schreiner, K. Microplastic abundance and composition in western Lake Superior as determined via microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. Technol. 2018, 52, 1787–1796. [Google Scholar] [CrossRef]
- Becker, R.; Altmann, K.; Sommerfeld, T.; Braun, U. Quantification of microplastics in a freshwater suspended organic matter using different thermoanalytical methods–outcome of an interlaboratory comparison. J. Anal. Appl. Pyrolysis 2020, 148, 104829. [Google Scholar] [CrossRef]
- Majewsky, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Bitter, H.; Lackner, S. First quantification of semi-crystalline microplastics in industrial wastewaters. Chemosphere 2020, 258, 127388. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Van Oyen, A.; Booth, A.M.; Meijboom, A.; Van Franeker, J.A. Marine microplastic: Preparation of relevant test materials for laboratory assessment of ecosystem impacts. Chemosphere 2018, 213, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; He, L.; Jiang, S.; Chen, J.; Zhou, C.; Qu, J.; Lu, Y.; Hong, P.; Sun, S.; Li, C. In situ surface-enhanced Raman spectroscopy for detecting microplastics and nanoplastics in aquatic environments. Sci. Total Environ. 2020, 728, 138449. [Google Scholar] [CrossRef]
- Mehdinia, A.; Dehbandi, R.; Hamzehpour, A.; Rahnama, R. Identification of microplastics in the sediments of southern coasts of the Caspian Sea, north of Iran. Environ. Pollut. 2020, 258, 113738. [Google Scholar] [CrossRef]
- Lin, J.; Xu, X.M.; Yue, B.Y.; Xu, X.P.; Liu, J.Z.; Zhu, Q.; Wang, J.H. Multidecadal records of microplastic accumulation in the coastal sediments of the East China Sea. Chemosphere 2021, 270, 128658. [Google Scholar] [CrossRef]
- Deng, J.; Guo, P.; Zhang, X.; Su, H.; Zhang, Y.; Wu, Y.; Li, Y. Microplastics and accumulated heavy metals in restored mangrove wetland surface sediments at Jinjiang Estuary (Fujian, China). Mar. Pollut. Bull. 2020, 159, 111482. [Google Scholar] [CrossRef]
- Tiwari, M.; Rathod, T.D.; Ajmal, P.Y.; Bhangare, R.C.; Sahu, S.K. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019, 140, 262–273. [Google Scholar] [CrossRef]
- Wang, J.; Peng, J.; Tan, Z.; Gao, Y.; Zhan, Z.; Chen, Q.; Cai, L. Microplastics in the surface sediments from the Beijiang River littoral zone: Composition, abundance, surface textures and interaction with heavy metals. Chemosphere 2017, 171, 248–258. [Google Scholar] [CrossRef]
- Wang, Z.M.; Wagner, J.; Ghosal, S.; Bedi, G.; Wall, S. SEM/EDS and optical microscopy analyses of microplastics in ocean trawl and fish guts. Sci. Total Environ. 2017, 603, 616–626. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Z.; Li, W.; Chang, X.; Yang, J.; Yang, F. Microplastics in the sediment of lake Ulansuhai of Yellow river basin, China. Water Environ. Res. 2020, 92, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Kannan, K. Polyethylene terephthalate and polycarbonate microplastics in pet food and feces from the United States. Environ. Sci. Technol. 2019, 53, 12035–12042. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Chen, J.P. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC Trends Anal. Chem. 2019, 110, 150–159. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Investigation of microplastics in aquatic environments: An overview of the methods used, from field sampling to laboratory analysis. Trends Anal. Chem. 2018, 108, 195–202. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Zhu, L.; Li, D. Comprehensive Comparison of Various Microplastic Sampling Methods in Sea Water: Implications for Data Compilation. Water 2023, 15, 1035. [Google Scholar] [CrossRef]
- Delgado-Gallardo, J.; Sullivan, G.L.; Esteban, P.; Wang, Z.; Arar, O.; Li, Z.; Watson, T.M.; Sarp, S. From sampling to analysis: A critical review of techniques used in the detection of micro-and nanoplastics in aquatic environments. ACS ES&T Water 2021, 1, 748–764. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A. Microplastics in the environment: Challenges in analytical chemistry-A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Mytilineou, C.; Smith, C.J.; Papadopoulou, K.N. Plastic debris ingested by deep-water fish of the Ionian Sea (Eastern Mediterranean). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2013, 74, 11–13. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Paradinas, L.M.; James, N.A.; Quinn, B.; Dale, A.; Narayanaswamy, B.E. A new collection tool-kit to sample microplastics from the marine environment (sediment, seawater, and biota) using citizen science. Front. Mar. Sci. 2021, 8, 657709. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Collection Techniques. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 179–202. [Google Scholar] [CrossRef]
- Brander, S.M.; Renick, V.C.; Foley, M.M.; Steele, C.; Woo, M.; Lusher, A.; Carr, S.; Helm, P.; Box, C.; Cherniak, S.; et al. Sampling and quality assurance and quality control: A guide for scientists investigating the occurrence of microplastics across matrices. Appl. Spectrosc. 2020, 74, 1099–1125. [Google Scholar] [CrossRef]
- Desforges, J.P.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Näkki, P.; Setälä, O.; Lehtiniemi, M. Seafloor sediments as microplastic sinks in the northern Baltic Sea–negligible upward transport of buried microplastics by bioturbation. Environ. Pollut. 2019, 249, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Zobkov, M.B.; Esiukova, E.E. Microplastics in a Marine Environment: Review of Methods for Sampling, Processing, and Analyzing Microplastics in Water, Bottom Sediments, and Coastal Deposits. Oceanology 2018, 58, 137–143. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Ashton, K.; Holmes, L.; Turner, A. Association of metals with plastic production pellets in the marine environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef]
- Blumenröder, J.; Sechet, P.; Kakkonen, J.E.; Hartl, M.G. Microplastic contamination of intertidal sediments of Scapa Flow, Orkney: A first assessment. Mar. Pollut. Bull. 2017, 124, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Arendt, N.; Foeldi, C.; Dierkes, G.; Wagner, M.; et al. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total Environ. 2020, 738, 139866. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; De Meester, S.; Van Landuyt, L.; De Clerck, K.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef]
- Hu, K.; Tian, W.; Yang, Y.; Nie, G.; Zhou, P.; Wang, Y.; Duan, X.; Wang, S. Microplastics remediation in aqueous systems: Strategies and technologies. Water Res. 2021, 198, 117144. [Google Scholar] [CrossRef]
- Chellasamy, G.; Kiriyanthan, R.M.; Maharajan, T.; Radha, A.; Yun, K. Remediation of microplastics using bionanomaterials: A review. Environ. Res. 2022, 208, 112724. [Google Scholar] [CrossRef]
- Poerio, T.; Piacentini, E.; Mazzei, R. Membrane processes for microplastic removal. Molecules 2019, 24, 4148. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Bian, K.; Wang, H.; Wang, C. A critical review of control and removal strategies for microplastics from aquatic environments. J. Environ. Chem. Eng. 2021, 9, 105463. [Google Scholar] [CrossRef]
- Ma, B.; Xue, W.; Hu, C.; Liu, H.; Qu, J.; Li, L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem. Eng. J. 2019, 359, 159–167. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, Z.R.; Li, W.H.; Yan, X.; Wang, L.K.; Zhang, L.; Jin, J.; Dai, X.; Ni, B.J. Revisiting microplastics in landfill leachate: Unnoticed tiny microplastics and their fate in treatment works. Water Res. 2021, 190, 116784. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, Y.; Zhu, J.; Shi, J.; Huang, H.; Xie, B. Distribution and removal characteristics of microplastics in different processes of the leachate treatment system. Waste Manag. 2021, 120, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, G.; Yu, H.; Xing, J. Dynamic membrane for micro-particle removal in wastewater treatment: Performance and influencing factors. Sci. Total Environ. 2018, 627, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Sci. Total Environ. 2021, 752, 141837. [Google Scholar] [CrossRef]
- Lu, S.; Liu, L.; Yang, Q.; Demissie, H.; Jiao, R.; An, G.; Wang, D. Removal characteristics and mechanism of microplastics and tetracycline composite pollutants by coagulation process. Sci. Total Environ. 2021, 786, 147508. [Google Scholar] [CrossRef]
- Sembiring, E.; Fajar, M.; Handajani, M. Performance of rapid sand filter–single media to remove microplastics. Water Supply 2021, 21, 2273–2284. [Google Scholar] [CrossRef]
- Alvim, C.B.; Bes-Piá, M.A.; Mendoza-Roca, J.A. An innovative approach to the application of ultrasounds to remove polyethylene microspheres from activated sludge. Sep. Purif. Technol. 2021, 264, 118429. [Google Scholar] [CrossRef]
- Fechine, G.J.; Souto-Maior, R.M.; Rabello, M.S. Structural changes during photodegradation of poly (ethylene terephthalate). J. Mater. Sci. 2002, 37, 4979–4984. [Google Scholar] [CrossRef]
- Wilken, R.; Holländer, A.; Behnisch, J. Vacuum ultraviolet photolysis of polyethylene, polypropylene, and polystyrene. Plasmas Polym. 2002, 7, 19–39. [Google Scholar] [CrossRef]
- Suhrhoff, T.J.; Scholz-Böttcher, B.M. Qualitative impact of salinity, UV radiation and turbulence on leaching of organic plastic additives from four common plastics—A lab experiment. Mar. Pollut. Bull. 2016, 102, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef] [PubMed]
- Tofa, T.S.; Ye, F.; Kunjali, K.L.; Dutta, J. Enhanced visible light photodegradation of microplastic fragments with plasmonic platinum/zinc oxide nanorod photocatalysts. Catalysts 2019, 9, 819. [Google Scholar] [CrossRef]
- Liu, G.; Liao, S.; Zhu, D.; Hua, Y.; Zhou, W. Innovative photocatalytic degradation of polyethylene film with boron-doped cryptomelane under UV and visible light irradiation. Chem. Eng. J. 2012, 213, 286–294. [Google Scholar] [CrossRef]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef]
- Piazza, V.; Uheida, A.; Gambardella, C.; Garaventa, F.; Faimali, M.; Dutta, J. Ecosafety screening of photo-fenton process for the degradation of microplastics in water. Front. Mar. Sci. 2022, 8, 791431. [Google Scholar] [CrossRef]
- Zafar, R.; Park, S.Y.; Kim, C.G. Surface modification of polyethylene microplastic particles during the aqueous-phase ozonation process. Environ. Eng. Res. 2021, 26, 200412. [Google Scholar] [CrossRef]
- Chen, R.; Qi, M.; Zhang, G.; Yi, C. Comparative Experiments on Polymer Degradation Technique of Produced Water of Polymer Flooding Oilfield. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 113, p. 012208. [Google Scholar] [CrossRef]
- Roager, L.; Sonnenschein, E.C. Bacterial candidates for colonization and degradation of marine plastic debris. Environ. Sci. Technol. 2019, 53, 11636–11643. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2021, 759, 143536. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, M.J.; Blanco, A.C.; Schmidgall, T.; Atanasoff-Kardjalieff, A.K.; Kappelmeyer, U.; Tischler, D.; Pieper, D.H.; Heipieper, H.J.; Eberlein, C. Toward biorecycling: Isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Front. Microbiol. 2020, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Ndahebwa Muhonja, C.; Magoma, G.; Imbuga, M.; Makonde, H.M. Molecular characterization of low-density polyethene (LDPE) degrading bacteria and fungi from Dandora dumpsite, Nairobi, Kenya. Int. J. Microbiol. 2018, 2018, 4167845. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K. Microbial deterioration and degradation of polymeric materials. J. Biochem. Technol. 2011, 2, 210–215. [Google Scholar]
- Thakur, S.; Mathur, S.; Patel, S.; Paital, B. Microplastic Accumulation and Degradation in Environment via Biotechnological Approaches. Water 2022, 14, 4053. [Google Scholar] [CrossRef]
- Ojha, N.; Pradhan, N.; Singh, S.; Barla, A.; Shrivastava, A.; Khatua, P.; Rai, V.; Bose, S. Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci. Rep. 2017, 7, 39515. [Google Scholar] [CrossRef]
- Yadav, V.; Dhanger, S.; Sharma, J. Microplastics accumulation in agricultural soil: Evidence for the presence, potential effects, extraction, and current bioremediation approaches. J. Appl. Biol. Biotechnol. 2022, 10, 38–47. [Google Scholar] [CrossRef]
- Raaman, N.; Rajitha, N.; Jayshree, A.; Jegadeesh, R. Biodegradation of plastic by Aspergillus spp. isolated from polythene polluted sites around Chennai. J. Acad. Indus. Res. 2012, 1, 313–316. [Google Scholar]
- Yoshida, S.; Hiraga, K.; Taniguchi, I.; Oda, K. Ideonella sakaiensis, PETase, and MHETase: From Identification of Microbial PET Degradation to Enzyme Characterization. In Methods in Enzymolog; Academic Press: Cambridge, MA, USA, 2021; Volume 648, pp. 187–205. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ouyang, D.; Lei, J.; Tan, Q.; Xie, L.; Li, Z.; Liu, T.; Xiao, Y.; Farooq, T.H.; et al. Systematical review of interactions between microplastics and microorganisms in the soil environment. J. Hazard. Mater. 2021, 418, 126288. [Google Scholar] [CrossRef]
- da Costa, A.M.; de Oliveira Lopes, V.R.; Vidal, L.; Nicaud, J.M.; de Castro, A.M.; Coelho, M.A. Poly (ethylene terephthalate)(PET) degradation by Yarrowia lipolytica: Investigations on cell growth, enzyme production and monomers consumption. Process Biochem. 2020, 95, 81–90. [Google Scholar] [CrossRef]
- Kiriyanthan, R.M.; Maharajan, T.; Radha, A.; Pandikumar, P. A Review on The Role of Nanotechnology in Enhancing Environmental Sustainability. Chem. Biol. Interface 2021, 11, 13–33. [Google Scholar]
- Jalvo, B.; Aguilar-Sanchez, A.; Ruiz-Caldas, M.X.; Mathew, A.P. Water filtration membranes based on non-woven cellulose fabrics: Effect of nanopolysaccharide coatings on selective particle rejection, antifouling, and antibacterial properties. Nanomaterials 2021, 11, 1752. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.; Sheng, J.; Zimba, P.V.; Zhu, L.; Fadare, O.O.; Haley, C.; Wang, M.; Phillips, T.D.; Conkle, J.; Xu, W. Testing an iron oxide nanoparticle-based method for magnetic separation of nanoplastics and microplastics from water. Nanomaterials 2022, 12, 2348. [Google Scholar] [CrossRef]
- Cao, B.; Wan, S.; Wang, Y.; Guo, H.; Ou, M.; Zhong, Q. Highly-efficient visible-light-driven photocatalytic H2 evolution integrated with microplastic degradation over MXene/ZnxCd1-xS photocatalyst. J. Colloid Interface Sci. 2022, 605, 311–319. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Ye, H.; Wang, Y.; Liu, X.; Xu, D.; Yuan, H.; Sun, H.; Wang, S.; Ma, X. Magnetically steerable iron oxides-manganese dioxide core–shell micromotors for organic and microplastic removals. J. Colloid Interface Sci. 2021, 588, 510–521. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suta, T.; Usuelli, M.; Handschin, S.; Canelli, G.; Bagnani, M.; Mezzenga, R. Sustainable removal of microplastics and natural organic matter from water by coagulation–flocculation with protein amyloid fibrils. Environ. Sci. Technol. 2021, 55, 8848–8858. [Google Scholar] [CrossRef]
- Kabir, M.S.; Wang, H.; Luster-Teasley, S.; Zhang, L.; Zhao, R. Microplastics in landfill leachate: Sources, detection, occurrence, and removal. Environ. Sci. Ecotechnol. 2023, 16, 100256. [Google Scholar] [CrossRef]
- Pico, Y.; Alfarhan, A.; Barcelo, D. Nano-and microplastic analysis: Focus on their occurrence in freshwater ecosystems and remediation technologies. TrAC Trends Anal. Chem. 2019, 113, 409–425. [Google Scholar] [CrossRef]
- Dey, T.K.; Jamal, M.; Uddin, M.E. Fabrication and performance analysis of graphene oxide-based composite membrane to separate microplastics from synthetic wastewater. J. Water Process Eng. 2023, 52, 103554. [Google Scholar] [CrossRef]
- Malankowska, M.; Echaide-Gorriz, C.; Coronas, J. Microplastics in marine environment: A review on sources, classification, and potential remediation by membrane technology. Environ. Sci. Water Res. Technol. 2021, 7, 243–258. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhu, Y.; Zeng, G.; Zhang, Y.; Yang, Y.; Wen, X.; Chen, M.; Yi, H. Removal of microplastics via drinking water treatment: Current knowledge and future directions. Chemosphere 2020, 251, 126612. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Xu, Y.; Wang, Q.; Wu, Z.; Grasmick, A. Organic matter recovery from municipal wastewater by using dynamic membrane separation process. Chem. Eng. J. 2013, 219, 190–199. [Google Scholar] [CrossRef]
- Saleem, M.; Alibardi, L.; Cossu, R.; Lavagnolo, M.C.; Spagni, A. Analysis of fouling development under dynamic membrane filtration operation. Chem. Eng. J. 2017, 312, 136–143. [Google Scholar] [CrossRef]
- Jiang, J.Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced oxidation processes (AOPs) involving ultrasound for wastewater treatment: A review with emphasis on cost estimation. Ultrason. Sonochem. 2010, 17, 990–1003. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Cvetnić, M.; Kušić, H.; Bolanča, T.; Kučić Grgić, D.; Ukić, Š. Potential of Advanced Oxidation as Pretreatment for Microplastics Biodegradation. Separations 2023, 10, 132–157. [Google Scholar] [CrossRef]
- Kim, S.; Sin, A.; Nam, H.; Park, Y.; Lee, H.; Han, C. Advanced oxidation processes for microplastics degradation: A recent trend. Chem. Eng. J. Adv. 2022, 9, 100213. [Google Scholar] [CrossRef]
- Moussavi, G.; Shekoohiyan, S. Simultaneous nitrate reduction and acetaminophen oxidation using the continuous-flow chemical-less VUV process as an integrated advanced oxidation and reduction process. J. Hazard. Mater. 2016, 318, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.I. (Ed.) Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2017; ISBN 978-1-78040-718-0. [Google Scholar]
- Wang, J.C.; Wang, H.; Huang, L.L.; Wang, C.Q. Surface treatment with Fenton for separation of acrylonitrile-butadiene-styrene and polyvinylchloride waste plastics by flotation. Waste Manag. 2017, 67, 20–26. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Y.; Sun, X. O3 and UV/O3 oxidation of organic constituents of biotreated municipal wastewater. Water Res. 2008, 42, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Pang, Z.; Wang, L.; Liu, X. Efficient degradation of acesulfame by ozone/peroxymonosulfate advanced oxidation process. Molecules 2019, 24, 2874. [Google Scholar] [CrossRef]
- Fischbacher, A.; von Sonntag, J.; von Sonntag, C.; Schmidt, T.C. The• OH radical yield in the H2O2+ O3 (peroxone) reaction. Environ. Sci. Technol. 2013, 47, 9959–9964. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment; IWA Publishing: London, UK, 2012; ISBN 978-1-78040-083-9. [Google Scholar]
- Zhou, Y.; Kumar, M.; Sarsaiya, S.; Sirohi, R.; Awasthi, S.K.; Sindhu, R.; Binod, P.; Pandey, A.; Bolan, N.S.; Zhang, Z.; et al. Challenges and opportunities in bioremediation of micro-nano plastics: A review. Sci. Total Environ. 2022, 802, 149823. [Google Scholar] [CrossRef]
- Tiwari, N.; Santhiya, D.; Sharma, J.G. Microbial remediation of micro-nano plastics: Current knowledge and future trends. Environ. Pollut. 2020, 265, 115044. [Google Scholar] [CrossRef]
- Rao, V.V.; Sonashree, R.; Halbavi, R.R. Review on Plastic Waste Disposal and Role of Microorganisms in Bioremediation of Plastics. In Research Anthology on Emerging Techniques in Environmental Remediation; IGI Global: Hershey, PA, USA, 2022; pp. 481–492. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, H. PMBD: A comprehensive plastics microbial biodegradation database. Database 2019, 2019, baz119. [Google Scholar] [CrossRef] [PubMed]
- Sanniyasi, E.; Gopal, R.K.; Gunasekar, D.K.; Raj, P.P. Biodegradation of low-density polyethylene (LDPE) sheet by microalga, Uronema africanum Borge. Sci. Rep. 2021, 11, 17233. [Google Scholar] [CrossRef] [PubMed]
- Bhuyar, P.; Sundararaju, S.; Feng, H.X.; Rahim, M.H.; Muniyasamy, S.; Maniam, G.P.; Govindan, N. Evaluation of Microalgae’s Plastic Biodeterioration Property by a Consortium of Chlorella sp. and Cyanobacteria sp. Environ. Res. Eng. Manag. 2021, 77, 86–98. [Google Scholar] [CrossRef]
- Samat, A.F.; Carter, D.; Abbas, A. Biodeterioration of pre-treated polypropylene by Aspergillus terreus and Engyodontium album. NPJ Mater. Degrad. 2023, 7, 28. [Google Scholar] [CrossRef]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent achievements in polymer bio-based flocculants for water treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.L.; Andou, Y. Detection and remediation of bisphenol A (BPA) using graphene-based materials: Mini-review. Int. J. Environ. Sci. Technol. 2022, 19, 6869–6888. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Hu, Y.; Wang, R.; Chen, M.; Wu, J.; Wang, Y.; Kang, S.; Sun, Y.; Zhu, M. Behavior, remediation effect and toxicity of nanomaterials in water environments. Environ. Res. 2019, 174, 54–60. [Google Scholar] [CrossRef]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J. Nanotechnology in wastewater management: A new paradigm towards wastewater treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic extraction of microplastics from environmental samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Sethi, B. Recycling of polymers in the presence of nanocatalysts: A green approach towards sustainable environment. Int. J. Chem. Mol. Eng. 2016, 10, 525–531. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, J.; Melo, J.S. Silica based bio-hybrid materials and their relevance to bionanotechnology. Austin J. Plant Biol. 2020, 6, 1024. [Google Scholar]
- Vázquez-Núñez, E.; Molina-Guerrero, C.E.; Peña-Castro, J.M.; Fernández-Luqueño, F.; de la Rosa-Álvarez, M.G. Use of nanotechnology for the bioremediation of contaminants: A review. Processes 2020, 8, 826. [Google Scholar] [CrossRef]
- Walker, T.R. (Micro) plastics and the UN sustainable development goals. Curr. Opin. Green Sustain. Chem. 2021, 30, 100497. [Google Scholar] [CrossRef]
- United Nations (UN). Resolution 70/1 in 2015: Transforming our World: The 2030 Agenda for Sustainable Development. United Nations General Assembly. 2015. Available online: https://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (accessed on 10 August 2023).
- Rai, M.; Pant, G.; Pant, K.; Aloo, B.N.; Kumar, G.; Singh, H.B.; Tripathi, V. Microplastic Pollution in Terrestrial Ecosystems and Its Interaction with Other Soil Pollutants: A Potential Threat to Soil Ecosystem Sustainability. Resources 2023, 12, 67. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Xu, S.; Ma, J.; Ji, R.; Pan, K.; Miao, A.J. Microplastics in aquatic environments: Occurrence, accumulation, and biological effects. Sci. Total Environ. 2020, 703, 134699. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Silva, A.L.; Prata, J.C.; Walker, T.R.; Duarte, A.C.; Ouyang, W.; Barcelò, D.; Rocha-Santos, T. Increased plastic pollution due to COVID-19 pandemic: Challenges and recommendations. Chem. Eng. J. 2021, 405, 126683. [Google Scholar] [CrossRef]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro) plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Shen, M.; Ye, S.; Zeng, G.; Zhang, Y.; Xing, L.; Tang, W.; Wen, X.; Liu, S. Can microplastics pose a threat to ocean carbon sequestration? Mar. Pollut. Bull. 2020, 150, 110712. [Google Scholar] [CrossRef]
- Walker, T.R.; McKay, D.C. Comment on “five misperceptions surrounding the environmental impacts of single-use plastic”. Environ. Sci. Technol. 2021, 55, 1339–1340. [Google Scholar] [CrossRef] [PubMed]
- UNFCCC. Paris Agreement to the United Nations Framework Convention on Climate Change. In Proceedings of the UN Climate Change Conference (COP21), Paris, France, 12 December 2015. T.I.A.S. No. 16-1104. [Google Scholar]
- Abdelhafeez, I.A.; Ramakrishna, S. Promising sustainable models toward water, air, and solid sustainable management in the view of SDGs. Mater. Circ. Econ. 2021, 3, 21. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Combating Marine Plastic Litter and Microplastics Summary for Policymakers: An Assessment of the Effectiveness of Relevant International, Regional and Subregional Governance Strategies and Approaches; United Nations: San Francisco, CA, USA, 2017. [Google Scholar]
- Environmental Investigation Agency (EIA). Convention on Plastic Pollution: Toward a New Global Agreement to Address Plastic Pollution; Center for International Environmental Law: Washington, DC, USA, 2020. [Google Scholar]




| Characteristics of Tested Particles | Particle Size | Target Cells | Effect of MPs and/orNPs on Organisms | Ref. |
|---|---|---|---|---|
| PS MPs | 0.5, 1, and 5 μm | Human umbilical vein endothelial cells (HUVECs) |
| [102] |
| PS NPs | 100 and 500 nm | HUVECs |
| [103] |
| PS MPs | 3 and 10 µm | Human intestinal cell line HT-29 |
| [104] |
| PS NPs | 50 nm | Human intestinal Caco-2 cells |
| [105] |
| PE, PP, PET, and PVC MPs | 1–4 μm and 10–20 μm | Human intestinal epithelial cell line Caco-2 |
| [106] |
| PS NPs. | 25, 100 and 1000 nm | Human small intestinal epithelium |
| [107] |
| PS MPs | 1 μm | Mice intestinal tissue and liver |
| [108] |
| PE MPs and PCBs | 40–48 µm | Human hepatoma cell line HepG2 |
| [109] |
| PS NPs | 50 nm | rat hepatocyte |
| [110] |
| PS NPs | 25 and 70 nm | Human alveolar type II epithelial cell line A549 |
| [111] |
| PS MPs | 1 and 10 μm | Human alveolar A549 cell line |
| [112] |
| PS NPs | 40 nm | Lung epithelial cells (BEAS-2B cell line) |
| [113] |
| PS NPs | 40 nm | Bronchial epithelium, pulmonary alveolar epithelial cells |
| [114] |
| PET NPs | ~62 nm | Human primary nasal epithelial cells |
| [115] |
| PE MPs beads and fragments | Beads: 10, 50 and 100 μm; Fragments: 25–75 and 75–200 μm | Peripheral blood mononuclear cells |
| [116] |
| PS, PMMA, and PVC NPs | 50–310 nm | Human monocytes and monocyte-derived dendritic cells. |
| [117] |
| PS NPs. | 50 nm | Human peripheral blood lymphocytes |
| [118] |
| PS NPs and functionalized PS pristine, PS-COOH, PS-NH2 | 100 nm | Mouse mononuclear macrophage (RAW264.7) cell line |
| [119] |
| PS NPs | 100 nm, 600 nm | Human gingival fibroblasts |
| [120] |
| PS MPs | 5–25, 25–75, 75–200 μm | Human dermal fibroblast, HeLa cell line, peripheral blood mononuclear cells (PBMCs) and KATO III cells |
| [121] |
| Method | Advantages | Disadvantages | Sample Mass | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Pyr-GC–MS | Different types of polymers, precise results, great sensitivity, and no sample preprocessing | Long processing times, sample degradation, high reaction temperatures, and manual placement. | 0.5 mg | 0.007 mg/g | [129,130] |
| TED-GC–MS | High sample mass, no blocking reaction cube, and no sample preprocessing | Excessive processing time, sample degradation, and high reaction temperatures | 100 mg | - | [131,132] |
| DSC | Accurate results, widely applied technique, inexpensive and straightforward analysis | Long processing times, sample damage, substrate influence that is easy to see | 3–15 mg | - | [133,134] |
| Method | Source of the Sample | Type of Sample | LOD | Sample Abundance | Ref. |
|---|---|---|---|---|---|
| Pressurized liquid extraction & Pyr-GC–MS | Soil and sediment | PE, PP | - | PE (3.3 ± 0.3 mg/g) and PP (0.08 ± 0.02 mg/g) | [135] |
| Pyr-GC–MS | Soil and sediments of freshwater | PE (fractionation ratio 15:2), PE (fractionation ratio 17:2), PE (fractionation ratio 18:2), PP, PS (pyrolysis product Sty), and PS (pyrolysis product aMeSty) | PE (fractionation ratio 15:2, 4800 μg/L), PE (Fractionation ratio 17:2, 2500 μg/L), PE (Fractionation ratio 18:2, 11,300 μg/L), PP (43,200 μg/L), PS (pyrolysis product Sty, 500 μg/L) and PS (pyrolysis product aMeSty, 1600 μg/L) | - | [136] |
| Pyr-GC–MS | Stomachs of marine fishes | PVC, PET, nylon, silica gel, and epoxy resin | - | - | [137] |
| Pyr-GC–MS | Surface water and wastewater | PS and PE | PS (30 μg/L) and PE (1000 μg/L) | - | [138] |
| Pyr-GC–MS and Nile red dye | Lagoon sludge | - | - | Fresh sludge (40.5 ± 11.9 × 103 particles/kg) and dehydrated sludge (36 ± 9.7 × 103 particles/kg) | [139] |
| Pyr-GC–MS and FTIR | Surface water | PVC, PP, and PE | 0–110,000 particles/km2 | [140] | |
| TED-GC–MS | Freshwater | PE, PS, PET, and PP | PE (20.0 μg/mg), PP (5.7 μg/mg), PS (2.2 μg/mg) and PET (18.0 μg/mg) | - | [141] |
| TGA, DSC | Wastewater | PE, PP, PET, PA, PES, PVC, and PU | - | [142] | |
| DSC | Wastewater | PE, PP, PA, and PET | - | [143] | |
| ATR-FTIR and DSC | Dutch beaches | - | - | - | [144] |
| Method | Particle Size | Advantages | Limitations |
|---|---|---|---|
| FTIR | ATR-FTIR can study particles >500 µm in size, while microscope coupled with FTIR can analyze particles <20 µm. | Nondestructive, reliable, quick, and credible. Significantly reduced analysis time using automatic FTIR imaging techniques like FPA, which enables the quick capture of thousands of spectra within an area using a single measurement. | Samples must be IR reactive; <20 µm may not provide interpretable spectra due to insufficient absorbance. Non-transparent particles are challenging to analyze. High in cost and needs skilled personnel to operate and process the data. The ambient matrix has an impact on the detection (e.g., biofilm growth on polymer), which makes it challenging to interpret the data. To get rid of IR active water, the sample needs to be processed. |
| Raman Spectroscopy | For particles >1 µm, the microscopy coupled Raman Spectroscopy (RS) approach is appropriate. For particles ranging in size from 1 to 20 µm, it is the only approach that works. | Enables the investigation of microscopic particles (1–20 µm) with excellent spatial resolution and relatively low sensitivity to water, analyze opaque and dark particles; perform fast chemical mapping, allowing for quick and automatic data gathering and processing. | Fluorescence from biological, organic, and inorganic contaminants interferes heavily and makes it difficult to identify MPs.Prior to analysis, the sample must be cleaned; crucial Raman acquisition parameters include wavelength, laser power, and photo bleaching. Micro-RS automated mapping is still being developed. The analysis by RS is time consuming. |
| Method | Particle Size | Advantages | Limitations |
|---|---|---|---|
| Scanning ElectronSpectroscopy | Analysis is possible for particles with diameters as small as a micron. | Creates a high-resolution picture of the samples. | High vacuum is required to cover the samples, and there is no precise identification data available. |
| Liquid Chromatography | The chemical extraction needs a sample size of several milligrams to carry out this examination. | Selected polymers have high recoveries. | Its uses are restricted to environmental samples since it is impossible to establish physical features, such as size information. Per run, only a few samples can be evaluated. By using this procedure, only particular polymers, such PS and PET, may be evaluated. |
| Method * | Sample Source | Sample Type | Element Type | Sample Abundance | Ref. |
|---|---|---|---|---|---|
| SEM-EDS 1, polarized light microscope and μ-Raman 2 | Caspian Sea | - | C, O, Fe, Ba, Na, Si, and Al | - | [146] |
| SEM-EDS and μ-FTIR 3 | East Coast | PP, PE, PS, PET, PVC and PP-PE copolymer | - | -- | [147] |
| SEM-EDS | Sediment | - | Cr, Ni, Cu, Zn, Pb, As, and Cd | - | [148] |
| SEM and XRD 4 | Aquatic environment | PA, PS, PE, PP, and PVC | - | - | [61] |
| Fluorescence microscopy, FTIR 5 and SEM-EDS | Beach | PE | - | 45 ± 12 particle/kg to 220 ± 50 particles/kg | [149] |
| μ-FTIR and SEM-EDS | Beijiang River | - | - | 178 ± 69 to 544 ± 107 particles/kg | [150,151] |
| FTIR and SEM-EDS | Sediment of Suhai lake | PE, PP, and PVC | - | 24 ± 7 to 14 ± 3 particles/kg | [152] |
| HPLC-MS 6 | Pet food | PET | - | 1500 ng/g to 12,000 ng/g | [153] |
| Sampling Approach | Criteria | Application | Advantages | Limitations |
|---|---|---|---|---|
| Selective Sampling | Utilized when plastic items are large enough for identification with the naked eye, extracted directly from environmental matrices. | Beach sampling | Simple & straightforward | Size limitation of detectable MPs is high and less obvious items are easily overlooked particularly when mixed with other debris. |
| Bulk Sampling | Involves collecting the entire sample without decreasing its volume during the sampling. | Sediment sampling & occasional water sampling | Collects all MPs- and NPs present within the sample regardless of size and visibility. | Sample collection is relatively small in amount which may negatively affect sample representativeness |
| Volume-Reduced Sampling | Used when the entire volume of a bulk sample needs to be reduced by fast filtration during sampling; thus, only small fraction of the sample is being preserved for further analysis. | Water sampling | Covers large quantities or areas of samples. | Substantial loss of MPs and NPs may occur as most of the sample is lost/discarded due to fast filtration, which is evident in the MPs’ size being smaller than the mesh size of sampling tool. |
| Material * | Type of Remediation | Used Techniques | Refs. |
|---|---|---|---|
| PE | Physical | Fe-Based Coagulation and UF | [182] |
| MPs | Physical | RO and Nanofiltration | [183] |
| Fiber MPs | Physical | UF | [184] |
| MPs | Physical | RO | [185] |
| MPs | Physical | Dynamic Membrane | [186] |
| polyacrylamide (PAM) | Physical | Sedimentation and Coagulation | [187] |
| PET | Physical | Primary Sedimentation | [179] |
| PS | Physical | Coagulation (FeCl3, PAC) and Sedimentation | [188] |
| PS and PE | Physical | Coagulation with PAC and FeCl3 | [189] |
| PET/weathered PET and TC | Physical | Coagulation with AlCl3 | [190] |
| MPs | Physical | Pre-sedimentation, Coagulation, Flocculation, and Sedimentation, RSF | [191] |
| PE | Physical | Ultrasound Treatment | [192] |
| PET | Chemical | Photolysis | [193,194] |
| PE, PS, PET, and PVC | Chemical | UV Radiation | [195] |
| PE and PS | Chemical | TiO2 Photocatalysts Under UV Illumination | [196] |
| LDPE | Chemical | (Pt)-Deposited ZnO Nanorods | [197] |
| PE | Chemical | Polypyrrole-Coated TiO2 Catalysts Under Solar Radiation | [198] |
| PVC | Chemical | Electro-Fenton-Like System with TiO2/C | [199] |
| PVC and PP | Chemical | Heterogeneous Photo-Fenton Degradation using ZnO Nanorods Coated with Oxide Layer and Fe0 Nanoparticles | [200] |
| PE, PS, PP, and PET | Chemical | Ozonation | [201,202] |
| Marine Plastics | Bioremediation | Kocuria palustris M16, Rhodococcus sp. 36, and Bacillus strains | [203] |
| PET | Bioremediation | Ideonella sakaiensis 201-F6 strain | [204] |
| Thermoset Polymers, PU | Bioremediation | Bacillus, Pseudomonas, and Micrococcus | [205,206] |
| LDPE | Bioremediation | B. gottheilii and B. cereus | [207,208] |
| PE | Bioremediation | Penicillum, Aspergillus, Basidiomycota and Zygomycota | [209,210] |
| LDPE and HDPE | Bioremediation | Aspergillus spp., Penicillum spp. | [211] |
| PE, PU, and PP | Bioremediation | A. clavatus, A. oryzae strain A5, A. fumigatus, and A. niger | [212,213] |
| PU | Bioremediation | A. tubingensis, Monascus ruber, M. sanguineus, Monascus sp., and Pestalotiopsis microspora | [214,215] |
| PET | Bioremediation | Fusarium, Humicola, and Penicillium | [216] |
| PAM and Small Size MP | Nanoremediation | Coagulation, Sedimentation and GAC Filtration | [187] |
| MPs | Nanoremediation | Green Nanoscale Semiconductors | [217] |
| MPs | Nanoremediation | Cellulose Nanocrystals, Chitin Nanocrystals, and Lignin-Zeolite Composite Nanofibers | [218] |
| MPs and NPs | Nanoremediation | IONPs with PDMS-based Hydrophobic Coatings | [219] |
| PE, PET, and PA | Nanoremediation | M-CNTs | [79] |
| PET | Nanoremediation | MXene/ZnxCd1-xS Nanocomposite Photocatalysts | [220] |
| LDPE | Nanoremediation | Deposited Platinum Nanoparticles on the Surface of ZnO Nanorods. | [197] |
| MPs | Nanoremediation | Carbon Nanosprings | [221] |
| MPs | Nanoremediation | Oxides-MnO2 Core-Shell Micromotors | [222] |
| MPs | Bionanoremediation | Lysozyme Amyloid Fibrils | [223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashed, A.H.; Yesilay, G.; Hazeem, L.; Rashdan, S.; AlMealla, R.; Kilinc, Z.; Ali, F.; Abdulrasool, F.; Kamel, A.H. Micro- and Nano-Plastics Contaminants in the Environment: Sources, Fate, Toxicity, Detection, Remediation, and Sustainable Perspectives. Water 2023, 15, 3535. https://doi.org/10.3390/w15203535
Rashed AH, Yesilay G, Hazeem L, Rashdan S, AlMealla R, Kilinc Z, Ali F, Abdulrasool F, Kamel AH. Micro- and Nano-Plastics Contaminants in the Environment: Sources, Fate, Toxicity, Detection, Remediation, and Sustainable Perspectives. Water. 2023; 15(20):3535. https://doi.org/10.3390/w15203535
Chicago/Turabian StyleRashed, Abdulkarim Hasan, Gamze Yesilay, Layla Hazeem, Suad Rashdan, Reem AlMealla, Zeynep Kilinc, Fatema Ali, Fatima Abdulrasool, and Ayman H. Kamel. 2023. "Micro- and Nano-Plastics Contaminants in the Environment: Sources, Fate, Toxicity, Detection, Remediation, and Sustainable Perspectives" Water 15, no. 20: 3535. https://doi.org/10.3390/w15203535
APA StyleRashed, A. H., Yesilay, G., Hazeem, L., Rashdan, S., AlMealla, R., Kilinc, Z., Ali, F., Abdulrasool, F., & Kamel, A. H. (2023). Micro- and Nano-Plastics Contaminants in the Environment: Sources, Fate, Toxicity, Detection, Remediation, and Sustainable Perspectives. Water, 15(20), 3535. https://doi.org/10.3390/w15203535







