Response of Forest Plant Diversity to Drought: A Review
Abstract
1. Introduction
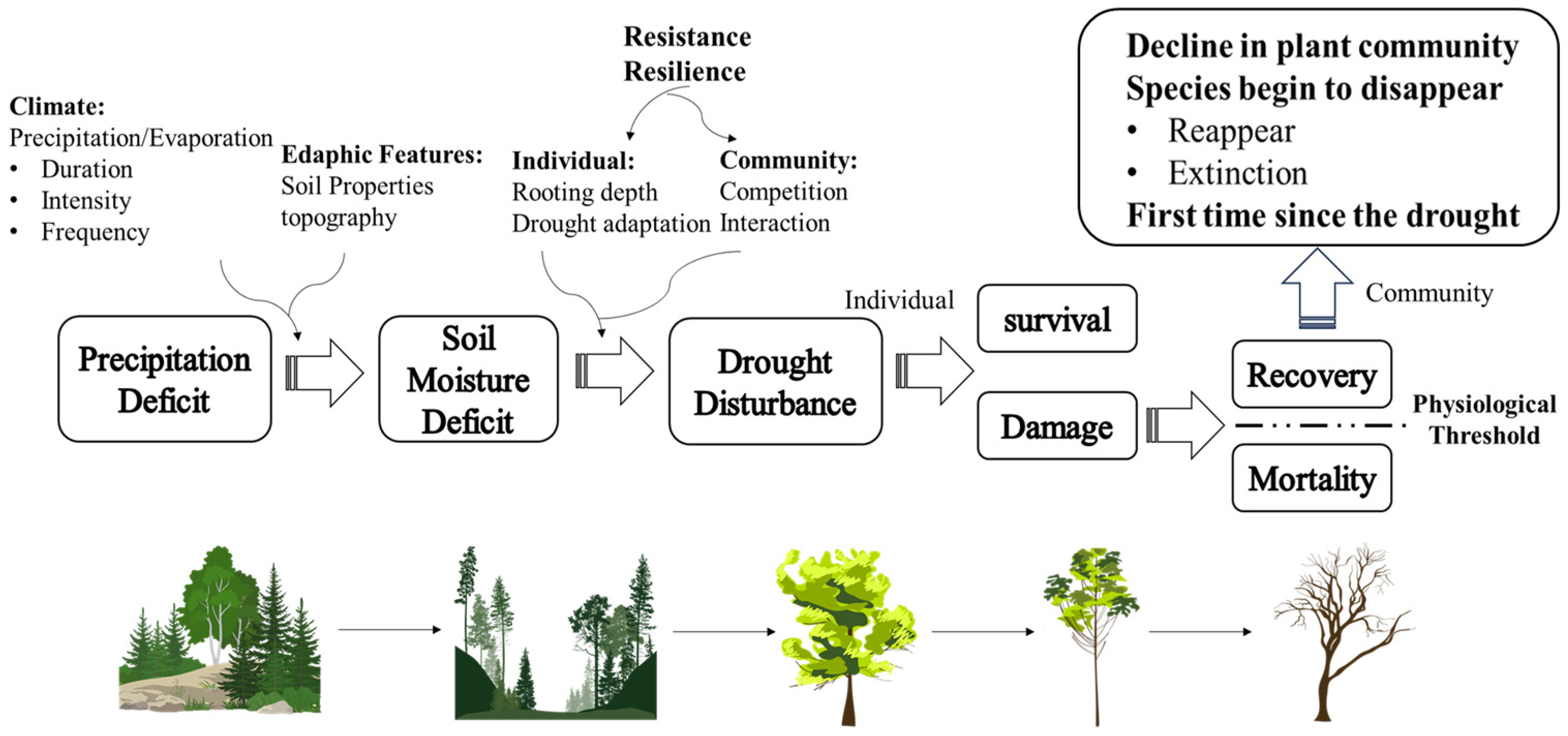
2. Drought and Forest Biodiversity
2.1. Selection of the Drought Definition and Index
2.2. Methods to Estimate and Measure Forest Biodiversity
2.2.1. Field Monitoring of Forest Plant Diversity
2.2.2. Remote Sensing Monitoring of Forest Plant Diversity
3. Mechanisms of Drought Effects on Forest Plant Diversity
3.1. Eco Physiological Responses to Drought
3.2. Species Resistance to Drought
3.3. Post-Drought Resilience of Species
4. Feedback of Forest Plant Diversity to Drought
4.1. The Role of Forest Biodiversity in Regulating Drought
4.2. Short- and Mid-Term Effects of Drought
4.3. Long-Term Effects of Drought and Global Climate Change
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yi, C.X.; Pendall, E.; Ciais, P. Focus on extreme events and the carbon cycle. Environ. Res. Lett. 2015, 10, 70201. [Google Scholar] [CrossRef]
- Diaz, S.; Fargione, J.; Chapin, F.S.; Tilman, D. Biodiversity loss threatens human well-being. PLoS Biol. 2006, 4, 1300–1305. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Berry, J.A. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Stephenson, N.L. Apparent climatically induced increase of tree mortality rates in a temperate forest. Ecol. Soc. Am. Annu. Meet. Abstr. 2007, 10, 909–916. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T. Diversity enhances carbon storage in tropical forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Morin, X.; Fahse, L. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef]
- Jactel, H.; Brockerhoff, E.G. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef]
- Redmond, K.T. The depiction of drought—A commentary. Bull. Am. Meteorol. Soc. 2002, 83, 1143–1147. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, V.P. A review of drought concepts. J. Hydrol. 2010, 391, 204–216. [Google Scholar] [CrossRef]
- Endt, P.M.; Patter, D.M.; Buechner, W.W. An objective approach to definitions and investigations of continental hydrologic droughts. VUJICA YEVJEVICH: Fort Collins, Colorado State University, 1967, 19 p. (Hydrology paper no. 23). J. Hydrol. 1951, 7, 491–494. [Google Scholar]
- Zelenhasic, E.; Salvai, A. A method of streamflow drought analysis. Water Resour. Res. 1987, 23, 156–168. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.P.; Dai, M.Q. Improving crop drought resistance with plant growth regulators and rhizobacteria: Mechanisms, applications, and perspectives. Plant Commun. 2022, 3, 100228. [Google Scholar] [CrossRef]
- Heim, R.R., Jr. A review of twentieth-century drought indices used in the United States. Bull. Am. Meteorol. Soc. 2002, 83, 1149–1165. [Google Scholar] [CrossRef]
- Mckee, T.B.; Doesken, N.J.; Kleist, J. The Relationship of Drought Frequency and Duration to Time Scales. In Proceedings of the 8th Conference on Applied Climatology, Anaheim, CA, USA, 17–22 January 1993. [Google Scholar]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A Multiscalar Drought Index Sensitive to Global Warming: The Standardized Precipitation Evapotranspiration Index. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Palmer, W.C. Meteorological Drought: US Department of Commerce; US Department of Commerce: Washington, DC, USA, 1965.
- Palmer, W.C. Keeping Track of Crop Moisture Conditions, Nationwide: The New Crop Moisture Index. Weatherwise 1968, 21, 156–161. [Google Scholar] [CrossRef]
- Sims, A.P.; Niyogi, D.D.S.; Raman, S. Adopting drought indices for estimating soil moisture: A North Carolina case study. Geophys. Res. Lett. 2002, 29, 24-1–24-4. [Google Scholar] [CrossRef]
- Oh, T.S.; Moon, Y.I.; Kim, S.S.; Gu, S.P. Frequency analysis of meteorologic drought indices using boundary kernel density function. J. Korean Soc. Civ. Eng. B 2011, 31, 87–98. [Google Scholar]
- Eslamian, S.; Ostad-Ali-Askari, K.; Singh, V.P.; Dalezios, N.R.; Matouq, M. A Review of Drought Indices. Int. J. Civ. Eng. 2017, 3, 48–66. [Google Scholar]
- Liu, D.; Wang, T.; Peñuelas, J.; Piao, S. Drought resistance enhanced by tree species diversity in global forests. Nat. Geosci. 2022, 15, 800–804. [Google Scholar] [CrossRef]
- Liang, J.; Crowther, T.W.; Picard, N.; Wiser, S. Positive biodiversity-productivity relationship predominant in global forests. Science 2016, 354, aaf8957. [Google Scholar] [CrossRef]
- Motz, K.; Sterba, H.; Pommerening, A. Sampling measures of tree diversity. For. Ecol. Manag. 2010, 260, 1985–1996. [Google Scholar] [CrossRef]
- Han, F. Sampling theory for forest inventory. For. Ecol. Manag. 1989, 26, 151. [Google Scholar] [CrossRef]
- Vos, P.; Meelis, E.; Keurs, W.J.T. A Framework for the Design of Ecological Monitoring Programs as a Tool for Environmental and Nature Management. Environ. Monit. Assess. 2000, 61, 317–344. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Davies, S.J.; Bennett, A.C.; Gonzalez-Akre, E.B. CTFS-ForestGEO: A worldwide network monitoring forests in an era of global change. Glob. Change Biol. 2015, 21, 528–549. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Kerr, J.T.; Ostrovsky, M. From space to species: Ecological applications for remote sensing. Trends Ecol. Evol. 2003, 18, 299–305. [Google Scholar] [CrossRef]
- Turner, W. Sensing biodiversity. Science 2014, 346, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Turner, W.; Spector, S.; Gardiner, N. Remote sensing for biodiversity science and conservation. Trends Ecol. Evol. 2003, 18, 306–314. [Google Scholar] [CrossRef]
- Ke, Y.H.; Quackenbush, L.J.; Im, J. Synergistic use of QuickBird multispectral imagery and LIDAR data for object-based forest species classification. Remote Sens. Environ. 2010, 114, 1141–1154. [Google Scholar] [CrossRef]
- Cross, M.; Scambos, T.; Pacifici, F.; Vargas-Ramirez, O.; Moreno-Sanchez, R.; Marshall, W. Classification of Tropical Forest Tree Species Using Meter-Scale Image Data. Remote Sens. 2019, 11, 1411. [Google Scholar] [CrossRef]
- Kokhan, S.; Vostokov, A. Using Vegetative Indices to Quantify Agricultural Crop Characteristics. J. Ecol. Eng. 2020, 21, 120–127. [Google Scholar] [CrossRef]
- Fairbanks, D.H.K.; McGwire, K.C. Patterns of floristic richness in vegetation communities of California: Regional scale analysis with multi-temporal NDVI. Glob. Ecol. Biogeogr. 2004, 13, 221–235. [Google Scholar] [CrossRef]
- Waring, R.H.; Coops, N.C.; Fan, W.; Nightingale, J.M. MODIS enhanced vegetation index predicts tree species richness across forested ecoregions in the contiguous USA. Remote Sens. Environ. 2006, 103, 218–226. [Google Scholar] [CrossRef]
- Carlson, K.M.; Asner, G.P.; Hughes, R.F.; Ostertag, R.; Martin, R.E. Hyperspectral remote sensing of canopy biodiversity in Hawaiian lowland rainforests. Ecosystems 2007, 10, 536–549. [Google Scholar] [CrossRef]
- Guo, Q.F. Progress in the application of remote sensing in biodiversity research. Biodiversity 2018, 26, 789–806. [Google Scholar]
- Duro, D.; Coops, N.C.; Wulder, M.A.; Han, T. Development of a large area biodiversity monitoring system driven by remote sensing. Prog. Phys. Geogr. Earth Environ. 2007, 31, 235–260. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Mwanamwenge, J.; Loss, S.P.; Siddique, K.H.M.; Cocks, P.S. Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 1999, 11, 1–11. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. Cmls 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Signarbieux, C.; Feller, U. Effects of an extended drought period on physiological properties of grassland species in the field. J. Plant Res. 2012, 125, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Bohnert, H.J.; Bo, S. Transformation and compatible solutes. Sci. Hortic. 1998, 78, 237–260. [Google Scholar] [CrossRef]
- Berriri, S.; Garcia, A.V.; Frey, N.F.D.; Rozhon, W.; Pateyron, S.; Leonhardt, N.; Montillet, J.L.; Leung, J.; Hirt, H.; Colcombet, J. Constitutively Active Mitogen-Activated Protein Kinase Versions Reveal Functions of Arabidopsis MPK4 in Pathogen Defense Signaling. Plant Cell 2012, 24, 4281–4293. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J.; Ye, N.H.; Cao, J.M.; Tan, M.P.; Zhang, J.H.; Jiang, M.Y. ZmMPK5 is required for the NADPH oxidase-mediated self-propagation of apoplastic H2O2 in brassinosteroid-induced antioxidant defence in leaves of maize. J. Exp. Bot. 2010, 61, 4399–4411. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C.; Doll, M. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef]
- Clauw, P.; Coppens, F.; De Beuf, K. Leaf Responses to Mild Drought Stress in Natural Variants of Arabidopsis. Plant Physiol. 2015, 168, 1180. [Google Scholar] [CrossRef]
- Fanizza, G.; Ricciardi, L. Influence of drought stress on shoot, leaf growth, leaf water potential, stomatal resistance in wine grape genotypes (Vitis vinifera L.). Vitis 1990, 29, 371. [Google Scholar]
- Comas, L.H.; Becker, S.R.; Cruz, V.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Sun, H.; Hakim; Yang, X.Y.; Zhang, X.L. A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol. Plant. 2018, 162, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Stael, S.; Wurzinger, B.; Mair, A.; Mehlmer, N.; Vothknecht, U.C.; Teige, M. Plant organellar calcium signalling: An emerging field. J. Exp. Bot. 2012, 63, 1525–1542. [Google Scholar] [CrossRef]
- Tanveer, M. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef]
- Lennon, J.T.; den Hollander, F.; Wilke-Berenguer, M.; Blath, J. Principles of seed banks and the emergence of complexity from dormancy. Nat. Commun. 2021, 12, 4807. [Google Scholar] [CrossRef]
- Treep, J.; de Jager, M.; Bartumeus, F.; Soons, M.B. Seed dispersal as a search strategy: Dynamic and fragmented landscapes select for multi-scale movement strategies in plants. Mov. Ecol. 2021, 9, 4. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, M.; Li, Q.; Liu, X.; Song, H.; Peng, X.; Wang, H.; Yang, N.; Fan, P.; Wang, R.; et al. Effects of defoliation modalities on plant growth, leaf traits, and carbohydrate allocation in Amorpha fruticosa L. and Robinia pseudoacacia L. seedlings. Ann. For. Sci. 2020, 77, 53. [Google Scholar] [CrossRef]
- Jentsch, A.; Kreyling, J.; Elmer, M.; Gellesch, E.; Beierkuhnlein, C. Climate Extremes Initiate Ecosystem-Regulating Functions While Maintaining Productivity; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Arfin Khan, M.A.S.; Grant, K.; Beierkuhnlein, C.; Kreyling, J.; Jentsch, A. Climatic extremes lead to species-specific legume facilitation in an experimental temperate grassland. Plant Soil 2014, 379, 161–175. [Google Scholar] [CrossRef]
- de Dios, V.R.; Weltzin, J.F.; Sun, W.; Huxman, T.E.; Williams, D.G. Transitions from grassland to savanna under drought through passive facilitation by grasses. J. Veg. Sci. 2014, 25, 937–946. [Google Scholar] [CrossRef]
- Keeley, J.E. Trace Gas Emissions and Smoke-Induced Seed Germination. J. Veg. Sci. 1997, 276, 1248–1250. [Google Scholar] [CrossRef]
- Cipriotti, P.A.; Flombaum, P.; Sala, O.E.; Aguiar, M.R. Does drought control emergence and survival of grass seedlings in semi-arid rangelands?: An example with a Patagonian species. J. Arid Environ. 2008, 72, 162–174. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Kemp, P.R. Ecology of Soil Seed Banks; Elsevier: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Kayes, L.J.; Tinker, D.B. Forest structure and regeneration following a mountain pine beetle epidemic in southeastern Wyoming. For. Ecol. Manag. 2012, 263, 57–66. [Google Scholar] [CrossRef]
- Yu, F.; Wang, D. Seed dispersal by small rodents favors oak over pine regeneration in the pine-oak forests of the Qinling mountains, China. Scand. J. For. Res. 2013, 28, 540–549. [Google Scholar] [CrossRef]
- Mező-Kricsfalusy, G.; Kricsfalusy, V. Population biology of plants. Popul. Biol. Plants 1977, 67. [Google Scholar]
- Prichard, S.J.; Hessburg, P.F.; Hagmann, R.K. Adapting western North American forests to climate change and wildfires: Ten common questions. Ecol. Appl. 2021, 31, e02433. [Google Scholar] [CrossRef]
- McDermid, S.S.; Cook, B.I.; De Kauwe, M.G.; Mankin, J.; Smerdon, J.E.; Williams, A.P.; Seager, R.; Puma, M.J.; Aleinov, I.; Kelley, M.; et al. Disentangling the Regional Climate Impacts of Competing Vegetation Responses to Elevated Atmospheric CO2. J. Geophys. Res.-Atmos. 2021, 126, e2020JD034108. [Google Scholar] [CrossRef]
- Lauvaux, C.A.; Skinner, C.N.; Taylor, A.H. High severity fire and mixed conifer forest-chaparral dynamics in the southern Cascade Range, USA. For. Ecol. Manag. 2016, 363, 74–85. [Google Scholar] [CrossRef]
- Fairman, T.A.; Bennett, L.T.; Nitschke, C.R. Short-interval wildfires increase likelihood of resprouting failure in fire-tolerant trees. J. Environ. Manag. 2019, 231, 59–65. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Aussenac, G. Influence des changements climatiques sur les peuplements forestiers et le cycle de l’eau. La Houille Blanche 2002, 88, 90–95. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Fay, P.A.; Carlisle, J.D.; Knapp, A.K.; Blair, J.M.; Collins, S.L. Altering Rainfall Timing and Quantity in a Mesic Grassland Ecosystem: Design and Performance of Rainfall Manipulation Shelters. Ecosystems 2000, 3, 308–319. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J. Effects of biodiversity on ecosystem functioning:A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Forrester, D.I.; Theiveyanathan, S.; Collopy, J.J.; Marcar, N.E. Enhanced water use efficiency in a mixed Eucalyptus globulus and Acacia mearnsii plantation. For. Ecol. Manag. 2010, 259, 1761–1770. [Google Scholar] [CrossRef]
- Kunert, N.; Schwendenmann, L.; Potvin, C.; Hölscher, D. Tree diversity enhances tree transpiration in a Panamanian forest plantation. J. Appl. Ecol. 2012, 49, 135–144. [Google Scholar] [CrossRef]
- Haberstroh, S.; Werner, C. The role of species interactions for forest resilience to drought. Plant Biol. 2022, 24, 1098–1107. [Google Scholar] [CrossRef]
- Grossiord, C.; Granier, A.; Ratcliffe, S.; Bouriaud, O.; Bruelheide, H.; Checko, E.; Forrester, D.I.; Dawud, S.M.; Finer, L.; Pollastrini, M.; et al. Tree diversity does not always improve resistance of forest ecosystems to drought. Proc. Natl. Acad. Sci. USA 2014, 111, 14812–14815. [Google Scholar] [CrossRef] [PubMed]
- Pourbabae, H.; Rahimi, V.; Adel, M.N. Effects of drought on plant species diversity and productivity in the Oak forests of Western Iran. Ecol. Balk. 2014, 6, 61–71. [Google Scholar]
- Brandao, D.O.; Barata, L.E.S.; Nobre, C.A. The Effects of Environmental Changes on Plant Species and Forest Dependent Communities in the Amazon Region. Forests 2022, 13, 466. [Google Scholar] [CrossRef]
- Ciríaco, E.; Albuquerque, M.B.D.; Dias, A. Drought and Its Consequences to Plants—From Individual to Ecosystem. In Responses of Organisms to Water Stress; IntechOpen: London, UK, 2013. [Google Scholar]
- Barnard, R.L.; Osborne, C.A.; Firestone, M.K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 2013, 7, 2229–2241. [Google Scholar] [CrossRef]
- Aubry-Kientz, M.; Rossi, V. A joint individual-based model coupling growth and mortality reveals that tree vigor is a key component of tropical forest dynamics. Ecol. Evol. 2015, 5, 2457–2465. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, I.; Voukkali, I.; Zorpas, A.A. Mediterranean: Main environmental issues and concerns. Euro-Mediterr J. Environ. 2022, 7, 477–481. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D. Drought-induced shift of a forest–woodland ecotone: Rapid landscape response to climate variation. Proc. Natl. Acad. Sci. USA 1998, 95, 14839–14842. [Google Scholar] [CrossRef]
- Cheng, H.; Sinha, A.; Cruz, F.W. Climate change patterns in Amazonia and biodiversity. Nat. Commun. 2013, 4, 1411. [Google Scholar] [CrossRef]
- Liiri, M.; Setl, H.; Haimi, J.; Fritze, P.H. Relationship between soil microarthropod species diversity and plant growth does not change when the system is disturbed. Oikos 2002, 96, 137–149. [Google Scholar] [CrossRef]
- Mulder, C.; Uliassi, D.D.; Doak, D.F. Physical stress and diversity-productivity relationships: The role of positive interactions. Proc. Natl. Acad. Sci. USA 2001, 98, 6704–6708. [Google Scholar] [CrossRef]
- Réjou-Méchain, M.; Tymen, B.; Blanc, L.; Fauset, S.; Chave, J. Using repeated small-footprint LiDAR acquisitions to infer spatial and temporal variations of a high-biomass Neotropical forest. Remote. Sens. Environ. 2015, 169, 93–101. [Google Scholar] [CrossRef]
- Lindh, M.; Zhang, L.; Falster, D. Plant diversity and drought: The role of deep roots. Ecol. Model. 2014, 290, 85–93. [Google Scholar] [CrossRef]
- Jonard, M.; Andre, F.; Dambrine, E.; Ponette, Q.; Ulrich, E. Temporal trends in the foliar nutritional status of the French, Walloon and Luxembourg broad-leaved plots of forest monitoring. Ann. For. Sci. 2009, 66, 412. [Google Scholar] [CrossRef][Green Version]
- Peterken, G.F.; Mountford, E.P. Effects of drought on beech in Lady Park Wood, an unmanaged mixed deciduous woodland. For. Int. J. For. Res. 1996, 69, 125–136. [Google Scholar] [CrossRef]
- Emperaire, G.; Laming-Emperaire, A. La grotte du Mylodon (Patagonie occidentale). J. Soc. Am. 1954, 43, 214–220. [Google Scholar] [CrossRef]
- Thuiller, W. Large-scale environmental correlates of forest tree distributions in Catalonia (NE Spain). Glob. Ecol. Biogeogr. 2010, 12, 313–325. [Google Scholar] [CrossRef]
- Landmann, G. Role of Climate, Stand Dynamics and Past Management in Forest Declines: A Review of Ten Years of Field Ecology in France; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Convey, P. Impacts of climate change on biota in the Arctic and Antarctic. In Proceedings of the 96th ESA Annual Convention, Austin, TX, USA, 7–12 August 2011. [Google Scholar]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2010, 32, 277–307. [Google Scholar] [CrossRef]
- Gutschick, V.P.; Bassirirad, H. Extreme Events as Shaping Physiology, Ecology, and Evolution of Plants: Toward a Unified Definition and Evaluation of Their Consequences; Wiley/Blackwell: Hoboken, NJ, USA, 2003. [Google Scholar]
- Aguirre-Gutiérrez, J.; Malhi, Y.; Lewis, S.L.; Fauset, S.; Adu-Bredu, S.; Affum-Baffoe, K.; Baker, T.R.; Gvozdevaite, A.; Hubau, W.; Moore, S.; et al. Long-term droughts may drive drier tropical forests towards increased functional, taxonomic and phylogenetic homogeneity. Nat. Commun. 2020, 11, 3346. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Konings, A.G.; Shaw, J. Divergent forest sensitivity to repeated extreme droughts. Nat. Clim. Change 2020, 10, 1091–1095. [Google Scholar] [CrossRef]
- Ackerly, D.D. Community assembly, niche conservatism, and adaptive evolution in changing environments. Int. J. Plant Sci. 2003, 164, S165–S184. [Google Scholar] [CrossRef]
- Li, C.; Barclay, H.; Roitberg, B.; Lalonde, R. Ecology and Prediction of Compensatory Growth: From Theory to Application in Forestry. Front. Plant Sci. 2021, 12, 655417. [Google Scholar] [CrossRef]
| Organism | Mechanisms | Specific Reaction | References |
|---|---|---|---|
| Individual | Morpho-physiological response mechanisms | Stomatal closure | [52] |
| Changing leaf structure and reducing shape | [53] | ||
| Development of longer and more dense root systems | [54] | ||
| Biochemical and molecular Adaptations | Compound changes in cell membrane composition | [49] | |
| Production of endogenous level of hormones | [55] | ||
| Calcium signaling induced drought resistance | [56] | ||
| External strategies to enhance drought resistance | Exogenous application of substances | [57] | |
| Seed bank | [58] | ||
| Seed dispersal | [59] | ||
| Prompt defoliation | [60] | ||
| Community | Plant interactions | Competitive effect | [61] |
| Species specificity | [62] | ||
| Passive promotion | [63] | ||
| The introduction of more drought-tolerant species | [64] | ||
| Community interactions | Animal–plant interactions | [65] | |
| Plant Microbe Interactions | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.-Y.; Di, D.-R.; Liao, X.-L.; Shi, W.-Y. Response of Forest Plant Diversity to Drought: A Review. Water 2023, 15, 3486. https://doi.org/10.3390/w15193486
Zhang T-Y, Di D-R, Liao X-L, Shi W-Y. Response of Forest Plant Diversity to Drought: A Review. Water. 2023; 15(19):3486. https://doi.org/10.3390/w15193486
Chicago/Turabian StyleZhang, Tian-Ye, Dong-Rui Di, Xing-Liang Liao, and Wei-Yu Shi. 2023. "Response of Forest Plant Diversity to Drought: A Review" Water 15, no. 19: 3486. https://doi.org/10.3390/w15193486
APA StyleZhang, T.-Y., Di, D.-R., Liao, X.-L., & Shi, W.-Y. (2023). Response of Forest Plant Diversity to Drought: A Review. Water, 15(19), 3486. https://doi.org/10.3390/w15193486







